Abstract
Invasive fungal infections are most commonly seen in immunocompromised patients and usually affect the respiratory system. Gastrointestinal system involvement of mucormycosis and invasive aspergillosis is rarely reported in childhood. Here we describe a 5 year old boy with acute lymphoblastic leukaemia who developed invasive fungal infection particularly affecting the lower gastrointestinal system to emphasise the difficulties in diagnosis and management of invasive fungal infections in immunocompromised patients.
Keywords: Invasive aspergillosis, Aspergillus flavus, Gastrointestinal bleeding, Mucor, Leukaemia, Child
1. Introduction
Invasive aspergillosis (IA) is a life-threatening opportunistic infection that usually affects immunocompromised patients [1]. Immunsuppressive therapies, high-dose corticosteroids, severe and prolonged neutropenia are the factors that facilitate the infection. Majority of the cases are caused by Aspergillus fumigatus, followed by Aspergillus flavus, Aspergillus niger, and Aspergillus terreus [2]. IA most commonly involves the respiratory tract, lung or sinus but the central nervous system, cardiovascular system, and other tissues may be infected as a result of hematogenous spread [3]. Gastrointestinal (GI) aspergillosis, which is associated with high mortality, is a rarely seen form of extra-pulmonary aspergillosis and is most often described in the setting of disseminated disease [3]. GI involvement is rarely seen in mucormycosis, and most reported cases are associated with malignant haematological diseases [4].
Here, we describe a child with acute lymphoblastic leukaemia (ALL) who developed probable invasive aspergillosis and diagnosed with GI mucormycosis by histopathologic examination simultaneously. He survived with aggressive antifungal therapy and surgery.
2. Case
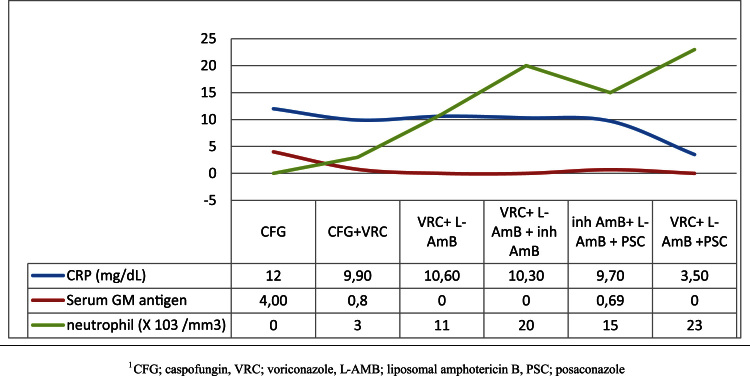
A 5-year-old boy was diagnosed with CALLA (+), pre-B cell ALL in July 2013. He was put on Standard risk arm of ALL BFM 2000 Protocol at day −30. The patient was referred to our intensive care unit because of clinical deterioration and severe lower gastrointestinal system bleeding. At the admission day, at day 0, the patient's general condition was poor, with tachycardia and respiratory distress present. His body temperature was 38.5 °C, and jaundice, abdominal distension and mucositis were also observed. Laboratory test results showed a white blood count of 48/mm3, an absolute neutrophil count of 6/mm3, haemoglobin of 6.9 g/dL, a thrombocyte count of 35,000/mm3and C-reactive protein of 11.7 mg/dL (N:0–0.5 mg/dL). The prothrombin time and activated partial thromboplastin time were prolonged, with a high international normalised ratio. The patient's biochemical values revealed a total bilirubin of 7.3 mg/dL, with direct bilirubin dominance, hypokalaemia and hypocalcaemia. He was intubated and required sedation, mechanical ventilation and haemodynamic support. Erythrocytes, platelet suspension and fresh frozen plasma were administered for continuing upper and lower GI bleeding. Broad-spectrum antibiotics (piperacillin–tazobactam, amikacin and vancomycin) were started initially. At day +4, the antibiotics were changed to teicoplanin and meropenem because of the ongoing fever, and caspofungin was added after yeast (Candida krusei) growth in a blood culture at day +6. Abdominal ultrasonography revealed nothing of note, except hepatomegaly and minimal abdominal plastering fluid. At day +8, the patient was still febrile but he was no longer neutropenic. Gancyclovir