Abstract
Background
The lifecourse perspective suggests a pathway may exist among maternal exposure to stressful life events prior to conception (PSLEs), infant birthweight, and subsequent offspring health, whereby PLSEs are part of a “chains-of-risk” that set children on a certain health pathway. No prior study has examined the link between PSLEs and offspring health in a nationally-representative sample of US mothers and their children. We used longitudinal, nationally-representative data to evaluate the relation between maternal exposure to PSLEs and subsequent measures of infant and toddler health, taking both maternal and obstetric characteristics into account.
Methods
We examined 6,900 mother-child dyads participating in two waves of the nationally-representative Early Childhood Longitudinal Study-Birth Cohort (n=6,900). Infant and toddler health outcomes assessed at 9 and 24 months included overall health status, special health care needs, and severe health conditions. Adjusted path analyses examined associations between PSLEs, birthweight, and child health outcomes.
Results
In adjusted analyses, PSLEs increased the risk for very low birthweight (VLBW, <1,500 grams), which, in turn, predicted poor health at both 9 and 24 months of age. Path analyses demonstrated that PSLEs had small indirect effects on children’s subsequent health that operated through VLBW.
Conclusion
Our analysis suggests a chains-of-risk model in which women’s exposure to PSLEs increases the risk for giving birth to a VLBW infant, which, in turn, adversely affects infant and toddler health. Addressing women’s preconception health may have important downstream benefits for their children, although more research is needed to replicate these findings.
Keywords: Birthweight, child health, psychosocial factors, lifecourse epidemiology, maternal and child health, path analyses
INTRODUCTION
Despite advances in health care and implementation of social service and early intervention programs, the prevalence of children with disabilities in the US remains high and may even be increasing among some groups. [1] Recent data from the National Health Interview Survey found that the prevalence of childhood disabilities rose 16% from 2001 to affect nearly 6 million US children in 2011, with notable increases observed for children with neurodevelopmental and mental health disorders. [1] Understanding factors that influence these trends is essential to inform intervention efforts that seek to improve children’s developmental and health trajectories.
The lifecourse perspective conceptualizes health as arising from cumulative effects of events that occur across the lifespan, as well as from intergenerational effects. [2–6] Disparities in child health are thus consequences of both exposures before and during pregnancy and early life exposures. In a previous study, Witt et al. found that a woman’s exposure to stressful life events prior to conception (PSLEs) increased her risk for having a very low birthweight (VLBW) infant. [7] In turn, a large body of literature demonstrates that low birthweight contributes to infant and childhood morbidity. [8–11] The lifecourse perspective suggests a pathway may exist among PSLEs, birthweight, and subsequent child health, whereby PLSEs are part of a “chains-of-risk” that set children on a certain health pathway. In this scenario, PSLEs increase a woman’s risk of having a VLBW baby, and VLBW, in turn, contributes to poor health in early childhood. [6] Understanding the direct and/or indirect relationships between PSLEs and children’s early health could elucidate mechanisms linking children’s health to the preconception experiences of their mothers, and thus lend insight into how these “chains-of-risk” might be interrupted.
Using a nationally-representative sample of Danish women, Li et al. found that maternal bereavement (e.g., death of a parent, spouse, or close relative) within 6 months prior to pregnancy was associated with attention deficit hyperactivity disorder[12] and adolescent overweight[12] in offspring. Class et al. noted associations between women’s preconception stress and infant mortality in a Swedish cohort. [13] While these European-based studies are among the first to suggest that women’s exposure to PSLEs may affect the immediate and long-term health of offspring, no study has examined the relation between PSLEs and obstetric and child health outcomes in a national cohort of US families.
We used longitudinal data from the nationally-representative Early Childhood Longitudinal Study, Birth Cohort (ECLS-B) to evaluate maternal exposure to PSLEs and subsequent measures of infant and toddler health (overall health status, diagnoses of special health care needs, and the presence of severe health conditions), taking both maternal and obstetric characteristics into account.
METHODS
Data Source
The ECLS-B is a nationally-representative, longitudinal cohort study of nearly 10,700 US live-born infants. The probability sample was drawn from the ~4 million US children born in 2001, with oversampling of children from minority groups, twins, and children born at low and very low birthweights, based on registered births from the National Center for Health Statistics vital statistics system. The sampling frame excluded births to mothers under 15 years and children adopted or deceased before the initial collection wave. Data for this study were from the first two waves, which occurred when children were 9 and 24 months of age. [14]
Parents of participating children in the ECLS-B provided informed consent and the data collection procedures were approved by National Center for Education Statistics (NCES) as ensuring confidentiality. We obtained a license agreement with NCES for analysis of ECLS-B’s restricted data. We rounded unweighted sample sizes to the nearest 50 to comply with NCES guidelines. [14]
Participants were eligible for this study if the survey respondent was the biological mother (n=10,550); we excluded 450 children missing birth certificate data. For each child sampled as a twin (n=1,500), we randomly selected one child from each pair to remain in the sample. Testing models with multiples removed did not influence our findings. Given the previously identified relationship between PSLEs and VLBW, [7] we then restricted our sample to children born with VLBW (<1,500 grams) and normal birthweight (NBW; between 2,500–3,999 grams). Our final sample thus consisted of 6,900 children and their mothers who participated in the second ECLS-B data wave and who had complete covariate information.
