Abstract
The use of natalizumab for highly active relapsing-remitting multiple sclerosis (MS) is influenced by the occurrence of progressive multifocal leukoencephalopathy (PML). Through measurement of the anti-JCV antibody index, and in combination with the presence or absence of other known risk factors, it may be possible to stratify patients with MS according to their risk of developing PML during treatment with natalizumab and detect early suspected PML using MRI including a diffusion-weighted imaging sequence. This paper describes a practical consensus guideline for treating neurologists, based on current evidence, for the introduction into routine clinical practice of anti-JCV antibody index testing of immunosuppressant-naïve patients with MS, either currently being treated with, or initiating, natalizumab, based on their anti-JCV antibody status. Recommendations for the frequency and type of MRI screening in patients with varying index-associated PML risks are also discussed. This consensus paper presents a simple and pragmatic algorithm to support the introduction of anti-JCV antibody index testing and MRI monitoring into standard PML safety protocols, in order to allow some JCV positive patients who wish to begin or continue natalizumab treatment to be managed with a more individualised analysis of their PML risk.
Keywords: MULTIPLE SCLEROSIS
Introduction
The use of natalizumab, for highly active relapsing-remitting MS, is influenced by the occurrence of progressive multifocal leukoencephalopathy (PML).1 The risk of developing PML requires the patient and the treating physician to carefully consider the benefits and risks of natalizumab. Although alternative licensed treatment options in this clinical scenario are available such as fingolimod2 3 and alemtuzumab,4 5 when given the choice some patients decide to start or remain on natalizumab and are willing to accept a certain level of risk of PML.
Clinical vigilance during treatment with natalizumab is essential to optimise physicians’ ability to identify PML in its early stages and to maximise the potential for favourable outcomes (see Berger et al6 for a discussion regarding the clinical features of PML). Additionally, a number of studies have reported that MRI detection of presymptomatic PML may be associated with improved outcomes, including potential reductions in morbidity and mortality, although as the number of patients identified in the asymptomatic stage remains small this needs further longitudinal confirmation.7–12
The development of PML during treatment with natalizumab is associated with the presence of anti-JCV antibodies, treatment duration, especially beyond 2 years, and immunosuppressant use before receiving natalizumab.13 Determination of anti-JCV antibody status and assessment of other risk factors has, in recent years, enabled a clearer understanding of the risk of PML occurring during therapy with natalizumab; which helps support decision-making regarding the risks of initiating, or continuing with, treatment.14 15 Pharmacovigilance in the UK and Ireland is well established. This paper uses data collected from worldwide clinical trial and postmarketing settings to support the notion that measuring the patient's anti-JCV antibody index, in combination with the presence or absence of the other known risk factors, might allow further stratification of these patients according to their risk of developing PML.16
A group of neurologists from the UK and Ireland were convened by Biogen at an advisory board with a view to examining the data and potential utility of the anti-JCV antibody index to help neurologists treat patients. Following this advisory board, the group independently convened and took the decision to ask Biogen to support the development of this practical consensus guideline for neurologists. The authors did not receive financial reimbursement for this work. The expert group recognised that current evidence was often not of high level, resulting in several areas of doubt and uncertainty. The group felt, however, that it would be of practical value to develop up-to-date guidelines for monitoring patients receiving natalizumab by stratifying PML risk based on anti-JCV antibody index. No data were available at the time of writing to support some of the guidance and, in such cases, recommendations were based on the clinical experience of the authors. In addition, the authors’ deliberations identified several areas that would benefit from further research.
Risks of PML associated with natalizumab treatment
PML is a subacute, evolving infectious disease of oligodendrocytes and astrocytes of the central nervous system (CNS).14 15 Typically, PML affects the juxtacortical and deep white matter of the frontal and parietal lobes, as well as the cortical and deep grey matter.17 PML is an opportunistic infection that is almost exclusively associated with immunosuppression. The condition occurs most commonly in HIV-infected individuals; but has also been reported in those with underlying malignancies, organ transplants, rheumatic diseases, and sarcoidosis; but not untreated immune-based diseases.15 More recently, PML has become associated with biological treatments that act via immune modulation, including natalizumab.15
It is estimated that almost 60% of the population of Europe have been infected with JCV.18 The virus is typically benign and resides in the gut, kidneys, bone marrow and lymphoreticular system.15 PML is caused by a mutating, neurotropic strain of JCV,14 but it is not entirely clear what drives this conversion within natalizumab-treated patients with MS. Adaptive genetic mutations allow for the development of a pathogenic JCV,14 15 which produces a lytic infection of oligodendrocytes, astrocytes and neuronal cells. It is thought that natalizumab may then facilitate the development of overt PML by affecting CNS immune surveillance at numerous levels.14
The presence of anti-JCV antibodies is a well-characterised factor for the development of PML among patients with MS treated with natalizumab. Early reports identified a prevalence of 100% for anti-JCV antibody-positivity among natalizumab-treated patients with MS who later developed PML.19 20 In postmarketing analyses, as of 3 June 2015, 138 800 patients worldwide had received natalizumab, with overall PML incidence of 3.96 per 1000 patients (Biogen MedInfo. https://medinfo.biogen.com accessed 1 July 2015). In patients without previous immunosuppressant use, PML incidence rose from 1/1000 after 1–24 months natalizumab exposure, to 5 and 6/1000 after 25–48 and 49–72 months. In patients with a history of immunosuppressant use, PML incidence was 2, 11 and 9/1000 after 1–24, 25–48 and 49–72 months, respectively.21 However, it should be noted that the absolute number of diagnosed PML cases globally may depend on the robustness of pharmacovigilance in each country. Additionally, while JCV antibody positivity is a risk factor, PML can potentially occur in JCV-antibody negative patients.
Of the 566 cases of PML reported to Biogen, 296 natalizumab-associated PML cases had serology samples tested for anti-JCV antibodies at least 6 months before a diagnosis of PML. Overall, 292 of these 296 samples (99.0%) tested positive for anti-JCV antibodies before PML diagnosis and four (1.0%) tested anti-JCV antibody-negative.
Of the latter four patients, two had a JCV negative status reported 8 and 9 months before the diagnosis or PML. The third patient had an anti-JCV antibody negative test result 5.7 months before PML diagnosis, and an anti-JCV antibody positive test result at the time of PML diagnosis. No additional pre-PML samples were available for testing (Biogen MedInfo (https://medinfo.biogen.com accessed 1 July 2015). The fourth was diagnosed with PML 2 weeks after last negative anti-JCV antibody testing. However, the patient tested positive for antibodies at diagnosis.22
Based on the available information at the time of European Medicines Agency (EMA) guideline issue and owing to the intrinsic variability of anti-JCV antibody status over time, the Committee for Medicinal Products for Human Use currently recommends six-monthly anti-JCV antibody testing to allow for earlier identification of patients who change their antibody status from negative to positive.23
Epidemiology of PML infection during natalizumab treatment
The currently known risk of developing PML during treatment with natalizumab is highest for patients with MS who have anti-JCV antibody-positive status, have received any prior immunosuppressant therapy of any duration at any time, and have been treated with natalizumab for ≥25 months. The estimated incidence of PML in this subgroup of patients is 11.1/1000 (or one in 90) patients.19
Patients who possess all three of these risk factors should be closely monitored for potential signs of PML onset during natalizumab treatment and for 6 months post-treatment.13–15 This time frame allows surveillance to capture PML cases potentially associated immunosuppression associated with natalizumab that persists beyond the 3-month wash out.24 25 The consensus group agreed that MRI scanning should be performed when patients discontinue natalizumab. The group suggested carefully examining the last on-natalizumab scan, followed by an additional ‘safety scan’ 3 months after the end of natalizumab treatment. The latter could act as a new baseline scan before starting the next treatment. Subsequent scanning should follow the clinician's practice or local practice for the new agent.
