Abstract
Background
Medicare data from acute hospitals do not contain information on functional status. This lack of information limits the ability to conduct rehabilitation-related health services research.
Objective
The purpose of this study was to examine the associations between 5 comorbidity indexes derived from acute care claims data and functional status assessed at admission to an inpatient rehabilitation facility (IRF). Comorbidity indexes included tier comorbidity, Functional Comorbidity Index (FCI), Charlson Comorbidity Index, Elixhauser Comorbidity Index, and Hierarchical Condition Category (HCC).
Design
This was a retrospective cohort study.
Methods
Medicare beneficiaries with stroke, lower extremity joint replacement, and lower extremity fracture discharged to an IRF in 2011 were studied (N=105,441). Data from the beneficiary summary file, Medicare Provider Analysis and Review (MedPAR) file, and Inpatient Rehabilitation Facility–Patient Assessment Instrument (IRF-PAI) file were linked. Inpatient rehabilitation facility admission functional status was used as a proxy for acute hospital discharge functional status. Separate linear regression models for each impairment group were developed to assess the relationships between the comorbidity indexes and functional status. Base models included age, sex, race/ethnicity, disability, dual eligibility, and length of stay. Subsequent models included individual comorbidity indexes. Values of variance explained (R2) with each comorbidity index were compared.
Results
Base models explained 7.7% of the variance in motor function ratings for stroke, 3.8% for joint replacement, and 7.3% for fracture. The R2 increased marginally when comorbidity indexes were added to base models for stroke, joint replacement, and fracture: Charlson Comorbidity Index (0.4%, 0.5%, 0.3%), tier comorbidity (0.2%, 0.6%, 0.5%), FCI (0.4%, 1.2%, 1.6%), Elixhauser Comorbidity Index (1.2%, 1.9%, 3.5%), and HCC (2.2%, 2.1%, 2.8%).
Limitation
Patients from 3 impairment categories were included in the sample.
Conclusions
The 5 comorbidity indexes contributed little to predicting functional status. The indexes examined were not useful as proxies for functional status in the acute settings studied.
Acute hospitalization often accelerates the loss of muscle mass and physiological declines in older adults due to prolonged immobilization.1–3 Many hospitalized older adults also have comorbid conditions that precipitate functional decline, thereby influencing their health outcome across the continuum of care. These functional declines are difficult to evaluate because no information regarding functional status is available in national acute care datasets such as Medicare claims files. We are not aware of any studies examining the association between comorbidity indexes and discharge functional status using administrative claims data from acute hospitals.
Despite significant associations of poor functional status with higher use of post–acute care services, institutionalization, readmission, and mortality,4–10 measurement of functional status is still missing in acute care Medicare claims data and is not considered a quality indicator for acute hospitals. In contrast, post–acute care settings (inpatient rehabilitation facilities [IRFs], skilled nursing facilities, and home health care agencies) are required to use and submit standardized functional status data to the Centers for Medicare & Medicaid Services (CMS).
Two provisions of the Affordable Care Act, the Hospital Readmissions Reduction Program and the acute care/post–acute care bundled payments initiative, warrant evaluation of the effects of functional status across the continuum of care, including the transitions from acute care to post–acute care settings.11,12 The absence of functional status information in the database during acute hospitalization highlights the challenges in addressing the effect of residual confounding in studies of discharge planning and post–acute care resource utilization. Previous studies have raised the issue of unmeasured or residual confounding effect of functional status in health service research based on administrative claims data in the Medicare population.13–15 Using Medicare Current Beneficiary Survey data, Schneeweiss and colleagues have shown the residual confounding effect of unmeasured functional status on adverse outcomes, such as mortality and risk of hip fracture, in older adults.14,15
Identifying limitations in functional status at the time of discharge from the acute hospital could improve discharge planning and care transitions. Based on clinical experience and previous research,13,16 we believe that it may be possible to identify a surrogate method of estimating functional status at acute care discharge by using administrative data on patient characteristics and comorbid health conditions. If this assumption is correct, a surrogate measure of comorbidity could prove useful in modeling discharge setting and other outcomes within specific medical diagnoses at discharge from acute care.
The majority of research using comorbidity indexes contained in administrative claims data collected during acute hospitalization has been conducted in the context of predicting health care cost and mortality, not functional status. The commonly used Charlson Comorbidity Index and Elixhauser Comorbidity Index were developed to predict health care utilization and mortality.17,18 In a longitudinal cohort study on 291 older adults, Bravo and colleagues19 reported that the Charlson Comorbidity Index performs slightly better for predicting 3-year mortality than functional decline in long-term care settings. The tier comorbidity system was developed to allocate resources under the prospective payment system for IRFs and has been used in rehabilitation health services research to predict the length of stay (LOS) and resource utilization.20 The CMS developed the Hierarchical Condition Category (HCC) to estimate health care costs for beneficiaries in the Medicare Advantage program, using demographic characteristics and diagnostic-based medical conditions.21 Recently, the CMS began using HCC conditions in risk prediction models of 30-day readmission. The Functional Comorbidity Index (FCI), developed by Groll and colleagues,16 is the only comorbidity index currently available that was designed to predict functional status.
