Abstract
Purpose of Review
To describe the mechanisms and consequences of both microbial translocation and microbial dysbiosis in HIV infection.
Recent Findings
Microbes in HIV are likely playing a large role in contributing to HIV pathogenesis, morbidities and mortality. Two major disruptions to microbial systems in HIV infection include microbial translocation and microbiome dysbiosis. Microbial translocation occurs when the bacteria (or bacterial products) that should be in the lumen of the intestine translocate across the tight epithelial barrier into systemic circulation, where they contribute to inflammation and pathogenesis. This is associated with poorer health outcomes in HIV infected individuals. In addition, microbial populations in the GI tract are also altered after HIV infection, resulting in microbiome dysbiosis, which further exacerbates microbial translocation, epithelial barrier disruption, inflammation, and mucosal immune functioning.
Summary
Altered microbial regulation in HIV infection can lead to poor health outcomes, and understanding the mechanisms underlying microbial dysbiosis and translocation may result in novel pathways for therapeutic interventions.
Keywords: Microbiome, microbial translocation, HIV/AIDS, inflammation, mucosal
Introduction
Immune activation is a hallmark of HIV infection and is stongly associated with AIDS and non-AIDS morbidity and mortality. There are several causes of immune activation in HIV infected individuals, however mucosal dysfunction is a major factor contributing to persistent inflammation and immunological dysfunction. Mucosal dysfunction in HIV infection is characterized by drastic immunological alterations, breaches in the epithelial barrier, translocation of microbial products into circulation, and microbiome dysbiosis [1,2**]. Thus the mucosal barrier of the gastrointestinal (GI) tract is a central component of HIV. As the GI tract contains the largest source of CD4+ T cells it is not surprising to be a primary site of ongoing viral replication [3,4]. However, despite antiretroviral therapy (ART) that restricts virus replication, complete restoration of barrier function does not occur and mucosal immune dysfunction persists [5,6].
The proper functioning of the mucosal barrier relies on protein structures that maintain epithelial integrity including tight junctions, adherens junctions, or desmosomes. These structures adhere cells to one another [7], which seals the paracellular space between epithelial cells and forms branching networks of strands that help form intact epithelial sheets [8]. Although the complete understanding and the etiology of epithelial disruption during HIV infection remains unclear, it is known that these tight junctions are breached during HIV infection [6], which has been linked to many immunological influences [9**]. For example, homeostatic T cell subsets are lost very early in infection, including Th17 cells, which are crucial in responding to bacterial antigens [10*,11*]. Indeed, Th17 cells in HIV infection are thought to aid in maintainence of the epithelial barrier by amplifying signals of microbial translocation [12]. Also, neutrophils, which are crucial in the immune response to bacterial and fungal pathogens [13], infiltrate into the GI tract in both HIV and the simian immunodeficiency virus (SIV) model of infection [2,14]. This coincides with damage to the epithelial barrier and microbial translocation, suggesting that neutrophils are responding to translocation of bacterial products but are unable to contribute to their containment, but could be releasing inflammatory factors that contribute to barrier damage [15*]. However, their contribution to mucosal dysfunction in the GI tissue is unknown [2**,14]. Many other effects on local immune cell milieu, epithelial cell responses, virus-cell interactions [16], altered molecular events [17], enhanced proinflammatory soluble products [18,19], and altered microbial regulation have been observed which suggest a multifacotral process [1,20]. While acknowledging there are many immunological contributions to this disruption, this review will focus on the contributing roles of microbial dysbiosis and translocation HIV-related inflammation.
Microbial Translocation in HIV infection
Microbial translocation (MT) is defined as the movement of microbial products from the gut mucosa into circulation. Though recognized in other fields, MT in the context of HIV was first characterized only 10 years ago [21], but the finding has since been replicated in many cohorts and is now accepted as a general phenomenon in HIV. In retrospect, MT in HIV should not be surprising, given that HIV is a gut tropic infection and a site of major and irreversible damage regardless of how infection is acquired [22,23]. In addition the gut represents one of the body's largest and most influential microbiomes, exerting an impact on a diverse range of human health and immunological conditions [24,25].
MT has been found to be a major driver of morbidity and mortality in HIV infection, likely due to the persistent inflammation it induces and sustains [26-28]. Importantly, the associations between microbial translocation and disease progression and mortality are independent of whether the HIV-infected individual is virally suppressed with ART [29]. A commonly identified translocating microbial product is lipopolysaccharide (LPS) from the surface of Gram-negative bacteria [21]. Several studies have demonstrated direct correlations between plasma LPS levels in HIV-infected individuals residual viremia, cellular activation including CD38+HLA-DR+ CD8+ T cells and activation of monocytes, interferon responsive genes such as MxA, and proinflammatory cytokines including IFN-α, IL-6, TNFα [21,23,30-34]. In addition, LPS levels and/or bacterial DNA levels directly correlate with other markers of microbial translocation and innate immune activation such as soluble CD14 (sCD14; released by monocytes in response to bacterial stimulation), LPS-binding protein (LBP) and endotoxin. While it is unclear how much inflammation during HIV infection is directly attributed to microbial translocation given the many inflammatory mechanisms which occur during HIV infection (including virus replication, opportunistic infections, etc.), studies in the absence of HIV demonstrate relationships between microbial translocation and inflammation. In idiopathic CD4 lymphocytopenia (ICL), LPS is elevated and associated with proliferating CD4+ T cells [35], and colon LPS levels in uninfected pigtail macaques correlate with interferon responsive gene MxA in the GI tract [36], demonstrating that microbial products can directly stimulate inflammatory responses.
Microbial translocation occurs early in infection, and in SIV models has been demonstrated to begin mainly in the post-peak viremia phase, or days 14-28 post-SIV infection [14]. However, prior to infection in the macaque SIV model, the extent of epithelial breaches and MT predicts the kinetics of progression to AIDS [33]. Additionaly, epithelial damage and local inflammation predicts mortality in HIV infection [2**,37**]. Indeed, a major mechanism which drives MT is focal breaches that occur to the epithelial barrier of the GI tract, which physically allows microbial products to translocate [14,36], however it is unclear what induces these breaches. One potential mechanism underlying damage to the epithelial barrier is alterations in the microbial communities of the GI tract. In HIV infection, there is an increased abundance of epithelial-adherent bacteria of the Proteobacteria family [38], and interestingly, it is this increased Proteobacteria that is found to predominantly translocate in SIV infection [39**]. This suggests that interactions between bacteria associated with the mucosa and the epithelium may contribute to MT. However, the relative contribution of various host and microbial factors to barrier damage are not well defined and studies examining these processes promise to provide novel targets for therapeutics designed to restore GI barrier function.
