Abstract
Background
Bactericidal antibiotics have been shown to stimulate reactive oxygen species (ROS) formation in mammalian cells through mitochondrial dysfunction. This results in oxidative tissue damage that may have negative consequences for long-term antibiotic use. Antibiotics are widely and heavily used in the treatment of acute and chronic sinusitis, however the relationship between antibiotics and ROS formation in sinonasal epithelial cells (SNECs) has not yet been demonstrated.
Methods
Human SNECs were collected from patients during endoscopic sinus surgery and grown in culture at the air-liquid interface. Differentiated SNECs were stimulated with the bactericidal antibiotics amoxicillin and levofloxacin and the bacteriostatic antibiotic clarithromycin for 24-hours. Reactive oxygen species were quantified via fluorescence. Cell death was quantified by LDH secretion. Expression of inflammatory markers such as TNF-α and Nrf2 mediated antioxidant genes were measured by RT-PCR.
Results
Cultured SNECs treated with the bactericidal antibiotics amoxicillin and levofloxacin resulted in a significant increase in production of ROS (p<0.05) and secretion of LDH (p<0.05). The increase in ROS formation correlated with an increase in expression of Nrf-2 mediated antioxidant genes as well as the expression and production of pro-inflammatory cytokine TNF- α, and IL-1β (p<0.05). SNECs treated with clarithromycin did not demonstrate statistically significant increases in ROS or pro-inflammatory cytokine production.
Discussion
In this study, we demonstrate that treatment of cultured human SNECs with bactericidal antibiotics leads to formation of ROS with an associated increase in inflammatory and antioxidant gene expression and cell death. This suggests that long term or inappropriate antibiotic use in the treatment of sinusitis, may result in oxidative tissue damage to the sinonasal epithelium. Future studies will explore the clinical implications of such damage to the sinonasal epithelium.
Keywords: Antibiotics, ROS, Rhinosinusitis, TNF, Innate Immunity, Inflammasome
Introduction
Antibiotics are widely used in the treatment of both acute and chronic rhinosinusitis1 (CRS). The relationship between the sinonasal bacterial flora and CRS remains unclear. The bacterial infection or colonization that is often seen in CRS may be the primary inflammatory stimulus or could instead occur secondarily as a result of the pre-existing inflammatory state, perhaps acting only as a disease modifier. Irrespective of the role of bacteria as a causal factor for CRS, antibiotics continue to have a central role in the treatment of active CRS2–4. While the primary role of antibiotics is to reduce the burden of a bacterial infection, some classes of antibiotics have been shown to have non-microbial functions.
Macrolides, quinolones and penicillins are the most common antibiotics used in the treatment of sinusitis5 and a diagnosis of acute or chronic sinusitis accounts for 11% of all antibiotics prescribed in an ambulatory setting6. The macrolide class of antibiotics targets translation in bacteria resulting in a cessation of growth (bacteriostatic). Macrolides also have anti-inflammatory properties thought to be related to the ring structure of this class of drugs that results in the reduction of pro-inflammatory cytokines7. These anti-inflammatory properties have been utilized to guide treatment of lung disease7 and may also be useful in the treatment of recalcitrant CRS8.
Quinolones and penicillins are bactericidal antibiotics – those which kill greater than 99.9% of bacteria. In addition to their classic mechanisms of action, bactericidal antibiotics also promote altered bacterial metabolism, respiration and iron homeostasis that results in reactive oxygen species (ROS) formation that contributes to a portion of the cell death9–16. Expanding upon this common mechanism of cell death, bactericidal antibiotics were also shown to induce reactive oxygen species formation in mammalian cells through mitochondrial dysfunction which resulted in accumulation of damaged DNA, proteins, and lipids that may have long term consequences to human systems17.
In this study, we examined the ability of bactericidal antibiotics to induce ROS formation in cultured human sinonasal epithelial cells (SNECs) from subjects without CRS. In addition, we also examined the ability of antibiotics to stimulate antioxidant production and inflammatory cytokines in SNECs. We also demonstrate that over time, continued treatment with antibiotics may also result in increased epithelial cell death.
Materials and Methods
Human Subjects
Thirteen control subjects were enrolled in the study. The research protocol was approved through our Institutional Review process, and all subjects gave signed informed consent. Mucosal tissue was collected from the ethmoid sinuses during endoscopic sinus surgery and grown in culture at the air-liquid interface (ALI). All control patients were defined as those without CRS who were undergoing endoscopic sinonasal surgery for dacrocystorhinostomy, cerebrospinal fluid leak repair or endoscopic skull base surgery.
The mucosal tissue was transferred to phosphate buffered saline (PBS) supplemented by penicillin (100 µg/mL, Gibco, Gaithersburg, MD), streptomycin (100 µg/mL, Gibco), amphotericin B (2.5 µg/mL, Gibco), and gentamicin (50µg/mL, Gibco). Samples were collected through a cell strainer (BD Falcon) and digested in 4° C overnight in Ham’s F12 media containing 0.01% protease Sigma Type XIV (Sigma, St. Louis, MO) supplemented with antibiotics as above. The cells were separated by straining into a conical tube to which fetal bovine serum (FBS, Sigma) was added to a final concentration of 10% to inactivate the protease. Cells were centrifuged at 1200 rpm for 10 minutes in 4°C, after which the supernatant was aspirated. The washed SNEC were re-suspended in Bronchial Epithelium Growth Medium (BEGM) and seeded at a density ≥ 1.5×104 cells/cm2 in collagen coated 100-mm culture dishes. The media was changed initially 24 hours after the cells were grown, and then every 48 hours until cells reached confluence.
