Abstract
Rationale
There is suggestive evidence that the neural mechanisms mediating one-trial and multi-trial behavioral sensitization differ, especially when the effects of various classes of dopamine (DA) agonists are examined.
Objective
The purpose of the present study was to determine the role of the D2 receptor for the induction of one-trial and multi-trial methamphetamine sensitization in preweanling rats.
Methods
In a series of experiments, rats were injected with saline or raclopride (a selective D2 receptor antagonist), either alone or in combination with SCH23390 (a selective D1 receptor antagonist), 15 min prior to treatment with the indirect DA agonist methamphetamine. Acute control groups were given two injections of saline. This pretreatment regimen occurred on either postnatal days (PD) 13–16 (multi-trial) or PD 16 (one-trial). On PD 17, rats were challenged with methamphetamine and locomotor sensitization was determined.
Results
Blockade of D2 or D1/D2 receptors reduced or prevented, respectively, the induction of multi-trial methamphetamine sensitization in young rats, while the same manipulations had minimal effects on one-trial behavioral sensitization.
Conclusions
DA antagonist treatment differentially affected the methamphetamine-induced sensitized responding of preweanling rats depending on whether a one-trial or multi-trial procedure was used. The basis for this effect is uncertain, but there was some evidence that repeated DA antagonist treatment caused nonspecific changes that produced a weakened sensitized response. Importantly, DA antagonist treatment did not prevent the one-trial behavioral sensitization of preweanling rats. The latter result brings into question whether DA receptor stimulation is necessary for the induction of psychostimulant-induced behavioral sensitization during early ontogeny.
Keywords: Behavioral sensitization, Methamphetamine, Raclopride, SCH23390, Ontogeny
Introduction
Behavioral sensitization is a form of neural plasticity in which repeated treatment with certain psychoactive compounds (e.g., cocaine, methamphetamine, or apomorphine) causes an augmented behavioral response after a challenge injection with the same drug (Robinson and Becker 1986). The neural mechanisms underlying behavioral sensitization have been studied intensively (for reviews, see Pierce and Kalivas 1997; Vanderschuren and Kalivas 2000), yet it remains uncertain whether D1 and/or D2 receptor stimulation is necessary for the induction (i.e., development) of behavioral sensitization (e.g., see White et al. 1998).
Complicating interpretation are studies showing that the dopamine (DA) receptor subtypes underlying the induction process differ in importance depending on both the sensitizing agent being used and the number of drug-environment pairings (i.e., whether the pretreatment phase consists of single or multiple drug exposures). For example, selective D1 and D2 receptor antagonists do not block the induction of multi-trial cocaine-induced behavioral sensitization in adult rats and mice (Kuribara and Uchihashi 1993; Mattingly et al. 1994; White et al. 1998); whereas, the induction of one-trial cocaine sensitization is prevented by both D1 (Fontana et al. 1993; Valjent et al. 2010) and D2 receptor antagonists (Weiss et al. 1989; Fontana et al. 1993; but see Valjent et al. 2010). In contrast, the sensitized responding evident after repeated daily injections of amphetamine-like compounds is prevented by pretreatment administration of either a D1 or D2 receptor antagonist (Kuczenski and Leith 1981; Kuribara and Uchihashi 1993, 1994; Kuribara 1995a; Kelly et al. 2008; but see Vezina 1996). In adult animals, studies assessing methamphetamine and amphetamine sensitization have almost exclusively relied on the multi-trial procedure.
Young rats also exhibit one-trial and multi-trial behavioral sensitization, but the sensitized responding of preweanling rats differs from adults in some key respects (for a review, see Tirelli et al. 2003). Most importantly, the sensitized responding of preweanling rats appears to be weaker and less persistent than in older animals (Smith and Morrell 2008; McDougall et al. 2009b). As with adults, the multi-trial behavioral sensitization of preweanling rats is stronger if drug pretreatment and testing occur in the same environmental context (i.e., context-dependent sensitization) (Wood et al. 1998; Zavala et al. 2000). Unlike adults, however, the one-trial behavioral sensitization of preweanling rats is context-independent (McDougall et al. 2009b; Herbert et al. 2010; Kozanian et al. 2012); whereas, the one-trial behavioral sensitization of adult rats is completely context-dependent (Weiss et al. 1989; Jackson and Nutt 1993; Battisti et al. 1999a, b). The relative importance of associative learning processes may explain why adult rats show a sensitized response for many months after a single exposure to a psychostimulant (Leith and Kuczenski 1982; Robinson et al. 1982; Valjent et al. 2010), while the one-trial behavioral sensitization of preweanling rats persists for only a few days (McDougall et al. 2009a).
When considered together, these age-dependent differences in sensitized responding may be reflective of ontogenetic changes in the neural mechanisms underlying behavioral sensitization. Few studies have examined the receptor systems mediating behavioral sensitization in preweanling rats (for an exception, see Duke et al. 1997), but those that have report some interesting ontogenetic differences. For example, pretreatment with the D1 receptor antagonist SCH23390 does not prevent the methamphetamine- or cocaine-induced one-trial behavioral sensitization of preweanling rats (Mohd-Yusof et al. 2014); whereas, SCH23390 blocks the one-trial behavioral sensitization of cocaine-treated adults (Fontana et al. 1993; Valjent et al. 2010). The importance of the D2 receptor for the one-trial and multi-trial behavioral sensitization of preweanling rats has not been assessed.
The purpose of the present study was to determine whether pretreating young rats with the D2 receptor antagonist raclopride, or a cocktail of raclopride+SCH23390, would prevent the induction of one-trial or multi-trial methamphetamine sensitization. On postnatal day (PD) 16 or PD 13–16, rats received daily pretreatment injections of the DA antagonist(s) 15 min prior to methamphetamine administration. Locomotor sensitization was assessed on PD 17. In a separate experiment, a one-trial procedure was conducted as just described, with the exception that rats were injected with D1 and/or D2 receptor antagonists in their home cage on PD 13–15. This general methodology (i.e., administering antagonists 15 min prior to DA agonist treatment and testing rats 24 hr later) is similar to studies reported by Fontana et al. (1993) and Mohd-Yusof et al. (2014). Lower doses of DA agonists were administered on the test day, relative to the pretreatment day, in order to preferentially induce locomotor activity rather than stereotypy (for a fuller discussion, see Robinson and Becker 1986).
Materials and methods
Subjects
Subjects were 168 (n = 8 subjects per group) young male and female rats of Sprague-Dawley descent (Charles River, Hollister, CA) that were born and raised at California State University, San Bernardino (CSUSB). Litters were culled to 10 pups on PD 3. Rats were housed in large polycarbonate maternity cages (30.5 × 43 × 19 cm) on a ventilated rack. Food and water were freely available. The colony room was maintained at 22–23°C and kept under a 12:12 light/dark cycle. Except during testing, rats were kept with the dam and littermates. Testing was done in a separate experimental room and was conducted during the light phase of the cycle. Subjects were cared for according to the “Guide for the Care and Use of Laboratory Animals” (National Research Council 2010) under a research protocol approved by the Institutional Animal Care and Use Committee of CSUSB.
Apparatus
Behavioral testing was done in activity monitoring chambers (25.5 × 25.5 × 41 cm) that consisted of acrylic walls, a plastic floor, and an open top (Coulbourn Instruments, Whitehall, PA). Each chamber included an X–Y photobeam array, with 16 photocells and detectors, that was used to determine distance traveled (a measure of locomotor activity).
Drugs
(+)-Methamphetamine hydrochloride, S(−)-raclopride (+)-tartrate salt, and R(+)-SCH23390 hydrochloride were dissolved in saline. Drugs were purchased from Sigma-Aldrich (St. Louis, MO) and injected intraperitoneally (IP) at a volume of 5 ml/kg.
Procedure
Experiment 1: Effects of D2 receptor blockade on methamphetamine-induced multi-trial behavioral sensitization
During the pretreatment phase (PD 13–16), rats were injected with raclopride (0, 0.1, 0.5, or 1 mg/kg) followed, 15 min later, by an injection of 4 mg/kg methamphetamine. Rats in the acute control groups were given two injections of saline. After the second injection, rats were placed in activity chambers and distance traveled was measured for 30 min. On the test day (PD 17), all rats (N = 40) were injected with 2 mg/kg methamphetamine and placed in activity chambers for 90 min. These doses of methamphetamine produce robust one-trial behavioral sensitization in preweanling rats (Kozanian et al. 2012).
