This cohort study assesses outcomes in patients who underwent CT-guided aspiration and injection of sacral Tarlov cysts at Johns Hopkins Hospital from 2003–2013. A total of 289 cysts were treated in 213 consecutive patients, with 83% followed for 3–6 years. One year postprocedure, excellent results were obtained in 104 patients (54.2% of patients followed), and good or satisfactory results were obtained in 53 patients (27.6%).
Abstract
BACKGROUND AND PURPOSE:
There has been a steady progression of case reports and a small surgical series that report successful surgical treatment of Tarlov cysts with concomitant relief of patients' symptoms and improvement in their neurological dysfunction, yet patients are still told that these lesions are asymptomatic by physicians. The purpose of this study was to analyze the efficacy and safety of intervention in 213 consecutive patients with symptomatic Tarlov cysts treated by CT-guided 2-needle cyst aspiration and fibrin sealing.
MATERIALS AND METHODS:
This study was designed to assess outcomes in patients who underwent CT-guided aspiration and injection of ≥1 sacral Tarlov cyst at Johns Hopkins Hospital between 2003 and 2013. In all, 289 cysts were treated in 213 consecutive patients. All these patients were followed for at least 6 months, 90% were followed for 1 year, and 83% were followed for 3–6 years. The aspiration-injection procedure used 2 needles and was performed with the patients under local anesthesia and intravenous anesthesia. In the fibrin-injection stage of the procedure, a commercially available fibrin sealant was injected into the cyst through the deep needle (Tisseel VH).
RESULTS:
One year postprocedure, excellent results were obtained in 104 patients (54.2% of patients followed), and good or satisfactory results were obtained in 53 patients (27.6%). Thus, 157 patients (81.8%) in all were initially satisfied with the outcome of treatment. At 3–6 years postprocedure, 74.0% of patients followed were satisfied with treatment. There were no clinically significant complications.
CONCLUSIONS:
The aspiration-injection technique described herein constitutes a safe and efficacious treatment option that holds promise for relieving cyst-related symptoms in many patients with very little risk.
Perineurial Tarlov cysts (TC) are extrathecal CSF-filled cavities in the perineurial recesses around dorsal spinal nerve roots. Composed of vascularized connective tissue lined with flattened arachnoid tissue, TCs characteristically contain nerve root fibers and ganglion cells in their walls or cavities and tend to be sacral.1,2 They are also notable for their restricted connection to the subarachnoid space and thus exhibit delayed filling during spinal myelography.1 The initial characterization of these structures was cadaveric anatomic variants of unknown clinical significance3,4; the neurosurgeon I.M. Tarlov recognized TCs in patients and established that some cause neurologic symptoms that can be cured neurosurgically.5–10 His 1953 monograph detailed their pathology, including compression and distortion of local nerves and hemorrhage.10 Ensuing work has established that TCs can cause axial sacrococcygeal pain; intrathecal hypotension; perineal pain; sensory loss; and bladder, bowel, and sexual dysfunction.11–13 Radicular symptoms have also been recognized,14 as have electrophysiologic correlates12 and links to collagen dysfunction (eg, Ehlers-Danlos and Marfan syndromes).15,16
Diagnosis preferentially begins with dedicated sacral MR imaging, which is more sensitive than CT or standard lumbosacral MR imaging.17 TCs are visualized (but not always reported) on 1%–2% of sacral MRIs, with approximately 25% of these believed to cause symptoms.18 It is often necessary to evaluate subarachnoid connectivity with myelography to distinguish TCs from intradural ectasias, subarachnoid cysts, and meningeal diverticula—cystic abnormalities that are frequently confused with TCs and for which intracystic injection is contraindicated to avoid intrathecal spread and arachnoiditis.
Despite misimpressions in many clinical circles that TCs are always asymptomatic, there has been steady progress in developing the interventional treatments required for definitive management of symptoms. Conservative approaches including analgesic/anti-inflammatory medication and physical therapy have achieved varying degrees of success in reducing cyst-associated symptoms.19 Surgical methods, meanwhile, include lumbar-peritoneal and cystosubarachnoidal drainage and shunting20,21; bipolar cautery to shrink cysts17; decompressive laminectomy22–24; and laminectomy with either total cyst resection,1,10,25,26 partial cyst wall resection,27 or duroplasty/plication of cyst walls.28 Despite standard limitations such as malfunction and infection after shunting, persistence of pain after laminectomy, and radicular deficits after ablative procedures, a prior review found that 88.6% of patients in the studies evaluated were satisfactorily relieved by surgery according to the outcome criteria of each report.14 Microsurgical techniques, such as laminectomies with electrophysiology-guided cyst wall fenestration/imbrication and myofascial flap repair and closure, are also used, and while these are potentially less effective, they appear to cause fewer adverse events and preserve neural tissue better.29–32
Minimally invasive percutaneous techniques have also emerged. In 1994, Paulsen et al13 reported immediate symptom relief lasting up to 3 months after single-needle aspiration of symptomatic TCs in 5 patients. Lee et al33 then described using a patient's temporary response to cyst aspiration as a diagnostic maneuver to select candidates for surgery. In 1997, Patel et al34 first described injecting autologous fibrin glue into aspirated cysts. Four of 4 patients exhibited marked improvement without recurrence during 23 months, with 1 achieving lasting relief. They postulated that fibrin deposition on cyst walls would impede CSF ingress, trigger fibrosis, and, ideally, promote cyst contracture. Zhang et al35 subsequently reported that 100% of 31 patients treated with intracystic fibrin glue injection achieved satisfactory relief without recurrence during 28 months of follow-up. This methodology was further refined by the development by Murphy et al36 of the 2-needle technique evaluated in this report. Maintaining an equilibrium of intracystic pressure during aspiration-injection, this procedure reduced pressure-related procedural radicular pain sometimes noted with the single-needle technique and perhaps improved fibrin sealant filling.
