Abstract
Prenatal drug exposure (PDE) can undermine subsequent health and development. In a prospective longitudinal study we examine whether PDE moderates the link between stress reactivity and cognitive functioning in adolescence. Participants were 76 prenatally drug exposed and 61 non-exposed (NE) community comparison African American youth (50% male, mean age 14.17 years) living in an urban setting. All participants completed neuropsychological and academic achievement tests (Children’s Memory Scales, the California Verbal Learning Test-Children’s Version, and the Wide Range Achievement Test 4) over the course of one day in a laboratory setting. Two mild stressors (Balloon Analogue Risk Task-Youth and Behavioral Indicator of Resilience to Distress) were administered with saliva samples (assayed for cortisol) collected pre and post stress task. A higher percentage in the NE group, compared to the PDE group [26% vs. 12%, χ2(df=1, N=137)=4.70, p=.03)], exhibited task-related increases in salivary cortisol. PDE moderated the association between stress reactivity and 11 of 15 cognitive performance scales. In each case, the NE-stress reactive group had better cognitive performance than either the NE-lower cortisol reactive group or the PDE group regardless of stress reactivity status. Stress-related reactivity and regulation of the hypothalamic-pituitary-adrenal axis in adolescence may be disrupted by PDE, and the disruption may be linked to lower cognitive performance.
Keywords: Hypothalamic-Pituitary-Adrenal axis, cortisol, psychobiology
Prenatal drug exposure (PDE; cocaine/heroin) is a recognized public health problem with more than 4% of women between the ages of 15 and 44 reporting drug use while pregnant [1]. Recent developmental theories suggest that prenatal stressors such as PDE may impact the building blocks of adult health and well-being through their influence on early brain development and the hypothalamic-pituitary-adrenal axis (HPA) [2–5]. In both human and animal studies, stress in various forms (e.g., psychopathology, natural disasters, pharmacological treatments, etc.) experienced by pregnant females increases activation of the HPA axis. Prolonged exposure to the chemical products (i.e., glucocorticoids) released by the HPA axis has the potential to alter fetal neurological and cognitive development [6,7], impacting brain regions that are involved in the development and regulation of the HPA axis (e.g., hippocampus, amygdala, and frontal cortex) and resulting in possible functional deficits in memory, learning, and executive functioning that can last a lifetime [3,4,6,8]. The association between stress and cognitive function is viewed as an inverted U-shape with moderate stress, versus low or high levels, as optimal [9], but chronic stress during the prenatal and postnatal period may lead to prolonged, repeated elevations in glucocorticoids resulting in the down-regulation of the HPA axis response. These disruptions may prevent an expected stress response, resulting in a blunted cortisol or atypical response to stress over time [10,11]. This process has been demonstrated in maltreated and deprived/neglected children [10–13] and in children with early life stress (e.g., harsh parenting, poverty) [14–16].
PDE has subtle, measurable consequences on children’s behavior and development through adolescence [17,18]. There is preliminary evidence that PDE is associated with compromised memory performance and academic achievement in adolescence [18]. In four of five recent studies, adolescents with PDE demonstrated worse performance on memory tasks [19–21] or were more likely to have an individualized education plan (be enrolled in special education) [22] than non-exposed adolescents. The fifth study found no association between cognitive functioning and PDE [23]. Several of these studies considered mechanisms, including psychopathology [22], neural connections [20], growth [19], and gender [21], but none examined the potential role of individual differences in the psychobiology of the stress response.
There is evidence that PDE may result in disruption of the HPA axis [24–27]. Two studies involving adolescents with PDE who were also exposed to domestic violence [25] or maltreatment [24], found a blunted cortisol response to stress, and a third study demonstrated a blunted cortisol increase in the overnight pattern [27]. All three suggest that PDE dulled the adolescents’ sensitivity to stress. Conversely, a fourth study found that adolescents with PDE had higher cortisol concentrations than non-exposed adolescents before and after exposure to stress [26].
Contemporary theories emphasize the environmental impact on biological systems that play a role in development [2–5]. The current study examines the environmental challenge of PDE and stress reactivity and the impact on adolescent development. Since evidence suggests that stress-related reactivity of the HPA axis maybe disrupted by PDE [25,28], it is plausible that this dysregulation may be a mechanism explaining some portion of the effects of PDE on cognitive performance in adolescents. This study examines the hypothesis that PDE disrupts the relationship between stress reactivity and adolescent performance on cognitive/memory tasks, such that among non-exposed adolescents, stress reactivity predicts high scores on cognitive/memory tasks, and among adolescents with PDE, stress reactivity is not related to cognitive/memory performance.
Method
Participants
One hundred and thirty seven adolescents were recruited from a longitudinal investigation of the effects of PDE. The participants were a mean age of 14.17 (SD=1.17; min-max=11.93–16.64 yrs), were evenly divided by gender with 50% male, and were 99% African American. Adolescents with PDE were significantly more likely than non-exposed adolescents to have been prenatally exposed to alcohol [54% vs. 18%, χ2(df=1, N=137) = 18.54, p<.01] and tobacco [79% vs. 21%, χ2(df=1, N=137) = 45.16, p<.01]. In the PDE sample, 33% were exposed to cocaine only, 13% to heroin only, and 54% to both cocaine and heroin.