The FCI was developed in a community-based sample to predict the self-reported physical component subscale of the 36-Item Short-Form Health Survey (SF-36).22 Compared with the Charlson Comorbidity Index and other common indexes, the FCI captures more chronic conditions (eg, arthritis, hearing impairment, degenerative disk disease) that have strong associations with physical performance.16 The FCI has been validated in acute care and intensive care settings for predicting self-reported physical function using medical records in a limited sample.23–25 Using medical records of 34 patients in intensive care unit settings, Haines and colleagues24 found that the FCI was a better predictor of self-reported physical function than an objective measure of physical function. Similarly, using prospective data from an observational study in 73 patients with acute respiratory distress syndrome, Groll and colleagues23 showed that the FCI was a better predictor of self-reported physical function than the Charlson Comorbidity Index after controlling for age and severity of illness.
The objective of our study was to examine the associations among 5 comorbidity indexes derived from acute care administrative claims data and functional status assessed at admission to inpatient rehabilitation. The study sample includes Medicare fee-for-service beneficiaries with stroke, lower extremity fracture, and lower extremity joint replacement. We considered functional status measured at IRF admission to be a reasonable proxy for discharge functional status from acute hospitals. We hypothesized that the Functional Comorbidity Index would have the strongest association with functional status.16
Method
Data Sources
A secondary analysis of Medicare data was conducted using the Medicare Provider Analysis and Review (MedPAR) data file, beneficiary summary file, and Inpatient Rehabilitation Facility–Patient Assessment Instrument (IRF-PAI) file for the calendar year 2011. The MedPAR file contains claims for all inpatient stays, including acute hospitals and IRFs and information about diagnostic conditions, surgical procedures, LOS, and amount of health services used during the short-term acute care stay. In this study, data from the MedPAR file associated with only inpatient short-term acute care stay was used to derive all variables for the comorbidity indexes. The beneficiary summary file contains Medicare enrollment indicators and the patient's sociodemographic characteristics. The IRF-PAI file includes data on the patient's functional status at the time of admission and discharge from IRF. In this study, the IRF-PAI file was used to retrieve patient's functional status at the time of admission. A data use agreement was established with the CMS.
Study Population
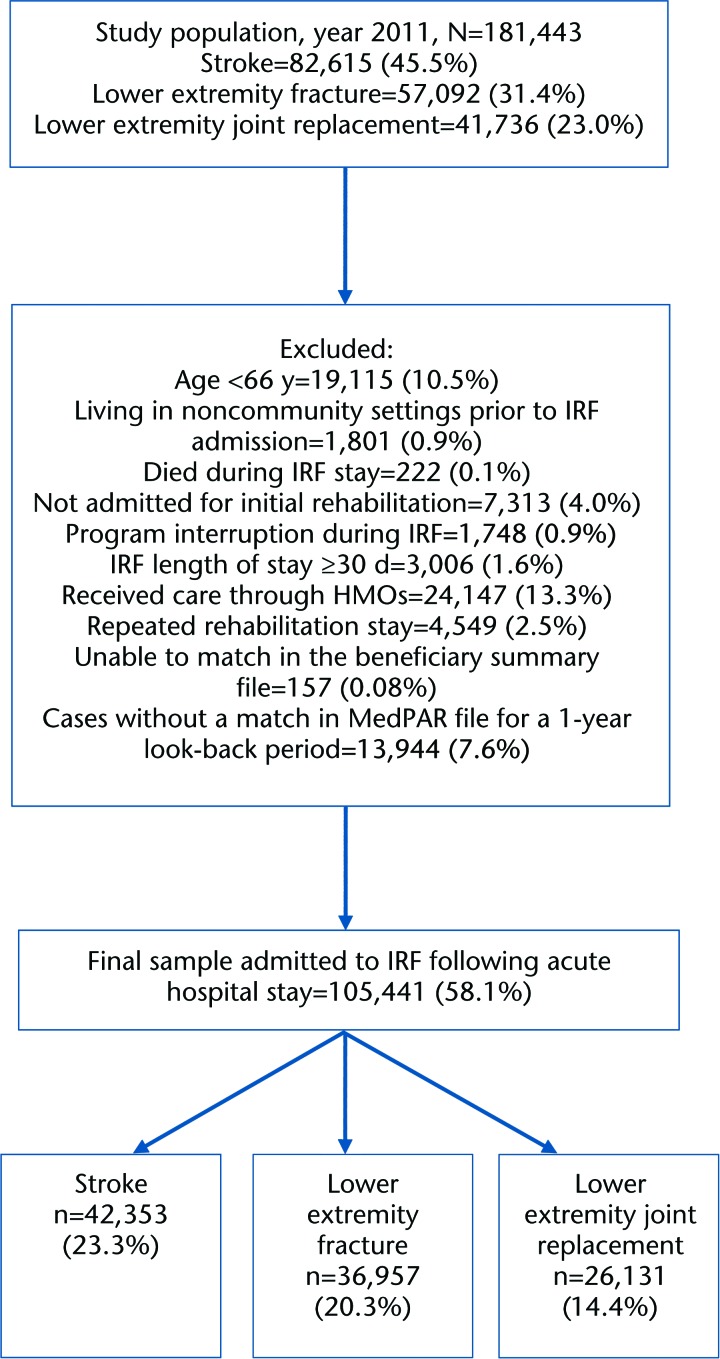
The population included Medicare beneficiaries, 66 years of age or older, on Medicare fee-for-service plans. We included patients 66 years and older in order to have look-back data for a 1-year period. The analytic sample included patients admitted to IRFs directly from acute hospitals in 1 of 3 rehabilitation impairment categories (RICs): stroke, lower extremity fracture, and lower extremity joint replacement. Patients from these 3 RICs cumulatively represented approximately 44% of all IRF admissions in 2012.26 The flow diagram (Fig. 1) shows the number of patients included and excluded. We excluded patients with the end-stage renal disease because they are not defined as a rehabilitation impairment category and are not primarily treated in IRFs. Patients in Medicare health maintenance organizations were excluded because their claims data were not available. Patients living in institutional settings prior to IRF admission, not admitted for initial rehabilitation, with repeated rehabilitation stays, and with LOS more than 30 days were excluded because they are atypical patients in IRFs with generally severe chronic or uncontrolled conditions. Patients with program interruption were excluded because they went back to acute care to receive intervention for a medical complication. The final sample comprised 105,441 patients discharged from the acute hospital to an IRF with stroke, lower extremity fracture, or lower extremity joint replacement.