Materials and Methods
Study Design
This study, initiated in 2003 after receiving institutional approval, assessed outcomes in all patients who underwent CT-guided 2-needle aspiration-injection of ≥1 symptomatic sacral TC at Johns Hopkins Hospital between 2003 and 2012. Assessments were repeated at 3 months postprocedure, 1 year postprocedure, and yearly thereafter.
Patient Selection
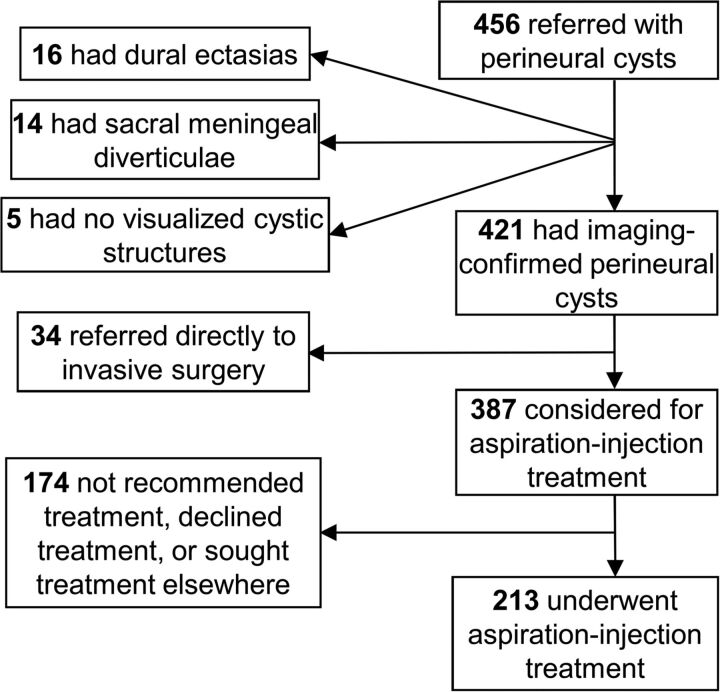
The study cohort was drawn from patients referred with apparently symptomatic sacral TCs. Although 6 patients were treated for cervical, thoracic, or lumbar TCs, these are not discussed here because they were too few to satisfactorily analyze. Referred patients were vetted for inclusion as illustrated in Fig 1. First, a diagnosis of TC was confirmed by scanning all patients with lumbar and sacral MR imaging to identify, characterize, and localize any cystic structures present. All studies were read independently by both an interventional neuroradiologist and a participating neurosurgeon. TCs were identified according to the classification criteria of Goyal et al2 and Wilkins.37 Patients in whom MR imaging inadequately defined subarachnoid connectivity underwent myelography to assess the rapidity of contrast spread into the cyst, and only those without significant connectivity (ie, those with slowly filling cysts) were considered for inclusion.
Fig 1.
Patient recruitment flowchart for aspiration-injection therapy.
Depending on the seriousness of their condition and their willingness to enter the study, patients with imaging-confirmed sacral TCs were either referred to invasive surgery or considered for aspiration-injection treatment. Those who remained in consideration underwent further examination and testing to determine whether their symptoms could be attributed to visualized cysts (ie, whether their TCs were symptomatic). Confirmation of symptomatic cysts required that axial pain be in the immediate anatomic vicinity of the cyst, that radicular symptoms and signs occur only in the appropriate distribution of cyst-bearing segments, and that other potential pain generators be excluded by imaging or other diagnostic testing. Additionally, 104 patients in whom the symptoms of cysts were particularly uncertain underwent diagnostic sacral blockade of cyst-bearing roots; this was performed in the standard fashion through the most convenient posterior sensory foramen and used fluoroscopic or CT guidance to place a needle on the cyst-bearing root, superior to the cyst, and near the next root above. Pain relief commensurate with the agent used and lack of pain relief with control blocks were required for attribution of symptoms to the blocked cyst.
Exclusion Criteria.
All patients with cystic abnormalities other than perineurial cysts and patients with cysts possessing considerable (wide-neck) direct communication with the subarachnoid space were rejected. Patients who lacked the ability to communicate in English; had the probability of inadequate follow-up (eg, those who lived outside North America); had any Diagnostic and Statistical Manual of Mental Disorders diagnosis; had unexplained symptoms that were not clearly related to cyst-bearing roots or (as assessed by a neurosurgery nurse practitioner) the presence of ≥2 Waddell signs (to exclude patients whose pain may have considerable nonorganic components); and patients with any other probable identified pain generator besides TCs were also all rejected for treatment.