Experiment 2: Effects of D1/D2 receptor blockade on methamphetamine-induced multi-trial behavioral sensitization
The procedures for this experiment are essentially the same as those described for Experiment 1, with the exception that during the pretreatment phase (PD 13–16) rats were injected with either saline or a cocktail of raclopride+SCH23390 (0.5 mg/kg each). After 15 min, rats were injected with 4 mg/kg methamphetamine (acute controls received a second saline injection) and behavior was assessed for 30 min. On the test day (PD 17), all rats (N = 24) were injected with 2 mg/kg methamphetamine and placed in activity chambers for 90 min.
Experiment 3: Effects of D2 receptor blockade on methamphetamine-induced one-trial behavioral sensitization
On the pretreatment day (PD 16), rats were injected with raclopride (0, 0.1, 0.5, or 1 mg/kg) followed, 15 min later, by an injection of 4 mg/kg methamphetamine. Rats in the acute control group were given two injections of saline. On the test day (PD 17), all rats (N = 40) were injected with 2 mg/kg methamphetamine. Behavioral testing on the pretreatment and test days was the same as described in Experiments 1 and 2.
Experiment 4: Effects of D1/D2 receptor blockade on methamphetamine-induced one-trial behavioral sensitization
The procedures for this experiment are the same as those described for Experiment 2, with the exception that the pretreatment phase occurred on a single day (PD 16). Specifically, rats (N = 24) were injected with saline or a cocktail of raclopride+SCH23390 (0.5 mg/kg each) followed, 15 min later, by an injection of 4 mg/kg methamphetamine. Rats in the acute control groups were given two injections of saline. Distance traveled was measured for 30 min. The test day was the same as described in Experiments 1–3.
Experiment 5: Effects of raclopride and SCH23390 preinjections on methamphetamine-induced one-trial behavioral sensitization
An inherent concern when screening antagonist compounds using a multi-trial sensitization procedure is that the DA antagonist must be given repeatedly across the pretreatment phase. In addition to transiently blocking the acute effects of agonist drugs, repeatedly administering a DA antagonist can cause DA receptor upregulation, DA supersensitivity, and persistent alterations in second messenger systems (Seeger et al. 1982; Staunton et al. 1982; Oda et al. 2015). Any of these antagonist-mediated effects could impact the induction process and make data interpretation difficult (White et al. 1998; Valjent et al. 2010). The purpose of Experiment 5 was to determine whether repeated treatment with a D1 and/or D2 receptor antagonist, with no agonist being present, would have a persistent inhibitory effect on the one-trial methamphetamine-induced behavioral sensitization of preweanling rats.
On PD 13–15, rats were injected with saline, raclopride (0.5 mg/kg), SCH23390 (0.5 mg/kg), or a cocktail of raclopride+SCH23390 and immediately returned to the home cage. On the pretreatment day (PD 16), rats were again treated with saline, raclopride (0.5 mg/kg), SCH23390 (0.5 mg/kg), or a cocktail of raclopride+SCH23390, but this time the first injection was followed, 15 min later, by an injection of 4 mg/kg methamphetamine. Rats in the acute control group were given two injections of saline. After the second injection, rats were placed in activity chambers and distance traveled was measured for 30 min. On the test day (PD 17), rats (N = 40) were injected with 2 mg/kg methamphetamine and placed in activity chambers for 90 min.
Data analysis
To minimize litter effects, no more than one subject per litter was assigned to a given group (for a discussion of litter effects, see Zorrilla 1997). To statistically analyze the pretreatment and test day data both single and multifactor analyses of variance (ANOVAs) were used. Typically, distance traveled scores on the pretreatment day were analyzed using either Group × Day repeated measures ANOVAs or Group ANOVAs; whereas, test day data were analyzed using Group × Time Block repeated measures ANOVAs. When informative, test day data were also analyzed using Treatment × Time Block repeated measures ANOVAs, in which the scores of saline-pretreated rats were compared to methamphetamine-pretreated rats (i.e., collapsed across the individual methamphetamine groups). In all cases, if the assumption of sphericity was violated, as determined by Mauchly’s test of sphericity, the Greenhouse-Geisser epsilon statistic was used to adjust degrees of freedom (Geisser and Greenhouse 1958). Corrected degrees of freedom were rounded to the nearest whole number and are indicated by a superscripted “a”. When further analyzing statistically significant higher order interactions, the mean square error terms (i.e., MSerror) used for the Tukey calculations were based on separate one-way ANOVAs at each time block.
Unlike adult rodents, prepubescent rats do not typically exhibit sex differences after treatment with DA agonists (Frantz et al. 1996; Bowman et al. 1997; Snyder et al. 1998; McDougall et al. 2013). Consistent with these past studies, preliminary analyses indicated that distance traveled data did not differ according to sex, therefore this variable was not included in the final statistical analyses.
Results
Experiment 1: Effects of D2 receptor blockade on methamphetamine-induced multi-trial behavioral sensitization
Pretreatment phase
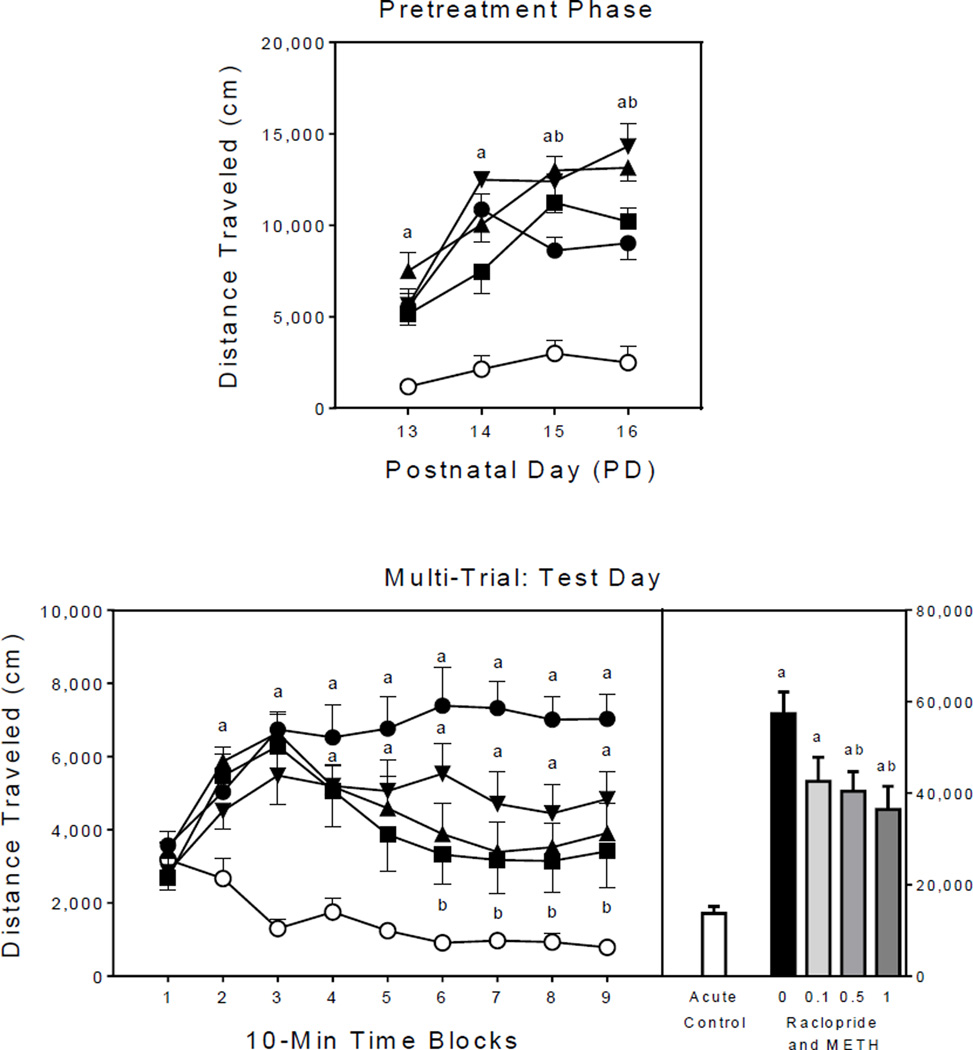
During the pretreatment phase (Fig. 1, upper graph), methamphetamine-pretreated rats (filled symbols) exhibited greater distance traveled scores than saline controls (i.e., SAL–SAL group; open circles) [Group main effect, F4,35 =35.67, P<0.001; and Tukey tests, P<0.05]. Differences between the methamphetamine- and saline-pretreated rats were statistically significant on all four pretreatment days [aGroup × Day interaction, F10,88 =5.16, P<0.001; and Tukey tests, P<0.05]. On no pretreatment day did raclopride (0.1, 0.5, or 1 mg/kg) significantly reduce methamphetamine-induced locomotor activity, although 0.1 and 0.5 mg/kg raclopride did potentiate the distance traveled scores of methamphetamine-treated rats on PD 15 and PD 16 (Tukey tests, P<0.05).