Figure 1.
Flowchart of the study sample discharged from acute care to inpatient rehabilitation facilities. IRF=inpatient rehabilitation facility, HMO=health maintenance organization, MedPAR=Medicare Provider Analysis and Review.
Variables
Outcome.
The primary outcome was motor functional status at admission to IRF, as measured by the Functional Independence Measure (FIM) and documented in the IRF-PAI.27 As noted earlier, we considered IRF admission functional status to be a proxy for discharge functional status from acute hospitals. The FIM is administered by rehabilitation clinicians within 3 days of inpatient rehabilitation admission and within 3 days of discharge date.27 In this study, we classified FIM motor items into 2 subscales: self-care and mobility. The self-care subscale assesses eating, grooming, bathing, upper body dressing, lower body dressing, toileting, bladder management, and bowel management. The mobility subscale assesses bed-to-chair transfer, toilet transfer, tub/shower transfer, walking or wheelchair use, and climbing stairs. We did not include cognitive FIM scores in this analysis. All FIM items are scored on a 7-point ordinal scale, ranging from complete dependence (1) to complete independence (7).27 The self-care subscale score ranges from 8 to 56, and the mobility score ranges from 5 to 35. The motor FIM score ranges from 13 to 91. The reliability and validity of the FIM instrument have been studied extensively in patients with stroke and other impairment categories.28
Demographic variables.
Patient demographic variables included: age, sex, race/ethnicity, the original reason for Medicare due to disability, and dual eligibility for Medicare and Medicaid. Age was entered as a categorical variable having 3 levels: 66–75, 76–85, and >85 years. Race/ethnicity was categorized as non-Hispanic white, non-Hispanic black, Hispanic, and other races. Disability and dual eligibility were dichotomized into “yes” and “no” categories. Disability was classified as “yes” if the beneficiary originally qualified for Medicare benefits due to disability rather than age. Beneficiaries enrolled in both Medicare and Medicaid were classified as “dual eligible.”
Comorbidity indexes.
Comorbidity indexes include medical conditions based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes listed in the MedPaR files for the specified acute hospitalization.
Tier comorbidity.
The tier comorbidity system was developed by the CMS as part of the IRF prospective payment system. It classifies medical conditions into 1 of 4 categories (tier 1, tier 2, tier 3, and no tier) based on the costs and projected utilization of resources during an IRF stay.20 Tier 3 includes 932 diagnostic codes that represent the lowest-cost category. Tier 1 represents the highest-cost category and includes 8 diagnostic codes that yield additional reimbursement. We computed tier categories for acute hospital stays using ICD-9-CM codes published in the Federal Register for the year 2011.20 The tier was included as a single 4-level variable in our analysis, and no tier was used as a reference category. The patients were assigned to a single tier category for reimbursement purposes during their inpatient rehabilitation stay.
Charlson Comorbidity Index.
The Charlson Comorbidity Index consists of 18 medical conditions and was developed to predict 1-year all-cause mortality in patients with breast cancer using hospital medical records.17 Deyo adapted the original Charlson Comorbidity Index to administrative data, using ICD-9-CM codes, into 17 comorbid categories for predicting health services.29 The Deyo version of the Charlson Comorbidity Index was used in this study. The ICD-9-CM codes for 17 conditions were based on those documented in the literature.17,29 Dichotomous indicators for each of the 17 comorbidity conditions, rather than the weighted index, were included in our analyses.
Elixhauser Comorbidity Index.
The Elixhauser Comorbidity Index consists of 30 medical conditions and is widely used in health service research for predicting mortality and health service utilization.18 We used the ICD-9-CM codes for the 30 conditions as described in the original study.18 Dichotomous indicators for each of the 30 comorbidity conditions were included in our analyses.
FCI.
The FCI includes 18 comorbid conditions and shows significant associations with physical functioning.16,25,30 To validate the use of the FCI in Medicare data, we mapped these 18 conditions to ICD-9-CM codes. After acquiring ICD-9-CM codes for each comorbid condition in the FCI, a physician, physical therapist, occupational therapist, or nurse was consulted to verify mapping accuracy. Additionally, validation of ICD-9-CM codes for the FCI medical conditions was done by consulting an experienced billing and claims specialist. The ICD-9-CM codes used to define each comorbid condition in the FCI are reported in eTable 1. Dichotomous indicators for each of the 18 comorbidity conditions were included in our analyses.
HCC.
The CMS calculates HCC using ICD-9-CM diagnostic codes from ambulatory and inpatient claims data. The HCC scores are calculated every year for each Medicare Advantage enrollee. Initially, the CMS developed 70 HCCs from 805 diagnostic groups and 14,000 ICD-9-CM diagnostic codes. The CMS recently revised the index to include 79 HCC condition categories.31 We used the ICD-9-CM codes for HCC conditions as reported in the CMS manual.21 Dichotomous indicators for each of the 70 HCC comorbidity conditions were included in our analyses using inpatient claims data specific to acute hospitalization. We did not have access to ambulatory claims data.