JCV positivity
Epidemiological analyses have uniformly shown that the prevalence of anti-JCV antibody-positivity is significantly associated with increasing MS patient age, male gender, and country of residence.18 26–28 In the JCV Epidemiology in MS (JEMS) trial—which included more than 7700 patients with MS from across 10 different countries—the prevalence of anti-JCV positivity across Europe ranged from 48.8% in the UK and 51.0% in Ireland, to 69.5% in Portugal.18 Cross-sectional data showing the relationship between rising anti-JCV antibody index and duration of natalizumab therapy shows no association.29 However, one of the co-authors of this consensus statement recently reported an increasing anti-JCV index over time in a UK cohort of natalizumab treated patients. This observation was greater than expected due to age alone and merits further evaluation in other populations.30 Vennegoor et al reported 4 of 193 patients treated with natalizumab who developed PML. All four patients with PML showed consistently high serum anti-JCV antibodies before the PML diagnosis. But, in contrast to some other studies,27 the anti-JCV antibody indices did not increase, possibly because of a ceiling in the assay.31
The risk of developing PML during natalizumab treatment is significantly lower among anti-JCV antibody-negative patients with MS compared with those who are seropositive. In STRATIFY-2, the estimated PML incidence was 0.09/1000 (or 1 in 11 111) patients among individuals who were anti-JCV antibody-negative when starting treatment, but who subsequently seroconverted prior to PML diagnosis.29 The presence of anti-JCV antibodies has been estimated to increase the risk of developing PML by more than 40-times, with an incidence of 3.9/1000 (or 1 in 263) patients (p<0.001) among those treated for at least 1 month with natalizumab.19
Duration of treatment with natalizumab
Elevated PML risk during natalizumab therapy is also associated with an increasing duration of natalizumab treatment. In JCV-positive patients, the greatest increase in PML risk appear after 24 months of natalizumab therapy: the risk of PML is 0.6/1000 (or 1 in 1667) patients from 1 to 24 months of natalizumab exposure and 5.2/1000 (or 1 in 192) patients from month 25 to 48 in immunosuppressant-naïve patients.16 Data beyond 4 years of therapy are limited in this cohort, although the risk of PML has been estimated to be 5.4/1000 (or 1 in 185) after 49–72 months of natalizumab exposure.16 A recent analysis reported that the risk of PML was 1.37 and 10.12/1000 anti-JCV antibody positive patients after 49–72 months of natalizumab exposure for patients with indices of ≤1.5 and >1.5, respectively, although relatively few patients received treatment for more than 49 months.32
Previous use of immunosuppressive therapies
Prior immunosuppressant therapies that were most commonly given to 68 patients who developed natalizumab-related PML included (some patients received more than one agent): mitoxantrone (38/68 (55.9%) confirmed PML cases), cyclophosphamide (14/68 (20.6%) confirmed PML cases), azathioprine (11/68 (16.2%) confirmed PML cases), methotrexate (9/68 (13.2%) confirmed PML cases), mycophenolate mofetil (6/68 (8.8%) confirmed PML cases), and other immunosuppressants (8/68 (11.8%) confirmed PML cases).19 More data are required regarding the mode of action of emerging disease-modifying therapies before their association with PML can be fully determined. However, the risk of PML has been reported to be 0.31/1000 (or 1 in 3226) patients for immunosuppressant-naïve versus 0.88/1000 (or 1 in 1136) patients with prior immunosuppressant therapy.19
Diagnosis of PML in asymptomatic stages may improve outcomes
The overall rate of survival among all patients diagnosed with natalizumab-associated PML is around 70–80%.15 33–35 Despite such observations, patients who survive PML often have serious morbidity, associated with substantial and permanent disability.33 34 In this regard, it has been reported that PML may be detected in the presymptomatic phase using routine surveillance MRI, and that early detection and treatment of suspected PML could lead to improved outcomes.7–11 35 36
An analysis of postmarketing surveillance data collected on 336 patients who developed natalizumab-associated PML reported that 76% survived during a mean follow-up from PML diagnosis of 16.1 months. The mean time from diagnosis to death was 4.7 months for non-survivors. Younger age at diagnosis, less functional disability before PML diagnosis, lower JC viral load (based on in situ hybridisation for JCV DNA) at diagnosis, and more localised brain involvement based on MRI at diagnosis seemed to predict improved survival. Patients rapidly stabilised following the initial phase of natalizumab-associated PML, and EDSS showed a high correlation with Karnofsky Performance Scale scores.35
Data suggest that patients who are asymptomatic at the time of diagnosis of PML, compared with those who are symptomatic, may have less functional disability and improved survival at 12 months. Mean Expanded Disability Status Scale (EDSS) scores were 4.1—that is, on average, fully independently ambulant—among patients asymptomatic at diagnosis and 5.4—not able to walk 200 m without aid or rest—in those who were symptomatic (p=0.038). Overall survival was 96.7% for asymptomatic and 75.4% for symptomatic patients, respectively, through a mean follow-up of 13.4 and 11.2 months.12
Early PML diagnosis is also critical in limiting the degree of permanent brain damage before immune reconstitution can be achieved.33 This further reinforces the need to put in place strategies to enable the earliest possible identification of potential cases of PML during treatment with natalizumab, alongside maintained clinical vigilance. However, the current data includes small numbers of patients and further data are needed to confirm the impact of early identification on clinical outcomes.
The anti-JCV antibody index and PML risk stratification
The anti-JCV antibody index is a function of a patient's anti-JCV antibody level. The presence or absence of anti-JCV antibodies in serum is qualitatively assessed using an ELISA method via the STRATIFY JCV DxSelect assay (Focus Diagnostics, Cypress, California, USA).37 The anti-JCV antibody index value is the optical density of an anti-JCV antibody-positive serum sample, normalised to an assay calibrator.16 This enables the specific anti-JCV antibody level to be quantified for individual patients.26–28 38 Laboratories in the UK and Ireland do not automatically report the anti-JCV antibody index, so the test must be requested by the ordering clinician.38 However, index data are available to clinicians across Europe via Unilabs.