Microbial dysbiosis in HIV infection
Microorganisms associated with the GI lumen and mucosa are mostly bacteria but also include Archaea, Fungi, other eukaryotes such as helminthes, and viruses, many of which are bacteriophages [40-43]. It has become exceedingly clear that GI resident microbes and their associated metabolites are required to maintain proper function of the immune system and general health [40]. Perturbation of these microbial communities can have dramatic impacts on the health of the host and recent studies have demonstrated that shifts in the composition and function of the GI microbiome are associated with many disease states including HIV [38,44**,45].
HIV-induced alterations to the gut microbiota are most commonly characterized by overall decreased diversity [46], with reduced abundances of the genus Bacteroides and increased abundances of the genus Prevotella [38,44-45, 46**, 47]. Bacteria from these two genera are known to be important drivers of gut ecology and function [48]. Thus, shifts in the abundances of these bacteria can have major consequences for the gut microbiome and host. Indeed, altered abundances of Bacteroides and Prevotella have been implicated in a number of disease states, such as inflammatory bowel diseases (IBD) [49-52]. Table 1 shows different genera of bacteria found to be altered in HIV infection and possible roles for these bacteria in the context of the diesease.
Table 1. Bacteria altered in HIV infection and potential role on health and disease progression.
| Bacteria enriched in HIV-infected individuals | ||
|---|---|---|
| Genus | Compounds produced | Potential Role(s) in HIV |
|
Prevotella [44,45] (P. copri) |
SCFAs | Increase activation of myeloid dendritic cells [53*] |
| Pseudomonas [38,54] (P. aeruginosa) |
Acetate, Lactate, NO2 [55] | Potential opportunistic pathogen [56], mucolytic [57] |
|
Desulfovibrio [45] (D. piger) |
H2S [58] | Colonic inflammation [58] |
| Acinetobacter [44**] (A. baumannii) |
LPS, α-ketoglutarate [59] | Can recruit neutrophils by induction of IL-8 production* [60] |
| Campylobacter [46**] (C. jejuni) |
Enterotoxin, Cytotoxin [61] | Induces mucosal inflammation [62] |
|
Ruminococcu s[45] (R. productus) |
Soluble oligosaccharides, SCFAS [63] | Mucolytic, Proinflammatory [64] |
|
Escherichia [46**] (E. coli) |
Indole derivatives [65] | Mucolytic [57], Increase HIV replication [66] |
| Bacteria depleted in HIV-infected individuals | ||
|
Bacteroides [44**-46] (B. fragilis) |
PSA, SCFAs | Mucolytic [64], Induces IL-10 production, protects against inflammation, increases proportion of memory CD4+ T cells† [67,68] |
| Lactobacillus [54] (L. acidopholous) |
Lactate, B, K Vitamins | Support health, increase colonic CD4+ T cells [69*,70] |
| Bifidobacterium [54] (B. breve) |
Lactate, B, K Vitamins [71] | Support health |
| Eubacterium [47] (E. rectale) |
Butyrate, other SCFAs [65,72] | Support colonic health |
|
Coprococcus [44**,46**,47] (C. eutactus) |
Butyrate, other SCFAs [72] | Support colonic health |
|
Blautia [44**,46**] (B. hydrogenotrophica) |
Acetate [73] | Support colonic health |
|
Ruminococcus [46,47] (R. productus) |
Soluble oligosaccharides, SCFAS [63] | Mucolytic, Proinflammatory [64] |
Shown in vitro
Shown in murine models
Loss of bacteria from the gut may reduce the ability of HIV-infected individuals to manage gastrointestinal inflammation, and may result in altered T cell subsets shown in HIV infection. For example, in murine models, polysaccharide A (PSA) produced by Bacteroides fragilis has been shown to increase the expression of the anti-inflammatory cytokine IL-10 and increase populations of memory CD4+ T cells [67]. Additionally, Bacteroides are typically associated with the gut microbiota of individuals that consume a diet rich in animal-derived fats and glycans [74] and reduced abundances of these bacteria may lead to a diet-microbiome mismatch in HIV-infected individuals, which can manifest as metabolic disease [75].
Other bacterial genera that are reduced in abundance in HIV infection are Lactobacillus, Bifidobacterium, Coprococcus, Eubacterium, Blautia, and Ruminococcus. Genera enriched in the gut microbiome of HIV-infected individuals include Pesudomonas, Acinetobacter, Campylobacter, Escherichia, and Desulfovibrio [38,44-47,54,76**]. Lactobacillus spp. and Bifidobacterium spp. are generally regarded as beneficial, especially in the context of mucosal immunity and administration of probiotic supplements containing these bacteria have been found to be highly beneficial in the context of GI disease and inflammation [77-80]. These bacteria are also known to provide B and K vitamins to the host which are necessary for a variety of metabolic processes [71]. Coprococcus spp., Eubacterium spp., Blautia spp., and Ruminococcus spp. are usually commensal bacteria that are rarely implicated in intestinal infections and ferment dietary polysaccharides to form short chain fatty acids (SCFAs) such as acetate and butyrate, which are important energy sources for intestinal epithelial cells [57,72,81]. The genera Pseudomonas, Acinetobacter, Campylobacter, and Escherichia include a variety of opportunistic pathogens [56,60,61,82] while Desulfovibrio spp. produce toxic hydrogen sulfide and are closely associated with the mucosa [83,84]. Expansion of these bacteria has been implicated in the pathogenesis of other inflammatory bowel diseases [85]. Thus, HIV-induced dysbiosis appears to be characterized by decreased abundances of bacteria that are generally regarded as commensal, fermentative, or protective accompanied by an expansion of bacteria that are potentially inflammatory or pathogenic, which agrees with the general phenotype of mucosal inflammation in HIV infection.
In the SIV model, Handley et al. showed that an overall decrease in bacterial populations and an increase in enteric viruses was linked to GI pathology [86]. Interestingly, the authors of this study did not detect significant dysbiosis of the bacterial component of the microbiome. However these studies were performed on fecal samples, which do not exhibit dysbiosis as clearly as tissue samples in HIV or SIV infection. These authors also demonstrated an increase in enteric viruses in viremic macaques, including altered bacteriophages, which could be a potential mechanism underlying altered microbiome in HIV infection, and should be researched further. Another recent study demonstrated shifts in the oral fungal microbiota in ART-treated, HIV-infected individuals characterized by increased Candida colonization, which may cause infection [87**]. Studies examining potential dysbiosis of fungal or Archaeal communities in the gut of HIV-infected individuals are lacking and warrant investigation. Further, given the unclear mechanisms underlying dysbiosis, the interactions between bacteria, viruses, fungi and Archaea should be better elucidated in order to develop a complete understanding of the nature of HIV-induced dysbiosis.