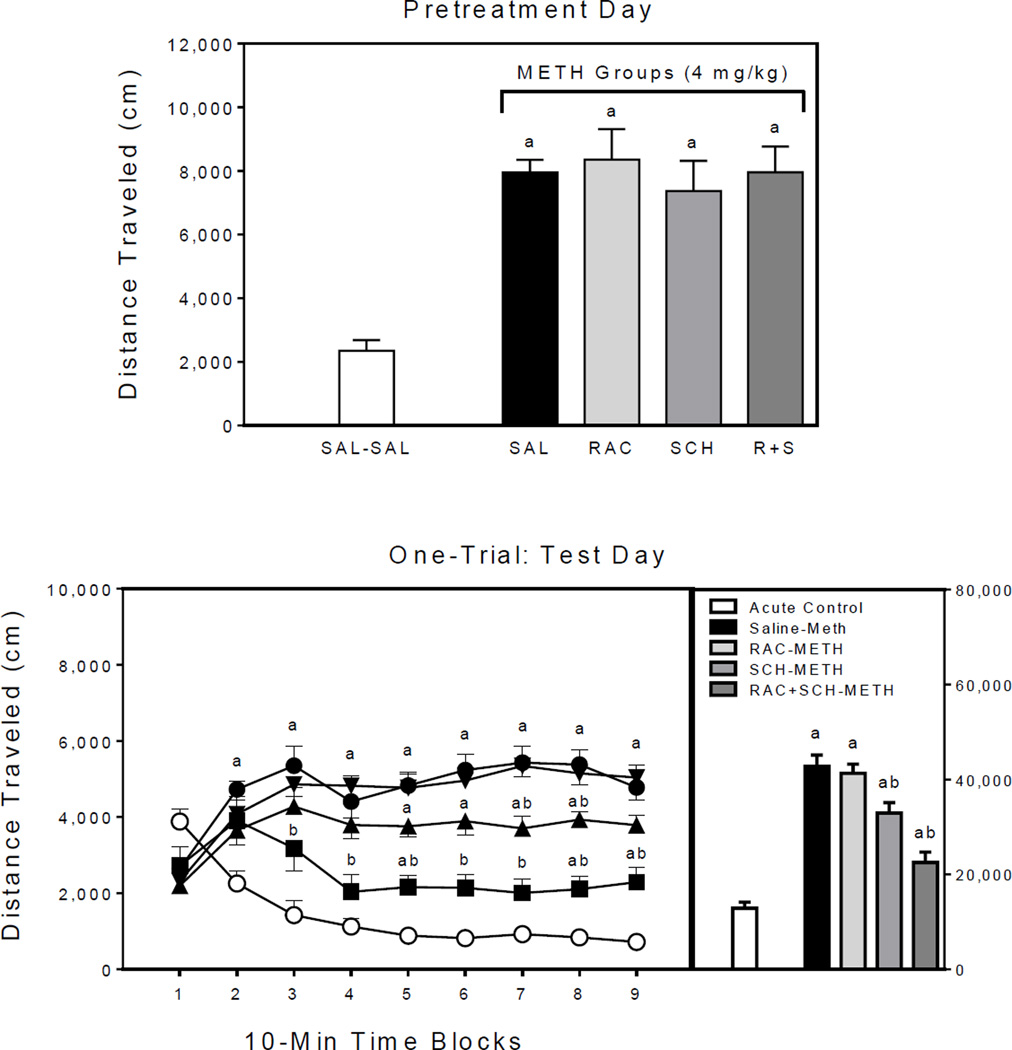
Fig. 1.
Mean distance traveled scores (±SEM) of rats (n = 8 per group) during the pretreatment phase (upper graph) and on the test day (lower graph) of Experiment 1. The right panel represents total distance traveled collapsed across the testing session. Open circle = SAL–SAL (acute control group); filled circle = 0 mg/kg RAC–METH (methamphetamine alone group); filled inverse triangle = 0.1 mg/kg RAC–METH; filled triangle = 0.5 mg/kg RAC–METH; and filled square = 1 mg/kg RAC–METH. (a) Significantly different from the SAL–SAL group (acute control group; open circles); (b) Significantly different from the 0 mg/kg RAC–METH group (methamphetamine alone group; filled circles).
Test day
Overall, methamphetamine-pretreated rats (filled symbols) had greater distance traveled scores than the acute control group (open circles), which is indicative of a sensitized response (Fig. 1, lower graph) [Treatment main effect, F1,38 =30.70, P<0.001]. The two highest doses of raclopride (0.5 and 1 mg/kg) significantly reduced distance traveled scores relative to rats pretreated with methamphetamine alone (i.e., the 0 mg/kg RAC–METH group) [Group main effect, F4,35 =13.00, P<0.001; and Tukey tests, P<0.05]. The latter result indicates that D2 receptor antagonism attenuated the sensitized responding of preweanling rats. Conversely, rats pretreated with both raclopride (0.1–1 mg/kg) and methamphetamine had significantly greater distance traveled scores than the acute control group, thus showing that the D1 antagonist did not fully attenuate methamphetamine sensitization (Tukey tests, P<0.05). Many of these effects varied across the testing session. On time blocks 2–4, all rats given methamphetamine, regardless of raclopride treatment, had greater distance traveled scores than the acute control group [aGroup × Time Block interaction, F11,92 =4.13, P<0.001; and Tukey tests, P<0.05]. On time block 5, rats in the 1 mg/kg RAC–METH group no longer differed significantly from the acute controls. On time blocks 6–9, only rats in the 0 mg/kg RAC–METH or 0.1 mg/kg RAC–METH groups exhibited significantly more locomotor activity than the acute control group; whereas, rats pretreated with the higher doses of raclopride (0.5 or 1 mg/kg) had significantly reduced distance traveled scores when compared to the methamphetamine alone group (Tukey tests, P<0.05).