SNEC Culture at the Air-Liquid Interface (ALI)
Confluent cells were washed with HBSS prior to trypsinization, then treated at 37°C for 2 minutes with a solution containing 0.2% Trypsin (Sigma), 1% polyvinylpyrrolidone (Sigma), and 0.02% EGTA (Sigma) in HBSS. The trypsin was then neutralized by the addition of an equal volume of cold soybean trypsin inhibitor at a concentration of 1 mg/mL in Ham’s F12 media. Dissociated cells were washed and re-suspended into BEGM media and plated into human type IV placental collagen (Sigma, Type VI) coated 12-well Falcon filter inserts (0.4-µm pore size; Becton Dickinson, Franklin Lakes, NJ). When confluent, media was removed from above the cultures and the media below the inserts was changed to LHC Basal Medium:DMEM-H (Gibco) (50:50) containing the same concentrations of additives as BEGM with the exception that the concentration of epidermal growth factor was reduced to 0.63 ng/mL, and penicillin, gentamicin, streptomycin and amphotericin B were omitted (ALI media). Each set of SNEC cultures came from a separate patient source and was maintained at the air-liquid interface with the apical surfaces remaining free of medium for at least 3 weeks prior to study. This differentiated cell culture model, with media in the basolateral compartment and air at the apical surface, is an established model for studying sinonasal epithelial cells that closely resembles nasal cavity mucosa18,19.
Treatment of SNECs with Antibiotics and Measurement of Reactive Oxygen Species Formation
SNECs were left untreated (control), or treated with 20 µg/ml clarithromycin, 80 µg/ml levofloxacin, 80 µg/ml amoxicillin, or 0.2% acetone (vehicle control - used to dissolve clarithromycin), respectively for 24 hours. RNA was extracted for qPCR analysis as described below. To measure ROS formation, SNECs at the ALI interface from thirteen patients were trypsinized and transferred to a 96 well plate in antibiotic-free ALI media and allowed to adhere overnight. These cells were then stained with 20uM DCFDA (Abcam, Cambridge, MA) for 45 minutes, washed with 1× PBS, and either left untreated (control) or stimulated with 20 µg/ml clarithromycin, 80 µg/ml levofloxacin, 80 µg/ml amoxicillin, or 0.2% acetone. Fluorescence readings were recorded in triplicate for each patient sample at 24 hours using an excitation wavelength of 485 nm and emission wavelength of 528 nm using a microplate reader.
Real Time Polymerase Chain Reaction (PCR)
Total RNA was extracted from the SNECs cultured from thirteen patients following 24 hours of treatment in the presence or absence of antibiotics using the RNeasy Kit by Qiagen according to the manufacturer’s directions. DNAse I (Qiagen) was used to treat RNA to remove contaminating genomic DNA. RNA concentration was determined by measuring the OD values at 260 nm. cDNA was synthesized from isolated mRNA by reverse transcribing 500 ng of RNA in a reaction volume of 20 µL using random hexamer primers (Invitrogen) and reagents from the Omniscript RT kit (Qiagen).
Real time PCR analysis was performed for each sample in duplicate using the Applied Biosystems StepOnePlus machine (Foster City, CA) under standard cycling parameters for Taqman per the manufacturer recommendations. For all genes of interest, the reaction mixture consisted of 100ng total RNA, Taqman primers (Life Technologies - see Table 1), and Taqman Fast Universal PCR Master Mix (Applied Biosystems) according to manufacturer recommendations. A Corresponding 18S Taqman control was also run using 5ng total RNA according to the manufacturer’s recommendations. Amplicon expression in each sample was normalized to its 18S RNA content, and the level of expression of target mRNA was determined as the delta CT (ΔCT), the difference in threshold cycles for each target and housekeeping gene. All primers were commercially synthesized by Life Technologies (Table 1).
Table 1.
RT-PCR Taqman primers
| Gene | Probe |
|---|---|
| GCLC | Hs00155249_m1 |
| GCLM | Hs00157694_m1 |
| HMOX1 | Hs01110250_m1 |
| NQO1 | Hs00168547_m1 |
| SOD1 | Hs00533490_m1 |
| IL8 | Hs00174103_m1 |
| TNF-α | Hs01113624_g1 |
| p62 | Hs01061917_g1 |
| CASP1 | Hs00354836_m1 |
| IL-1β | Hs01555410_m1 |
| IL18 | Hs01038788_m1 |
| 18S | Hs99999901_s1 |
Cytokine Quantification
TNF-α and IL-1β were quantified in basal media and from SNEC lysates by ELISA using commercially available kits (Abcam, Cambridge MA). Lysates and basal media were collected 48 hours after treatment with antibiotics. Antibiotics were re-dosed in fresh media applied to the basal compartment every 24 hours until samples were collected. Basal media was centrifuged for 1 minute at 15,000 rpm and supernatants were collected and frozen at −80°C. Lysates were incubated on ice with shaking for 15 minutes, then centrifuged for 10 minutes at 13,000 rpm. Supernatants were collected and stored at −80°C. The assay was performed according to manufacturer’s instructions using the provided pre-coated 96-well plates with samples from five patients run in triplicate.
Cell Death (LDH Assay)
1 × PBS was incubated and then collected from the apical surface of SNECs from five patients both with and without antibiotic treatment at both 48 and 96-hour time points. Antibiotics were re-dosed in fresh media applied to the basal compartment every 24 hours until samples were collected. Lactate Dehydrogenase (LDH) release was quantified by using a commercially available kit (Life Technologies) with 50 µL of sample in triplicate in 96-well plates (Corning, New York). Absorbance from each well was read using a microplate reader at 450 nm (signal) and 570 nm (background). Negative control (1 × PBS) and positive control (cell lysates) samples were performed in triplicate for each run.
Statistical Analysis
Raw data from real-time PCR, CM-H2DCFDA assays were entered into a spreadsheet (Excel; Microsoft Corp, Redmond, WA). Statistical analysis was performed using a software program (Excel; Microsoft Corp, Redmond, WA). Data are expressed as mean ± SEM. Statistical significance of differences between the same populations with and without antibiotic treatment was determined using the Student’s paired t-test. Differences between different populations were evaluated by employing the two-tailed t-test for sample means with unequal variance. Differences were considered statistically significant at p<0.05.