Data Analysis
Linear regression models were constructed to evaluate the association of the 5 comorbidity indexes with functional status at admission to post-acute inpatient rehabilitation. Separate analyses were run for the 3 impairment categories. We included 3 outcomes for each comorbidity model: motor FIM and the 2 subscales (mobility and self-care). All baseline models included age, sex, race/ethnicity, disability, dual eligibility, and acute LOS. In each of the 5 subsequent models, one of the comorbidity indexes was added separately to the base models. Adjusted R2 statistics were then compared across all the models. The R2 statistic reflects goodness-of-fit of a linear model and measures how well the regression line approximates the real data points in the model and the amount of total variance explained in the outcome variable by the predictors or covariates.32 The R2 values can range from 0 to 1, with 0 indicating no statistical correlation between the data points and the regression line and 1 indicating a perfect fit.32
Role of the Funding Source
The study was supported by the National Institutes of Health (R24-HD065702, R01-HD069443, and K12 HD055929; Principal Investigator: Dr Ottenbacher) and the National Institute for Disability, Independent Living, and Rehabilitation Research (90IF0071; Principal Investigtor: Dr Ottenbacher). Study sponsors had no role in the study design, analysis, or interpretation of the data. Study sponsors did not have any role in the writing of the manuscript or the submission to a journal.
Results
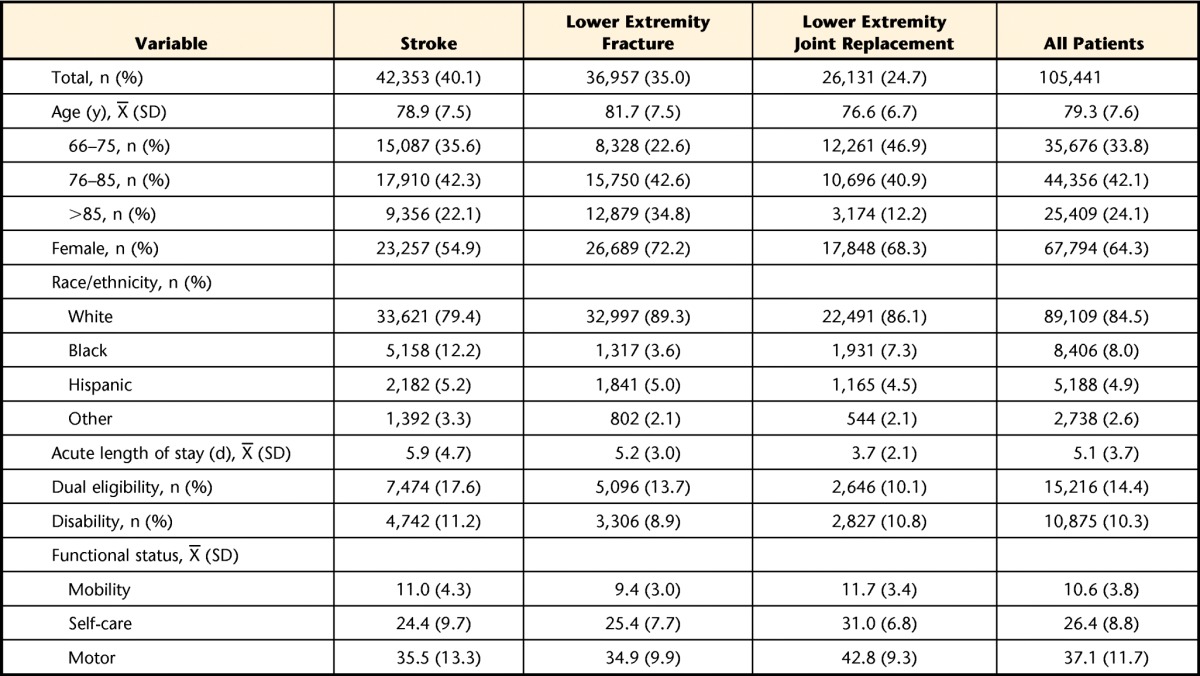
The study sample included 105,441 patients discharged from acute hospitals who were admitted directly to the IRF on the same day. The mean age was 79.3 years (SD=7.6). The majority were non-Hispanic white (85%) and female (64%). The mean LOS in an acute hospital was 5.1 days. Only 14% were dual-eligible (Medicare and Medicaid), and 10% were eligible for Medicare due to disability. Stroke was the largest impairment category, representing 40% (n=42,353) of the study sample, followed by lower extremity fracture (35%, n=36,957) and lower extremity joint replacement (25%, n=26,131). Demographic and clinical characteristics stratified by impairment groups are shown in the Table. The mean functional score of the total sample at the time of IRF admission was 10.6 (SD=3.8) for mobility FIM, 26.4 (SD=8.8) for self-care FIM, and 37.1 (SD=11.7) for motor FIM. The distributions of individual conditions across the 5 comorbidity indexes in each of the 3 impairment categories are shown in eTables 2–6.
Table.
