Abstract
Background
There is a discrepancy in clinical outcomes and the achieved recanalization rates with stent retrievers in the endovascular treatment of ischemic stroke. It is our hypothesis that procedural release of embolic particulate may be one contributor to poor outcomes and is a modifiable risk. The goal of this study is to assess various treatment strategies that reduce the risk of distal emboli.
Methods
Mechanical thrombectomy was simulated in a vascular phantom with collateral circulation. Hard fragment-prone clots (HFC) and soft elastic clots (SECs) were used to generate middle cerebral artery (MCA) occlusions that were retrieved by the Solitaire FR devices through (1) an 8 Fr balloon guide catheter (BGC), (2) a 5 Fr distal access catheter at the proximal aspect of the clot in the MCA (Solumbra), or (3) a 6 Fr guide catheter with the tip at the cervical internal carotid artery (guide catheter, GC). Results from mechanical thrombectomy were compared with those from direct aspiration using the Penumbra 5MAX catheter. The primary endpoint was the size distribution of emboli to the distribution of the middle and anterior cerebral arteries.
Results
Solumbra was the most efficient method for reducing HFC fragments (p<0.05) while BGC was the best method for preventing SEC fragmentation (p<0.05). The risk of forming HFC distal emboli (>1000 µm) was significantly increased using GC. A non-statistically significant benefit of direct aspiration was observed in several subgroups of emboli with size 50–1000 µm. However, compared with the stent-retriever mechanical thrombectomy techniques, direct aspiration significantly increased the risk of SEC fragmentation (<50 µm) by at least twofold.
Conclusions
The risk of distal embolization is affected by the catheterization technique and clot mechanics.
Keywords: Device, Intervention, Stroke, Thrombectomy
Introduction
Recent advances in technology, including the introduction of stent retrievers, have substantially increased efficacy outcomes and reduced adverse events in comparison with earlier generation technologies.1 2 Superior clinical outcomes in protocol-controlled studies where a balloon guide catheter (BGC) was required have been reported,3 4 and other access techniques are the subject of ongoing investigation.5 6
Clot debris generated during mechanical thrombectomy caused by variation in guide catheter utilization strategies may be a possible contributor to patient outcome.7 Arterial recanalization does not necessarily lead to brain tissue reperfusion,8 and lack of tissue reperfusion can happen as a result of clot fragmentation. Disrupted clots can cause distal embolization in the previously unaffected area or blockage of collateral flow to the potentially salvageable tissue.9 10 Procedural-related embolic complications have been noted with various thrombectomy devices.11–14
Previous in vitro studies showed that thousands of clot fragments were generated during the thrombectomy procedure.15 16 The majority of these clot fragments were relatively small (∼10 μm); therefore, the resulting small occlusions may not be appreciated with standard digitally subtracted angiography, resulting in inability to diagnose distal embolization.
Our goal is to assess various treatment strategies to determine which techniques can reduce the risk of distal embolization. In this study, the Solitaire thrombectomy procedure was modified using the three most common access catheter set-ups to evaluate distal emboli during the procedure. Additionally, we included a direct aspiration as a first pass technique (ADAPT) that uses a large-bore distal access catheter and primary aspiration for vessel recanalization.6 11 17 18 The frequency of emboli to the middle cerebral artery (MCA) and anterior cerebral artery (ACA) was assessed in an in vitro occlusion model.
Materials and methods
In vitro MCA occlusion model
A cerebrovascular model was built from clinical imaging data using a small-batch manufacturing process previously described.19 In brief, rapid prototyping was used to create a core shell mold for silicone infusion (Sylgard 184; Dow Corning, Midland, Michigan, USA). The core shell mold was dissolved in xylene after silicone curing, resulting in a transparent vascular replica.
Hard fragment-prone clot (HFC) and soft elastic clot (SEC) models were used in this study. The HFC was generated using thrombin-induced clotting of bovine blood (2.5 NIHU thrombin/mL blood) with addition of barium sulfate (1 g/10 mL blood). This clot model mimicked the aged cholesterol-rich clot that was prone to fragmentation during thrombectomy or aspiration.20 Mixing human blood with thrombin (2.5 NIHU thrombin/mL blood) formed the SEC model. The composition and mechanical behavior of the SEC were similar to those of the fresh red clots found commonly in patients with stroke.20 The clot (length −1 cm, diameter −4 mm) was injected into the silicone replica to form a MCA occlusion.