The utility of the anti-JCV antibody index in defining PML risk among anti-JCV antibody-positive patients with MS has been investigated in a longitudinal study. The study included data from 2522 non-PML and 71 patients with PML included in natalizumab clinical studies—including AFFIRM, STRATIFY-1, and STRATIFY-2—and from postmarketing sources.16 Data from 51 patients with PML in this study indicates that the anti-JCV antibody index value was significantly higher in patients who developed PML at least 6 months prior to diagnosis, compared with non-PML patients (P<0.0001). This pattern was not seen in those who had previously used immunosuppressants (for the purposes of clarity, glatiramer acetate and β interferon are not classified as immunosuppressants). The anti-JCV antibody index value appears to predict the risk of subsequently developing PML during treatment with natalizumab, with high sensitivity but low specificity because many patients not developing PML also have high anti-JCV antibody levels (eg, 57.1% of non-PML patients have an index >1.5).16
Based on their anti-JCV antibody index and duration of natalizumab treatment, anti-JCV antibody-positive, immunosuppressant-naïve patients with MS may be stratified into one of two practical risk management groups: those with an index value ≤1.5 and those with an index >1.5 (see online supplementary data, table S1A). Patients with an anti-JCV antibody index ≤1.5 have a lower PML risk compared with the total population of anti-JCV antibody-positive patients. All those with an index value of ≤1.5 carry a 0.17/1000 (or 1 in 5882) or lower PML risk in months 1–24 of natalizumab therapy. In months 25–48 of therapy, an index value of ≤0.9 carries a risk of 0.51/1000 (or one in 1961), rising to a risk of 1.13/1000 (or 1 in 885) if index values increase to 1.5. During months 49–72, an index value of ≤0.9 carries a risk of 0.58/1000 (or 1 in 1724), rising to 1.37/1000 (or 1 in 730) if index values increase to 1.5.32
The PML risk among patients with an anti-JCV antibody index >1.5 is also low during months 1–24 of natalizumab therapy—with an estimated risk of 1.17/1000—but increases markedly to 8.83/1000 (or 1 in 113) in months 25–48 and rises to a level of 10.12/1000 (or 1 in 99) from months 49–72.32
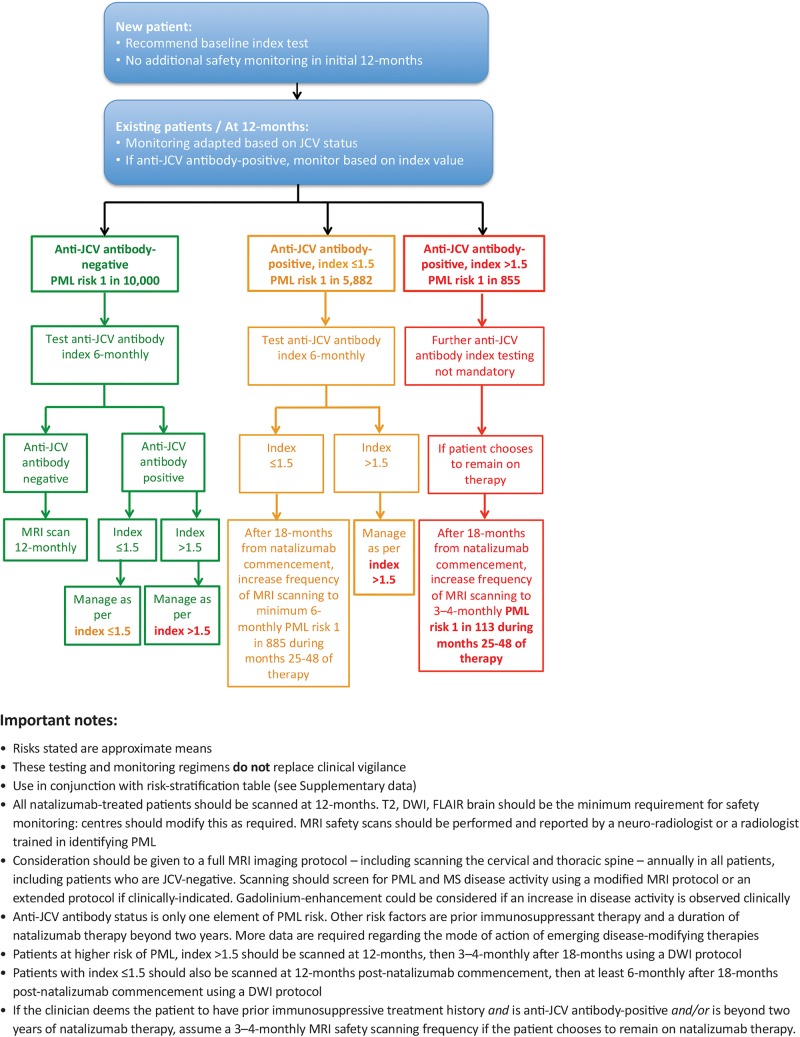
While index values and their relation to risk of PML exist on a spectrum and are not absolute, the consensus group concluded that the ≤1.5 and >1.5 index values provide a reasonable numerical cut-off for implementation of a practical protocol, based on the large increase in risk based on an index value of greater than 1.5 beyond 2 years of treatment. Based on critical assessment of the limited data available, combined with the opinion and experience of the author group, it is suggested that patients with an anti-JCV antibody index >1.5 are considered to require high-frequency MRI monitoring to mitigate their risk of developing PML during natalizumab therapy, and those with an anti-JCV antibody index ≤1.5 are considered to require moderate-frequency MRI monitoring, as outlined in the algorithm for MRI-based PML safety monitoring described in the following section (figure 1). When compared with the risk calculation without index (as shown in see online supplementary data, table S1B), application of anti-JCV antibody index data in the stratification of PML risk seems to improve the accuracy with which overall risk can be estimated. This protocol allows more patients who are anti-JCV antibody-positive to continue on natalizumab, albeit incurring a high-frequency MRI monitoring scheme in the 57.1% of anti-JCV antibody positive patients who have >1.5 index and a moderate-frequency MRI monitoring scheme in the remaining 42.9% of patients with ≤1.5 index.16
Figure 1.
Algorithm for MRI-based PML safety monitoring during natalizumab therapy, utilising anti-JCV antibody status and anti-JCV antibody index level. DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery; MS, multiple sclerosis; PML, progressive multifocal leukoencephalopathy.
Longitudinal data suggest that anti-JCV antibody index values may fluctuate over time,16 although an upper-limit to the assay may limit the ability to track changes at high indices.31 Over a period of 18 months, 87% of patients who tested anti-JCV antibody-negative at baseline remained negative during subsequent testing and 97% of patients who tested anti-JCV antibody-negative at baseline remained below an anti-JCV antibody index threshold of 1.5 (at 18 months). The remainder of patients (3%) seroconverted to an index of >1.5 during 18 months of follow-up.16
Among patients who were anti-JCV antibody-positive, 84% who had an index >1.5 maintained this level for at least 6 months prior to a diagnosis of PML and during subsequent tests. Longitudinal data were relatively similar for index thresholds of 0.9, 1.2 and 1.5.16 Thus, the consensus panel concluded that while there is no evidence that a rising level indicates an increased risk of PML, there are some data to support the practical protocol cut-off of 1.5. Serial measurement of JCV status and index in JCV-positive patients also seems sensible based on these observations.
By providing an additional level of sensitivity compared with previous estimates of PML risk, these data appear to be extremely useful in guiding treatment decisions in immunosuppressant-naïve patients with MS. As such, it is strongly recommended that serial anti-JCV antibody index testing is incorporated into standard safety monitoring protocols for patients with MS considering or undergoing treatment with natalizumab.