Descriptive Characteristics of the Study Population by Rehabilitation Impairment Categories
Findings for Stroke
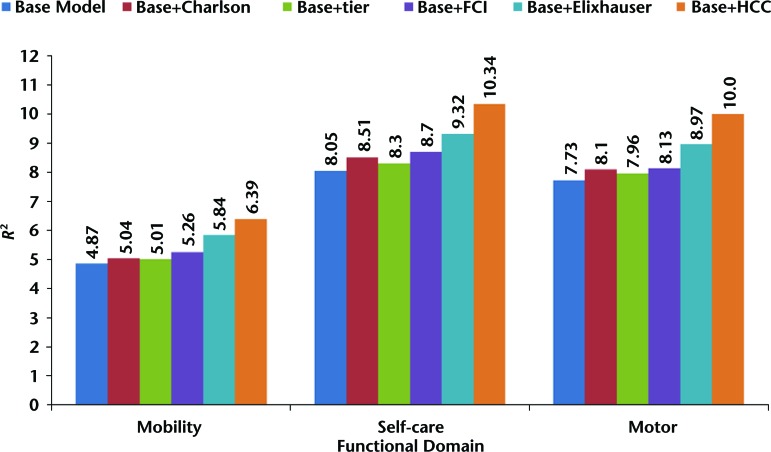
Figure 2 shows the R2 estimates from the linear regression models predicting mobility, self-care, and motor FIM scores among patients with stroke. The base model (which includes age, gender, race/ethnicity, disability, dual eligibility, and acute LOS) explained 4.87% of the variance in IRF admission mobility scores. The increases in variance explained with the addition of the comorbidity indexes to the base model were: Charlson Comorbidity Index (0.17%), tier comorbidity (0.14%), FCI (0.39%), Elixhauser Comorbidity Index (0.97%), and HCC (1.52%). The base model explained 8.05% of the variance in IRF admission self-care scores. The increases in variance explained with the addition of the comorbidity indexes to the base model were: Charlson Comorbidity Index (0.46%), tier comorbidity (0.25%), FCI (0.65%), Elixhauser Comorbidity Index (1.27%), and HCC (2.29%). The base model explained 7.73% of the variance in predicting IRF admission motor FIM scores. The increases in variance explained with the addition of the comorbidity indexes to the base model were: Charlson Comorbidity Index (0.37%), tier comorbidity (0.23%), FCI (0.40%), Elixhauser Comorbidity Index (1.24%), and HCC (2.27%).
Figure 2.
R2 statistics for predicting mobility, self-care, and motor Functional Independence Measure performance in patients with stroke. Charlson=Charlson Comorbidity Index, FCI=Functional Comorbidity Index, tier=tier comorbidity system, Elixhauser=Elixhauser Comorbidity Index, HCC=Hierarchical Condition Category.
Findings for Lower Extremity Joint Replacement
Figure 3 shows the R2 estimates from the linear regression models predicting mobility, self-care, and motor FIM scores among patients with lower extremity joint replacement. The base model (including age, gender, race/ethnicity, disability, dual eligibility, and acute LOS) explained 2.04% of the variance in predicting IRF admission mobility scores. The increases in variance explained with the addition of the comorbidity indexes to the base model were: Charlson Comorbidity Index (0.59%), tier comorbidity (0.70%), FCI (1.24%), Elixhauser Comorbidity Index (1.93%), and HCC (1.80%). The base model explained 3.92% of the variance in predicting IRF admission self-care scores. The increases in variance explained with the addition of the comorbidity indexes to the base model were: Charlson Comorbidity Index (0.33%), tier comorbidity (0.44%), FCI (1.03%), Elixhauser Comorbidity Index (1.53%), and HCC (1.91%). The base model explained 3.83% of the variance in predicting IRF admission motor FIM score. The increases in variance explained with the addition of the comorbidity indexes to the base model were: Charlson Comorbidity Index (0.50%), tier comorbidity (0.63%), FCI (1.28%), Elixhauser Comorbidity Index (1.97%), and HCC (2.18%).
Figure 3.
R2 statistics for predicting mobility, self-care, and motor Functional Independence Measure scores in patients with lower extremity joint replacement. Charlson=Charlson Comorbidity Index, FCI=Functional Comorbidity Index, tier=tier comorbidity system, Elixhauser=Elixhauser Comorbidity Index, HCC=Hierarchical Condition Category.
Findings for Lower Extremity Fracture
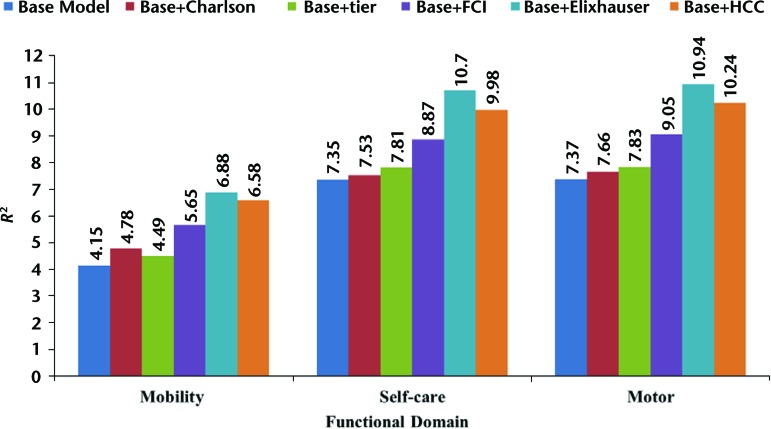
Figure 4 shows the R2 estimates from the linear regression models predicting mobility, self-care, and motor FIM scores among patients with lower extremity fracture. The base model (including age, gender, race/ethnicity, disability, dual eligibility, and acute LOS) explained 4.15% of the variance in predicting IRF admission mobility scores. The increases in variance explained with the addition of the comorbidity indexes to the base model were: Charlson Comorbidity Index (0.63%), tier comorbidity (0.34%), FCI (1.50%), Elixhauser Comorbidity Index (2.73%), and HCC (2.43%). The base model explained 7.35% of the variance in predicting IRF admission self-care scores. The increases in variance explained with the addition of the comorbidity indexes to the base model were: Charlson Comorbidity Index (0.18%), tier comorbidity (0.46%), FCI (1.52%), Elixhauser Comorbidity Index (3.35%), and HCC (2.63%). The base model explained 7.37% of the variance in predicting IRF admission motor FIM score. The increases in variance explained with the addition of the comorbidity indexes to the base model were: Charlson Comorbidity Index (0.29%), tier comorbidity (0.46%), FCI (1.68%), Elixhauser Comorbidity Index (3.57%), and HCC (2.87%).