This study used existing data from a randomized, controlled trial of a home-based intervention for substance abusing women and their infants recruited at delivery from an urban University Hospital that catered to a largely African American population [29]. Eligibility criteria included gestational age ≥ 32 weeks, birth weight ≥ 1,750g, no admission to the neonatal intensive care unit, and positive maternal and/or infant urine toxicology (cocaine and/or heroin) at delivery and/or maternal self-report of cocaine and/or heroin use during pregnancy. The study was conducted during a time when toxicology screens were conducted routinely during delivery. The study was approved by the University’s Institutional Review Board. Seventy-two percent of potentially eligible mothers (N=265) agreed to participate [30].
Two groups of non-exposed (NE) children and their caregivers were recruited to serve as community comparisons. The first group was recruited at age 5, (N=70) [29,31] and the second group was recruited in early adolescence (N=24). All NE participants were recruited from a primary care clinic serving the University Hospital. Medical records were reviewed to identify children delivered at the University Hospital at the same time period as children from the PDE group who had negative toxicology screens and no evidence of substance use. Participants in the NE group resided in the same community as participants in the PDE group and were matched for socioeconomic status (e.g., maternal education), maternal age at first pregnancy, and child age, gender, and race [31].
Procedures
Participating families were re-recruited for assessments in adolescence. In the intervening years, there was a gap in funding, and many families were assigned to other health care providers through changes in Medicaid Managed Care, and there was significant housing relocation/demolition in the area. Families lost to follow up did not differ from retained families on birth weight, maternal education, maternal age at first pregnancy, maternal age at the target child’s birth, neonatal abstinence scores, child gender, or receipt of public assistance.
The adolescent protocol took place in a university-based laboratory where each caregiver and adolescent completed a comprehensive protocol that included questionnaires, neuropsychological and cognitive tasks, and three assessments of cortisol (collected over a 4.5–6 hour period). The adolescents fasted for three hours prior to their appointment, as cortisol can be influenced by glucose levels [32]. Participants and their caregivers were scheduled for morning appointments (85% arrived at 10:30 am or earlier). Experimenters established rapport, discussed consent forms, and collected the first cortisol sample (pre task; M=9:41am, SD=.85 hrs). Participants then were presented with mild stressors on the computer and a questionnaire. Approximately 30 minutes after the mild stressors, the second cortisol sample was collected (post task; M=10:42 am, SD=.86 hrs). Both cortisol samples were collected before noon (99% of pre and 97% of post task collections before noon). After the completion of the pre and post task cortisol collections, adolescents received breakfast, completed structured tasks and assessments, had lunch, and completed more structured tasks. The third cortisol collection occurred at the end of the visit. Since the focus of this investigation is the response to stress, only cortisol collections at pre and post task are used in the analyses.
Measures
Mild stressors
Two computer tasks were meant to impose mild stress and provoke an individual difference in stress reactivity measured by the change in salivary cortisol. Both tasks are impossible to complete at times. The first, the Balloon Analogue Risk Task-Youth (BART-Y) [33] was designed to measure risk-taking propensity from a cognitive decision making perspective, in a mildly stressful task. To earn a prize, the BART-Y requires respondents to inflate a computerized balloon over multiple trials to become as large as possible without breaking. Accumulated points are lost if the balloon explodes, and the balloon can explode at any time, making a loud bursting noise. Participants always received at least one small prize. The second task, the Behavioral Indicator of Resiliency to Distress (BIRD) [34] was developed based on the adult computerized distress tolerance task. Ten numbered boxes (1–10) are presented on a computer screen. To earn a prize, respondents use the computer’s mouse to click a numbered box when a green dot appears above it, but before the green dot jumps to another box. The green dot moves quickly between the boxes, seemingly at random, and frequently changes speed.
Prenatal alcohol and tobacco exposure
For the PDE group, alcohol and tobacco exposure were determined through maternal self-report at delivery. In the NE group, alcohol and tobacco exposure were determined through retrospective self-report at recruitment. Youth received a “0” if they were not exposed and a “1” if they were exposed.
Salivary cortisol
Following Granger and colleagues [35,36], whole saliva samples were collected by passive drool and frozen at −20° C until transported on dry ice via overnight delivery to Salimetrics Laboratories (State College, PA). Saliva samples were assayed in duplicate using a commercially available immunoassay specifically designed for use with saliva without modification to the manufacturers recommended protocol. Test volume was 25 ul and range of sensitivity was from .007 to 3.0 μg/dL. On average, intra- and inter-assay coefficients of variation were less than 5% and 15% respectively. All samples were assayed in duplicate and the average of the duplicate tests was used in the analyses. As expected, salivary cortisol values were skewed and kurtotic; therefore, pre and post task values were subjected to ln transformation.
California Verbal Learning Test-Children’s Version (CVLT-C)
The CVLT-C measures strategies and processes involved in learning and recalling verbal material. Participants were asked to remember a shopping list of 15 items (List A). The same list was recited to participants for 5 consecutive trials, and they were asked to recall words after each presentation. An interference list (List B), was then presented, and participants were asked to recall words from List B. Participants were then asked to recall List A words without an additional presentation of List A. The 15 words on List A were categorized as fruits, clothing, or toys. For the final recall, these categories were used as cues to elicit words from List A. This assessment resulted in measures of immediate recall (List A–Trial 1), learning (List A–Trial 5), proactive interference (List B and percent change from List A–Trial 1 to List B–Trial 1), free recall (short-delay free recall), and cued recall (short delay cued recall and semantic and serial clustering) [37]. Higher scores are optimal on all subscales except serial clustering.
