Abstract
Objectives
Dysbiosis of the intestinal microbiota is associated with Crohn's disease (CD). Functional evidence for a causal role of bacteria in the development of chronic small intestinal inflammation is lacking. Similar to human pathology, TNFdeltaARE mice develop a tumour necrosis factor (TNF)-driven CD-like transmural inflammation with predominant ileal involvement.
Design
Heterozygous TNFdeltaARE mice and wildtype (WT) littermates were housed under conventional (CONV), specific pathogen-free (SPF) and germ-free (GF) conditions. Microbial communities were analysed by high-throughput 16S ribosomal RNA gene sequencing. Metaproteomes were measured using LC-MS. Temporal and spatial resolution of disease development was followed after antibiotic treatment and transfer of microbial communities into GF mice. Granulocyte infiltration and Paneth cell function was assessed by immunofluorescence and gene expression analysis.
Results
GF-TNFdeltaARE mice were free of inflammation in the gut and antibiotic treatment of CONV-TNFdeltaARE mice attenuated ileitis but not colitis, demonstrating that disease severity and location are microbiota-dependent. SPF-TNFdeltaARE mice developed distinct ileitis-phenotypes associated with gradual loss of antimicrobial defence. 16S analysis and metaproteomics revealed specific compositional and functional alterations of bacterial communities in inflamed mice. Transplantation of disease-associated but not healthy microbiota transmitted CD-like ileitis to GF-TNFdeltaARE recipients and triggered loss of lysozyme and cryptdin-2 expression. Monoassociation of GF-TNFdeltaARE mice with the human CD-related Escherichia coli LF82 did not induce ileitis.
Conclusions
We provide clear experimental evidence for the causal role of gut bacterial dysbiosis in the development of chronic ileal inflammation with subsequent failure of Paneth cell function.
Keywords: INTESTINAL MICROBIOLOGY, SMALL BOWEL DISEASE, TNF, CROHN'S DISEASE, IBD BASIC RESEARCH
Significance of this study.
What is already known on this subject?
IBD is associated with changes in intestinal bacterial composition.
Germ-free mouse models of IBD-related colitis are established.
Paneth cell function is associated with ileal disease phenotypes of Crohn's disease.
What are the new findings?
Crohn's disease-like ileitis development and location in TNFdeltaARE mice are microbiota dependent, and completely absent in germ-free conditions.
Antibiotics reduce disease severity, and recurrence of inflammation follows the relapse of microbiota composition.
Ileitis severity is associated with compositional and functional dysbiosis of the intestinal commensal microbiota.
Transfer of dysbiotic microbiota to germ-free recipients causes Crohn's disease-like inflammation in genetically susceptible recipients.
Loss of Paneth cell-related antimicrobial defence is subsequent to the development of ileitis in TNFdeltaARE mice.
How might it impact on clinical practice in the foreseeable future?
This study provides a basal understanding for the causality of microbe-host interaction in Crohn's disease, based on dynamic alterations of the microbial ecosystem (dysbiosis) in the gut.
This study will help to elucidate the therapeutic relevance of microbiota transplantation in Crohn's disease.
Introduction
Crohn's disease (CD) and ulcerative colitis (UC) are the two main forms of inflammatory bowel disease (IBD). While inflammation in patients with CD mainly occurs in the terminal ileum and is found in the proximal colon only occasionally, inflammation in UC is confined to the colon.1 2 Despite unknown aetiologies, chronic activation of the mucosal immune system is triggered by gut commensals.3 4 Genome-wide association studies identified links between disease and genes involved in microbial signalling, underlining the role of gut microbes in IBD pathogenesis.5 Genetic predispositions and inflammation of the host were shown to induce altered composition and metabolic activity of microbial communities, defined as dysbiosis,6–8 and, as expected, patients with IBD are characterised by dysbiotic changes in the gut microbial ecosystem.9–11 Changes in bacterial communities were shown to be shaped by Paneth cell (PC)-derived antimicrobial peptides.12 PCs are located in the crypt base of the small intestine in close proximity to the epithelial stem cell compartment and loss of PC function is associated with ileal phenotypes of CD.12–14 In IBD treatment, the clinical benefit of antibiotics or faecal-stream diversion supports the pathological role of bacteria.15 Faecal microbiota transplantation (FMT) is effective in restoring homoeostasis in Clostridium difficile infections.16–19 Consequently, there is rising interest for a therapeutical implementation of FMT in UC and CD. However, evidence from controlled clinical trials is still limited and characteristics of a successful donor microbiota are not yet defined. Moreover, the rationale of introducing new antigen pools in a milieu that has been overreacting to microbial stimuli is questionable. For colitis, the importance of bacteria in disease development has been extensively studied in animal models, for example, by showing reduction of colitis in IL-10−/− mice after antibiotic treatment or the ability of various bacterial strains to induce inflammation in germ-free (GF) colitis models.20–23 Due to the lack of GF models for CD-like ileitis, proof for causality of microbes or dysbiosis in the onset of ileitis is lacking.
In the present study, we assessed the impact of intestinal bacterial communities in a spontaneous model of chronic CD-like ileitis. TNFdeltaARE mice carry a deletion in the tumour necrosis factor (TNF) AU-rich (adenosin-uracil) elements (ARE) leading to Tnf driven transmural inflammation in the distal ileum.24 We previously showed that iron-induced modulation of the microbiota is associated with dramatic changes in disease activity of TNFdeltaARE mice.25 However, mechanistic proof for a causal role of microbe-host interactions in the pathogenesis of CD-like ileitis is missing. Here, we used antibiotics and different hygienic conditions (GF, specific pathogen-free (SPF) or conventional (CONV) housing) to dissect the relationship between microbiota changes and ileitis development in TNFdeltaARE mice. To assess the causal role of dysbiotic microbial communities in ileal inflammation, we performed microbiota transplant experiments with GF-TNFdeltaARE mice and characterised PC functions.
Methods
Ethics statement
Animal use was approved by the local institution in charge (Regierung von Oberbayern, approval no. 55.2-1-54-2531-75-10 and 55.2-1-54-2531-99-13). All animals were housed in mouse facilities at the Technische Universität München (School of Life Sciences Weihenstephan).
Housing
TNFdeltaARE mice were provided by George Kollias (Alexander Fleming BSRC, Greece), bred in our CONV facility and transferred to SPF via embryo transfer. TNFdeltaARE mice were made GF by hysterectomy (Institute for Laboratory Animal Science; Hannover). Sterility was checked by cultivation of faeces in Luria broth (LB) or wilkins chalgren agar (WCA) broth (OXOID) and by microscopic observation of Gram-stained faecal smears every 10–14 days and at sampling. A mould-trap was used to indicate the presence of mold. No contaminations were observed during the experiments. Heterozygous TNFdeltaARE and WT littermates (C57BL/6N) were kept in CONV, SPF or GF conditions (12 h light/dark cycles at 24–26°C) until the age of 18 weeks. Mice were fed a standard diet (autoclaved R/M-H for SPF and CONV, or M-Z V1124-300 for GF-animals, Ssniff, Soest, Germany) ad libitum and were sacrificed by CO2.
Antibiotic treatment
CONV-TNFdeltaARE and CONV-WT mice received antibiotics (VM: 0.25 g/L vancomycin and 1.0 g/L metronidazole, Sigma-Aldrich and Fluka) from 8 weeks to 12 weeks of age. Antibiotics were prepared fresh twice a week and administered ad libitum via drinking water in light-protected bottles. Mice were sacrificed 0 weeks, 2 weeks, 4 weeks and 6 weeks after cessation of VM therapy.
Colonisation of GF mice
Caecal content from SPF mice was collected and immediately suspended (1:10, weight/volume) in filter-sterilised phosphate buffered saline (PBS)/glycerol (20%), snap-frozen and stored (−80°C). Aliquots were centrifuged (300 g/3 min/4°C) to pellet debris. Supernatants were centrifuged (8000 g/10 min/4°C) and pellets were resuspended in equal volumes of PBS. Each mouse was gavaged at 8 weeks of age with 100 µL caecal microbiota-suspensions of one SPF-donor (approximately 1–5×108 cells per mouse, as determined by THOMA counting-chamber). Mice were housed in microbiota-specific isolators with mixed genotypes per cage and sacrificed 4 weeks after colonisation. To investigate ileitis development over time, additional mice were colonised as described above, and samples were collected 1 week, 2 weeks and 4 weeks after colonisation. See online supplementary methods for association of mice with Escherichia coli LF-82.