To evaluate the robustness of the results, we repeated analyses comparing children born LBW versus NBW (Appendix 1) and included children born LBW and high birthweight (HBW; ≥4000 grams) with NBW children as the reference outcome (Appendix 2).
Measures
Stressful Life Events Prior to Conception
Our approach to defining PSLEs is detailed elsewhere. [7, 15] Briefly, we derived the date of conception using birth certificate information on the length of gestation and the infant’s date of birth. We coded women as having experienced a PSLE if they reported that one or more of the following events occurred prior to conception: (1) death of their mother; (2) death of their father; (3) death of a previous live born child; (4) divorce; (5) separation from partner; (6) death of a spouse; or (7) problems with infertility (Appendix 3). These experiences are considered stressful life events and/or have been operationalized as such in previous research. [16–19] In our sample, nearly 75% of women who experienced any PSLE had an event occur within 1 year prior to conception (data not shown).
Very Low Birthweight
Birthweight (continuous grams) was derived from the birth certificate. We used a cutpoint of <1,500 grams to denote VLBW and the 2,500–3,999 gram range to denote NBW.
Infant and Toddler Health
We examined three child health binary indicators, assessed by maternal report at 9 and 24 months: (1) overall health status, reported on a 5-point Likert scale and dichotomized as “excellent, very good, or good” versus “fair or poor;” (2) a clinically diagnosed special health care need (SHCN); and (3) any severe health condition, defined as asthma, respiratory illness, ear infection or any gastrointestinal-related illness that required an emergency room visit or overnight hospitalization. SHCNs included blindness, difficulty seeing, difficulty hearing or deafness, problem with mobility or using legs, and heart defects. Additional SHCNs assessed at 9 months included diagnoses of cleft lip or palate, failure to thrive, problem with arms or hands, Down syndrome, Turner syndrome, Spina Bifida, and any other special needs or limitations and at 24 months included diagnoses of developmental delay, epilepsy or seizures, mental retardation, lactose intolerance, and food allergies or sensitivities. Children whose mothers endorsed any of these conditions at either time point were considered to have SHCNs. As a sensitivity analysis, we excluded genetic disorders/chromosomal abnormalities from our definition of SHCN; results were consistent with the main findings.
Maternal Health, Stress, and Sociodemographic Factors
Birth certificate data determined if women had experienced any pregnancy complication (e.g., anemia, eclampsia) or had chronic a condition (e.g., cardiac disease, chronic hypertension). At 9 months, self-reported maternal health was coded on a 5-point Likert scale ranging from 1 (excellent) to 5 (poor). Pre-pregnancy body mass index (BMI) was calculated from measured height and self-reported weight prior to pregnancy (dichotomized as overweight or obese [BMI ≥ 25] versus normal or underweight [BMI <25] or unknown). We evaluated timing of initiation of prenatal care (first trimester; second or third trimester; or no prenatal care), whether the child was a singleton or multiple, and the number of prior live births/parity (0 versus ≥1). Women were coded as having experienced a stressful life event during pregnancy if they indicated that the death of a close relative, divorce, or partner separation occurred during their pregnancy.
Birth certificate data also provided maternal age in years, race/ethnicity (non-Hispanic white; non-Hispanic black; non-Hispanic Asian/Pacific Islander; non-Hispanic other race; or Hispanic); marital status (married or living with partner; separated, divorced or widowed; or never married); and health insurance (private; any public; or no insurance). At 9 months, we assessed socioeconomic status (SES; defined using composite index generated by NCES that incorporated parental education, occupation and household income), and US region of residence.
Analyses
Descriptive analyses were conducted using survey procedures from SAS (v9.2, Cary, NC); we generated summary statistics to describe sample characteristics and used chi-square and t-tests to evaluate differences in covariates between women who did and did not experience any PSLE and by children’s VLBW status. We then used MPlus (v7.1, Los Angeles, CA) to conduct path analyses using linear probability models (with binary outcomes) that assessed direct and indirect effects of PSLEs and VLBW on: (1) overall child health; (2) SHCN; and (3) severe health conditions. This approach enabled us to account for multiple sequential and temporal pathways and to specify and test both direct effects (e.g., of PSLEs on infant and toddler health) and indirect effects (e.g., of PSLEs on infant and toddler health via birthweight) of PSLEs on the outcomes of interest. [20] Linear probability path analysis models were used to derive interpretable estimates of direct and indirect effects, specifically so that coefficients represented the percent change in the probability each child health outcome for a one-unit change in each independent variable, holding everything else constant (e.g., the change in the probability of having a child in fair/poor health when the mother was exposed to any PSLE compared to none). Analyses adjusted for appropriate covariates (see Figure 1) and accounted for ECLS-B’s complex sampling design.