Consensus guideline proposal
Recommended guidelines for anti-JCV antibody index testing
The recommended algorithm for the management of immunosuppressant-naïve patients with MS based on anti-JCV antibody status and index value is shown in figure 1. Note that this algorithm should be used in conjunction with the risk stratification table (see online supplementary data, table S1A).
It is recommended that all new patients being assessed for natalizumab are tested for anti-JCV antibody index status before (or around the time of, in highly active disease) initiating treatment to aid treatment duration decisions. Index testing should be performed in the event of positive anti-JCV antibody status. Given that the initial year of therapy is associated with a low risk of developing PML (1 in 10 000 to 1 in 1000),16 patients—irrespective of their baseline anti-JCV antibody status—may not then require further anti-JCV antibody monitoring for up to 12 months after initiating natalizumab.
For existing natalizumab patients, or those who have been on treatment for 12 months or longer, the consensus panel recommends that monitoring should be adapted based on anti-JCV antibody status with the aim of guiding subsequent patient management. In the absence of real-world data, it is suggested that extending the use of index into the current six-monthly anti-JCV antibody testing schedule is a pragmatic approach for anti-JCV antibody-negative patients or antibody-positive patients found to have anti-JCV antibody index levels ≤1.5. For those with an anti-JCV antibody index >1.5, further index testing is not mandatory.
The stability of the anti-JCV antibody test over time and the prognostic significance of changes in the index are not known. Therefore, the frequency of the testing may need adaptation as evidence emerges. The consensus group suggests reviewing this recommendation as more data emerges. As there are insufficient data at the time of writing to predict PML risk based on changing antibody level, the consensus group recommends that the cut-offs described here are used and ongoing risk calculation should be based on the highest level obtained. As shown in figure 1, if at any time anti-JCV antibody-negative patients seroconvert to positive status, the consensus panel recommends reverting to the testing schedule recommended in this algorithm for their index level. Likewise, if anti-JCV antibody-positive patients with an index value ≤1.5 switch to an index value >1.5 during natalizumab therapy, that is, if they move from a lower to a higher PML risk, they should be managed as per the algorithm (ie, further index testing is not mandatory). The consensus group recommends that patients with an index value >1.5 should be managed as per this high-positive value irrespective of whether they subsequently convert to a lower index value, in the absence of clinical data supporting index testing beyond this marker.38 There are currently insufficient data to determine the prevalence and clinical significance of patients who may move from high-index to low-index levels.38
Given the clinical impact of natalizumab, a positive test for anti-JCV antibodies is not a contraindication for natalizumab treatment; neither is an anti-JCV antibody index >1.5 a contraindication to continue natalizumab treatment. Indeed, many patients who do not develop PML are likely to have high levels, although such patients should have intensive MRI monitoring (as described in the next section). The risks and benefits of treatment should be re-evaluated at appropriate intervals, which will be determined by the patient's disease course and wishes.
The consensus algorithm outlined here emphasises the determination of a patient's JCV status—either anti-JCV antibody-positive or negative. The STRATIFY JCV DxSelect ELISA has been reported to have a false-negative rate of 2.2%.37 However, some studies suggest that the false-negative rate may be higher.39 40
The false-negative rate reinforces the necessity for continued clinical vigilance alongside scheduled paraclinical monitoring. Furthermore, sensitive methods for the detection of JCV DNA, such as PCR-based analysis of CSF, may be utilised alongside serological testing, particularly for natalizumab-treated patients with an increased risk of PML development.
Recommendations for the frequency and type of MRI screening in patients with varying index-associated PML risks
Data are emerging that suggest MRI evidence of PML may be evident up to 6 months before the onset of symptoms.7 12 The majority of current data, however, support the brain MRI-based detection of PML at 3–4 months prior to symptoms.8 10 17 36 41 42 Such lesions generally occur in brain areas not previously affected by MS and, depending on the location of lesions, patients may remain asymptomatic for several months.7 33 36 Emerging data have characterised the lesion pattern in early scans from symptomatic patients with PML (see below),43 44 although the characteristics of asymptomatic lesions are poorly characterised because of the small numbers of patients. A study of 18 patients with natalizumab-associated PML lesions reported frontal and parietal lobe involvement in 77.8% and 22.2% of patients, respectively. Most patients presented with focal lesions (72.2%), usually in one lobe (66.7%), with cortical grey matter (83.3%) and cortical grey and white matter involvement (72.2%). Forty per cent did not show high-signal-intensity lesions.45
It is not yet known whether all patients developing PML pass through a presymptomatic phase with MRI abnormalities. de Toffol et al46 reported the case of a patient with AIDS-related PML who had a normal MRI. In addition, PML associated with natalizumab has occurred with JC granule cell neuronopathy of the cerebellum,47 although based on present evidence this presentation appears to be rare. In the latter case, the MRI would not necessarily show any classic PML lesions.48 49
Fluid-attenuated inversion recovery (FLAIR) has been shown to be more sensitive than T2-weighted MRI for the detection of PML-associated lesions. Diffusion-weighted imaging (DWI) may be useful for detection of PML within confluent MS lesions.1 Gadolinium-enhancement has been reported in up to 43% of MRI scans at diagnosis of natalizumab-associated PML, typically evident in the vicinity of T2-hyperintense lesions, and may be present in the early phases (ie, when T2 hyperintensity is already present) or later in the disease course.1 14 33 50 In general, contrast enhancement is variable and is not diagnostic of PML.1 14
A recent report has additionally suggested that frontal, parietal and thalamic punctuate gadolinium-enhancing lesions may be apparent prior to T2 hyperintensity. These lesions were transient, disappearing after 6 weeks, and preceded the onset of PML symptoms and new T2 hyperintense lesions by around 2 months.50 Gadolinium-enhancement is not recommended for routine MRI safety monitoring of patients receiving natalizumab; although it is useful for disease activity scans.
Evidence from 40 natalizumab-treated patients with MS diagnosed with PML suggests that a PML lesion may be defined by the following MRI criteria: subcortical location; a T2/FLAIR hyperintense, T1 hypointense, and DW hyperintense signal; a border well-defined within the cortical grey matter and ill-defined in the white matter; with punctuate T2-hyperintense lesions in the immediate vicinity of the main lesion.43 Better outcomes have been reported when early PML lesions are localised to a single lobe of the brain and affect the frontal lobes.7 17
The findings described above suggest that it may be possible to identify presymptomatic PML by performing more frequent MRI scans, especially in higher PML risk patients. It is important to bear in mind that no new activity on an MRI does not exclude PML. If clinical suspicion of PML remains, the consensus panel recommends that the physician should continue to serially assess the patient clinically and perform CSF analysis and MRI until satisfactory evidence is obtained. Hence, ongoing clinical vigilance remains paramount and should not be relegated or replaced by the testing and monitoring regimens suggested in the algorithm.