Cultivation of intestinal bacteria
See online supplementary methods.
Histopathology
H&E stained terminal ileal and proximal colonic tissue sections were scored (blindly) by assessing lamina propria mononuclear cell infiltration, crypt hyperplasia, goblet cell depletion and architectural distortion resulting in a score from 0 to 12.26 27 Images were acquired using Digital microscope M8 (PreciPoint GmbH).
Immunofluorescence staining
See online supplementary methods.
Gene expression analysis
RNA of total ileal tissue was isolated according to manufacturer's instructions (NucleoSpin RNAII kit; Macherey-Nagel GmbH, KG). Complementary DNA was synthetised from 500 ng RNA using random hexamers and moloney murine leukemia virus (M-MLV) reverse transcriptase (RT) Point Mutant Synthesis System (Promega). Quantification was performed using the LightCycler 480 Universal Probe Library System (Roche). For primer sequences and respective probes see supplementary methods. Calculations (2-ΔΔCt method28) were done normalised to gapdh.
16S ribosomal RNA gene sequence analysis
See online supplementary methods.
Mass spectrometry and metaproteome analysis
See online supplementary methods.
Statistics
Statistical analyses were performed with R or Sigma Plot 11.0 using analysis of variance (ANOVA) followed by pairwise comparison testing (Holm-Sidak test). Graphics were created using GraphPad Prism V.5.00. Unless otherwise stated, data are presented as mean±SD and p values below 0.05 were considered to be statistically significant. The Benjamini-Hochberg method was used for adjustment after multiple testing. For visualisation of the relationships between bacterial profiles, non-parametrical multiple dimensional scaling plots were computed using the packages vegan and ade4.
Results
Inflammation in TNFdeltaARE mice is microbiota-dependent
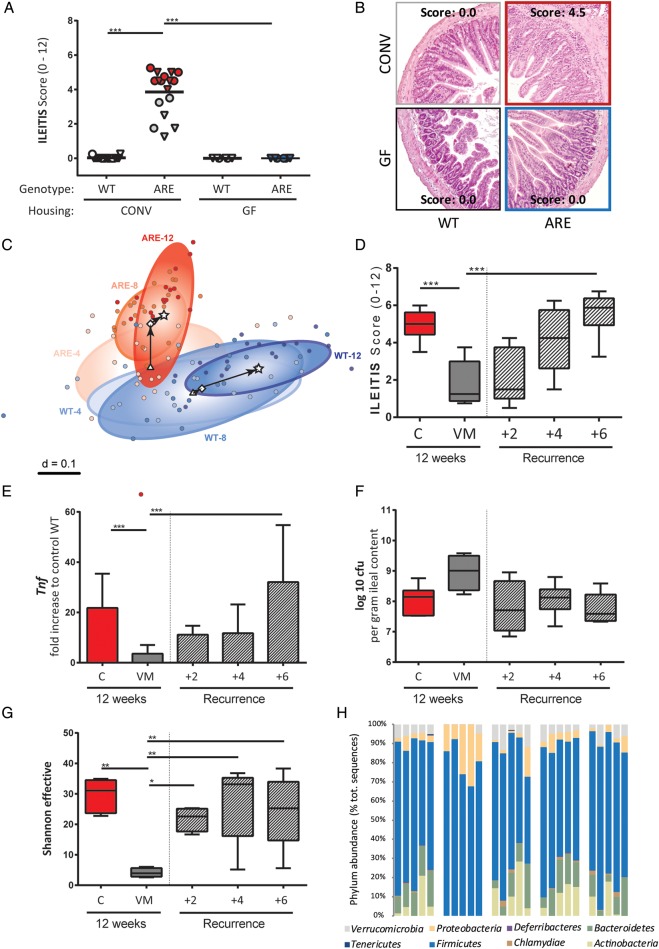
To assess the role of the gut microbiota in the development of CD-like inflammation, we generated GF-TNFdeltaARE mice or treated CONV-TNFdeltaARE with antibiotics. Under CONV-housing, TNFdeltaARE mice developed tissue pathology in the terminal ileum at the age of 18 weeks (figure 1A, B). In the absence of microorganisms (GF conditions), TNFdeltaARE mice were free of ileal (figure 1A, B) and colonic disease (see online supplementary figure S1A). Although TNFdeltaARE mice are known as model for CD-like ileitis,24 inflammation of the proximal colon was observed in all CONV-mice (see online supplementary figure S1A). There was no correlation between ileitis and colitis severity, as for example several mice with severe ileitis (score >4) developed moderate colitis (score <4).
Figure 1.
Changes in the gut microbial ecosystem are associated with inflammation in TNFdeltaARE mice. (A) Ileitis scores in 18-week-old TNFdeltaARE (ARE) and WT littermates in CONV or GF housing. Ileitis-score groups are colour-coded according to inflammation severity: score=0 (blue); score <4 (grey); score >4 (red). Male mice are displayed as triangles, female mice as circles. (B) Representative H&E-stained sections of the distal ileum. (C) NMDS plot showing shifts of centroids (indicated by arrows) and variance of faecal bacterial profiles (circled areas) of CONV-TNFdeltaARE and WT mice at 4 weeks, 8 weeks and 12 weeks of age. 16S ribosomal RNA gene amplicons of the V3/V4 region (407 bp) in faeces were sequenced on a MiSeq platform. (D) Ileitis scores in TNFdeltaARE mice treated for 4 weeks with vancomycin and metronidazole (VM), starting at 8 weeks of age. Recurrence of ileitis after VM therapy was followed for 6 weeks (n=5–6/group). (E) Ileal expression of Tnf as fold-increase to CONV-WT mice (normalised to Gapdh). (F) Ileal bacterial density of cultivable anaerobes (as log 10 of colony forming units (cfu) per gram ileal content). (G) Intestinal bacterial diversity (Shannon effective counts). (H) Changes in caecal bacterial composition (relative abundance of total sequences) at the phylum level. *p<0.05; **p<0.01; ***p<0.001 (Two-way-ANOVA followed by Holm-Sidak test). CONV, conventional; GF, germ-free; ARE, AU-rich elements; TNF, tumour necrosis factor; NMDS, non-parametrical multiple dimensional scaling.
We previously showed that ileitis develops gradually in CONV-TNFdeltaARE mice and reaches maximum levels at 12 weeks.29 Using 16S ribosomal RNA sequencing, we characterised faecal bacterial diversity and composition at different ages in TNFdeltaARE and WT littermates. In the absence of inflammation (age of 4 weeks), bacterial phylogenetic make-ups in TNFdeltaARE and WT mice overlapped (figure 1C). Shifts in community structure started to be distinct at the age of 8 weeks, when tissue pathology reaches significance (ileitis score of 3.92±0.52; p<0.01). Parallel to the age-related increase in inflammation, microbial communities from WT diverged from TNFdeltaARE mice and interindividual differences decreased within genotypes. Main significant differences in composition included increased abundance of Bacteroidaceae, Erysipelotrichaceae, Peptostreptococcaceae and Verrucomicrobiaceae in TNFdeltaARE mice (p<0.001; see online supplementary figure S1B). The increase in Bacteroidaceae was mainly due to three operational-taxonomic units (OTUs) closely related to Bacteroides acidifaciens and Bacteroides sartorii.
To assess whether changes in microbial communities can antagonise established inflammation, we treated inflamed CONV-TNFdeltaARE mice with antibiotics starting at 8 weeks of age. Ileitis was significantly reduced after VM-treatment and complete relapse of ileitis was achieved 6 weeks after ending the antibiotic treatment (figure 1D). Colitis activity remained unaltered over the whole period (see online supplementary figure S1C). Tnf-expression levels reflected the dynamic changes in ileal disease activity (figure 1E). Numbers of colony-forming units grown under anaerobic conditions (figure 1F) and total cell counts (see online supplementary figure S1D) showed no change after VM treatment, suggesting growth of VM-resistant bacterial species. However, bacterial diversity was drastically reduced in VM-treated mice (figure 1G). Analysis of phyla distribution in TNFdeltaARE mice showed the predominance of Firmicutes and Bacteroidetes in controls, whereas VM treatment led to a reduction of Bacteroidetes and a bloom in Firmicutes (Lactobacillus spp) and Proteobacteria (E. coli) (figure 1H). Interestingly, bacterial community composition showed rapid resilience in overall composition, that is, phyla distribution returned to pre-VM values 2 weeks after cessation of treatment preceding the return of inflammation.