Physical Examination
All patients were seen by an experienced nurse practitioner who obtained a typical neurosurgical history with review of systems and medical history. All patients were also seen by a neurosurgeon and underwent history, physical evaluation of the spine, and neurologic evaluation, focusing on the symptomatic area of the spine. In addition, all patients underwent a detailed history and physical evaluation of the territories supplied by cyst-bearing nerve roots. All patients with pelvic, abdominal, or genital symptoms were also seen by a gynecologist and/or urologist. Urodynamics were obtained if recommended by the urologist.
Pain and function were evaluated by the Lumbar Spine Outcomes Questionnaire scale, a validated outcome measure in spinal disease that permits quantification of pain and neurologic loss (including bowel, bladder, or sexual dysfunction) and can be administered satisfactorily by telephone.38 In addition to the severity measures, pain was also described in terms of its spatial and temporal characteristics, with aggravating and alleviating factors taken into account.
Treatment
All patients had a detailed discussion of treatment options including no treatment, pharmacologic pain management, invasive surgery, and aspiration-injection. That aspiration-injection was a new and novel technique was emphasized. All patients were given written material explaining these points in accordance with institutional review board guidelines. Patients who elected the aspiration-injection procedure were seen by the interventional neuroradiologist and were engaged in a second discussion of the procedure and its risks and benefits.
Method of Aspiration-Injection
All patients included in our cohort were treated by using the aspiration-injection technique previously described by Murphy et al.36 Briefly, aspiration was preceded by performance of diagnostic CT (Aquilion 16-detector row multidetector CT unit; Toshiba Medical Systems, Tokyo, Japan) to select the level providing access to the cyst through the thinnest overlying bone. Sedation was induced intravenously. The back was prepared in the usual sterile fashion, with local anesthesia infiltrated into the skin, fat, muscles, and periosteum overlying the sacrum. Two 18-ga needles were advanced into the cyst with CT fluoroscopy (13 frames per second, 3 adjacent 2-, 4-, and 2-mm sections) (Fig 2). The tip of the first needle was typically placed deep in the cyst, while the second was placed more superficially. The stylets were removed from both needles, and fluid was aspirated via the deeper needle. The more superficial needle served as a venting tube during the aspiration process, allowing air to enter the cyst. Following aspiration, an air-fluid level developed; this was monitored intermittently with CT fluoroscopy for evidence of rapid cyst refilling, which would indicate a connection to the thecal sac. (We avoided using iodinated contrast agents to monitor cyst fluid levels because these fill up the cyst, complicate fibrin injection, and impair fibrin binding while providing no appreciable additional information compared with air-fluid levels). A commercially available fibrin sealant composed of human/bovine fibrin, fibrinolysis inhibitor, thrombin, and calcium chloride was next injected into the cyst through the deep needle (Tisseel VH; Baxter Healthcare, Deerfield, Illinois).
Fig 2.
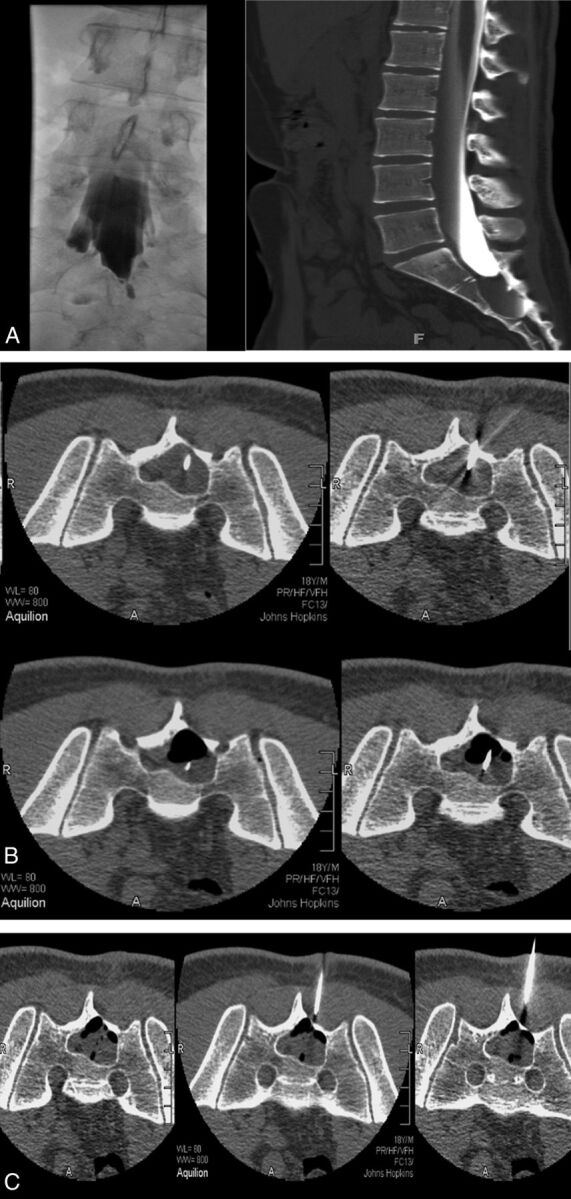
A, CT myelogram and myelogram showing minimal filling of a large right S2 Tarlov cyst with a narrow neck. There is significant remodelling of S2 and S3. B, With 18-ga spinal needles in place, one deeper than the other, partial aspiration has developed an air-fluid level. C, Injection of fibrin fills the cyst 80%. The volume of fibrin injected is close to the volume of CSF aspirated.
