Abstract
Sleep benefits memory consolidation across a variety of domains in young adults. However, while declarative memories benefit from sleep in young children, such improvements are not consistently seen for procedural skill learning. Here we examined whether performance improvements on a procedural task, although not immediately observed, are evident after a longer delay when augmented by overnight sleep (24-hrs after learning). We trained forty-seven children, aged 33–71 months, on a serial reaction time task and, using a within-subject design, evaluated performance at three time points: immediately after learning, after a daytime nap (nap condition) or equivalent wake opportunity (wake condition), and 24-hours after learning. Consistent with previous studies, performance improvements following the nap did not differ from performance improvements following an equivalent interval spent awake. However, significant benefits of the nap were found when performance was assessed 24-hrs after learning. This research demonstrates that motor skill learning is benefited by sleep, but that this benefit is only evident after an extended period of time.
Keywords: Sleep, nap, children, procedural memory, motor skill learning
Introduction
Sleep has been shown to protect and enhance newly formed memories across a variety of memory systems in young adults (Walker and Stickgold 2004; Stickgold 2005). Gains in declarative memory performance are thought to be the result of sleep-dependent memory consolidation, or the process by which labile, short-term memories are transferred during sleep to more robust stores for long-term retrieval (Stickgold and Walker 2007). Procedural memories have likewise been shown to benefit from both overnight sleep and daytime naps in young adults (Fischer et al. 2002; Spencer et al. 2006; Backhaus and Junghanns 2006; Wilson et al. 2012) particularly when learning is explicit (see Song, Howard, & Howard, 2007; Spencer, Sunm, & Ivry, 2006). Such benefits may reflect consolidation of the episodic memory associated with learning (Rauchs et al. 2004) or the consolidation of sensory and motor skill components of a task specifically during non-REM stage 2 (Walker et al. 2002).
One commonly used probe of procedural memory consolidation is a motor sequence learning task such as the serial reaction time task (SRTT; Nissen and Bullemer 1987). In this task, subjects press buttons in response to a sequence of cues. Improvement in performance is expressed as faster and more accurate button presses, particularly relative to performance when cues are randomized. In young adults, participants exhibit significant improvements in sequence-specific movement speed following sleep but not after an equivalent interval spent awake (Spencer et al. 2006; Spencer et al. 2007).
While a growing body of research supports sleep-dependent consolidation of declarative memories in children (Backhaus et al. 2008; Wilhelm et al. 2008; Kurdziel et al. 2013), the role of sleep in consolidating procedural memories in early childhood is less clear. Children constantly and rapidly acquire new procedural skills (Haywood and Getchell 2009). Moreover, total sleep time for children is greater than that of young adults (Weissbluth 1995; Iglowstein et al. 2003; Montgomery-Downs et al. 2006). Yet, in spite of procedural skill learning abilities at a young age (before 4 yrs; e.g., Thomas & Nelson, 2001), several studies have failed to find evidence of sleep-dependent procedural memory consolidation in children. Prehn-Kristensen and colleagues (2009) demonstrated that children received no benefit of nocturnal sleep compared to daytime wake using a mirror-tracing task in 10–13 year old children. Additionally, Fischer and colleagues (2007) trained children, aged 7–11 years, on an SRTT and then evaluated their performance across a 12-hour period that contained either overnight sleep or daytime wake. Children not only showed a lack of improvement in procedural skill following sleep, but were actually significantly worse compared to performance following wake. Similar observations have been made using a motor sequence task in 6–8 year old children (Wilhelm et al. 2008). One study demonstrated sleep-dependent consolidation of procedural learning in preschool children aged 4–6 years, but only when extensive training was provided. Specifically, Wilhelm and colleagues (2012) examined consolidation of motor sequence learning following a nap and equivalent wake for two groups, one of which had a standard amount of learning and another group who had additional exposure to the task for three days prior. The nap was beneficial compared to wake only for the group that had additional exposure to the task. The authors posited that, with low levels of learning, there might not be sufficient engagement of the hippocampus for consolidation to occur.
While it is possible that sleep offers little benefit to procedural skills in the absence of extensive practice in young children, it is also possible that sleep-dependent improvements are not evident immediately. Rather, as suggested by Deregnaucort and colleagues (2005), lability of memories shortly after a sleep episode may prevent performance benefits from being evident in the short term but nonetheless result in performance enhancement following a delay. For this reason, we examined whether children’s motor skill performance, although unchanged (Wilhelm et al. 2008) or impaired (Fischer et al. 2007) immediately after the initial sleep bout, may benefit from sleep in the longer term. Performance on an SRTT was assessed within-subjects following a daytime nap and an equivalent interval of wake and again 24-hours after initial training. Our focus was on preschool-aged children, 3.5–6 yrs of age, who nap frequently but not habitually making both the nap and wake conditions within the range of normal behavior. We hypothesized that children would not exhibit any gains in performance following the nap compared to the wake interval consistent with prior studies, but that performance in the nap condition would be superior to the wake condition when probed 24-hours after initial learning.
Methods
Participants
Forty-seven children (30 males) between the ages 33–71 months (M = 57.30 months, SD = 6.04) participated in the study. Children were recruited from local preschools in western Massachusetts. Children were deemed ineligible to participate if they were diagnosed with any sleep disorder other than mild parasomnias, using sleep-affecting or psychotropic medications, had diagnosed developmental, hearing, or vision impairments, had traveled across time zones within two weeks prior to the study, or were suffering from fever or respiratory illness at the time of testing.