Children’s Memory Scales (CMS)
Memory was evaluated using CMS Stories subscale. The CMS measures learning and memory across a variety of memory dimensions to assess free recall and recognition of story narratives [38]. Experimenters read two short stories to participants who were asked to recall them immediately and after a 15-minute delay. This assessment resulted in measures of immediate and delayed recall of verbatim and thematic information as well as delayed recognition. The authors report adequate reliability coefficients (Cronbach’s alpha=0.76–0.81) for children ages 11 to 16 [38].
Wide Range Achievement Test 4 (WRAT)
The WRAT measures basic skills in reading and arithmetic [39]. The Word Reading and Math Computation subscales were administered to adolescents. Raw scores are converted into standard scores (M=100, SD=15). The WRAT 4 is correlated with the Wechsler Individual Achievement Test II and the Woodcock Johnson III. Authors report the reliability coefficients for this test as high for 11 to 16 year-olds (Word Reading: 0.96–0.97; Math Computation: 0.94–0.95). Higher scores are optimal.
Analytic Strategy
Two cortisol reactivity variables were calculated, one continuous (a change score) and one dichotomous. Duplicate samples were assayed with “reactivity” defined following Granger and colleagues’ methods [35]. First, a 10% difference between pre and post task cortisol levels was required because this is twice the intra-assay coefficient of variation (i.e., the error inherent in the assay when comparing results from the same samples assayed twice). Second, an absolute difference of at least 0.02 μg/dL between pre task and post task cortisol collections was required (i.e., the lower limit of salivary cortisol assay sensitivity). If participants met both conditions, they were coded as “reactive;” otherwise they were coded “less reactive.” In psychological science, the use of mild to moderate stressors typically produces 20–30% of participants who have a salivary cortisol increase from time 1 to time 2 of at least 10% [35]. The dichotomous variable representing reactive (1) or less reactive (0) was used to determine participant reactivity to the mild stressors and to describe the sample. To test whether adolescents with PDE are less reactive to mild stressors than NE adolescents, a chi square analysis was conducted using the dichotomous cortisol stress reactivity variable.
Following data transformation, a continuous cortisol stress response change score was calculated for each participant by subtracting pre task cortisol from post task cortisol so that a higher positive change score indicates a larger salivary cortisol reaction to the mild stressors. Using this variable, we tested whether PDE modified the association between cortisol stress and cognitive performance during adolescence by following procedures for testing interactions described in Aiken and West [40] and Holmbeck [41], adjusting for covariates. After the cortisol change variable was centered [40], each criterion variable was regressed upon the predictor, the moderator variable, the covariates, and the interaction term of the predictor and the moderator [40,41]. When the interaction term was statistically significant (p<.05) or marginally significant (p<.10), the outcome variables were plotted in bar graphs by PDE status and reactivity status [40,41].
Covariates were selected based on their theoretical and statistical associations to cortisol. There is a wide range of ages in this sample, and adolescents with PDE were significantly more likely than NE adolescents to have been prenatally exposed to alcohol and tobacco. Also, the time of the first cortisol sample collection varied (see Procedures), and the time of day saliva is collected can affect cortisol concentrations because cortisol follows a diurnal rhythm over the course of a day [35]. Finally, there is a theoretical difference between males and females in their responses to stress and their cortisol response [42,43]. In this sample, males were significantly more reactive to the stressor than females [25% vs. 12%, χ2(df=1, N=137)=3.80, p=05)]. Therefore, covariates for all regression analyses were gender, adolescent age, prenatal tobacco exposure, prenatal alcohol exposure, and time of first cortisol collection (pre task).
Results
Cortisol Production and Stress Reactivity
There were no group differences in cortisol levels at pre or post task. For pre task, the NE group mean cortisol value was 0.23 μg/dL (SD=0.16) and for the PDE group was 0.23 μg/dL (SD=0.19). For post task, the mean was 0.19 μg/dL (SD=0.20) for the NE group and 0.15 μg/dL (SD=0.12) for the PDE group. Overall, 19% of the sample demonstrated a measurable reaction to the mild stressors. A higher percentage in the NE group exhibited stress reactivity, compared to the PDE group [26% vs. 12%, χ2(df=1, N137)=4.70, p=.03).
Cognitive Performance
Similar to findings in Riggins et al. [20] using the same participants as the current study, compared to the NE group, adolescents with PDE scored significantly to marginally lower on 7 of 15 cognitive tests in raw comparisons (Table 1). After the inclusion of covariates, only the CVLT-C list B to A percent change score remained significant, F(1,129)=5.83, p=.02. Across both groups, most cognitive test mean scores were low. CMS scores were at the 25th percentile and WRAT math computation scores were at near the 35th percentile (Table 1). For the PDE group, WRAT mean word reading scores were at the 35th percentile, with slightly higher scores for the NE group (Table 1).