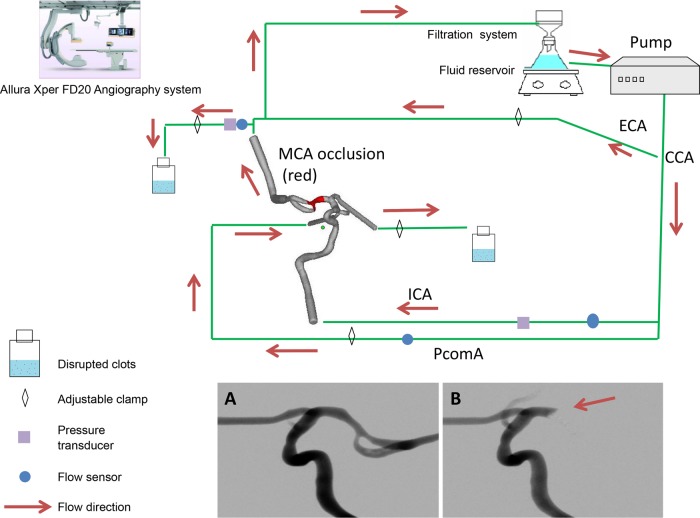
The silicone replica was connected into a flow loop driven by a peristaltic pump delivering oscillatory flow of normal saline. Adjustable clamps were used to mimic the peripheral resistance and maintain a physiologically representative pressure gradient. The system was fitted with flow (Transonic Systems, Ithaca, New York, USA) and pressure (Validyne Engineering, Northridge, California, USA) sensors for the real-time recording of internal carotid artery (ICA), MCA, ACA and posterior communicating artery (PComA) flow and pressure, respectively. The quantitative measure of procedure success was defined as the ratio of MCA flow following the thrombectomy divided by the pre-occlusion flow. Saline that traveled through the external carotid artery (ECA) was passed through the filtration system before re-entering the reservoir. A filter funnel with a pore size of 51 μm was attached to the saline reservoir to reduce the number of particles introduced into the flow loop.15 16 A schematic illustration of the flow loop used in this study is shown in figure 1. Notably, a small anastomosis was created between the ECA circuit and the distal MCA to simulate leptomeningeal collaterals. The PComA flow was derived from an anastomosis with the proximal ICA. By adjusting each of the resistors, the baseline flow conditions were matched to normal human phase contrast MR measurements for each of the vessels creating a hemodynamically representative cerebrovascular model.21 22
Figure 1.
Schematic illustration of the flow loop (arrows indicate the direction of flow). The common carotid artery (CCA) is connected to the flow pump and divided to form internal and external carotid arteries (ICA and ECA, respectively). The vascular phantom includes collateral circulation via the posterior communicating artery (PComA) and a small anastomosis with the distal middle cerebral artery that represents leptomeningeal collateral supply. Inset shows digital subtraction angiography before (A) and after (B) clot introduction. Note that the PComA flow is toward the anterior circulation during the occlusion and is transiently opacified due to a forceful injection of contrast. All distal emboli are captured in the effluent of the middle and anterior cerebral arteries.
Particulate analysis of both the MCA and ACA territories was performed. For each experiment, 700 mL saline was collected as a baseline for particle characterization to account for particles introduced into the system from the ambient environment. After thrombectomy or direct aspiration, clot fragments (>1000 µm) were first separated from the collected sample by a 10 mL graduated pipette. The number and the Feret diameter of these fragments were recorded. Characterization of the emboli smaller than 1000 µm was conducted using the Coulter principle (Multisizer 4 Coulter Counter; Beckman Coulter, Brea, California, USA). The average number of clot fragments beyond the baseline sample from eight replicate experiments in each group was used to plot the size distribution of the clot fragments. The results of particulate analysis were further grouped into three categories based on fragment diameter: 100–200 μm, 50–100 μm, and <50 μm. The number of the clot fragments with size >200 µm was summed to yield the size distribution due to the infrequency of the observation.
Flow restoration procedure
Four treatment groups were used. The Solitaire FR device was used to achieve recanalization according to the manufacturer's instructions for use (IFU) in groups 1, 2 and 3.