Disease severity in the distal ileum is associated with loss of PC-associated antimicrobial defence
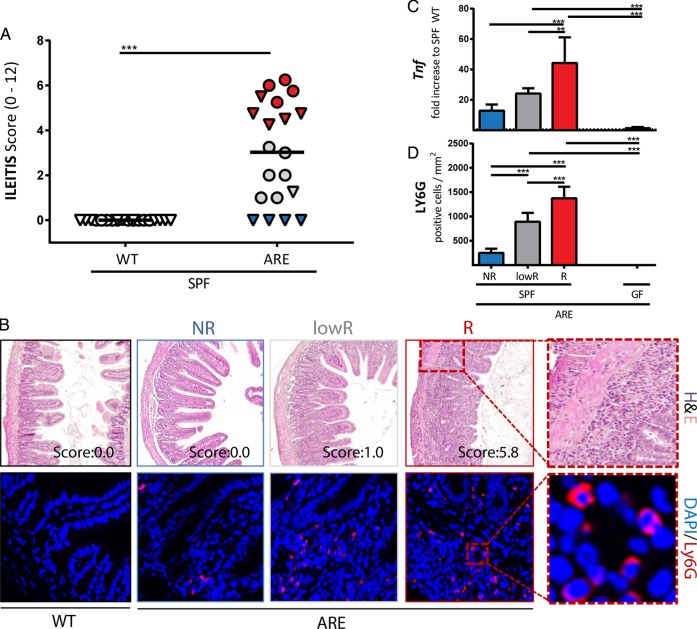
Based on sequencing, culture and Federation of Laboratory Animal Science Associations (FELASA)-recommended analyses, we observed the presence of murine norovirus,30 Helicobacter, Pasteurella, Syphacia, Trichomonas, Candida, Kazachstania and Chlamydiae spp in the CONV-housing facility (see online supplementary table S1). Hence, to study the role of microbial communities in a strictly controlled environment, we transferred CONV-TNFdeltaARE mice by embryo transfer to a specific pathogen-free (SPF) environment. In contrast to age-matched CONV-mice, SPF-TNFdeltaARE mice developed CD-like ileitis but not colitis (figure 2A, B and see online supplementary figure S2A). Interestingly, we observed a gradient of ileitis severity in animals from the SPF facility, including three main categories of TNFdeltaARE mice: (1) 4 mice out of 20 showed no ileitis, hereon referred to as ‘non-responders’ (NRs); (2) 9 mice had an ileitis score >4 and were defined as ‘responders’ (Rs); and (3) 7 mice with a score between 1 and 4 were defined as ‘low responders’ (lowR). Of note, all non-inflamed mice were male and no cage effect was observed. Inflammation-related markers, such as cytokine expression, granulocyte infiltration and PC function were measured in the three ileitis phenotypes and in GF-TNFdeltaARE mice. Similar to CONV-TNFdeltaARE mice, Tnf expression levels (figure 2C) and granulocyte-infiltration into the ileal mucosa (figure 2B, D) reflected the gradual increase in disease activity in SPF-TNFdeltaARE mice. Tnf-transcript levels were reduced and Ly6G-positive granulocytes were totally absent in GF-TNFdeltaARE mice.
Figure 2.
Ileitis in the specific pathogen-free (SPF) environment shows different grades of severity. (A) Ileitis scores in 18-week-old TNFdeltaARE (AU-rich elements, ARE) and WT littermates in the SPF facility. The three ileitis score groups are colour-coded as ‘non-responders’ (NRs) in blue (score=0), ‘low-responders’ in grey (score <4) and ‘responders’ in red (score >4). Male mice are displayed as triangles, female mice as circles. (B) Upper panel: Representative H&E-stained sections of the distal ileum. Lower panel: Representative terminal ileum sections stained against Ly-6G (red). (C) Ileal expression (normalised to Gapdh) of Tnf as fold-increase to SPF-WT-mice (dotted line) in NRs, low-responders (lowRs) and responders (Rs). (D) Quantification of Ly6G-positive granulocytes per mm2 in tissue sections of the terminal ileum.
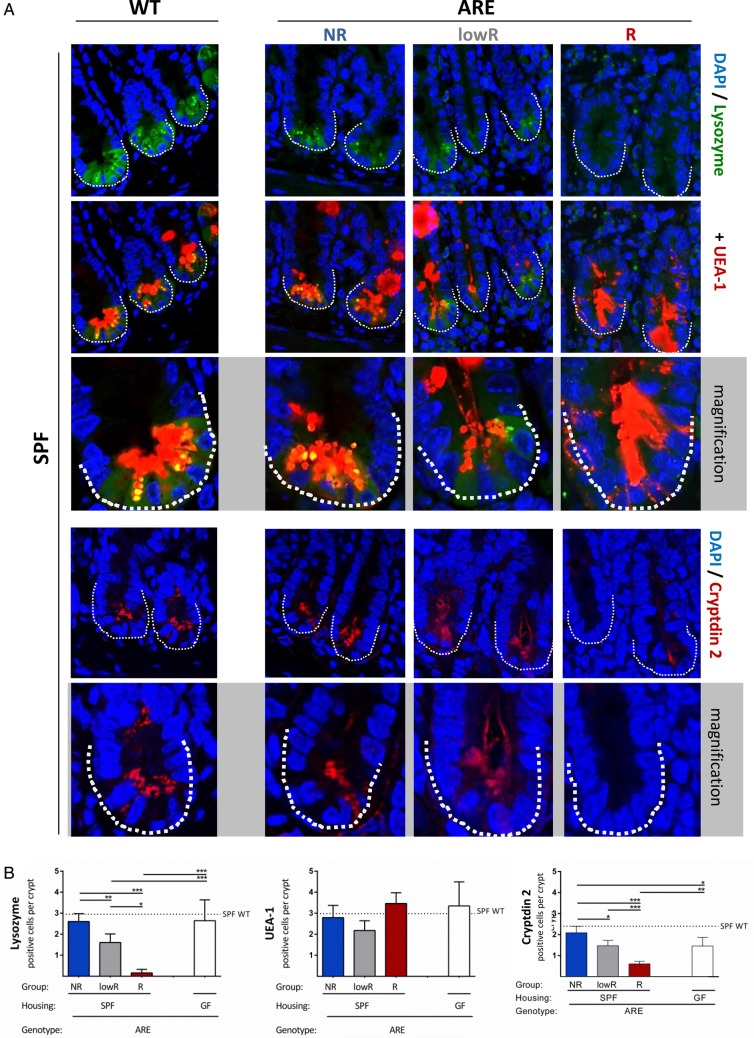
To assess PC function, lysozyme and α-fucosylated proteins (as detected by Ulex europaeus agglutinin-1 (UEA-1)) were stained in the ileal mucosa of SPF-TNFdeltaARE and WT littermates (figure 3A and see online supplementary figure S2B). Immunofluorescence analysis clearly demonstrated a significant loss of lysozyme but not UEA-1 positive cells in the crypt base of inflamed lowR and R SPF-TNFdeltaARE mice compared with GF controls (figure 3B), while numbers of lysozyme and UEA-1 positive cells in NR TNFdeltaARE mice were comparable to WTs (dotted line). These data suggest a loss of PC activity but not PC number in the presence of inflammation. Besides the loss of lysozyme expression, signals for Cryptdin 2-positive cells were also reduced in severe ileitis (figure 3A, B). Consistent with a loss of PC function in R mice, transcript levels of the antimicrobial peptide Angiogenin 4 was significantly reduced compared with NRs (see online supplementary figure S2C). While Cryptdin 5 (Defensin a5) showed high interindividual variations but the same trend towards reduction under inflammation (p>0.05), Reg3 γ expression remained unchanged (see online supplementary figure S2C). GF mice showed lower transcript levels of the tested antimicrobial peptides, which accords previous publications.31 32 An increase in the number of Caspase-3-positive cells in the ileal crypts could not be observed (figure S2D).
Figure 3.
Disease severity is associated with loss of PC function. (A) Paneth cell staining of lysozyme (green; upper panel) combined with UEA-1 (red). A representative crypt is shown in the magnification. Lower panel: Paneth cell staining of cryptin 2 (red). A representative crypt is shown in the magnification. (B) Quantification of lysozyme, UEA-1 and cryptdin-2 positive cells per crypt base in ARE-mice compared with WT mice (dotted line). **p<0.01; ***p<0.001; Two-way ANOVA followed by Holm-Sidak test.