Figure 4.
R2 statistics for predicting mobility, self-care, and motor Functional Independence Measure scores in patients with lower extremity fracture. Charlson=Charlson Comorbidity Index, FCI=Functional Comorbidity Index, tier=tier comorbidity system, Elixhauser=Elixhauser Comorbidity Index, HCC=Hierarchical Condition Category.
Discussion
To our knowledge, this is the first study to compare associations among commonly used comorbidity indexes derived from acute hospitalization and functional status measures in Medicare beneficiaries using large administrative data. We used IRF admission self-care, mobility, and motor FIM scores as proxy measures of discharge functional status from acute hospitals in a sample of patients receiving rehabilitation services for stroke, lower extremity joint replacement, and lower extremity fractures.
We found that tier comorbidity, Charlson Comorbidity Index, FCI, Elixhauser Comorbidity Index, and HCC added little explanatory power to the base model in predicting functional status at IRF admission. In models containing patient demographic characteristics, the addition of various comorbidity indexes explained approximately 2% to 3% additional variance in functional status measures across all 3 impairment categories. The explanatory power of the best models for motor FIM score for each RIC was still low, with R2 values of 10% for stroke, approximately 6% for joint replacement, and 10% for lower extremity fracture. The results suggest that models containing these comorbidity indexes cannot substitute for functional measurement at acute care discharge or IRF admission.
The study results did not support the research hypothesis that the FCI would outperform the other indexes. The weak association between FCI and IRF admission functional status was unexpected. This finding may be partially explained by the fact that the FCI was developed and validated in a community-based sample that may have different types and prevalence of comorbid conditions than those found in patients in acute hospital settings. Our sample included older patients with stroke and lower extremity fracture or joint replacement who received intense medical care or surgical procedures during hospitalization and were admitted to an IRF. Additionally, the FCI was developed and validated using a self-reported measure of functional status, based on the SF-36 measure,22 whereas our study utilized performance-based functional measures of self-care, mobility, and motor FIM (self-care and mobility).27
Based on R2 estimates, we found that the Elixhauser Comorbidity Index and HCC performed marginally better in models predicting functional status relative to models containing the Charlson Comorbidity Index, tier comorbidity, and FCI across all 3 impairment categories and for all 3 FIM measures. Several explanations may exist for this finding. First, the HCC incorporates more medical conditions than the other indexes. The HCC includes 70 comorbidities; the next most complete index, the Elixhauser Comorbidity Index, includes 30, the FCI has 18, the Deyo version of Charlson Comorbidity Index includes 17 comorbidities, and the tier comorbidity system has 4 categories.16,18,29,31 Furthermore, each of the comorbid conditions for the HCC has the largest number of associated ICD-9-CM codes compared with the other comorbidity indexes. Nevertheless, the association between the HCC and all functional status scores was very modest. These results are consistent with a recent study on risk adjustment models for Medicare capitation that showed the HCC lacks comorbidities that can affect patients' functional status and thus the models underpredicted Medicare capitation payments for patients with certain chronic conditions.33
None of the comorbidity indexes we examined were considered robust enough to use as a surrogate measure of functional status. Thus, our results underscore the need to add a standardized measure of functional status to the data set associated with acute hospitalization. Periodic assessment of patient functional status during acute hospitalization is recommended in the clinical practice guidelines for rehabilitation after critical illness34 and the Institute of Medicine report on performance-based quality measures.35 There is a growing interest in the development of a uniform functional measure that can be used across post–acute care settings as mandated in the recent Improving Medicare Post-Acute Care Transformation (IMPACT) Act.36
In the absence of standardized functional status measurement in the data set associated with the acute care setting, further research to develop a new index or surrogate method to predict functional status is warranted. There may also be factors beyond comorbidities that play an influential role in patient functional status, which should be considered in risk adjustment models. For instance, using claims and survey data from the Medicare Current Beneficiary Survey, Faurot and colleagues13 identified variables other than medical conditions, such as durable medical equipment, wheelchairs, and portable oxygen, as the strongest predictors of functional impairment. Furthermore, immobility and prolonged bed rest associated with acute care hospitalization, particularly care in the intensive care unit and critical care unit, are known to contribute to hospital-associated disability.
Our study has some limitations. First, we used functional assessment conducted at admission to IRF as a proxy for functional status at discharge from acute care. We believe that this is a reasonable approach, given that IRFs are required to document functional status within 3 days of admission. However, we recognize the possibility that some patients may experience a change in functional status between discharge and the first IRF functional status assessment. Nevertheless, because no standardized functional status data are captured at acute care discharge, there is no other viable method of determining functional status at acute care discharge. Future research should explore the consistency of functional status assessment between acute care discharge and first IRF assessment.
