Abstract
Aim
To estimate the prevalence of, and assess factors associated with, diabetes and prediabetes in three South Asian cities.
Methods
Using a multi-stage cluster random sample representative of each city, 16,288 subjects aged ≥20 years (Chennai: 6906, Delhi: 5365 and Karachi: 4017) were recruited to the Centre for cArdiometabolic Risk Reduction in South-Asia (CARRS) Study. Fasting plasma glucose (FPG) and glycosylated hemoglobin (HbA1c) were measured in 13720 subjects. Prediabetes was defined as FPG 100-125mg/dl (5.6-6.9 mmol/l) and/or HbA1c 5.7-6.4% (39-46mmol/mol) and diabetes as self-report and/or drug treatment for diabetes and/or FPG ≥126 mg/dl (≥7.0mmol/l) and/or HbA1c ≥6.5% (48mmol/mol). We assessed factors associated with diabetes and prediabetes using polytomous logistic regression models.
Results
Overall 47.3-73.1% of the population had either diabetes or prediabetes: Chennai 60.7% [95%CI: 59.0-62.4%] (diabetes-22.8% [21.5-24.1%], prediabetes-37.9% [36.1-39.7%]); Delhi 72.7% [70.6-74.9%] (diabetes-25.2% [23.6-26.8%], prediabetes-47.6% [45.6-49.5%]); and Karachi 47.4% [45.7-49.1%]; (diabetes-16.3% [15.2-17.3%], prediabetes-31.1% [29.5-32.8%], respectively). Proportions of self-reported diabetes were 55.1%, 39.0%, and 48.0% in Chennai, Delhi, and Karachi, respectively. City, age, family history of diabetes, generalized obesity, abdominal obesity, body fat, high cholesterol, high triglyceride, and low HDL cholesterol levels were each independently associated with prediabetes, while the same factors plus waist-to-height ratio and hypertension were associated with diabetes.
Conclusion
Six in ten adults in large South Asian cities have either diabetes or prediabetes. These data call for urgent action to prevent diabetes in South Asia.
Keywords: Diabetes, prediabetes, dysglycemia, urban, South Asians, CARRS Surveillance
Diabetes is a costly and growing global health challenge and the recent International Diabetes Federation (IDF) Atlas1 counts 387 million people with diabetes worldwide, with 246 million residing in urban areas. Furthermore, South Asia is at the epicenter of this pandemic with 73.8 million people with diabetes residing in India and Pakistan alone.
Diabetes is frequently undiagnosed, because hyperglycemia is often asymptomatic, but carries significant long-term morbidity. Diabetes complications like retinopathy were already present at the time of diagnosis among 1.5-31%2 of people with and 7-8% of those with prediabetes.3,4 Although the risk factors associated with diabetes and prediabetes are largely similar across populations, their expression and intensity may vary widely between ethnic groups and countries.5 This heterogeneity highlights the importance of sound ongoing epidemiological assessments of diabetes and its risk factors on a regular basis. We present data from the population-based representative Center for Cardio-metabolic Risk Reduction in South Asia (CARRS) Study6 in three large cities in south Asia, namely Chennai and Delhi in India and Karachi in Pakistan to provide up-to-date data for 2011 on the prevalence of and risk factors associated with diabetes and prediabetes among south Asians using standardized methods, venous plasma glucose plus glycated hemoglobin (HbA1c).
Research Design and Methods
Population
The CARRS Study recruited representative cohorts of three metropolitan urban cities, namely Chennai, Delhi and Karachi.6 In 2010-2011, the populations of these three cities totaled over 35 million people, and these sites were chosen as they are metropolitan cities with large, growing, and heterogeneous populations with diversity in socio-economic, demographic, epidemiologic, and nutrition/lifestyle transitions.
The detailed CARRS protocol that includes the study design and methods has been published elsewhere.6 Households were selected in each of the three cities using multi-stage cluster random sampling techniques. Each city has its own distinctive municipal sub-divisions, encompassing municipal corporations, wards, and Census Enumeration Blocks (CEB) which were used sequentially as sampling frames to randomly select households. While wards were the primary sampling units (PSUs) for Chennai and Delhi, CEBs or clusters were the PSUs for Karachi. STATA version 10.1 (Statacorp, TX) and data from the census (2001 for Chennai and Delhi; 1998 for Karachi) were used to randomly select the wards, CEBs, and households. To give each household an equal chance of being selected for the study and to identify households constructed after the last census survey, manual listing and mapping of all households in each CEB was done before randomly selecting them.
Two participants, one male and one female, aged 20 years or older, were selected from each household. Those excluded from the study were pregnant women and bed-ridden individuals. Two methods were used for within household sampling. First, for households with one to two adults (≥20 years), the sampling strategy described in the 2002 Health Information National Trends Study (HINTS)7 was used. According to HINTS, one or both individuals (one male, one female) were selected and enrolled into the study based on eligibility criteria and informed consent. Second, for households with more than two eligible adults, the “Kish method” used in the WHO's STEPS surveys8 was applied. There are two main steps in KISH method. First, all eligible participants from the household are ranked according to age in decreasing orders (males followed by females). Participants are then selected using KISH table identifying the last digit of household and number of eligible participants.
The study was approved by the Institutional Review Boards (IRBs) of the Public Health Foundation of India, New Delhi, All India Institute of Medical Sciences, New Delhi, Madras Diabetes Research Foundation, Chennai, India, Aga Khan University, Karachi, Pakistan, and Emory University, Atlanta, USA. In addition, the study has received regulatory approval from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), USA, and the Health Ministry Screening Committee of India, New Delhi. All respondents gave written informed consent, themselves or through a next of kin/family member in the case of illiterate respondents, prior to enrollment and participation in the study.
Sample size determination
Utilizing risk factor prevalence estimates from previously published Indian and Pakistani studies and anticipating a response rate of 80% with a design effect factor of 1.5 (to account for cluster sampling), the sample size estimates were generated for males and females in three age strata in each urban setting. The highest required sample size was 3,983 rounded-off to 4000 participants, which permits each site to reliably estimate one or more of the CMD risk factors for each of the gender and age strata leading to a total sample size of 12,000.
A total of 17,274 individuals in 10,002 households were approached in the three study sites (7,596 participants in Chennai, 5,420 in Delhi, 4,258 in Karachi). From these, a total of 16,288 participants were recruited (the overall response rate was 94.3% at the participant level; 6,906 Chennai [90.9%], 5,365 Delhi [98.9%], and 4,017 Karachi [94.3%]). 13,720 of the participants (84.2%) recruited into the study contributed bio-specimens (fasting plasma glucose and HbA1c) [Chennai (n=5961) 86.3%, Delhi (n=4244) 79.1% and Karachi (n= 3515) 87.5%].
