Abstract
Background
Emergency department (ED) visits involving benzodiazepines have increased in the United States. Most states have created prescription monitoring programs (PMPs) to improve drug prescribing safety. To determine the association between PMP implementation and ED visits involving benzodiazepine misuse, we conducted a retrospective analysis of data from 11 metropolitan areas in the United States from 2004 to 2011.
Methods
We estimated rates of ED visits per 100,000 residents involving benzodiazepine misuse from the Drug Abuse Warning Network dataset. Dates of PMP implementation were obtained from program administrators. We used linear regression models to assess whether PMP implementation was associated with a change in ED visits involving benzodiazepines. Models were adjusted for calendar quarter, metropolitan area, and metropolitan area-specific linear time trends.
Results
Rates of ED visits involving benzodiazepine misuse increased in all metropolitan areas during the study period. PMP implementation was not associated with a change in ED visits (mean difference: 0.9 [95% CI: −0.09 to 1.9] visits per 100,000 population per quarter; p=0.08). When analyzed by number of years after implementation, PMPs were associated with a higher visit rate in year one (0.8 [95% CI: 0.2 to 1.5]; p = 0.01]), but not in year two (0.3 [95% CI: −2.1 to 2.8]; p = 0.78) or year three or later (2.1 [95% CI: −0.4 to 4.7]; p = 0.10).
Conclusion
We did not find evidence that PMP implementation was associated with reductions in ED visits involving benzodiazepine misuse. Future work should identify PMP features and capabilities that improve benzodiazepine safety.
Keywords: benzodiazepines, prescription drug misuse, health policy, public policy, public health
Introduction
Benzodiazepines are medications with sedative, anxiolytic, and anticonvulsant effects that are commonly used to treat anxiety disorders, insomnia, muscle spasms, and seizure disorders. In 2008, an estimated 5.2% of American adults (over 11 million) filled one or more prescription for a benzodiazepine (Olfson, King, & Schoenbaum, 2015). While use of benzodiazepines for panic disorder and insomnia is supported by clinical practice guidelines (Bandelow et al., 2008; Baldwin et al., 2005; Schutte-Rodin, Broch, Buysse, Dorsey, & Sateia, 2008; Morgenthaler et al., 2007), it can be associated with several risks, including misuse and dependence (Fenton, Keyes, Martins, & Hasin, 2010), falls and fractures (Xing et al., 2014), and motor vehicle crashes (Smink, Egberts, Lusthof, Uges, & de Gier, 2010). Furthermore, concurrent benzodiazepine use is associated with a much higher risk of opioid overdose and in 2011, benzodiazepines were estimated to be involved in approximately one third (31%) of opioid overdoses (Park, Saitz, Ganoczy, Ilgen, & Bohnert, 2015; Jones & McAninch, 2015). Many of these adverse events can lead to emergency department (ED) care and a recent study found that ED visits involving benzodiazepines approximately doubled between 2005 and 2011 (Substance Abuse and Mental Health Services Administration Center for Behavioral Health Statistics and Quality., 2014).
In the United States, prescription monitoring programs (PMPs) are state-level registries of prescriptions for controlled substances. Early PMPs were designed primarily for law enforcement use but more recent PMPs have made prescription data accessible to prescribers (Clark, Eadie, Kreiner, & Strickler, 2012). These programs aim to improve prescription safety by helping providers to identify individuals filling prescriptions from multiple providers or pharmacies (i.e., “doctor shopping” or “pharmacy shopping”), which has previously been documented among some people taking benzodiazepines (Wilsey et al., 2010). As of 2015, all states but one have an operational PMP (Prescription Drug Monitoring Program Training and Technical Assistance Center, 2015). The impact of prescriber-accessible PMPs on benzodiazepine safety, specifically ED visits involving benzodiazepine misuse, is unknown. To examine this association, we conducted a retrospective study of PMP implementation and ED visits involving benzodiazepine misuse in 11 major metropolitan areas in the United States.
Methods
To estimate the rate of ED visits involving benzodiazepine misuse, we used Drug Abuse Warning Network (DAWN) data from 2004 to 2011. DAWN is a survey administered by the Substance Abuse and Mental Health Services Administration to identify ED visits in which illicit or prescription drugs were a cause or contributing factor (Center for Behavioral Health Statistics and Quality, 2013); data are collected by trained chart reviewers. DAWN can be used to study these ED visits on a national level as well as for certain large metropolitan areas in which sufficient data are available to produce reliable estimates. For the period 2004 to 2011, data were available for the following metropolitan areas: Boston, Chicago, Denver, Detroit, Houston, Miami-Dade County, Minneapolis-St. Paul, New York City, Phoenix, San Francisco, and Seattle.