Another limitation is that we did not use index-specific or generic weights in computing composite scores for comorbidity indexes. Instead, we used dichotomous indicators for each condition included in the indexes. We chose not to use weights because, in most cases, condition weights were developed to predict outcomes other than functional status. For example, Charlson Comorbidity Index weights were developed to predict mortality, HCC weights were developed to estimate the cost in the Medicare Advantage population, and weights for tier comorbidity are used for prospective allocation of resources in patients in IRFs. The Elixhauser Comorbidity Index does not use weights. It is possible that our conclusions about the relative contributions of each comorbidity index would differ if weights were applied. However, overall conclusions about the magnitude of variance explained by risk adjustment models would be unchanged. The use of indicator variables instead of weights for each comorbid condition may have resulted in a slight increase in explanatory power for all indexes.30 Traditionally, HCC scores have been calculated based on medical conditions documented in inpatient and ambulatory data. We used only inpatient claims specific to acute hospitalization that may explain the underperformance of the HCC. Our analytic sample was limited to patients from 3 rehabilitation impairment categories admitted to IRFs directly from acute care. The findings cannot be generalized to patients with other types of impairments or to those admitted to skilled nursing facilities or discharged to another setting. Our study excluded patients with more severe conditions, based on longer IRF stay, not living in the community, program interruption, repeated rehabilitation stays, and death during the IRF stay. These exclusion criteria may have influenced the distribution of functional status scores by limiting the spectrum at the lower range. Our study may have underestimated the influence of other severity indicators. We also did not include other types of severity indicators, such as a number of days in an intensive care unit or critical care unit, in any of the analytical models.
Our study also has several strengths. We believe that linking patients' hospital claims records with their immediate inpatient rehabilitation functional assessment data is a powerful approach and can be applied in future studies to obtain a reasonable measure of functional status at acute care discharge. We used national data on all Medicare fee-for-service beneficiaries admitted to an IRF from an acute hospital with a stroke, lower extremity joint replacement, or fracture in 2011. Thus, our sample was diverse and captured a substantial proportion of all IRF admissions.
In conclusion, the primary findings of this study suggest that adding the tier comorbidity system, Charlson Comorbidity Index, FCI, Elixhauser Comorbidity Index, or HCC to risk adjustment models predicting functional status at discharge from acute care contributes very little explanatory power. Our results suggest that the 5 comorbidity indexes examined in our study cannot substitute for functional status measures in acute care hospitals. Additional research is needed to identify functional assessment and improve our ability to conduct outcomes research and establish quality metrics across the continuum of acute and postacute care.
Footnotes
Dr Kumar and Dr Karmarkar had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Dr Kumar, Dr Karmarkar, Dr Graham, Dr Al Snih, and Dr Ottenbacher. Data acquisition, analysis, or interpretation of data: Dr Kumar, Dr Resnik, Dr Karmarkar, and Dr Deutsch. Drafting of the manuscript: Dr Kumar, Dr Graham, Dr Al Snih, Dr Ottenbacher, and Dr Resnik. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: Dr Kumar and Dr Tan. Administrative, technical, or material support: Dr Ottenbacher, Dr Graham, Dr Karmarkar, Dr Deutsch, and Dr Resnik. Obtaining funding: Dr Ottenbacher. Study supervision: Dr Graham and Dr Ottenbacher. The authors acknowledge the assistance of Sarah Toombs Smith, PhD, ELS, in manuscript preparation.
The study was approved by the University of Texas Medical Branch, Galveston Institutional Review Board.
The study was supported by the National Institutes of Health (R24-HD065702, R01-HD069443, and K12 HD055929; Principal Investigator: Dr Ottenbacher) and the National Institute for Disability, Independent Living, and Rehabilitation Research (90IF0071; Principal Investigator: Dr Ottenbacher).
The views expressed herein are those of the authors and do not reflect the official policy or position of the Department of the Army, the US Department of Defense, the US Department of Veterans Affairs, or the US government.
References
- 1. Graf C. Functional decline in hospitalized older adults. Am J Nurs. 2006;106:58–67. [DOI] [PubMed] [Google Scholar]
- 2. Hoenig HM, Rubenstein LZ. Hospital-associated deconditioning and dysfunction. J Am Geriatr Soc. 1991;39:220–222. [DOI] [PubMed] [Google Scholar]
- 3. Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52:1263–1270. [DOI] [PubMed] [Google Scholar]
- 4. Fields SD, MacKenzie CR, Charlson ME, Sax FL. Cognitive impairment: can it predict the course of hospitalized patients? J Am Geriatr Soc. 1986;34:579–585. [DOI] [PubMed] [Google Scholar]
- 5. Inouye SK, Peduzzi PN, Robison JT, et al. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279:1187–1193. [DOI] [PubMed] [Google Scholar]
- 6. Narain P, Rubenstein LZ, Wieland GD, et al. Predictors of immediate and 6-month outcomes in hospitalized elderly patients: the importance of functional status. J Am Geriatr Soc. 1988;36:775–783. [DOI] [PubMed] [Google Scholar]
- 7. Sabartes O, Miralles R, Ferrer M, et al. Factors predicting return home of hospitalized aged patients. An Med Interna. 1999;16:407–414. [PubMed] [Google Scholar]
- 8. Kansagara D, Englander H, Salanitro A, et al. Risk prediction models for hospital readmission: a systematic review. JAMA. 2011;306:1688–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ottenbacher KJ, Graham JE, Ottenbacher AJ, et al. Hospital readmission in persons with stroke following postacute inpatient rehabilitation. J Gerontol A Biol Sci Med Sci. 2012;67:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim DH, Schneeweiss S. Measuring frailty using claims data for pharmacoepidemiologic studies of mortality in older adults: evidence and recommendations. Pharmacoepidemiol Drug Saf. 2014;23:89–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centers for Medicare & Medicaid Services. Readmissions Reduction Program 2012. Available at: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html Accessed April 2015.