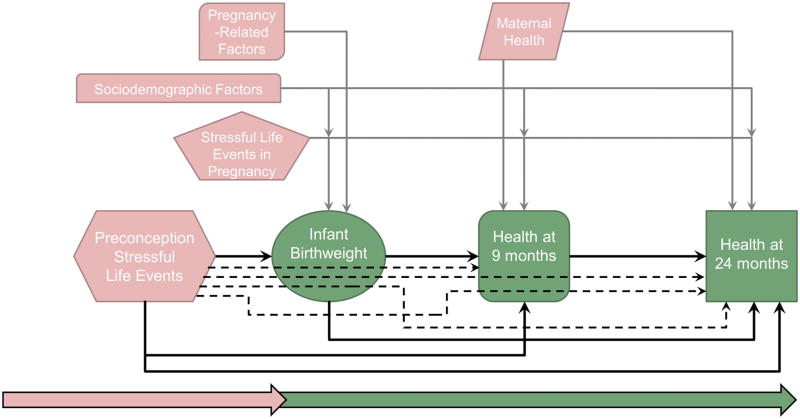
Figure 1.
Figure 1 displays the direct and indirect effects included in the path models. Primary direct effects of interest (depicting relationships among exposure to stressful life events prior to conception, infant birthweight, and child health outcomes) are shown with solid black arrows. Direct effects for groups of adjustment variables are shown with solid gray arrows. Maternal factors are depicted in pink and infant and child outcomes are depicted in green. Variables included as sociodemographic factors are: maternal chronic conditions, number of children born, parity, maternal age, race/ethnicity, marital status, health insurance status, socioeconomic status, and region of residence. Variables included as pregnancy-related factors are: pregnancy complications, prepregnancy body mass index, and initiation of prenatal care. Specific indirect effects are shown as dashed black arrows, and map the hypothesized pathway through intermediate variables.
RESULTS
Tables 1 and 2 present descriptive statistics and bivariate associations of covariates and child health outcomes to PSLEs and VLBW status. In the entire sample, 19.0% of women experienced any PSLE; 28.6% of children who were born VLBW had mothers who experienced any PSLE (Table 1). At 9 months, 2.4% of infants were in fair/poor health, 6.4% had SHCN, and 7.5% had severe health conditions (Table 2); by 24 months, the percentage of toddlers with SHCN increased to 15.5%. VLBW children were more likely to have poor health, SHCN, and severe health conditions at 9 and 24 months than NBW children (all p<0.01).
Table 1.
Descriptive statistics by maternal exposure to stressful life events prior to conception and infant birthweight status, national data from the Early Childhood Longitudinal Study-Birth Cohort (ECLS-B), 2001–2003
| Total | Stressful Life Events Prior to Conception | Infant Birthweight Status | |||||
|---|---|---|---|---|---|---|---|
| None | Any | p-value | Very Low | Normal | p-value | ||
| TOTAL (weighted) | 3,182,840 | 2,577,421 | 605,419 | 44,732 | 3,138,108 | ||
| % | 81.0% | 19.0% | 1.4% | 98.6% | |||
| TOTAL (unweighted)a | 6,900 | 5,450 | 1,400 | 900 | 5,950 | ||
| Infant Birthweight Status | <.001 | - | |||||
| Very Low | 1.4% | 1.2% | 2.1% | 100.0% | - | ||
| Normal | 98.6% | 98.8% | 97.9% | - | 100.0% | ||
| Stressful Life Events Prior to Conception | - | <.001 | |||||
| None | 81.0% | 100.0% | - | 71.4% | 81.1% | ||
| Any | 19.0% | - | 100.0% | 28.6% | 18.9% | ||
| Health, Stress, and Sociodemographic Factors | |||||||
| Pregnancy Complications | 0.36 | <.001 | |||||
| None | 87.4% | 87.7% | 86.6% | 57.0% | 87.9% | ||
| Any | 12.6% | 12.3% | 13.4% | 43.0% | 12.1% | ||
| Maternal Chronic Conditions | 0.23 | <.001 | |||||
| None | 80.1% | 80.5% | 78.5% | 60.8% | 80.4% | ||
| Any | 19.9% | 19.5% | 21.5% | 39.2% | 19.6% | ||
| Maternal Health Status, mean (SD)b | 2.0 (0.95) | 2.0 (0.95) | 2.1 (0.97) | 0.80 | 2.3 (3.1) | 2.0 (0.89) | <.001 |
| Prepregnancy Body Mass Index | 0.29 | 0.01 | |||||
| Normal, underweight, or unknown | 56.2% | 56.5% | 54.5% | 51.2% | 56.2% | ||
| Overweight or obese | 43.8% | 43.5% | 45.5% | 48.8% | 43.8% | ||
| Initiation of Prenatal Care | 0.22 | <.001 | |||||
| In the first trimester | 95.5% | 95.5% | 95.3% | 93.6% | 95.5% | ||
| In the second or third trimester | 4.2% | 4.2% | 4.0% | 4.5% | 4.2% | ||
| Did not receive prenatal care | 0.3% | 0.3% | 0.7% | 1.9% | 0.3% | ||
| Plurality | <.001 | <.001 | |||||
| Singleton | 98.8% | 99.1% | 97.7% | 81.7% | 99.1% | ||
| Multiple | 1.2% | 0.9% | 2.3% | 18.3% | 0.9% | ||
| Parity | <.001 | 0.01 | |||||
| None | 41.0% | 44.8% | 24.8% | 44.9% | 41.0% | ||
| One or more | 59.0% | 55.2% | 75.2% | 55.1% | 59.0% | ||
| Stressful Life Events During Pregnancy | 0.02 | 0.66 | |||||
| None | 94.1% | 93.8% | 95.6% | 93.7% | 94.2% | ||
| Any | 5.9% | 6.2% | 4.4% | 6.3% | 5.8% | ||
| Maternal Age, Years, mean (SD) | 27.2 (6.1) | 26.3 (5.8) | 30.8 (6.2) | <.001 | 27.1 (19.7) | 27.2 (5.7) | 0.85 |