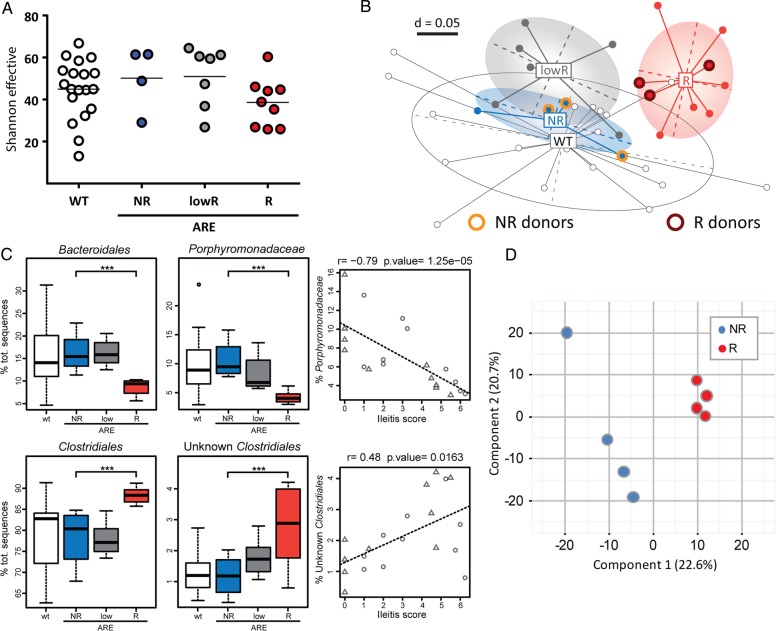
Ileitis severity is linked to compositional and functional dysbiosis
To investigate the relationship between ileitis severity and the gut microbiota in SPF mice, we performed 16S ribosomal RNA gene sequencing. There was no change in α-diversity between the three ileitis phenotypes (figure 4A). However, β-diversity analysis revealed clear separation of R TNFdeltaARE-mice and their WT littermates (figure 4B). Most interestingly, bacterial community structures of NR TNFdeltaARE-mice clustered together with WT mice but were separated from R TNFdeltaARE-mice. This clearly indicates a link between inflammation and bacterial communities. Relative abundance of taxonomic groups revealed that changes in bacterial diversity were associated with changes in composition (figure 4C). Ileitis scores negatively correlated with relative abundances of Porphyromonadacaeae (r=−0.79; p<0.001). In contrast, unknown members of the order Clostridiales showed significantly higher proportions in R mice and positively correlated with the degree of histopathology (r=0.48; p=0.016). Additional sequence analysis of faecal samples directly after weaning demonstrated that the transfer of microbiota from mother to offspring is not related to the disease susceptibility later in life and disease-related changes in microbiota are independent of littermate effects. TNFdeltaARE mice of the same litter were found in the three groups of ileitis severity and thus developed the dysbiotic or non-dysbiotic caecal microbiota post weaning (see online supplementary figure S3A).
Figure 4.
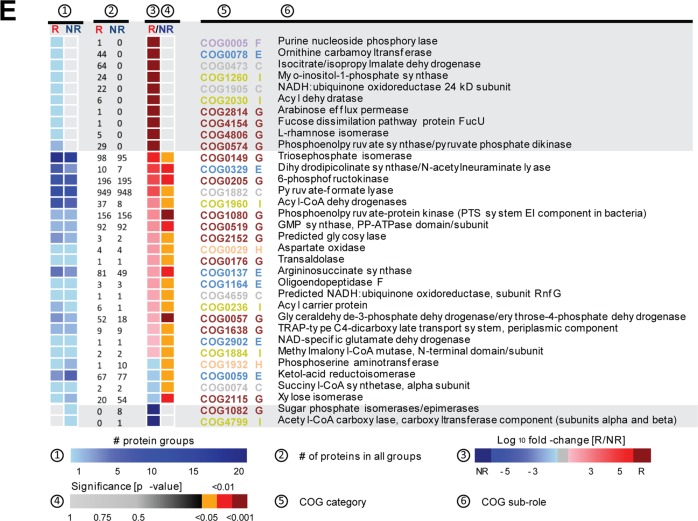
Bacterial profiles and functional dysbiosis mirror ileitis severity. (A) Shannon effective species counts in SPF-WT and SPF-TNFdeltaARE mice. (B) Non-parametrical multiple dimensional scaling analysis showing separation of caecal bacterial communities according to genotype and ileitis severity. Responders (R; red) were clearly separated from TNFdeltaARE with low ileitis scores (low-responder, lowR; grey) and from non-responders (NR; blue). Microbiota donor-mice used in colonisation experiments are shown with halos. (C) Correlation of bacterial taxa with ileitis scores. (D) Metaproteome analysis of the colonic microbiota. Partial least squared (PLS) analysis differentiated responder (red) and NR (blue) TNFdeltaARE-mice. (E) Significant changes in abundance of protein functions belonging to the clusters of orthologous groups (COGs) main role metabolism between R and NR samples. The heatmap represents differences in the abundance of COGs. Protein group data were log10 transformed and normalised by median of bacteria protein data. Protein functions that are unique to either R or NR are shown in the grey squares. COG subroles are: C, Energy production and conversion; E, amino acid transport and metabolism; F, nucleotide transport and metabolism; G, carbohydrate transport and metabolism; H, coenzyme transport and metabolism; I, lipid transport and metabolism. ***p<0.001; Two-way ANOVA followed by Holm-Sidak test.
These differences in composition were underlined by functional changes in microbial communities as determined by LC-MS/MS metaproteome analysis. Partial least squared analysis showed a clear separation of R and NR mice (figure 4D). Proteins were assigned to functions using clusters of orthologous groups. The subroles translation ribosomal structure and biogenesis (fold change (fc) 103, p=0.028), carbohydrate transport and metabolism (fc 59.8, p=0.018) as well as amino acid transport and metabolism (fc 23.4, p=0.027) showed significant increase in the R group. Proteins that were significantly different between the two groups of mice within the categories metabolism or cellular processes and signalling are shown in figure 4E and see online supplementary figure S3B. Ten proteins were exclusively found in Rs, while only two were specific for NRs.
Ileitis is transmissible via dysbiotic gut microbiota
GF-TNFdeltaARE mice are free of intestinal inflammation, and the occurrence of ileitis in SPF-housing is associated with dysbiosis. We first tested monocolonisation of GF-TNFdeltaARE mice with the Gram-negative adherent invasive bacterium E coli LF82, a well-known strain associated with CD.33 34 After 4 weeks of colonisation, LF82 did not induce ileitis in TNFdeltaARE mice (p=0.07), although cell density reached 10.2±0.3 log10 cfu/g in the caecum (see online supplementary figure S4A).
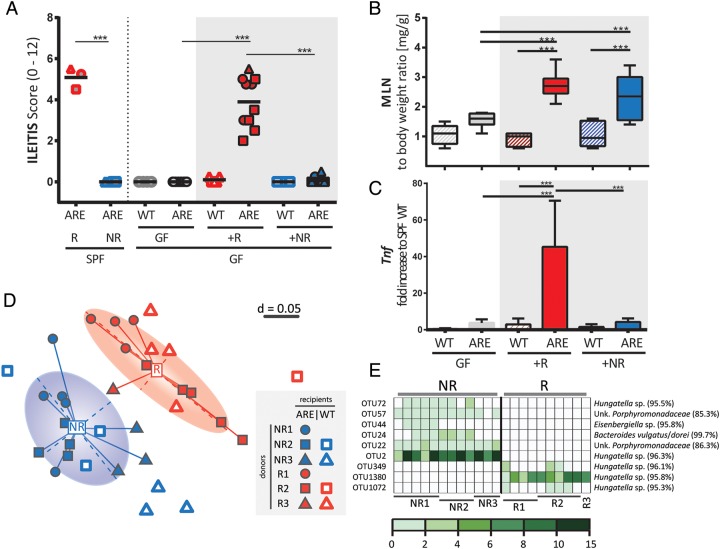
To test the causal relationship of a dysbiotic microbiota associated with development of CD-like ileitis in TNFdeltaARE mice, we next transferred caecal microbial communities from SPF-TNFdeltaARE mice into GF-recipients (figure 5A). Therefore, GF-TNFdeltaARE and GF-WT mice were colonised with donor-microbiota from either Rs or NRs. Donor-microbiota is highlighted with halos in figure 4B. TNFdeltaARE mice colonised with dysbiotic microbiota from Rs (+R; red) developed inflammation after 4 weeks, mimicking ileitis severity of corresponding donors (recipient mice and respective donors are shown with the same symbols). WT littermates showed no signs of inflammation, supporting the fact that genetic susceptibility was required for the development of ileitis after transfer. Most remarkably, GF-TNFdeltaARE mice colonised with the microbiota from NRs also showed no signs of ileitis (+NR; blue). GF-mice were colonised by comparable densities of bacteria in both recipient groups (approximately 109 cfu/g) and tissue maturation was confirmed by reduced caecum weight (see online supplementary figure S4B). Mesenteric lymph node weight to body weight ratios were significantly increased in both recipient groups when compared with GF-TNFdeltaARE mice (figure 5B), suggesting the presence of mucosal immune cell activation independent of tissue pathology. Increased Tnf-transcript levels (p<0.001) correlated with the induction of tissue pathology (figure 5C).