Table 1.
Comparison of Raw Means (Standard Deviations) of Adolescent Memory and Academic Performance
| Overall | Drug Exposed | Non-Drug Exposed |
p | |
|---|---|---|---|---|
| School Achievement | ||||
| WRAT Math Computation | 91.02 (14.47) | 90.36 (14.60) | 91.81 (14.04) | ns |
| WRAT Word Reading | 93.83 (15.06) | 91.47 (13.80) | 96.56 (16.09) | .04 |
| Memory Performance | ||||
| CMS Immediate Recall | 7.99 (3.05) | 7.50 (2.68) | 8.61 (3.38) | .03 |
| CMS Delayed Recall | 7.60 (2.98 | 7.09 (2.63) | 8.21 (3.30) | .03 |
| CMS Delayed Recognition | 7.33 (3.29) | 7.11 (3.30) | 7.59 (3.31) | ns |
| CMS Immediate Thematic | 7.28 (2.99) | 6.70 (2.76) | 8.00 (3.14) | .01 |
| CMS Delayed Thematic | 7.11 (2.99) | 6.69 (2.80) | 7.62 (3.17) | .06 |
| CVLT Trials 1–5 | 46.39 (10.66) | 46.22 (11.16) | 46.61 (10.08) | ns |
| CVLT Trial 5 | −0.37 (1.11) | −0.34 (1.17) | −0.41 (1.05) | ns |
| CVLT B vs. A % change | −7.06 (39.20) | −13.99 (35.77) | 1.57 (41.83) | .02 |
| CVLT List B | −0.50 (1.08) | −0.70 (1.05) | −0.25 (1.08) | .02 |
| CVLT Short Delay Free Recall | −0.38 (.95) | −0.36 (1.01) | −0.42 (0.87) | ns |
| CVLT Short Delay Cued Recall | −0.40 (1.04) | −0.45 (1.07) | −0.33 (1.02) | ns |
| CVLT Serial Cluster | −0.45 (0.79) | −0.39 (0.87) | −0.53 (0.69) | ns |
| CVLT Semantic Cluster | 0.25 (1.06) | 0.30 (1.07) | 0.19 (1.07) | ns |
Note. WASI=Wechsler Scales of Intelligence; IQ=intelligence quotient; WRAT=Wide Range Achievement Test; CMS=Children’s Memory Scales; CVLT=California Verbal Learning Test
PDE Moderates the Association between Stress Reactivity and Cognitive Functioning
PDE either significantly or marginally moderated the association between stress reactivity and 11 of the 15 analyses of cognitive performance. In each case, stress reactivity predicted academic achievement and memory performance, but the findings and direction varied by PDE status (Table 2). In academic achievement, the stress reactivity and PDE interaction significantly predicted word reading and math computation scores on the WRAT 4 (Table 2; Figure 1). In memory performance, the interaction significantly predicted immediate recall, delayed recall, delayed recognition, immediate thematic memory, and delayed thematic memory on the CMS (Table 2; Figure 2). Finally, the stress reactivity and PDE interaction significantly predicted the short delay and cued recall scores on the CVLT, and marginally predicted performance on list A trials 1–5 and serial clustering (Table 2; Figure 3). In Figure 3, a constant of 10 was added to the cued recall, delayed recall, and serial clustering scores for graphing clarity. Interactions were probed to examine the effects of stress reactivity on cognitive performance in each drug exposure group.
Table 2.
Prenatal Drug Exposure Interacts with Cortisol Change from Pre task to Post task to Predict Cognitive Outcomes
| Outcome Variable of PDE by Cortisol Change Score Interaction | b | t | p | Effect Size (f2) |
|---|---|---|---|---|
| School Achievement | ||||
| WRAT Math Computation | 28.15 | 2.98 | .003 | .07 |
| WRAT Reading Score | 22.99 | 2.28 | .02 | .04 |
| Memory Performance | ||||
| CMS Immediate Recall | 6.25 | 3.12 | .002 | .08 |
| CMS Delayed Recall | 5.41 | 2.76 | .01 | .06 |
| CMS Delayed Recognition | 4.97 | 2.27 | .03 | .04 |
| CMS Immediate Thematic | 5.68 | 2.92 | .004 | .07 |
| CMS Delayed Thematic | 5.52 | 2.76 | .01 | .06 |
| CVLT Trials 1–5 | −13.38 | −1.92 | .06 | .03 |
| CVLT Trial 5 | −0.34 | −0.47 | ns | – |
| CVLT B vs. A % change | −10.08 | −0.39 | ns | – |
| CVLT List B | −1.01 | −1.45 | ns | – |
| CVLT Short Delay Free Recall | −1.37 | −2.21 | .03 | .04 |
| CVLT Short Delay Cued Recall | −1.88 | −2.72 | .01 | .06 |
| CVLT Serial Cluster | 0.92 | 1.74 | .08 | .02 |
| CVLT Semantic Cluster | −0.26 | −0.36 | ns | – |
Note. PDE = Prenatal Drug Exposure; WASI=Wechsler Scales of Intelligence; IQ=intelligence quotient; WRAT=Wide Range Achievement Test; CMS=Children’s Memory Scales. All regression equations included gender, adolescent age, prenatal tobacco exposure, prenatal alcohol exposure, and time of first cortisol sample collection as covariates.