Microbial dysbiosis in immunity and inflammation
The alterations to the microbiome in HIV infection have also been demonstrated to result in direct effects on immunity and increased inflammation (summarized in Figure 1). Vujkovic-Cvijin et al. demonstrated that enrichment of Proteobacteria and loss of Bacteroides in HIV infection results in increased adherent bacteria to the mucosal epithelium, which could be a mechanism for barrier damage, although this has not been directly demonstrated [38]. In addition, the authors of this study found that dysbiosis of the microbiome was associated with increased gut and peripheral T cell activation, and plasma inflammatory soluble factors, which have independently been found to be associated with HIV pathogenesis [38,88,89]. Furthermore, dysbiosis was associated with an increased Kynurenine:Tryptophan ratio (Kyn:Trp) and increased indoleamine-2,3-dioxygenase (IDO) activity, which were previously demonstrated to be associated with loss of mucosal IL-17 producing cells [90], also a pathogenic consequence of HIV infection [91-93].
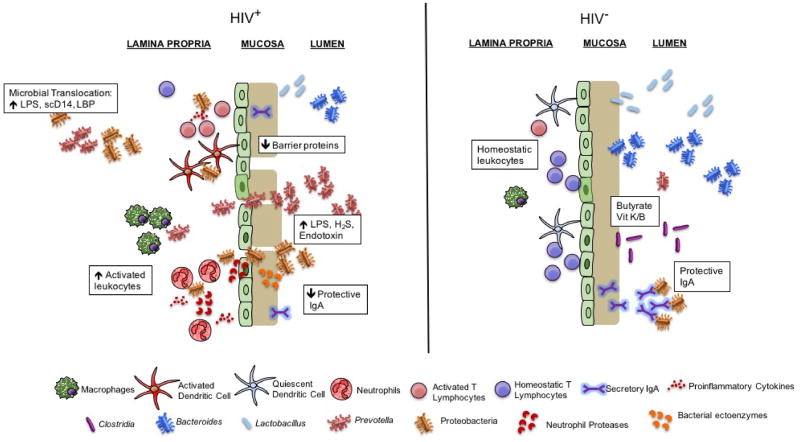
Figure 1.
Microbial dysbiosis in HIV is characterized by decreased abundances of Bacteroides, Lactobacillus, and beneficial Clostridia with increased abundances of Prevotella and pathogenic Proteobacteria, increasing T cell and DC activation. Loss of secretory IgA may help to explain the outgrowth of pathogenic bacteria. Increased neutrophil and macrophage accumulation in the LP, bacterial activity in the mucosa, and other mechanisms destabilize the mucosa and GI epithelium, leading to MT and further accumulation of inflammatory microbial products and cytokines in circulation. Together, these mechanisms perpetuate an inflammatory cycle that leads to chronic immune activation in ART treated HIV-infected individuals.
Dillon et al. recently demonstrated that HIV-altered mucosal bacteria (HAMBs) are associated with inflammatory responses from immune cells. Here the authors found that increased abundance of Prevotella in HIV-infected individuals was associated with increased mucosal T cell and dendritic cell (DC) activation [44]. Furthermore, the co-culturing of HAMBs with colonic lamina propria mononuclear cells, including Prevotella copri and Prevotella stercore, directly stimulated activation of CD1c+ DCs, compared to bacteria lost in HIV including Rumminococcus bromii [94**]. This may affect HIV pathogenesis given that the authors also demonstrated DC activation in mucosal tissues was associated with increased mucosal viral load, increased mucosal and systemic T cell activation, and increased plasma and mucosal cytokine production, including several cytokines associated with mortality in HIV infection IL-6, TNFα, IL-10, IL-1β, and IFNγ [94**]. Thus, there is a clear relationship between microbial dysbiosis on immunity and inflammation in HIV infection, and preventing or reversing this altered GI microbiome in HIV infection may result in beneficial effects on immunity. However, whether altered immunity after HIV infection drives dsybiosis or vice versa is unclear, and cause and effect of microbe dysregulation relative to immune dysfunction needs to be clarified.
Another role of the microbiome in HIV that is not as clear is the ability of the microbiome to drive IgA responses. It is well established that secretory IgA in the lamina propria is essential for proper management and containment of intestinal microbiota [95]. Indeed, IgA in mucosal tissues can act to protect from pathogens, block microbe-epithelium interactions, clear microbes from lamina propria if translocated, and can itself act to shape the microbiota [95-97]. Given the loss of CD4+ T cells, and specifically T follicular helper (Tfh) cells which induce class switching of B cells, during HIV infection, a potential mechanism underlying altered microbiome could be loss of IgA control in mucosa, but these mechanisms have not been clearly defined. Of interest, recent studies have demonstrated that there is a dual specificity of IgA for the microbiome and HIV [98**,99]. Thus, altered IgA responses in the presence of HIV infection could be an underlying mechanism of microbiome dysbiosis or lack of immune control of the microbiota. Interestingly, a link between IgA and neutrophil responses has recently been demonstrated whereby neutrophils negatively regulated mucosal IgA after vaccination [100**]. Given the accumulation of neutrophils during HIV infection the interactions bewtween neutrophils and IgA depletion in this context may be a novel avenue of exploration..
Therapeutic approaches to improve microbiome and microbial translocation in HIV infection
Novel therapies to restore the microbiome and repair damage to the GI epithelial barrier can improve mucosal function and may reduce morbidity and mortality in ART-treated HIV-infected individuals. Oral administration of beneficial microbes in the form of probiotic supplements have attracted attention as a method to enhance mucosal function by increasing mucus production, preventing apoptosis, or stabilizing tight junctions [101]. In a recent study, intake of a probiotic containing several Lactobacillus, Bifidobacterium, and Streptococcus spp. significantly reduced CD4+ and CD8+ T cell activation and plasma concentrations of high-sensitivity C-reactive protein, a biomarker associated with cardiovascular disease risk, in ART treated HIV-infected adults [102**]. Interestingly, these patients also reported fewer episodes of diarrhea and constipation. However, plasma concentrations of sCD14, a biomarker of MT, did not decrease after sustained probiotic intake [102**]. Another study demonstrated that intake of a probiotic yeast, Saccharomyces boulardii, reduced plasma concentrations of LBP and IL-6 in ART-treated HIV-infected individuals [103*]. Similarly, intake of prebiotic oligosaccharides significantly increased populations of beneficial Bifidobacterium spp., reduced plasma concentrations of sCD14, and reduced CD4+ T cell activation in a cohort of untreated HIV-infected individuals [104]. Probiotic therapy in SIV-infected non-human primates treated with ART results in multiple benefits to mucosal immunity including increased mucosal CD4+ T cell frequency and functionality, which was attributed to decreased fibrosis in the GI tract [70]. In addition, the authors observed improved functionality of antigen presenting cells (APCs) without overt DC activation, suggesting that innate immunity can also be enhanced by probiotic supplementation [70]. In a follow up study, ART-treated SIV-infected macaques were given a combination of probiotics and IL-21, which also improved frequencies of Th17 cells, potentially due to an observed decrease in Kyn:Trp ratios and decreased IDO activity in plasma [105**].
