Abstract
Background and Objectives:
Probiotic yeasts are used in production of functional foods and pharmaceutical products. They play an important role in promoting and maintaining human health. Until now, little work has been published on improving the survival of Saccharomyces in stimulated gastrointestinal condition.
Material and Methods:
In this study the exposure of the yeast in the capsulate and free forms to artificial gastrointestinal conditions was assessed and the number of viable Saccharomyces cerevisiae cells during 0 to 120 mines in these conditions was evaluated by a pour plate method using sabouraud dextrose agar.
Results:
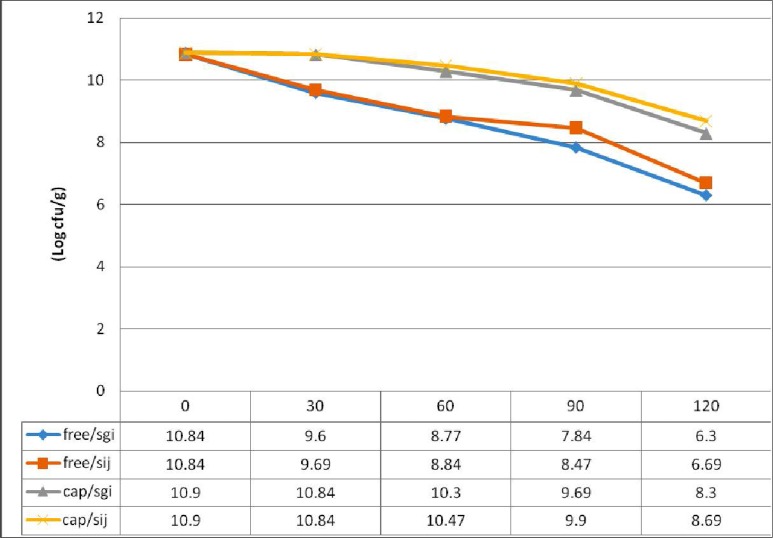
Results showed the shape of the beads was generally spherical, sometimes elliptical with a mean diameter of about 50–90 μm. Also count of viable probiotic cells obtained for all the microcapsules were above the recommended levels for a probiotic food. Also decrease of approximately 4 logs was noted in the number of free cells after 2 h of incubation at pH 2 and 8, when compared to decreases of about 2 logs in the all microencapsulated S. cerevisiae under similar conditions.
Conclusion:
It is concluded that microencapsulation process was significantly able to increase the survival rate of Saccharomyces in a simulated gastrointestinal condition (p<0.05)..
Keywords: Calcium alginate, survival, Saccharomyces, repeated condition
INTRODUCTION
Microencapsulation is one of the most important forms of controlled release of substances that is widely used in several fields of the industry, particularly the food industry (1). The encapsulated microbial cells have been used in wide range of fields for optimum activity benefiting the suitable conditions for growth and metabolism and at the same time protection from harsh environmental conditions (2, 3).Over the years, there has grown a great interest in developing suitable eukaryotic probiotics in general and probiotic yeasts in particular for human and animal practice (4). However, nowadays yeasts are part of nutritional supplements and healthy food realms due to their established beneficial probiotic effects (5). yeasts especially the genus Saccharomyces are widely used probiotics in human and animals throughout the world (6, 7). Providing probiotic living cells with a physical barrier against adverse environmental conditions is therefore an approach currently receiving considerable interest (8, 9). In addition microencapsulation via calcium alginate has been used extensively for immobilization of probiotic because of its simplicity of handling, its non-toxic nature, and its low cost (9–11). The aim of this study was to evaluate the survival rate of free and microencapsulated S. cerevisiae by calcium alginate in simulated gastrointestinal conditions.
MATERIALS AND METHODS
Yeast strain and growth conditions.
The yeast strain used in this study was S. cerevisiae (ATCC code 9763). Yeast cells were activated by transferring cells from refrigerated slant to 50 ml of liquid culture medium sabouraud dextrose (SD) broth (Merck Co., Darmstadt, Germany) and incubated overnight at 28 °C with orbital shaking of 180 rpm. 1 ml of the incubation was inoculated into another 50 ml of the same medium and incubated for 24 h under the same culture conditions. Cells were harvested by centrifugation at 10,000 rpm for 10 min and washed five times with distilled water to remove the culture medium from the cells thoroughly.
Preparation of simulated gastric and intestinal juices.