Results
Bactericidal Antibiotics Stimulate Reactive Oxygen Species Formation
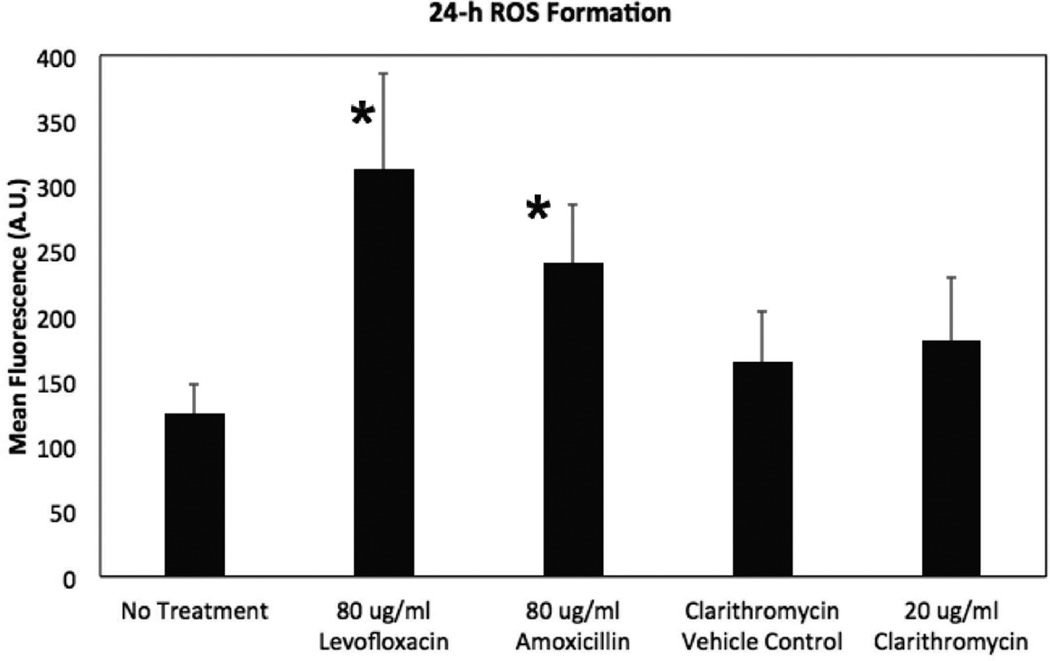
Cultured SNECs from control subjects were left untreated or were treated with 20µg/ml clarithromycin, 80µg/ml levofloxacin or 80µg/ml amoxicillin for 24-hours and ROS formation was quantified using the ROS-sensitive dye DCFDA. There was a significant increase in ROS formation following treatment with either levofloxacin (p = 0.013) or amoxicillin (p = 0.008) whereas treatment with clarithromycin did not lead to a significant increase in ROS formation (Figure 1). These results are consistent with the prior study demonstrating that bactericidal antibiotics stimulate ROS formation in mammalian cells17 and demonstrates that this phenomena also occurs in human sinonasal epithelial cells.
Figure 1.
Bactericidal Antibiotics Stimulate ROS Formation in SNECs. ROS formation was detected using the fluorescent probe CM-H2DCFDA in SNEC after treatment for 24 hours with no antibiotic (No treatment), 0.2% acetone (clarithromycin vehicle Control), 20µg/mL clarithromycin, 80µg/mL amoxicillin, and 80µg/mL levofloxacin, respectively. Shown are mean fluorescence +/− standard error of the mean (s.e.m.). * indicates p <0.05 relative to untreated samples.
Bactericidal Antibiotics Stimulate Expression of Nrf-2-Regulated Antioxidant Genes
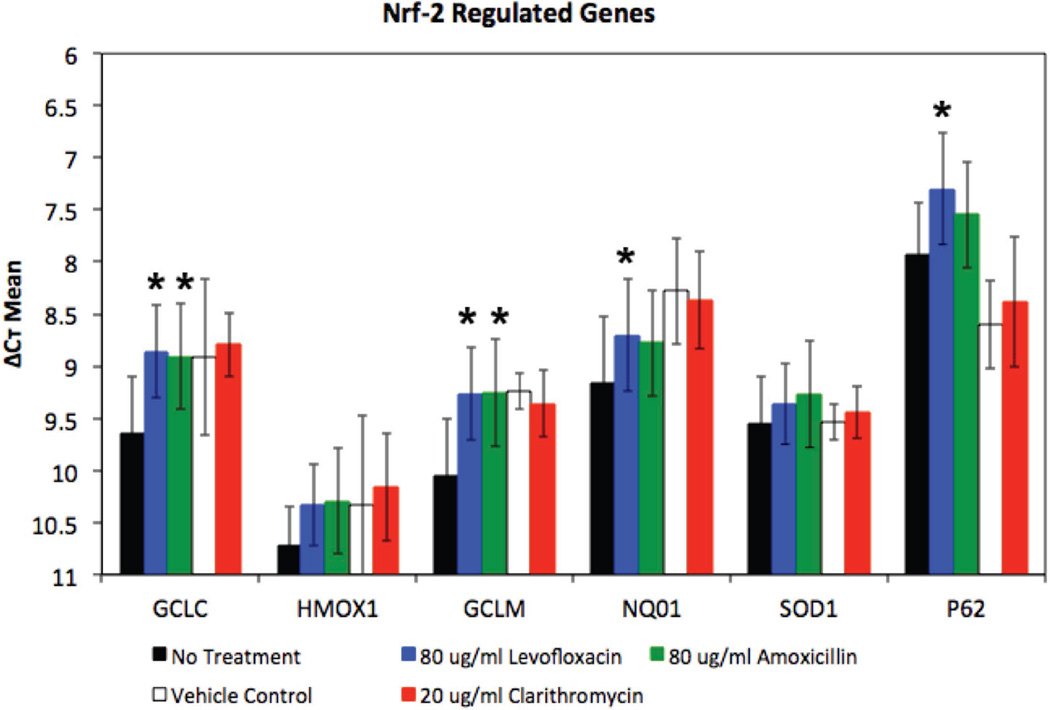
Left unchecked, ROS formation results in oxidative stress and accumulation of DNA, protein and lipid damage that can lead to a breakdown in normal cellular processes17,20. Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor that binds to antioxidant responsive elements and activates expression of systems that can mitigate the damage caused by ROS20,21. We examined expression of Nrf-2-regulated genes GCLC (glutamate-cysteine ligase catalytic), GCLM (glutamate-cysteine ligase modifier), HOX1 (heme oxygenase-1), SOD1 (superoxide dismutase-1), NQO1 (NAD(P)H quinone oxidoreductase-1), and p62 (nucleoporin 62) in SNECs following 24-hours of treatment with no antibiotics, clarithromycin, levofloxacin or amoxicillin (Figure 2).