treatment was started following a positive result of a polymerase chain reaction (PCR) analysis for cytomegalovirus, and voriconazole was added following the detection of mould in a nasopharyngeal swab. At this time, a thoraco-abdominal computed tomography (CT) scan showed colonic mural thickening in the splenic flexure and infarct formation in the kidney and spleen that were suggestive of IA. Nodular lesions compatible with IA were detected in the lung (Fig. 1). Serum galactomannan antigen was positive (>4.73), and fungal cultures of sputum, tracheal aspirate were positive for A. flavus. At day +14, he was afebrile, and teicoplanin and meropenem were stopped. As no further bleeding had occurred over the previous 48 h, he was taken to the haematology department. Despite an initial improvement (reduced GI symptoms and haemorrhage) with the voriconazole treatment, the patient developed massive abdominal distension at day +16. A new abdominal CT revealed progression of the fungal lesions and peripheral invasion, with a covered perforation in the splenic flexure and a new hypodense lesion in the liver that resembled an aspergilloma (Fig. 2). The patient underwent an immediate bowel resection and splenectomy. After the surgery, he was taken to the paediatric intensive care department. Metronidazole was added to the treatment, caspofungin was stopped, and intravenous liposomal amphotericin B (AmB) (5 mg/kg/day) was started. The patient showed a clinical improvement after the surgery, with resolution of the abdominal distension and rectal bleeding. However, at day +20, he was febrile again, and teicoplanin was added due to the growth of coagulase-negative staphylococci in a blood culture. At day +22, he was reintubated because of increased respiratory distress, and a repeat CT scan showed no change in the lesions, which were compatible with aspergillus. At this point, inhaled AmB (25 mg twice weekly) was added (Fig. 3.). At day +29, a histopathological analysis of resected specimens of the bowel and spleen showed ulceration, with intense inflammation, and non-septate, thick, variable in diameter fungal hyphae, which were compatible with mucormycosis (Fig. 4). Culture or PCR for pathogenic fungi from resected material could not be performed. The dose of liposomal AmB was increased to 10 mg/kg/day, posaconazole was started, and voriconazole was stopped. At day +34, bone marrow aspiration was performed and resulted in haematological remission. At day +37, a tracheostomy was performed due to prolonged ventilator dependence. His lower GI symptoms did not show persistence with this therapy. A galactomannan test positivity showed increment. Repeated thoracal tomography revealed an increase in the number and size of the nodules in the lung. Thus, voriconazole was added to liposomal AmB and posaconazole. The patient had fever intermittently between the +40 and +54th days of hospitalisation, but blood cultures remained sterile. At the end of a 62-day follow-up, he was afebrile, no further gastrointestinal bleeding episodes were seen, a galactomannan test was negative, and the liposomal AmB dose was reduced to 5 mg/kg/day (Fig. 5). The patient was referred to the centre that he had been followed up for his haematological malignancy with triple anti-fungal therapy. Posaconazole was ceased 15 days after the patient became afebrile.
Fig. 1.
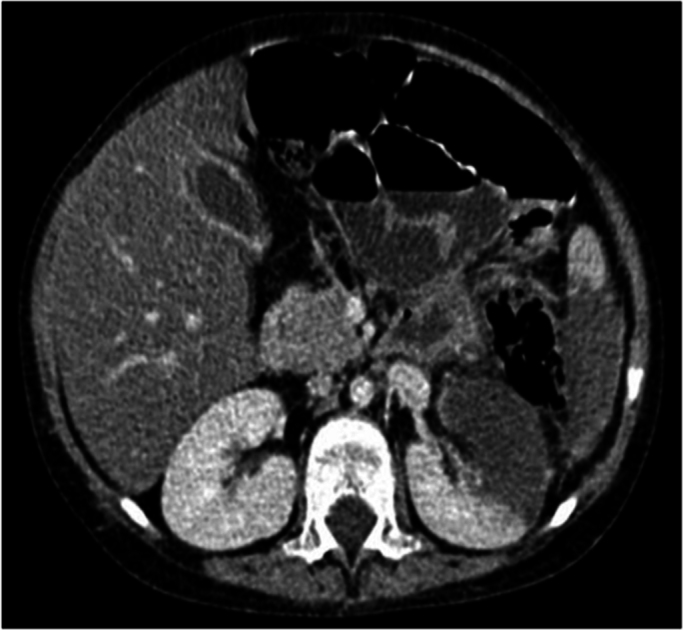
CT scan showed colonic mural thickening in the splenic flexure, infarct formation in the kidney and spleen which were suggestive of IA on the 8th day.