Experiment 2: Effects of D1/D2 receptor blockade on methamphetamine-induced multi-trial behavioral sensitization
Pretreatment phase
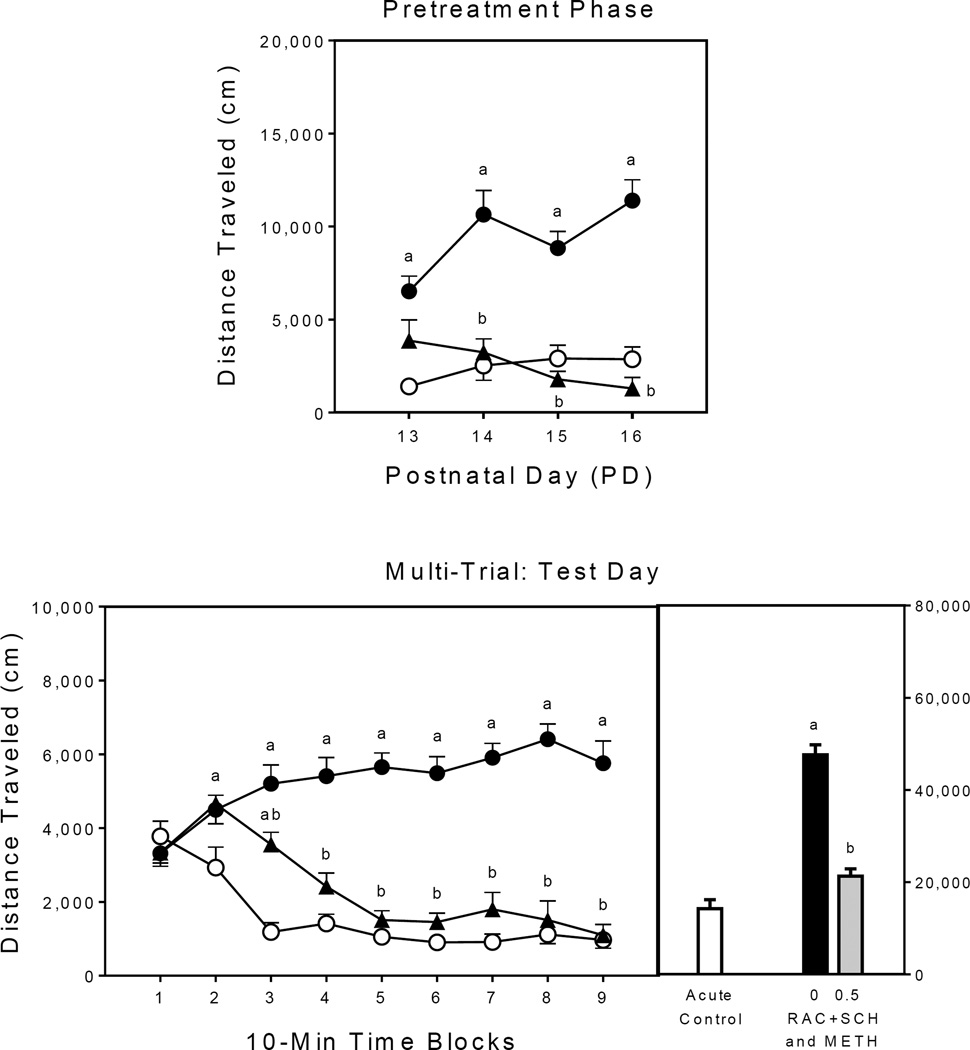
When given alone, methamphetamine significantly increased the distance traveled scores of rats on all four days of the pretreatment phase (Fig. 2, upper graph) [Group main effect, F2,21 =52.98, P<0.001; aGroup × Day interaction, F5,47 =4.81, P<0.01; and Tukey tests, P<0.05]. Administering a cocktail of raclopride+SCH23390 prior to agonist treatment completely blocked methamphetamine-induced locomotor activity.
Fig. 2.
Mean distance traveled scores (±SEM) of rats (n = 8 per group) during the pretreatment phase (upper graph) and on the test day (lower graph) of Experiment 2. The right panel represents total distance traveled collapsed across the testing session. Open circle = SAL–SAL (acute control group); filled circle = 0 mg/kg RAC+SCH–METH (methamphetamine alone group); filled triangle = 0.5 mg/kg RAC+SCH–METH. (a) Significantly different from the SAL–SAL group (acute control group; open circles); (b) Significantly different from the 0 mg/kg RAC+SCH–METH group (methamphetamine alone group; filled circles).
Test day
Rats pretreated with methamphetamine alone (i.e., the 0 mg/kg RAC+SCH–METH group), had greater distance traveled scores than the acute control group on time blocks 2–9 (Fig. 2, lower graph) [Group main effect, F2,21 =84.70, P<0.001; aGroup × Time Block interaction, F6,60 =13.07, P<0.001; and Tukey tests, P<0.05]. Pretreating rats with raclopride+SCH23390 blocked sensitized responding, as the 0.5 mg/kg RAC+SCH–METH group did not differ from the acute control group on time blocks 4–9; whereas, the methamphetamine alone group had greater distance traveled scores on time blocks 3–9 than rats pretreated with the D1 and D2 antagonists (Tukey tests, P<0.05).
Experiment 3: Effects of D2 receptor blockade on methamphetamine-induced one-trial behavioral sensitization
Pretreatment day
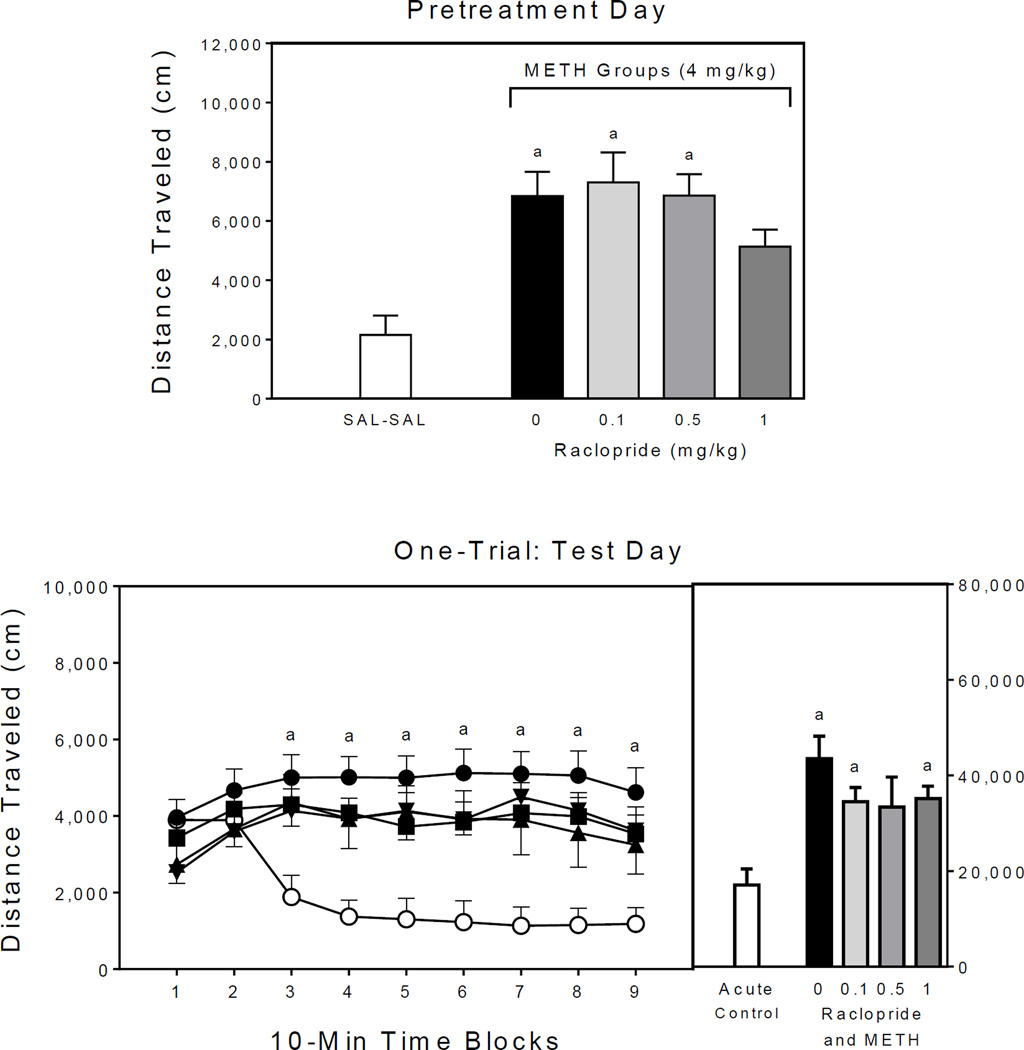
On the single pretreatment day, rats given methamphetamine alone (i.e., the 0 mg/kg RAC–METH group) or raclopride (0.1 and 0.5 mg/kg) plus methamphetamine had significantly greater distance traveled scores than the saline control group (Fig. 3, upper graph) [Group main effect, F4,21 =7.61, P<0.001; and Tukey tests, P<0.05].
Fig. 3.