Figure 2.
Bactericidal Antibiotics Stimulate Expression of Nrf-2-Regulated Genes in SNECs. Expression of Nrf-2-regulated genes, GCLC, GCLM, HMOX1, NQO1, SOD1 and p62 were measured by RT-PCR in SNEC after treatment for 24 hours with no antibiotic (No treatment, black fill rectangles), 0.2% acetone (clarithromycin vehicle Control, No fill rectangles), 20µg/mL clarithromycin (red rectangles), 80µg/mL levofloxacin (blue rectangles), and 80µg/mL amoxicillin (red rectangles), respectively. Shown are mean ΔCt +/− s.e.m. * indicates p <0.05 relative to untreated samples.
Treatment with clarithromycin did not result in a significant increase in Nrf-2-regulated gene expression compared to vehicle control (Table 2, Figure 2). Treatment with amoxicillin resulted in a significant increase in expression for 2 of the 6 tested genes – GCLC (p= 0.024) and GCLM (p= 0.024) (Figure 2, Table 2). Treatment with levofloxacin resulted in a significant increase in expression relative to untreated SNECs for 4 of the 6 tested genes - Glutamate-Cysteine ligase genes [GCLC (p = 0.001), GCLM (p = 0.005)], NQO1 (p = 0.004) and p62 (p = 0.005) (Table 2, Figure 2). It is possible that the increased production of ROS following levofloxacin treatment compared to amoxicillin treatment (Figure 1), may account for the increased expression of Nrf-2-regulated genes with levofloxacin compared to amoxicillin. These data suggest that bacteriostatic antibiotics do not stimulate antioxidant gene expression and that bactericidal antibiotics which stimulate ROS production can stimulate expression of some Nrf-2-regulated antioxidant genes.
Table 2.
Gene expression changes following treatment with no antibiotic (no treatment), 80µg/ml levofloxacin, and 80µg/ml amoxicillin, respectively. Shown are average ΔCt, standard error (s.e.) ΔCt, and p-values comparing levofloxacin or amoxicillin treatment to no treatment. Statistically significant p-values highlighted gray.
| No Treatment |
80 µg/ml Levofloxacin | 80 µg/ml Amoxicillin | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | ΔCt mean | ΔCt s.e.m. | ΔCt mean | ΔCt s.e | p- value |
ΔCt mean | ΔCt s.e. | p- value |
|||
| GCLC | 9.64 | 0.54 | 8.86 | 0.45 | 0.001 | 8.91 | 0.68 | 0.024 | |||
| GCLM | 10.06 | 0.55 | 9.27 | 0.44 | 0.005 | 9.26 | 0.63 | 0.024 | |||
| HMOX1 | 10.72 | 0.37 | 10.34 | 0.39 | 0.423 | 10.30 | 0.49 | 0.268 | |||
| NQO1 | 9.16 | 0.63 | 8.70 | 0.54 | 0.004 | 8.78 | 0.76 | 0.125 | |||
| SOD1 | 9.55 | 0.45 | 9.36 | 0.39 | 0.596 | 9.27 | 0.49 | 0.405 | |||
| p62 | 7.93 | 0.50 | 7.30 | 0.53 | 0.005 | 7.55 | 0.51 | 0.275 | |||
| IL-8 | 7.42 | 0.62 | 7.91 | 0.51 | 0.235 | 7.23 | 0.60 | 0.258 | |||
| TNF | 34.94 | 3.39 | 25.90 | 4.12 | 0.037 | 20.45 | 3.86 | 0.002 | |||
| CASP1 | 8.64 | 0.29 | 8.16 | 0.30 | 0.006 | 8.10 | 0.31 | 0.019 | |||
| IL1B | 9.99 | 0.42 | 9.04 | 0.53 | 0.0002 | 8.87 | 0.40 | 0.0002 | |||
| IL18 | 11.99 | 0.74 | 11.43 | 0.89 | 0.013 | 11.50 | 0.75 | 0.004 | |||
Bactericidal Antibiotics Stimulate expression of Pro-inflammatory Genes in SNECs
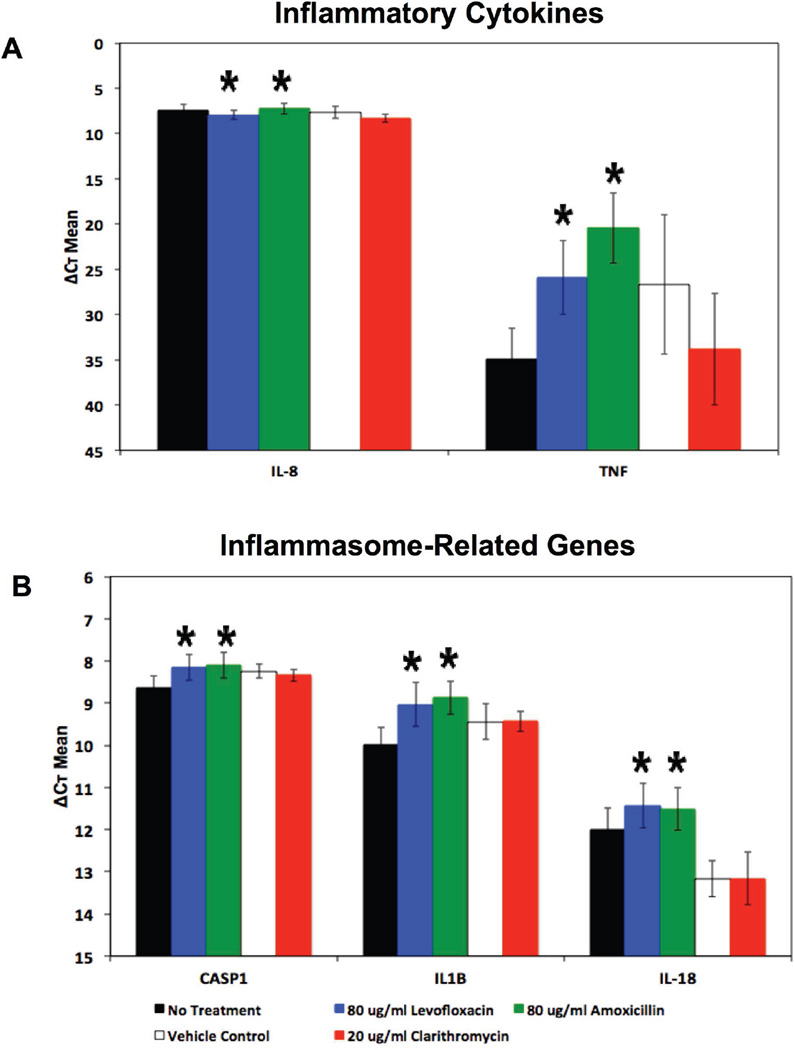