We calculated the ED visit rate per calendar quarter, per 100,000 metropolitan area residents, where benzodiazepine “misuse or abuse” (henceforth “misuse”) was coded as causing or contributing to the visit. We only included visits involving benzodiazepines that were coded as specifically related to misuse, such as visits resulting from taking a higher-than-prescribed benzodiazepine dose, taking benzodiazepine medication prescribed for another individual, requesting detoxification services, attempting suicide, or being maliciously poisoned by another individual (Center for Behavioral Health Statistics and Quality, 2013). ED visit rates were calculated using methods to account for DAWN’s complex sampling (i.e., weights, strata, and replicates) (Center for Behavioral Health Statistics and Quality, 2013). We contacted PMP administrators to determine dates when prescriber-accessible PMPs began recording data on benzodiazepine (Schedule IV) prescriptions. We classified a PMP as present for all calendar quarters that included or followed the PMP implementation date; if a PMP was present in any part of a quarter, we counted it as present for the entire quarter. We coded the presence of a PMP in two ways. First, to account for the fact that metropolitan areas can be composed of counties from several states, the presence of a PMP in each metropolitan area was coded to reflect the proportion of the population residing in a state with a PMP present. For example, in the first quarter of 2011, Massachusetts had a provider-accessible PMP, but New Hampshire did not. Because 91% of the population in the Boston metropolitan area resides in Massachusetts, the value of the PMP variable in this quarter for this metropolitan area was 0.91. Second, to determine the association between PMPs and ED visit rates in each year after PMP implementation, we coded the PMP variable as the number of years the PMP has been operational. Given the relatively short follow-up time for several of the metropolitan areas that implemented PMPs, we categorized the years since PMP implementation as: 1, 2, or 3 or later.
First, we examined unadjusted ED visit rates by grouping metropolitan areas by year of PMP implementation. Next, we fit multivariable linear regression models under a generalized estimating equations framework with a first-order autoregressive (AR1) working covariance matrix to account for correlation over time. The ED visit rate for benzodiazepine misuse in a given metropolitan area in a given calendar quarter was the dependent variable. The main independent variable was the presence of a prescriber-accessible PMP. We included several covariates: calendar quarter (to adjust for time trends common to all metropolitan areas), metropolitan area (to adjust for time-invariant differences between metropolitan areas), and an interaction term between quarter and metropolitan area (metropolitan area-specific linear time trends, to adjust for differential effects of time in each metropolitan area). Previous work has found an association between higher rates of drug use during periods of unemployment both on an individual and a state level (Henkel, 2011; Merline, O'Malley, Schulenberg, Bachman, & Johnston, 2004; Spiller, Lorenz, Bailey, & Dart, 2009); however, adjustment for metropolitan area-specific quarterly unemployment rates did not alter the significance, direction, or magnitude of the association between PMPs and ED visit rates and we therefore did not include it. With this model specification, the coefficient on the main independent variable (PMP) represents the mean difference in ED visits associated with PMP implementation, relative to pre-implementation trends and trends in metropolitan areas without PMPs. In these analyses, we weighted by the inverse variance of the estimated ED visit rate to incorporate uncertainty around DAWN estimates (French & Heagerty, 2008). Analyses were conducted with SAS 9.4 (SAS Institute, Cary, NC) and STATA 13.1 (College Station, TX). This study was determined to be exempt by the University of Pennsylvania Institutional Review Board.
Results
Of the 11 metropolitan areas in our sample, Detroit was the first to be in a state implementing a PMP that collected information on benzodiazepine prescriptions (2003). Between 2008–2009, such PMPs were implemented in states that contained the majority of residents in the Phoenix, San Francisco, and Denver metropolitan areas. During 2010–2011, PMPs were implemented in states that contained the majority of residents in the Boston, Miami, Minneapolis, and New York City metropolitan areas. Residents of Chicago, Houston, and Seattle were not covered by a PMP until after 2011.