Procedure
All procedures were approved by the University of Massachusetts Amherst Institutional Review Board. Parents and guardians provided informed consent and children provided assent before testing. To test procedural memory consolidation, children performed a modified SRTT across multiple sessions, which took place in their preschool classroom with experimenters working one-on-one with enrolled children. Using a within-subjects design, children were randomly assigned to begin with either the nap or wake condition with the alternate condition taking place approximately one week later.
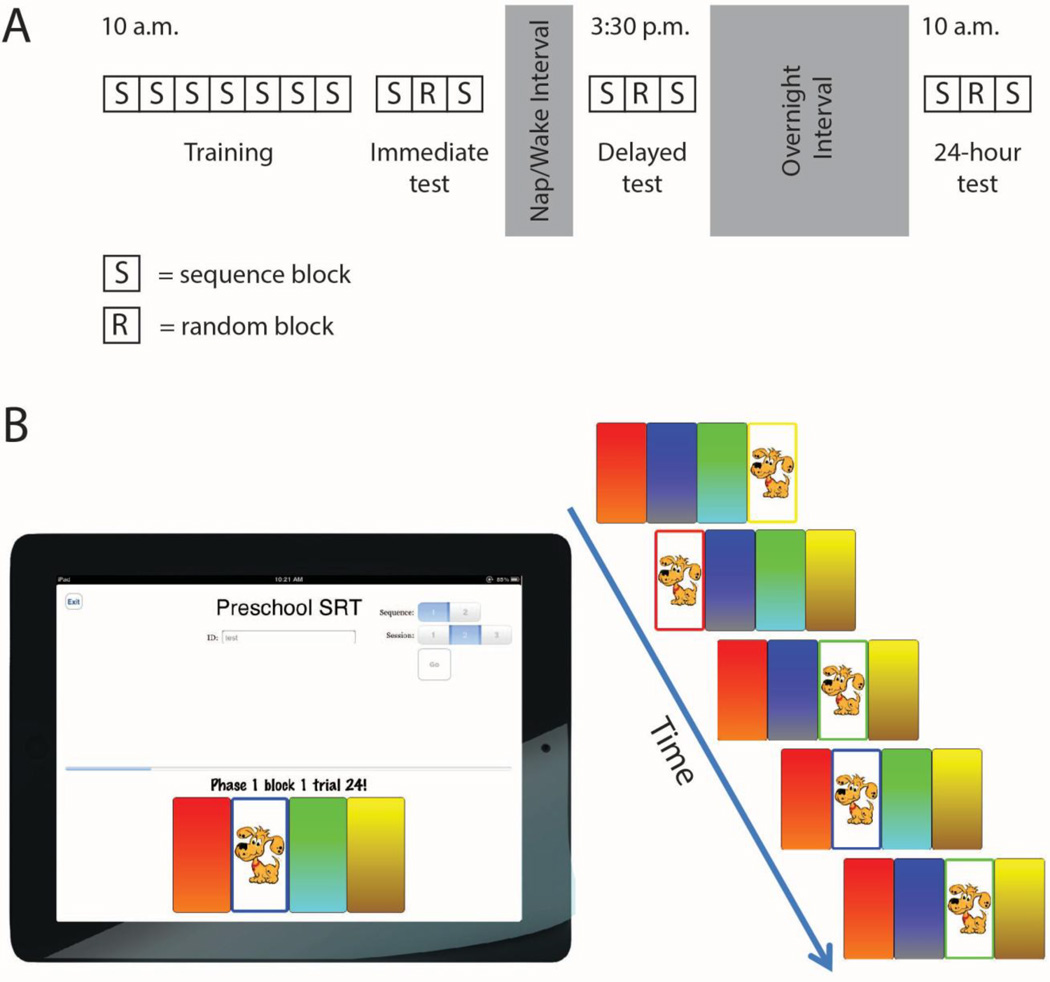
In the morning (approximately 10:00 AM; Figure 1A), the experimenter evaluated handedness by observing the hand which the children used to draw a picture. Children then completed the Training and Immediate Test phases of the SRTT. Later in the day, during the classroom's typical nap opportunity (approximately 1:00–3:00 PM), children either napped (nap condition) or were kept quietly awake (wake condition). In both conditions, the room was darkened and quiet and children remained on their individual cots/mats as per the typical classroom naptime routine. In the nap condition, children were nap-promoted via traditional classroom procedures (e.g., verbal encouragement, back rubbing, soothing music), and experimenters recorded nap and wake onset time (and any mid-nap wakings) for all enrolled children. In the wake condition, children were kept in a state of restful wake by reading books and doing quiet activities (e.g., puzzles), as necessary, on their cots/mats.
Fig 1.
Performance on a modified serial reaction time task (SRTT) was assessed in three testing phases (A). The task was executed using on iPad (B). Children responded to a repeating sequence of cues by pressing a virtual "button".
Following the nap/wake period, children were given 30 minutes before subsequent testing to allow any residual sleep inertia to dissipate in the nap condition. Children were then evaluated on their performance on the SRTT in the Delayed Test phase. The final testing phase, the 24- hour Test, took place at 10:00 AM the following morning. At all three test sessions, children reported their subjective sleepiness (Maldonado 2004) and mood (Folstein and Luria 1973) using pictorial Likert scales. Experimenters also rated their impression of the child's sleepiness and mood using the same scale.