The drug sevelamer, which sequesters bacterial LPS, has been shown to reduce plasma concentrations of LPS and sCD14 while also decreasing immune activation when administered to macaques during acute SIV infection [106*]. Administration of sevelamer did not reduce plasma LPS concentrations in patients in the chronic stage of HIV infection, but did produce potential cardiovascular benefits [107*]. Similarly, treatment of HIV-infected individuals with hyperimmue bovine colostrum containing anti-LPS antibodies did not reduce plasma LPS concentrations [108]. In SIV-infected macaques, blockade of the immune inhibitory receptor programmed death-1 (PD-1), which plays a critical role in determining the functionality of HIV-specific T cells, improved gut barrier function by increasing expression of tight junction proteins and reduced plasma LPS concentrations. Furthermore, PD-1 blockade enhanced immune responses against potentially pathogenic bacteria [109]. Together, these studies demonstrate that therapies designed to restore the structure and function of the microbiome and reduce microbial translocation can be effective for reducing inflammation in ART-treated HIV-infected individuals. However, further studies are needed to properly assess the efficacy of these approaches and need for combinational approaches, and whether restoration of the GI tract is even possible after the extensive damage that HIV induces in mucosal tissues rapidly after infection.
Conclusion
It is clear that HIV infection results in damage to the GI mucosa and epithelium leading to translocation of microbes and microbial products into the lamina propria and systemic circulation and that these events are accompanied by dysbiosis of the GI-resident microbes. However, whether this dysbiosis is a cause or a symptom of immune dysregulation in HIV remains unclear. The intestinal microbiome is heavily regulated by the innate and adaptive arms of the immune system and the microbiota contribute to its maintainance [40]. Persistent activation of the immune system may lead to an inability of the host to properly regulate the microbiome. This, in turn, may result in microbial dysbiosis and outgrowth of potentially pathogenic bacteria, which creates an inflammatory cycle. Thus, in order to identify novel targets for therapeutic intervention, it is imperative to better understand the interplay between microbial community changes and mucosal dysfunction in HIV infection.
Key Points.
HIV infection is associated with persistent inflammation caused by translocation of microbes and/or microbial products from the GI lumen to the lamina propria and circulation; this process remains elevated even with successful antiretroviral treatment
HIV is also associated with bacterial dysbiosis is characterized by reduced abundances of beneficial bacteria and increased abundances of potentially inflammatory bacteria
Both bacterial dysbiosis and translocation have been linked to altered immune function and/or persistent inflammation, and are likely causative contributors
Novel therapies to restore gut ecology and function are necessary to reduce inflammation and improve prognosis of ART-treated HIV-infected individuals
Acknowledgments
Financial support and sponsorship: Personnel time and support provided by University of Washington (AZ, NK), CAPRISA (LM) and University of Manitoba (AB).
Footnotes
Conflicts of interest: None to declare
References
- 1.Burgener A, McGowan I, Klatt NR. HIV and mucosal barrier interactions: consequences for transmission and pathogenesis. Curr Opin Immunol. 2015;36:22–30. doi: 10.1016/j.coi.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 2**.Somsouk M, Estes JD, Deleage C, Dunham RM, Albright R, Inadomi JM, Martin JN, Deeks SG, McCune JM, Hunt PW. Gut epithelial barrier and systemic inflammation during chronic HIV infection. AIDS. 2015;29:43–51. doi: 10.1097/QAD.0000000000000511. • The authors find that in ART-treated HIV infected individuals, there is increased neutrophil accumulation and epithelial apoptosis in the GI tract which may contribute to GI immunological dysfunction and immune activation in HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science. 1998;280:427–431. doi: 10.1126/science.280.5362.427. [DOI] [PubMed] [Google Scholar]
- 4.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 5.Macal M, Sankaran S, Chun TW, Reay E, Flamm J, Prindiville TJ, Dandekar S. Effective CD4+ T-cell restoration in gut-associated lymphoid tissue of HIV-infected patients is associated with enhanced Th17 cells and polyfunctional HIV-specific T-cell responses. Mucosal Immunol. 2008;1:475–488. doi: 10.1038/mi.2008.35. [DOI] [PubMed] [Google Scholar]
- 6.Brenchley JM, Douek DC. HIV infection and the gastrointestinal immune system. Mucosal Immunol. 2008;1:23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol. 2007;127:2525–2532. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- 8.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 9**.Arnold KB, Burgener A, Birse K, Romas L, Dunphy LJ, Shahabi K, Abou M, Westmacott GR, McCorrister S, Kwatampora J, et al. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.51. • The authors find increased inflammatory proteins, including neutrophil proteases, in mucosal barriers is associated with increased HIV acquisition. [DOI] [PubMed] [Google Scholar]
- 10*.Schuetz A, Deleage C, Sereti I, Rerknimitr R, Phanuphak N, Phuang-Ngern Y, Estes JD, Sandler NG, Sukhumvittaya S, Marovich M, et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog. 2014;10:e1004543. doi: 10.1371/journal.ppat.1004543. • The authors find that Th17 cells in the GI tract are lost early in HIV infection and associated with mucosal and systemic inflammation; Th17 could be preserved only by early (Fiebig I/II) initiation of ART. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.McKinnon LR, Nyanga B, Kim CJ, Izulla P, Kwatampora J, Kimani M, Shahabi K, Mugo N, Smith JS, Anzala AO, et al. Early HIV-1 infection is associated with reduced frequencies of cervical Th17 cells. J Acquir Immune Defic Syndr. 2015;68:6–12. doi: 10.1097/QAI.0000000000000389. • The authors found loss of CCR6+ CD4+ T cells early in HIV infection in the cervix, association with high levels of RANTES and MIP3a in cervicovaginal fluids. [DOI] [PubMed] [Google Scholar]