| Maternal Race/Ethnicity | 0.01 | <.001 | |||||
| Non-Hispanic white | 57.0% | 56.1% | 61.0% | 46.0% | 57.2% | ||
| Non-Hispanic black | 14.3% | 14.3% | 13.9% | 27.5% | 14.1% | ||
| Non-Hispanic Asian/Pacific Islander | 3.6% | 3.7% | 3.1% | 2.8% | 3.6% | ||
| Non-Hispanic other race | 2.6% | 2.5% | 3.1% | 2.1% | 2.6% | ||
| Hispanic | 22.5% | 23.3% | 18.9% | 21.7% | 22.5% | ||
| Marital Status | <.001 | <.001 | |||||
| Married or living with partner | 83.4% | 83.1% | 84.7% | 76.7% | 83.5% | ||
| Separated/divorced/widowed | 3.1% | 2.5% | 5.6% | 3.1% | 3.1% | ||
| Never married | 13.5% | 14.4% | 9.7% | 20.2% | 13.4% | ||
| Health Insurance | 0.10 | <.001 | |||||
| Private only | 58.9% | 58.2% | 62.0% | 51.6% | 59.0% | ||
| Any public | 37.5% | 38.3% | 34.2% | 44.6% | 37.4% | ||
| None | 3.6% | 3.5% | 3.8% | 3.8% | 3.5% | ||
| Socioeconomic Status, mean (SD)c | −0.08 (0.81) | −0.09 (0.82) | −0.04 (0.80) | 0.20 | −0.25 (2.4) | −0.08 (0.76) | <.001 |
| Region of Residence | 0.30 | 0.01 | |||||
| Northeast | 17.0% | 17.2% | 15.8% | 17.7% | 17.0% | ||
| Midwest | 22.2% | 22.2% | 22.0% | 21.0% | 22.2% | ||
| South | 36.9% | 36.3% | 39.6% | 42.1% | 36.8% | ||
| West | 24.0% | 24.3% | 22.7% | 19.2% | 24.0% | ||
Weighted estimates. P-values derived from t-tests for continuous variables and chi-square tests for categorical variables; SD, standard deviation. National Center for Education Statistics (NCES) rounding rules applied to unweighted Ns;
Unweighted subgroup Ns may not add to the total due to rounding.
Self-reported health was measured on a 5-point Likert scale with higher scores indicating worse health.
Higher scores indicate higher socioeconomic status.
Table 2.
Child health at 9 and 24 months by maternal exposure to stressful life events prior to conception and infant birthweight status, national data from the Early Childhood Longitudinal Study-Birth Cohort (ECLS-B), 2001–2003
| Total | Stressful Life Events Prior to Conception | Infant Birthweight Status | |||||
|---|---|---|---|---|---|---|---|
| None | Any | p-value | Very Low | Normal | p-value | ||
| Child Health Outcomes | |||||||
| Fair or Poor Health Status | |||||||
| At 9 Months | 2.4% | 2.6% | 1.4% | 0.03 | 8.2% | 2.3% | <.001 |
| At 24 Months | 2.0% | 1.9% | 2.3% | 0.51 | 7.6% | 1.9% | <.001 |
| Any Special Health Care Need | |||||||
| At 9 Months | 6.4% | 6.5% | 6.0% | 0.58 | 33.5% | 6.0% | <.001 |
| At 24 Months | 15.5% | 15.6% | 15.0% | 0.69 | 45.5% | 15.0% | <.001 |
| Any Severe Health Condition | |||||||
| At 9 Months | 7.5% | 7.6% | 7.3% | 0.80 | 17.2% | 7.4% | <.001 |
| At 24 Months | 8.1% | 7.8% | 9.6% | 0.16 | 20.2% | 7.9% | <.001 |
N=6,900. Weighted estimates. Tables show prevalence estimates for each child health outcome by exposure to stressful life events prior to conception and infant birthweight status. The reference categories are “excellent, very good, or good” health, no special health care need, and no severe health condition at each time point.
Table 3 and Figure 2 summarize path model coefficients for the direct and indirect effects between PSLEs, VLBW and child health outcomes at 9 and 24 months. Path models for each of the three health outcomes demonstrated good model fit (Table 3), suggesting that no important paths had been omitted. As expected, there was a significant direct effect of PSLEs on VLBW, such that women exposed to PSLEs had a higher probability of delivering a VLBW infant. In turn, being born VLBW showed direct effects on all three child health measures at 9 and 24 months, ranging from a 4.1% increase in the probability of fair/poor health at 24 months to a 26.5% increase in the probability of SHCN at 9 months. Fair/poor health status, SHCN, and severe health conditions at 9 months were significantly associated with increased probability of similar adverse health status at 24 months (fair/poor health β=13.2%, 95% CI: 6.0–20.4; SHCN β=28.3%, 95% CI: 22.4–34.1; severe health conditions β=20.6%, 95% CI: 15.7–25.4).