Mean distance traveled scores (±SEM) of rats (n = 8 per group) on the single pretreatment day (upper graph) and test day (lower graph) of Experiment 3. The right panel represents total distance traveled collapsed across the testing session. Open circle = SAL–METH (acute control group); filled circle = 0 mg/kg RAC–METH (methamphetamine alone group); filled inverse triangle = 0.1 mg/kg RAC–METH; filled triangle = 0.5 mg/kg RAC–METH; and filled square = 1 mg/kg RAC–METH. (a) Significantly different from the SAL–SAL group (acute control group; open circles).
Test day
A single pretreatment injection of methamphetamine was sufficient to induce behavioral sensitization (Fig. 3, lower graph), because the distance traveled scores of methamphetamine-pretreated rats were greater than the acute controls [Treatment main effect, F1,38 =17.20, P<0.001]. Although raclopride produced a slight diminution in locomotor activity, differences between the methamphetamine alone group (i.e., the 0 mg/kg RAC–METH group) and the raclopride groups did not approach statistical significance (Ps = 0.62–0.44); instead, rats pretreated with both methamphetamine and 0, 0.1, or 1 mg/kg raclopride had greater distance traveled scores than the acute control group when collapsed across the testing session [Group main effect, F4,35 =5.30, P<0.01; and Tukey tests, P<0.05]. On time blocks 3–8, all of the methamphetamine-pretreated groups, regardless of raclopride dose (0, 0.1, 0.5, or 1 mg/kg), had greater distance traveled scores than the acute control group [aGroup × Time Block interaction, F12,108 =5.10, P<0.001; and Tukey tests, P<0.05]. On time block 9, only the methamphetamine alone group differed from the acute controls (Tukey tests, P<0.05).
Experiment 4: Effects of D1/D2 receptor blockade on methamphetamine-induced one-trial behavioral sensitization
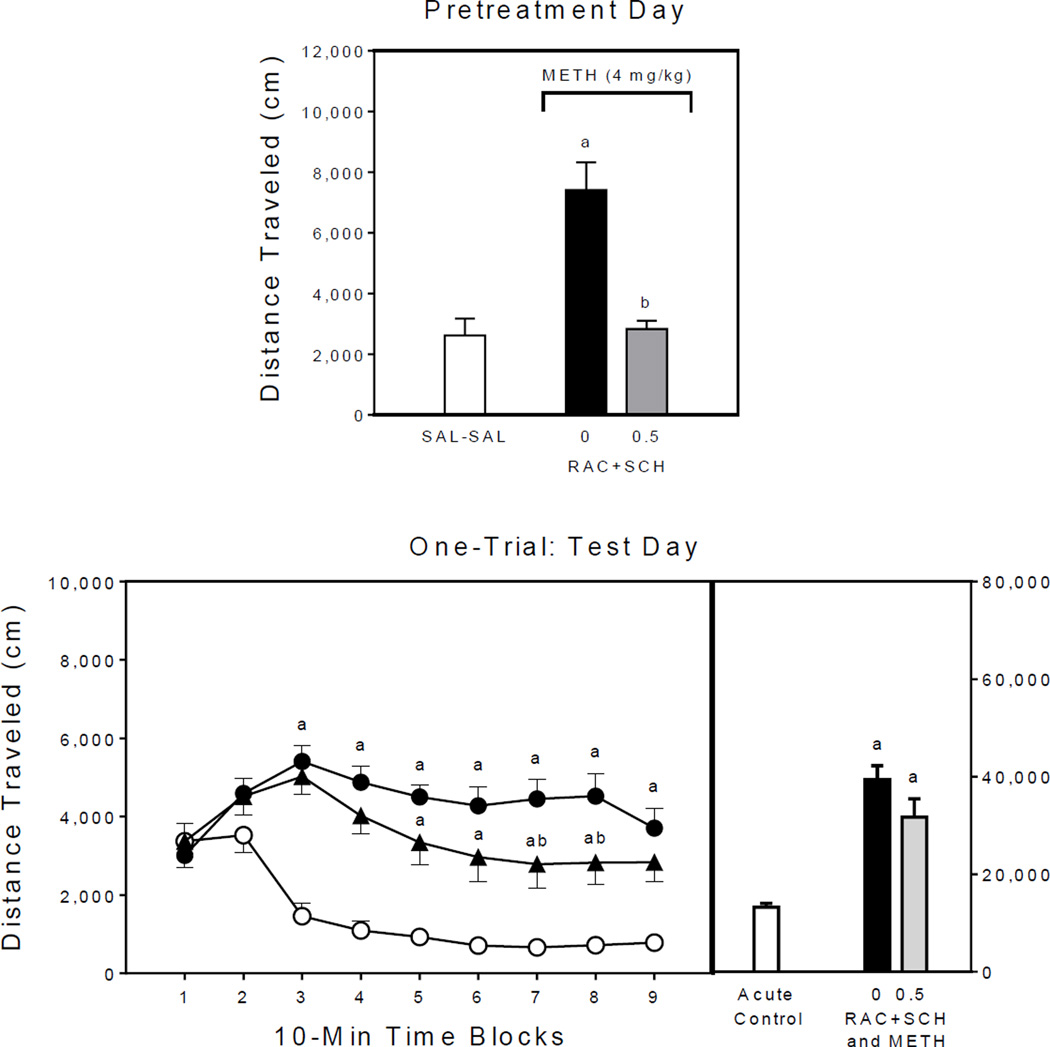
Pretreatment day
On the single pretreatment day, rats treated with methamphetamine alone (i.e., the 0 mg/kg RAC+SCH–METH group) had greater distance traveled scores than the saline controls (Fig. 4, upper graph) [Group main effect, F2,21 =18.06, P<0.001; and Tukey tests, P<0.05]. Administering a cocktail of raclopride+SCH23390 fully attenuated methamphetamine-induced locomotor activity.
Fig. 4.
Mean distance traveled scores (±SEM) of rats (n = 8 per group) on the single pretreatment day (upper graph) and test day (lower graph) of Experiment 4. The right panel represents total distance traveled collapsed across the testing session. Open circle = SAL–SAL (acute control group); filled circle = 0 mg/kg RAC+SCH–METH (methamphetamine alone group); and filled triangle = 0.5 mg/kg RAC+SCH–METH. (a) Significantly different from the SAL–SAL group (acute control group; open circles); (b) Significantly different from the 0 mg/kg RAC+SCH–METH group (methamphetamine alone group; filled circles).
Test day
Analysis of the session totals showed that methamphetamine-pretreated rats, regardless of raclopride+SCH23390 condition, had greater distance traveled scores than the acute control group (Fig. 4, lower graph) [Group main effect, F2,21 =22.75, P<0.001; and Tukey tests, P<0.05]. A somewhat different picture emerges when the analysis takes into account time block, because rats pretreated with raclopride+SCH23390 exhibited less locomotor activity than the methamphetamine alone group on time blocks 7 and 8 [aGroup × Time Block interaction, F7,70 =7.24, P<0.001; and Tukey tests, P<0.05]. All of the methamphetamine-pretreated rats had greater distance traveled scores than the acute controls on time blocks 3–9 (Tukey tests, P<0.05).
Experiment 5: Effects of raclopride and SCH23390 preinjections on methamphetamine-induced one-trial behavioral sensitization
Pretreatment day
On the single pretreatment day (Fig. 5, upper graph), rats treated with methamphetamine alone (i.e., the SAL–METH group) had greater distance traveled scores than rats injected with only saline (i.e., the SAL–SAL group) [Group main effect, F4,35 =11.44, P<0.001; and Tukey tests, P<0.05]. Neither raclopride, SCH23390, nor a cocktail of raclopride+SCH23390 reduced methamphetamine-induced locomotor activity.
Fig. 5.
Mean distance traveled scores (±SEM) of rats (n = 8 per group) on the single pretreatment day (upper graph) and test day (lower graph) of Experiment 5. The right panel represents total distance traveled collapsed across the testing session. Open circle = SAL–SAL (acute control group); filled circle = SAL–METH (methamphetamine alone group); filled inverse triangle = RAC–METH; filled triangle = SCH–METH; and filled square = RAC+SCH–METH. (a) Significantly different from the SAL–SAL group (acute control group; open circles); (b) Significantly different from the SAL–METH group (methamphetamine alone group; filled circles).