Fig. 2.

CT scan showed progression in fungallesions, peripheral invasion, a covered perforation in the splenic flexure and a new hypodense lesion in the liver suspecting an aspergilloma on the 16th day.
Fig. 3.
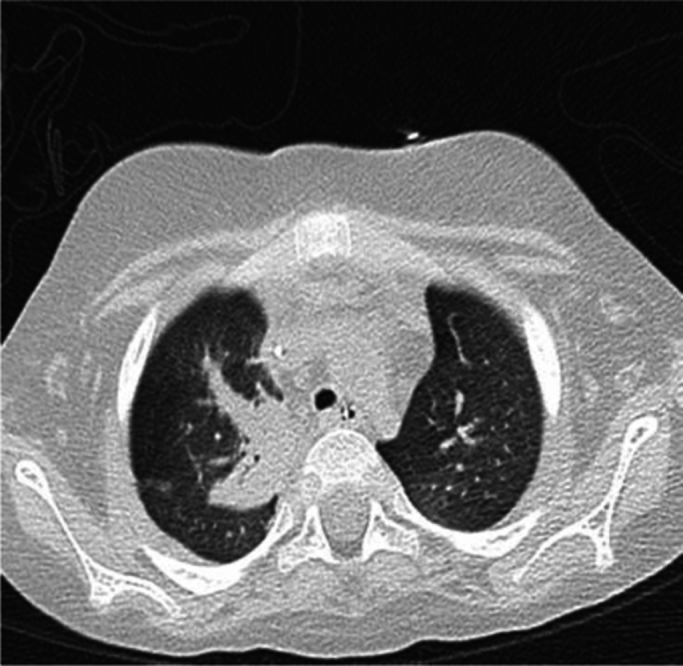
CT scan revealed lesions compatible with IA.
Fig. 4.
Histopathological examination showed the non-septate, thick, variable in diameter fungal hyphae,which were compatible with mucormycosis. (A; jejunum, Hematoxylin and eosin, ×20. B; spleen, d-PAS, ×20.).
Fig. 5.
Changes in laboratory parameters with the treatment of different antifungal agents.
3. Discussion
The frequency of IFI has increased over the last years because of the more intensive cytotoxic agents. With the widespread use of azole prophylaxis candidiasis became less frequent while IA and mucormycetes increased in patients with haematologic diseases [5].
IA most commonly affects the lower respiratory system and nasal sinuses. Other extrapulmonary locations of the infection include the central nervous system, eyes, skin, kidneys, heart, bones, joints and GI tract [6]. Aspergillosis is a highly lethal infection in the GI tract, which is the second most common site of IA. It usually occurs via dissemination from a primary pulmonary infection, and its frequency is estimated to be about 17% [7]. Primary GI aspergillosis, causing focal invasion as a primary site of inoculation is rarely reported. Symptoms are often nonspecific. Patients with GI aspergillosis can present as neutropenic enterocolitis (typhlitis), appendicitis, ileus, colonic ulcers, abdominal pain or GI bleeding [7].
Mucormycosis is a systemic opportunistic, fatal infection in immunocompromised patients which most commonly affects the paranasal sinuses (39%) and lungs (24%), more likely skin (19%), brain (9%) and GI tract (7%) [8]. Due to the invasion of mesenteric arteries and intravascular thrombosis, tissue ischaemia leading to infarction and perforation of the intestine in both GI aspergillosis and mucormycosis can occur.
Early diagnosis of IFI in GI tract is difficult in neutropenic patients. The diagnosis is delayed because the organism can seldom be isolated from blood or tissue culture. It is difficult to discriminate between IA and mucormycosis on the basis of a radiological examination alone. Therefore, histological confirmation is often essential [9]. However, for an accurate diagnosis, clinicians are unable to provide appropriate conditions at all times and cannot get sufficient material for examination. There are no specific radiological findings that suggest GI aspergillosis or mucor. Abdominal CT scans may show diffuse thickening of the intestinal wall or diffuse bowel distention. Ulcerative or necrotic lesions are commonly detected with endoscopy or surgery [10].
The choice of antifungal agent must be based on the clinical characteristics of the IFI and the local epidemiology with considering the increase in the incidence of mucormycetes [9]. Voriconazole is the primary therapy for IA while high doses of liposomal AmB are recommended for GI mucormycosis. Adjuvant surgery is essential in the management of Mucor and recommended in case of intestinal aspergillosis to avoid perforation or obstruction [9].