Figure 5.
Ileitis is transmissible via dysbiotic gut microbiota. (A) GF-WT and GF-TNFdeltaARE (ARE) mice were colonised at the age of 8 weeks with the complex caecal microbiota from SPF responder (+R) or non-responder (+NR) donor-TNFdeltaARE mice (orange halos in figure 4; each n=3). Age-matched and time-matched GF-WT and GF-TNFdeltaARE-mice served as controls. Ileitis was scored after 4 weeks of colonisation. SPF-donor mice and respective recipients are shown with identical symbols. (B) Increase in mesenteric lymph node (MLN) weight. (C) Ileal expression (normalised to Gapdh) of Tnf as fold-increase to SPF-WT mice. (D) NMDS plot showing clear separation between R (red) and NR (blue) recipients according to respective donor (R1–3 or NR1–3; donor and recipient are displayed using identical symbols). ARE and WT are shown as filled and open symbols, respectively. (E) OTUs associated with R or NR colonisation. Each column represents one recipient ARE mouse. Donors and percentages of total sequences (coloured scale) are indicated below the table. ***p<0.001; Two-way ANOVA followed by Holm-Sidak test. ARE, AU-rich elements; GF, germ-free; NMDS, non-parametrical multiple dimensional scaling.
To verify the hypothesis that transmissible ileitis was due to the transfer of dysbiotic bacterial communities, we analysed caecal content in recipient mice using high-throughput sequencing. α-Diversity analysis showed no differences between genotypes and donor groups (see online supplementary figure S4C). β-Diversity analysis displayed again a clear separation between inflamed and non-inflamed recipients (figure 5D). Differences within the recipient groups could still be assigned to the respective donors including WT and TNFdeltaARE mice. The increase in Clostridiales spp and decrease in Porphyromonadacaeae observed in inflamed donor SPF-TNFdeltaARE mice also occurred in recipients, but results did not reach significance (see online supplementary figure S4D). Differential abundance analysis identified two OTUs classified as Hungatella sp exclusively present in NRs, with relative abundance of up to 15%. Three other OTUs of the same genus were only present in Rs (figure 5E), indicating species-specific differences between R and NR mice.
Inflammation precedes loss of PC function
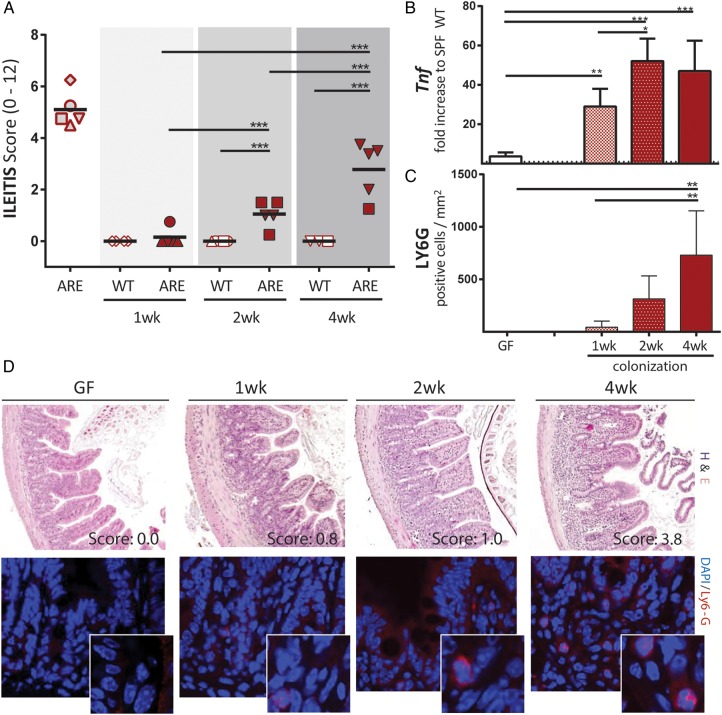
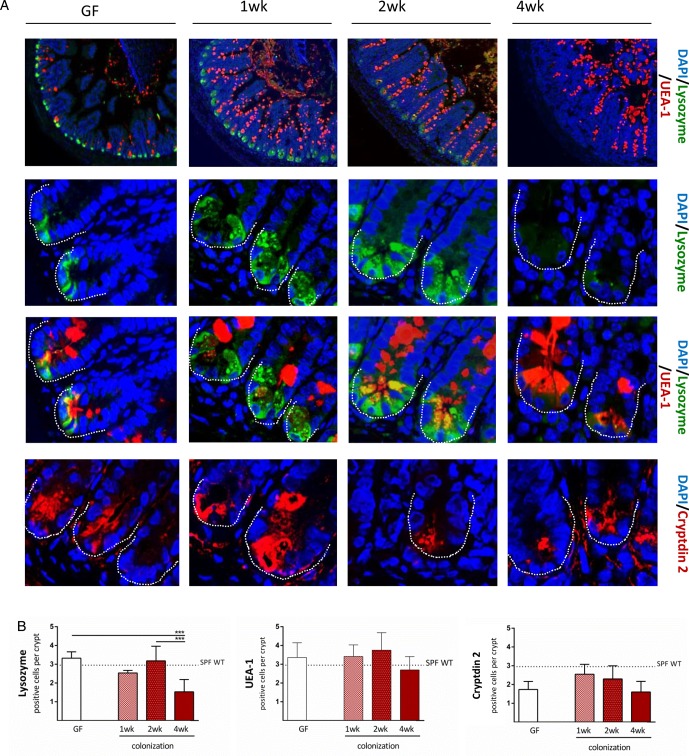
To better understand the role of PCs in ileitis development, we analysed time series of tissue pathology and PC function in the transfer model of CD-like ileitis. GF-TNFdeltaARE and GF-WT recipients were colonised at the age of 8 weeks with dysbiotic microbiota from R SPF-TNFdeltaARE mice for 1 week, 2 weeks and 4 weeks. As shown in figure 6A, ileitis was absent after 1 week (0.13±0.28), but increased with the duration of colonisation in TNFdeltaARE but not in WT mice. Tnf mRNA expression levels were already significantly increased relative to GF-controls after 1 week of colonisation (figure 6C; p<0.001), whereas granulocyte infiltration and mesenteric lymph node weight increased later, parallel to disease severity in TNFdeltaARE mice (figure 6D and see online supplementary figure S5A). The influence of colonisation on caecum-weight and mucus-fucosylation was already visible 1 week post colonisation showing increased staining across the crypt-villus axis (figure 7A; upper panel and see online supplementary figure S5B). Numbers of UEA-1-positive crypt cells remained constant over the entire colonisation period. Most importantly, loss of lysozyme-positive crypt cells of recolonised TNFdeltaARE mice did not precede the onset of tissue pathology (figure 7B), suggesting that loss of PC function is associated but not causal for the development of CD-like ileitis. Quantification of Cryptdin 2-positive cells per crypt (figure 7A lower panel and 7B right panel) showed no reduction in Cryptdin 2 expression in mice after 4 weeks of colonisation, despite the presence of moderate histopathology and inflammatory activation (increased Tnf expression levels and Ly6G-positive granulocyte infiltration).
Figure 6.