Figure 1.
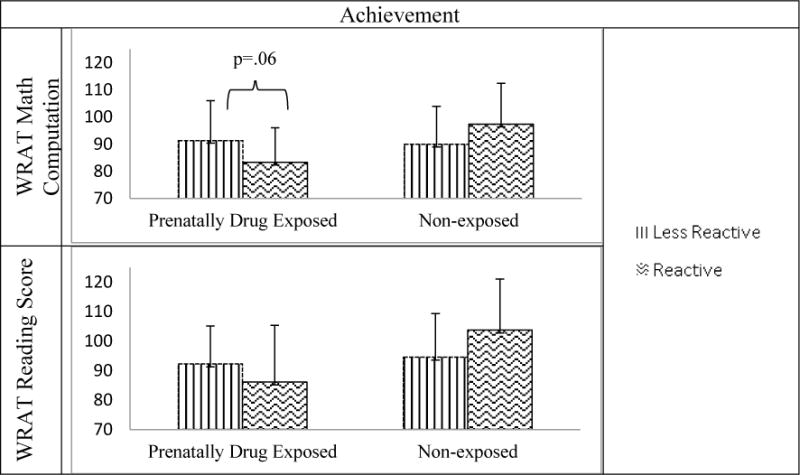
Prenatal Drug Exposure Moderates the Association between Stress Reactivity and Academic Achievement
Note. WRAT = Wide Range Achievement Test
Figure 2.

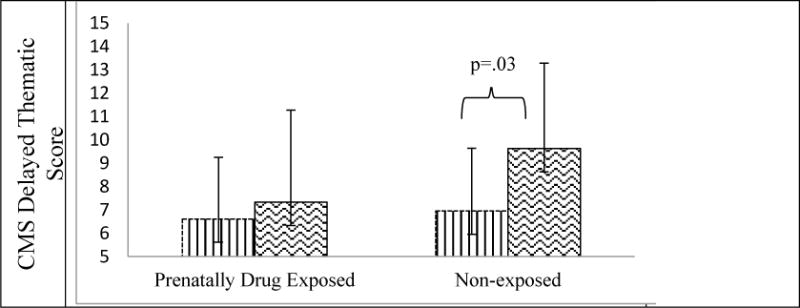
Prenatal Drug Exposure Moderates the Association between Stress Reactivity and Memory Performance on the Children’s Memory Scales
Note. CMS = Children’s Memory Scales
Figure 3.

Prenatal Drug Exposure Moderates the Association between Cortisol Change and CVLT Scores
Note: CVLT = California Verbal Learning Test; A higher score is optimal for Lists 1–5, cued and delayed recall while a lower score is optimal for serial clustering; A constant of 10 was added to the cued recall, delayed recall and serial clustering scores for graphing purposes to enhance clarity.
Probing the interactions
In the NE group, stress reactivity (versus NE less reactivity) significantly predicted higher CMS immediate recall (b=4.36, p=.01), delayed recall (b=4.03, p=.02), delayed recognition (b=5.11, p=.002), immediate thematic (b=4.89, p=.002), delayed thematic (b=3.47, p=.03), and CVLT short delay cued recall (b=1.22, p=.01) memory scales. No other associations were detected in the NE group. In the PDE group, cortisol stress reactivity (versus PDE less reactivity) predicted lower recall scores on trials 1–5 (b=−10.81, p=.04), lower short delay free recall (b=−.96, p=.05), and higher (less optimal) serial clustering (b=.89, p=.03) on the CVLT, and marginally predicted lower WRAT 4 math computation (b=−13.48, p=.06). No other associations were detected in the PDE group.
Discussion
Our observations suggest that PDE and stress-related change in the activity of the HPA axis interact to predict cognitive performance. The findings are particularly noteworthy given the effects of PDE were observed when individuals reached adolescence, and that the nature of the effects manifested across a range of cognitive performance scales. Small but significant differences in the effects of PDE on adolescent cognitive performance may, at least partially, be attributable to the effects of PDE on individual differences in biological sensitivity to context. Previous reviews have identified the need to explore the distal effects of PDE on functioning, with an emphasis on examining mechanisms [18,44,45]. PDE is an established prenatal teratogen, but the process of how it affects later development is not fully understood. It is thought that stressors during pregnancy, such as PDE, alter the fetal development of physiological systems such as the HPA axis, which may influence later stress reactivity [2–7]. In the current study, although there were no differences between the PDE and NE groups on cortisol levels at pre or post task, there were differences in reaction to the presentation of mild stressors, with more adolescents in the NE group demonstrating the expected reactivity, compared to the PDE group. Together with the lack of association between stress reactivity and cognitive performance in the PDE group, the results support the findings of earlier studies [24,25,27] and suggest the possibility of dysregulation of the HPA axis in adolescents with PDE. These findings also support current developmental theories [2–5] and expand them to include the special population of youth with PDE.