Actigraphy
Children wore actigraphy watches (Actiwatch Spectrum; Philips Respironics Inc., Bend, OR) on their non-dominant wrist for 16 days (beginning approximately 2–3 day prior to the first experimental condition) to obtain information regarding overnight sleep and napping. The Actiwatch sampled activity at 32 Hz; activity was stored in 15-second epochs. The caregiver and child were instructed to press an event marker on the Actiwatch to mark the beginning and end of each sleep bout during the 16-day testing period. Caregivers also completed a sleep diary for the child in which they were asked to note the beginning and end of sleep bouts occurring in the home.
Serial Reaction Time Task
The task was a modified SRTT similar to that reported by Savion-Lemieux and colleagues (2009). Children executed a sequence of movements across four distinctly colored virtual "buttons" on an iPad that was positioned approximately 16 inches from the edge of the table, adjusting for the reach and comfort of the child. The four 1 × 2 inch buttons were arranged horizontally and positioned at the bottom of the screen. A cartoon dog appeared on one button at a time (Figure 1B), and children were told to "catch the dog" by pressing the button as quickly as possible without making mistakes. The dog remained on the button until the child made a response, at which point the dog moved to the next button in the sequence. Children were instructed to use only the index finger of their non-dominant hand (thus requiring a sequence of arm movement responses). No information was given regarding the order of the button presses, but children were told at the start of the task that the dog would repeatedly move to the buttons in the same order. The trained sequence was unique for the nap and wake conditions, and sequences were counterbalanced across subjects.
The Training phase was composed of seven blocks (Figure 1A). The five-item sequence (e.g., 4-1-3-2-3) repeated eight times in each block. Each test phase (Immediate Test, Delayed Test, and 24-hour Test) consisted of three blocks; the first and third blocks consisted of presentation of the trained sequence repeated eight times. In the second block of each test phase, 40 cues were presented in a semi-random order. Both the sequence and random blocks were designed such that no button was cued consecutively. As children performed the task, they were given verbal praise for their effort and attention, but did not receive performance-based feedback.
Explicit awareness
Since the extraction of explicit sequence knowledge was intended to be enhanced as children were told that buttons would be cued in a repeating order, we probed the level of sequence awareness in a portion of the participants (n = 16 as this measure was added after the study commenced). Following each 24-hour Test session, children were asked to reproduce the sequence on the iPad without being cued. The experimenter prompted children to execute all five items of the sequence consecutively, guessing if necessary.
Data Analyses
Data were scored with Actiware software (Philips Respironics, Bend, OR). Data from children with less than 3 days of actiwatch data was excluded (. If records lacked parental report information or event markers, onsets of sleep bouts were determined using the first three uninterrupted minutes of sleep, while offsets of sleep bouts were determined using the last five uninterrupted minutes of sleep (Acebo et al., 2005). Experimenters evaluated actigraphy data for nap duration, overnight sleep duration, and total sleep duration (nap duration plus overnight duration) on the nap and wake days.
Reaction time was computed as the time between the stimulus presentation and the subsequent button touch. In each block, individual trials with a reaction time (RT) two standard deviations above or below the mean for the respective block were removed to eliminate lapses in attention or inadvertent button presses. Of the remaining trials, accuracy (# of correct trials / # of trials) was computed for each block. Paired samples t-tests were used to examine differences in accuracy between nap and wake conditions at each test session. Remaining analyses were based on correct trials only.
Block RTs were calculated as the median trial RT for each block. To assess general motor learning across the training session, a paired samples t-test was used to compare block RTs between blocks 1 and 7. A decrease in block RTs across the training session indicated learning.
Two measurements were calculated to assess learning across intervals. First, median block RT was calculated for the sequence blocks (blocks 1 and 3) in each test phase. Second, a Learning Score (LS), reflecting sequence-specific learning, was calculated as the median RT for the random block in each test phase (block 2) minus the average of the median RTs for the surrounding sequence blocks (blocks 1 and 3), as is common in studies using the SRTT (e.g., Nissen and Bullemer 1987). Sequence-specific learning was evaluated with a one-sample t-test to determine whether the LS was significantly greater than zero. Participants with a score greater than 2 SD above or below the mean were excluded from both conditions for each of these analyses.
RT and the LS were used to examine the change in performance between testing sessions across the nap/wake interval (Immediate Test to Delayed Test) and the overnight interval (Delayed Test to 24-hour Test). To assess the change in performance across these two intervals, a Percent Improvement (PI) score was calculated. Performance at the first test session was subtracted from the second test session, and then divided by performance at the first test session. These scores were then multiplied by 100. We report the inverse of the PI scores for the RT PI only to maintain the premise that positive PI values indicate improvement. A repeated measures ANOVA was used to determine differences between Nap/Wake conditions at each test session. Further, a repeated measures ANCOVA was used to control for age of the participants. Participants with a score greater than 2 SD above or below the mean were excluded for the RT PI and the LS PI. To control for family-wise error rates, we performed a Sidak correction for the two ANOVAs (across the nap/wake interval and overnight interval) and we determined a new alpha cutoff to be 0.025.
Finally, explicit knowledge of the sequence was compared between conditions. Explicit knowledge was measured as the number of identified transitions that matched the trained sequence, starting from any given point in the sequence. Each number of correct transitions was given a score from zero to four, with zero signifying no correct transitions and four signifying all correct transitions as compared to the trained sequence. A paired samples t-test was used to compare children's explicit knowledge of the sequence between nap and wake conditions. A post-hoc linear regression was used to determine how a nap benefit of explicit recall predicted performance across the overnight interval in the nap condition.