The consensus reached regarding the frequency of MRI scanning within the PML monitoring algorithm is based on the expert opinion of authors, in the absence of peer-reviewed evidence of the positive and negative predictive values of MRI scanning at various time points prior to PML diagnosis (figure 1). It is recommended that all new patients are scanned prior to initiating natalizumab and at least annually on treatment. This provides a recent reference MRI, which may be used to distinguish new lesions in patients that develop new or worsening neurological symptoms or signs once on therapy. Any new lesions on subsequent scans that were not evident on the previous most recent scan should trigger clinical consideration as to whether further investigation is necessary (ie, CSF analysis).1
For those already being treated with natalizumab, or after 12 months of treatment, the frequency of MRI monitoring should be adapted based on JCV status and—for anti-JCV antibody-positive patients—their index levels. MRI should, the consensus panel recommends, be performed in the event of unexpected clinical worsening or a suspicious clinical course.
Anti-JCV antibody-positive patients who choose to remain on natalizumab therapy should have annual MRI scans, until they have been on treatment for 18 months. After this time, the frequency of MRI is recommended to be increased to a minimum 6-monthly for index ≤1.5 and 3–4 monthly for index >1.5. Based on the available data among natalizumab-related PML cases, the risk of PML increases after 24 months of natalizumab therapy. However, in the opinion of the consensus group, in order to detect early asymptomatic evolving PML lesions on MRI, the frequency of scanning should increase after 18 months of natalizumab therapy.
A limited cerebral MRI is recommended for safety monitoring for those requiring at least 6-monthly, or 3 to 4-monthly MRI scans using a minimum of an axial FLAIR and DWI, which could be extended if a suspicious finding is discovered.38 43 These scans could be performed alongside annual MRI scans to assess efficacy, with or without gadolinium, based on local protocols. Imaging should be performed and reviewed in all cases by a neuroradiologist or a radiologist trained in identifying PML.
Diagnosis and initial management of suspected PML
The differential diagnosis of new lesions is key in the early identification of PML. Any new lesions beyond 18 months of therapy, or in the appropriate clinical context, should be treated with suspicion. Any MRI-associated changes suspected of being caused by PML that occur in immunocompromised patients should be classified as being ‘radiologically-suspected PML’.36 If PML is suspected, further dosing of natalizumab should be delayed and the patient and lesion(s) carefully monitored until PML has been, as far as clinically possible, excluded.13 A negative CSF does not exclude PML, and each clinician needs to determine the optimal management based on each patient's clinical characteristics. However, because of a lack of evidence the consensus group was unable to make definitive recommendations on the exact timings or number of repeat CSF tests where clinical suspicion of PML persists, or regarding alternative methods such as CSF index testing and local practices should be followed until further information emerges on this issue.51
The differential diagnosis of MS progression and PML may be further assisted based on specific MRI-associated features (table 1).1 17 43 Clinicians should evaluate the patient to determine whether symptoms indicative of neurological dysfunction are present and, if so, whether these symptoms are typical of MS and, therefore, indicative of treatment failure, or possibly suggestive of PML.13
Table 1.
Clinical and MRI features that may be considered in the differential diagnosis of MS and PML
| Feature | Multiple sclerosis | Progressive multifocal leukoencephalopathy |
|---|---|---|
| Clinical features | ||
| Onset | Acute | Subacute |
| Evolution |
|
|
| Clinical presentation |
|
|
| MRI features | ||
| Aspect and location of new lesions | Focal, generally periventricular in location. Lesions occur in all areas of the brain particularly the corpus callosum and spinal cord | Diffuse. Generally large >3 cm lesions in a unifocal, multifocal or widespread distribution. Subcortical location rather than periventricular. Affecting U fibres and extending into the gyrus. Cortical GM involvement in 50% of cases. Posterior fossa less frequent site. Spinal cord presentation rare |
| Borders | Sharp edges; mostly round or flame shaped (especially periventricular lesions), confluent with other lesions; U-fibres may be involved | Irregular in shape. Ill-defined border toward the white matter, sharp border toward the cortical grey matter |
| Mode of extension | Initially focal, lesions enlarge within days or weeks and later decrease in size within months | Lesion volume increases continuously, and sometimes rapidly to contiguous (multifocal) and non-contiguous regions (widespread) confined to white matter tracts, sparing the cortex |
| Mass effect | Large acute lesions may have mass effect | No mass effect even in large lesions apart from when inflammatory response is present |
| On T2-weighted sequence | Homogeneous hyperintensity | Diffuse hyperintensity, little irregular signal intensity within the lesions, can have a punctate microcystic appearance. Small punctate T2 lesions may be seen in proximity to the lesion |
| On T1-weighted sequence | Acute lesions: hypointense (due to oedema) or isointense. Increasing signal intensity over time in 80%; decreasing signal intensity (axonal loss) in about 20% | Slightly hypointense at onset, with signal intensity decreasing over time in the affected area; no reversion to isointense signal intensity |
| On FLAIR sequence | Hyperintense, sharply delineated | FLAIR is the preferred sequence for PML diagnosis, because of the subcortical location |
| Contrast enhancement | Acute lesions: homogeneous nodular or ring enhancement, with sharp edges eventual resolution over 1–2 months. Chronic lesions: no enhancement | Less than half of the cases to date have shown some enhancement at the time of presentation often with a patchy or punctate appearance. Rim enhancement at leading edge can be seen in larger lesions |
| Diffusion-weighted imaging | Acute lesions hyperintense. Chronic lesions isointense. Conforms to shape of lesions on FLAIR and T2W | Acute PML lesions are hyperintense but not specific for PML. Helpful to detect new PML lesions within confluent areas of chronic WM disease. ADC maps not helpful |
| Atrophy | Focal atrophy possible, due to focal white-matter degeneration; no progression | No focal atrophy initially, but can be seen in late stages of PML progression |
It should be noted that none of the MRI features are pathognomonic of MS or PML. Adapted from Physician information and management guidelines for multiple sclerosis patients on Tysabri therapy, Biogen, V.14, 22 May 2015 (permission obtained).
ADC, Apparent diffusion coefficient; FLAIR, fluid-attenuated inversion recovery; GM, grey matter; MS, multiple sclerosis; PML, progressive multifocal leukoencephalopathy; T2W, T2 weighted; WM, white matter.