Table 3.
Path analysis results of the association between maternal exposure to stressful life events prior to conception and offspring overall health, special health care needs, and severe health conditions in early childhood through very low birthweight
| Fair or Poor Health | Special Health Care Needs | Severe Health Conditions | ||||
|---|---|---|---|---|---|---|
| β | 95% CI | β | 95% CI | β | 95% CI | |
| Direct Effects | ||||||
| From PSLEs to VLBW | 0.55% | (0.23, 0.87) | 0.55% | (0.23, 0.87) | 0.55% | (0.23, 0.87) |
| From PSLEs to CH at 9 mo | −0.81% | (−1.75, 0.14) | −0.98% | (−2.86, 0.90) | 1.03% | (−1.27, 3.34) |
| From PSLEs to CH at 24 mo | 0.56% | (−0.48, 1.60) | −0.44% | (−3.34, 2.45) | 1.99% | (−0.75, 4.73) |
| From VLBW to CH at 9 mo | 5.33% | (2.97, 7.69) | 26.53% | (22.53, 30.53) | 8.92% | (5.91, 11.92) |
| From VLBW to CH at 24 mo | 4.10% | (2.01, 6.19) | 21.50% | (17.50, 25.51) | 9.04% | (5.96, 12.12) |
| From CH at 9 mo to CH at 24 mo | 13.19% | (5.96, 20.42) | 28.25% | (22.40, 34.10) | 20.55% | (15.68, 25.42) |
|
| ||||||
| Indirect Effectsa | ||||||
| PSLEs --> VLBW --> CH at 9 mo | 0.03% | (0.01, 0.05) | 0.15% | (0.06, 0.24) | 0.05% | (0.01, 0.08) |
| PSLEs --> CH at 24 mo | −0.08% | (−0.22, 0.06) | −0.12% | (−0.69, 0.46) | 0.27% | (−0.21, 0.75) |
| PSLEs --> CH at 9 mo --> CH at 24 mo | −0.11% | (−0.25, 0.04) | −0.28% | (−0.82, 0.26) | 0.21% | (−0.27, 0.70) |
| PSLEs --> VLBW --> CH at 24 mo | 0.02% | (0.01, 0.04) | 0.12% | (0.04, 0.20) | 0.05% | (0.02, 0.08) |
| PSLEs --> VLBW --> CH at 9 mo --> CH at 24 mo | 0.01% | (0.00, 0.01) | 0.04% | (0.02, 0.07) | 0.01% | (0.00, 0.02) |
|
| ||||||
| Model Fit Statistics | ||||||
| Chi-square statistic & p-value | 14.3 | 0.11 | 15.9 | 0.11 | 11.2 | 0.26 |
| CFI | 0.991 | 0.991 | 0.997 | |||
| RMSEA | 0.009 | 0.011 | 0.006 | |||
N=6,900. Weighted estimates. Beta coefficients reported as the percent change in the probability of having each child health outcome for a one-unit change in each independent variable. Models adjust for pregnancy complications, maternal chronic conditions, prepregnancy body mass index, initiation of prenatal care, plurality, parity, exposure to any stressful life event during pregnancy, maternal race/ethnicity, maternal age, marital status at birth, health insurance coverage, family socioeconomic status, and US region of residence (see Figure 1).
Specific indirect effects are listed below the total indirect effects, which are in bold. CI, confidence interval; PSLEs, stressful life events prior to conception; VLBW, very low birthweight; CH, child health; CFI, comparative fit index; RMSEA, root mean square error of approximation.
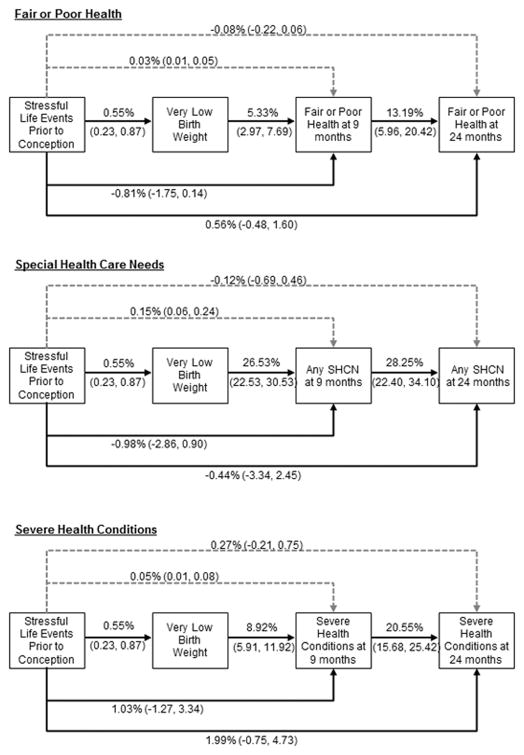
Figure 2.
