Abstract
Background:
Coagulase negative Staphylococci (CoNS) are one of the most common bacteria found on human skin and on mucous membranes as a component of normal flora. The presence of CoNS in clinical specimens is frequently associated with an infectious aetiology or contamination.
Objectives:
We aimed to evaluate CoNS species distribution and susceptibility patterns in specimens obtained from clinics and hospitals in the Northern area of Jordan.
Methods:
Standard identification methods showed the presence of CoNS in 223 specimens at different local hospitals. Susceptibility testing was performed using 18 antibiotics in accordance with the Clinical and Laboratory Standards Institute (CLSI) recommendations.
Results:
Staphylococcus epidermidis and S. haemolyticus were found to be the most common species isolated from all specimens representing 122 (54.7%) and 52 (23.4%) of all CoNS species, respectively. Antibiotic susceptibility testing of CoNS species revealed their sensitivity to vancomycin, linozolid, rifampin and nitrofurantin, while showing a highly resistant pattern to ampicillin, penicillin, ceftriaxone, cefazolin, amoxicillin-clavulanic acid and erythromycin. Some variation of the susceptibility pattern of CoNS species were identified in specimens isolated from the ICU and paediatric hospital wards as well as from clinical specimens of urine, blood and catheter tips.
Conclusion:
The most common CoNS isolates were found to be S. epidermidis and S. haemolyticus with variable percentages according to the specimen source. Moreover, a high susceptibility CoNS to vancomycin, rifampin, and linezolid showed resistance to amoxicillin and penicillin.
Keywords: CoNS, Antibiotic, Hospital, Intensive Care Units, Hospital Wards, Nosocomial Infection
INTRODUCTION
Coagulase Negative Staphylococci (CoNS) are a major component of the normal flora of human skin and the oral flora found on mucous membranes (1, 2). These Gram positive bacteria have been found to be the third most common causative agent of nosocomial infections (1, 2). In addition to their virulence, CoNS possess the ability to survive on synthetic medical devices and equipment and also on various surfaces in hospitals for weeks to months (3). While this species of Gram-positive bacteria have been reported to colonize patients and hospital staff, they can also be introduced or transmitted on to the body through the use of contaminated equipment and devices such as intravenous catheters, prosthetic heart valves, orthopaedic implants and others (4, 5).
CoNS are a heterogeneous group of bacteria, consisting of approximately 40 species, of which, several species have been recognized as potential pathogens to humans (6). The most common species isolated from human specimens that result in disease are S. epidermidis, S. haemolyticus, S. hominis, and S. saprophyticus. Other species such as S. warneri, S. lugdunensis, S. capitis, S. simulans, S. cohnii, S. saccharolyticus, and S. xylosus have been considered as significant opportunistic pathogens but rarely isolated (7, 8).
CoNS species have been recognised as etiologic agents of a wide variety of infections especially in immune-compromised patients, patients with indwelling or implanted foreign bodies (9). They can play an important role in bacteraemia (10), central nervous system shunt infection, intravascular catheter-related infections, endocarditic (11), urinary tract infections (12), surgical site infections (13), endophthalmitis, foreign body infection, peritonitis and wound, bone and joint infections as well as infections in neonates (14, 15). Distinction between a clinically significant pathogenic infection and contaminating CoNS isolates is difficult and remains a major challenge for clinicians (8, 16).
Moreover, the susceptibility pattern of CoNS has varied to antimicrobial agents between species and countries due to wide use of antibiotics for prophylaxis or therapy especially in hospitalized patients (17, 18). The main aim of this study was to determine the most common CoNS species isolated from different clinical specimens in the Northern Province of Jordan and to evaluate the susceptibility pattern of these species.
MATERIALS AND METHODS
Specimens were collected from all patient populations and consisted of inpatient hospitalized patients as well as outpatient samples. The type of specimen obtained for analysis consisted of the following: blood culture, abscess fluid, catheter tip, urine, wound culture, respiratory samples, nasal samples, body fluids, urethral samples, eye cultures, cerebro-spinal fluid, tissue samples, semen, ear, and cervical swabs. The seven hospital wards from which samples were obtained included the intensive care unit (ICU), nursery intensive care unit (NICU), emergency department (ED), department of surgery, internal medicine, paediatrics, and the outpatient clinics (OPD).