The dysregulation of the HPA axis has serious consequences for development throughout the lifespan. Prior research suggests that early life stressors compound the effects of prenatal stressors by chronically over-activating physiological systems such as the HPA axis, eventually leading to down-regulation of the response until an individual demonstrates a blunted response [10–16]. Two recent studies found that the effects of poverty, financial instability, and caregiver instability accumulated over time resulting in a decline in cortisol [14] and time in poverty along with household chaos were associated with a flattened cortisol change trajectory [16]. PDE is often associated with multiple stressors such as non-supportive or absent caregivers, few financial resources, neighborhood and/or home violence exposure, continued caregiver drug use, multiple out-of-home placements, and increased likelihood of various forms of abuse [46]. Therefore, the significant stressors often associated with PDE may have compounded the prenatal effects of PDE. Future research could address this possibility as an additional explanatory mechanism.
In the brain, an extensive circuitry coordinates the HPA axis in response to stressors with the hippocampus, amygdala, and prefrontal cortex playing major parts. These areas of the brain are also integral to functions such as cognition, emotion, and impulse control because they help to interpret events on the basis of prior experience, determining whether an event is, in fact, stressful [8]. When there is repeated activation of this circuitry, glucocorticoid levels increase which can disrupt the functioning of the hippocampus (i.e., glucocorticoid neurotoxicity) [8,43]. Both the CMS and CVLT were designed to assess skills that are regulated by the hippocampus, amygdala, and prefrontal cortex (e.g., memory and attention) [37,38]; thus, disruption to the HPA axis should be detectable. Furthermore, the WRAT 4 assesses academic achievement which is dependent upon skills such as memory and attention. In previous research on this sample, hippocampal volume in the PDE group was larger compared to the NE group, and this was associated with poorer memory performance [20] and is consistent with other research on hippocampal volume and memory performance [47]. The findings in the current study suggest that PDE, acting as a stressor, may have an effect on brain development (as measured through assessments such as the CMS, CVLT, and WRAT 4) that has lasting psychobiological and cognitive consequences.
Examination of the cortisol response across the cognitive functioning domains revealed that the NE stress reactive group performed better on each cognitive task than the NE less reactive group and the PDE groups, regardless of reactivity. Three cognitive tests with a total of 15 subscales were examined measuring rote memorization with immediate and delayed recall and recognition (CVLT), recall of stories and story themes (CMS), and academic skills (WRAT 4), and the pattern held across each type of cognitive performance. In the PDE group, there were no consistent patterns with the scores varying between the two reactivity groups, possibly reflecting individual variability in the development of stress-related reactivity and regulation of the HPA axis. Findings are similar to those of children from other types of disadvantaged backgrounds [10,11]. Because these findings indicate a robust association between a dysregulated HPA axis and multiple domains of cognitive functioning, there is an implication for the impacts on other areas of functioning. A dysregulated HPA axis has been associated with negative physical health consequences [43,48] and poor performance on memory and cognitive tasks [9]. Over time, individuals with a dysregulated HPA axis often experience increased risk of metabolic and cardiovascular diseases as well as lowered life expectancy and cognitive impairments [49]. These associations have negative biomedical and quality of life implications.
This study has several limitations to acknowledge. Future research could examine the temporality of dysregulated stress reactivity and subsequent memory and cognitive functioning to develop a greater understanding of the specificity of effects on these functions in a PDE sample. The sample size is small and racially homogenous, limiting generalizability. Additionally, we did not collect data regarding participant waking time, and this information should be collected in future research to control for the natural diurnal rhythm. Finally, findings may be influenced by the poverty present in both groups [50]. Poverty and associated stresses have been shown to impact both the development of the HPA axis and cognitive development [6,7]. Performance scores for both groups were extremely low with means below the 50th percentile and some as low as the 25th percentile [37,38].
This study has several strengths to note. First, this multi-method study and its hypotheses were informed by current developmental theories [2–5] and examined the psychobiological mechanisms underlying cognitive performance among a high risk sample of adolescents followed since birth. The findings support the hypothesized association between stress reactivity and cognitive performance, and the extension to an additional at-risk population, children with PDE. Second, findings were replicated across three objective measures of cognitive functioning, yielding similar patterns within the stress reactivity groups. This replication indicates robustness in the association between stress reactivity and cognitive function. Finally, the current study provides a psychobiological explanation for the individual differences in cognitive functioning, particularly memory, that may extend to other areas of functioning.
In conclusion, this investigation demonstrated dysregulation of the HPA axis in a sample of adolescents with PDE and an association with poor cognitive performance. Due to the negative consequences of a dysregulated HPA axis and PDE, further investigations of protective mechanisms that may reduce either the dysregulation of the HPA axis or the consequences of dysregulation are warranted.
Acknowledgments
This study was supported by the National Institute on Drug Abuse (R01-DA07432, R01-DA021059, and 1F32DA036274-01). In the interest of full disclosure, we note that DAG is founder and Chief Scientific and Strategy Advisor at Salimetrics (Carlsbad, CA) and SalivaBio (State College, PA), and these relationships are managed by the policies of the committee on conflict of interest at the Johns Hopkins School of Medicine and the Office of Research Integrity and Adherence at Arizona State University.