Tumor Necrosis Factor (TNF-α) and Interleukin-8 (IL-8) are important mediators of inflammation and play a role in the early recruitment of neutrophils and other lymphocytes in response to bacterial infection of mucosal surfaces22,23, and varying levels of these cytokines has been demonstrated in tissue and mucus from those with CRS24. Previous work demonstrated an increase in expression of these mediators as a result of infection of cultured SNECs with S. aureus25. To explore if antibiotic-mediated ROS formation promotes a pro-inflammatory state, we looked at the expression of TNF-α and IL-8 in SNECs following 24-hours of treatment with no antibiotics, clarithromycin, levofloxacin or amoxicillin (Figure 3A). Neither clarithromycin nor the bactericidal antibiotics tested resulted in a significant increase in IL-8 expression (Figure 3A, Table 2, Table 3). There was a significant increase in TNF-α expression following treatment with the bactericidal antibiotics, levofloxacin (p = 0.013) and amoxicillin (p = 0.004), but not with the bacteriostatic antibiotic, clarithromycin (Figure 3A, Table 2).
Figure 3.
Bactericidal Antibiotics Stimulate Expression of Pro-Inflammatory Genes in SNECs. Expression of inflammatory markers (A) TNF-α, IL-8, and (B) IL-1β, IL-18 and CASP1 were measured by RT-PCR in SNEC after treatment for 24 hours with no antibiotic (No treatment, black fill rectangles), 0.2% acetone (clarithromycin vehicle Control, No fill rectangles), 20µg/mL clarithromycin (red rectangles), 80µg/mL levofloxacin (blue rectangles), and 80µg/mL amoxicillin (red rectangles), respectively. Shown are mean ΔCt +/− s.e.m. * indicates p <0.05 relative to untreated samples.
Table 3.
Gene expression changes following treatment with 0.2% acetone (Clarithromycin Vehicle Control), and 20µg/ml clarithromycin, respectively. Shown are average ΔCt, standard error (s.e.) ΔCt, and p-values comparing clarithromycin treatment to clarithromycin vehicle control treatment. Statistically significant p-values highlighted gray.
| Vehicle Control | 20 µg/ml Clarithromycin | ||||
|---|---|---|---|---|---|
| Gene | ΔCt mean | ΔCt s.e. | ΔCt mean | ΔCt s.e. |
p- value |
| GCLC | 8.91 | 0.75 | 8.80 | 0.30 | 0.81 |
| GCLM | 9.24 | 0.17 | 9.36 | 0.32 | 0.30 |
| HMOX1 | 10.34 | 0.86 | 10.16 | 0.51 | 0.64 |
| NQO1 | 8.28 | 0.51 | 8.37 | 0.47 | 0.52 |
| SOD1 | 9.53 | 0.17 | 9.44 | 0.25 | 0.44 |
| p62 | 8.60 | 0.43 | 8.39 | 0.62 | 0.24 |
| IL-8 | 7.66 | 0.65 | 8.31 | 0.45 | 0.32 |
| TNF | 26.66 | 7.71 | 33.84 | 6.16 | 0.28 |
| CASP1 | 8.24 | 0.17 | 8.34 | 0.14 | 0.45 |
| IL1B | 9.44 | 0.42 | 9.43 | 0.24 | 0.87 |
| IL18 | 13.17 | 0.25 | 13.17 | 0.05 | 0.67 |
Inflammasomes are a part of the innate immune response and consist of protein complexes containing a sensor protein, an adaptor protein (ASC) and caspase 1. These complexes can sense cellular stress from internal and external sources and activate a pro-inflammatory state through activation of IL-1β and IL-1826. We measured expression of IL-1β, IL-18 and caspase-1 (CASP1) in SNECs following 24-hours of treatment with no antibiotics, clarithromycin, levofloxacin or amoxicillin (Figure 3B). Treatment with clarithromycin did not result in a significant increase in expression of IL-1β, IL-18 or caspase-1 (Figure 3B, Table 3). Treatment of SNECs with levofloxacin or amoxicillin resulted in a significant increase in expression of IL-1β (p= 0.0002, p = 0.0002), IL-18 (p= 0.013, p = 0.004) and caspase-1 (p= 0.006, p = 0.01) (Figure 3B, Table 2). These results suggest that treatment of SNECs with bactericidal antibiotics promotes a pro-inflammatory state.