Clinical specimens were collected using standard collection techniques (9, 13), and inoculated on appropriate bacteriological media, including 10% Sheep Blood agar, Chocolate agar, Thioglycollate, MacConkey agar media. Inoculated plates were incubated aerobically at 37 °C for 18–24 hours while blood cultures were analysed using an automated blood culture system (BACT / ALERT 3D. Bio-Merieux) to detect bacterial growth.
Identification of isolated CoNS was made according to the standard methods for any potentially clinically significant growth appearing on the culture media based on quantity, feature of growth, source and site of specimens (19).
Primary identification was initiated using basic microbiological features and biochemical characteristics such as colony morphology, Gram staining, catalase and coagulase tests (19). Final identification and antibiotic susceptibility testing of the bacteria isolated from clinical specimens was performed using different auto-analyzing systems. The results of autoanalyzers were confirmed manually using biochemical tests and the Kirby – Bauer disk diffusion technique according to Clinical and Laboratory Standards Institute (CLSI) guidelines on Mueller-Hinton agar (20, 21).
In vitro antibiotic susceptibility patterns of all isolated CoNS was determined against 18 antibiotics (μg): penicillin, ampicillin (30), amoxicillin-clavulanic acid (30), cefazolin (30), trimethoprim-sulfa-methoxazole (1.25/23.75), oxacillin (30), gentamicin (120), erythromycin (15), ceftriaxon (30), ciprofloxacin (5), norfloxacin (5), nitrofurantoin (300), linezolid (30), levofloxacin (5), tetracycline (30), clindamycin (2), rifampicin (5) and vancomycin (30). All antibiotic medication was purchased from Oxoid Microbiology Products, Thermo Scientific (Hampshire, UK).
Statistical analysis consisted of descriptive statistics where data were expressed as a number and corresponding percentage as appropriate.
RESULTS
Based on standard manual and automated methods, 223 specimens showed the presence of CoNS species, which were isolated and identified from various clinical specimens collected from in patients and out-patients of different ages and sexes during the study period.
Of the fifteen samples sources, the most common source of CoNS species was blood culture (n=69, 30.9%) isolates, followed by abscesses (n=36, 16.2%), catheter tip (n=34, 15.2%), urine (n=27, 12.2%) and wound (n=14, 6.3%) (Table 1). The remaining ten sources, when combined together, accounted for 43(19.2%) of all obtained isolates.
Table 1:
Number and percentage of isolated CoNS according to the source of specimens.
| Type of Sample | Number of Isolates | Percentage % |
|---|---|---|
| Blood | 69 | 30.9 |
| Abscess | 36 | 16.2 |
| Catheter tip | 34 | 15.2 |
| Urine | 27 | 12.2 |
| Wound | 14 | 6.3 |
| Respiratory | 8 | 3.6 |
| Nasal | 6 | 2.7 |
| Body fluids | 6 | 2.7 |
| Urethral | 5 | 2.2 |
| Eye | 4 | 1.8 |
| CSF | 4 | 1.8 |
| Tissue | 3 | 1.3 |
| Semen | 3 | 1.3 |
| Ear | 3 | 1.3 |
| Cervical | 1 | 0.5 |
| Total | 223 | 100 |
Samples were obtained from seven different hospital wards and showing a varied distribution of CoNS sources as shown in (Table 2).