Results
Eleven children were unavailable to complete one or more test session, leaving a total of 36 children (23 males, M = 57.45 months, SD = 6.07) with complete data for both nap and wake conditions. There were no outliers for measures of RT. However, for the LS, three participants were excluded from analysis. Six additional participants were outliers for the LS PI measure and were excluded from those analyses only. One child’s parents did not report their child’s exact age, and thus was not included in ANCOVAs controlling for age.
Of the 36 children included in the final analysis, 27 children had actigraphy data for three or more weekdays. Twenty-six children had record of their overnight sleep duration on the nap day, while twenty-seven children had record of their overnight sleep duration on the wake day. All 36 children had record of their nap duration. Children napped 66.3% of weekdays (SD = 24.3%). Naps lasted an average of 92.3 mins (SD = 28.5). Overnight sleep duration was significantly greater on the wake day (M = 9 hours 29 mins, SD = 59.1) than on the nap day (M = 8 hours 55 mins, SD = 77.2; t(23) = −2.94, p > 0.01). However, total sleep duration (nap + overnight sleep) was significantly greater on the nap day (M = 10 hours 20 mins, SD = 90.3) than on the wake day (M = 9 hours 29 mins, SD = 59.1; t(24) = 3.69, p > 0.01).
Participants' ratings of mood and sleepiness did not differ between conditions (paired samples t-tests; all p's > 0.05). Subjects performed with a high degree of accuracy (>90% correct on average) and accuracy did not significantly differ across nap and wake conditions for any test phase (Immediate Test: t(35) = 0.64, p = 0.53; Delayed Test: t(35) = 0.74, p = 0.46; 24-hour Test: t(35) = 0.22, p = 0.83) as is often the case in studies using the SRTT (Savion-Lemieux et al. 2009; Pace-Schott and Spencer 2013). Thus, the following results focus on RT and LS.
Initial Learning (Training session)
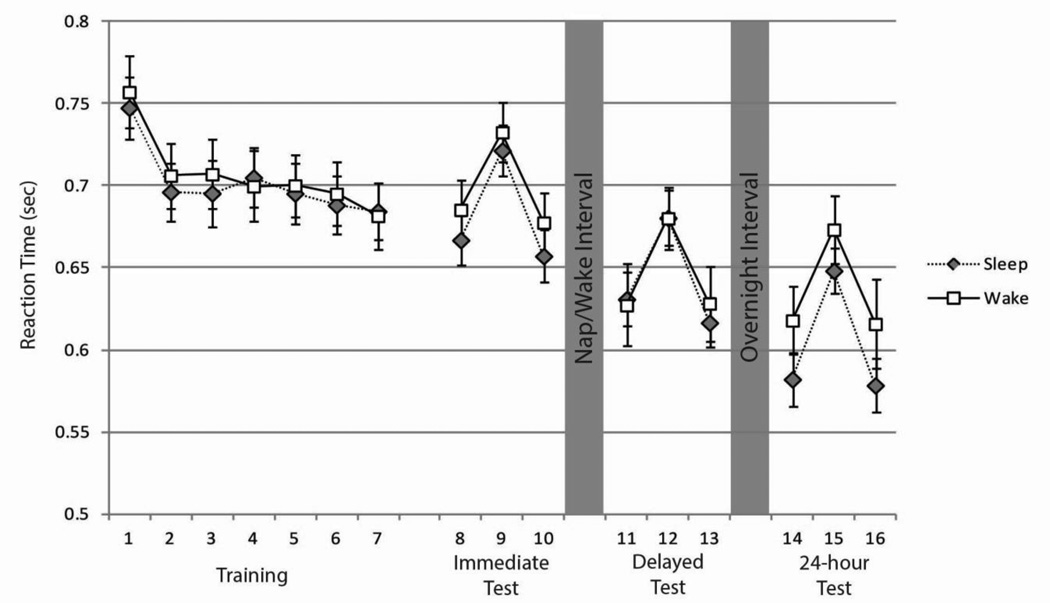
RT improved significantly from block 1 to 7 of the Training phase in both the wake condition (t(35) = 4.60, p < 0.001) and the nap condition (t(35) = 6.02, p < 0.001; Figure 2), indicative of motor learning. There were no differences between the nap and wake conditions at the Immediate Test for measures of RT (average of the median RTs from blocks 1 and 3; t(35) = 0.69, p = 0.49). Likewise, there were no differences in LS between conditions (t(32) = 1.33, p = 0.19). Moreover, the LS was significantly greater than zero at the Immediate Test (nap: t(32) = 7.08, p < 0.001; wake: t(32) = 5.00, p < 0.001) indicative of sequence-specific learning.
Fig 2.
Reaction times across blocks in both the nap and wake conditions. Error bars represent standard error.
Off-line Change in Performance
The LS remained significantly greater than 0 at Delayed Test (nap: t(32) = 5.63, p < 0.001; wake: t(32) = 5.22, p < 0.001) and 24-hour Test (nap: t(32) = 6.07, p < 0.001; wake: t(32) = 7.21, p < 0.001), indicating that significant sequence-specific learning was maintained. Of interest was whether performance changes over the nap and overnight intervals differed based on whether a nap took place.