Transfer of dysbiotic microbiota induces a gradual disease onset. (A) GF-WT and GF-TNFdeltaARE (ARE) mice were colonised at the age of 8 weeks with the complex caecal microbiota from inflamed SPF responder mice (open symbols). After 1 week, 2 weeks and 4 weeks of colonisation, ileitis was assessed. (B) Upper panel: Representative H&E stained terminal ileum sections of ARE-mice colonised with responder microbiota for 1 week, 2 weeks and 4 weeks (wk). Age matched GF-AREs (left) served as controls. Lower panel: Representative terminal ileum sections stained against Ly-6G (red). (C) Ileal expression (normalised to Gapdh) of Tnf as fold-increase to SPF-WT mice. Tnf expression of GF-ARE-mice is displayed as dotted line. (D) Quantification of Ly6G-positive granulocytes per mm2 in tissue sections of the terminal ileum after 1 week, 2 weeks and 4 weeks of colonisation with R microbiota. ARE, AU-rich elements; GF, germ-free.
Figure 7.
Loss of antimicrobial peptides is secondary to the development of inflammation. (A) Paneth cell staining of lysozyme, UEA-1 and Cryptdin 2, as labelled in the figure (upper panel, 10× magnification; others, 120×). (B) Quantification of lysozyme-positive, UEA-1-positive and cryptdin 2-positive cells per crypt base in AU-rich elements (ARE) mice compared with WT mice (dotted line). *p<0.05; ***p<0.001 (One-way-ANOVA followed by Tukey's test).
Discussion
In the present work, we provide for the first time evidence for disease-related causality of bacterial dysbiosis in experimental CD-like ileitis. We showed that GF-TNFdeltaARE mice are protected from intestinal inflammation, and thereby demonstrated the essential role of microbial triggers in a model that shares main clinical features of CD. In several models of colitis, the disease-free status under GF conditions has been shown, for example, in IL-10−/− or T-bet(−/−) ×Rag2(−/−) ulcerative colitis (TRUC) mouse model (TRUC) mice and HLA/B27-β2m transgenic rats.23 35 36 However, there are only few IBD-related ileitis models including TNFdeltaARE and SAMP1/YitFc mice.37 GF housing induced attenuated or no disease activity in SAMP1/YitFc mice, but information about the specific contribution of microbial communities in ileitis development is lacking.38 39 Sequential sampling of faecal microbiota demonstrated the gradual influence of inflammation, as illustrated by increasing divergence of bacterial communities over time in TNFdeltaARE versus WT littermates. Several taxa were significantly more abundant in TNFdeltaARE mice, including members of the family Bacteroidaceae, which are frequently reported to increase the risk for the development of intestinal inflammation in humans and mice.7 9 23 40–42
Changes in microbial composition induced by antibiotic treatment established a disease course with recurrent inflammation, similar to observations in patients with IBD.43 The clinical efficacy of microbial therapy in CD, including antibiotics, probiotics and FMT is not well established. Meta-analyses show no clear advantage of probiotics in maintenance of remission in patients with CD, but hint towards a beneficial effect of antibiotics.44 45 Interestingly, the development of colitis in TNFdeltaARE mice was only detected in the presence of a variety of mouse-related pathogens in the CONV housing and was not affected by antibiotic treatment, suggesting different mechanisms in the pathogenesis of ileitis and colitis in this model of CD-like inflammation.
Embryo transfer of CONV-TNFdeltaARE mice to a barrier-controlled environment led to the progression of extreme ileal phenotypes, that is, approximately half of SPF-TNFdeltaARE mice developed severe ileitis, whereas 20% remained ileitis-free. Disease activity and inflammatory tissue activation, measured at the level of granulocyte infiltration and Tnf-expression, significantly correlated with the reduction in lysozyme-positive or cryptin 2-positive PCs and ileal ANG4 expression. PCs play an important role in mucosal defence and were shown to be functionally impaired in IBD, especially in patients with CD.46–49 Genetic risk variants like autophagy-associated ATG16L1-T300A and endoplasmic reticulum stress responses contribute to PC dysfunction and epithelial cell-specific knockout models, that is, Caspase-8−/− or ATG16L1−/− and XBP-1−/− mice, allowed a mechanistic understanding of CD-like disease phenotypes.50–52 Interestingly, these newly generated mouse models share loss of PC functions as an important mechanism of ileal pathogenesis. However, lineage-specific ablation of PCs showed no specific IBD-associated phenotype, suggesting that loss of PC function alone is not sufficient to initialise the onset of disease, but rather facilitates the loss of complex regulatory circuits in the epithelium.53 Interferon-γ inhibits the release of PC-derived antimicrobial peptides,54 supporting our observation that inflammation-associated mediators abrogate PC functions. Therefore, loss of PC functions associated with reduced production of antimicrobial peptides in the small intestine might act as a disease accelerator. We showed that TNF-driven ileitis severity in TNFdeltaARE mice is linked to reduced PC function rather than numbers using combined lysozyme-UEA-1 and cryptdin-2 staining. Time-series analysis of transfer experiments with disease-conditioning microbiota in GF recipients clearly demonstrated that loss of PC function was subsequent to TNF-driven development of inflammation, suggesting that loss in PC function might contribute to, but seems not to be causal for the divergence of disease-related bacterial communities or development of dysbiosis.
SPF-TNFdeltaARE mice with severe ileitis and loss of antimicrobial peptides were characterised by a taxonomically and functionally dysbiotic gut microbiota. Analysis of intestinal communities showed an ileitis-associated increase in the relative abundance of unknown Clostridiales and reduced abundance of Porphyromonadacaeae (order of Bacteroidales). While members of Clostridiales were found in lower abundance in patients with CD, they were increased in TNFdeltaARE ileitis and TM-IEC-C1galt1−/− colitis models, indicating model-specific traits of this very broad taxonomic group.55 56 Consistent with changes in our metaproteome analysis, shotgun metagenomic analysis of microbiota from patients with CD and UC showed functional shifts especially in ileal CD, driven by pathways of carbohydrate transport and mechanism and amino acid biosynthesis.57 Metaproteomics revealed that, among others, the abundance of fucose-utilising enzyme was exclusive for the dysbiotic ecosystem in SPF-TNFdeltaARE mice. Inflammation-driven increase in host-derived fucose was shown to affect metabolic functions of fucosidase-expressing commensal bacteria.58 Together with the fact that loss of function in the human Fut2 gene was shown to be associated with variations in the gut microbiota of patients with CD, we might speculate a role for the fucose metabolising enzymes in generating a disease-associated microbial environment in the gut.59 60
We showed that inflammation is associated with the development of dysbiosis and, most importantly, microbiota transfer experiments confirmed a causal relationship between microbial dysbiosis and disease initiation in TNFdeltaARE mice. We demonstrated for the first time that a compositionally and functionally diverse microbiota, which originates from donors with the same genetic background but different pathologies, transmits ileal inflammation. Garrett et al demonstrated in TRUC mice that a genotype-specific microbiota transferred colitis to RAG2−/− recipient mice. However, WT cagemates were also inflamed, suggesting a rather pathogenic than dysbiotic trait.36 61 Interestingly, Powell et al62 identified Helicobacter typhlonius as a key driver of pathogenesis in TRUC mice. In our experiments, inflammation occurred only after transfer of disease-related microbiota to GF-TNFdeltaARE and not WT recipient mice, supporting the necessity of genetic susceptibility for ileitis development, as well as the non-infectious nature of the dysbiotic microbial communities. Vice versa, the microbiota from NR animals failed to induce CD-like inflammation in GF-TNFdeltaARE mice, clearly demonstrating the disease-related specificity of the dysbiotic microbiota.
Again, the overall community structure between recipients of R and NR microbiota were very distinct. OTU-based analysis confirmed that members of the family Porphyromonadacaeae were increased in NRs, but also that the relative abundance of Hungatella spp was significantly different. Of note, phylogeny of the genus Hungatella is not yet well established and ambiguous delineation from neighbouring species (eg, Clostridium clostridioforme, Clostridium aerotolerans, Clostridium bolteae and Clostridium xylanolyticum) calls for taxonomic amendments.63 Hence, different representatives of Hungatella were found in higher abundances in R and NR TNFdeltaARE mice, which hints at dysbiotic conditions characterised by subtle differences in bacterial profiles down to at least the species level. There is rising evidence that protective or deleterious effects of intestinal bacteria are strain-specific or species-specific, for example, PrtP expression in Lactobacillus casei, the presence of polysaccharide A in Bacteroides fragilis, or gelatinase E production by colitogenic Enterococcus faecalis.22 64 65 Some specific pathobionts, for example, Bilophila wadsworthia in IL-10−/− mice, have been selected from the commensal microbiota with the capability to transfer colitis into susceptible GF hosts.66 However, we failed to induce ileitis in monoassociated TNFdeltaARE mice using the CD-related pathobiont E. coli LF82.67 68 All our results point towards a community effect of the complex microbiota and loss of aggressive, or gain of protective mechanisms, rather than the selection of aggressive phylotypes as single agents causing the development of CD-like ileitis in TNFdeltaARE mice.