Measurements
Comprehensive and uniform data collection instruments were used to capture measurements in all three sites. A summary of all surveillance indicators, measures, methods and instruments used in the study has been published in detail.6 In brief, a questionnaire was administered to collect information regarding demographic, socio-economic, behavioral, and past and present health status of the participant. Household data were collected through interviewer administered questionnaires, which was pilot tested for face and construct validity prior to use in the study.
Anthropometric measurements included height, weight, and waist measurements, using standardized techniques. Body mass index (BMI) was calculated as weight in kilograms divided by height in meter squared. The waist measurement was taken at the midpoint between the lowest rib and top of the hip bone (iliac crest). Two readings were taken and the mean of the two was used. Blood pressure was recorded in the sitting position using electronic sphygmomanometer; Omron HEM-7080 and HEM-7080IT-E; Omron Corporation, Tokyo, Japan. Two readings were taken in the resting position, five minutes apart, and the mean of the two was used. All the anthropometric devices and procedures were standardized. Centralized training of trainers was conducted in English for the team leaders and coordinators from three sites before the start of the survey. Coordinators and team leaders in-turn trained the respective field staff in local language at sites using standardized training manuals. Field staff were regularly monitored using check lists and re-trained every three months (more frequently if there were deviations from protocol during monitoring visits) to maintain constant and high quality data collection throughout the study recruitment.
Fasting blood samples were collected from each participant and transported from field sites in cold chain to the site-specific laboratories for analysis. Sample aliquots were also stored in cryo-vials at -80 degrees Celsius for future studies. Fasting plasma glucose was estimated by hexokinase/kinetic in Chennai and Delhi site and by glucose oxidase/endpoint method in Karachi. Intra-class correlation (ICC) between Chennai and Delhi lab for glucose values in a subset of randomly chosen 73 subjects was 0·99 (95%CI: 0.98–0.99), implying excellent agreement. The external quality assessment of the three laboratories was performed periodically by RIQAS (Randox International Quality Assessment Scheme).
Total cholesterol was estimated by Cholesterol Oxidase Peroxidase (CHOD-POD) method in Chennai and Delhi and by enzymatic Colorimetric (CHOD-PAP) method in Karachi. Triglycerides were estimated by enzymatic (GPO-PAP) methods, HDL cholesterol by direct method, LDL cholesterol by Friedwald formula,9 and glycated hemoglobin (HbA1c) by high performance liquid chromatography (HPLC) method in all three sites.
Definitions
Self-reported diabetes was defined as subjects with self-reported history of diabetes and/or on drug treatment for diabetes. Self-reported history of diabetes was checked against medical records for validity, which also helped to define the date and year of diagnosis. Newly diagnosed diabetes was defined as no prior diagnosis of diabetes, but fasting plasma glucose (FPG) ≥126mg/dl (7.0mmol/l) and/or HbA1c ≥6.5% (48mmol/mol). Total diabetes was defined as known and newly diagnosed diabetes. Prediabetes was defined as no prior diagnosis of diabetes and FPG 100-125 mg/dl (5.6-6.9 mmol/l) (impaired fasting glucose [IFG]) and/or HbA1c 5.7-6.4% (39-46 mmol/mol).10-12 Dysglycemia was defined as the presence of either prediabetes or total diabetes.
Hypertension was defined as being on antihypertensive medications or a systolic blood pressure ≥140 mmHg and/or a diastolic blood pressure ≥90 mmHg.13 Generalized obesity was defined as BMI ≥25 kg/m2 and abdominal obesity was defined as waist ≥90cm (males), ≥80cm (females) using Asia-Pacific guidelines for South Asians.14 Waist-to-height ratio, index for assessing central fat distribution, of ≥0.5 is considered as risk for cardiovascular disease and diabetes.15
Occupation was categorized as professional [e.g., doctor, lawyer, large business owner], trained [e.g., clerical, teacher, middle-level farmer], skilled [e.g., small business owner, skilled manual labourer, small farmer], semi-skilled [e.g., semi-skilled manual laborer, carpenter], unskilled [e.g., landless laborer, unskilled manual laborer).
Statistical analysis
Statistical analyses were done using STATA (version 12.1, TX: StataCorp LP, USA). This analysis included 16,287 non-pregnant adults after excluding a single transgender participant. Weighted prevalence was calculated with the crude prevalence adjusted for sampling weights. For age-standardized prevalence, the prevalence estimates were age-and sex- standardized to the 2010 South Asia regional population. To achieve, this, the five-year population projection tables (that account for age, sex, mortality, fertility, and migration) were used separately for India and Pakistan for 2010, provided by the World Bank. The 95% confidence intervals (CI) for diabetes and prediabetes were estimated. Estimates of continuous variables were summarized as mean±standard error (SE) for normally distributed data or median (IQR; interquartile range) for non-normal data; whereas categorical variables as proportions. Comparison of means of quantitative variables across different diabetes categories was done by analysis of variance (ANOVA) for normal data or Kruskal Wallis test for non-normal data. Association between categorical variables was checked using Chi-square test.
Polytomous logistic regression models were used to assess the risk factors associated with diabetes and prediabetes using normoglycemia as the reference category. Bivariate regression analysis was performed first to identify the variables associated with diabetes status and those variables shown a Wald test p-value<0.2 were considered for multivariate analysis. Odds ratio (OR) of diabetes and prediabetes and their 95%CI were estimated for all cities together after controlling for city, gender, age per ten year increment, current use of smoking, family history, generalized obesity, abdominal obesity, waist-to-height ratio, body fat%, hypertension, hypercholesterolemia, hypertriglyceridemia, and low HDL cholesterol.
It is common to discard individuals with missing variables, but imputing missing values of the variable frequently leads to more efficient estimates of the regression coefficients when the imputation is based on the non-missing predictor variables.16 Hence, we generated ten imputed datasets using multiple imputation using chained equations (MICE) for imputing outcomes (FPG and HbA1c) along with missing observations in the exposure variables of interest.
Results
Table 1 shows the age-standardized and weighted prevalence of diabetes, prediabetes and dysglycemia (diabetes/prediabetes) for the three cities. The age-standardized prevalence of total diabetes (based on either self-reported or FPG or HbA1c) was 22.8% (95%CI: 21.5-24.1%) in Chennai, 25.2% (23.6-26.8%) in Delhi and 16.3% (15.2-17.3%) in Karachi and of which the prevalence of newly diagnosed diabetes were 11.7%(10.5-12.8%), 17.5% (16.1-19.0%) and 9.4% (8.5-10.3%) in Chennai, Delhi, and Karachi respectively. The prevalence of prediabetes was 37.9% (36.1-39.7%) in Chennai, 47.6% (45.6-49.5%) in Delhi, and 31.1% (29.5-32.8%) in Karachi. In Chennai, females had significantly higher prevalence of diabetes, prediabetes and dysglycemia compared to males, except for newly diagnosed diabetic subjects. However, in Delhi & Karachi, no gender difference in the prevalence was observed, except for newly diagnosed diabetes based on FBS alone, where males had significantly higher prevalence. Among the self-reported diabetic subjects, the proportion of those with glucose or HbA1c levels above the diagnostic criteria was 86.5% (Chennai–83.1%; Delhi–90.8% and Karachi–85.6%).