Group 1: Thrombectomy through a BGC positioned at the cervical ICA
An 8 Fr BGC was placed proximal to the occlusion at the cervical ICA through which the microcatheter (Rebar 18; Covidien, Irvine CA) was navigated over the wire (Xpedion 14; Covidien) beyond the distal end of the occlusive clot. The Solitaire device (4×20 mm) was deployed and allowed to engage the clot for 5 min. Prior to retriever retraction, the balloon was inflated to arrest antegrade flow in the ICA and the procedure continued per the IFU. Notably, during retrieval, aspiration with 20 mL syringes was applied.
Group 2: Solitaire thrombectomy in conjunction with thromboaspiration via a 5 Fr intracranial guide catheter at the origin of the MCA (Solumbra)
A triaxial assembly was used comprising of an 8 Fr guide catheter in the cervical ICA and a 5 Fr intracranial guide catheter (Navien 058; Covidien) positioned with the tip in the origin of the MCA.23 The microcatheter was navigated and placed distal to the clot via the intermediate catheter. Once the Solitaire FR device was deployed, the microcatheter was removed, leaving the aforesaid coaxial system in place. Aspiration was applied through the intermediate catheter during the thrombectomy.
Group 3: Thrombectomy through a 6 Fr guide catheter with the tip placed at the cervical ICA
The same protocol as that used in group 1 was applied in this group with the exception that a 6 Fr guide catheter (GC) was placed at the cervical ICA with aspiration being applied during clot retrieval.
Group 4: ADAPT
A Penumbra 5MAX aspiration catheter (Penumbra, Alameda, California, USA) was delivered via a 6 Fr shuttle sheath (Cook Medical, Bloomington, Indiana, USA) and navigated to the proximal aspect of the clot. Aspiration was applied through the catheter via a 20 mL syringe which offered favorable local aspiration. The devices were used in a non-IFU setting and the Penumbra aspiration pump was not used.
Eight experiments were carried out for each group. The maximum number of thrombectomy attempts was limited to three.
Endpoints
The primary endpoint was assessed by the risk of the embolic shower as indicated by the number and size distribution of the clot fragments. The secondary endpoints were the recanalization rate and the amount of flow restored.
Statistical analysis
Results were expressed as mean±SD. Data were analyzed using the programs SAS and Prism (Graphpad Software, San Diego, California, USA). A check was first made for normality of distribution. If it was passed, an unpaired t test or one-way analysis of variance was used. If the distributions were not normal, a non-parametric Kruskal–Wallis test was used. Significance was concluded when p<0.05.
Results
Characteristics of clot fragments
The number and size distribution of the disrupted clots generated during the thrombectomy procedure are shown in figure 2.
Figure 2.
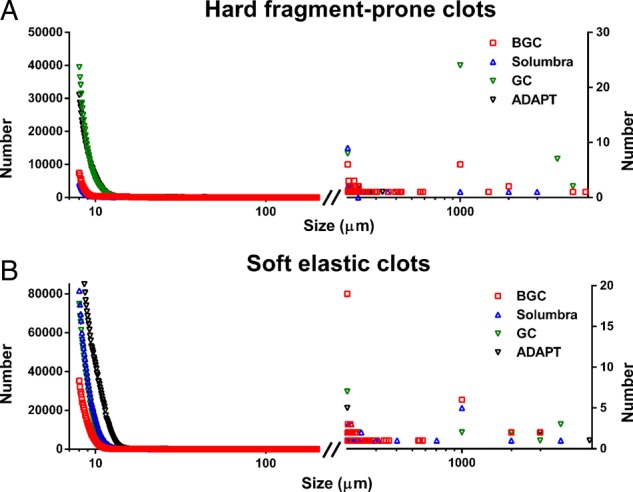
Cumulative size distribution of (A) hard and (B) soft clot fragments generated during the procedure. The majority of the clot fragments have size <20 µm. The x-axis is shown in log scale to indicate the large range of the data.