Following our observation that completely filling cysts with sealant often temporarily exacerbated postprocedural radicular pain (likely due to swelling sealant compressing the affected nerve root or nearby nociceptors), we adjusted the technique so that sealant injection was halted when CT fluoroscopy indicated that the cavity was approximately 75% full rather than 100% full. This step was found to address the postoperative pain issue without compromising efficacy. Both needles were then withdrawn, and the puncture sites were covered with an antibiotic ointment and a sterile dressing. Patients were observed for 2 hours after the completion of the procedure before being discharged. They were asked to stay locally for 1 night and were permitted to return to their normal physical activity immediately but were advised not to undertake strenuous physical exercise for 2–3 weeks. If multiple cysts were present, typically only 2 were treated per session, so some patients returned for repeat sessions.
Outcome Assessments
Outcomes were evaluated in the clinic or by telephone at 1 day and at 6 and 12 weeks postprocedure by an experienced nurse practitioner or a neurosurgeon. Lumbar sacral MR imaging was repeated at 12 weeks and 1 year postprocedure. Subsequent yearly MRIs were recommended but not required. The primary outcomes, pain and function, were assessed by using the Lumbar Spine Outcomes Questionnaire. An “excellent” outcome required complete pain relief according to the Lumbar Spine Outcomes Questionnaire scale, discontinuation of all pain medications and treatments, and improvement or stabilization of other cyst-linked neurologic signs and symptoms so that no further treatments were required. Patients also had to report satisfaction with the outcome of their procedure and willingness to undergo it again for the same result. A “good/satisfactory” outcome required pain to have “improved quite a bit” on the Lumbar Spine Outcomes Questionnaire scale plus discontinuation of narcotic analgesics. Neurologic deficits had to be commensurate with adequate function or no further progression. Patients also had to be satisfied with the results and not seeking further treatment. All outcomes other than “excellent” or “good/satisfactory” were reported as treatment failures even if some improvement was noted.
Results
The 213 patients described in this article had all been followed for at least 6 months by the time of submission, with 192 (90.1%) of these patients having been followed for 1 year and 177 (83.1%) having been followed for between 3 and 6 years.
Characterization of Cysts
Overall, the 213 patients treated had 289 cysts among them. One hundred forty-four patients had unilateral cysts and symptoms, and 69 patients had bilateral cysts. Multiple cysts ranged from 2 to 9, and the average was 3 for each patient in whom multiple cysts were treated bilaterally. Nine patients had S4 or S5 cysts, but none were symptomatic in isolation. These imaging findings are summarized in Table 1.
Table 1:
Imaging findings
| Findings | No. of Patients |
|---|---|
| Position/No. of root nerves involved | |
| Unilateral, single root | 113 |
| Bilateral, single root | 78 |
| Bilateral, ≥2 roots | 22 |
| Nerve root localization | |
| L4 | 1 |
| L5 | 1 |
| S1 | 16 |
| S2 | 142 |
| S3 | 120 |
| S4–S5 | 9 |
| Aspirated cyst size, cm | |
| 1–2 | 1 |
| 2–3 | 111 |
| 3–4 | 32 |
| >4 | 8 |
Neurologic Symptoms and Signs
Local pain in the region of the cyst that was aggravated by sitting down was the most common symptom in our cohort, though S1, S2 sciatica and neuropathy were also prevalent. Patients with isolated S2 root cysts and no evidence of S1 nerve root compression characteristically had sciatica proceeding no further than the medial heel and immediately adjacent sole. Additionally, many patients had generalized sacral and/or lumbar pain; pelvic and/or perineal pain; and sexual, bladder, or bowel dysfunctions. Common bladder symptoms included incontinence, urinary frequency or urgency, and an inability to fully empty the bladder, while bowel dysfunction tended to involve urgency or mild incontinence. The most common physical neurologic abnormality observed in our patients was an absent ankle reflex. Weakness of plantar flexion was also prevalent, as were cyst-related sensory loss, reduced rectal sphincter tone, and bladder sphincter impairment. Sensory loss most frequently occurred in the perineal region and usually comanifested with perineal pain. Two patients with cysts that compressed the L5 nerve root exhibited weakness of dorsiflexion and complete footdrop. Table 2 summarizes the presenting signs and symptoms in treated patients.
Table 2:
Neurologic symptoms and signs
| Symptom/Sign | No. of Presenting Patients |
|---|---|
| Local pain | 210 |
| L4, L5 neuropathy | 2 |
| S1, S2 sciatica | 151 |
| S1, S2 neuropathy | 137 |
| Generalized sacral/lumbar pain | 189 |
| Pelvic/perineal pain | 209 |
| Bladder dysfunction | 92 |
| Sexual dysfunction | 92 |
| Bowel dysfunction | 62 |
| Absent Achilles reflex | 130 |
| Weakness of plantar flexion | 87 |
| Paralysis of plantar flexion | 2 |
| Paralysis of dorsiflexion | 2 |
| Rectal sphincter tone reduction | 61 |
| Bladder sphincter impairment | 92 |
| Cyst-related sensory loss | |
| L4 | 1 |
| L5 | 1 |
| S1 | 16 |
| S2 | 137 |
| S3, S4, S5 | 97 |
Aspiration-Injection Outcome
At 1 year postprocedure, “excellent” results had been obtained in 104 patients (54.2%), and “good/satisfactory” results had been obtained in 53 patients (27.6%). Thus, 157 patients (81.8%) in all were initially satisfied with the outcome of treatment. In 7 of the patients who were not satisfactorily treated, the aspiration-injection procedure was not technically feasible. In the remainder, the intervention was performed but failed to yield satisfactory results at 1 year postprocedure.