Figure 2 summarizes the path analyses results of selected direct and total indirect effects of maternal exposure to stressful life events in the preconception period on very low birthweight and child health at 9 and 24 months of age. Weighted results are presented as percent changes and corresponding 95% confidence intervals, controlling for pregnancy complications, maternal chronic conditions, prepregnancy body mass index, initiation of prenatal care, plurality, parity, exposure to any stressful life event during pregnancy, maternal race/ethnicity, maternal age, marital status at birth, health insurance coverage, family socioeconomic status, and US region of residence. Direct effects from very low birthweight status to each child health variable at 24 months are also included. Solid lines represent direct effects and dashed lines indicate total indirect effects. SHCN = Special health care need.
Although PSLEs did not display significant direct effects on any health outcome at 9 or 24 months, small but statistically significant indirect effects were observed such that PSLEs affected the probability of fair/poor health (β=0.03%, 95% CI: 0.01–0.05), SHCN (β=0.15%, 95% CI: 0.06–0.24) and severe health conditions (β=0.05%, 95% CI: 0.01–0.08) at 9 months through the effect of PSLEs on VLBW. While the total indirect effect of PSLEs on child health at 24 months was not significant, the specific indirect effects of PSLEs on child health at 24 months that operated through VLBW were statistically significant (Table 3).
In sensitivity analyses, neither the direct effect from PSLEs to LBW nor the indirect effects from PSLEs to outcomes at 9 or 24 months through LBW were significant (Appendix 1). Comparing VLBW children to all other children weighing >1,500 grams yielded similar results (Appendix 2).
DISCUSSION
In this population-based cohort of US families, we found evidence for a small indirect association between women’s exposure to stressful life events in the preconception period and the likelihood of fair or poor health, special health care needs, or severe health conditions in offspring. As hypothesized, the effects of PSLEs on these early markers of children’s health operated via a “chain-of-risk” model, [6] whereby PSLEs increased the risk of VLBW, which, in turn, increased the risk of children’s poor health. These findings are consistent with our previous study in the ECLS-B, which indicated that exposure to PSLEs increases the risk for VLBW, [7] and with a large body of evidence demonstrating the deleterious effects of VLBW on children’s long-term health. [8–11] The path models identified here suggest that women’s exposure to PSLEs may not exert a direct effect on early child health but could, via an indirect pathway, place very young children at risk through the increased risk of being born VLBW. Although the effects were relatively small, our findings highlight the potential importance of women’s preconception environment and suggest avenues for future research.
Preconception stress has been previously examined as a determinant of perinatal outcomes. For example, using data from the ECLS-B, Witt et al. found that exposure to at least one PSLE increased the odds of VLBW by 38%. [7] In separate studies, the authors also found associations between PSLEs and the likelihoods of PTB among teenage mothers[15] and of delivering via medically-indicated Cesarean section. [15] Similar associations have also been noted in Danish populations. [21] Fewer studies have examined the prospective effect of preconception stress with outcomes after the perinatal period, although some European-based evidence ties preconception stress to the risk of infant mortality, [13] childhood ADHD, [12] overweight status in adolescence, [12] and adult affective disorder in men. [22] Still, other studies have reported null associations. [23–25] To our knowledge, the present study is the first to expand on this work by comprehensively testing the relations among maternal exposure to PSLEs, VLBW, and early child health in a national cohort of US families.
Several mechanisms may explain our findings. The accumulation of stress across the lifecourse has been theorized to increase allostatic load or “weathering,” leading to a decline in reproductive health. [2, 26] High cortisol levels or immune dysfunction during pregnancy could contribute to neuroendocrine, immune, and inflammatory processes that are associated with poor birth outcomes. [27, 28] Women’s exposure to PSLEs may also have epigenetic effects in utero that, in turn, increase children’s susceptibility to disease. [29] This hypothesis is supported by research showing heightened levels of total Immunoglobulin E (IgE, a biomarker of atopic risk) in cord blood of children born to mothers exposed to interpersonal trauma. [30] Alternatively, women exposed to stressors might be more likely to engage in negative health behaviors, such as smoking, a correlate of both infant birthweight[31] and children’s health. [32] However we note that the percent differences we found in this study were, in most cases, small, as exposure to PSLEs is likely only one component of a more comprehensive set of intergenerational pathways linking maternal and child health. Other factors may play important roles in these pathways (e.g., gestational age, shared environmental risks, maternal stress reactivity) that may spillover to influence the child. Research should identify additional preconception risk factors and determine how they influence social, behavioral, and physiological processes that contribute to health outcomes in the next generation, and pinpoint specific critical periods (e.g. in utero, neonatal period, etc.) in which the effects of PSLEs are most salient. This may help us better understand the biological mechanisms connecting PSLEs and child health and the potential intergenerational transmission of stress.