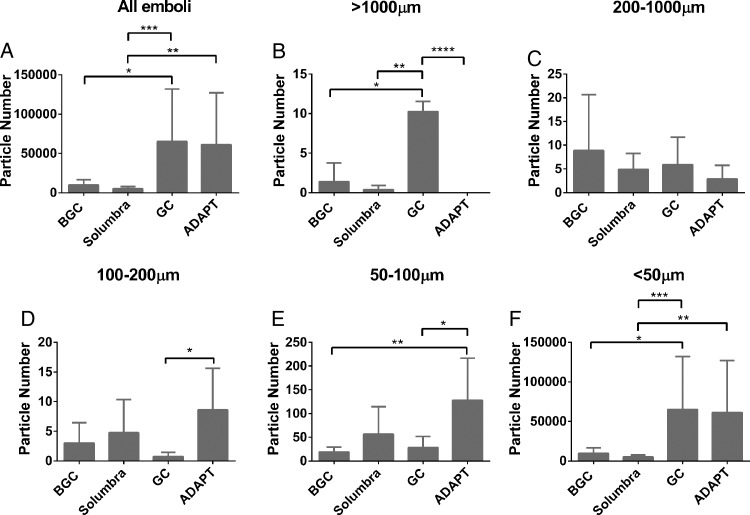
The Solumbra technique was the most efficient method for reducing the rate of clot fragmentation from the HFC model compared with the BGC, GC and ADAPT methods (figure 3A, p=0.0002). Further analysis on particles within different size ranges showed that using GC during mechanical thrombectomy significantly increased the risk of large hard emboli formation (>1000 µm) (figure 3B, p<0.0001). On average, the total number of hard clot fragments >1000 µm generated in the GC group was 27 times more than in the Solumbra group. Clot removal via ADAPT trended to be more efficient for reducing large HFC fragments among all four treatment strategies (size 200–1000 µm) (figure 3C, p>0.05). However, ADAPT led to high numbers of distal emboli in the size ranges 100–200 µm, 50–100 µm, and <50 µm (figure 3D–F).
Figure 3.
(A) Total number of hard fragment-prone clot (HFC) emboli. (B, C) Number of HFC clot fragments with size >1000 µm (B) and 200–1000 µm (C). The risk of large clot fragmentation (>1000 µm) is increased with the use of the guide catheter (GC). Average number of HFC fragments with size <200 µm collected during the experiments shown is in D (100–200 µm), E (50–100 µm) and F (<50 µm). The ADAPT technique generates more clot fragments than the other three mechanical thrombectomy techniques (D, E). *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
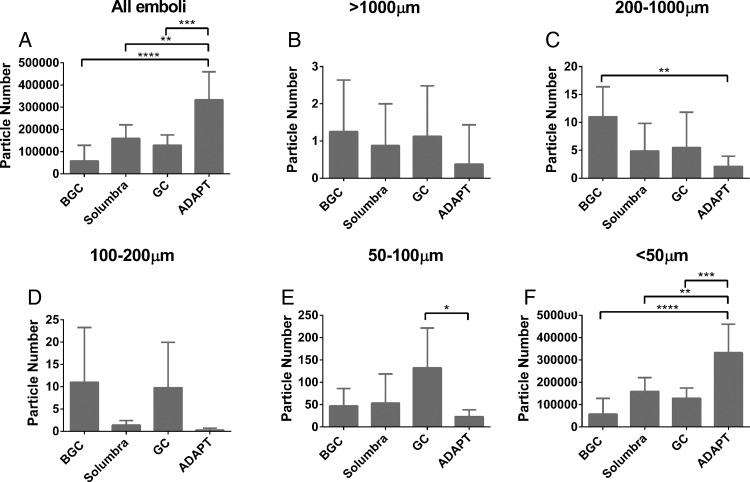
Temporary flow arrest during clot removal with the use of BGC offered at least twofold improvement in SEC fragmentation (figure 4A, p<0.0001). There were few large particle emboli (>1000 µm) in the SEC group with no statistical difference between the four different access techniques (figure 4B). In the size range 200–1000 µm the BGC technique generated the most emboli, followed by the GC, Solumbra, and ADAPT techniques (figure 4C, p<0.05 BGC versus ADAPT). For SEC fragments with size 100–200 µm (figure 4D) and 50–100 µm (figure 4E) there was a trend for ADAPT to offer a benefit. Figure 4F shows that the chance of SEC fragmentation was reduced by five times when employing thrombectomy with BGC compared to ADAPT (p<0.0001).
Figure 4.