In our case, the patient’s main symptoms were abdominal distension and rectal bleeding. The strongly positive galactomannan test (>4.73), together with the growth of A. flavus in sputum and tracheal aspirate, and the radiological findings, especially the lung involvement, pointed to IA. Urgent surgical intervention played a key role in controlling the disease. However, it was surprising that the histological findings were consistent with mucormycosis because Mucor was not detected in the previous microbiological examinations. This raises questions about the actual diagnosis: Was it only IA, co-infection or breakthrough infection under voriconazole therapy? Tissue culture could help to provide a definitive diagnosis.
In conclusion, early diagnosis of IFI is difficult but essential to reduce mortality. Clinicians must be aware of the unexpected involvements and also the possibility of coinfection and breakthrough infection. Survival of these patients will improve with early surgical intervention in addition to aggressive antifungal treatments.
Conflict of interest
There are none.
Acknowledgement
There are none.
References
- 1.Mohite U., Kell J., Haj M.A., O'Brien C., Kundu S., Rees J., Burnett A.K. Invasive aspergillosis localised to the colon presenting as toxic megacolon. Eur. J. Haematol. 2007;78:270–273. doi: 10.1111/j.1600-0609.2006.00812.x. [DOI] [PubMed] [Google Scholar]
- 2.Walsh T.J., Groll A.H. Overview: non-fumigatus species of Aspergillus: perspectives on emerging pathogens in immunocompromised hosts. Curr. Opin. Investig. Drugs. 2001;2:1366–1367. [PubMed] [Google Scholar]
- 3.Lehrnbecher T., Becker M., Schwabe D., Köhl U., Kriener S., Hunfeld K.P., Schmidt H., Beyer P., Klingebiel T., Bader P., Sörensen J. Primary intestinal aspergillosis after highdose chemotherapy and autologous stem cell rescue. Pediatr. Infect. Dis. J. 2006;25:465–466. doi: 10.1097/01.inf.0000217475.83393.f6. [DOI] [PubMed] [Google Scholar]
- 4.Karanth M., Taniere P., Barraclough J., Murray J.A. A rare presentation of zygomycosis (mucormycosis) and review of the literature. J. Clin. Pathol. 2005;58:879–881. doi: 10.1136/jcp.2004.021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leventakos K., Lewis R.E., Kontoyiannis D.P. Fungal infections in leukemia patients: how do we prevent and treat them? Clin. Infect. Dis. 2010;50:405–415. doi: 10.1086/649879. [DOI] [PubMed] [Google Scholar]
- 6.Bizet J., Cooper C.J., Zuckerman M.J., Torabi A., Mendoza-Ladd A. A bleeding colonic ulcer from invasive Aspergillus infection in an immunocompromised patient: a case report. J. Med. Case Rep. 2014;8:407. doi: 10.1186/1752-1947-8-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González-Vicent M., Díaz M.A., Colmenero I., Sevilla J., Madero L. Primary gastrointestinal aspergillosis after autologous peripheral blood progenitor cell transplantation: an unusual presentation of invasive aspergillosis. Transpl. Infect. Dis. 2008;10:193–196. doi: 10.1111/j.1399-3062.2007.00288.x. [DOI] [PubMed] [Google Scholar]
- 8.Pagano L., Offidani M., Fianchi L., Nosari A., Candoni A., Piccardi M., Corvatta L., D’Antonio D., Girmenia C., Martino P., Del Favero A. GIMEMA (Gruppo Italiano Malattie EMatologiche dell'Adulto) infection program. Mucormycosis in hematologic patients. Haematologica. 2004;89:207–214. [PubMed] [Google Scholar]
- 9.Bergantim R., Rios E., Trigo F., Guimarães J.E. Invasive coinfection with Aspergillus and Mucor in a patient with acute myeloid leukemia. Clin. Drug Investig. 2013;33(Suppl 1):S51–S55. doi: 10.1007/s40261-012-0022-4. [DOI] [PubMed] [Google Scholar]
- 10.Trésallet C., Nguyen-Thanh Q., Aubriot-Lorton M., Akakpo J.P., Al Jijakli A., Cardot V., Chigot J.P., Menegaux F. Small-bowel infarction from disseminated aspergillosis. Dis. Colon Rectum. 2004;47:1515–1518. doi: 10.1007/s10350-004-0625-9. [DOI] [PubMed] [Google Scholar]