During the 3- to 6-year follow-up, “excellent” results were obtained in 106 patients (59.9%). This outcome represented an increase from the equivalent statistic at 1 year; all those patients who were classified as having excellent results at 1 year maintained this status into the latter follow-up period, and an additional 2 patients in the “good/satisfactory” category at 1 year now qualified as having excellent results. Twenty-five patients (14.1%) now rated their outcome as “good/satisfactory,” a decline from the number that did so at 1 year. Overall, 131 patients (74.0%) were satisfied with treatment at 3- to 6-year follow-up. Only 2 patients have reported recurrence of symptoms after >6 years.
Table 3 presents a breakdown of the aspiration-injection therapy outcomes with respect to individual signs and symptoms.
Table 3:
Outcome of aspiration-injection therapy
| Symptom/Sign | Outcome |
||
|---|---|---|---|
| Excellent | Good | Unchanged | |
| Local pain | 87 (41.4%) | 72 (34.3%) | 51 (24.3%) |
| Sciatica/neuropathy | 81 (53.6%) | 32 (21.2%) | 38 (25.2%) |
| Perineal pain/sensory loss | 86 (41.4%) | 70 (33.5%) | 53 (25.4%) |
| Bladder/sexual dysfunction | 30 (32.6%) | 38 (41.3%) | 24 (26.1%) |
| Bowel dysfunction | 22 (35.5%) | 23 (37.1%) | 17 (27.4%) |
| Plantar flexion weakness | 44 (50.6%) | 20 (23.0%) | 23 (26.4%) |
| Plantar flexion paralysis | 0 (0%) | 0 (0%) | 2 (100%) |
| Dorsiflexion paralysis | 0 (0%) | 0 (0%) | 2 (100%) |
| Rectal sphincter reduction | 22 (36.1%) | 23 (37.7%) | 16 (26.2%) |
Re-Aspiration
Twenty-three patients (10.8% of the treated cohort) underwent re-aspiration within the first 6 months due to recurrent symptoms after immediate short-term relief. Thirteen patients (6.1% of the treated cohort) underwent re-aspiration after 6 months. Two of these had the procedure between 6 and 12 months after the initial aspiration. In 7, re-aspiration was undertaken after the first year, and in 3, after 2 years. One re-aspiration failed, but all the others conferred satisfactory relief again. One patient underwent 3 re-aspirations for recurrent symptoms at 2-year intervals. Repeat MR imaging demonstrated fluid-filled cysts in all patients who underwent re-aspiration, indicating cyst reaccumulation following the initial procedure.
Complications
There were no documented infections or nerve injuries in the treated cohort. One patient had a mild nonspecific allergic reaction with systemic hives, which led to overnight hospitalization as a precaution, but they resolved without incident. Three patients appeared to experience elevated inflammation; all inflammation resolved without treatment during 2–4 weeks, however.
Seven patients had symptoms of spinal fluid leak and required a postprocedure blood patch for control. Twenty patients had increased sciatica following the procedure, which resolved within <3 months. One patient had increased sciatica that persisted for >3 months but eventually resolved. Seven patients had severely increased local pain; this resolved in 6 of these patients within 3 months. Three patients described an increase in all symptoms, including bowel and bladder dysfunction, immediately following injection, though all were transient and resolved within 3 months. There was no incidence of aseptic meningitis.
Surgical Outcome
Patients who had been referred for surgery initially and 11 patients who failed aspiration and were subsequently referred for surgery were followed on the same schedule used for patients undergoing aspiration. Of the initial 34 patients referred for surgery, 31 (91.2%) achieved excellent or satisfactory relief and were satisfied with their surgical outcome. The remaining 3 patients underwent surgery that proved to be partially successful, but they continued to have symptoms afterward. Nonetheless, no worsening of patients' conditions or significant increases in neurologic deficits resulted from surgery in these cases. Of the 11 patients who failed aspiration and were referred to surgery, all had successful relief of symptoms.