(A) The total number of soft elastic clot (SEC) emboli is reduced with the use of the balloon guide catheter (BGC). Similar to the findings observed in the hard fragment-prone clots groups, the least number of SEC fragments with size >1000 µm (B) and 200–1000 µm (C) is found in the ADAPT group. The average number of SEC fragments with size 100–200 µm, 50–100 µm, and <50 µm is presented in D, E, and F, respectively. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
The distribution of clot fragments varied depending on the treatment strategy. Migration of clot fragments into the ACA territory was more frequently observed when the GC or ADAPT was used.
Flow restoration and recanalization rate
The pre-occlusion MCA, PcomA, and ACA flow rate was 131.6±3.2, 23.3±9.4, and 67±5.1 mL/min, respectively. Complete occlusion of the MCA was confirmed by both flow reduction acquired by the flow sensor and angiography (figure 1 inset).
The Penumbra 5MAX catheter was successfully delivered to the proximal aspect of the clot in the in vitro bench model of cerebral circulation. Complete flow restoration was achieved at the first attempt in all cases regardless of clot type in the Solumbra and ADAPT groups. Although there was no statistical difference in the mean number of pass attempts in the BGC, Solumbra, GC, and ADAPT groups (1.1, 1.0, 1.6, and 1.0, respectively), the number of attempts was, in general, one of the determinants of embolic shower produced during mechanical thrombectomy.
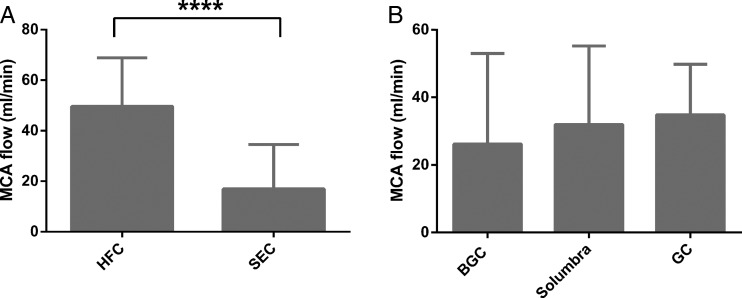
Deployment of Solitaire FR devices was found to be more effective for immediate MCA flow restoration in the HFC group than in the SEC group (49.6±19.2 mL/min vs 16.9±17.6 mL/min, figure 5A). An increase in immediate flow restoration was noted when the GC was used (34.8±14.9 mL/min) in place of the BGC (26.2±26.8 mL/min) or Solumbra (32.0±23.2 mL/min) (p>0.05, figure 5B).
Figure 5.
(A) Partial flow restoration after deployment of stentriever. There is a significant increase in partial flow restoration after deployment of the Solitaire FR device in the hard fragment-prone clots (HFC) group compared with the soft elastic clot (SEC) group. (B) Using the guide catheter (GC) achieves the greatest middle cerebral artery (MCA) flow restoration after device deployment.****p<0.0001.
A mean flow rate of 16.6±24.2 mL/min was measured in the MCA after balloon occlusion. There was no significant difference between the MCA flow before and after balloon inflation (26.2±26.8 vs 16.6±24.2 mL/min) when Solitaire FR devices were employed (figure 6A). Temporary occlusion with the BGC and direct aspiration through the 5MAX resulted in flow reversal in the MCA (figure 6B).
Figure 6.
(A) With the representative collateral circulation, temporary internal carotid artery (ICA) occlusion provided by the balloon guide catheter (BGC) during mechanical thrombectomy does not significantly change the restored middle cerebral artery (MCA) flow. (B) Flow reversal in the MCA due to aspiration during clot retrieval is observed in the BGC and ADAPT groups. *p<0.05, ****p<0.0001.
Discussion
Emboli (clot fragments) to previously unaffected areas or distal territories may occlude or reduce the collateral flow into the penumbra region and worsen the clinical outcome in patients with acute ischemic stroke. This embolic complication has been addressed in previous clinical studies as well as in prior in vitro work.9 24–26 We found that thousands of emboli were generated during the thrombectomy procedure in an experimental MCA occlusion model.15 16 To date, different stroke treatments have been developed to prevent distal embolization.11 27 In this study we quantified the number and size of the emboli and also studied the emboli distribution produced during the thrombectomy procedure. Our results suggest that the Solumbra and BGC techniques are efficient in reducing the risk of HFC and SEC fragmentation, respectively.