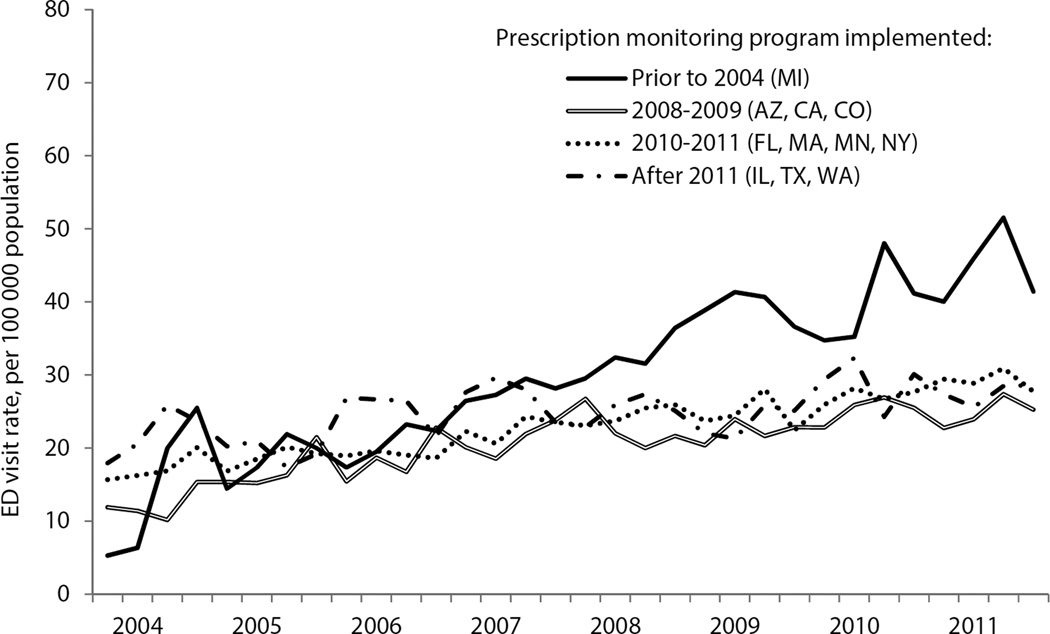
During the study period, unadjusted rates of ED visits involving benzodiazepines increased in all metropolitan areas; increases were similar when grouped by year of PMP implementation (Figure). In adjusted analyses, PMP implementation was not associated with a significant difference in the rate of ED visits involving benzodiazepines (mean difference: 0.9 [95% CI: −0.09 to 1.9] visits per 100,000 population per quarter; p=0.08; Table). When ED visits were analyzed by year after program implementation, PMPs were associated with a significantly higher visit rate in the first year, followed by no significant difference in subsequent years (Table).
Figure.
Emergency department visits involving benzodiazepine misuse in 11 metropolitan areas grouped by year of prescription monitoring program implementation, 2004–2011
Table.
Implementation of a prescriber-accessible prescription monitoring program and rates of emergency department visits involving benzodiazepine misuse in 11 United States metropolitan areas, 2004–2011
| Mean absolute difference in quarterly emergency department visits per 100 000 population (95% CI) |
|||
|---|---|---|---|
| Model 1a | Model 2 | ||
| PMP implementation | 0.8 (−0.1, 1.8) | — | |
| Years since PMP implementation | — | ||
| 1 | 0.8 (0.2, 1.5)* | ||
| 2 | 0.3 (−2.1, 2.8) | ||
| 3 or later | 2.1 (−0.4, 4.7) | ||
Both models adjust for metropolitan area, calendar quarter, and metropolitan area-specific linear time trends. Coefficients for these terms are available in Supplementary Table 1.
P ≤ 0.05
Discussion
In this retrospective study, we did not find PMP implementation to be associated with a decrease in rates of ED visits involving benzodiazepine misuse. Although not reaching statistical significance, the point estimate of the association between PMP implementation and ED visits involving benzodiazepine misuse was positive, suggesting an increase in the post-implementation period. In our model examining time after PMP implementation, we also found a higher rate of ED visits involving benzodiazepine misuse in the first year.
By increasing the difficulty of obtaining benzodiazepines from a physician, PMPs may potentially drive some individuals to obtain diverted medication, which may be associated with an increased risk of adverse events. For opioid analgesics, recent research has found that changes in the supply of these medications may have led some individuals to use alternative opioids, including heroin (Unick, Rosenblum, Mars, & Ciccarone, 2013; Cicero, Ellis, & Surratt, 2012; Coplan, Kale, Sandstrom, Landau, & Chilcoat, 2013). The extent to which this may occur among people taking benzodiazepines is unknown. Alternatively, our findings may be due to enhanced identification of benzodiazepine use where PMPs may have allowed medical providers to identify benzodiazepine misuse more readily, creating an apparent increase in ED visits when there was, in fact, no true increase.