Table 1. Prevalence of diabetes and prediabetes among Chennai, Delhi, and Karachi residents aged ≥20 years, CARRS (n=16,287).
| Prevalence rates | Chennai (n=6906) | Delhi (n=5364) | Karachi (n=4017) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Male (n=3188) | Female (n=3718) | Male (n=2680) | Female (n=2684) | Male (n=1892) | Female (n=2125) | |||||||
|
| ||||||||||||
| % | 95%CI | % | 95%CI | % | 95%CI | % | 95%CI | % | 95%CI | % | 95%CI | |
| Age-standardized prevalence (based on HbA1c & FBS definition) | ||||||||||||
| NDD: FPG ≥7.0 mmol/l and/or HbA1c ≥48 mmol/mol | 11.4 | [9.7,13.1] | 11.9 | [9.9,13.9] | 16.8 | [14.9,18.7] | 18.2 | [16.2,20.1] | 9.8 | [8.4,11.1] | 9.0 | [7.8,10.2] |
| DM: FPG ≥7.0 mmol/l and/or HbA1c ≥48 mmol/mol or self-report | 20.8 | [18.9,22.8] | 24.7* | [22.5,26.9] | 24.3 | [22.2,26.5] | 26.0 | [23.9,28.2] | 15.8 | [14.5,17.2] | 16.8 | [15.1,18.5] |
| PreDM: FPG 5.6-6.9 mmol/l and/or HbA1c 39-46 mmol/mol | 35.2 | [32,38.3] | 40.7** | [38.4,42.9] | 48.0 | [45.2,50.8] | 47.1 | [44.3,49.9] | 31.7 | [28.8,34.7] | 30.5 | [28.3,32.7] |
| PreDM: FPG 6.1-6.9 mmol/l and/or HbA1c 39-46 mmol/mol | 31.8 | [28.6,34.9] | 36.8** | [34.6,39.1] | 39.8 | [36.5,43.2] | 39.1 | [35.9,42.3] | 28.1 | [25.4,30.8] | 26.0 | [23.9,28.1] |
| Dyglycemia: DMor PreDM | 56.0 | [53,59] | 65.4** | [63.3,67.5] | 72.3 | [69.1,75.6] | 73.1 | [69.7,76.6] | 47.5 | [44.6,50.5] | 47.3 | [44.9,49.7] |
|
| ||||||||||||
| Age-standardized prevalence (based on FBS definition) | ||||||||||||
| NDD: FPG ≥7.0 mmol/l | 5.2 | [4.1,6.3] | 4.7 | [3.6,5.9] | 6.1 | [5,7.3] | 5.6* | [4.5,6.7] | 5.7 | [4.8,6.7] | 4.4* | [3.4,5.3] |
| DM: FPG ≥7.0 mmol/l or self-report | 15.8 | [14.2,17.5] | 19.0* | [17.5,20.4] | 14.9 | [13.4,16.3] | 14.5 | [13,15.9] | 12.5 | [11.1,13.9] | 12.8 | [11.3,14.3] |
| PreDM: IFG 5.6-6.9 mmol/l | 15.6 | [13.7,17.4] | 19.5** | [17.8,21.3] | 32.2 | [29.4,35] | 35.1 | [32.4,37.8] | 13.8 | [11.9,15.7] | 15.4 | [13.4,17.5] |
| PreDM: IFG 6.1-6.9 mmol/l | 4.8 | [3.6,5.9] | 6.0* | [5.0,7.1] | 9.7 | [8.3,11.1] | 10.6 | [8.9,12.3] | 4.5 | [3.5,5.5] | 5.2 | [4.1,6.3] |
| Dysglycemia: DMor PreDM | 31.3 | [29,33.6] | 38.3** | [36.3,40.3] | 47.0 | [44.1,49.8] | 49.4 | [46.5,52.3] | 26.2 | [24.4,28] | 28.0 | [26,30.1] |
|
| ||||||||||||
| Weighted prevalence (based on HbA1c & FBS definition) | ||||||||||||
| NDD: FPG ≥7.0 mmol/l and/or HbA1c ≥48 mmol/mol | 13.1 | [11.2,15.0] | 12.1 | [10.4,13.7] | 19.9 | [17.8,22.1] | 20.9 | [18.2,23.6] | 11.7 | [9.8,13.7] | 10.7 | [9.2,12.2] |
| DM: FPG ≥7.0 mmol/l and/or HbA1c ≥48 mmol/mol or self-report | 23.8 | [20.8,26.8] | 24.1 | [21.5,26.6] | 29.7 | [26.7,32.6] | 30.2 | [26.5,33.9] | 19.9 | [16.6,23.2] | 19.3 | [15.6,23.0] |
| PreDM: FPG 5.6-6.9 mmol/l and/or HbA1c 39-46 mmol/mol | 37.1 | [34.2,40.0] | 42.8 | [40.4,45.3] | 47.8 | [45.5,50.2] | 47.6 | [45.4,49.8] | 33.3 | [30.5,36.0] | 32.5 | [30.4,34.7] |
| PreDM: FPG 6.1-6.9 mmol/l and/or HbA1c 39-46 mmol/mol | 33.5 | [30.6,36.3] | 38.6 | [36.0,41.2] | 40.2 | [37.5,42.8] | 39.8 | [37.1,42.6] | 29.4 | [26.9,31.8] | 27.7 | [25.8,29.6] |
| Dysglycemia: DMor PreDM | 60.9 | [57.4,64.3] | 66.9 | [63.9,69.8] | 77.5 | [74.4,80.6] | 77.7 | [73.9,81.5] | 53.2 | [49.0,57.5] | 51.8 | [47.7,55.9] |
|
| ||||||||||||
| Weighted prevalence (based on FBS definition) | ||||||||||||
| NDD: FPG ≥7.0 mmol/l | 6.0 | [4.8,7.3] | 5.0 | [3.9,6.1] | 7.3 | [5.9,8.7] | 6.3 | [5.2,7.4] | 6.7 | [5.5,7.9] | 5.4 | [4.5,6.4] |
| DM: FPG ≥7.0 mmol/l or self-report | 18.2 | [15.5,20.9] | 18.3 | [16.1,20.4] | 18.7 | [16.4,20.9] | 16.8 | [14.4,19.1] | 15.9 | [12.8,18.9] | 14.8 | [11.9,17.8] |
| PreDM: IFG 5.6-6.9 mmol/l | 16.8 | [14.8,18.9] | 21.0 | [19.1,22.9] | 34.2 | [31.4,36.9] | 36.9 | [34.6,39.3] | 15.5 | [13.2,17.8] | 16.5 | [14.1,18.9] |
| PreDM: IFG 6.1-6.9 mmol/l | 5.2 | [4.1,6.3] | 6.5 | [5.4,7.6] | 10.9 | [9.5,12.3] | 11.7 | [10.1,13.3] | 5.1 | [4.0,6.3] | 5.5 | [4.2,6.9] |
| Dyglycemia: DMor PreDM | 35.0 | [31.7,38.2] | 39.0 | [36.0,42.0] | 52.7 | [49.4,55.9] | 53.5 | [50.4,56.6] | 31.2 | [27.6,34.8] | 31.1 | [26.8,35.4] |
Abbreviations: CARRS, Center for cArdiometabolic Risk Reduction in South Asia; NDD, Newly diagnosed diabetes; DM, Diabetes Mellitus (newly diagnosed + self-reported diabetes); PreDM–Prediabetes mellitus; IFG–Impaired Fasting Glucose; FPG–Fasting Blood Glucose; HbA1c–glycosylated hemoglobin; 95%CI–95% Confidence Interval; Age-standardized prevalence–based on the regional [India and Pakistan] projection data for the year 2010 by the World Bank; Weighted prevalence–crude prevalence adjusted for sampling weights;
p<0.05 and
p<0.001 compared to males.