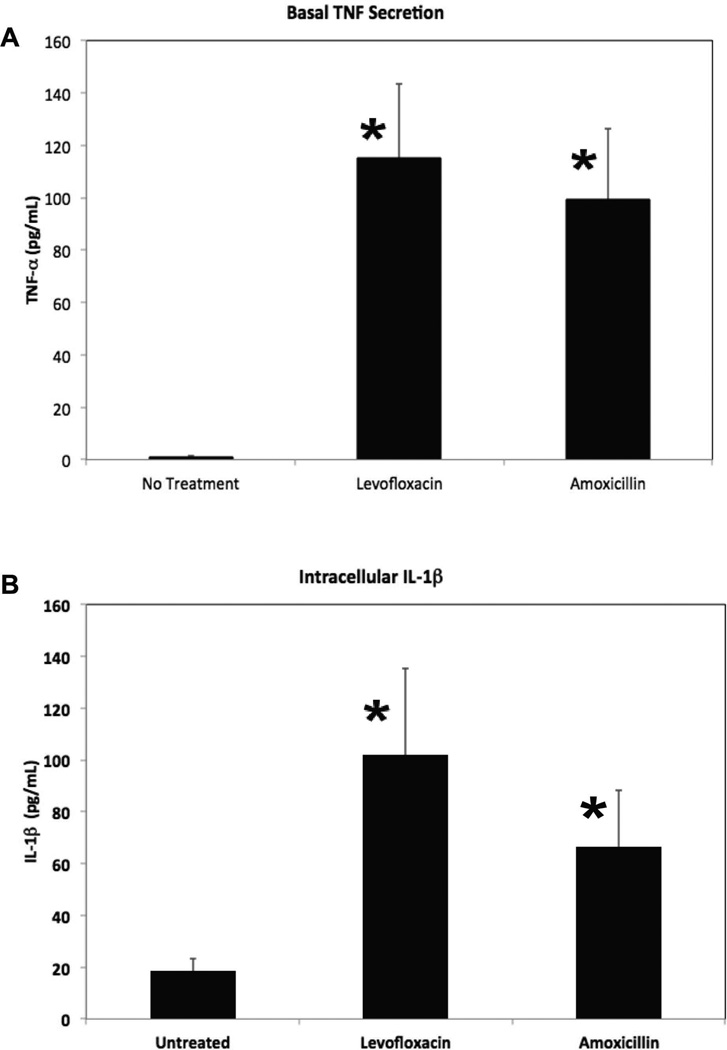
We next measured basal secretion of TNF-α as well as intracellular levels of the mature form of IL-1β after 48-hours of treatment with levofloxacin or amoxicillin to determine if treatment with bactericidal antibiotics triggers an increase in production of these inflammatory proteins. We found that there was a significant increase in basal secretion of TNF-α by SNECs following treatment with either levofloxacin (115 pg/mL, p=0.01) or amoxicillin (99.5 pg/mL, p = 0.01) compared to untreated (0.9 pg/mL) SNECs (Figure 4A). We also found a significant increase in intracellular levels of IL-1β in SNEC following treatment with levofloxacin (102 pg/mL, p = 0.03) or amoxicillin (67 pg/mL, p = 0.04) compared to untreated (18 pg/mL) SNECs (Figure 4B).
Figure 4.
Bactericidal Antibiotics Stimulate Production of Pro-Inflammatory Proteins in SNECs. (A) Basal secretion of TNF-α was quantified by ELISA in SNEC after 48 hours of treatment with no antibiotic (No treatment), 80µg/mL levofloxacin, and 80µg/mL amoxicillin, respectively. (B) Intracellular concentration of IL-1β was quantified by ELISA in SNEC after 48 hours of treatment with no antibiotic (No treatment), 80µg/mL levofloxacin, and 80µg/mL amoxicillin, respectively. Shown are mean concentration (pg/mL) +/− s.e.m. of TNF-α and IL-1β, respectively. *indicates p<0.05 relative to untreated samples.
SNEC cell death following treatment with Bactericidal Antibiotics
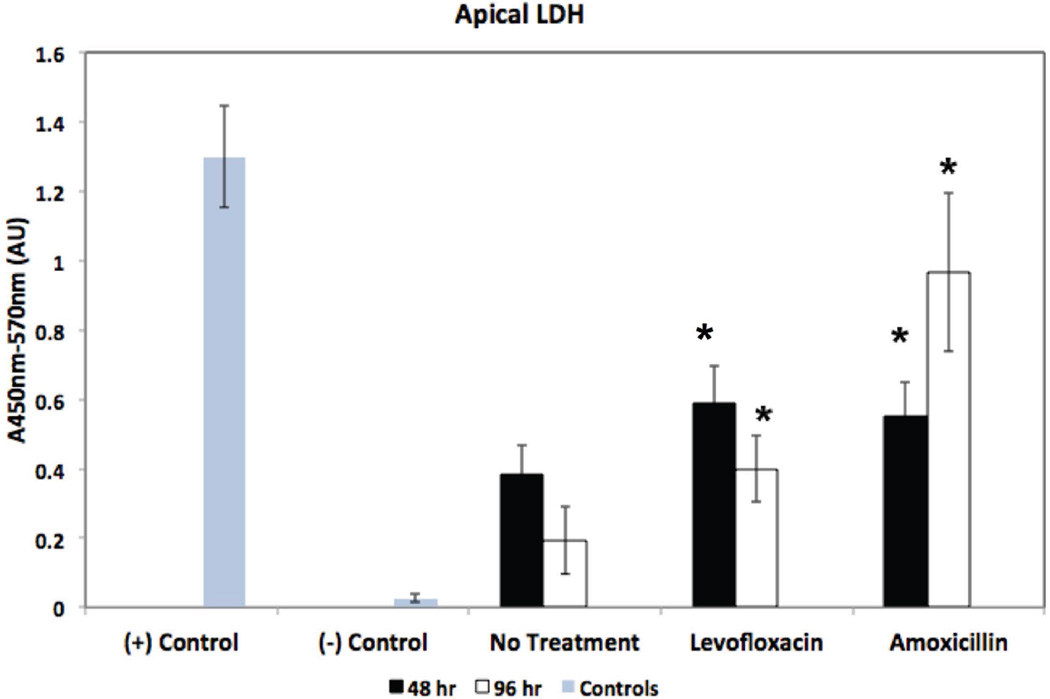
In addition to their pro-inflammatory properties, both TNF-α and Il-1β are associated with cell death. TNF-α can activate pro-apoptotic pathways27 and production and release of mature Il-1β may occur when the permeability of the cell membrane is increased, potentially triggering cell necrosis28. To determine if bactericidal antibiotic treatment is also associated with cell death, we measured excretion of LDH by SNECs - LDH is released by dying mammalian cells and has been used as a marker of cytotoxicity. We measured apical excretion of LDH in SNECs that were untreated or treated with 80µg/ml levofloxacin, or 80µg/ml amoxicillin for 48 and 96-hours, respectively (Figure 5). We found a significant increase in LDH secretion for both levofloxacin and amoxicillin-treated SNEC compared to untreated SNEC at both 48-hours and 96-hours (Figure 5). These data suggest that bactericidal antibiotic treatment are associated with SNEC cell death.