Efficient clot retrieval observed in the Solumbra group implied that aspiration location was related to suction efficacy. The tip of the 5 Fr Navien catheter used in the Solumbra group was placed in the origin of the MCA in this study, proximal to the occlusion. Although the 5 Fr Navien catheter had the smallest luminal diameter (compared with 8 Fr BGC and 6 Fr GC), the Solumbra group had the shortest distance between the occlusion and catheter tip and the greatest catheter inner diameter/vessel diameter ratio. The efficacy of the Solumbra versus the BGC approaches appeared to depend on clot mechanics and assays are needed to assess clot properties in vivo to enlist the best technique. Aspiration with temporary flow arrest using the 8 Fr BGC increased suction efficacy, thereby facilitating clot removal and minimizing the chance of distal embolization. These in vitro findings are consistent with the results reported in a recent analysis of the North American Solitaire Stent-Retriever Acute Stroke Registry (NASA).4 Shorter procedure time and less rescue therapy were found in the patients with BGC compared with those without BGC.
The main disparity between our results and the NASA results is the number of distal emboli generated during the procedure. The NASA data showed no difference in distal emboli between the BGC and non-BGC groups. However, we found that the risk of forming clot fragments was affected by the treatment strategies. The disparity may be due to the inability of the current imaging modalities to observe microemboli <20 µm, which account for more than 90% of the clot fragments generated during the procedure. The presence of microemboli <20 µm may occlude possible collateral routes and cerebral microcirculation with a diameter <10 µm, and that can worsen clinical outcomes.
The neurologic complications related to emboli release in conjunction with mechanical thrombectomy are little known. In the previous carotid artery stenting work it was demonstrated that the severity of neurologic complications is associated with the degree of embolization.28 In addition, the risk of neurologic events associated with plaque fragments >1000 μm increases as these fragments can occlude the cerebral arteries or the communicating arteries of the circle of Willis.29 In general, a large number of small particles are necessary to produce the same effect as a small number of large particles. Rapp and other researchers attempted to correlate emboli character with neural deficit by injecting human plaque fragments <200 μm into male Sprague-Dawley rats. Their MRI results showed that fragments <200 μm resulted in infarction. Moreover, one of five animals died 24 h after injection of 100–200 μm calcified fragments.30
Preliminary comparisons of the performance of direct thromboaspiration against mechanical thrombectomy coupled with thromboaspiration are presented in this study. In this model, the direct aspiration technique produced rapid and reliable recanalization, but with increased risk of distal embolization. The results revealed the potential of ADAPT as an alternative treatment for acute ischemic stroke.
Our study has several limitations. The cerebrovascular model described employs hemodynamically representative collateral pathways via the PComA and leptomeningeal supply. However, since flow through the ACA is collected for particle analysis, there is no anterior communicating artery in this model. Consequently, there may be an increased risk of ACA distal emboli. Moreover, since the vascular replica does not interact with the occlusive clot, efficacy measures for different techniques are likely to be overestimated. Finally, microemboli from the clot may be subject to autolysis that is not modeled in this in vitro experiment.
Conclusion
The risk of distal embolization is affected by the catheterization technique and clot mechanics. Use of the BGC technique during Solitaire FR thrombectomy was associated with a lower rate of SEC embolization. The Solumbra technique was shown to reduce the risk of distal embolization in several hard clot subgroups. When encountering hard clot, use of the Solumbra or ADAPT techniques in addition to the BGC may provide an additional reduction in distal emboli and may be considered for comprehensive distal emboli reduction. Future work will seek to determine if such combined methods provide a benefit for the reduction of distal emboli.
Footnotes
Contributors: J-YC: study design, data acquisition, data analysis, data interpretation, manuscript preparation. ASP: study design, data acquisition, data interpretation, manuscript revision. AKW: study design, data interpretation, manuscript revision. MJG: study design, data acquisition, data analysis, data interpretation, manuscript preparation.
Funding: This work was supported in part by eV3 Neurovascular/Covidien and Philips Healthcare. The content is solely the responsibility of the authors and does not represent the official views of eV3 Neurovascular or Philips Healthcare.
Competing interests: AKW is a consultant for Stryker Neurovascular; research grant: Philips Healthcare, Wyss Institute; speaker: Harvard Postgraduate Course, Miami Cardiovascular Institute. MJG is a consultant for Codman Neurovascular and Stryker Neurovascular; research grants: NIH, eV3/Covidien Neurovascular, Codman Neurovascular, Fraunhofer Institute, Wyss Institute, Philips Healthcare, Stryker Neurovascular, Silk Road, Lazarus-Effect.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Unpublished data may be available to academic researchers on a per request basis to the corresponding author.