Current criteria for definitive diagnosis of PML, from which the PML risk figures referred to in this paper are derived, are: (A) based on clinical presentation, the presence of JCV DNA in the CSF, and MRI findings suggestive of PML; or (B) via the detection of PML on histopathology of biopsy material in combination with the presence of JCV by electron microscopy and/or immunohistochemistry, or JCV and/or JCV DNA via (quantitative) PCR (qPCR).17 6
There is emerging opinion that a tiered approach to PML case classification based on the degree of diagnostic certainty may be more appropriate than the current diagnostic criteria. This includes the proposal that in asymptomatic patients, a ‘probable PML’ diagnosis can be made (which should be managed as PML), based on pathological MRI findings consistent with PML, and, JCV-positive CSF.6 12 52 53 However, it is important to emphasise that if radiological results are suggestive of PML, a negative CSF JCV result cannot rule out the potential for later diagnosis.6 52 53 In the early stages of the natural history of PML, JCV may not be evident in the cerebrospinal fluid (CSF) because viral loads are below the sensitivity of the virus assay. In one study of 28 patients with natalizumab-associated PML, 16 initially had undetectable JCV DNA as the CSF viral load was below the threshold of 500 copies/mL.33
Furthermore, even small lesions associated with JCV-negative CSF can be symptomatic.54 Thus, if a negative result is obtained but clinical suspicion of PML remains high based on MRI findings and/or symptomatic presentation, the consensus panel recommends that CSF testing should be repeated over several months if necessary, or brain biopsy performed.36 A reference laboratory that can detect a low CSF copy number (ie, 50 copies/mL) should be utilised.33
Early detection of PML, followed by rapid cessation of natalizumab therapy and plasma exchange to restore immune function, appears to be associated with a good prognosis,7–11 36 although definitive evidence is needed. Immune reconstitution inflammatory syndrome (IRIS) occurs in almost all natalizumab-associated patients with PML within days or several weeks after withdrawal or removal of natalizumab by plasma exchange.13 55 While there is no conclusive evidence that an early immune reconstitution produces better outcomes compared to natalizumab washout, plasmapheresis with immunoadsorption could be considered, which would theoretically remove drug more rapidly. It is noteworthy that in an attempt to mitigate the risk of IRIS, some centres are not currently using plasma exchange in favour of allowing natural wash-out of natalizumab.
Once the clinician has excluded PML (if necessary, by repeating clinical, imaging and/or laboratory investigations if clinical suspicion remains), dosing of natalizumab may resume.13
Conclusions
The overall aim of this consensus paper was to present a simple and pragmatic algorithm to support the introduction of anti-JCV antibody index testing into standard PML safety protocols, in order to allow those JCV positive patients who wish to continue treatment to be managed with a more individualised analysis of their risk. Fundamental to these revised protocols is enhanced MRI monitoring, which should be guided by anti-JCV antibody index levels. This consensus has been generated by clinicians to facilitate the evolution of the management of patients with MS. While not a scientific paper, the authors have conducted rigorous analysis of the limited data available, and have had considerable discussion regarding the application of index in the context of current practice. No data were available at the time of writing to support some of the guidance and, in such cases, recommendations were based instead on the clinical experience of the authors. There is a need for more data around the positive and negative predictive value of MRI in the detection of presymptomatic PML, to better inform safety protocols. At the time such data were available, this guideline may need to be updated. Additionally, this recommendation is intended to outline best practice and does not take into account local variations in MRI resource provision.
The introduction of the anti-JCV antibody index into standard clinical practice should provide patients with MS and clinicians with more tangible evidence on which to base the decision whether or not to continue natalizumab treatment. For instance, further studies should assess the stability of the index value over time using serial data and ascertain the specificity and selectivity of other emerging and potential biomarkers.
The greater understanding of individual patient risk provided by the new test may help balance the concern of PML with the benefits that natalizumab offers to patients: particularly those who are anti-JCV antibody-positive with a low index value (≤1.5) in whom, before the application of stratification by index, the decision whether or not to continue natalizumab treatment would be difficult. At the same time, the improved understanding of PML risk among anti-JCV antibody-positive patients with a high index value (>1.5) may assist decisions to discontinue therapy, based on the views of unacceptable levels of risk. The stability of the anti-JCV antibody test over time and the prognostic significance of a rising index are not known. Therefore, the consensus group suggests reviewing this recommendation as new data emerges.
PML is a severe and well-documented complication of natalizumab treatment. There is evidence to show PML treatment outcomes are better if it can be detected in a presymptomatic state, through clinical vigilance, and appropriate MRI monitoring protocols applied according to JCV risk and stratified using the index test result.
The aim of these guidelines is to support clinicians in counselling patients on the risk benefit profile of starting natalizumab and once on treatment, early detection of PML before it can cause significant disability or death, although further data are needed to fully characterise the impact of early identification on clinical outcomes. Such early or presymptomatic diagnosis requires improved identification of at-risk patients, sustained clinical vigilance, and evidence-led MRI monitoring protocols, such as those proposed by the algorithm presented here.
Supplementary Material
Acknowledgments
The authors thank Chris Incles, of CMI Medical Writing Limited, Cambridge, UK, and Mark Greener of Mark Greener Communications, who provided medical writing services. Tracy Willmott, TW1 Healthcare Consulting Limited, London, UK, provided editorial assistance and project management support. Medivents Limited, Letchworth, UK, provided logistical support for the organisation of an author-publication development meeting and teleconference(s).
Footnotes
Contributors: All authors have met all four authorship criteria listed in the ICJME Recommendations 2013. A group of neurologists from the UK and Ireland were convened by Biogen at an advisory board with a view to examining the data and potential utility of the anti-JCV antibody index to help neurologists treat patients. Following this advisory board, the group independently convened and took the decision to ask Biogen to support the development of this practical consensus guideline for neurologists. CMcG is the primary author and guarantor.
Funding: Funding for medical writing support, project management coordination, and logistical support—including the provision of teleconference services, meeting venue, and transport costs—was supplied by Biogen. No authorship fees were paid. Biogen reviewed and provided feedback on the paper to the authors. The authors had full editorial control of the paper, and provided their final approval of all content.