Test day
All methamphetamine-pretreated groups (filled symbols) had greater distance traveled scores than the acute control group (Fig. 5, lower graph) [Group main effect, F4,35 =39.88, P<0.001; and Tukey tests, P<0.05]. Unlike what was observed when DA antagonists were administered acutely (see Experiment 3), rats given four pretreatment injections of SCH23390 or raclopride+SCH23390 exhibited less locomotor activity than the methamphetamine alone group (i.e., the SAL–METH group) on time blocks 7–8 and time blocks 3–9, respectively [aGroup × Time Block interaction, F16,137 =8.98, P<0.001; and Tukey tests, P<0.05]. Conversely, methamphetamine-treated rats given multiple preinjections of saline, raclopride, or SCH23390 (i.e., the SAL–METH, RAC–METH, and SCH–METH groups) had greater distance traveled scores than the acute controls on time blocks 3–9. Rats pretreated with raclopride+SCH23390 (i.e., the RAC+SCH–METH group) exhibited more locomotor activity than the acute controls on time blocks 5, 8, and 9 (Tukey tests, P<0.05).
Discussion
Although the characteristics of behavioral sensitization vary substantially across ontogeny, age-dependent changes in the neural mechanisms responsible for sensitized responding have gone largely uninvestigated. During adulthood, D2 and D1 receptor antagonists block the induction of multi-trial methamphetamine sensitization in both rats and mice (Kuribara and Uchihashi 1993, 1994; Kuribara 1995a; Kelly et al. 2008). We now report that D2 or D1/D2 receptor antagonism has similar actions during early ontogeny as raclopride partially attenuated, while raclopride+SCH23390 fully attenuated, the induction of multi-trial methamphetamine sensitization in preweanling rats. A different pattern of effects was observed when using a one-trial procedure, as D2 or D1/D2 receptor antagonism did not prevent the induction of methamphetamine-induced behavioral sensitization. These results suggest that the neural mechanisms mediating the initial induction of behavioral sensitization in young rats differ depending on whether a one-trial or multi-trial procedure is used (see also Mohd-Yusof et al. 2014). Consistent with this possibility, researchers using adult rodent models have proposed that one-trial and multi-trial behavioral sensitization varies in terms of second messenger signaling mechanisms, associative learning factors, and the role of DA receptors (Fontana et al. 1993; White et al. 1998; Battisti et al. 2000; Valjent et al. 2010).
Why DA antagonists differentially affect the one-trial and multi-trial behavioral sensitization of preweanling rats is uncertain, but one possibility is that D2 receptors may partially mediate the associative components of methamphetamine behavioral sensitization. If true, raclopride pretreatment should attenuate multi-trial behavioral sensitization, which relies on contextual conditioning (Wood et al. 1998; Zavala et al. 2000), but not affect the one-trial behavioral sensitization of preweanling rats, which is context-independent (Herbert et al. 2010; McDougall et al. 2011). Since D1 receptor stimulation is often necessary for the full expression of D2-mediated behaviors (Clark and White 1987), it is not surprising that co-administration of raclopride+SCH23390 would have an exaggerated inhibitory effect on the multi-trial methamphetamine sensitization of preweanling rats. An alternative explanation for these findings is that repeated exposure to DA antagonists may cause nonspecific neural changes (i.e., changes unrelated to modifying the acute effects of psychostimulant drugs) that weaken the sensitized response. Experiment 5 provides evidence for this possibility since three daily preinjections of raclopride+SCH23390 intensified the inhibitory effects of a subsequent raclopride+SCH23390 injection given prior to methamphetamine treatment. This explanation leaves open the possibility that the neural mechanisms underlying one-trial and multi-trial behavioral sensitization are the same; and the impaired sensitized responding, which was evident when using the multi-trial procedure, may have been an artifact of repeated antagonist administration. If the latter explanation proves to be correct then the one-trial procedure would be a superior methodology for assessing the acute effects of DA antagonists on psychostimulant-induced behavioral sensitization.
When relevant adult rodent sensitization studies are considered together, one of the most perplexing questions is why do the DA receptor subtypes appear to have different roles in behavioral sensitization depending on the DA agonist being administered? As an example, neither D1 nor D2 receptor antagonists prevent the induction of cocaine sensitization in adult rats (one-trial sensitization is an exception), while both antagonists block methamphetamine sensitization. No such dichotomy exists when the one-trial behavioral sensitization of preweanling rats is assessed, as neither D1 nor D2 receptor antagonists block the induction of cocaine- or methamphetamine-induced behavioral sensitization. This pattern of results suggests that for one-trial behavioral sensitization, at least, other receptor systems may be responsible for mediating the induction process during the preweanling period.
To explain the inconsistent actions of selective DA antagonists on the behavioral sensitization of adult rats, White et al. (1998) speculated that redundant DA and serotonin (5-HT) pathways may be capable of triggering the induction process after cocaine treatment. Consistent with this line of reasoning, antagonists of various serotonergic (e.g., 5-HT1A, 5-HT2C, and 5-HT3) and adrenergic (α1B) receptors prevent the induction of cocaine-induced sensitization in adult rats and mice (King et al. 1997; Ago et al. 2006a; Craige and Unterwald 2013). In terms of the present study, it is also possible that redundant pathways, perhaps involving the 5-HT or adrenergic system, may mediate the induction of one-trial behavioral sensitization in preweanling rats. Such redundant pathways would explain why neither SCH23390 (Mohd-Yusof et al. 2015) nor raclopride (present study) blocked the initial induction of methamphetamine-induced one-trial behavioral sensitization in preweanling rats. On first appraisal, methamphetamine’s mechanism of action and selectivity at monoamine transporters may seem to argue against a ‘redundant pathway’ explanation. Specifically, methamphetamine enhances neurotransmitter release through actions at plasma membrane transporters and the vesicular monoamine transporter type 2 (for reviews, see McMillen 1983; Fleckenstein and Hanson 2003). Methamphetamine has an approximately equal affinity for DA and norepinephrine transporters, but a much lesser affinity for the 5-HT transporter (Howell and Kimmel 2008). Despite the relatively low affinity for 5-HT transporters, methamphetamine and amphetamine sensitization is blocked by 5-HT1A and 5-HT2 receptor antagonists (Auclair et al. 2004; Ago et al. 2006b, 2007), suggesting that the induction process is not exclusively mediated by DA pathways.
Data gathered during the pretreatment phase are also informative. When administered across a one- or four-day pretreatment phase, raclopride (0.1–1 mg/kg) was unable to block the acute locomotor activating effects of methamphetamine in preweanling rats. Indeed, co-administration of raclopride and methamphetamine caused a potentiated locomotor response on the final two days of the pretreatment phase. Similar effects have been observed in adult rats and ascribed to DA supersensitivity (White et al. 1998), which at times is indistinguishable from a sensitized response (see also Mattingly et al. 1996). Unlike raclopride alone, combined treatment with raclopride+SCH23390 fully attenuated the acute locomotor activating effects of methamphetamine, regardless of whether the pretreatment phase lasted one or four days (Fig. 2 and 4). The only exception occurred when rats had been given pretreatment injections of raclopride+SCH23390 in the home cage (Fig. 5). In this circumstance neither raclopride, SCH23390, nor raclopride+SCH23390 attenuated the acute stimulatory effects of methamphetamine. Although an adequate explanation for this effect remains elusive, these results show that the home injection procedure caused persistent alterations in DA receptor functioning and behavior.
Importantly, the ability (or inability) of each antagonist to block agonist-induced locomotor activity on the pretreatment day did not determine whether a sensitized response was expressed on the test day. For example, co-administration of raclopride+SCH23390 fully attenuated methamphetamine-induced locomotion on the pretreatment day, but did not prevent sensitization from being expressed on the test day (Fig. 4). Conversely, raclopride (0.1 and 0.5 mg/kg) actually potentiated methamphetamine-induced locomotor activity by the end of the pretreatment phase, yet the sensitized response was reduced on the test day (Fig. 1). Other lines of research also suggest that the occurrence or non-occurrence of agonist-induced locomotor activity on the pretreatment day does not determine whether a sensitized response will be evident on the test day. As examples, preweanling and adult rats anesthetized during the pretreatment phase (i.e., no locomotor activity was possible) exhibited behavioral sensitization on the test day (Wang and Hsiao 2003; Herbert et al. 2010), while adult mice injected with SCH23390 up to 5 h after methamphetamine pretreatment (i.e., a full locomotor response was evident on the pretreatment day) did not exhibit behavioral sensitization (Kuribara 1995b). Therefore, the induction of behavioral sensitization is independent of the overt manifestation of drug-induced locomotor activity during the pretreatment phase (see also Fontana et al. 1993).