Table 2:
Number and percentage of isolated CoNS according to the hospital wards.
| Wards | Number of Isolates | Percentage % |
|---|---|---|
| ICU | 92 | 41.2 |
| OPD clinics | 61 | 27.5 |
| NICU | 26 | 11.7 |
| Pediatrics | 21 | 9.3 |
| Emergency | 17 | 7.6 |
| Surgery | 4 | 1.8 |
| Internal | 2 | 0.9 |
| Total | 223 | 100 |
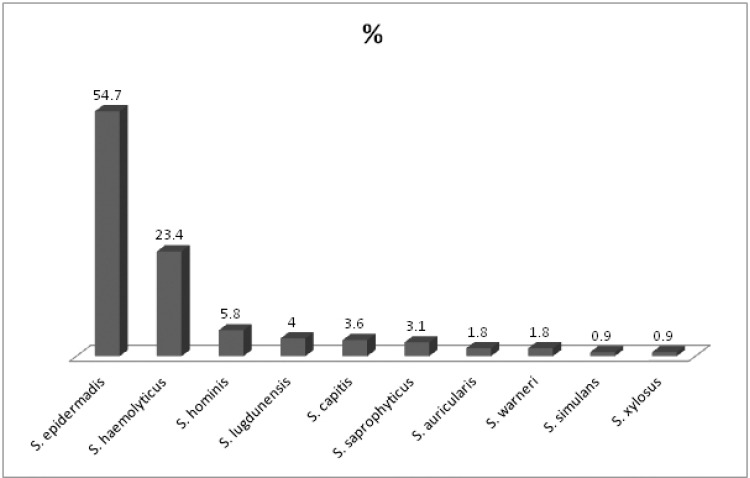
Ten species of CoNS were isolated and identified during the study from various body sites, the most common species isolated from all specimens were Staphylococcus epidermidis and S. haemolyticus which comprised 122 (54.7%) and 52 (23.4%), respectively. Species identification showed the presence of S. hominis in 13 (5.8%) isolates followed by S. lugdunensis in 9 (4%), S. capitis in 8(3.6%) and S. saprophyticus in 7 (3.1%). Four other less common isolates of CoNS consisted of S. auricularis, S. warneri, S. simulans and S. Xylosus collectively represented the remaining 12 (5.4%) (Fig.1).
Fig 1.
Percentage of isolated CoNS species.
Analysis of each individual isolated species of CoNS showed a variable distribution of all species among the different sources of clinical specimens and the different inpatient and outpatient hospital wards (Table 3). S. epidermidis, the most common species of CoNS isolated, was isolated from all types of specimens and was present on all hospital wards. However, the majority of the bacteria were found growing in blood cultures (34%) and on catheter tips (21%). Despite the ubiquitous presence of this species on all wards, the majority was found in ICU (46%) isolates while having a high presence in specimens received from OPD clinics (21%) and the NICU (17%).
Table 3:
Distribution of CoNS isolates in clinical specimens and hospital wards
| CoNS species | S. epidermadis | S. haemolyticus | S. hominis | S. lugdunensis | S. capitis | S. saprophyticus | S. auricularis | S. warneri | S. simulans | S. xylosus | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| NO. of Isolates (%) | 122 (54.7) | 52 (23.4) | 13 (5.8) | 9 (4) | 8 (3.6) | 7 (3.1) | 4 (1.8) | 4 (1.8) | 2 (0.9) | 2 (0.9) | |
| Distributed of CoNS isolates in clinical specimens | |||||||||||
| Clinical specimens | Blood | 41 | 13 | 8 | 0 | 1 | 1 | 2 | 2 | 1 | 0 |
| Abscess | 19 | 6 | 1 | 4 | 4 | 0 | 1 | 0 | 0 | 1 | |
| Tip of catheter | 26 | 5 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | |
| Urine | 12 | 5 | 0 | 2 | 1 | 5 | 1 | 1 | 0 | 0 | |
| Wound | 8 | 3 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | |
| Respiratory | 8 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Other* | 11 | 17 | 2 | 3 | 1 | 1 | 0 | 0 | 0 | 0 | |
| Distributed of CoNS isolates in hospital wards | |||||||||||
| Hospital Wards | ICU | 56 | 27 | 3 | 0 | 2 | 0 | 2 | 1 | 0 | 1 |
| OPD clinic | 26 | 16 | 2 | 4 | 3 | 6 | 1 | 1 | 1 | 1 | |
| NICU | 21 | 3 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| Pediatrics | 11 | 1 | 5 | 0 | 2 | 0 | 0 | 1 | 1 | 0 | |
| Emergency | 5 | 4 | 2 | 3 | 1 | 1 | 1 | 0 | 0 | 0 | |
| Other** | 3 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
Nasal swab, Body fluids, Urethral, Eye, CSF, Tissue, Semen, Ear, Cervical swab
Surgary, Internal Wards
The second most commonly isolated CoNS species was S. haemolyticus. The majority of these bacteria were isolated from blood (25%) and abscess (11%) specimens although they were also identified to be in high presence on catheter tips (10%) and in urine specimens (10%) (Table 3). S. haemolyticus was found to be dominant in ICU isolates (52%) and present in high number of samples received from OPD clinics (31%) when compared to the other hospital wards. The other eight species of CoNS had a sporadic distribution among the sources of the individual specimens and between the various hospital wards; their distribution patterns can be seen in Table 3.