In summary, we report that TNF-driven chronic inflammation with CD-like ileal pathology depends on microbial triggers and that inflammation is transmitted via dysbiotic communities of commensal bacteria. Consistent with previously published data, maternally inherited factors seem not relevant to the initial development of dominant dysbiotic bacterial communities and disease susceptibility later in life.69 Understanding the true nature of a dysbiotic and disease-conditioning microbiota seems of essential importance to judge the risk of relapse in patients with IBD after therapeutic intervention or to achieve best possible clinical efficacy in FMT trials. The transfer of IBD-related microbiota in GF-TNFdeltaARE mice will be an excellent tool to gain translational insights into the role of complex microbial communities in the progression of CD-like inflammation.
Supplementary Material
Acknowledgments
The authors thank Arlette Darfeuille-Michaud for providing Escherichia coli LF82, Sigrid Kisling for histopathological scoring, Melanie Klein, Caroline Ziegler and Kathleen Eismann for outstanding technical work, Benjamin Scheer for help with maintenance of the mass-spectrometer, and Ludovica Butto and Elena Lobner for critical proofreading of the manuscript. The authors are grateful for using the facilities of the Centre for Chemical Microscopy (ProVIS) at the Helmholtz Centre for Environmental Research (supported by European Regional Development Funds (EFRE–Europe funds Saxony)) and the Helmholtz Association.
Footnotes
Correction notice: This article has been corrected since it was published Online First. The first author affiliation and the author correspondence address have both been corrected. The affiliation details for Aline Dupont and Mathias Hornef have also been updated.
Contributors: MS, TC and DH designed experiments and wrote the manuscript. MS, TC and JC performed experiments. MS, TC and IL analysed sequencing data. SBH, NJ and MvB performed metaproteomic studies. MB and AB generated germ-free mice. AD and MH performed Cryptdin-2 staining. TC and DH supervised and coordinated the project.
Funding: Generation and housing of germ-free TNFdeltaARE mice was financed by the Priority Program SPP 16565 of the German Research Foundation (DFG) granted to DH (HA3148/10-1) and to AB (953/5-1). All experiments with germ-free TNFdeltaARE were financed by DFG SPP1656 granted to DH (HA3148/8-1).
Competing interests: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: We would be happy to make our sequencing data available.
Ethics: The animal experiments were performed according to the German guidelines for animal care.
References
- 1.Baumgart DC, Sandborn WJ. Crohn's disease. Lancet 2012;380:1590–605. 10.1016/S0140-6736(12)60026-9 [DOI] [PubMed] [Google Scholar]
- 2.Ordas I, Eckmann L, Talamini M, et al. Ulcerative colitis. Lancet 2012;380:1606–19. 10.1016/S0140-6736(12)60150-0 [DOI] [PubMed] [Google Scholar]
- 3.Huttenhower C, Kostic AD, Xavier RJ. Inflammatory bowel disease as a model for translating the microbiome. Immunity 2014;40:843–54. 10.1016/j.immuni.2014.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol 2010;28:573–621. 10.1146/annurev-immunol-030409-101225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jostins L, Ripke S, Weersma RK, et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491:119–24. 10.1038/nature11582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mondot S, Barreau F, Al Nabhani Z, et al. Altered gut microbiota composition in immune-impaired Nod2(−/−) mice. Gut 2012;61:634–5. 10.1136/gutjnl-2011-300478 [DOI] [PubMed] [Google Scholar]
- 7.Elinav E, Strowig T, Kau AL, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 2011;145:745–57. 10.1016/j.cell.2011.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lupp C, Robertson ML, Wickham ME, et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007;2:204 10.1016/j.chom.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 9.Frank DN, St Amand AL, Feldman RA, et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 2007;104:13780–5. 10.1073/pnas.0706625104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010;464:59–65. 10.1038/nature08821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe 2014;15:382–92. 10.1016/j.chom.2014.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salzman NH, Hung K, Haribhai D, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol 2010;11:76–83. 10.1038/ni.1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clevers HC, Bevins CL. Paneth cells: maestros of the small intestinal crypts. Annu Rev Physiol 2013;75:289–311. 10.1146/annurev-physiol-030212-183744 [DOI] [PubMed] [Google Scholar]
- 14.Wehkamp J, Salzman NH, Porter E, et al. Reduced Paneth cell alpha-defensins in ileal Crohn's disease. Proc Natl Acad Sci USA 2005;102:18129–34. 10.1073/pnas.0505256102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sartor RB. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008;134:577–94. 10.1053/j.gastro.2007.11.059 [DOI] [PubMed] [Google Scholar]
- 16.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013;368:407–15. 10.1056/NEJMoa1205037 [DOI] [PubMed] [Google Scholar]
- 17.Angelberger S, Reinisch W, Makristathis A, et al. Temporal bacterial community dynamics vary among ulcerative colitis patients after fecal microbiota transplantation. Am J Gastroenterol 2013;108:1620–30. 10.1038/ajg.2013.257 [DOI] [PubMed] [Google Scholar]
- 18.Kump PK, Grochenig HP, Lackner S, et al. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis 2013;19:2155–65. 10.1097/MIB.0b013e31829ea325 [DOI] [PubMed] [Google Scholar]
- 19.Cui B, Feng Q, Wang H, et al. Fecal microbiota transplantation through mid-gut for refractory Crohn's disease: Safety, feasibility and efficacy trial results. J Gastroenterol Hepatol 2015;30:51–8. 10.1111/jgh.12727 [DOI] [PubMed] [Google Scholar]
- 20.Madsen KL, Doyle JS, Tavernini MM, et al. Antibiotic therapy attenuates colitis in interleukin 10 gene-deficient mice. Gastroenterology 2000;118:1094–105. 10.1016/S0016-5085(00)70362-3 [DOI] [PubMed] [Google Scholar]
- 21.Kim SC, Tonkonogy SL, Albright CA, et al. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology 2005;128:891–906. 10.1053/j.gastro.2005.02.009 [DOI] [PubMed] [Google Scholar]
- 22.Steck N, Hoffmann M, Sava IG, et al. Enterococcus faecalis metalloprotease compromises epithelial barrier and contributes to intestinal inflammation. Gastroenterology 2011;141:959–71. 10.1053/j.gastro.2011.05.035 [DOI] [PubMed] [Google Scholar]
- 23.Rath HC, Herfarth HH, Ikeda JS, et al. Normal luminal bacteria, especially Bacteroides species, mediate chronic colitis, gastritis, and arthritis in HLA-B27/human beta2 microglobulin transgenic rats. J Clin Invest 1996;98:945–53. 10.1172/JCI118878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kontoyiannis D, Pasparakis M, Pizarro TT, et al. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity 1999;10:387–98. 10.1016/S1074-7613(00)80038-2 [DOI] [PubMed] [Google Scholar]
- 25.Werner T, Wagner SJ, Martinez I, et al. Depletion of luminal iron alters the gut microbiota and prevents Crohn's disease-like ileitis. Gut 2011;60:325–33. 10.1136/gut.2010.216929 [DOI] [PubMed] [Google Scholar]
- 26.Erben U, Loddenkemper C, Doerfel K, et al. A guide to histomorphological evaluation of intestinal inflammation in mouse models. Int J Clin Exp Pathol 2014;7:4557–76. [PMC free article] [PubMed] [Google Scholar]
- 27.Katakura K, Lee J, Rachmilewitz D, et al. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest 2005;115:695–702. 10.1172/JCI22996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–8. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 29.Baur P, Martin FP, Gruber L, et al. Metabolic phenotyping of the Crohn's disease-like IBD etiopathology in the TNF(DeltaARE/WT) mouse model. J Proteome Res 2011;10:5523–35. 10.1021/pr2007973 [DOI] [PubMed] [Google Scholar]
- 30.Mahler Convenor M, Berard M, Feinstein R, et al. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. Lab Anim 2014;48:178–92. 10.1177/0023677213516312 [DOI] [PubMed] [Google Scholar]
- 31.Moghadamrad S, McCoy KD, Geuking MB, et al. Attenuated portal hypertension in germ-free mice: Function of bacterial flora on the development of mesenteric lymphatic and blood vessels. Hepatology 2015. doi:10.1002/hep.27698 10.1002/hep.27698 [DOI] [PubMed] [Google Scholar]