- 12.Raffatellu M, Santos RL, Verhoeven DE, George MD, Wilson RP, Winter SE, Godinez I, Sankaran S, Paixao TA, Gordon MA, et al. Simian immunodeficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 14.Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, Paiardini M, Barclay GR, Smedley J, Pung R, Oliveira KM, et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 2010;6:e1001052. doi: 10.1371/journal.ppat.1001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15*.Sereti I, Estes JD, Thompson WL, Morcock DR, Fischl MA, Croughs T, Beq S, Lafaye de Micheaux S, Yao MD, Ober A, et al. Decreases in colonic and systemic inflammation in chronic HIV infection after IL-7 administration. PLoS Pathog. 2014;10:e1003890. doi: 10.1371/journal.ppat.1003890. • The authors found that that administering IL-7 to HIV-infected individuals with low CD4+ T cell counts improved both CD4+ and CD8+ T cells in blood, as well as increased the gut homing marker α4β7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sankaran S, George MD, Reay E, Guadalupe M, Flamm J, Prindiville T, Dandekar S. Rapid onset of intestinal epithelial barrier dysfunction in primary human immunodeficiency virus infection is driven by an imbalance between immune response and mucosal repair and regeneration. J Virol. 2008;82:538–545. doi: 10.1128/JVI.01449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGowan I, Radford-Smith G, Jewell DP. Cytokine gene expression in HIV-infected intestinal mucosa. AIDS. 1994;8:1569–1575. doi: 10.1097/00002030-199411000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Hirao LA, Grishina I, Bourry O, Hu WK, Somrit M, Sankaran-Walters S, Gaulke CA, Fenton AN, Li JA, Crawford RW, et al. Early mucosal sensing of SIV infection by paneth cells induces IL-1beta production and initiates gut epithelial disruption. PLoS Pathog. 2014;10:e1004311. doi: 10.1371/journal.ppat.1004311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poles MA, Fuerst M, McGowan I, Elliott J, Rezaei A, Mark D, Taing P, Anton PA. HIV-related diarrhea is multifactorial and fat malabsorption is commonly present, independent of HAART. Am J Gastroenterol. 2001;96:1831–1837. doi: 10.1111/j.1572-0241.2001.03879.x. [DOI] [PubMed] [Google Scholar]
- 21.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 22.Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat Immunol. 2006;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 23.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013;21:6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474:327–336. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489:220–230. doi: 10.1038/nature11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, Ledergerber B, Lundgren J, Neuhaus J, Nixon D, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodger AJ, Fox Z, Lundgren JD, Kuller LH, Boesecke C, Gey D, Skoutelis A, Goetz MB, Phillips AN. Activation and coagulation biomarkers are independent predictors of the development of opportunistic disease in patients with HIV infection. J Infect Dis. 2009;200:973–983. doi: 10.1086/605447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, Pedersen C, Ruxrungtham K, Lewin SR, Emery S, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ancuta P, Kamat A, Kunstman KJ, Kim E-Y, Autissier P, Wurcel A, Zaman T, Stone D, Mefford M, Morgello S, et al. Microbial Translocation Is Associated with Increased Monocyte Activation and Dementia in AIDS Patients. PLoS ONE. 2008;3:e2516. doi: 10.1371/journal.pone.0002516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassol E, Malfeld S, Mahasha P, van der Merwe S, Cassol S, Seebregts C, Alfano M, Poli G, Rossouw T. Persistent Microbial Translocation and Immune Activation in HIV-1-Infected South Africans Receiving Combination Antiretroviral Therapy. J Infect Dis. 2010 Sep 1;202:723–733. doi: 10.1086/655229. [DOI] [PubMed] [Google Scholar]
- 32.Jiang W, Lederman MM, Hunt P, Sieg SF, Haley K, Rodriguez B, Landay A, Martin J, Sinclair E, Asher AI, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral-Treated HIV infection. J Infect Dis. 2009;199:1177–1185. doi: 10.1086/597476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canary LA, Vinton CL, Morcock DR, Pierce JB, Estes JD, Brenchley JM, Klatt NR. Rate of AIDS progression is associated with gastrointestinal dysfunction in simian immunodeficiency virus-infected pigtail macaques. J Immunol. 2013;190:2959–2965. doi: 10.4049/jimmunol.1202319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Funderburg N, Luciano AA, Jiang W, Rodriguez B, Sieg SF, Lederman MM. Toll-like receptor ligands induce human T cell activation and death, a model for HIV pathogenesis. PLoS One. 2008;3:e1915. doi: 10.1371/journal.pone.0001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee PI, Ciccone EJ, Read SW, Asher A, Pitts R, Douek DC, Brenchley JM, Sereti I. Evidence for translocation of microbial products in patients with idiopathic CD4 lymphocytopenia. J Infect Dis. 2009;199:1664–1670. doi: 10.1086/598953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klatt NR, Harris LD, Vinton CL, Sung H, Briant JA, Tabb B, Morcock D, McGinty JW, Lifson JD, Lafont BA, et al. Compromised gastrointestinal integrity in pigtail macaques is associated with increased microbial translocation, immune activation, and IL-17 production in the absence of SIV infection. Mucosal Immunol. 2010;3:387–398. doi: 10.1038/mi.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Hunt PW, Sinclair E, Rodriguez B, Shive C, Clagett B, Funderburg N, Robinson J, Huang Y, Epling L, Martin JN, et al. Gut Epithelial Barrier Dysfunction and Innate Immune Activation Predict Mortality in Treated HIV Infection. J Infect Dis. 2014 doi: 10.1093/infdis/jiu238. • Here the authors find that intestinal fatty acid binding protein, zonulin-1, soluble CD14, kynurenine/tryptohpan ratio, soluble tumor necrosis factor receptor 1, high-sensitiivty C-reactive protein, and D-dimer levels all predicted mortality. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38**.Vujkovic-Cvijin I, Dunham RM, Iwai S, Maher MC, Albright RG, Broadhurst MJ, Hernandez RD, Lederman MM, Huang Y, Somsouk M, et al. Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med. 2013;5:193ra191. doi: 10.1126/scitranslmed.3006438. • The authors find that dysbiosis of the microbiome in the colon occurs during HIV infection, described by increased adherent bacteria, and is associated with increased tryptophan catabolism that may underlie immune activation during HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39**.Klase Z, Ortiz A, Deleage C, Mudd JC, Quinones M, Schwartzman E, Klatt NR, Canary L, Estes JD, Brenchley JM. Dysbiotic bacteria translocate in progressive SIV infection. Mucosal Immunol. 2015 doi: 10.1038/mi.2014.128. • The authors find that the bacteria which translocate during SIV infection in macaques is predominantly Proteobacteria, which is also assocaited with dysbiosis of the microbiome in HIV infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thaiss CA, Levy M, Suez J, Elinav E. The interplay between the innate immune system and the microbiota. Curr Opin Immunol. 2014;26:41–48. doi: 10.1016/j.coi.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Dalmasso M, Hill C, Ross RP. Exploiting gut bacteriophages for human health. Trends Microbiol. 2014;22:399–405. doi: 10.1016/j.tim.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 42.Oever JT, Netea MG. The bacteriome-mycobiome interaction and antifungal host defense. Eur J Immunol. 2014;44:3182–3191. doi: 10.1002/eji.201344405. [DOI] [PubMed] [Google Scholar]