Susceptibility patterns of CoNS showed interesting results. In general, the species showed high sensitivity to vancomycin (100%), linozolid (98.2%), rifampin (95.5%) and nitrofurantin (92.8%). Meanwhile, the lowest sensitivity rate was observed with ampicillin (1.8%), penicillin (2.7%), ceftriaxon (22%), cefazolin (22.4%), amoxicillin-clavulanic acid (24.2%) and erythromycin (24.2%). The most commonly isolated species, S. epidermidis, showed a similar susceptibility pattern as described above for all CoNS (Table 4).
Table 4:
Susceptibility pattern of CoNS of the most common isolated species, specimen types and hospital wards.
| All CoNS | Most common species | Specimens type | Hospital wards | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| S. epidermadis | S. haemolyticus | Blood | Abscess | TIP* | Urine | ICU | OPD | NICU | Pediatrics | ||
| % | % | % | % | % | % | % | % | % | % | % | |
| Amoxicillin-clav. acid | 24.2 | 17.2 | 19.2 | 10.1 | 58.3 | 0 | 37 | 5.4 | 52.5 | 15.4 | 9.5 |
| Ampicillin | 1.8 | 0.8 | 2 | 1.4 | 2.8 | 0 | 0 | 0 | 3.3 | 3.8 | 0 |
| Cefazolin | 22.4 | 17.2 | 19.2 | 10.1 | 58.3 | 0 | 26 | 4.4 | 47.5 | 15.4 | 9.5 |
| Cefriaxone | 22 | 16.4 | 19.2 | 10.1 | 58.3 | 0 | 26 | 4.4 | 47.5 | 11.5 | 9.5 |
| Ciprofloxacin | 45.7 | 44.3 | 23.1 | 37.7 | 66.7 | 20.6 | 77.8 | 19.6 | 72.1 | 50 | 61.9 |
| Clindamycin | 45.3 | 42.6 | 42.3 | 33.3 | 61.1 | 41.2 | 51.9 | 30.4 | 54.1 | 53.8 | 42.9 |
| Erythromycin | 24.2 | 20.5 | 17.3 | 16 | 38.9 | 14.7 | 48.1 | 10.9 | 36.1 | 23.1 | 19.9 |
| Gentamicin | 50.7 | 50.8 | 32.7 | 31.9 | 86.1 | 26.5 | 92.6 | 31.5 | 85.2 | 27 | 33.3 |
| Levofloxacin | 45.3 | 41.8 | 25 | 36.2 | 66.7 | 14.7 | 77.8 | 17.4 | 73.8 | 50 | 61.9 |
| Linezolid | 98.2 | 97.5 | 98 | 100 | 100 | 91.2 | 96.3 | 96.7 | 100 | 100 | 100 |
| Nitrofurantoin | 92.8 | 92.6 | 96.2 | 97.1 | 94.4 | 82.4 | 92.3 | 93.5 | 91.8 | 96.2 | 100 |
| Norfloxacin | 45.3 | 45.1 | 19.2 | 37.7 | 63.9 | 17.6 | 85.2 | 18.5 | 70.5 | 50 | 66.7 |
| Oxaciliin | 25.1 | 19 | 19.2 | 10.1 | 58.3 | 0 | 37 | 5.4 | 55.7 | 15.4 | 9.5 |
| Penicillin | 2.7 | 0.8 | 19.2 | 2.9 | 5.6 | 0 | 0 | 0 | 3.3 | 3.8 | 0 |
| Rifampin | 95.5 | 93.4 | 96.2 | 94.2 | 100 | 94.1 | 100 | 92.4 | 96.7 | 100 | 100 |
| Tetracyclin | 80.7 | 86.9 | 75 | 84.1 | 86.1 | 88.2 | 70.4 | 88 | 72.1 | 80.8 | 76.2 |
| Tri-sulfamethoxazole | 64.1 | 59.1 | 61.5 | 53.6 | 75 | 61.8 | 77.8 | 57.6 | 77 | 53.8 | 66.7 |
| Vancomycin | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Catheter tip
Results of susceptibility testing of the most common specimen sources of CoNS (blood culture, abscess, catheter tip, and urine culture) and the most common hospital wards where specimens were obtained (ICU, OPD, NICU, Paediatrics) using the set of antibiotics can be seen in (Table 4). CoNS obtained from blood cultures were susceptible to linezolid (100%) and vancomycin (100%) followed by rifampin (94%), and tetracyclin (84%). Antibiotics with the least activity against the Gram positive bacteria were ampicillin (1.4%) and penicillin (2.9%). Specimens obtained from abscess, catheter tips, and urine cultures mimicked the susceptibility that was described from blood cultures although specimens from catheter tips had additional antibiotics which were ineffective at eradicating infection. In addition to ampicillin and penicillin, catheter tip CoNS infections had very low susceptibility to amoxicillin-clavulonic acid, cefazolin, ceftriaxone, and oxacillin.