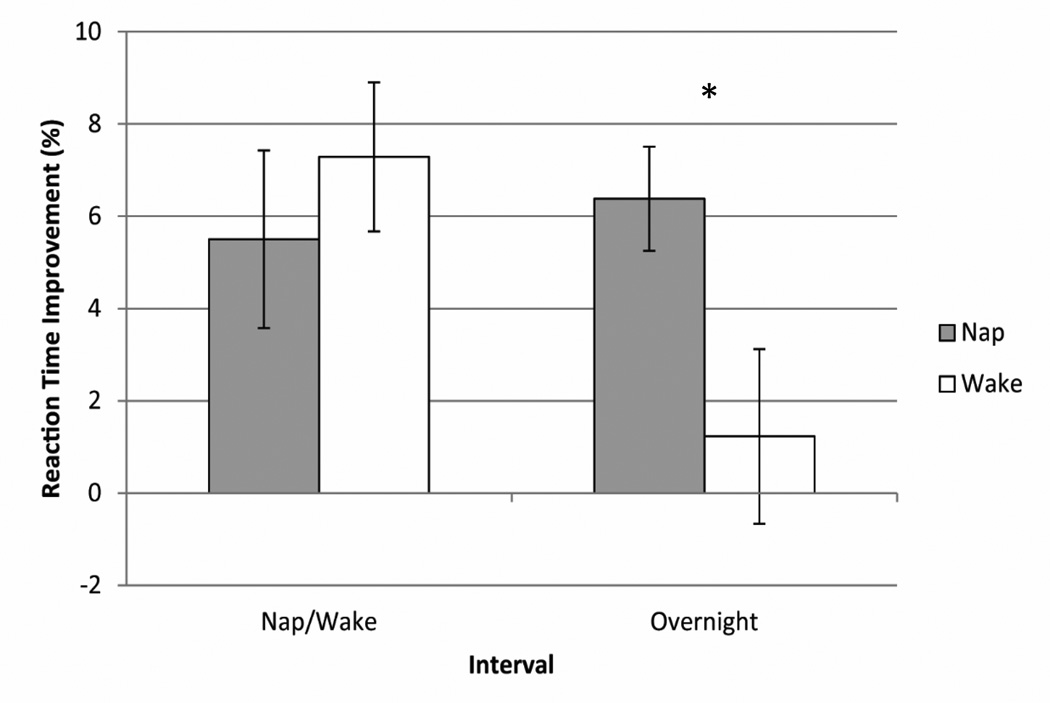
Consistent with prior studies (Fischer et al. 2007; Wilhelm et al. 2008; Prehn-Kristensen et al. 2009), there was no significant difference between conditions in RT PI across the nap/wake interval (F(1, 35) = 0.48, p = 0.50). There was, however, a significant effect of condition on the RT PI across the overnight interval (F(1, 35) = 5.88, p = 0.02). Children's performance improved more overnight when they had napped (M = 6.38%, SD = 6.77) than when they stayed awake during the nap opportunity (M = 1.23%, SD = 11.35; Figure 3). Given that sleep quality changes across this age span, we performed a subsequent ANCOVA controlling for age. Effects remained such that there was no significant difference in RT PI between conditions across the nap/wake interval (F(1, 33) = 0.02, p = 0.94), but there was a significant difference in RT PI between conditions across the Overnight Interval (F(1, 33) = 6.25, p = 0.02). In the nap condition, neither the nap duration (r(36) = 0.26, p = 0.13), nor overnight sleep duration (r(26) = −0.16, p = 0.44) was correlated with the Overnight Interval RT PI.
Fig 3.
Reaction times improved across the nap/wake interval in both conditions, but across the overnight interval, RTs improved significantly more in the nap condition than in the wake condition. Error bars represent standard error. *p < 0.05
Since trials > 2 SD from the mean of each block were excluded as they reflect lapses in attention, we confirmed that these exclusions did not impact the observed effects. With these trials included in our analyses, we still observe a benefit of sleep across the Overnight Interval (F(1, 35) = 5.83, p = 0.02) and not across the Nap/Wake Interval (F(1, 35) = 0.48, p = 0.50). Likewise, if trials with an RT < 2 SD from the mean of each block (which were few, <1 trial per block on average, as most were excluded as errors) were included, we still observe a sleep benefit across the Overnight Interval (F(1, 35) = 4.45, p = 0.04) and not across the Nap/Wake Interval (F(1, 35) = 0.217, p = 0.644). The number of trials excluded as errors did not differ between blocks (all p’s > 0.05).