Age-standardized prevalence of diabetes, using only FPG definition was, as expected, lower: 17.4% (16.3-18.5%) in Chennai, 14.7% (13.6-15.7%) in Delhi, and 12.7% (11.6-13.7%) in Karachi. The prevalence of newly diagnosed diabetes based on FPG alone were 5.0% (4.2-5.7%), 5.9% (5.0-6.7%) and 5.0% (4.4-5.7%) in Chennai, Delhi, and Karachi respectively. The prevalence of prediabetes was 17.6% (16.3-18.8%) in Chennai, 33.7% (31.8-35.6%) in Delhi, and 14.6% (13.3-16.0%) in Karachi. Thus, adding HbA1c to the diagnostic criteria increases the prevalence of diabetes in all three sites.
Figure 1 shows the age- sex-specific prevalence of diabetes and prediabetes. The prevalence of diabetes was higher with age (p<0.001). At all three sites, prevalence of diabetes rose sharply at 25-34 years. For prediabetes, the rise in prevalence was at 20-24 years, with a decline or stabilization after age 45 in Chennai and Delhi and after the age of 55 in Karachi. The prevalence of diabetes and prediabetes were similar in both sexes at every age interval, except in Chennai, where females had slightly higher prevalence of prediabetes.
Figure 1.
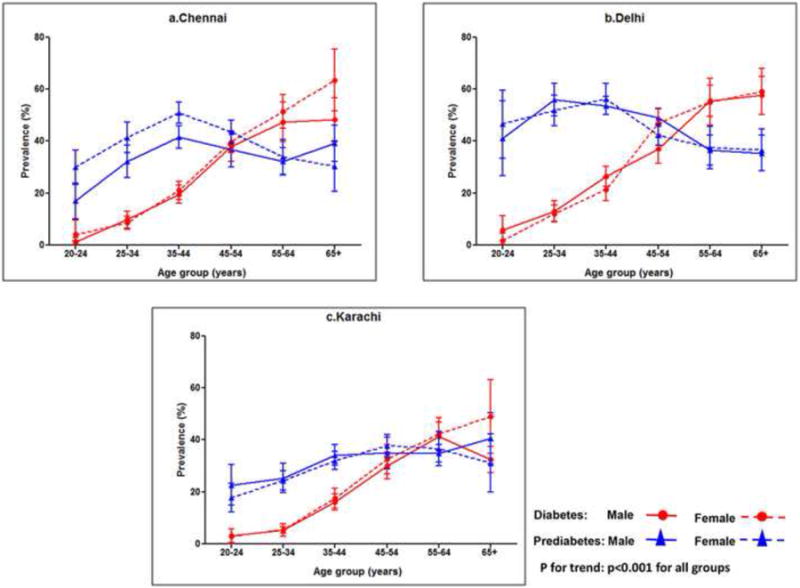
a-c: Age-, sex- and site-specific prevalence of diabetes and prediabetes. Diabetes, males–circles, solid line; diabetes females–circles, dotted line; prediabetes, males–triangles, solid line; prediabetes, females–triangles, dotted line; p for trend: p<0.001 for all groups.
The mean age of the study population (all three sites) was 42.2 years with the male: female ratio of 0.91. About 84.7% of them were literates with 47.3% of the subjects involved in some kind of occupation (professional–3.1%; trained or skilled occupation–21.3%; semi-skilled or unskilled occupation–22.9%). 72.4% of the subjects were in the income level of <10,000 Indian Rupees (INR) or <16,684 Pakistani Rupees (PKR) or <USD $165 per month; 16.1% were in the income range of 10,000-20,000 INR (16,684-33,368 PKR or USD$ 165-330) and 11.4% were earning income >20,000 INR (>33,368 PKR or >USD$330) per month. While 87.9% of the population was married, 6.9% was single, 4.9% was widow/widower and 0.3% was separated/divorced.
Table 2 summarizes the characteristics of the study population based on their glycemic status (diabetes, prediabetes, and no diabetes). Participants with normal fasting glucose were younger, had lower body mass index, waist circumference, systolic blood pressure, diastolic blood pressure, cholesterol, triglycerides, LDL-C, cholesterol to HDL-C ratio, triglyceride to HDL-C ratio, and higher HDL-C than those with prediabetes or diabetes in all three sites.
Table 2. Characteristics of subjects by diabetes status.