Competing interests: CMcG received honoraria, participated in advisory boards and/or received research funding from Biogen, Merck Serono, Novartis, Genzyme and Bayer. MC was involved in research support administered via institutional fund, fees for speaking, advisory boards, or funding to attend scientific meetings in past 5 years received from Biogen, Teva, Genzyme, and Novartis. JG and ORP gave honoraria and support to attend scientific meetings, speakers’ fees, and advisory boards from Biogen, Genzyme, Novartis, Teva, and Merck Serono. RK gave honoraria and support to attend scientific meetings from Biogen, Genzyme, Novartis, Teva, and Merck Serono. GM served as an advisor for Biogen. MS Department received research and service development grants from Biogen, Novartis, Merck Serono, Teva, and Bayer Schering. PM gave fees for speaking, advisory boards or funding to attend scientific meetings in past 5 years received from Biogen, Novartis, Teva, Merck Serono and Roche. RN was involved in the research support administered via institutional fund, fees for speaking, advisory boards, or funding to attend scientific meetings in past 5 years received from Bayer, Biogen, Genzyme, Merck Serono, Novartis, Roche, and Teva. JP partly funded by highly specialised services to run a National congenital myasthenia service and a neuromyelitis service. Received support for scientific meetings and honorariums for advisory work from Merck Serono, Biogen, Novartis, Teva, Chugai Pharma, Bayer Schering, and Alexion; and unrestricted grants from Merck Serono, Novartis, Biogen, and Bayer Schering. Hospital trust receives funds for her role as clinical lead for the RSS and she has received grants from the MS society and Guthie Jackson Foundation for unrelated research studies. Board member for the charitable European MS foundation ‘The Charcot Foundation’ and on the steering committee for a European collaborative MS imaging group ‘MAGNIMS’. DR was involved in the research support administered via institutional fund, fees for speaking, advisory boards, or funding to attend scientific meetings in past 5 years received from Biogen, Teva, Genzyme, Mitsubishi, Sanofi, Novartis and Roche. CAY was involved in the research support administered via institutional fund, fees for speaking, advisory boards, or funding to attend scientific meetings in last 5 years received from Biogen, Genzyme, Novartis, Roche, and Teva.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kappos L, Bates D, Edan G, et al. Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring. Lancet Neurol 2011;10:745–58. 10.1016/S1474-4422(11)70149-1 [DOI] [PubMed] [Google Scholar]
- 2.Kappos L, Radue EW, O'Connor P, et al. , FREEDOMS Study Group. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med 2010;362:387–401. 10.1056/NEJMoa0909494 [DOI] [PubMed] [Google Scholar]
- 3.Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med 2010;362:402–15. 10.1056/NEJMoa0907839 [DOI] [PubMed] [Google Scholar]
- 4.Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 2012;380:1819–28. 10.1016/S0140-6736(12)61769-3 [DOI] [PubMed] [Google Scholar]
- 5.Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 2012;380:1829–39. 10.1016/S0140-6736(12)61768-1 [DOI] [PubMed] [Google Scholar]
- 6.Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: consensus statement from the AAN neuroinfectious disease section. Neurology 2013;80:1430–8. 10.1212/WNL.0b013e31828c2fa1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blair NF, Brew BJ, Halpern JP. Natalizumab-associated PML identified in the presymptomatic phase using MRI surveillance. Neurology 2012;78:507–8. 10.1212/WNL.0b013e318246d6d8 [DOI] [PubMed] [Google Scholar]
- 8.Lindå H, von Heijne A. Presymptomatic diagnosis with MRI and adequate treatment ameliorate the outcome after natalizumab-associated progressive multifocal leukoencephalopathy. Front Neurol 2013;4:11 10.3389/fneur.2013.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mc Govern E, Hennessy M. Asymptomatic progressive multifocal leukoencephalopathy associated with natalizumab. J Neurol 2013;260:665–7. 10.1007/s00415-012-6759-0 [DOI] [PubMed] [Google Scholar]
- 10.Phan-Ba R, Belachew S, Outteryck O, et al. The earlier, the smaller, the better for natalizumab-associated PML: In MRI vigilance veritas? Neurology 2012;79:1067–9. 10.1212/WNL.0b013e31826846b4 [DOI] [PubMed] [Google Scholar]
- 11.Phan-Ba R, Lommers E, Tshibanda L, et al. MRI preclinical detection and asymptomatic course of a progressive multifocal leucoencephalopathy (PML) under natalizumab therapy. J Neurol Neurosurg Psychiatry 2012;83:224–6. 10.1136/jnnp-2011-300511 [DOI] [PubMed] [Google Scholar]
- 12.Dong-Si T, Richman S, Wattjes MP, et al. Outcome and survival of asymptomatic PML in natalizumab-treated MS patients. Ann Clin Transl Neurol 2014;1:755–64. 10.1002/acn3.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biogen. Tysabri 300 mg concentrate for solution for infusion. Summary of medical product characteristics. http://www.medicines.org.uk/EMC/medicine/18447/SPC/TYSABRI+300+mg+concentrate+for+solution+for+infusion/ (accessed 24 Jun 2015).
- 14.Hunt D, Giovannoni G. Natalizumab-associated progressive multifocal leucoencephalopathy: a practical approach to risk profiling and monitoring. Pract Neurol 2012;12:25–35. 10.1136/practneurol-2011-000092 [DOI] [PubMed] [Google Scholar]
- 15.Sørensen PS, Bertolotto A, Edan G, et al. Risk stratification for progressive multifocal leukoencephalopathy in patients treated with natalizumab. Mult Scler 2012;18:143–52. 10.1177/1352458511435105 [DOI] [PubMed] [Google Scholar]
- 16.Plavina T, Subramanyam M, Bloomgren G, et al. Anti–JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol 2014;76:802–12. 10.1002/ana.24286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wattjes MP, Richert ND, Killestein J, et al. The chameleon of neuroinflammation: magnetic resonance imaging characteristics of natalizumab-associated progressive multifocal leukoencephalopathy. Mult Scler 2013;19:1826–40. 10.1177/1352458513510224 [DOI] [PubMed] [Google Scholar]
- 18.Bozic C, Subramanyam M, Richman S, et al. Anti-JC virus (JCV) antibody prevalence in the JCV Epidemiology in MS (JEMS) trial. Eur J Neurol 2014;21:299–304. 10.1111/ene.12304 [DOI] [PubMed] [Google Scholar]
- 19.Bloomgren G, Richman S, Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 2012;366:1870–80. 10.1056/NEJMoa1107829 [DOI] [PubMed] [Google Scholar]
- 20.Gorelik L, Lerner M, Bixler S, et al. Anti-JC virus antibodies: Implications for PML risk stratification. Ann Neurol 2010;68:295–303. 10.1002/ana.22128 [DOI] [PubMed] [Google Scholar]
- 21. Biogen. Physician Information and Management Guidelines for Multiple Sclerosis Patients on TYSABRI Therapy. Version 14. Maidenhead: Biogen 2015.
- 22.Brosseau M-S, Stobbe G, Cramer D, et al. Natalizumab-Related PML Is Possible Even With Recent Negative JCV Antibody Testing. Poster presented at the 67th American Academy of Neurology Annual Meeting April 18–25, Washington: DC, USA, 2015. http://www.abstracts2view.com/aan/view.php?nu=AAN15L1_P4.030 [Google Scholar]
- 23.EMA. (European Medicines Agency). Tysabri: Procedural steps taken and scientific information after authorisation 2014. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Procedural_steps_taken_and_scientific_information_after_authorisation/human/000603/WC500044692.pdf (accessed 24 Jun 2015).