We note several limitations. Children who died before 9 months of age were not eligible to participate in the ECLS-B, potentially leading to conservative estimates of the effect of PSLEs on birthweight and child health. The ECLS-B retrospectively collected limited data on PSLEs and may not have comprehensively captured the spectrum of stressors women experience. This might have resulted in misclassification. The number of individuals who endorsed specific PSLEs was small so we were unable to examine the independent effects of each type of event. The null finding of an indirect effect of PSLEs on child health through LBW could be explained by several mechanisms, including misclassification of infant birthweight[33, 34] or undetected dose-response effects. Alternatively, this finding may indicate that the effects of stressors on child health operate only via extreme effects on fetal growth. These hypothesized pathways could be examined in future research. Birth certificates may incorrectly report some information. [35] Our SCHNs measure may not have included important conditions; conditions were also reported by mothers, potentially resulting in misclassification. Mothers who experienced PSLEs and/or gave birth to VLBW babies may have different expectations for their child’s health. Although not available in the ECLS-B, future work validating our findings using medical record data is warranted.
Despite these limitations, our findings suggest that a pathway may exist between PSLEs, VLBW, and early indicators of child health, thereby serving as an important first step to understanding an intergenerational link between maternal and child health that has not been previously examined among US populations. Our path analyses allowed for the decomposition of direct and indirect effects of preconception and obstetric factors on early child health, a notable advancement over previous work. Although the effects we found were mostly small and potentially conservative given data limitations, they complement those in other populations by suggesting that women’s preconception stress exposure is associated with a slightly elevated prevalence of poor health in offspring. Future work, utilizing more complete data from prospectively designed studies, is needed to replicate these findings and elucidate the mechanisms by which these processes might occur.
Current approaches to understanding the link between maternal and child health have traditionally focused on women’s health during pregnancy and postpartum, but growing evidence indicates that there may be opportunities to focus research and prevention efforts further upstream. Women experiencing stress before their pregnancies may need more support than is provided in standard care. While PSLEs themselves may not be preventable, making appropriate mental health and social services accessible to vulnerable women could buffer their effect on obstetric outcomes, thus also potentially benefiting the health of their children.
In conclusion, our analyses suggest that women’s exposure to PSLEs not only increases the risk for having a VLBW infant, but may also be associated with children’s future health. Better understanding the intergenerational risk pathways connecting maternal and child health may enhance efforts to reduce children’s health disparities. One strategy – targeting women’s preconception health – would not only address women’s own unmet needs, but may also indirectly benefit the early health of their children. Future research should explore this hypothesis and investigate the extent to which children’s early health outcomes are associated with specific sensitive developmental periods (e.g., maternal adverse childhood events, the immediate preconception period, or the perinatal period). This may offer insight into etiologic mechanisms leading to these outcomes and help pave the way for preventive interventions. Our findings emphasize that adopting an intergenerational lifecourse approach may aid such efforts.
Supplementary Material
What is already known on this subject?
Emerging evidence suggests that women’s exposure to stressful life events prior to conception (PSLEs) may affect the immediate and long-term health of offspring. Such evidence supports a lifecourse approach to understanding health and development. No study has examined the relationship between PSLEs and obstetric and child health outcomes in a nationally-representative sample of US mothers and their children.
What this study adds?
This study provides an empirical application of the lifecourse approach using nationally representative longitudinal data from the US. Using path analyses, we show that women’s exposure to PSLEs may not exert a direct effect on early child health but, via an indirect pathway, place very young children at risk through the increased risk of being born very low birthweight.
References
- 1.Houtrow AJ, Larson K, Olson LM, et al. Changing Trends of Childhood Disability, 2001–2011. Pediatrics. 2014;134:530–8. doi: 10.1542/peds.2014-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu MC, Halfon N. Racial and ethnic disparities in birth outcomes: a life-course perspective. Maternal and child health journal. 2003;7:13–30. doi: 10.1023/a:1022537516969. [DOI] [PubMed] [Google Scholar]
- 3.Halfon N, Hochstein M. Life course health development: an integrated framework for developing health, policy, and research. Milbank Quarterly. 2002;80:433–79. doi: 10.1111/1468-0009.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elder GH., Jr The life course as developmental theory. Child development. 1998;69:1–12. [PubMed] [Google Scholar]
- 5.Pickles A, De Stavola B. An overview of models and methods for life course analysis. In: Pickles A, Maughan B, Wadsworth M, editors. Epidemiological Methods in Life Course Research. Oxford: Oxford University Press; 2007. pp. 181–220. [Google Scholar]