Simulated gastric juices (SGJ) were prepared by suspending pepsin (P7000, 1:10,000) in sterile sodium chloride solution (0.5%, w/v) to a final concentration of 3 g/l (1038 U/ml) and adjusting the pH to 2.0 with concentrated HCl or sterile 0.1 mol/l NaOH. Simulated intestinal juices (SIJ) were prepared by suspending pancreatin USP (P-1500) in sterile sodium chloride solution (0.5%, w/v) to a final concentration of 1 g/l, with 4.5% bile salts (Oxoid, Basingstoke, UK) and adjusting the pH to 8.0 with sterile 0.1 mol/l NaOH. Both solutions were filtered for sterilization through a 0.22 μm membrane.
Microencapsulation procedure.
In this study extrusion technique was performed for microencapsulation process described earlier (11). A 2% Na–alginate mixture in distilled water. Then, the mixture of cell suspension and Na–alginate were injected into a 0.1 M CaCl2 solution through a sterile insulin syringe. The droplets formed gel spheres immediately. The distance between the syringe and CaCl2 solution was 25 cm.
Cell tolerance to gastrointestinal and determination of total viable counts.
The tolerance of free and immobilized cells of S. cerevisiae on simulated gastric and intestinal juices was determined using the adapted method described by Charteris et al. (1998). The tests were performed using a series of 10ml sterile falcon tubes, one for each sampling point. Two different conditions were tested: in the first and second tests, 0.4 ml of the suspension of either immobilized or free cells were mixed with 1.8 ml of SGJ or SIJ, gently mixed and incubated for 120 min at 28 °C. The control for these tests was done by incubating 0.4 ml of either free or immobilized cells in 1.8 ml sterile sodium chloride solution (0.5 %, w/v) for 120 min at 28 °C. After the addition of free cells or immobilized to SGJ and SIJ, the pH of these were corrected to 2.0 and 8.0, respectively, with sterile 0.1 mol/l NaOH or concentrated HCl. Aliquots of 1 ml were removed at 0, 30, 60, and 120 min (for all trials) for the determination of total viable counts. Total viable counts of S. cerevisiae were determined by a pour plate method using SC agar (Merck Co., Darmstadt, Germany) after serial 10-fold dilutions in peptone water. Plates were incubated at 28 °C for 72 h.
Statistical analysis.
The chi-square (χ2) test was used to assess statistical differences between the groups. A P value less than 0.05 was statistically considered significant.
RESULTS
In this study the size, surface morphology and shape of the 50 randomly selected calcium alginate beads were determined with light microscope at a magnification of 400. The shape of the beads was generally spherical, sometimes elliptical with a mean diameter of about 50–90 μm. On the other hand internal, appearance of coated bead at 400× magnification showed that yeasts were distributed randomly in the alginate matrix (Figs. 1 and 2). The loss during encapsulation was very low due to the gentle methods used. The results of survival rate of encapsulated cells in simulated gastric pH and intestinal bile salt solutions showed in Table 1. No significant reduction in viable count was observed in free as well encapsulated cells in distilled water (pH 6.5) on incubation for up to 2 h (control condition). But variations in counts of free and microencapsulated S. cerevisiae during incubation period at SGJ and SIJ were significant (Table. 1). However, results show there were significant reductions of free yeast in immediate exposure to pH 2 and 8. After 120 min incubation in simulated gastric and intestinal condition there was a significant increase (p < 0.05) in survival of yeast cells in the capsulated compared with free cells.
Fig. 1.
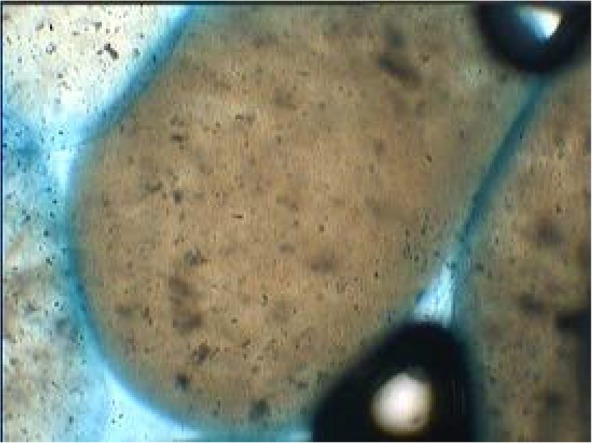
Optical microscope image of coated yeast breads at 400× magnification.
Fig. 2.