There are several reasons why PMPs may not have reduced benzodiazepine misuse during the time period studied. First, the expansion of PMPs has primarily focused on opioid use among patients being treated for chronic pain, and PMP use by prescribers of benzodiazepines (primarily psychiatrists) may still be low. More recently, some states have started requiring providers to check PMPs prior to prescribing a controlled substance; however, no states in this study had this provision (Clark et al., 2012). Second, even if utilization was high in some states, limitations of PMP data such as delays in pharmacy reporting to PMPs or lack of data on prescriptions filled in neighboring states could limit their effectiveness. Finally, adverse events involving benzodiazepines may not be concentrated among individuals whose PMP record would raise prescriber concerns. For example, if most benzodiazepine-related morbidity and mortality occurs among individuals obtaining prescriptions from a single provider, obtaining medications from a friend or family member, or concurrently using alcohol, then even an ideal PMP might do little to reduce adverse events.
Furthermore, there are important differences in PMP content, functionality, and utilization that are potentially associated with improvements in benzodiazepine safety that we could not evaluate with our dataset. For example, PMPs sending unsolicited reports notifying prescribers of patients with potentially dangerous prescription patterns were linked with lower rates of opioid analgesic prescribing in a previous report (Simeone & Holland, 2006). However, only two metropolitan areas in our sample were in a state requiring such reports (Phoenix, Arizona and Boston, Massachusetts) for which there were only a small number of quarters of follow-up data after PMP implementation (Prescription Drug Monitoring Program Center of Excellence at Brandeis, 2014). Therefore, attempts to evaluate the effectiveness of that provision with our data might reflect the idiosyncrasies of those metropolitan areas during those time periods, as opposed to a true association. More generally, the small sample size and relatively short follow-up period after PMP implementation for several metropolitan areas at this time limits our ability to determine the impact of specific PMPs in each metropolitan area. Future research will be needed to investigate the impacts of specific PMP capabilities on benzodiazepine safety.
Research on an early PMP in New York State that used triplicate prescriptions suggested that the program was associated with reduced benzodiazepine use and fewer ED visits involving benzodiazepines in the early 1990s (Lurie, Kahn, & Wolfe, 1992; Ross-Degnan et al., 2004; Weintraub, Singh, Byrne, Maharaj, & Guttmacher, 1991; Fisher, Sanyal, Frail, & Sketris, 2012). However, this program differed markedly from current PMPs by requiring that physicians purchase and use official triplicate prescription forms, providing a structural barrier to writing prescriptions. While this program was associated with reduced benzodiazepine misuse, it was also associated with reduced appropriate use of benzodiazepines, including the substitution of patients’ benzodiazepine prescriptions with alternative medications that had worse safety profiles (e.g., chloral hydrate) (Weintraub et al., 1991; Ross-Degnan et al., 2004; Fisher et al., 2012). Future evaluations of prescription monitoring programs regarding benzodiazepine misuse should also consider impacts on medically appropriate use and substitution of potentially less safe alternatives.
This study has several limitations. First, this is an ecological study of a small number of metropolitan areas over a relatively short time period; results may not be generalizable to other PMPs. Second, measures of PMP utilization were not consistently available for inclusion in our models so our estimates therefore represent the associations between ED visits and PMPs as they were implemented and not the potential impact of an ideally designed, implemented, and utilized PMP. Finally, we may not have accounted for important confounders in our regression models such as patterns of medical and psychiatric diagnoses among people taking benzodiazepines and co-ingestion with other medications (e.g., opioids), alcohol, or both.
While we did not find an association between PMP implementation and a decrease in benzodiazepine misuse in this analysis, current PMPs vary markedly in capabilities and utilization and it is possible that some will improve benzodiazepine safety. Outside of the United States, several countries have implemented PMPs that are associated with reductions in potentially problematic prescribing; however, their impact on benzodiazepine safety (e.g., ED visits) is unclear (Gomes et al., 2014; Pradel et al., 2009; Dormuth, Miller, Huang, Mamdani, & Juurlink, 2012; Islam & McRae, 2014). As morbidity and mortality related to benzodiazepines has increased, urgent investigation is needed to identify PMP structures and capabilities, as well as other potential policy interventions, to improve prescribing safety.