- 6.Kuh D, Ben-Shlomo Y, Lynch J, et al. Life course epidemiology. Journal of epidemiology and community health. 2003;57:778. doi: 10.1136/jech.57.10.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witt WP, Cheng ER, Wisk LE, et al. Maternal stressful life events prior to conception and the impact on infant birth weight in the United States. American Journal of Public Health. 2014;104:S81–S9. doi: 10.2105/AJPH.2013.301544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heron M. Deaths: leading causes for 2008. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2012;60:1–94. [PubMed] [Google Scholar]
- 9.Bhushan V, Paneth N, Kiely JL. Impact of improved survival of very low birth weight infants on recent secular trends in the prevalence of cerebral palsy. Pediatrics. 1993;91:1094–100. [PubMed] [Google Scholar]
- 10.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. The New England journal of medicine. 1985;312:82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- 11.Vohr BR, Wright LL, Dusick AM, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993–1994. Pediatrics. 2000;105:1216–26. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 12.Li J, Olsen J, Vestergaard M, et al. Prenatal Stress Exposure Related to Maternal Bereavement and Risk of Childhood Overweight. PLoS ONE. 2010;5 doi: 10.1371/journal.pone.0011896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Class QA, Khashan AS, Lichtenstein P, et al. Maternal stress and infant mortality: the importance of the preconception period. Psychological science. 2013;24:1309–16. doi: 10.1177/0956797612468010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Early Childhood Longitudinal Study, Birth Cohort, Nine-Month Data Collection. Washington, D.C: U.S. Department of Education, National Center For Education Statistics; 2001. [Google Scholar]
- 15.Witt WP, Wisk LE, Cheng ER, et al. Determinants of Cesarean Delivery in the US: A Lifecourse Approach. Maternal and Child Health Journal. 2014:1–10. doi: 10.1007/s10995-014-1498-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eugster A, Vingerhoets A. Psychological aspects of in vitro fertilization: a review. Social science & medicine. 1999;48:575–89. doi: 10.1016/s0277-9536(98)00386-4. [DOI] [PubMed] [Google Scholar]
- 17.Holmes TH, Rahe RH. The social readjustment rating scale. Journal of Psychosomatic Research. 1967;11:213–8. doi: 10.1016/0022-3999(67)90010-4. [DOI] [PubMed] [Google Scholar]
- 18.Rahe CRH, Arthur DRJ. Life change and illness studies: Past history and future directions. Journal of Human Stress. 1978;4:3–15. doi: 10.1080/0097840X.1978.9934972. [DOI] [PubMed] [Google Scholar]
- 19.Rahe RH, Veach TL, Tolles RL, et al. The stress and coping inventory: an educational and research instrument. Stress Medicine. 2000;16:199–208. [Google Scholar]
- 20.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford Press; 2013. [Google Scholar]
- 21.Khashan AS, McNamee R, Abel KM, et al. Reduced infant birthweight consequent upon maternal exposure to severe life events. Psychosomatic Medicine. 2008;70:688–94. doi: 10.1097/PSY.0b013e318177940d. [DOI] [PubMed] [Google Scholar]
- 22.Khashan AS, McNamee R, Henriksen TB, et al. Risk of affective disorders following prenatal exposure to severe life events: a Danish population-based cohort study. Journal of psychiatric research. 2011;45:879–85. doi: 10.1016/j.jpsychires.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Class QA, Abel KM, Khashan AS, et al. Offspring psychopathology following preconception, prenatal and postnatal maternal bereavement stress. Psychological medicine. 2014;44:71–84. doi: 10.1017/S0033291713000780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Vestergaard M, Obel C, et al. A nationwide study on the risk of autism after prenatal stress exposure to maternal bereavement. Pediatrics. 2009;123:1102–7. doi: 10.1542/peds.2008-1734. [DOI] [PubMed] [Google Scholar]
- 25.Zhu JL, Olsen J, Sorensen HT, et al. Prenatal maternal bereavement and congenital heart defects in offspring: a registry-based study. Pediatrics. 2013;131:e1225–30. doi: 10.1542/peds.2012-3024. [DOI] [PubMed] [Google Scholar]
- 26.Geronimus AT. Black/white differences in the relationship of maternal age to birthweight: a population-based test of the weathering hypothesis. Social science & medicine. 1996;42:589–97. doi: 10.1016/0277-9536(95)00159-x. [DOI] [PubMed] [Google Scholar]
- 27.Dunkel Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annual review of psychology. 2011;62:531–58. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- 28.Wadhwa PD, Culhane JF, Rauh V, et al. Stress and preterm birth: neuroendocrine, immune/inflammatory, and vascular mechanisms. Matern Child Health J. 2001;5:119–25. doi: 10.1023/a:1011353216619. [DOI] [PubMed] [Google Scholar]
- 29.Wadhwa PD, Buss C, Entringer S, et al. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Seminars in reproductive medicine. 2009;27:358–68. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sternthal MJ, Enlow MB, Cohen S, et al. Maternal interpersonal trauma and cord blood IgE levels in an inner-city cohort: a life-course perspective. The Journal of allergy and clinical immunology. 2009;124:954–60. doi: 10.1016/j.jaci.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valero De Bernabe J, Soriano T, Albaladejo R, et al. Risk factors for low birth weight: a review. European journal of obstetrics, gynecology, and reproductive biology. 2004;116:3–15. doi: 10.1016/j.ejogrb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 32.DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children’s health. Pediatrics. 2004;113:1007–15. [PubMed] [Google Scholar]
- 33.Emmerson AJ, Roberts SA. Rounding of birth weights in a neonatal intensive care unit over 20 years: an analysis of a large cohort study. BMJ Open. 2013;3:e003650. doi: 10.1136/bmjopen-2013-003650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edouard L, Senthilselvan A. Observer error and birthweight: digit preference in recording. Public Health. 1997;111:77–9. doi: 10.1016/s0033-3506(97)90004-4. [DOI] [PubMed] [Google Scholar]
- 35.Martin J, Hamilton B, Ventura S, et al. National Vital Statistics Reports. 1. Vol. 61. Hyattsville, MD: National Center for Health Statistics; 2012. Births: Final data for 2010. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.