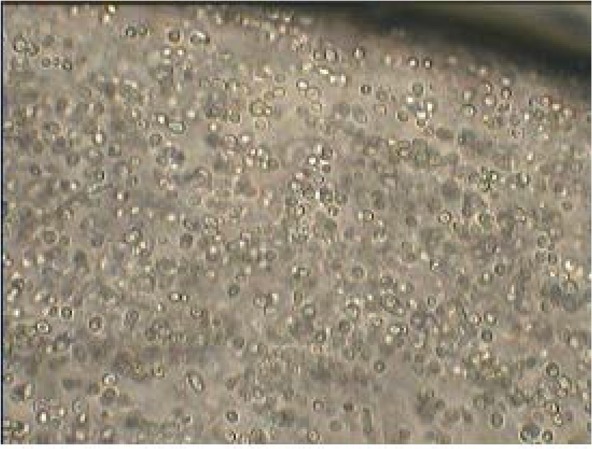
Vertical cross-section and internal appearance of yeast coated bead at 400× magnification.
Table 1.
Number of alive cells after expose to SGI and SIJ for different times (mines) mean ± Sea
| Condition | Type of cell | Hardening time | ||||
|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 90 | 120 | ||
| distilled water | free | 9±.2 × 1010 | 9±.4 × 1010 | 9±.4 × 1010 | 8±.1 ×1010 | 8±.3 ×1010 |
| Capsulated | 9±.2 × 1010 | 9±.3 ×1010 | 9±.2 ×1010 | 8±.4 ×1010 | 7±.4 ×1010 | |
| SGJ | free | 7±.2 ×1010 | 4±.1 ×109 | 6±.3 ×108 | 7±×107 | 2±.2 ×106 |
| Capsulated | 8±.3 ×1010 | 7±.2 ×1010 | 2±.3 ×1010 | 5±.7 ×109 | 2±.6 ×108 | |
| SIJ | free | 7±.3 ×1010 | 5±.2 ×109 | 7±.4 ×108 | 3±.3 ×108 | 5±.2 ×106 |
| Capsulated | 8±.4 ×1010 | 7±.2 ×1010 | 3±.2 ×1010 | 8±.1 ×109 | 5±.1 ×108 | |
Fig. 3.
Survival of microencapsulated and free Sacchromyces cerevisiae in simulated gastric conditions
DISCUSSION
Over the years, probiotics have gained scientific and commercial interest and have now quite commonplace in our daily life starting from health promoting functional foods to therapeutic, prophylactic, and growth supplements (11, 12). On the contrary to prokaryotic cells, the use of eukaryotic as probiotics is limited and only a few probiotics of eukaryotic origin are commercially available for human and animal practices. Survival of the S. cerevisiae was found to increase on alginate encapsulation. Also there was no significant difference in the viability of free cells in distilled water, indicating that water had no effect on the survival (13, 14).
Among the eukaryotic probiotics, yeasts especially Saccharomyces species are dominant and routinely used in a broad range of hosts that can tolerate a wide range of temperature, salt concentration, and pH depending upon the strain especially S. cerevisiae(15).
In addition, the count of viable probiotic cells obtained for all the microcapsules was above the recommended levels for a probiotic food, i.e., equal to or greater than 7 log CFU/g of the product, which is in accordance with earlier reports (15, 16).
On the other hand, the free strain showed a steady loss in viability when exposed to acid conditions. However, the microcapsules containing S.cerevisiae survived very well (P < 0.05) after exposure to in vitro acid conditions when compared to free cells. Also, a decrease of approximately 4 log was noted in the number of free cells after 2 h of incubation at pH 2, when compared to decreases of about 2 log in the all microencapsulated S.cerevisiae under similar conditions (11, 17).
However, the literature report mentioned the effect of Ox gall bile on the viability of the probiotics, in this study the capsulated yeast had a significant survival rate in SIJ. More recently, Saccharomyces probiotics have been found to aid in controlling the pathogenesis of diabetes and other metabolic diseases. It is necessary to mentioned that high tolerance of yeast in different conditions such as intestinal and functional food showed the good potential of this cell as probiotic. Regarding the above mentioned points and agreement of this study results with many other reports about microencapsulation of bacteria, Ca alginate could be good material for protection of probiotic cells (18–20).
CONCLUSION
S. cerevisiae yeast cells, fundamental in the fermentation industry, comprise a natural, safe (food grade), cheap and abundant food material. The present study has shown that Ca alginate microencapsulation significantly improve the yeast survival in simulated gastric environment, and allow viable cells to reach a beneficial level as probiotic. In order to obtain the other aspect of yeast probiotics, this study should be continued by different material and in functional food condition with defined variable in to the future.
Acknowledgements
This work was supported by the Research Council of the University of Tehran. The results described in this paper were part of student PhD thesis. The authors would like to thank Mr. Asad Balal for his kind cooperation during the project.