Valjent et al. (2010) proposed that the one-trial sensitization procedure, which they call a two-injection protocol (TIPS), has some important advantages when compared to traditional multi-trial sensitization procedures. For example, the one-trial procedure largely circumvents issues of tolerance and dependence, while permitting an unbiased measure of both the induction and expression of behavioral sensitization (Valjent et al. 2010). If DA antagonists are tested, interpretive problems involving receptor up-regulation and DA supersensitivity are also avoided. The one-trial procedure provides special advantages to researchers examining the ontogeny of behavioral sensitization because the entire procedure can be accomplished in as little as two days. As a consequence, sensitized responding is less likely to be confounded with maturational changes in motoric ability or programmed alterations in CNS functioning. Unlike with adult rats, the sensitized response of preweanling rats shows little persistence (McDougall et al. 2009a), so the one-trial procedure is not appropriate when long-term sensitization is of interest.
Conclusion
In conclusion, we believe that the one-trial procedure is ideally suited for assessing the effects of antagonist drugs on the induction of behavioral sensitization. Using a one-trial protocol, researchers testing adult rats and mice have reported that D1 (Fontana et al. 1993; Valjent et al. 2010) and D2 receptor antagonists (Weiss et al. 1989; Fontana et al. 1993) block psychostimulant-induced behavioral sensitization. Conversely, neither D1 nor D2 receptor antagonists prevent the induction of methamphetamine sensitization in preweanling rats (present study; Mohd-Yusof et al. 2014). This pattern of results suggests that the neural mechanisms underlying the induction of behavioral sensitization, perhaps including the availability of ‘redundant pathways’, differs between early ontogeny and adulthood. Additional “downstream” neuroadaptations involving glutamate and DA neurotransmission are necessary for both the induction and expression of behavioral sensitization in adult rats (for a review, see Vanderschuren and Kalivas 2000). Whether similar neural mechanisms mediate the behavioral sensitization of preweanling rats has yet to be determined.
Acknowledgments
Funding sources:
This research was supported by NIGMS training grant GM083883 (AEG), and NIDA research training grants DA025319 (AMY) and DA033877 (AMY).
Footnotes
Conflict of interest:
All authors declare no conflict of interest.
References
- Ago Y, Nakamura S, Hayashi A, Itoh S, Baba A, Matsuda T. Effects of osemozotan, ritanserin and azasetron on cocaine-induced behavioral sensitization in mice. Pharmacol Biochem Behav. 2006a;85:198–205. doi: 10.1016/j.pbb.2006.07.036. [DOI] [PubMed] [Google Scholar]
- Ago Y, Nakamura S, Uda M, Kajii Y, Abe M, Baba A, Matsuda T. Attenuation by the 5-HT1A receptor agonist osemozotan of the behavioral effects of single and repeated methamphetamine in mice. Neuropharmacology. 2006b;51:914–922. doi: 10.1016/j.neuropharm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Ago Y, Nakamura S, Kajita N, Uda M, Hashimoto H, Baba A, Matsuda T. Ritanserin reverses repeated methamphetamine-induced behavioral and neurochemical sensitization in mice. Synapse. 2007;61:757–763. doi: 10.1002/syn.20421. [DOI] [PubMed] [Google Scholar]
- Auclair A, Drouin C, Cotecchia S, Glowinski J, Tassin JP. 5-HT2A and α1b-adrenergic receptors entirely mediate dopamine release, locomotor response and behavioural sensitization to opiates and psychostimulants. Eur J Neurosci. 2004;20:3073–3084. doi: 10.1111/j.1460-9568.2004.03805.x. [DOI] [PubMed] [Google Scholar]
- Battisti JJ, Chang CH, Uretsky NJ, Wallace LJ. Sensitization of stereotyped behavior to amphetamine is context and response dependent. Pharmacol Biochem Behav. 1999a;63:263–269. doi: 10.1016/s0091-3057(98)00259-7. [DOI] [PubMed] [Google Scholar]
- Battisti JJ, Uretsky NJ, Wallace LJ. Sensitization of apomorphine-induced stereotyped behavior in mice is context dependent. Psychopharmacology (Berlin) 1999b;146:42–48. doi: 10.1007/s002130051086. [DOI] [PubMed] [Google Scholar]
- Battisti JJ, Uretsky NJ, Wallace LJ. Importance of environmental context in the development of amphetamine- or apomorphine-induced stereotyped behavior after single and multiple doses. Pharmacol Biochem Behav. 2000;66:671–677. doi: 10.1016/s0091-3057(00)00214-8. [DOI] [PubMed] [Google Scholar]
- Bowman BP, Blatt B, Kuhn CM. Ontogeny of the behavioral response to dopamine agonists after chronic cocaine. Psychopharmacology (Berlin) 1997;129:121–127. doi: 10.1007/s002130050171. [DOI] [PubMed] [Google Scholar]
- Clark D, White FJ. Review: D1 dopamine receptor the search for a function: a critical evaluation of the D1/D2 dopamine receptor classification and its functional implications. Synapse. 1987;1:347–388. doi: 10.1002/syn.890010408. [DOI] [PubMed] [Google Scholar]
- Craige CP, Unterwald EM. Serotonin (2C) receptor regulation of cocaine-induced conditioned place preference and locomotor sensitization. Behav Brain Res. 2013;238:206–210. doi: 10.1016/j.bbr.2012.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke MA, O'Neal J, McDougall SA. Ontogeny of dopamine agonist-induced sensitization: role of NMDA receptors. Psychopharmacology (Berlin) 1997;129:153–160. doi: 10.1007/s002130050175. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Hanson GR. Impact of psychostimulants on vesicular monoamine transporter function. Eur J Pharmacol. 2003;479:283–289. doi: 10.1016/j.ejphar.2003.08.077. [DOI] [PubMed] [Google Scholar]
- Fontana D, Post RM, Weiss SRB, Pert A. The role of D1 and D2 dopamine receptors in the acquisition and expression of cocaine-induced conditioned increases in locomotor activity. Behav Pharmacol. 1993;4:375–387. [PubMed] [Google Scholar]
- Frantz K, Babcock D, Van Hartesveldt C. The locomotor effects of a putative dopamine D3 receptor agonist in developing rats. Eur J Pharmacol. 1996;302:1–6. doi: 10.1016/0014-2999(96)00014-3. [DOI] [PubMed] [Google Scholar]
- Geisser S, Greenhouse SW. An extension of Box’s results on the use of the F distribution in multivariate analysis. Ann Math Statist. 1958;29:885–891. [Google Scholar]
- Herbert MS, Der-Ghazarian T, Palmer AG, McDougall SA. One-trial cocaine-induced behavioral sensitization in preweanling rats: role of contextual stimuli. Exp Clin Psychopharm. 2010;18:284–295. doi: 10.1037/a0019142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochem Pharmacol. 2008;75:196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Jackson HC, Nutt DJ. A single preexposure produces sensitization to the locomotor effects of cocaine in mice. Pharmacol Biochem Behav. 1993;45:733–735. doi: 10.1016/0091-3057(93)90533-y. [DOI] [PubMed] [Google Scholar]
- Kelly MA, Low MJ, Rubinstein M, Phillips TJ. Role of dopamine D1-like receptors in methamphetamine locomotor responses of D2 receptor knockout mice. Genes Brain Behav. 2008;7:568–577. doi: 10.1111/j.1601-183X.2008.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King GR, Xiong Z, Ellinwood EH., Jr Blockade of cocaine sensitization and tolerance by the co-administration of ondansetron, a 5-HT3 receptor antagonist, and cocaine. Psychopharmacology (Berlin) 1997;130:159–165. doi: 10.1007/s002130050224. [DOI] [PubMed] [Google Scholar]