- 12. Centers for Medicare & Medicaid Services. Bundled Payments for Care Improvement (BPCI) Initiative 2013. Available at: http://innovation.cms.gov/initiatives/bundled-payments/ Accessed December 2014.
- 13. Faurot KR, Jonsson Funk M, Pate V, et al. Using claims data to predict dependency in activities of daily living as a proxy for frailty. Pharmacoepidemiol Drug Saf. 2015;24:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schneeweiss S, Setoguchi S, Brookhart MA, et al. Assessing residual confounding of the association between antipsychotic medications and risk of death using survey data. CNS Drugs. 2009;23:171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schneeweiss S, Wang PS. Association between SSRI use and hip fractures and the effect of residual confounding bias in claims database studies. J Clin Psychopharmacol. 2004;24:632–638. [DOI] [PubMed] [Google Scholar]
- 16. Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58:595–602. [DOI] [PubMed] [Google Scholar]
- 17. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 18. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 19. Bravo G, Dubois MF, Hebert R, et al. A prospective evaluation of the Charlson Comorbidity Index for use in long-term care patients. J Am Geriatr Soc. 2002;50:740–745. [DOI] [PubMed] [Google Scholar]
- 20. Centers for Medicare & Medicaid Services. Medicare Program; Inpatient Rehabilitation Facility Prospective Payment System for federal fiscal year 2011: final rule. Fed Regist. 2011;75:42835–42884. [Google Scholar]
- 21. Pope GC, Kautter J, Ellis RP, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25:119–141. [PMC free article] [PubMed] [Google Scholar]
- 22. Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36), I: conceptual framework and item selection. Med Care. 1992;30:473–283. [PubMed] [Google Scholar]
- 23. Groll DL, Heyland DK, Caeser M, Wright JG. Assessment of long-term physical function in acute respiratory distress syndrome (ARDS) patients: comparison of the Charlson Comorbidity Index and the Functional Comorbidity Index. Am J Phys Med Rehabil. 2006;85:574–581. [DOI] [PubMed] [Google Scholar]
- 24. Haines K, Berney S, Warrillow S, Denehy L. Predicting physical function and health related quality of life following intensive care. Int J Phys Med Rehabil. 2014;2:1–6. [Google Scholar]
- 25. Fan E, Gifford JM, Chandolu S, et al. The Functional Comorbidity Index had high inter-rater reliability in patients with acute lung injury. BMC Anesthesiol. 2012;12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. The Medicare Payment Advisory Commission (MedPAC). Report to the Congress: Medicare and the health care delivery system. Inpatient Rehabilitation Facility Services. July 2013. Available at: http://www.medpac.gov/documents/reports/mar13_entirereport.pdf?sfvrsn=0 Accessed June 2015.
- 27. Inpatient Rehabilitation Facility Patient Assessment Instrument (IRF-PAI) Training Manual. Buffalo, NY: Uniform Data System for Medical Rehabilitation; 2001. [Google Scholar]
- 28. Ottenbacher KJ, Hsu Y, Granger CV, Fiedler RC. The reliability of the Functional Independence Measure: a quantitative review. Arch Phys Med Rehabil. 1996;77:1226–1232. [DOI] [PubMed] [Google Scholar]
- 29. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 30. Resnik L, Gozalo P, Hart DL. Weighted index explained more variance in physical function than an additively scored functional comorbidity scale. J Clin Epidemiol. 2011;64:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Centers for Medicare & Medicaid Services. Risk Adjustment: Evaluation of the CMS-HCC Risk Adjustment Model 2015. Available at: https://www.cms.gov/Medicare/Health-Plans/MedicareAdvtgSpecRateStats/downloads/Evaluation_Risk_Adj_Model_2011.pdf Accessed August 2015.
- 32. Kleinbaum D, Kupper L, Nizam A, et al. Applied Regression Analysis and Other Multivariable Methods. 5th ed Boston, MA: Cengage Learning; 2013. [Google Scholar]
- 33. Noyes K, Liu H, Temkin-Greener H. Medicare capitation model, functional status, and multiple comorbidities: model accuracy. Am J Manag Care. 2008;14:679–690. [PMC free article] [PubMed] [Google Scholar]
- 34. Centre for Clinical Practice at NICE (UK). Rehabilitation After Critical Illness 2009. Available at: http://www.ncbi.nlm.nih.gov/books/NBK11658/ Accessed July 2015.
- 35. Institute of Medicine. Performance Measurement: Accelerating Improvement (Pathways to Quality Health Care Series). Washington, DC: The National Academics Press; 2006. Available at: https://www.iom.edu/Reports/2005/Performance-Measurement-Accelerating-Improvement.aspx Accessed October 2014. [Google Scholar]
- 36. US Congress. The Improving Medicare Post-Acute Care Transformation Act of 2014, HR 4994 (113th) 2014. Available at: https://www.govtrack.us/congress/bills/113/hr4994 Accessed March 2015.