Supplementary Material
Highlights.
Emergency department visits involving benzodiazepines have increased in the US
States have implemented prescription monitoring programs to improve safety
We examine changes in emergency department visits after implementation
We do not find evidence that prescription monitoring programs reduced visits
Acknowledgments
This study was supported by funding from the National Institute on Drug Abuse (K23DA027719) and a Matt Slap Pilot Research Award from the Division of General Internal Medicine, Perelman School of Medicine, University of Pennsylvania. The funding bodies had no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors declare no potential conflicts of interest.
Contributor Information
Marcus A. Bachhuber, Email: marcus.bachhuber@gmail.com.
Brandon C. Maughan, Email: bmaughan@gmail.com.
Nandita Mitra, Email: nanditam@mail.med.upenn.edu.
Jordyn Feingold, Email: jordyn.feingold@gmail.com.
Joanna L Starrels, Email: jostarre@montefiore.org.
References
- Baldwin DS, Anderson IM, Nutt DJ, Bandelow B, Bond A, Davidson JR, et al. Evidence-based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. J.Psychopharmacol. 2005;19:567–596. doi: 10.1177/0269881105059253. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Zohar J, Hollander E, Kasper S, Moller HJ, Zohar J, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders - first revision. World J.Biol.Psychiatry. 2008;9:248–312. doi: 10.1080/15622970802465807. [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. Drug Abuse Warning Network Methodology Report, 2011 Update. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2013. [Google Scholar]
- Cicero TJ, Ellis MS, Surratt HL. Effect of abuse-deterrent formulation of OxyContin. N.Engl.J.Med. 2012;367:187–189. doi: 10.1056/NEJMc1204141. [DOI] [PubMed] [Google Scholar]
- Clark T, Eadie J, Kreiner P, Strickler G. Prescription drug monitoring programs: An assessment of the evidence for best practices. Waltham, MA: The Prescription Drug Monitoring Program Center of Excellence, Heller School for Social Policy and Management, Brandeis University; 2012. [Google Scholar]
- Coplan PM, Kale H, Sandstrom L, Landau C, Chilcoat HD. Changes in oxycodone and heroin exposures in the National Poison Data System after introduction of extended-release oxycodone with abuse-deterrent characteristics. Pharmacoepidemiol.Drug Saf. 2013;22:1274–1282. doi: 10.1002/pds.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormuth CR, Miller TA, Huang A, Mamdani MM, Juurlink DN. Effect of a centralized prescription network on inappropriate prescriptions for opioid analgesics and benzodiazepines. CMAJ. 2012;184:E852–E856. doi: 10.1503/cmaj.120465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton MC, Keyes KM, Martins SS, Hasin DS. The role of a prescription in anxiety medication use, abuse, and dependence. Am.J.Psychiatry. 2010;167:1247–1253. doi: 10.1176/appi.ajp.2010.09081132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J, Sanyal C, Frail D, Sketris I. The intended and unintended consequences of benzodiazepine monitoring programmes: a review of the literature. J.Clin.Pharm.Ther. 2012;37:7–21. doi: 10.1111/j.1365-2710.2011.01245.x. [DOI] [PubMed] [Google Scholar]
- French B, Heagerty PJ. Analysis of longitudinal data to evaluate a policy change. Stat.Med. 2008;27:5005–5025. doi: 10.1002/sim.3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes T, Juurlink D, Yao Z, Camacho X, Paterson JM, Singh S, et al. Impact of legislation and a prescription monitoring program on the prevalence of potentially inappropriate prescriptions for monitored drugs in Ontario: a time series analysis. CMAJ.Open. 2014;2:E256–E261. doi: 10.9778/cmajo.20140027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel D. Unemployment and substance use: a review of the literature (1990–2010) Curr.Drug Abuse Rev. 2011;4:4–27. doi: 10.2174/1874473711104010004. [DOI] [PubMed] [Google Scholar]
- Islam MM, McRae IS. An inevitable wave of prescription drug monitoring programs in the context of prescription opioids: pros, cons and tensions. BMC.Pharmacol.Toxicol. 2014;15:46. doi: 10.1186/2050-6511-15-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, McAninch JK. Emergency Department Visits and Overdose Deaths From Combined Use of Opioids and Benzodiazepines. Am.J.Prev.Med. 2015 doi: 10.1016/j.amepre.2015.03.040. [DOI] [PubMed] [Google Scholar]