Discussion
Contrary to popular sentiments in the medical community, current data indicate that at least some TCs are symptomatic. Beyond the simple correlational finding that a consistent set of symptoms is associated with certain cysts, perhaps the strongest evidence for this position is the well-documented effectiveness of surgical treatment of apparently symptomatic cysts. Since Tarlov described the successful resection of symptomatic perineurial cysts in 1953,10 there has been a steady progression of case reports and small surgical series that reported successful surgical treatment of such cysts with concomitant relief of patient symptoms and improvement in their neurologic dysfunction.19–32 If one accepts the authors' criteria for success, collation of the outcomes of these studies indicates that 88.6% of patients were satisfactorily relieved of symptoms with relatively low morbidity and mortality (no deaths or serious neurologic worsening were reported). Similarly, this study found that 91.2% of the 34 patients referred directly for surgical obliteration (as opposed to aspiration-injection treatment) of their cysts achieved excellent or satisfactory outcomes, while the remainder reported at least some improvement and had no worsening of symptoms or neurologic signs. Relief of symptoms and signs following treatment has long been used in neurosurgery to justify attribution of both to specific pathology; the success of herniated disc excision and decompression of spinal stenosis, for instance, has (supported by excellent clinical research data) helped cement symptomatic attribution to their respective pathologies. As such, this body of evidence strongly supports the contentions that some TCs are symptomatic and that these symptoms can be relieved by surgical intervention.
Such a position is also bolstered by the favorable results obtained by several studies13,33–37 that treated symptomatic TCs with variants of the aspiration-injection technique, including the current article. Moreover, we believe that our data help justify the image-guided aspiration of probable symptomatic cysts and their injection with fibrin sealant as a less-invasive alternative treatment to surgical measures. The initial and long-term success rates for this technique are high enough to hold promise for its adoption as an adequate and efficacious therapy option, with 81.8% of treated patients satisfied with the intervention in terms on its effects on their symptoms at 1 year postprocedure and 74.0% satisfied at 3- to 6-year follow-up. Additionally, our data indicate that aspiration-injection treatment is associated with low morbidity and adverse events: No instances of neurologic injury occurred among those treated in our cohort, and only 8 patients had minor complications (all of which resolved without need for further operations). Only 4 patients reported worsened pain following the procedure but 2 of these recovered shortly afterward and a third was lost to follow-up.
Of special note is the utter lack of instances of postprocedural aseptic meningitis (reported by Patel et al34 to have occurred in 75% of patients) in our series; we surmise this may be because we treated only narrow-neck cysts, thus avoiding fibrin reflux into the thecal sac and subarachnoid space. That all of the 11 patients who failed aspiration treatment were subsequently successfully treated with surgery also indicates that the former procedure does not reduce the chance or extent of success for the latter; this indication reinforces the potential of aspiration-injection as a useful first-option treatment for TC.
The simple discovery of a cyst does not justify attribution of the symptoms to that cyst, but cysts of <10 mm have, in our experience, occasionally been symptomatic. Attribution should be based on the location of the cyst and the ability of these factors to explain (via likely effects on cyst-bearing or neighboring nerve roots) local pain and associated signs and symptoms, as well as temporary relief of symptoms with controlled diagnostic blockade of cyst-bearing nerve roots and the absence of another plausible generator to explain pain and other signs or symptoms. One should not overstate the prevalence of symptomatic Tarlov cysts, especially the prevalence of symptomatic Tarlov cysts that require surgical or percutaneous intervention. In this study, a considerable proportion of patients of the larger group of referred patients were found to be adequately managed by pharmacologic means alone without significant evidence of progression.
Why are these cysts symptomatic in the first place? Certainly, the pathologic changes associated with perineurial cysts are rather striking: Nerves within the cyst-bearing dorsal nerve roots, structures notoriously sensitive to compression, may be distorted, compressed, and injured by the bulging cyst, and adjacent nerves are often also compressed.1,10 Given that afferent nerves populate this area, one may make a direct link between such pathologic changes and the (not infrequently) accompanying radicular symptoms, such as neuropathic pain, paresthesias, and sensory loss/neurophysiologic abnormalities. Additionally, cyst pressure is known to be great enough to erode sacral bone and has been hypothesized to sensitize dural and/or periosteal nociceptors.10,39 It is possible that this second mechanism is primarily responsible for the local, nociceptive pain that often characterizes symptomatic cysts. Meanwhile, the worsening of both radicular and local symptoms with time could be attributed to the gradual enlargement that some cysts may undergo.1,17
Finally, one should wonder why the fibrin sealant used in the aspiration-injection procedure would be effective in treating symptomatic TCs for the long-term, because the compound is slowly absorbed in the body so that permanent cyst obliteration would not occur. Our underlying hypothesis (shared by Patel et al34) was that the injection of sealant into the cyst would thicken the wall of the cyst via fibrosis and block the 1-way valve at the neck of the cyst, reducing the entry of spinal fluid (and thereby preventing the cyst from distending and compressing local nerves or stimulating nearby nociceptors). This hypothesis remains unproven from these data. Still, some support exists for such a position. In the case of subsequent neurosurgical procedure after the injection of fibrin sealant, the formation of a thin impermeable membrane is reported to be evident in the operative site.
Conclusions
Despite widespread belief to the contrary, it has been known for some 70 years that perineurial cysts are sometimes symptomatic and that associated symptoms and signs may be relieved by successful treatment of the troublesome cyst. Surgical methods are effective but are often complicated by infection, postoperative CSF leak, or damage to neural tissue; these make them an imperfect first-option treatment and suggest the need for a percutaneous image-guided approach. The aspiration-injection technique described herein constitutes a safe and efficacious treatment option, and one that holds promise for relieving cyst-related symptoms in many patients with very small risk.