| Characteristics | Chennai (n=6906) | Delhi (n=5364) | Karachi (n=4017) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Row % or Mean [95% CI] | Row % or Mean [95% CI] | Row % or Mean [95% CI] | |||||||
|
| |||||||||
| Diabetes (n=1,648) |
Prediabetes (n=2,755) |
Normals (n=2,503) |
Diabetes (n=1,633) |
Prediabetes (n=2,544) |
Normals (n=1,187) |
Diabetes (n=776) |
Prediabetes (n=1,336) |
Normals (n=1,905) |
|
| Age (years) | 49.0[47.8,50.3] | 41.3[40.3,42.2] | 35.7[34.9,36.5]* | 51.2[49.5,52.9] | 42.7[41.2,44.3] | 37.5[36.2,38.9]* | 50.9[48.4,53.3] | 43.4[40.7,46.1] | 36.7[34.8,38.5]* |
| Gender | |||||||||
| Male | 20.8[18.9,22.8] | 35.2[32.5,37.8] | 44.0[41.4,46.6]* | 24.3[22.3,26.3] | 48.0[45.2,50.8] | 27.7[24.6,30.7] | 15.8[14.5,17.1] | 31.7[29.1,34.4] | 52.5[49.9,55] |
| Female | 24.7[22.8,26.6] | 40.7[38.3,43.1] | 34.6[32.4,36.8] | 26.0[23.9,28.2] | 47.1[44.2,50] | 26.9[23.8,29.9] | 16.8[15.1,18.4] | 30.5[28.4,32.6] | 52.7[50.5,54.9] |
| Body Mass Index (kg/m2) | 27.1[26.4,27.7] | 25.5[25.3,25.8] | 23.3[23,23.6]* | 26.7[26,27.4] | 24.6[24.3,24.9] | 23.1[22.7,23.5]* | 26.5[25.7,27.4] | 25.2[24.9,25.6] | 23.7[23.5,24]* |
| Waist circumference (cm) | 88.5[86.8,90.1] | 84.3[83.6,84.9] | 78.9[78.2,79.5]* | 90.4[88.6,92.2] | 84.7[84,85.3] | 81.4[80.5,82.3]* | 91.6[89.5,93.7] | 87.2[86.4,88.1] | 83.6[83,84.2]* |
| Systolic Blood Pressure (mmHg) | 125[124,127] | 122[121,123] | 118[117,119]* | 127[125,128] | 123[123,124] | 123[121,124]* | 122[120,125] | 119[118,120] | 117[116,118]* |
| Diastolic Blood Pressure (mmHg) | 83[82,84] | 81[80,82] | 78[77,79]* | 84[83,85] | 82[82,83] | 81[80,82]* | 81[79,83] | 79[79,80] | 78[77,79]* |
| Fasting Plasma Glucose(mmol/l) | 8.50[7.88,9.16] | 5.33[5.33,5.39] | 4.89[4.89,4.89]* | 7.94[7.50,8.33] | 5.56[5.50,5.56] | 5.0[5.0,5.06]* | 8.78[7.89,9.61] | 5.33[5.28,5.39] | 4.89[4.83,4.89]* |
| HbA1c (mmol/mol) | 70[64,76] | 41[41,41] | 34[34,34]* | 61[56,64] | 41[40,41] | 34[33,34]* | 63[57,69] | 40[40,40] | 33[33,33]* |
| Total cholesterol (mmol/l) | 4.9[4.8,5.0] | 4.8[4.7,4.9] | 4.5[4.5,4.6]* | 4.8[4.6,4.9] | 4.6[4.5,4.6] | 4.4[4.3,4.5]* | 4.5[4.2,4.7] | 4.5[4.4,4.6] | 4.3[4.2,4.3]* |
| Triglycerides (mmol/l)# | 4.1 [2.8] | 3.2[2.1] | 2.6[1.7]* | 3.7[2.5] | 3.2[1.9] | 2.7 [1.6]* | 3.7[2.5] | 3.3[2.3] | 2.5[1.7]* |
| HDL Cholesterol (mmol/l) | 1.04[0.98,1.06] | 1.06[1.06,1.09] | 1.11[1.09,1.13]* | 1.14[1.09,1.17] | 1.19[1.17,1.22] | 1.22[1.90,1.24]* | 1.04[0.98,1.06] | 1.09[1.09,1.11] | 1.14[1.14,1.17]* |
| LDL Cholesterol (mmol/l) | 2.98[2.85,3.13] | 3.0[2.95,3.06] | 2.80[2.75,2.85]* | 2.77[2.67,2.90] | 2.67[2.62,2.72] | 2.51[2.43,2.59]* | 2.80[2.64,2.95] | 2.80[2.75,2.87] | 2.64[2.59,2.69]* |
| Cholesterol to HDL-C ratio | 4.9[4.7,5.1] | 4.7[4.6,4.7] | 4.2[4.2,4.3]* | 4.4[4.3,4.6] | 4.0[4.0,4.1] | 3.8[3.7,3.9]* | 4.6[4.3,4.9] | 4.3[4.2,4.4] | 3.9[3.9,4]* |
| Triglyceride to HDL-C ratio# | 4.1 [3.4] | 3.0 [2.5] | 2.4 [1.9]* | 3.3 [2.9] | 2.8 [2.3] | 2.4 [1.8]* | 3.8 [2.9] | 3.0 [2.7] | 2.3 [2.0]* |
Values are presented as mean [95% CI]; #Median [interquartile range];
p<0.001 using analysis of variance (ANOVA) for normal data or Kruskal Wallis test for non-normal data; Diabetes–self-report and/or drug treatment for diabetes and/or fasting plasma glucose (FPG) ≥126 mg/dl (≥7.0 mmol/l) and/or glycosylated hemoglobin (HbA1c) ≥6.5% (≥48 mmol/mol); Prediabetes–FPG 100–125 mg/dl (5.6-6.9 mmol/l) and/or HbA1c 5.7–6.4% (39-46 mmol/mol); Normals–Neither diabetes nor prediabetes; HDL Cholesterol–High density lipoprotein cholesterol; LDL Cholesterol–Low density lipoprotein cholesterol; 95%CI–95% Confidence Interval; Except for age, all other variables are age-standardized–based on the regional [India and Pakistan] projection data for the year 2010 by the World Bank
Table 3 summarizes the risk factors for diabetes and prediabetes from a standardized polytomous logistic regression with normoglycemia as the reference group. Overall, older age, family history of diabetes, generalized obesity, abdominal obesity, body fat, high cholesterol, high triglyceride, and low HDL-C were significantly associated with higher odds of both diabetes and prediabetes. In addition, waist-to-height ratio and hypertension were significantly associated with diabetes.
Table 3. Risk factors for diabetes and prediabetes.