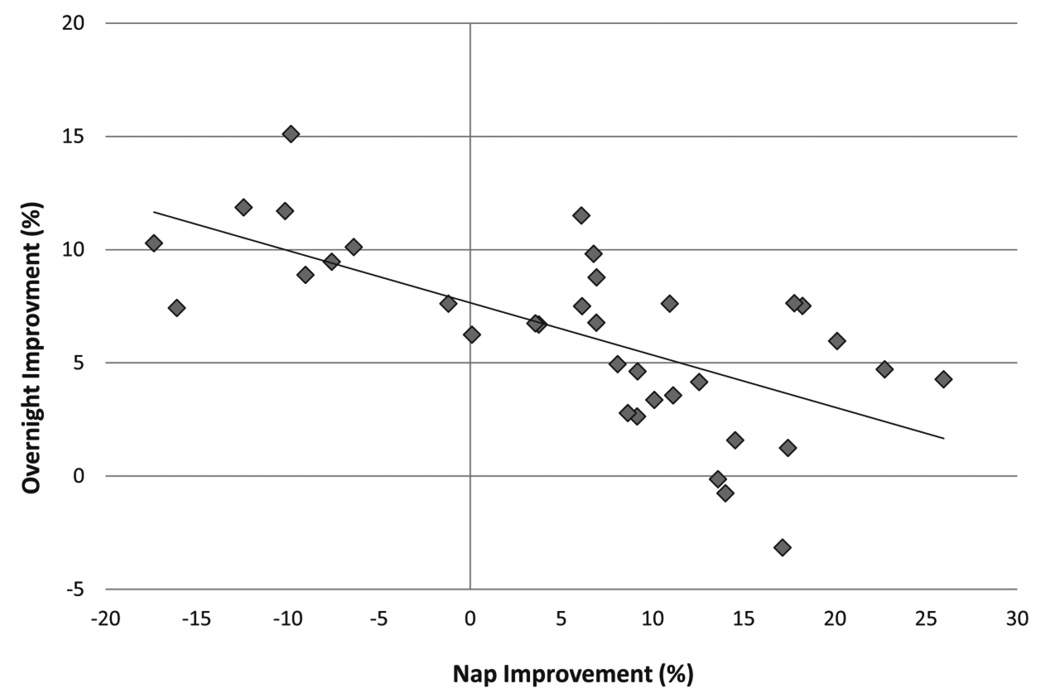
Given this evidence of a nap benefit across the Overnight Interval but not across the nap interval, we performed a post-hoc linear regression to probe whether the change over the nap predicted the change over the subsequent night. Indeed, RT PI alone across the Nap/Wake interval significantly predicted 14.5% of the variability of RT PI across the Overnight Interval in the Nap condition (R2 = 0.15, p = 0.02; B = −0.23, p = 0.02). Age alone predicted 11.6% of the variability in the model (R2 = 0.12, p = 0.05; B = −0.004, p = 0.05), such that older children had faster reaction times. When this regression was performed controlling for age, the model predicted 33.2% of the variability in RT PI across the Overnight Interval (R2 = 0.33, p < 0.01; B = −0.30, p < 0.01). In other words, those children with worse performance over the nap had better performance over the subsequent overnight interval.
Surprisingly, we also found no significant difference between nap and wake conditions in the LS PI across the Nap/Wake interval (F(1, 26) = 1.59, p = 0.22) or the overnight interval (F(1, 26) = 0.26, p = 0.62). Likewise, when controlling for age, we found no benefit of the nap on LS PI across the nap/wake interval (F(1,24) = 1.85, p = 0.19) and across the overnight interval (F(1, 24) = 0.80, p = 0.38).
Evaluation of Explicit Sequence Knowledge
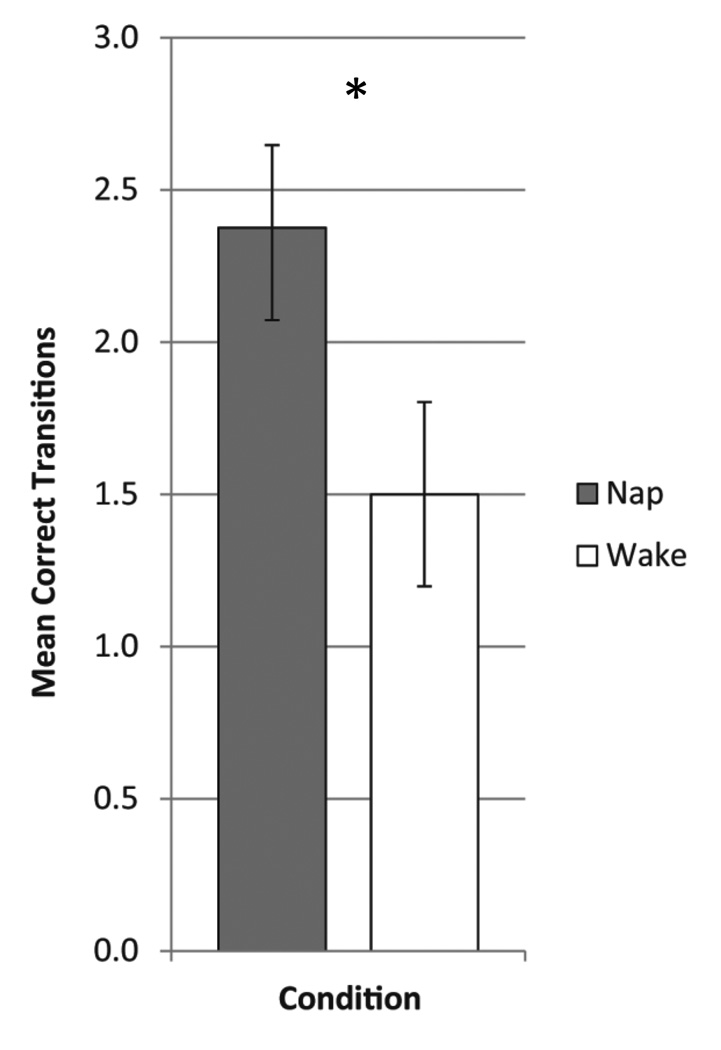
When evaluating the ability of children to reproduce the trained sequence in a free recall period, children were more successful when they napped as opposed to when they stayed awake (Figure 5; t(15) = 2.57, p = 0.02). On the whole, complete replication of the sequence was low, with only two children able to successfully replicate the full sequence in both conditions and one child who successfully reproduced the sequence in the nap condition only. To determine whether the RT PI was predicted by the level of explicit awareness in the nap condition, we used a linear regression. In the model predicting the overnight interval RT PI in the nap condition, the main effect of explicit recall was not significant (B = −0.43, p = 0.80). Moreover, age was not correlated with explicit sequence extraction in the nap condition (r(35) = 0.24, p = 0.38) or in the wake condition (r(35) = 0.48, p = 0.86). However, we recognize that the small number of participants who completed the explicit recall probe may hinder our ability to detect an effect.