References
- 1.Nogueira RG, Lutsep HL, Gupta R, et al. . Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet 2012;380:1231–40. 10.1016/S0140-6736(12)61299-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saver JL, Jahan R, Levy EI, et al. . Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet 2012;380:1241–9. 10.1016/S0140-6736(12)61384-1 [DOI] [PubMed] [Google Scholar]
- 3.Nguyen TN, Malisch T, Castonguay A, et al. . Balloon guide catheter improves recanalisation, procedure time, and clinical outcomes with Solitaire in acute stroke: analysis of the NASA Registry. J Neurointerv Surg 2013;5:A2–3. 10.1136/neurintsurg-2013-010870.4 [DOI] [Google Scholar]
- 4.Nguyen TN, Malisch T, Castonguay AC, et al. . Balloon guide catheter improves revascularization and clinical outcomes with the Solitaire device: analysis of the North American Solitaire Acute Stroke Registry. Stroke 2013;45:141–5. 10.1161/STROKEAHA.113.002407 [DOI] [PubMed] [Google Scholar]
- 5.Humphries W, Hoit D, Doss VT, et al. . Distal aspiration with retrievable stent assisted thrombectomy for the treatment of acute ischemic stroke. J Neurointerv Surg 2015;7:90–4.. [DOI] [PubMed] [Google Scholar]
- 6.Kang DH, Kim YW, Hwang YH, et al. . Switching strategy for mechanical thrombectomy of acute large vessel occlusion in the anterior circulation. Stroke 2013;44:3577–9. 10.1161/STROKEAHA.113.002673 [DOI] [PubMed] [Google Scholar]
- 7.Costalat V, Lobotesis K, Machi P, et al. . Prognostic factors related to clinical outcome following thrombectomy in ischemic stroke (RECOST study). 50 patients prospective study. Eur J Radiol 2012;81:4075–82. 10.1016/j.ejrad.2012.07.012 [DOI] [PubMed] [Google Scholar]
- 8.Molina CA. Reperfusion therapies for acute ischemic stroke: current pharmacological and mechanical approaches. Stroke 2010;42:S16–19. 10.1161/STROKEAHA.110.598763 [DOI] [PubMed] [Google Scholar]
- 9.Kurre W, Vorlaender K, Aguilar-Perez M, et al. . Frequency and relevance of anterior cerebral artery embolism caused by mechanical thrombectomy of middle cerebral artery occlusion. AJNR Am J Neuroradiol 2013;34:1606–11. 10.3174/ajnr.A3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gascou G, Lobotesis K, Machi P, et al. . Stent retrievers in acute ischemic stroke: complications and failures during the perioperative period. AJNR Am J Neuroradiol 2013;35:734–40. 10.3174/ajnr.A3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turk AS, Spiotta A, Frei D, et al. . Initial clinical experience with the ADAPT technique: a direct aspiration first pass technique for stroke thrombectomy. J Neurointerv Surg 2013;6:231–7. 10.1136/neurintsurg-2013-010713 [DOI] [PubMed] [Google Scholar]
- 12.Tomsick TA, Khatri P, Jovin T, et al. . Equipoise among recanalization strategies. Neurology 2010;74:1069–76. 10.1212/WNL.0b013e3181d76b8f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mokin M, Kass-Hout T, Levy EI. Solitaire FR—a promising new device for acute ischemic stroke treatment. World Neurosurg 2012;78:557–8. 10.1016/j.wneu.2012.10.012 [DOI] [PubMed] [Google Scholar]
- 14.Dorn F, Stehle S, Lockau H, et al. . Endovascular treatment of acute intracerebral artery occlusions with the solitaire stent: single-centre experience with 108 recanalization procedures. Cerebrovasc Dis 2012;34:70–7. 10.1159/000338903 [DOI] [PubMed] [Google Scholar]
- 15.Chueh JY, Kuhn AL, Puri AS, et al. . Reduction in distal emboli with proximal flow control during mechanical thrombectomy: a quantitative in vitro study. Stroke 2013;44:1396–401. 10.1161/STROKEAHA.111.670463 [DOI] [PubMed] [Google Scholar]