| Factors | Overall (n=16287) | Male (n=7760) | Female (n=8527) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prediabetes | Diabetes | Prediabetes | Diabetes | Prediabetes | Diabetes | |||||||
| OR | 95%CI | OR | 95%CI | OR | 95%CI | OR | 95%CI | OR | 95%CI | OR | 95%CI | |
| Karachi* | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||
| Chennai | 1.69 | [1.47, 1.95] | 2.05 | [1.71, 2.46] | 1.36 | [1.12, 1.65] | 1.70 | [1.32, 2.18] | 2.08 | [1.70, 2.56] | 2.49 | [1,93, 3.20] |
| Delhi | 3.32 | [2.84, 3.88] | 4.03 | [3.32, 4.95] | 3.17 | [2.57, 3.91] | 3.97 | [3.08, 5.13] | 3.45 | [2.77, 4.30] | 4.08 | [3.07, 5.42] |
| Age, per 10 year increment | 1.41 | [1.34, 1.48] | 2.27 | [2.15, 2.40] | 1.38 | [1.28, 1.49] | 2.17 | [2.02, 2.34] | 1.43 | [1.34, 1.53] | 2.35 | [2.17, 2.55] |
| Current smoking | 0.99 | [0.85, 1.15] | 0.90 | [0.75, 1.07] | 1.02 | [0.85, 1.22] | 0.92 | [0.75, 1.12] | 1.03 | [0.63, 1.68] | 0.82 | [0.41, 1.62] |
| Family history of diabetes | 1.20 | [1.06, 1.36] | 2.17 | [1.88, 2.50] | 1.16 | [0.97, 1.38] | 2.35 | [1.92, 2.89] | 1.24 | [1.04, 1.47] | 2.00 | [1.65, 2.44] |
| Generalized obesity [BMI ≥25 kg/m2] | 1.34 | [1.15, 1.57] | 1.62 | [1.33, 1.98] | 1.11 | [0.86, 1.43] | 1.31 | [0.97, 1.76] | 1.54 | [1.28, 1.85] | 1.91 | [1.48, 2.47] |
| Abdominal obesity [Waist ≥90 for male and ≥80 cm for female] | 1.22 | [1.03, 1.44] | 1.82 | [1.48, 2.24] | 1.29 | [1.00, 1.67] | 1.74 | [1.31, 2.32] | 1.20 | [0.94, 1.54] | 2.13 | [1.58, 2.88] |
| Waist to height ratio [ratio ≥0.5] | 1.15 | [0.99, 1.34] | 1.46 | [1.18, 1.79] | 1.24 | [1.01, 1.52] | 1.65 | [1.27, 2.13] | 1.04 | [0.81, 1.35] | 1.13 | [0.79, 1.63] |
| Body fat (%) [male ≥25% and female ≥30%] | 1.25 | [1.08, 1.43] | 1.21 | [1.02, 1.44] | 1.19 | [0.92, 1.55] | 1.25 | [0.97, 1.61] | 1.30 | [1.06, 1.58] | 1.30 | [0.96, 1.77] |
| Hypertension [self-reported and/or BP ≥140/90 mm Hg] | 1.09 | [0.90, 1.31] | 1.38 | [1.13, 1.68] | 1.09 | [0.86, 1.37] | 1.24 | [0.97, 1.60] | 1.16 | [0.85, 1.58] | 1.64 | [1.17, 2.28] |
| Hypercholesterolemia [Cholesterol ≥200 mg/dl] | 1.26 | [1.12, 1.43] | 1.30 | [1.12, 1.49] | 1.29 | [1.07, 1.55] | 1.29 | [1.04, 1.59] | 1.21 | [1.02, 1.44] | 1.28 | [1.03, 1.57] |
| Hypertriglyceridemia [Triglycerides ≥150 mg/dl] | 1.43 | [1.26, 1.64] | 2.56 | [2.20, 2.98] | 1.39 | [1.17, 1.64] | 2.17 | [1.76, 2.67] | 1.57 | [1.27, 1.95] | 3.06 | [2.40, 3.89] |
| Low HDL cholesterol [HDL-C <40 mg/dl for males and <50 mg/dl for females] | 1.20 | [1.08,1.35] | 1.34 | [1.17, 1.53] | 1.22 | [1.06, 1.41] | 1.43 | [1.19, 1.72] | 1.15 | [0.96, 1.37] | 1.23 | [1.00, 1.52] |
| Adjusted F statistic with p value | 81.2; p<0.001 | 48.57; p<0.001 | 51.29; p<0.001 | |||||||||
Reference category; Polytomous logistic regression models used to assess the risk factors associated with diabetes and prediabetes using normoglycemia as the reference category; Odds ratio (OR) of diabetes and prediabetes and their 95% confidence intervals (CI) were estimated for all cities together after controlling for the above mentioned variables; Diabetes–self-report and/or drug treatment for diabetes and/or fasting plasma glucose (FPG)≥126 mg/dl (≥7.0 mmol/l) and/or glycosylated hemoglobin (HbA1c) ≥6.5% (≥48 mmol/mol); Prediabetes–FPG 100–125 mg/dl (5.6-6.9 mmol/l) and/or HbA1c 5.7–6.4% (39-46 mmol/mol).
Discussion
In this representative study of three large cities in South Asia where we were able to characterize diabetes by biochemical testing, one in every five adults had diabetes and two out of every five adults had prediabetes.
Though other epidemiological studies are available in India,17–21 they are not directly comparable to our study due to the following reasons: these other studies included both urban and rural areas and the results for large metropolitan cities were not presented separately;18,20 smaller urban settings were studied,18 the studies used only capillary blood glucose for diagnosing diabetes,19,20 or used different methodology and sampling techniques.
Earlier studies carried out in six major urban metropolitan cities in India, the National Urban Diabetes Study (NUDS) in 2000, among adults aged ≥20 years, reported an overall prevalence of 12.1%, with the highest prevalence of 16.6% in Hyderabad (south India) followed by 13.5% in Chennai (south India), 12.4% in Bangalore (south India), 11.7% in Calcutta (east India), 11.6% in Delhi (north India) and 9.3% in Mumbai (central India),19 indicating a higher prevalence of diabetes in south Indian cities. Another study from south India, Chennai carried out in 2001-2004, the Chennai Urban Rural Epidemiological Study (CURES)21 reported the prevalence of diabetes as 14.3% among urban adults aged ≥20 years. Though earlier data had consistently showed that Chennai had higher diabetes prevalence, in the current study, Delhi has a substantially higher prevalence.
Other regional studies in urban cities of India, such as the Chandigarh Urban Diabetes Study (CUDS) carried out in 2009, a survey among adults aged ≥20 years, reported the prevalence of diabetes and prediabetes as 11.1% and 13.2%, respectively.22 In a study among 1,178 adults aged 20-80 years in the urban residents of Orissa of Eastern India in 2012, the crude and age-standardized prevalence of diabetes were 15.7% and 11.1% respectively.23 In a cross sectional study among subjects aged 20-40 years in urban Kashmir (in 2000), the age-adjusted prevalence of diabetes was reported as 2.4% in adults aged 20-40 years24 and 6.2% in those aged ≥40 years.25 Previous studies in Pakistan have reported a high prevalence of diabetes.26,27 The Pakistan National Diabetes Survey carried out in 2000, reported the overall prevalence of diabetes as 10.6% in urban areas of all four provinces of Pakistan in adults aged ≥25 years.26 In the second phase of Pakistan National Diabetes Survey in Punjab province (2010) with a sample size of 1,852 adults aged ≥25 years, the prevalence of diabetes was 12.1% in males and 9.3% in females.28 The prevalence reported in the current study are higher than those reported earlier from various studies in India and Pakistan.
Compared to the prevalence based on fasting and/or HbA1c, using only FPG to define diabetes, the overall age standardized prevalence of diabetes was lower, 14.9% (Chennai:17.4%, Delhi:14.7%, Karachi:12.7%), so was the prevalence of prediabetes, 21.9% (Chennai:17.6%, Delhi:33.7%, Karachi:14.6%). A recent study in China on a nationally representative sample (n=98,658),29 also reported a higher prevalence of diabetes and prediabetes using HbA1c criteria compared to only fasting glucose parameters. Other studies among Asian Indians, have also reported that, use of HbA1c criteria resulted in markedly higher prevalence of diabetes.30 It is of interest to note that based on fasting glucose measurements alone, 36.9% of urban residents in three large South Asian cities have dysglycemia (14.9% with diabetes, 21.9% with prediabetes). If 2-hour post-load glucose measurements were done, an even higher proportion of the population would likely have dysglycemia. It is worrying that if the HbA1c criteria is used, 60.3% of study population had dysglycemia (21.4% with diabetes, 38.9% with prediabetes).