References
- 1.Substance Abuse and Mental Health Services Administration. Results from the 2010 national survey on drug use and health: Summary of national findings. 2011. (NSDUH Series H-41, HHS Publication No. (SMA) 11-4658). [Google Scholar]
- 2.Shonkoff JP. Leveraging the biology of adversity to address the roots of disparities in health and development. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17302–17307. doi: 10.1073/pnas.1121259109. doi: 10.1073/pnas.1121259109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shonkoff JP, Garner AS. Committee on Psychosocial Aspects of Child and Family Health, Committee on Early Childhood, Adoption, and Dependent Care, Section on Developmental and Behavioral Pediatrics. The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–46. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 4.Shonkoff JP. Building a new biodevelopmental framework to guide the future of early childhood policy. Child Dev. 2010;81(1):357–367. doi: 10.1111/j.1467-8624.2009.01399.x. doi: 10.1111/j.1467-8624.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- 5.Johnson SB, Riley AW, Granger DA, Riis J. The science of early life toxic stress for pediatric practice and advocacy. Pediatrics. 2013;131(2):319–327. doi: 10.1542/peds.2012-0469. doi: 10.1542/peds.2012-0469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lupien S, McEwen B, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature reviews Neuroscience. 2009;10(6):434. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 7.Davis EP, Sandman CA. Prenatal exposure to stress and stress hormones influences child development. Infants and young children. 2006;19(3):246. doi: 10.1097/00001163-200607000-00008. [DOI] [Google Scholar]
- 8.McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwabe L, Wolf O. Stress and multiple memory systems: From ‘thinking’ to ‘doing’. Trends Cogn Sci (Regul Ed) 2013;17(2):60. doi: 10.1016/j.tics.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Loman M, Gunnar MR. Early experience and the development of stress reactivity and regulation in children. Neuroscience & biobehavioral reviews. 2010;34(6):867. doi: 10.1016/j.neubiorev.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: Potential indices of risk in human development. Dev Psychopathol. 2001;13(3):515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- 12.Bruce J, Gunnar MR, Pears K, Fisher PA. Early adverse care, stress neurobiology, and prevention science: Lessons learned. Prevention science. 2013;14(3):247. doi: 10.1007/s11121-012-0354-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cicchetti D, Rogasch F, Toth S, Sturge-Apple M. Normalizing the development of cortisol regulation in maltreated infants through preventive interventions. Dev Psychopathol. 2011;23(3):789. doi: 10.1017/S0954579411000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blair C, Raver C, Granger DA, Mills-Koonce R, Hible L. Allostasis and allostatic load in the context of poverty in early childhood. Dev Psychopathol. 2011;23(3):845. doi: 10.1017/S0954579411000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldman-Mellor S, Hamer M, Steptoe A. Early-life stress and recurrent psychological distress over the lifecourse predict divergent cortisol reactivity patterns in adulthood. Psychoneuroendocrinology. 2012;37(11):1755. doi: 10.1016/j.psyneuen.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Blair C, Berry D, Mills-Koonce R, Granger DA. Cumulative effects of early poverty on cortisol in young children: Moderation by autonomic nervous system activity. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lester BM, Lagasse LL. Children of addicted women. Journal of Addictive Diseases. 2010;29(2):259–276. doi: 10.1080/10550881003684921. doi: http://dx.doi.org/10.1080/10550881003684921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buckingham-Howes S, Berger SS, Scaletti LA, Black MM. Systematic review of prenatal cocaine exposure and adolescent development. Pediatrics. 2013;131:e1917–e1936. doi: 10.1542/peds.2012-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Betancourt LM, Yang W, Brodsky NL, et al. Adolescents with and without gestational cocaine exposure: Longitudinal analysis of inhibitory control, memory and receptive language. Neurotoxicol Teratol. 2011;33(1):36–46. doi: 10.1016/j.ntt.2010.08.004. doi: http://dx.doi.org/10.1016/j.ntt.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riggins T, Cacic K, Buckingham-Howes S, Scaletti LA, Salmeron BJ, Black MM. Memory ability and hippocampal volume in adolescents with prenatal drug exposure. Neurotoxicol Teratol. 2012;34(4):434–441. doi: 10.1016/j.ntt.2012.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Minnes S, Singer LT, Min MO, et al. Comparison of 12-year-old children with prenatal exposure to cocaine and non-exposed controls on caregiver ratings of executive function. Journal of youth and adolescence. 2013 doi: 10.1007/s10964-013-9927-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levine TP, Lester B, Lagasse L, et al. Psychopathology and special education enrollment in children with prenatal cocaine exposure. J Dev Behav Pediatr. 2012;33(5):377–386. doi: 10.1097/DBP.0b013e3182560cd9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurt H, Betancourt LM, Malmud EK, et al. Children with and without gestational cocaine exposure: A neurocognitive systems analysis. Neurotoxicol Teratol. 