ABBREVIATION:
- TC
Tarlov cyst
Footnotes
Disclosures: Donlin M. Long—UNRELATED: Consultancy: SKK Corp, Comments: consulting related to lumbar disc herniation treatment; Expert Testimony: medicolegal consultant for medical malpractice and personal injury.
This work was supported in part by research grants from the US Public Health Service (NIH K24NS9892).
References
- 1. Tarlov IM. Spinal perineurial and meningeal cysts. J Neurol Neurosurg Psychiatry 1970;33:833–43 10.1136/jnnp.33.6.833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goyal RN, Russell NA, Benoit BG, et al. Intraspinal cysts: a classification and literature review. Spine (Phila Pa 1976) 1987;12:209–13 10.1097/00007632-198704000-00003 [DOI] [PubMed] [Google Scholar]
- 3. Rexed B. Arachnoidal proliferations with cyst formation in human spinal nerve roots at their entry into the intervertebral foramina: preliminary report. J Neurosurg 1947;4:414–21 10.3171/jns.1947.4.5.0414 [DOI] [PubMed] [Google Scholar]
- 4. Tarlov IM. Perineurial cysts of the spinal nerve roots. Arch Neurol Psychiatry 1938;40:1067–74 10.1001/archneurpsyc.1938.02270120017001 [DOI] [Google Scholar]
- 5. Tarlov IM. Cysts (perineurial) of the sacral roots: another cause (removable) of sciatic pain. J Am Med Assoc 1948;138:740–44 10.1001/jama.1948.02900100020005 [DOI] [PubMed] [Google Scholar]
- 6. Tarlov IM. Cysts of the sacral nerve roots: clinical significance and pathogenesis. AMA Arch Neurol Psychiatry 1952;68:94–108 10.1001/archneurpsyc.1952.02320190100010 [DOI] [PubMed] [Google Scholar]
- 7. Tarlov IM. Cysts (perineurial) of the sacral nerve roots: another cause of the sciatic or sacral cauda equina syndrome. J Neuropathol Exp Neurol 1952;11:88–89 [PubMed] [Google Scholar]
- 8. Tarlov IM. Cysts of sacral nerve roots: pathogenesis and clinical significance. AMA Arch Neurol Psychiatry 1953;69:391–92 [PubMed] [Google Scholar]
- 9. Tarlov IM. Sacral nerve-root cysts: pathogenesis and clinical significance. J Nerv Ment Dis 1953;117:156–57 10.1097/00005053-195302000-00009 [DOI] [PubMed] [Google Scholar]
- 10. Tarlov IM. Sacral Nerve-Root Cysts: Another Cause of the Sciatic or Cauda Equina Syndrome. Springfield: Charles C. Thomas; 1953 [Google Scholar]
- 11. Abbott KH, Leimbach WH, Retter RH. The role of perineurial sacral cysts in the sciatic and sacrococcygeal syndromes: a review of the literature and report of 9 cases. J Neurosurg 1957;14:5–21 10.3171/jns.1957.14.1.0005 [DOI] [PubMed] [Google Scholar]
- 12. Cattaneo L, Pavesi G, Mancia D. Sural nerve abnormalities in sacral perineural (Tarlov) cysts. J Neurol 2001;248:623–24 10.1007/s004150170144 [DOI] [PubMed] [Google Scholar]
- 13. Paulsen RD, Call GA, Murtagh FR. Prevalence and percutaneous drainage of cysts of the sacral nerve root sheath (Tarlov cysts). AJNR Am J Neuroradiol 1994;15:293–97; discussion 298–99 [PMC free article] [PubMed] [Google Scholar]
- 14. Oaklander AL, Long DM, Larvie M, et al. Case records of the Massachusetts General Hospital: Case 7–2013—a 77-year-old woman with long-standing unilateral thoracic pain and incontinence. N Engl J Med 2013;368:853–61 10.1056/NEJMcpc1114034 [DOI] [PubMed] [Google Scholar]
- 15. Isono M, Hori S, Konishi Y, et al. Ehlers-Danlos syndrome associated with multiple spinal meningeal cysts: case report. Neurol Med Chir (Tokyo) 1999;39:380–83 10.2176/nmc.39.380 [DOI] [PubMed] [Google Scholar]
- 16. Doi H, Sakurai S, Ida M, et al. A case of sacral meningeal cyst with Marfan syndrome [in Japanese]. No Shinkei Geka 1999;27:847–50 [PubMed] [Google Scholar]
- 17. Rodziewicz GS, Kaufman B, Spetzler RF. Diagnosis of sacral perineural cysts by nuclear magnetic resonance. Surg Neurol 1984;22:50–52 10.1016/0090-3019(84)90228-3 [DOI] [PubMed] [Google Scholar]
- 18. Langdown AJ, Grundy JR, Birch NC. The clinical relevance of Tarlov cysts. J Spinal Disord Tech 2005;18:29–33 10.1097/01.bsd.0000133495.78245.71 [DOI] [PubMed] [Google Scholar]
- 19. Hiers RH, Long D, North RB, et al. Hiding in plain sight: a case of Tarlov perineurial cysts. J Pain 2010;11:833–37 10.1016/j.jpain.2010.07.007 [DOI] [PubMed] [Google Scholar]