In the current study, the prevalence of diabetes rose sharply at 25-34 years while for prediabetes, the take off point was 20-24 years, which is very worrisome. It is of interest that the age at which prediabetes plateaus is similar in the three cities, whereas the peak prevalence of prediabetes is very different. A similar take-off point in age for the prevalence of diabetes was reported in the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study.20 Studies have shown that though the prevalence of diabetes and prediabetes increased with age, marked variations in the prevalence occur in the older populations in different countries. In the current study, the decline or stabilization in the prevalence of diabetes and prediabetes in the older age (65+years), may be partly due to the premature deaths associated with diabetes reflecting survival bias or may reflect an age cohort effect of the epidemic. However, the fact that diabetes is so prevalent even among younger adults is of concern as this could potentially lead to a huge burden of complications by middle age due to longer exposure to hyperglycemia.
Comparison of the three metro cities shows that, Chennai had highest proportion of self-reported diabetes compared to newly diagnosed. In the recent ICMR-INDIAB study20 also, Tamilnadu in south India was reported to have highest proportions of self-reported diabetes compared to Chandigarh in north India, Maharashtra in west India, and Jharkhand in east India. Reasons for this could be the better awareness in Tamilnadu due to periodic diabetes screening carried out in this state by several agencies including the government.20 Of the three sites, using fasting and/or HbA1c criteria, the prevalence of prediabetes was almost twofold higher than the diabetes prevalence in all three cities. However, using FPG criteria, the prevalence of diabetes and prediabetes was similar in Chennai, slightly higher prevalence of prediabetes than diabetes in Karachi, while in Delhi the prevalence of prediabetes was twofold higher than the diabetes prevalence. Probable reasons could be slower rates of progression to diabetes in Delhi or ethnic differences, though the exact reason for this is not known.
In the current study, the factors positively associated with diabetes and prediabetes included older age, city of residence (Chennai/Delhi), family history of diabetes, obesity, body fat, high cholesterol, high triglyceride, and low HDL cholesterol levels. Similar risk factors have been reported in other studies as well.19,20,22,26,28 In the NUDS, age, BMI, waist-to-hip ratio, monthly income, family history of diabetes, retired, and unemployed category of occupation and sedentary lifestyle showed significant associations with diabetes.19 In ICMR-INDIAB study, age, male sex, family history of diabetes, urban residence, abdominal obesity, generalized obesity, hypertension, and income status were significantly associated with diabetes.20 In the urban Chandigarh population, older age, family history of diabetes, obesity, and hypertension were positively associated with diabetes.22 In the Pakistan National Diabetes Survey, the major risk factors identified were age, positive family history, and central obesity.26 In the second phase of Pakistan National Diabetes Survey in Punjab province, central obesity, hypertension, and positive family history were strongly associated with diabetes.28
One of the limitations of this study is its cross-sectional nature. However, with follow up of the CARRS cohort, which is currently in progress, the causal pathways underlying the reported relationships with risk factors could be investigated further, and rate of progression to prediabetes and to diabetes can be investigated. Another limitation is that extrapolation of the study results to the whole country is not possible; as only metropolitan cities were studied and no rural areas were included. Finally, only fasting plasma glucose was used instead of an oral glucose tolerance test (OGTT), which is considered the gold standard method for diagnosis of diabetes. However, the current study has included diabetes prevalence based on the recent international expert committee recommended HbA1c criterion for diagnosis of diabetes. The strengths are that it is population-based, large (n=16,287), representative of the general population of three large metropolitan cities, and has an excellent response rate (94.3%).
In conclusion, diabetes and prediabetes prevalence are alarmingly high among the south Asian urban population with the disease burden beginning even at a young age. It is a matter of concern that even in these large cities studied, almost half of people with diabetes were not aware of their condition. This warrants raising awareness regarding diabetes, a focus on efficient means to appropriately screen for diabetes and prediabetes in the population, and also organized systems to improve quality of diabetes care and control. In addition to improving awareness, focusing on healthy lifestyle is urgently needed to prevent the disorder in this high risk population.
Supplementary Material
Acknowledgments
This study is coordinated by CoE-CARRS (Center of Excellence - Center for CArdio-metabolic Risk Reduction in South Asia) based at Public Health Foundation of India (PHFI), New Delhi, India in collaboration with Centre for Chronic Disease Control (CCDC), New Delhi, Emory University, Atlanta, U.S.A, All India Institute of Medical Sciences (AIIMS), New Delhi, Madras Diabetes Research Foundation (MDRF), Chennai, India and Aga Khan University, Karachi, Pakistan. We hereby, acknowledge the contributions of the field and research staff of the “CARRS Surveillance Investigators’ Group”. This project is funded in part by the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), Department of Health and Human Services, under Contract No. HHSN268200900026C, and the United Health Group, Minneapolis, Mn, USA.
Several members of the research team at PHFI, Emory University, and CCDC were/are supported by the Fogarty International Clinical Research Scholars – Fellows programme (FICRS-F) through Grant Number 5R24TW007988 from NIH, Fogarty International Center (FIC) through Vanderbilt University; D43 NCDs in India Training Program through Award Number D43HD05249 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) and FIC; and Wellcome Trust (grant number 096735/A/11/Z). Ajay was supported by a Wellcome Trust Capacity Strengthening Strategic Award to the Public Health Foundation of India and a consortium of UK universities. However, the contents of this paper are solely the responsibility of the writing group and do not necessarily represent the official views of FIC, Vanderbilt University, Emory University, PHFI, NICHD, or the NIH.
Footnotes
Guarantor's Statement: “KM Venkat Narayan is the guarantor of this work and, takes full responsibility for the work as a whole, including the study design, access to data, and the decision to submit and publish the manuscript”.
Author Contributions: MMK, MKA, DP, NT, VM and KMVN conceived the study, its design, and revised all drafts of the manuscript. MD, RP, SR, HMK, ZF, IN, VSA and RMA coordinated the study, checked the integrity and accuracy of results. MD wrote the first draft of the article and carried out the corrections in consecutive drafts. MG and BB were responsible for data management and statistical analysis. All authors read and approved the final manuscript.