Furthermore, susceptibility pattern of CoNS species according to the hospital wards showed similar results as seen when examining susceptibility for all species of CoNS. However, two hospital locations, the ICU and paediatric wards, showed high rates of resistance to amoxicillin, clavulonic acid, cefazolin, ceftriaxone, and oxacillin, similar to isolates cultured from catheter tips.
DISCUSSION
Coagulase-negative staphylococci (CoNS) are a group of micro-organisms that have largely been considered as contaminants because of the role in the normal flora. Recently, some studies have implicated this group as one of the more important causative agents of human infections, especially in immune-compromised patients, premature newborns and patients with indwelling medical devices (4, 5).
The various species of CoNS are one of the most commonly isolated bacteria from clinical specimens in a clinical microbiology laboratory (22). They are the most common agents of nosocomial bloodstream infections as well as other type of infections either in healthy individuals or hospitalized patients (23). We have demonstrated that the highest percentage of CoNS species isolates were found in blood cultures and in patients located in the ICU, which is consistent with other similar studies (4, 5, 24). Our results also show that while the most common source of CoNS species isolation was in blood cultures (30%), other sources including abscess, catheter tip, and urine cultures combined for a total of 43%. Unsurprisingly, the ICU accounted for 41%, or the majority, of all CoNS isolates obtained by laboratories. However, an interesting finding was that the outpatient clinics (27%) accounted for slightly less than the adult ICU and more than the Nursery ICU. This high number can possibly be explained by looking further into the population of patients being seen in the outpatient departments. For instance, urinary tract infections and wound infections are high among out-patients population, which could potentially clarify the increased use of microbiology specimens to manage these outpatient conditions. Another possible reason for the OPD specimens having such a high percentage of CoNS species isolates may be related to poor sample collection techniques and/or multi-sample sources and clinics (8, 11).
Increase use of central venous catheters (CVC) and other indwelling vascular devices in hospitals, especially in ICU patients, create a potential for increased rates of infection. The possibility of colonization of these catheters and/or medical devices with CoNS as a result of skin contamination of patients and staff may explain the high percentage of isolates obtained from ICUs and the high number of infected catheter tips cultures (4, 25–27). Due to these circumstances, CoNS species must be considered as an important causative agent of nosocomial bacteraemia and catheter related infection (1, 28).
In our study, S. epidermidis is the major isolated species followed by S. haemolyticus, and both were isolated from all clinical specimens at all studied hospital wards. Our results are consistent with studies performed in other countries (8, 28–33).