- 24.Fine AJ, Sorbello A, Kortepeter C, et al. Progressive multifocal leukoencephalopathy after natalizumab discontinuation. Ann Neurol 2014;75:108–15. 10.1002/ana.24051 [DOI] [PubMed] [Google Scholar]
- 25.Gheuens S, Smith DR, Wang X, et al. Simultaneous PML-IRIS after discontinuation of natalizumab in a patient with MS. Neurology 2012;78:1390–3. 10.1212/WNL.0b013e318253d61e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Outteryck O, Zéphir H, Salleron J, et al. JC-virus seroconversion in multiple sclerosis patients receiving natalizumab. Mult Scler 2014;20:822–9. 10.1177/1352458513505353 [DOI] [PubMed] [Google Scholar]
- 27.Warnke C, Ramanujam R, Plavina T, et al. Changes to anti-JCV antibody levels in a Swedish national MS cohort. J Neurol Neurosurg Psychiatry 2013;84:1199–205. 10.1136/jnnp-2012-304332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trampe AK, Hemmelmann C, Stroet A, et al. Anti-JC virus antibodies in a large German natalizumab-treated multiple sclerosis cohort. Neurology 2012;78:1736–42. 10.1212/WNL.0b013e3182583022 [DOI] [PubMed] [Google Scholar]
- 29.Bozic C, Richman S, Plavina T, et al. Anti-JCV antibody status in MS patients: baseline results of STRATIFY-2. Poster presented at the 26th Annual Meeting of the Consortium of Multiple Sclerosis Centers, Orlando, FL, USA; May 30–June 2, 2012. Poster No. DX71 http://cmsc.omnibooksonline.com/2012/index.html (accessed 24 Jun 2015). [Google Scholar]
- 30.Raffel J, Gafson A, Malik O, et al. Anti-JC virus antibody titres increase over time with natalizumab treatment. Mult Scler 2015. (Accepted for publication). doi:10.1177/1352458515599681. [DOI] [PubMed] [Google Scholar]
- 31.Vennegoor A, van Rossum JA, Polman CH, et al. Longitudinal JCV serology in multiple sclerosis patients preceding natalizumab-associated progressive multifocal leukoencephalopathy. Mult Scler 2015;21:1600–3.. [DOI] [PubMed] [Google Scholar]
- 32.Kuesters G, Plavina T, Lee S, et al. Anti–JC virus (JCV) Antibody Index Differentiates Risk of Progressive Multifocal Leukoencephalopathy in Natalizumab-treated Multiple Sclerosis Patients with no Prior Immunosuppressant Use: an Updated Analysis Poster presented at the 67th American Academy of Neurology Annual Meeting April 18–25, Washington: DC, USA: http://www.abstracts2view.com/aan/view.php?nu=AAN15L1_P4.031 [Google Scholar]
- 33.Clifford DB, DeLuca A, Simpson DM, et al. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol 2010;9:438–46. 10.1016/S1474-4422(10)70028-4 [DOI] [PubMed] [Google Scholar]
- 34.Dahlhaus S, Hoepner R, Chan A, et al. Disease course and outcome of 15 monocentrically treated natalizumab-associated progressive multifocal leukoencephalopathy patients. J Neurol Neurosurg Psychiatry 2013;84:1068–74. 10.1136/jnnp-2013-304897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong-Si T, Gheuens S, Gangadharan A, et al. Predictors of survival and functional outcomes in natalizumab-associated progressive multifocal leukoencephalopathy. J Neurovirol 2015. (Accepted for publication) doi: 10. 1007/ s13365-015-0316-4. 10.1007/s13365-015-0316-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayzenberg I, Lukas C, Trampe N, et al. Value of MRI as a surrogate marker for PML in natalizumab long-term therapy. J Neurol 2012;259:1732–3. 10.1007/s00415-012-6426-5 [DOI] [PubMed] [Google Scholar]
- 37.Focus. Diagnostics. STRATIFY JCV DxSelectTM: Enzyme-linked immunosorbent assay (ELISA) for the qualitative detection of human antibodies to JC virus. CA, USA: Focus Diagnostics, Cypress, 2013. http://www.focusdx.com/pdfs/pi/US/EL1950.pdf (accessed 24 Jun 2015). [Google Scholar]
- 38.Nicholas JA, Racke MK, Imitola J, et al. First-line natalizumab in multiple sclerosis: rationale, patient selection, benefits and risks. Ther Adv Chronic Dis 2014;5:62–8. 10.1177/2040622313514790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berger JR, Houff SA, Gurwell J, et al. JC virus antibody status underestimates infection rates. Ann Neurol 2013;74:84–90. 10.1002/ana.23893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frohman EM, Monaco M, Remington G, et al. JC virus in cd34+ and cd19+ cells in patients with multiple sclerosis treated with natalizumab. JAMA Neurol 2014;71:596–602. 10.1001/jamaneurol.2014.63 [DOI] [PubMed] [Google Scholar]
- 41.Lindå H, von Heijne A, Major EO, et al. Progressive multifocal leukoencephalopathy after natalizumab monotherapy. N Engl J Med 2009;361:1081–7. 10.1056/NEJMoa0810316 [DOI] [PubMed] [Google Scholar]
- 42.Vennegoor A, Wattjes MP, van Munster ET, et al. Indolent course of progressive multifocal leukoencephalopathy during natalizumab treatment in MS. Neurology 2011;76:574–6. 10.1212/WNL.0b013e31820b7644 [DOI] [PubMed] [Google Scholar]
- 43.Yousry TA, Pelletier D, Cadavid D, et al. Magnetic resonance imaging pattern in natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol 2012;72:779–87. 10.1002/ana.23676 [DOI] [PubMed] [Google Scholar]
- 44.Richert N, Bloomgren G, Cadavid D, et al. Imaging findings for PML in natalizumab-treated MS patients. Mult Scler 2012;18(suppl 4):27–8. Oral 99. [Google Scholar]
- 45.Wattjes MP, Vennegoor A, Steenwijk MD, et al. MRI pattern in asymptomatic natalizumab-associated PML. J Neurol Neurosurg Psychiatry 2015;86:793–8. 10.1136/jnnp-2014-308630 [DOI] [PubMed] [Google Scholar]
- 46.de Toffol B, Vidailhet M, Gray F, et al. Isolated motor control dysfunction related to progressive multifocal leukoencephalopathy during AIDS with normal MRI. Neurology 1994;44:2352 10.1212/WNL.44.12.2352 [DOI] [PubMed] [Google Scholar]
- 47.Agnihotri SP, Dang X, Carter JL, et al. JCV GCN in a natalizumab-treated MS patient is associated with mutations of the VP1 capsid gene. Neurology 2014;83:727–32. 10.1212/WNL.0000000000000713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schippling S, Kempf C, Büchele F, et al. JC virus granule cell neuronopathy and GCN–IRIS under natalizumab treatment. Ann Neurol 2013;74:622–6. 10.1002/ana.23973 [DOI] [PubMed] [Google Scholar]
- 49.Wijburg M, van Oosten B, Murk JL, et al. Heterogeneous imaging characteristics of JC virus granule cell neuronopathy (GCN): a case series and review of the literature. J Neurol 2015;262:65–73. 10.1007/s00415-014-7530-5 [DOI] [PubMed] [Google Scholar]
- 50.Taieb G, Renard D, Thouvenot E, et al. Transient punctuate enhancing lesions preceding natalizumab-associated progressive multifocal leukoencephalopathy. J Neurol Sci 2014;346:364–5. 10.1016/j.jns.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 51.Warnke C, von Geldern G, Markwerth P, et al. Cerebrospinal fluid JC virus antibody index for diagnosis of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol 2014;76:792–801. 10.1002/ana.24153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wattjes M, Vennegoor A, Mostert J, et al. Diagnosis of asymptomatic natalizumab-associated PML: are we between a rock and a hard place? J Neurol 2014;261:1139–43. 10.1007/s00415-014-7336-5 [DOI] [PubMed] [Google Scholar]
- 53.Mentzer D, Prestel J, Adams O, et al. Case definition for progressive multifocal leukoencephalopathy following treatment with monoclonal antibodies. J Neurol Neurosurg Psychiatry 2012;83:927–33. 10.1136/jnnp-2012-302478 [DOI] [PubMed] [Google Scholar]
- 54.Kuhle J, Gosert R, Bühler R, et al. Management and outcome of CSF-JC virus PCR-negative PML in a natalizumab-treated patient with MS. Neurology 2011;77:2010–16. 10.1212/WNL.0b013e31823b9b27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan IL, McArthur JC, Clifford DB, et al. Immune reconstitution inflammatory syndrome in natalizumab-associated PML. Neurology 2011;77:1061–7. 10.1212/WNL.0b013e31822e55e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.