Figure 5.
Bactericidal Antibiotic treatment decreases viability of human SNECs. SNEC viability as measured by apical LDH secretion (Absorbance 450nm–570nm) after 48-hours (black bars) and 96-hours (white bars), respectively, of treatment with no antibiotic (no treatment), 80µg/ml levofloxacin or 80µg/ml amoxicillin. Gray bars indicate LDH measured from lysed cells [positive control, (+) control], and LDH measured in 1× PBS [ negative control, (−) control]. Shown are mean values +/− s.e.m. *indicates p<0.05 relative to untreated samples at respective time-point.
Discussion
The use have antibiotics in sinus disease has been steadily increasing with a recent study showing that antibiotics are prescribed in over 80% of visits for acute sinusitis5 and a diagnosis of acute or chronic sinusitis accounts for 11% of all antibiotics prescribed in an ambulatory setting6. Since most acute sinus infections tend to be viral, widespread antibiotic use early in the course of sinusitis may lead to microbial resistance and put patients at risk for adverse effects of antibiotics such as colitis, nephrotoxicity, and tendinopathies in the case of fluoroquinolones29. Recent studies by Kalghatgi, et al. have demonstrated that bactericidal antibiotics can induce mitochondrial dysfunction and oxidative tissue damage in various mammalian cell types17. Previous studies, however, have not explored whether bactericidal antibiotics may cause oxidative tissue damage to the sinonasal epithelium.
In this study, we demonstrate for the first time that treatment of cultured human SNECs with bactericidal antibiotics leads to formation of ROS with an associated increase in inflammatory and antioxidant gene expression and protein production. Treatment with the bacteriostatic antibiotic, clarithromycin did not result in ROS formation or up-regulation of pro-inflammatory or antioxidant gene expression. The resultant exposure of mammalian cells to bactericidal antibiotics can lead to the accumulation of damaged DNA, proteins and lipids which may have deleterious consequences17.
In this manuscript, we used an epithelial culture-based system to test that treatment with bactericidal antibiotics stimulates human SNECs to generate ROS, upregulate antioxidant defense systems, increase production of inflammatory cytokines and increase cellular cytotoxicity.
Mitochondria are the major source of ROS formation in mammalian cells30 and are the source of ROS formation following treatment with bactericidal antibiotics17. Mitochondria-derived ROS can activate the NLRP-3 inflammasome31 thereby stimulating an inflammatory response through cleavage of pro-IL-1β and pro-IL-18 by Caspase 126. In the lower respiratory system, activation of NLRP-3 has been associated with both the ability to mount a strong host response against viral lung infection as well as chronic inflammation of the lung such as seen in pulmonary fibrosis32. The increased expression of TNF-α, IL-1β, IL-18 and caspase 1 and the increased secretion of TNF-α and production of mature Il-1β following treatment of SNECs with bactericidal antibiotics together with the increase in ROS formation also suggests that the sinonasal epithelial cells may be primed by bactericidal antibiotics toward a pro-inflammatory state.
In addition to their roles in inflammation, both TNF-α and IL-1β are also associated with activation of pro-apoptotic or necrosis death pathways27,28. In this study, we also demonstrate that treatment with levofloxacin and amoxicillin for 48 and 96-hours is cytotoxic (as measured by LDH secretion) to human SNECs compared to untreated SNECs (Figure 5). There is no causal link at this time between antibiotic-driven ROS formation and cell death in cultured human SNECs, and future studies will explore this relationship in terms of varying antibiotic concentrations, duration of treatment as well as working to further elucidate the inflammatory/death pathways stimulated by bactericidal antibiotics. The clinical relevance of this model remains un-explored at this time, and further efforts to understand bactericidal antibiotic-mediated ROS formation may give us insight into the potential clinical relevance of this model, including possible deleterious side effects to sinonasal epithelial tissue from the long-term or inappropriate use of antibiotics in the treatment of sinusitis.
ROS formation by bactericidal antibiotics also resulted in increased expression of some antioxidant genes regulated by Nrf2 (Figure 2). It is possible that pre-treatment with compounds that activate the Nrf2 system prior to treatment with bactericidal antibiotics could protect against the deleterious properties of ROS while maintaining antimicrobial efficacy.
In future studies, it will be interesting to explore how the basal inflammatory differences among SNECs from control, CRS without polyps, and CRS with polyp patients may influence the formation of ROS and the activation of inflammatory pathways following stimulation with bactericidal antibiotics.
While the potential consequences of bactericidal antibiotic-mediated ROS accumulation have yet to be demonstrated in SNECs from those with CRS, the use of repetitive and extended courses of bactericidal antibiotics as part of maximal medical therapy may lead to long-term damage to the sinonasal mucosa in these patients. In particular, this should be considered when treating CRS with nasal polyp patients where the underlying disease state is believed to be propagated by a Th2-biased cytokine milieu33,34.
Conclusions
Treatment of sinonasal epithelial cells with bactericidal antibiotics results in ROS formation, cell death, and up-regulation of antioxidant defense systems as well as pro-inflammatory mediators. These changes are not seen with the bacteriostatic antibiotic clarithromycin. The results from this tissue culture system suggests a potentially clinically relevant model whereby long-term or inappropriate antibiotic use in the treatment of sinusitis, may result in oxidative tissue damage to the sinonasal epithelium. Future studies will explore the clinical implications of such damage to the sinonasal epithelium.
Acknowledgments
Research supported by NIH AI072502 (A.P.L.). and NIH ES020859(M.R.)
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Presented at the Spring Meeting of the American Rhinologic Society, April 24, 2015, Boston, MA.