CoNS species are one of the most common causative agents of sepsis and nosocomial infections for newborns and infants. They have been isolated from various medical devices used in these units, from NICU environment, and paediatric wards (14, 31). The percentage of isolated CoNS from the NICU and paediatric wards in our study is 9.3% and 7.3%, respectively, which is consistent with other findings while some of the inter-study differences are possibly due to the isolated species, the type of infection and clinical case of the patients (10, 14, 28, 33).
Different antibiotics were used to evaluate the susceptibility pattern of isolated CoNS according to recommendations of CLSI 2009 guidelines for Gram Positive Bacteria (21). CoNS isolates showed high sensitivity to vancomycin, linozolid, rifampin and nitrofurantoin. These four antibiotics may play an important role in the treatment and prevention of nosocomial infections of CoNS. However, CoNS species showed remarkable resistance to ampicillin, penicillin and other types of antibiotics listed in (Table 4). While bacteria continue to acquire resistance to antibiotics, selection of the appropriate agents is of paramount importance. Our results from the specimens obtained from Northern population of Jordan are consistent with the results observed by other institutions and studies (18, 28, 31, 32, 34–36).
We demonstrated that the predominant isolated CoNS species were S. epidermidis and S. haemolyticus, which makes the susceptibility pattern of these two species closely resembling the susceptibility pattern of all other isolated CoNS. Long hospitalization periods, extensive use of antibiotics and the ability of these organisms to create multi-layered bio-films on artificial surfaces are the possible reasons of a high resistance rate in general and to oxacillin specifically; especially in the species that were isolated from catheter tips and blood cultures (11, 16, 18, 27, 30, 34, 36). The low resistance rate of isolated CoNS bacteria from urine samples may be due to presence of a high number of S. saprophyticus species (9, 12, 28, 32). On the other hand, the high resistance rate of species that were isolated from the ICU and the paediatric wards may be due to the patients’ clinical case, age and type of causative agent identified (24, 29, 31, 37).
CONCLUSION
The most common CoNS isolates in the Northern Province of Jordan were found to be S. epidermidis and S. haemolyticus and varied in percentage according the specimen that was obtained. While this group of organisms shows high susceptibility to vancomycin, rifampin, and linezolid, high rates of resistance to amoxicillin and penicillin and other variability exists and warrants further evaluation. Infections originating in blood cultures, catheter tips, or from patients in the ICU or paediatric wards should undergo careful susceptibility testing because of the decreased efficacy of additional antibiotics in these situations.
Acknowledgements
We would like to thank King Abdullah University Hospital and Department of Biological Sciences at Yarmouk University for their financial support.
REFERENCES
- 1. Javadpour S KE, Karmostaji A. Frequency and anti-biogram pattern of coagulase negative Staphylococcus in clinical specimens of Shahid Mohammadi Hospital in patients Bandar-Abbas, Iran. Afric J Microbiol Res 2010;4: 1581– 1583. [Google Scholar]
- 2. Couto I, Pereira S, Miragaia M, Sanches IS, de Lencastre H. Identification of clinical staphylococcal isolates from humans by internal transcribed spacer PCR. J Clin Microbiol 2001;39: 3099– 3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malik M RK. Significance of coagulase negative Staphylococcus species in blood culture. J Clin Diagn Res 2012;6: 632– 635. [Google Scholar]