- Kozanian OO, Gutierrez A, Mohd-Yusof A, McDougall SA. Ontogeny of methamphetamine- and cocaine-induced one-trial behavioral sensitization in preweanling and adolescent rats. Behav Pharmacol. 2012;23:367–379. doi: 10.1097/FBP.0b013e32835651c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczenski R, Leith NJ. Chronic amphetamine: Is dopamine a link in or a mediator of the development of tolerance and reverse tolerance? Pharmacol Biochem Behav. 1981;15:405–413. doi: 10.1016/0091-3057(81)90270-7. [DOI] [PubMed] [Google Scholar]
- Kuribara H. Dopamine D1 receptor antagonist SCH 23390 retards methamphetamine sensitization in both combined administration and early posttreatment schedules in mice. Pharmacol Biochem Behav. 1995a;52:759–763. doi: 10.1016/0091-3057(95)00173-t. [DOI] [PubMed] [Google Scholar]
- Kuribara H. Inhibition of methamphetamine sensitization by post-methamphetamine treatment with SCH 23390 or haloperidol. Psychopharmacology (Berlin) 1995b;119:34–38. doi: 10.1007/BF02246051. [DOI] [PubMed] [Google Scholar]
- Kuribara H, Uchihashi Y. Dopamine antagonists can inhibit methamphetamine sensitization, but not cocaine sensitization, when assessed by ambulatory activity in mice. J Pharm Pharmacol. 1993;45:1042–1045. doi: 10.1111/j.2042-7158.1993.tb07177.x. [DOI] [PubMed] [Google Scholar]
- Kuribara H, Uchihashi Y. Effects of dopamine antagonism on methamphetamine sensitization: evaluation by ambulatory activity in mice. Pharmacol Biochem Behav. 1994;47:101–106. doi: 10.1016/0091-3057(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Leith NJ, Kuczenski R. Two dissociable components of behavioral sensitization following repeated amphetamine administration. Psychopharmacology (Berlin) 1982;76:310–315. doi: 10.1007/BF00449116. [DOI] [PubMed] [Google Scholar]
- Mattingly BA, Hart TC, Lim K, Perkins C. Selective antagonism of dopamine D1 and D2 receptors does not block the development of behavioral sensitization to cocaine. Psychopharmacology (Berlin) 1994;114:239–242. doi: 10.1007/BF02244843. [DOI] [PubMed] [Google Scholar]
- Mattingly BA, Rowlett JK, Ellison T, Rase K. Cocaine-induced behavioral sensitization: effects of haloperidol and SCH 23390 treatments. Pharmacol Biochem Behav. 1996;53:481–486. doi: 10.1016/0091-3057(95)02101-9. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Charntikov S, Cortez AM, Amodeo DA, Martinez CE, Crawford CA. Persistence of one-trial cocaine-induced behavioral sensitization in young rats: regional differences in Fos immunoreactivity. Psychopharmacology (Berlin) 2009a;203:617–628. doi: 10.1007/s00213-008-1407-1. [DOI] [PubMed] [Google Scholar]
- McDougall SA, Cortez AM, Palmer AG, Herbert MS, Martinez CE, Charntikov S, Amodeo DA. Importance of environmental context for one- and three-trial cocaine-induced behavioral sensitization. Psychopharmacology (Berlin) 2009b;206:377–388. doi: 10.1007/s00213-009-1616-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SA, Nuqui CM, Quiroz AT, Martinez CM. Early ontogeny ofd-amphetamine-induced one-trial behavioral sensitization. Pharmacol Biochem Behav. 2013;104:154–162. doi: 10.1016/j.pbb.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SA, Pothier AG, Der-Ghazarian T, Herbert MS, Kozanian OO, Castellanos KA, Flores AT. Importance of associative learning processes for the one-trial behavioral sensitization of preweanling rats. Behav Pharmacol. 2011;22:693–702. doi: 10.1097/FBP.0b013e32834affb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen BA. CNS stimulants: two distinct mechanisms of action for amphetamine-like drugs. Trends Pharmacol Sci. 1983;4:429–432. [Google Scholar]
- Mohd-Yusof A, Gonzalez AE, Veliz A, McDougall SA. Role of the D1 receptor for the dopamine agonist-induced one-trial behavioral sensitization of preweanling rats. Psychopharmacology (Berlin) 2014;231:4167–4177. doi: 10.1007/s00213-014-3561-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington, DC: National Academies Press; 2010. [Google Scholar]
- Oda Y, Tadokoro S, Takase M, Kanahara N, Watanabe H, Shirayama Y, Hashimoto K, Iyo M. G protein-coupled receptor kinase 6/β-arrestin 2 system in a rat model of dopamine supersensitivity psychosis. J Psychopharmacol. 2015 doi: 10.1177/0269881115593903. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Rev. 1986;11:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB, Presty SK. Long-term facilitation of amphetamine-induced rotational behavior and striatal dopamine release produced by a single exposure to amphetamine: sex differences. Brain Res. 1982;571:330–337. doi: 10.1016/0006-8993(82)90690-4. [DOI] [PubMed] [Google Scholar]
- Seeger TF, Thal L, Gardner EL. Behavioral and biochemical aspects of neuroleptic-induced dopaminergic supersensitivity: studies with chronic clozapine and haloperidol. Psychopharmacology (Berl) 1982;76:182–187. doi: 10.1007/BF00435275. [DOI] [PubMed] [Google Scholar]
- Smith KS, Morrell JI. Behavioral responses during the initial exposures to a low dose of cocaine in late preweanling and adult rats. Neurotoxicol Teratol. 2008;30:202–212. doi: 10.1016/j.ntt.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder KJ, Katovic NM, Spear LP. Longevity of the expression of behavioral sensitization to cocaine in preweanling rats. Pharmacol Biochem Behav. 1998;60:909–914. doi: 10.1016/s0091-3057(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Staunton DA, Magistretti PJ, Koob GF, Shoemaker WJ, Bloom FE. Dopaminergic supersensitivity induced by denervation and chronic receptor blockade is additive. Nature. 1982;299:72–74. doi: 10.1038/299072a0. [DOI] [PubMed] [Google Scholar]
- Tirelli E, Laviola G, Adriani W. Ontogenesis of behavioral sensitization and conditioned place preference induced by psychostimulants in laboratory rodents. Neurosci Biobehav Rev. 2003;27:163–178. doi: 10.1016/s0149-7634(03)00018-6. [DOI] [PubMed] [Google Scholar]
- Valjent E, Bertran-Gonzalez J, Aubier B, Greengard P, Hervé D, Girault JA. Mechanisms of locomotor sensitization to drugs of abuse in a two-injection protocol. Neuropsychopharmacology. 2010;35:401–415. doi: 10.1038/npp.2009.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology (Berlin) 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Vezina P. D1 dopamine receptor activation is necessary for the induction of sensitization by amphetamine in the ventral tegmental area. J Neurosci. 1996;16:2411–2420. doi: 10.1523/JNEUROSCI.16-07-02411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Hsiao S. Amphetamine sensitization: nonassociative and associative components. Behav Neurosci. 2003;117:961–969. doi: 10.1037/0735-7044.117.5.961. [DOI] [PubMed] [Google Scholar]
- Weiss SRB, Post RM, Pert A, Woodward R, Murman D. Context-dependent cocaine sensitization: differential effect of haloperidol on development versus expression. Pharmacol Biochem Behav. 1989;34:655–661. [PubMed] [Google Scholar]
- White FJ, Joshi A, Koeltzow TE, Hu X-T. Dopamine receptor antagonists fail to prevent induction of cocaine sensitization. Neuropsychopharmacology. 1998;18:26–40. doi: 10.1016/S0893-133X(97)00093-6. [DOI] [PubMed] [Google Scholar]
- Wood RD, Tirelli E, Snyder KJ, Heyser CJ, LaRocca TM, Spear LP. Evidence for behavioral sensitization to cocaine in preweanling rat pups. Psychopharmacology (Berlin) 1998;138:114–123. doi: 10.1007/s002130050653. [DOI] [PubMed] [Google Scholar]
- Zavala AR, Nazarian A, Crawford CA, McDougall SA. Cocaine-induced behavioral sensitization in the young rat. Psychopharmacology (Berlin) 2000;151:291–298. doi: 10.1007/s002130000377. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30:141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]