Fig 5.
Explicit recall of the sequence measured as the number of correct transitions reproduced. Error bars represent standard error. *p < 0.05
Discussion
In this study, we replicate findings from previous research showing that children do not receive a benefit of a nap compared to restful wake in a motor skill learning task when performance is assessed shortly after the sleep (Fischer et al. 2007; Wilhelm et al. 2008; Prehn-Kristensen et al. 2009). Importantly however, performance benefits from the nap emerged following an additional delay, 24-hours after training. This study demonstrates that sleep does in fact promote the consolidation of general skill acquisition in preschool children, which clarifies a previously controversial question in research examining procedural memory consolidation in children. Instead of occurring across a single sleep bout, as has been shown in adults, we show that this benefit is instead reliant upon an extended delay that includes additional sleep.
There are, of course, several other factors that must be considered when interpreting these findings. While it is possible that napping compared to wake promotion lead to subsequent differences in alertness or emotionality, these are unlikely to have affected the results of this study. First, there were no differences in subjective ratings by the child or experimenter. Second, the significant effects of the nap manipulation were not observed until the following morning, after all children had slept overnight. These findings are also unlikely to be caused by time-of-day effects, as children were tested at the same time of day in both conditions.
We also considered whether superior performance in the nap condition reflects interference of waking activities on the skill memory, thus impairing it in the wake condition as opposed to a direct benefit in the nap condition. We consider this alternative hypothesis unlikely. First, the tasks engaged in during the wake conditions – puzzles, reading – would seem most likely to interfere with declarative, not motor skill learning. Yet, we have demonstrated a nap-specific benefit for declarative learning even when these quiet activities were used in the wake condition (Kurdziel et al., 2013). Second, since, the SRTT was learned at 10:00 am and post-nap/wake performance was probed at 3:30 pm, only the culminating 90 mins of this interval differed. In other words, both conditions faced the possibility of extensive interference – 4 hrs of reading, puzzles, outdoor play, lunch. If these activities were to cause interference, the greatest interfering effects take place early, shortly after learning (Wozniak, 1999), and equated across the two groups. Third, by this explanation, one would expect an immediate difference between nap and wake conditions when, in fact, no difference was observed immediately. Rather, benefits were found the following day. Fourth, if interference from waking activities during the nap accounted for diminished performance in the wake relative to the nap condition, the longer naps should protect memories to the greatest extent. Yet, we found no significant association between nap or overnight sleep time and the performance benefit. Nonetheless, future studies using polysomnography during sleep intervals would be beneficial in supporting the direct benefit of sleep on performance outcomes.
Performance benefits were not found directly after a nap compared to wake, rather performance gains attributable to the nap are observed the next day. Furthermore, we find that poorer performance following the nap strongly predicts better performance after an extended period of time (especially when controlling for age). Since these findings were not correlated with nap or overnight sleep duration, this suggests that the observed sleep benefit is tied more to the quality and architecture of sleep rather than the amount of sleep children received. These findings may be the result of neuroplastic processes occurring during sleep, similar to what has been observed in juvenile zebra finches (Deregnaucourt et al. 2005). In children, molecular and cellular mechanisms of plasticity (i.e. protein synthesis, dendritic spine formation) that are modulated by sleep (Graves et al. 2001; Benington et al. 2003; Yang et al. 2014) may impede procedural memory benefits directly following the nap, but may ultimately facilitate successful consolidation. Thus, when sleep is distributed across the day, as in early childhood, sleep-dependent cognitive processes may similarly occur over multiple sleep bouts.
Children exhibit extensive differences in neuroplasticity across several brain regions as compared to adults, including the motor and sensorimotor cortices (Casey et al. 2000; Rapoport and Gogtay 2008). Preschool children have greater synaptic density than adults (Huttenlocher, 1979) and have greater gray matter volume (Casey et al. 2000). Meanwhile, white matter tracts and myelination of axons are still developing (Caviness et al. 1996; Geidd et al. 1996). Sleep-dependent memory consolidation is thought to be dependent on changes in connectivity in the brain (for review see Bennington and Frank 2003). However, since children have vastly more synaptic connections, the increased neural reorganization resulting from consolidation may hinder immediate benefits of sleep on performance. Furthermore, explicit or declarative memory domains may be prioritized over implicit procedural skill across a single sleep bout, resulting in additional time or sleep bouts to impact behavior. Conversely, the more efficient neural systems of young adults may not need additional time awake or further sleep bouts to consolidate procedural skill (Walker et al. 2005). Whether such consecutive memory processes occur over further sleep bouts and whether they may be found in adult humans is worthy of future research.