References
- 1.Bhattacharyya N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the United States. The Annals of otology, rhinology, and laryngology. 2011;120:423–427. doi: 10.1177/000348941112000701. [DOI] [PubMed] [Google Scholar]
- 2.Lund VJ. Maximal medical therapy for chronic rhinosinusitis. Otolaryngol Clin North Am. 2005;38:1301–1310. x. doi: 10.1016/j.otc.2005.07.003. doi:S0030-6665(05)00093-9 [pii] 10.1016/j.otc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Mandal R, Patel N, Ferguson BJ. Role of antibiotics in sinusitis. Curr Opin Infect Dis. 2012;25:183–192. doi: 10.1097/QCO.0b013e328350f728. [DOI] [PubMed] [Google Scholar]
- 4.Soler ZM, et al. Antimicrobials and chronic rhinosinusitis with or without polyposis in adults: an evidenced-based review with recommendations. International forum of allergy & rhinology. 2013;3:31–47. doi: 10.1002/alr.21064. [DOI] [PubMed] [Google Scholar]
- 5.Fairlie T, Shapiro DJ, Hersh AL, Hicks LA. National trends in visit rates and antibiotic prescribing for adults with acute sinusitis. Archives of internal medicine. 2012;172:1513–1514. doi: 10.1001/archinternmed.2012.4089. [DOI] [PubMed] [Google Scholar]
- 6.Smith SS, et al. National burden of antibiotic use for adult rhinosinusitis. J Allergy Clin Immunol. 2013;132:1230–1232. doi: 10.1016/j.jaci.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanoh S, Rubin BK. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clinical microbiology reviews. 2010;23:590–615. doi: 10.1128/CMR.00078-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soler ZM, Smith TL. What is the role of long-term macrolide therapy in the treatment of recalcitrant chronic rhinosinusitis? The Laryngoscope. 2009;119:2083–2084. doi: 10.1002/lary.20739. [DOI] [PubMed] [Google Scholar]
- 9.Dwyer DJ, et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E2100–2109. doi: 10.1073/pnas.1401876111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 11.Brynildsen MP, Winkler JA, Spina CS, MacDonald IC, Collins JJ. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nature biotechnology. 2013;31:160–165. doi: 10.1038/nbt.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dwyer DJ, Camacho DM, Kohanski MA, Callura JM, Collins JJ. Antibiotic-induced bacterial cell death exhibits physiological and biochemical hallmarks of apoptosis. Molecular cell. 2012;46:561–572. doi: 10.1016/j.molcel.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dwyer DJ, Kohanski MA, Hayete B, Collins JJ. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Molecular systems biology. 2007;3:91. doi: 10.1038/msb4100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science. 2012;336:315–319. doi: 10.1126/science.1219192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kohanski MA, DePristo MA, Collins JJ. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Molecular cell. 2010;37:311–320. doi: 10.1016/j.molcel.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohanski MA, Dwyer DJ, Wierzbowski J, Cottarel G, Collins JJ. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell. 2008;135:679–690. doi: 10.1016/j.cell.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kalghatgi S, et al. Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in Mammalian cells. Science translational medicine. 2013;5:192ra185. doi: 10.1126/scitranslmed.3006055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramanathan M, Jr, Turner JH, Lane AP. Technical advances in rhinologic basic science research. Otolaryngol Clin North Am. 2009;42:867–881. x. doi: 10.1016/j.otc.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramanathan M, Jr, Lane AP. A comparison of experimental methods in molecular chronic rhinosinusitis research. American journal of rhinology. 2007;21:373–377. doi: 10.2500/ajr.2007.21.3034. [DOI] [PubMed] [Google Scholar]
- 20.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annual review of pharmacology and toxicology. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain A, et al. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. The Journal of biological chemistry. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckmann L, Kagnoff MF, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khair OA, et al. Effect of Haemophilus influenzae endotoxin on the synthesis of IL-6, IL-8, TNF-alpha and expression of ICAM-1 in cultured human bronchial epithelial cells. Eur Respir J. 1994;7:2109–2116. doi: 10.1183/09031936.94.07122109. [DOI] [PubMed] [Google Scholar]
- 24.Oyer SL, Mulligan JK, Psaltis AJ, Henriquez OA, Schlosser RJ. Cytokine correlation between sinus tissue and nasal secretions among chronic rhinosinusitis and controls. The Laryngoscope. 2013;123:E72–E78. doi: 10.1002/lary.24305. [DOI] [PubMed] [Google Scholar]
- 25.Kohanski MA, Lane AP. Sinonasal Epithelial Cell Response to Staphylococcus aureus Burden in Chronic Rhinosinusitis. JAMA otolaryngology-- head & neck surgery. 2015 doi: 10.1001/jamaoto.2014.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nature reviews. Immunology. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikoletopoulou V, Markaki M, Palikaras K, Tavernarakis N. Crosstalk between apoptosis, necrosis and autophagy. Biochimica et biophysica acta. 2013;1833:3448–3459. doi: 10.1016/j.bbamcr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Cullen SP, Kearney CJ, Clancy DM, Martin SJ. Diverse Activators of the NLRP3 Inflammasome Promote IL-1beta Secretion by Triggering Necrosis. Cell reports. 2015;11:1535–1548. doi: 10.1016/j.celrep.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Poetker DM, Smith TL. What rhinologists and allergists should know about the medico-legal implications of antibiotic use: a review of the literature. International forum of allergy & rhinology. 2015;5:104–110. doi: 10.1002/alr.21433. [DOI] [PubMed] [Google Scholar]
- 30.Turrens JF. Mitochondrial formation of reactive oxygen species. The Journal of physiology. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 32.De Nardo D, De Nardo CM, Latz E. New insights into mechanisms controlling the NLRP3 inflammasome and its role in lung disease. The American journal of pathology. 2014;184:42–54. doi: 10.1016/j.ajpath.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ramanathan M, Jr, Lane AP. Innate immunity of the sinonasal cavity and its role in chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2007;136:348–356. doi: 10.1016/j.otohns.2006.11.011. doi:S0194-5998(06)03446-2 [pii] 10.1016/j.otohns.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Hamilos DL, et al. Evidence for distinct cytokine expression in allergic versus nonallergic chronic sinusitis. J Allergy Clin Immunol. 1995;96:537–544. doi: 10.1016/s0091-6749(95)70298-9. doi:S0091674995002661 [pii] [DOI] [PubMed] [Google Scholar]