- 4. Nzeako B AS, Neilson F, Albalkhair A. Type of bacteria on some medical devices used in sultan qaboos university hospital wards. Middle-East J Scientific Res 2010;5: 449– 53. [Google Scholar]
- 5. Sharma V, Jindal N, Devi P. Prevalence of methicillin resistant coagulase negative staphylococci in a tertiary care hospital. Iran J Microbiol 2010;2: 185– 188. [PMC free article] [PubMed] [Google Scholar]
- 6. Sarathbabu R RN, Ramani M TV. Characterization of coagulase negative staphylococci isolated from urine, pus, sputum and blood samples. Int J Pharma Sci Inv 2013;2: 37– 46. [Google Scholar]
- 7. Bouchami O AW, Ben Hassen A. Species distribution and antibiotic sensitivity pattern of coagulase-negative Staphylococci other than Staphylococcus epidermidis isolated from various clinical specimens. Afri Jf Microbiol Res 2011;5: 298– 1305. [Google Scholar]
- 8. Nowak T, Balcerczak E, Mirowski M, Szewczyk EM. Detection of methicillin resistance in hospital environmental strains of coagulase-negative staphylococci. P J Microbiol 2006;55: 339– 343. [PubMed] [Google Scholar]
- 9. Longauerova A. Coagulase negative staphylococci and their participation in pathogenesis of human infections. Bratisl Lek Listy 2006;107: 448– 52. [PubMed] [Google Scholar]
- 10. Mert G KA, Bedir O, Basustaoglu A. Clinical significance and staphylococcal cassette chromosome mec (SCCmec) characterization of coagulase-negative staphylococci isolated from blood cultures. Turk J Med Sci 2011; 41: 859– 865. [Google Scholar]
- 11. Jain A, Agarwal A, Verma RK, Awasthi S, Singh KP. Intravenous device associated blood stream staphylococcal infection in paediatric patients. Indian J Med Res 2011;134: 193– 199. [PMC free article] [PubMed] [Google Scholar]
- 12. Agbede O KO, Ogunleye V, Adegoke A. Incidence of novobiocin resistant coagulase negative staphylococcus saprophyticus in urinary tract infection in UITH, ilorin, nigeria. E3 J Med Res 2012;1: 44– 51. [Google Scholar]
- 13. Javed IHR, Zubair M, Anwar MS, Tayyib M, Husnain S. Microbiological surveillance of operation theatres and ICUs of a tertiary care hospital, Lahore. Afric J Biotechnol 2008;7: 3535– 3539. [Google Scholar]
- 14. Bhamare S KA, Dohe V, Rajput AG, Bhardwaj R, Kagal A. Study of sources of coagulase negative staphylococci infection from NICU environment. Int J of Healthcare & Biomed Res 2013;1: 26– 31. [Google Scholar]
- 15. Vackova M HI, Smetana J, Chlibek R, Bostikova V, Splino M. Microbial air load at the transplant intensive care unit. Mil Med Sci Lett 2011;80: 52– 57. [Google Scholar]
- 16. Gheibi S KM, Ilkhanizadeh B, AsghariSana F, Mahmoodzadeh H. Coagulase negative Staphylococcus; the most common cause of neonatal septicemia in urmia, Iran. Iran J Pediatr 2008;18: 237– 43. [Google Scholar]
- 17. El Kholy ABH, Hall GS, Procop GW, Longworth DL. Antimicrobial resistance in Cairo, Egypt 1999–2000: A survey of five hospitals. J Antimicrobial Chemother 2003;51: 625– 30. [DOI] [PubMed] [Google Scholar]
- 18. El Kholy A, Baseem H, Hall GS, Procop GW, Longworth DL. Antimicrobial resistance in Cairo, Egypt 1999–2000: a survey of five hospitals. J Antimicrobial Chemother 2003;51: 625– 630. [DOI] [PubMed] [Google Scholar]
- 19. Goyal R, Singh NP, Kumar A, Kaur I, Singh M, Sunita N, et al. Simple and economical method for speciation and resist typing of clinically significant coagulase negative staphylococci. Indian J Med Microbiol 2006;24: 201– 204. [PubMed] [Google Scholar]
- 20. Tiwari HK SD, Sen MR. Evaluation of different tests for detection of Staphylococcus aureus using coagulase (coa) gene PCR as the gold standard. Nepal Med Coll J 2008;10: 129– 31. [PubMed] [Google Scholar]
- 21. Clinical and Laboratory Standards Institute Performance standards for antimicrobia susceptibility testing: Nineteenth informational supplement. CLSI document no M100-S19 2009; CLSI. [Google Scholar]