- 43.Pimentel M, Gunsalus RP, Rao SSC, Zhang H. Methanogens in Human Health and Disease. The American Journal of Gastroenterology Supplements. 2012;1:28–33. [Google Scholar]
- 44**.Dillon SM, Lee EJ, Kotter CV, Austin GL, Dong Z, Hecht DK, Gianella S, Siewe B, Smith DM, Landay AL, et al. An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014;7:983–994. doi: 10.1038/mi.2013.116. • The authors find that HIV results in dysbiosis and contributes to T cell and dendritic cell activation as well as endotoxemia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lozupone CA, Li M, Campbell TB, Flores SC, Linderman D, Gebert MJ, Knight R, Fontenot AP, Palmer BE. Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe. 2013;14:329–339. doi: 10.1016/j.chom.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46**.Mutlu EA, Keshavarzian A, Losurdo J, Swanson G, Siewe B, Forsyth C, French A, Demarais P, Sun Y, Koenig L, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014;10:e1003829. doi: 10.1371/journal.ppat.1003829. • The authors find that HIV infection results in decreased diversity of bacterial communities in the colon and ileum. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McHardy IH, Li X, Tong M, Ruegger P, Jacobs J, Borneman J, Anton P, Braun J. HIV Infection is associated with compositional and functional shifts in the rectal mucosal microbiota. Microbiome. 2013;1:1–12. doi: 10.1186/2049-2618-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arumugam M, Raes J, Pelletier E, Le Paslier D, Yamada T, Mende DR, Fernandes GR, Tap J, Bruls T, Batto JM, et al. Enterotypes of the human gut microbiome. Nature. 2011;473:174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang DW, Park JG, Ilhan ZE, Wallstrom G, Labaer J, Adams JB, Krajmalnik-Brown R. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8:e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Scher JU, Sczesnak A, Longman RS, Segata N, Ubeda C, Bielski C, Rostron T, Cerundolo V, Pamer EG, Abramson SB, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife. 2013;2:e01202. doi: 10.7554/eLife.01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DuPont AW, DuPont HL. The intestinal microbiota and chronic disorders of the gut. Nat Rev Gastroenterol Hepatol. 2011;8:523–531. doi: 10.1038/nrgastro.2011.133. [DOI] [PubMed] [Google Scholar]
- 52.Schippa S, Conte MP. Dysbiotic events in gut microbiota: impact on human health. Nutrients. 2014;6:5786–5805. doi: 10.3390/nu6125786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dillon SM, Lee EJ, Kotter CV, Austin GL, Gianella S, Siewe B, Smith DM, Landay AL, McManus MC, Robertson CE, et al. Gut dendritic cell activation links an altered colonic microbiome to mucosal and systemic T-cell activation in untreated HIV-1 infection. Mucosal Immunol. 2015;7:983–994. doi: 10.1038/mi.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gori A, Tincati C, Rizzardini G, Torti C, Quirino T, Haarman M, Ben Amor K, van Schaik J, Vriesema A, Knol J, et al. Early impairment of gut function and gut flora supporting a role for alteration of gastrointestinal mucosa in human immunodeficiency virus pathogenesis. J Clin Microbiol. 2008;46:757–758. doi: 10.1128/JCM.01729-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eschbach M, Schreiber K, Trunk K, Buer J, Jahn D, Schobert M. Long-term anaerobic survival of the opportunistic pathogen Pseudomonas aeruginosa via pyruvate fermentation. J Bacteriol. 2004;186:4596–4604. doi: 10.1128/JB.186.14.4596-4604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Bentzmann S, Plesiat P. The Pseudomonas aeruginosa opportunistic pathogen and human infections. Environ Microbiol. 2011;13:1655–1665. doi: 10.1111/j.1462-2920.2011.02469.x. [DOI] [PubMed] [Google Scholar]
- 57.McGuckin MA, Linden SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011;9:265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 58.Rey FE, Gonzalez MD, Cheng J, Wu M, Ahern PP, Gordon JI. Metabolic niche of a prominent sulfate-reducing human gut bacterium. Proc Natl Acad Sci. 2013;110:13582–13587. doi: 10.1073/pnas.1312524110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peleg AY, de Breij A, Adams MD, Cerqueira GM, Mocali S, Galardini M, Nibbering PH, Earl AM, Ward DV, Paterson DL, et al. The success of acinetobacter species; genetic, metabolic and virulence attributes. PLoS One. 2012;7:e46984. doi: 10.1371/journal.pone.0046984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.March C, Regueiro V, Llobet E, Moranta D, Morey P, Garmendia J, Bengoechea JA. Dissection of host cell signal transduction during Acinetobacter baumannii-triggered inflammatory response. PLoS One. 2010;5:e10033. doi: 10.1371/journal.pone.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walker RI, Caldwell MB, Lee EC, Guerry P, Trust TJ, Ruiz-Palacios GM. Pathophysiology of Campylobacter Enteritis. Microbiological Reviews. 1986;50:81–94. doi: 10.1128/mr.50.1.81-94.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bereswill S, Fischer A, Plickert R, Haag LM, Otto B, Kuhl AA, Dasti JI, Zautner AE, Munoz M, Loddenkemper C, et al. Novel murine infection models provide deep insights into the “menage a trois” of Campylobacter jejuni, microbiota and host innate immunity. PLoS One. 2011;6:e20953. doi: 10.1371/journal.pone.0020953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flint HJ, Bayer EA, Rincon MT, Lamed R, White BA. Polysaccharide utilization by gut bacteria: potential for new insights from genomic analysis. Nat Rev Microbiol. 2008;6:121–131. doi: 10.1038/nrmicro1817. [DOI] [PubMed] [Google Scholar]
- 64.Png CW, Linden SK, Gilshenan KS, Zoetendal EG, McSweeney CS, Sly LI, McGuckin MA, Florin TH. Mucolytic bacteria with increased prevalence in IBD mucosa augment in vitro utilization of mucin by other bacteria. Am J Gastroenterol. 2010;105:2420–2428. doi: 10.1038/ajg.2010.281. [DOI] [PubMed] [Google Scholar]