- 20. Bartels RH, van Overbeeke JJ. Lumbar cerebrospinal fluid drainage for symptomatic sacral nerve root cysts: an adjuvant diagnostic procedure and/or alternative treatment? Technical case report. Neurosurgery 1997;40:861–64; discussion 864–65 10.1097/00006123-199704000-00044 [DOI] [PubMed] [Google Scholar]
- 21. Morio Y, Nanjo Y, Nagashima H, et al. Sacral cyst managed with cyst-subarachnoid shunt: a technical case report. Spine 2001;26:451–53 10.1097/00007632-200102150-00025 [DOI] [PubMed] [Google Scholar]
- 22. Siqueira EB, Schaffer L, Kranzler LI, et al. CT characteristics of sacral perineurial cysts: report of two cases. J Neurosurg 1984;61:596–98 10.3171/jns.1984.61.3.0596 [DOI] [PubMed] [Google Scholar]
- 23. Sá MC, Sá RC. Tarlov cysts: report of four cases [in Portuguese]. Arq Neuropsiquiatr 2004;62:689–94 10.1590/S0004-282X2004000400023 [DOI] [PubMed] [Google Scholar]
- 24. Tanaka M, Nakahara S, Ito Y, et al. Surgical results of sacral perineurial (Tarlov) cysts. Acta Med Okayama 2006;60:65–70 [DOI] [PubMed] [Google Scholar]
- 25. Nishiura I, Koyama T, Handa J. Intrasacral perineurial cyst. Surg Neurol 1985;23:265–69 10.1016/0090-3019(85)90093-X [DOI] [PubMed] [Google Scholar]
- 26. Voyadzis JM, Bhargava P, Henderson FC. Tarlov cysts: a study of 10 cases with review of the literature. J Neurosurg 2001;95:25–32 [DOI] [PubMed] [Google Scholar]
- 27. Strully KJ, Heiser S. Lumbar and sacral cysts of meningeal origin. Radiology 1954;62:544–49 10.1148/62.4.544 [DOI] [PubMed] [Google Scholar]
- 28. Yücesoy KK, Naderi S, Ozer H, et al. Surgical treatment of sacral perineural cysts: a case report. Kobe J Med Sci 1999;45:245–50 [PubMed] [Google Scholar]
- 29. Mummaneni PV, Pitts LH, McCormack BM, et al. Microsurgical treatment of symptomatic sacral Tarlov cysts. Neurosurgery 2000;47:74–78; discussion 78–79 10.1227/00006123-200007000-00016 [DOI] [PubMed] [Google Scholar]
- 30. Guo D, Shu K, Chen R, et al. Microsurgical treatment of symptomatic sacral perineurial cysts. Neurosurgery 2007;60:1059–65; discussion 1065–66 10.1227/01.NEU.0000255457.12978.78 [DOI] [PubMed] [Google Scholar]
- 31. Rasmussen MM, Clemmensen D, Karabeqovic S, et al. A novel microsurgical method for the treatment of spinal nerve root cysts. Dan Med J 2012;59:A4539 [PubMed] [Google Scholar]
- 32. Caspar W, Papavero L, Nabhan A, et al. Microsurgical excision of symptomatic sacral perineurial cysts: a study of 15 cases. Surg Neurol 2003;59:101–05; discussion 105–06 10.1016/S0090-3019(02)00981-3 [DOI] [PubMed] [Google Scholar]
- 33. Lee JY, Impekoven P, Stenzel W, et al. CT-guided percutaneous aspiration of Tarlov cyst as a useful diagnostic procedure prior to operative intervention. Acta Neurochir (Wien) 2004;146:667–70 10.1007/s00701-004-0274-8 [DOI] [PubMed] [Google Scholar]
- 34. Patel MR, Louie W, Rachlin J. Percutaneous fibrin glue therapy of meningeal cysts of the sacral spine. AJR Am J Roentgenol 1997;168:367–70 10.2214/ajr.168.2.9016209 [DOI] [PubMed] [Google Scholar]
- 35. Zhang T, Li Z, Gong W, et al. Percutaneous fibrin glue therapy for meningeal cysts of the sacral spine with or without aspiration of the cerebrospinal fluid. J Neurosurg Spine 2007;7:145–50 10.3171/SPI-07/08/145 [DOI] [PubMed] [Google Scholar]
- 36. Murphy K, Wyse G, Schnupp S, et al. Two-needle technique for the treatment of symptomatic Tarlov cysts. J Vasc Interv Radiol 2008;19:771–73 10.1016/j.jvir.2008.02.001 [DOI] [PubMed] [Google Scholar]
- 37. Wilkins RH. Intraspinal cysts. In: Wilkins RH, Rengachary SS, eds. Neurosurgery. New York: McGraw-Hill; 1985:2061–70 [Google Scholar]
- 38. Bendebba M, Dizerega GS, Long DM. The Lumbar Spine Outcomes Questionnaire: its development and psychometric properties. Spine J 2007;7:118–32 10.1016/j.spinee.2006.06.382 [DOI] [PubMed] [Google Scholar]
- 39. Dastur HM. The radiological appearances of spinal extradural arachnoid cysts. J Neurol Neurosurg Psychiatry 1963;26:231–35 10.1136/jnnp.26.3.231 [DOI] [PMC free article] [PubMed] [Google Scholar]