REFERENCES
- 1. Anal AK, Singh H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci Technol 2007; 18: 240– 251. [Google Scholar]
- 2. Cook MT, Tzortzis G, Charalampopoulos D, Khutoryanskiy VV. Microencapsulation of probiotics for gastrointestinal delivery. Control Release 2012; 162: 56– 67. [DOI] [PubMed] [Google Scholar]
- 3. Wohlgemuth S, Loh G, Blaut M. Recent developments and perspectives in the investigation of probiotic effects. Int J Med Microbiol 2010; 300: 3– 10. [DOI] [PubMed] [Google Scholar]
- 4. Paramera EI, Konteles SJ, Karathanos VT. Microencapsulation of curcumin in cells of Saccharomyces cerevisiae. Food Chem 2011; 125: 892– 902. [Google Scholar]
- 5. Pham-Hoang BN, Romero-Guido C, Phan-Thi H, Waché Y. Encapsulation in a natural, preformed, multi-component and complex capsule: yeast cells. Apple Microbiol Biotechnol 2013; 97: 6635– 6645. [DOI] [PubMed] [Google Scholar]
- 6. Wen-Tao Q, Wei-Ting Y, Yu-Bing X, Xiaojun M. Optimization of Saccharomyces cerevisiae culture in alginate–chitosan–alginate microcapsule. Biochem Eng J 2005; 25: 151– 157. [Google Scholar]
- 7. Graff S, Hussain S, Chaumeil J-C, Charrueau C. Increased intestinal delivery of viable Saccharomyces boulardii by encapsulation in microspheres. Pharm. Res 2008; 25 (6): 1290– 6. [DOI] [PubMed] [Google Scholar]
- 8. Salari R, Bazzaz BSF, Rajabi O, Khashyarmanesh Z. New aspects of Saccharomyces cerevisiae as a novel carrier for berberine. DARU J Pharm Sci 2013; 21: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Buts J-P, De Keyser N. Effects of Saccharomyces boulardii on intestinal mucosa. Dig Dis Sci 2006;51: 1485– 92. [DOI] [PubMed] [Google Scholar]
- 10. Homayouni A, Ehsani M, Azizi A, Razavi S, Yarmand M. Growth and survival of some probiotic strains in simulated ice cream conditions. J Appl Sci 2008; 8: 379– 382. [Google Scholar]
- 11. Fratianni F, Cardinale F, Russo I, Iuliano C, Tremonte P, Coppola R, et al. Ability of synbiotic encapsulated Saccharomyces cerevisiae boulardii to grow in berry juice and to survive under simulated gastrointestinal conditions. J Microencap 2013; 31: 299– 305. [DOI] [PubMed] [Google Scholar]
- 12. Fietto JL, Araújo RS, Valadão FN, Fietto LG, Brandão RL, Neves MJ, et al. Molecular and physiological comparisons between Saccharomyces cerevisiae and Saccharomyces boulardii. Can J Microbiol 2004; 50: 615– 621. [DOI] [PubMed] [Google Scholar]
- 13. Song D, Hayek S, Ibrahim S. Recent application of probiotics in food and agricultural science. INTECH Open Access Publisher; 2012.
- 14. Liouni M, Drichoutis P, Nerantzis ET. Studies of the mechanical properties and the fermentation behavior of double layer alginate–chitosan beads, using Saccharomyces cerevisiae entrapped cells. World J Microbiol Biotechnol 2008; 24: 281– 288. [Google Scholar]
- 15. Mitropoulou G, Nedovic V, Goyal A, Kourkoutas Y. Immobilization technologies in probiotic food production. J Nutr Metab 2013; 2013: 716861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Messaoudi S, Madi A, Prevost H, Feuilloley M, Manai M, Dousset X, et al. In vitro evaluation of the probiotic potential of Lactobacillus salivarius SMXD51. Anaerobe 2012; 18: 584– 589. [DOI] [PubMed] [Google Scholar]
- 17. Sultana K, Godward G, Reynolds N, Arumugaswamy R, Peiris P, Kailasapathy K. Encapsulation of probiotic bacteria with alginate–starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. Int J Food Microbiol 2000; 62: 47– 55. [DOI] [PubMed] [Google Scholar]
- 18. Chandramouli V, Kailasapathy K, Peiris P, Jones M. An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. J Microbiol Methods 2004; 56: 27– 35. [DOI] [PubMed] [Google Scholar]
- 19. Chavarri M, Maranon I, Ares R, Ibanez FC, Marzo F, del Carmen Villaran M. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int J Food Microbiol 2010; 142: 185– 189. [DOI] [PubMed] [Google Scholar]
- 20. Mirzaei H, Pourjafar H, Homayouni A. Effect of calcium alginate and resistant starch microencapsulation on the survival rate of Lactobacillus acidophilus La5 and sensory properties in Iranian white brined cheese. Food Chem 2012; 132: 1966– 1970. [Google Scholar]