- 65.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 66.Dillon SM, Manuzak JA, Leone AK, Lee EJ, Rogers LM, McCarter MD, Wilson CC. HIV-1 infection of human intestinal lamina propria CD4+ T cells in vitro is enhanced by exposure to commensal Escherichia coli. J Immunol. 2012;189:885–896. doi: 10.4049/jimmunol.1200681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 68.Troy EB, Kasper DL. Beneficial effects of Bacteroides fragilis polysaccharides on the immune system. Front Biosci. 2010;15:25–34. doi: 10.2741/3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69*.Perez-Santiago J, Gianella S, Massanella M, Spina CA, Karris MY, Var SR, Patel D, Jordan PS, Young JA, Little SJ, et al. Gut Lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921–1931. doi: 10.1097/qad.0b013e3283611816. • The authors find that levels of Lactobacillales in anal swabs of HIV-infected individuals associated with decreased microbial translocation, higher CD4+ T cell levels and decreased viral load prior to ART. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klatt NR, Canary LA, Sun X, Vinton CL, Funderburg NT, Morcock DR, Quinones M, Deming CB, Perkins M, Hazuda DJ, et al. Probiotic/prebiotic supplementation of antiretrovirals improves gastrointestinal immunity in SIV-infected macaques. J Clin Invest. 2013 doi: 10.1172/JCI66227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. 2013;24:160–168. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 72.Louis P, Flint HJ. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1–8. doi: 10.1111/j.1574-6968.2009.01514.x. [DOI] [PubMed] [Google Scholar]
- 73.Rey FE, Faith JJ, Bain J, Muehlbauer MJ, Stevens RD, Newgard CB, Gordon JI. Dissecting the in vivo metabolic potential of two human gut acetogens. J Biol Chem. 2010;285:22082–22090. doi: 10.1074/jbc.M110.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen Y-Y, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, et al. Linking Long-Term Dietary Patterns with Gut Microbial Enterotypes. Science. 2011;334:105–108. doi: 10.1126/science.1208344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lozupone CA, Rhodes ME, Neff CP, Fontenot AP, Campbell TB, Palmer BE. HIV-induced alteration in gut microbiota: driving factors, consequences, and effects of antiretroviral therapy. Gut Microbes. 2014;5:562–570. doi: 10.4161/gmic.32132. [DOI] [PubMed] [Google Scholar]
- 76**.Vazquez-Castellanos JF, Serrano-Villar S, Latorre A, Artacho A, Ferrus ML, Madrid N, Vallejo A, Sainz T, Martinez-Botas J, Ferrando-Martinez S, et al. Altered metabolism of gut microbiota contributes to chronic immune activation in HIV-infected individuals. Mucosal Immunol. 2015;8:760–772. doi: 10.1038/mi.2014.107. • The authors characterized the functional gene content after alterations in the microbiome in HIV-infected individuals via meta-analysis and found an altered functional profile including enrichment of genes associated with LPS biosynthesis, microbial translocation, and inflammatory pathways, with loss of genes associated with amino acid metabolisma and energy processes. [DOI] [PubMed] [Google Scholar]
- 77.Guandalini S, Cernat E, Moscoso D. Prebiotics and probiotics in irritable bowel syndrome and inflammatory bowel disease in children. Benef Microbes. 2014:1–9. doi: 10.3920/BM2014.0067. [DOI] [PubMed] [Google Scholar]
- 78.Tojo R, Suarez A, Clemente MG, de los Reyes-Gavilan CG, Margolles A, Gueimonde M, Ruas-Madiedo P. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol. 2014;20:15163–15176. doi: 10.3748/wjg.v20.i41.15163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kandasamy S, Chattha KS, Vlasova AN, Rajashekara G, Saif LJ. Lactobacilli and Bifidobacteria enhance mucosal B cell responses and differentially modulate systemic antibody responses to an oral human rotavirus vaccine in a neonatal gnotobiotic pig disease model. Gut Microbes. 2014 doi: 10.4161/19490976.2014.969972. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heilpern D, Szilagyi A. Manipulation of intestinal microbial flora for therapeutic benefit in inflammatory bowel diseases: review of clinical trials of probiotics, pre-biotics and synbiotics. Rev Recent Clin Trials. 2008;3:167–184. doi: 10.2174/157488708785700302. [DOI] [PubMed] [Google Scholar]
- 81.Murphy EC, Frick IM. Gram-positive anaerobic cocci--commensals and opportunistic pathogens. FEMS Microbiol Rev. 2013;37:520–553. doi: 10.1111/1574-6976.12005. [DOI] [PubMed] [Google Scholar]
- 82.Kumar P, Luo Q, Vickers TJ, Sheikh A, Lewis WG, Fleckenstein JM. EatA, an immunogenic protective antigen of enterotoxigenic Escherichia coli, degrades intestinal mucin. Infect Immun. 2014;82:500–508. doi: 10.1128/IAI.01078-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nava GM, Carbonero F, Croix JA, Greenberg E, Gaskins HR. Abundance and diversity of mucosa-associated hydrogenotrophic microbes in the healthy human colon. ISME J. 2012;6:57–70. doi: 10.1038/ismej.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Levitt MD, Furne J, Springfield J, Suarez F, DeMaster E. Detoxification of hydrogen sulfide and methanethiol in the cecal mucosa. The Journal of Clinical Investigation. 1999;104:1107–1114. doi: 10.1172/JCI7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Loubinoux J, Bronowicki JP, Pereira IAC, Mougenel JL, Le Faou AE. Sulfate-reducing bacteria in human feces and their association with infammatory bowel diseases. FEMS Microbiol Ecol. 2002;40:107–112. doi: 10.1111/j.1574-6941.2002.tb00942.x. [DOI] [PubMed] [Google Scholar]
- 86.Handley SA, Thackray LB, Zhao G, Presti R, Miller AD, Droit L, Abbink P, Maxfield LF, Kambal A, Duan E, et al. Pathogenic simian immunodeficiency virus infection is associated with expansion of the enteric virome. Cell. 2012;151:253–266. doi: 10.1016/j.cell.2012.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87*.Mukherjee PK, Chandra J, Retuerto M, Sikaroodi M, Brown RE, Jurevic R, Salata RA, Lederman MM, Gillevet PM, Ghannoum MA. Oral mycobiome analysis of HIV-infected patients: identification of Pichia as an antagonist of opportunistic fungi. PLoS Pathog. 2014;10:e1003996. doi: 10.1371/journal.ppat.1003996. • The authors characterized the fungal mycobiome in HIV infected vs uninfected individuals and found that the oral mycobiome was altered after HIV infection, and dominated in both groups by Candida species. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Giorgi JV, Hultin LE, McKeating JA, Johnson TD, Owens B, Jacobson LP, Shih R, Lewis J, Wiley DJ, Phair JP, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 89.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, Deeks SG. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 90.Favre D, Mold J, Hunt PW, Kanwar B, Loke P, Seu L, Barbour JD, Lowe MM, Jayawardene A, Aweeka F, et al. Tryptophan catabolism by indoleamine 2,3-dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2:32ra36. doi: 10.1126/scitranslmed.3000632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, Cervasi B, Yokomizo LK, Pan L, Vinton CL, et al. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol. 2012;5:646–657. doi: 10.1038/mi.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Klatt NR, Brenchley JM. Th17 cell dynamics in HIV infection. Curr Opin HIV AIDS. 2010;5:135–140. doi: 10.1097/COH.0b013e3283364846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, Scheinberg P, Price DA, Hage CA, Kholi LM, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94**.Dillon SM, Lee EJ, Kotter CV, Austin GL, Gianella S, Siewe B, Smith DM, Landay AL, McManus MC, Robertson CE, et al. Gut dendritic cell activation links an altered colonic microbiome to mucosal and systemic T-cell activation in untreated HIV-1 infection. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.33. • The authors find that HIV-altered mucosal bacteria increase activation of gut dendritic cells which contributes to gut and systemic T cell activation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Macpherson AJ, Geuking MB, McCoy KD. Homeland security: IgA immunity at the frontiers of the body. Trends Immunol. 2012;33:160–167. doi: 10.1016/j.it.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 96.Macpherson AJ, Koller Y, McCoy KD. The bilateral responsiveness between intestinal microbes and IgA. Trends Immunol. 2015;36:460–470. doi: 10.1016/j.it.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 97.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98**.Trama AM, Moody MA, Alam SM, Jaeger FH, Lockwood B, Parks R, Lloyd KE, Stolarchuk C, Scearce R, Foulger A, et al. HIV-1 envelope gp41 antibodies can originate from terminal ileum B cells that share cross-reactivity with commensal bacteria. Cell Host Microbe. 2014;16:215–226. doi: 10.1016/j.chom.2014.07.003. • The authors find that ileum B cell respones are predominantly to Env gp41 during HIV infection, but the majority of these antibodies cross react to commensal bacteria. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seaton KE, Ballweber L, Lan A, Donathan M, Hughes S, Vojtech L, Moody MA, Liao HX, Haynes BF, Galloway CG, et al. HIV-1 specific IgA detected in vaginal secretions of HIV uninfected women participating in a microbicide trial in Southern Africa are primarily directed toward gp120 and gp140 specificities. PLoS One. 2014;9:e101863. doi: 10.1371/journal.pone.0101863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jee J, Bonnegarde-Bernard A, Duverger A, Iwakura Y, Cormet-Boyaka E, Martin TL, Steiner HE, Bachman RC, Boyaka PN. Neutrophils negatively regulate induction of mucosal IgA responses after sublingual immunization. Mucosal Immunol. 2015;8:735–745. doi: 10.1038/mi.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bron PA, van Baarlen P, Kleerebezem M. Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol. 2012;10:66–78. doi: 10.1038/nrmicro2690. [DOI] [PubMed] [Google Scholar]
- 102.d'Ettorre G, Ceccarelli G, Giustini N, Serafino S, Calantone N, De Girolamo G, Bianchi L, Bellelli V, Ascoli-Bartoli T, Marcellini S, et al. Probiotics Reduce Inflammation in Antiretroviral Treated, HIV-Infected Individuals: Results of the “Probio-HIV” Clinical Trial. PLoS One. 2015;10:e0137200. doi: 10.1371/journal.pone.0137200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Villar-Garcia J, Hernandez JJ, Guerri-Fernandez R, Gonzalez A, Lerma E, Guelar A, Saenz D, Sorli L, Montero M, Horcajada JP, et al. Effect of probiotics (Saccharomyces boulardii) on microbial translocation and inflammation in HIV-treated patients- a double-blind, randomized, placebo-controlled trial. J Acquir Immune Defic Syndr. 2015;68:256–263. doi: 10.1097/QAI.0000000000000468. [DOI] [PubMed] [Google Scholar]
- 104.Gori A, Rizzardini G, Van't Land B, Amor KB, van Schaik J, Torti C, Quirino T, Tincati C, Bandera A, Knol J, et al. Specific prebiotics modulate gut microbiota and immune activation in HAART-naive HIV-infected adults: results of the “COPA” pilot randomized trial. Mucosal Immunol. 2011;4:554–563. doi: 10.1038/mi.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105**.Ortiz AM, Klase ZA, DiNapoli SR, Vujkovic-Cvijin I, Carmack K, Perkins MR, Calantone N, Vinton CL, Riddick NE, Gallagher J, et al. IL-21 and probiotic therapy improve Th17 frequencies, microbial translocation, and microbiome in ARV-treated, SIV-infected macaques. Mucosal Immunol. 2015 doi: 10.1038/mi.2015.75. • Authors find that combination probiotic and IL-21 treatment in SIv-infected, Art-suppressed macaques decreases microbial translocation and enahnces immunity, specifically with increased Th17 cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106*.Kristoff J, Haret-Richter G, Ma D, Ribeiro RM, Xu C, Cornell E, Stock JL, He T, Mobley AD, Ross S, et al. Early microbial translocation blockade reduces SIV-mediated inflammation and viral replication. J Clin Invest. 2014;124:2802–2806. doi: 10.1172/JCI75090. • The authors find that blocking microbial translocation early in infection with sevelamer improves prognosis of SIV-infected macaques. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107*.Sandler NG, Zhang X, Bosch RJ, Funderburg NT, Choi AI, Robinson JK, Fine DM, Coombs RW, Jacobson JM, Landay AL, et al. Sevelamer does not decrease lipopolysaccharide or soluble CD14 levels but decreases soluble tissue factor, low-density lipoprotein (LDL) cholesterol, and oxidized LDL cholesterol levels in individuals with untreated HIV infection. J Infect Dis. 2014;210:1549–1554. doi: 10.1093/infdis/jiu305. • The authors find that 8 weeks of sevelamer therapy did not alter microbial translcoation, but reduced inflammatory factors such as soluble tissue factor, LDL and oxidized LDS cholesterol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Byakwaga H, Kelly M, Purcell DF, French MA, Amin J, Lewin SR, Haskelberg H, Kelleher AD, Garsia R, Boyd MA, et al. Intensification of antiretroviral therapy with raltegravir or addition of hyperimmune bovine colostrum in HIV-infected patients with suboptimal CD4+ T-cell response: a randomized controlled trial. J Infect Dis. 2011;204:1532–1540. doi: 10.1093/infdis/jir559. [DOI] [PubMed] [Google Scholar]
- 109.Dyavar Shetty R, Velu V, Titanji K, Bosinger SE, Freeman GJ, Silvestri G, Amara RR. PD-1 blockade during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques. J Clin Invest. 2012;122:1712–1716. doi: 10.1172/JCI60612. [DOI] [PMC free article] [PubMed] [Google Scholar]