- Lurie P, Kahn JG, Wolfe SM. Regulation of benzodiazepine prescription. JAMA. 1992;268:472–473. [PubMed] [Google Scholar]
- Merline AC, O'Malley PM, Schulenberg JE, Bachman JG, Johnston LD. Substance use among adults 35 years of age: prevalence, adulthood predictors, and impact of adolescent substance use. Am.J.Public Health. 2004;94:96–102. doi: 10.2105/ajph.94.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenthaler TI, Lee-Chiong T, Alessi C, Friedman L, Aurora RN, Boehlecke B, et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007;30:1445–1459. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfson M, King M, Schoenbaum M. Benzodiazepine use in the United States. JAMA.Psychiatry. 2015;72:136–142. doi: 10.1001/jamapsychiatry.2014.1763. [DOI] [PubMed] [Google Scholar]
- Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015;350:h2698. doi: 10.1136/bmj.h2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradel V, Frauger E, Thirion X, Ronfle E, Lapierre V, Masut A, et al. Impact of a prescription monitoring program on doctor-shopping for high dosage buprenorphine. Pharmacoepidemiol.Drug Saf. 2009;18:36–43. doi: 10.1002/pds.1681. [DOI] [PubMed] [Google Scholar]
- Prescription Drug Monitoring Program Center of Excellence at Brandeis. Guidance on PDMP Best Practices: Options for Unsolicited Reporting. 2014 Retrieved 7-9-2015, from www.pdmpexcellence.org/sites/all/pdfs/Brandeis_COE_Guidance_on_Unsolicited_Reporting_final.pdf.
- Prescription Drug Monitoring Program Training and Technical Assistance Center. State Profiles. 2015 Retrieved 5-29-2015, from http://www.pdmpassist.org/content/state-profiles.
- Ross-Degnan D, Simoni-Wastila L, Brown JS, Gao X, Mah C, Cosler LE, et al. A controlled study of the effects of state surveillance on indicators of problematic and non-problematic benzodiazepine use in a Medicaid population. Int.J.Psychiatry.Med. 2004;34:103–123. doi: 10.2190/8FR4-QYY1-7MYG-2AGJ. [DOI] [PubMed] [Google Scholar]
- Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J.Clin.Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- Simeone R, Holland L. An evaluation of prescription drug monitoring programs. 2006 Retrieved 7-9-2015, from www.simeoneassociates.com/simeone3.pdf.
- Smink BE, Egberts AC, Lusthof KJ, Uges DR, de Gier JJ. The relationship between benzodiazepine use and traffic accidents: A systematic literature review. CNS.Drugs. 2010;24:639–653. doi: 10.2165/11533170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Spiller H, Lorenz DJ, Bailey EJ, Dart RC. Epidemiological trends in abuse and misuse of prescription opioids. J.Addict.Dis. 2009;28:130–136. doi: 10.1080/10550880902772431. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration Center for Behavioral Health Statistics and Quality. The DAWN Report: Benzodiazepines in Combination with Opioid Pain Relievers or Alcohol: Greater Risk of More Serious ED Visit Outcomes. Rockville, MD: 2014. [PubMed] [Google Scholar]
- Unick GJ, Rosenblum D, Mars S, Ciccarone D. Intertwined epidemics: national demographic trends in hospitalizations for heroin- and opioid-related overdoses, 1993–2009. PLoS.One. 2013;8:e54496. doi: 10.1371/journal.pone.0054496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub M, Singh S, Byrne L, Maharaj K, Guttmacher L. Consequences of the 1989 New York State triplicate benzodiazepine prescription regulations. JAMA. 1991;266:2392–2397. [PubMed] [Google Scholar]
- Wilsey BL, Fishman SM, Gilson AM, Casamalhuapa C, Baxi H, Zhang H, et al. Profiling multiple provider prescribing of opioids, benzodiazepines, stimulants, and anorectics. Drug Alcohol.Depend. 2010;112:99–106. doi: 10.1016/j.drugalcdep.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Xing D, Ma XL, Ma JX, Wang J, Yang Y, Chen Y. Association between use of benzodiazepines and risk of fractures: a meta-analysis. Osteoporos.Int. 2014;25:105–120. doi: 10.1007/s00198-013-2446-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.