Conflict of Interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.International Diabetes Federation. 2014 Update International Diabetes Federation. 6th. Brussels, Belgium: International Diabetes Federation; 2014. IDF Diabetes Atlas. [Google Scholar]
- 2.Ruta LM, Magliano DJ, Lemesurier R, Taylor HR, Zimmet PZ, Shaw JE. Prevalence of diabetic retinopathy in Type 2 diabetes in developing and developed countries. Diabet Med. 2013;30:387–398. doi: 10.1111/dme.12119. [DOI] [PubMed] [Google Scholar]
- 3.Wong TY, Barr EL, Tapp RJ, Harper CA, Taylor HR, Zimmet PZ, et al. Retinopathy in persons with impaired glucose metabolism: the Australian Diabetes Obesity and Lifestyle (AusDiab) study. American Journal of Ophthalmology. 2005;140:1157–1159. doi: 10.1016/j.ajo.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Diabetes Prevention Program Research Group. The prevalence of retinopathy in impaired glucose tolerance and recent-onset diabetes in the Diabetes Prevention Program. Diabetic Medicine. 2007;24:137–144. doi: 10.1111/j.1464-5491.2007.02043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DeCourten M, Bennett PH, Tuomilehto J, Zimmet P. Epidemiology of NIDDM in non-Europids. In: Alberti KGMM, Zimmet P, DeFronzo RA, editors. International textbook of diabetes mellitus. 2nd. Wiley & Sons; Chichester: 1997. pp. 143–170. [Google Scholar]
- 6.Nair M, Ali MK, Ajay VS, Shivashankar R, Mohan V, Pradeepa R, et al. CARRS Surveillance study: design and methods to assess burdens from multiple perspectives. BMC Public Health. 2012;12:701. doi: 10.1186/1471-2458-12-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizzo L, Brick J, Park I. A minimally intrusive method for sampling persons in Random Digit Dial surveys. Public Opin Q. 2004;68:267–274. [Google Scholar]
- 8. [Accessed on June 15th 2014];STEPwise approach to surveillance (STEPS) http://www.who.int/chp/steps/manual/en/index.html.
- 9.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 10.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S11–S12. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Report of a World Health Organization Consultation: Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Diabetes Research and Clinical Practice. 2011;93:299–309. [Google Scholar]
- 12.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 13.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC-7) JAMA. 2003;289:2560–2567. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. The Asia Pacific perspective Redefining obesity and its treatment World Health Organization. International Association for the study of Obesity and International Obesity Task Force. Melbourne International Diabetes Institute; 2000. [Google Scholar]
- 15.Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as a screening tool for the prediction of cardiovascular disease and diabetes: 0.5 could be a suitable global boundary value. Nutr Res Rev. 2010;23:247–269. doi: 10.1017/S0954422410000144. [DOI] [PubMed] [Google Scholar]
- 16.Harrell FE., Jr . Regression modeling strategies with applications to linear models, Logistic regression and survival analysis. New York: Springer-Verlag; 2001. p. 43. [Google Scholar]
- 17.Mohan V, Mathur P, Deepa R, Deepa M, Shukla DK, Menon GR, et al. Urban rural differences in prevalence of self-reported diabetes in India-the WHO-ICMR Indian NCD risk factor surveillance. Diabetes Res Clin Pract. 2008;80:159–168. doi: 10.1016/j.diabres.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Sadikot SM, Nigam A, Das S, Bajaj S, Zargar AH, Prasannakumar KM, et al. The burden of diabetes and impaired glucose tolerance in India using the WHO 1999 criteria: Prevalence of Diabetes in India Study (PODIS) Diabetes Res Clin Pract. 2004;66:301–307. doi: 10.1016/j.diabres.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Ramachandran A, Snehalatha C, Kapur A, Vijay V, Mohan V, Das AK, et al. for the Diabetes Epidemiology Study Group in India (DESI) High prevalence of diabetes and impaired glucose tolerance in India. National Urban Diabetes Survey (NUDS) Diabetologia. 2001;44:1094–1101. doi: 10.1007/s001250100627. [DOI] [PubMed] [Google Scholar]
- 20.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. ICMR–INDIAB Collaborative Study Group. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research-India DIABetes (ICMR-INDIAB) study. Diabetologia. 2011;54:3022–3027. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 21.Mohan V, Deepa M, Deepa R, Shantirani CS, Farooq S, Ganesan A, et al. Secular trends in the prevalence of diabetes and impaired glucose tolerance in urban south India – the Chennai Urban Rural Epedimology Study (CURES-17) Diabetologia. 2006;49:1175–1178. doi: 10.1007/s00125-006-0219-2. [DOI] [PubMed] [Google Scholar]
- 22.Ravikumar P, Bhansali A, Ravikiran M, Bhansali S, Walia R, Shanmugasundar G, et al. Prevalence and risk factors of diabetes in a community based study in North India: the Chandigarh Urban Diabetes Study (CUDS) Diabetes Metab. 2011;37:216–221. doi: 10.1016/j.diabet.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Prasad DS, Kabir Z, Dash AK, Das BC. Prevalence and risk factors for diabetes and impaired glucose tolerance in Asian Indians: a community survey from urban eastern India. Diabetes Metab Syndr. 2012;6:96–101. doi: 10.1016/j.dsx.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Zargar AH, Wani AA, Laway BA, Masoodi SR, Wani AI, Bashir MI, et al. Prevalence of diabetes mellitus and other abnormalities of glucose tolerance in young adults aged 20-40 years in North India (Kashmir Valley) Diabetes Res Clin Pract. 2008;82:276–281. doi: 10.1016/j.diabres.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Zargar AH, Khan AK, Masoodi SR, Laway BA, Wani AI, Bashir MI, et al. Prevalence of type 2 diabetes mellitus and impaired glucose tolerance in the Kashmir Valley of the Indian subcontinent. Diabetes Res Clin Pract. 2000;47:135–146. doi: 10.1016/s0168-8227(99)00110-2. [DOI] [PubMed] [Google Scholar]
- 26.Shera AS, Jawad F, Maqsood A. Prevalence of diabetes in Pakistan. Diabetes Res Clin Pract. 2007;76:219–222. doi: 10.1016/j.diabres.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Jafar TH, Levey AS, White FM, Gul A, Jessani S, Khan AQ, et al. Ethnic differences and determinants of diabetes and central obesity among South Asians of Pakistan. Diabet Med. 2004;21:716–723. doi: 10.1111/j.1464-5491.2004.01140.x. [DOI] [PubMed] [Google Scholar]
- 28.Shera AS, Basit A, Fawwad A, Hakeem R, Ahmedani MY, Hydrie MZ, et al. Pakistan National Diabetes Survey: prevalence of glucose intolerance and associated factors in the Punjab Province of Pakistan. Prim Care Diabetes. 2010;4:79–83. doi: 10.1016/j.pcd.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. for the 2010 China Non communicable Disease Surveillance Group. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. doi: 10.1001/jama.2013.168118. [DOI] [PubMed] [Google Scholar]
- 30.Nazir A, Papita R, Anbalagan VP, Anjana RM, Deepa M, Mohan V. Prevalence of diabetes in Asian Indians based on glycated hemoglobin and fasting and 2-H post-load (75-g) plasma glucose (CURES-120) Diabetes Technol Ther. 2012;14:665–658. doi: 10.1089/dia.2012.0059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