2009;31(6):334–341. doi: 10.1016/j.ntt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher PA, Kim HK, Bruce J, Pears KC. Cumulative effects of prenatal substance exposure and early adversity on foster children’s HPA axis reactivity during a psychosocial stressor. Int J Behav Dev. 2012;36(1):29–35. doi: 10.1177/0165025411406863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lester BM, LaGasse LL, Shankaran S, et al. Prenatal cocaine exposure related to cortisol stress reactivity in 11-year-old children. J Pediatr. 2010;157(2):288–295. doi: 10.1016/j.jpeds.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaplin TM, Freiburger MB, Mayes LC, Sinha R. Prenatal cocaine exposure, gender, and adolescent stress response: A prospective longitudinal study. Neurotoxicol Teratol. 2010;32(6):595–604. doi: 10.1016/j.ntt.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bauer CR, Lambert BL, Bann CM, et al. Long-term impact of maternal substance use during pregnancy and extrauterine environmental adversity: Stress hormone levels of preadolescent children. Pediatr Res. 2011;70(2):213–219. doi: 10.1203/PDR.0b013e3182291b13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eiden RD, Veira Y, Granger DA. Prenatal cocaine exposure and infant cortisol reactivity. Child Dev. 2009;80(2):528–543. doi: 10.1111/j.1467-8624.2009.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuler ME, Nair P, Black MM. Ongoing maternal drug use, parenting attitudes, and a home intervention: Effects on mother-child interaction at 18 months. J Dev Behav Pediatr. 2002;23(2):87–94. doi: 10.1097/00004703-200204000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schuler ME, Nair P, Black MM, Kettinger L. Mother-infant interaction: Effects of a home intervention and ongoing maternal drug use. J Clin Child Psychol. 2000;29(3):424–431. doi: 10.1207/S15374424JCCP2903_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nair P, Black MM, Ackerman JP, Schuler ME, Keane VA. Children’s cognitive-behavioral functioning at age 6 and 7: Prenatal drug exposure and caregiving environment. Ambul Pediatr. 2008;8(3):154–162. doi: 10.1016/j.ambp.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gonzalez-Bono E, Rohleder N, Hellhammer DH, Salvador A, Kirschbaum C. Glucose but not protein or fat load amplifies the cortisol response to psychosocial stress. Horm Behav. 2002;41(3):328–333. doi: 10.1006/hbeh.2002.1766. [DOI] [PubMed] [Google Scholar]
- 33.Lejuez CW, Aklin W, Daughters S, Zvolensky M, Kahler C, Gwadz M. Reliability and validity of the youth version of the balloon analogue risk task (BART-Y) in the assessment of risk-taking behavior among inner-city adolescents. J Clin Child Adolesc Psychol. 2007;36(1):106–111. doi: 10.1080/15374410709336573. [DOI] [PubMed] [Google Scholar]
- 34.Lejuez CW, Daughters S, Danielson CW, Ruggiero K. The behavioral indicator of resiliency to distress (BIRD) 2006. Unpublished manual. [Google Scholar]
- 35.Granger DA, Fortunato CK, Beltzer EK, Virag M, Bright MA, Out D. Focus on methodology: Salivary bioscience and research on adolescence: An integrated perspective. J Adolesc. 2012;35(4):1081–1095. doi: 10.1016/j.adolescence.2012.01.005. doi: 10.1016/j.adolescence.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 36.Granger DA, Kivlighan KT, Fortunato C, et al. Integration of salivary biomarkers into developmental and behaviorally-oriented research: Problems and solutions for collecting specimens. Physiol Behav. 2007;92(4):583–590. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Delis DC, Kramer JH, Kaplan E, Ober BA. California verbal learning test – second edition. San Antonio, TX: Pearson; 2000. [Google Scholar]
- 38.Cohen M. Children’s memory scale: Manual. San Antonio, TX: Harcourt; 1997. [Google Scholar]
- 39.Wilkinson GS, Robertson GJ. Wide range achievement test 4: Professional manual. Lutz, FL: Psychological Assessment Resources, Inc; 2006. [Google Scholar]
- 40.Aiken LA, West SG. Multiple regression: Testing and interpreting interactions. Sage; 1991. [Google Scholar]
- 41.Holmbeck GN. Toward terminological, conceptual, and statistical clarity in the study of mediators and moderators: Examples from the child-clinical and pediatric psychology literatures. J Consult Clin Psychol. 1997;65(4):599–610. doi: 10.1037//0022-006x.65.4.599. [DOI] [PubMed] [Google Scholar]
- 42.Taylor SE, Klein LC, Lewis BP, Gruenewald TL, Gurung RA, Updegraff JA. Biobehavioral responses to stress in females: Tend-and-befriend, not fight-or-flight. Psychol Rev. 2000;107(3):411–429. doi: 10.1037/0033-295x.107.3.411. [DOI] [PubMed] [Google Scholar]
- 43.Sapolsky RM. Why zebras don’t get ulcers: The acclaimed guide to stress, stress-related diseases, and coping. 3. New York, NY: St. Martin’s Griffin; 2004. [Google Scholar]
- 44.Frank DA, Augustyn M, Knight WG, Pell T, Zuckerman B. Growth, development, and behavior in early childhood following prenatal cocaine exposure: A systematic review. JAMA. 2001;285(12):1613–1625. doi: 10.1001/jama.285.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ackerman JP, Riggins T, Black MM. A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics. 2010;125(3):554–565. doi: 10.1542/peds.2009-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.The National Center on Addiction and Substance Abuse at Columbia University. Family matters: Substance abuse and the american family. 2005. [Google Scholar]
- 47.Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: Review and meta-analysis. Neuropsychologia. 2004;42:1394–413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 48.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 49.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Farah MJ, Shera DM, Savage JH, et al. Childhood poverty: Specific associations with neurocognitive development. Brain Res. 2006;1110(1):166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]