- 32.Inoue R, Tsuruta T, Nojima I, et al. Postnatal changes in the expression of genes for cryptdins 1–6 and the role of luminal bacteria in cryptdin gene expression in mouse small intestine. FEMS Immunol Med Microbiol 2008;52:407–16. 10.1111/j.1574-695X.2008.00390.x [DOI] [PubMed] [Google Scholar]
- 33.Boudeau J, Glasser AL, Masseret E, et al. Invasive ability of an Escherichia coli strain isolated from the ileal mucosa of a patient with Crohn's disease. Infect Immun 1999;67:4499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Conte MP, Longhi C, Marazzato M, et al. Adherent-invasive Escherichia coli (AIEC) in pediatric Crohn's disease patients: phenotypic and genetic pathogenic features. BMC Res Notes 2014;7:748 10.1186/1756-0500-7-748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 1998;66:5224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garrett WS, Gallini CA, Yatsunenko T, et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe 2010;8:292–300. 10.1016/j.chom.2010.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bamias G, Okazawa A, Rivera-Nieves J, et al. Commensal bacteria exacerbate intestinal inflammation but are not essential for the development of murine ileitis. J Immunol 2007;178:1809–18. 10.4049/jimmunol.178.3.1809 [DOI] [PubMed] [Google Scholar]
- 38.Matsumoto S, Okabe Y, Setoyama H, et al. Inflammatory bowel disease-like enteritis and caecitis in a senescence accelerated mouse P1/Yit strain. Gut 1998;43:71–8. 10.1136/gut.43.1.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bamias G, Dahman MI, Arseneau KO, et al. Intestinal-specific TNFalpha overexpression induces Crohn's-like ileitis in mice. PLoS One 2013;8:e72594 10.1371/journal.pone.0072594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Couturier-Maillard A, Secher T, Rehman A, et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J Clin Invest 2013;123:700–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bloom SM, Bijanki VN, Nava GM, et al. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell Host Microbe 2011;9:390–403. 10.1016/j.chom.2011.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flavell RA. Commensal Bacteroides species induce colitis in host-genotype-specific fashion in a mouse model of inflammatory bowel disease. Cell 2011;9:390–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scribano ML, Prantera C. Antibiotics and inflammatory bowel diseases. Dig Dis 2013;31:379–84. 10.1159/000354704 [DOI] [PubMed] [Google Scholar]
- 44.Khan KJ, Ullman TA, Ford AC, et al. Antibiotic therapy in inflammatory bowel disease: a systematic review and meta-analysis. Am J Gastroenterol 2011;106:661–73. 10.1038/ajg.2011.72 [DOI] [PubMed] [Google Scholar]
- 45.Ghouri YA, Richards DM, Rahimi EF, et al. Systematic review of randomized controlled trials of probiotics, prebiotics, and synbiotics in inflammatory bowel disease. Clin Exp Gastroenterol 2014;7:473–87. 10.2147/CEG.S27530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cadwell K, Liu JY, Brown SL, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 2008;456:259–63. 10.1038/nature07416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wehkamp J, Koslowski M, Wang G, et al. Barrier dysfunction due to distinct defensin deficiencies in small intestinal and colonic Crohn's disease. Mucosal Immunol 2008;1(Suppl 1):S67–74. 10.1038/mi.2008.48 [DOI] [PubMed] [Google Scholar]
- 48.Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol 2011;9:356–68. 10.1038/nrmicro2546 [DOI] [PubMed] [Google Scholar]
- 49.Cunliffe RN, Rose FR, Keyte J, et al. Human defensin 5 is stored in precursor form in normal Paneth cells and is expressed by some villous epithelial cells and by metaplastic Paneth cells in the colon in inflammatory bowel disease. Gut 2001;48:176–85. 10.1136/gut.48.2.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adolph TE, Tomczak MF, Niederreiter L, et al. Paneth cells as a site of origin for intestinal inflammation. Nature 2013;503:272–6. 10.1038/nature12599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaser A, Lee AH, Franke A, et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 2008;134:743–56. 10.1016/j.cell.2008.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gunther C, Martini E, Wittkopf N, et al. Caspase-8 regulates TNF-alpha-induced epithelial necroptosis and terminal ileitis. Nature 2011;477:335–9. 10.1038/nature10400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garabedian EM, Roberts LJ, McNevin MS, et al. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J Biol Chem 1997;272:23729–40. 10.1074/jbc.272.38.23729 [DOI] [PubMed] [Google Scholar]
- 54.Farin HF, Karthaus WR, Kujala P, et al. Paneth cell extrusion and release of antimicrobial products is directly controlled by immune cell-derived IFN-gamma. J Exp Med 2014;211:1393–405. 10.1084/jem.20130753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manichanh C, Rigottier-Gois L, Bonnaud E, et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut 2006;55:205–11. 10.1136/gut.2005.073817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perez-Munoz ME, Bergstrom K, Peng V, et al. Discordance between changes in the gut microbiota and pathogenicity in a mouse model of spontaneous colitis. Gut Microbes 2014;5:286–95. 10.4161/gmic.28622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morgan XC, Tickle TL, Sokol H, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 2012;13:R79 10.1186/gb-2012-13-9-r79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pickard JM, Maurice CF, Kinnebrew MA, et al. Rapid fucosylation of intestinal epithelium sustains host-commensal symbiosis in sickness. Nature 2014;514:638–41. 10.1038/nature13823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rausch P, Rehman A, Kunzel S, et al. Colonic mucosa-associated microbiota is influenced by an interaction of Crohn disease and FUT2 (Secretor) genotype. Proc Natl Acad Sci USA 2011;108:19030–5. 10.1073/pnas.1106408108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGovern DP, Jones MR, Taylor KD, et al. Fucosyltransferase 2 (FUT2) non-secretor status is associated with Crohn's disease. Hum Mol Genet 2010;19:3468–76. 10.1093/hmg/ddq248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garrett WS, Lord GM, Punit S, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell 2007;131:33–45. 10.1016/j.cell.2007.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Powell N, Walker AW, Stolarczyk E, et al. The transcription factor T-bet regulates intestinal inflammation mediated by interleukin-7 receptor+ innate lymphoid cells. Immunity 2012;37:674–84. 10.1016/j.immuni.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kaur S, Yawar M, Kumar PA, et al. Hungatella effluvii gen. nov., sp. nov., an obligately anaerobic bacterium isolated from an effluent treatment plant, and reclassification of Clostridium hathewayi as Hungatella hathewayi gen. nov., comb. nov. Int J Syst Evol Microbiol 2014;64(Pt 3):710–18. 10.1099/ijs.0.056986-0 [DOI] [PubMed] [Google Scholar]
- 64.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 2008;453:620–5. 10.1038/nature07008 [DOI] [PubMed] [Google Scholar]
- 65.von Schillde MA, Hormannsperger G, Weiher M, et al. Lactocepin secreted by Lactobacillus exerts anti-inflammatory effects by selectively degrading proinflammatory chemokines. Cell Host Microbe 2012;11:387–96. 10.1016/j.chom.2012.02.006 [DOI] [PubMed] [Google Scholar]
- 66.Devkota S, Wang Y, Musch MW, et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10-/- mice. Nature 2012;487:104–8. 10.1038/nature11225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Carvalho FA, Barnich N, Sivignon A, et al. Crohn's disease adherent-invasive Escherichia coli colonize and induce strong gut inflammation in transgenic mice expressing human CEACAM. J Exp Med 2009;206:2179–89. 10.1084/jem.20090741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Darfeuille-Michaud A, Boudeau J, Bulois P, et al. High prevalence of adherent-invasive Escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology 2004;127:412–21. 10.1053/j.gastro.2004.04.061 [DOI] [PubMed] [Google Scholar]
- 69.Hemmerling J, Heller K, Hormannsperger G, et al. Fetal exposure to maternal inflammation does not affect postnatal development of genetically-driven ileitis and colitis. PLoS One 2014;9:e98237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.