- 16.Chueh JY, Wakhloo AK, Gounis MJ. Effectiveness of mechanical endovascular thrombectomy in a model system of cerebrovascular occlusion. AJNR Am J Neuroradiol 2012;33:1998–2003. 10.3174/ajnr.A3103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Turk AS, Frei D, Fiorella D, et al. . ADAPT FAST study: a direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg 2014;6:260–4. 10.1136/neurintsurg-2014-011125 [DOI] [PubMed] [Google Scholar]
- 18.Jankowitz B, Aghaebrahim A, Zirra A, et al. . Manual aspiration thrombectomy: adjunctive endovascular recanalization technique in acute stroke interventions. Stroke 2012;43:1408–11. 10.1161/STROKEAHA.111.646117 [DOI] [PubMed] [Google Scholar]
- 19.Chueh JY, Wakhloo AK, Gounis MJ. Neurovascular modeling: small-batch manufacturing of silicone vascular replicas. AJNR Am J Neuroradiol 2009;30:1159–64. 10.3174/ajnr.A1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chueh JY, Wakhloo AK, Hendricks GH, et al. . Mechanical characterization of thromboemboli in acute ischemic stroke and laboratory embolus analogs. AJNR Am J Neuroradiol 2011;32:1237–44. 10.3174/ajnr.A2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Ooij P, Zwanenburg JJ, Visser F, et al. . Quantification and visualization of flow in the Circle of Willis: time-resolved three-dimensional phase contrast MRI at 7T compared with 3T. Magn Reson Med 2013;69:868–76. 10.1002/mrm.24317 [DOI] [PubMed] [Google Scholar]
- 22.Wahlin A, Ambarki K, Birgander R, et al. . Measuring pulsatile flow in cerebral arteries using 4D phase-contrast MR imaging. AJNR Am J Neuroradiol 2013;34:1740–5. 10.3174/ajnr.A3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mokin M, Dumont TM, Veznedaroglu E, et al. . Solitaire Flow Restoration thrombectomy for acute ischemic stroke: retrospective multicenter analysis of early postmarket experience after FDA approval. Neurosurgery 2013;73:19–25; discussion 25–6 10.1227/01.neu.0000429859.96652.57 [DOI] [PubMed] [Google Scholar]
- 24.Akins PT, Amar AP, Pakbaz RS, et al. . Complications of endovascular treatment for acute stroke in the SWIFT trial with solitaire and Merci devices. AJNR Am J Neuroradiol 2013;35:524–8. 10.3174/ajnr.A3707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soize S, Barbe C, Kadziolka K, et al. . Predictive factors of outcome and hemorrhage after acute ischemic stroke treated by mechanical thrombectomy with a stent-retriever. Neuroradiology 2013;55:977–87. 10.1007/s00234-013-1191-4 [DOI] [PubMed] [Google Scholar]
- 26.Todo A, Minaeian A, Sahni R, et al. . Incidence and outcome of procedural distal emboli using the Penumbra thrombectomy for acute stroke. J Neurointerv Surg 2012;5:135–8. 10.1136/neurintsurg-2011-010216 [DOI] [PubMed] [Google Scholar]
- 27.Deshaies EM. Tri-axial system using the Solitaire-FR and Penumbra Aspiration Microcatheter for acute mechanical thrombectomy. J Clin Neurosci 2013;20:1303–5. 10.1016/j.jocn.2012.10.037 [DOI] [PubMed] [Google Scholar]
- 28.Sprouse LR II, Peeters P, Bosiers M. The capture of visible debris by distal cerebral protection filters during carotid artery stenting: is it predictable? J Vasc Surg 2005;41:950–5. 10.1016/j.jvs.2005.02.048 [DOI] [PubMed] [Google Scholar]
- 29.Coggia M, Goeau-Brissonniere O, Duval JL, et al. . Embolic risk of the different stages of carotid bifurcation balloon angioplasty: an experimental study. J Vasc Surg 2000;31:550–7. 10.1067/mva.2000.102730 [DOI] [PubMed] [Google Scholar]
- 30.Rapp JH, Pan XM, Yu B, et al. . Cerebral ischemia and infarction from atheroemboli <100 micron in size. Stroke 2003;34:1976–80. 10.1161/01.STR.0000083400.80296.38 [DOI] [PubMed] [Google Scholar]