- 22. Shin JH, Jung HJ, Lee HR, Kim JH, Kim HR, Lee JN. Prevalence, identification, and antimicrobial susceptibility of Staphylococcus lugdunensis from various clinical specimens in Korea. Jpn J Infect Dis 2007;60: 312– 313. [PubMed] [Google Scholar]
- 23. Krause R, Haberl R, Wolfler A, Daxbock F, Auner HW, Krejs GJ, et al. Molecular typing of coagulase-negative staphylococcal blood and skin culture isolates to differentiate between bacteremia and contamination. Eur J Clin Microbiol Infect Dis 2003;22: 760– 763. [DOI] [PubMed] [Google Scholar]
- 24. Akpaka PE, Christian N, Bodonaik NC, Smikle MF. Epidemiology of coagulase-negative staphylococci isolated from clinical blood specimens at the University Hospital of the West Indies. West Indian Med J 2006;55: 170– 173. [DOI] [PubMed] [Google Scholar]
- 25. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev 2006;19: 788– 802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Findik UY, Otkun MT, Erkan T, Sut N. Evaluation of handwashing behaviors and analysis of hand flora of intensive care unit nurses. Asian Nursing Res 2011;5: 99– 107. [DOI] [PubMed] [Google Scholar]
- 27. Agvald-Ohman C LB, Edlund C. Multiresistant coagulase-negative staphylococci disseminate frequently between intubated patients in a multidisciplinary intensive care unit. Crit Care Med 2004;8: R42– 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singhal R, Dhawan S, Mohanty S, Sood S, Dhawan B, Das B, et al. Species distribution & antimicrobial susceptibility of coagulase negative Staphylococci in a tertiary care hospital. Indian J Med Res 2006;123: 569– 570. [PubMed] [Google Scholar]
- 29. Akinkunmi E OA, Lamikanra A. Species distribution and antibiotic resistance in coagulase-negative staphylococci colonizing the gastrointestinal tract of children in ile-ife, nigeria. Trop J Pharm Res 2010;9: 35– 43. [Google Scholar]
- 30. Cuevas O, Cercenado E, Vindel A, Guinea J, Sanchez-Conde M, Sanchez-Somolinos M, et al. Evolution of the antimicrobial resistance of Staphylococcus spp. in Spain: five nationwide prevalence studies, 1986 to 2002. Antimicrob Agents Chemother 2004;48: 4240– 4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. de Oliveira A, Sanches P, Lyra JC, Bentlin MR, Rugolo LM, de Lourdes Ribeiro de Souza da Cunha M. Risk factors for infection with coagulase-negative staphylococci in newborns from the neonatal unit of a brazilian university hospital. Clin Med Insights Pediatr 2012;6: 1– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sheikh A MM. Identification and determination of coagulase-negativeStaphylococci species and antimicrobial susceptibility pattern of isolates from clinical specimens. Afric J Microbiol Res 2012;6: 1669– 74. [Google Scholar]
- 33. Perez-Gonzalez LF, Ruiz-Gonzalez JM, Noyola DE. Nosocomial bacteremia in children: a 15-year experience at a general hospital in Mexico. Infect Control Hosp Epidemiol 2007;28: 418– 22. [DOI] [PubMed] [Google Scholar]
- 34. Cimolai N, Carter JE. Clinical validation for oxacillin susceptibility testing of coagulase negative staphylococci. Arch Dis Child 2002;86: 446– 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Khadri H AM. Prevalence and antibiotic susceptibility pattern of methicillin-resistant and coagulase-negative staphylococci in a tertiary care hospital in India. Int J Med Medical Sci 2010;2: 116– 20. [Google Scholar]
- 36. Huang SY, Tang RB, Chen SJ, Chung RL. Coagulase-negative staphylococcal bacteremia in critically ill children: risk factors and antimicrobial susceptibility. J Microbiol Immunol Infect 2003;36 (1): 51– 5. [PubMed] [Google Scholar]
- 37. Jean-Baptiste N, Benjamin DK, Cohen-Wolkowiez M, Fowler VG, Laughon M, Clark RH, et al. Coagulase-negative staphylococcal infections in the neonatal intensive care unit. Infect Control Hosp Epidemiol 2011;32: 679– 686. [DOI] [PMC free article] [PubMed] [Google Scholar]