It has been suggested that explicit sequence learning is consolidated over sleep while implicit sequence learning receives little or no benefit (Fischer et al. 2006; Spencer et al. 2006), perhaps due to the hippocampus’ role in explicit, episodic memory processing. For this reason, in this study, children were told that the cues would follow a sequence. Consequently, it was at the discretion of the child to discover and utilize the explicit sequence knowledge. In a subsample of children, we found that children demonstrated more explicit awareness of the sequence in the nap condition compared to the wake condition. Consistent with this, Wilhelm and colleagues (2013) reported that children extracted explicit knowledge of a motor sequence following sleep and, impressively, at a greater rate than adults. Moreover, extraction of explicit knowledge was associated with slow wave activity (SWA). Our finding that children exhibit greater sequence knowledge following sleep, although not reaching full awareness, nonetheless demonstrates that children at an even younger age may be capable of explicit sequence knowledge extraction. Interestingly, this coincides with a time where hippocampal growth is beginning to slow, approaching adult volume (Uematsu et al., 2012). Sleep, therefore, may contribute to the ability of a maturing hippocampus to extract these sequences in children. Age (as a proxy for hippocampal maturation) was not correlated with explicit sequence extraction in either the nap or wake conditions. However, it is notable that the short sequence length and small sample size for this measure may limit such conclusions. Moreover, awareness was only probed at 24-hour recall, thus we cannot ascertain whether such knowledge was extracted during the nap or overnight. Although naps lack rapid eye movement (REM) sleep in children (Kurdziel et al. 2013), 46% of the naps are spent in slow wave sleep (SWS), providing a mechanism by which the awareness could emerge. However, given that explicit awareness is enhanced by sleep in adults (Robertson et al 2004), and children (Wilhelm et al 2013), we posit that the emergence of explicit awareness was also delayed. Interestingly, some have shown that explicit awareness of a task actually impedes task performance in older adults, which suggests the interplay between explicit awareness and motor task performance develops along a trajectory that extends past young adulthood (Howard et al 2001). Future research may expound upon how explicit extraction of implicit motor sequences ties to the hippocampus, and the time course of explicit sequence extraction.
It is important to recognize that we found the benefit of sleep in the measure of RT but not in LS. Improvements in RT are the typical measure of sleep-dependent consolidation in studies using the motor sequence task in which the sequence is presented on a screen in full during a block (Walker et al. 2002; Wilhelm et al. 2008). Consistent with the typical SRTT paradigm, we included random blocks that allow for a probe of sequence-specific learning: if learning was sequence-specific, changes should be seen on sequence and not random blocks. The lack of significance for the LS PI over sleep compared to wake suggests that delayed nap-dependent improvements may not have been associated with consolidation of the sequence memory per se, but rather a general gain of motor efficiency on the task. Improvements in efficiency from a subsequent nap could reflect consolidation of learning of the general underlying skill required for the task (ballistic responses to a visual cue).
This study demonstrates that a nap during the day promotes the consolidation of procedural learning relative to an equivalent interval of wake, but that the benefits of the nap are only observed after an extended period of time. Furthermore, we show that sleep promotes the extraction of explicit knowledge in our SRTT. Neuroplastic processes of sleep in the immature brain may contribute to the lack of procedural memory benefit that has been documented in previous research.
Fig 4.
Less improvement across the nap interval predicted greater improvement across the overnight interval.
Acknowledgements
The authors would like to thank Tim Miller for programming the iPad task. This work was supported in part by National Institute of Health grant R01 HL111695 (to R.M.C.S.).
References
- Acebo C, Sadeh A, Seifer R, Tzischinsky O, Hafer A, Carskadon MA. Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1-to 5-year-old children. SLEEP. 2005;28(12):1568. doi: 10.1093/sleep/28.12.1568. [DOI] [PubMed] [Google Scholar]
- Albouy G, Sterpenich V, Vandewalle G, Darsaud A, Gais S, Rauchs G, Maquet P. Interaction between hippocampal and striatal systems predicts subsequent consolidation of motor sequence memory. PLoS ONE. 2013;8(3):1–10. doi: 10.1371/journal.pone.0059490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhaus J, Hoeckesfeld R, Born J, Hohagen F, Junghanns K. Immediate as well as delayed post learning sleep but not wakefulness enhances declarative memory consolidation in children. Neurobiology of Learning and Memory. 2008;89(1):76–80. doi: 10.1016/j.nlm.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Backhaus J, Junghanns K. Daytime naps improve procedural motor memory. Sleep Medicine. 2006;7(6):508–512. doi: 10.1016/j.sleep.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Benington JH, Frank MG. Cellular and molecular connections between sleep and synaptic plasticity. Progress in Neurobiology. 2003;69(2):71–101. doi: 10.1016/s0301-0082(03)00018-2. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54(1–3):241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Kennedy DN, Richelme C, Rademacher J, Filipek PA. The human brain age 7–11 years: A volumetric analysis based on magnetic resonance images. Cerebral Cortex. 1996;6(5):726–736. doi: 10.1093/cercor/6.5.726. [DOI] [PubMed] [Google Scholar]
- Deregnaucourt S, Mitra PP, Feher O, Pytte C, Tchernichovski O. How sleep affects the developmental learning of bird song. Nature. 2005;433(7027):710–716. doi: 10.1038/nature03275. [DOI] [PubMed] [Google Scholar]
- Fischer S, Drosopoulos S, Tsen J, Born J. Implicit learning–explicit knowing: a role for sleep in memory system interaction. Journal of Cognitive Neuroscience. 2006;18(3):311–319. [PubMed] [Google Scholar]
- Fischer S, Hallschmid M, Elsner AL, Born J. Sleep forms memory for finger skills. Proceedings of the National Academy of Sciences. 2002;99(18):11987–11991. doi: 10.1073/pnas.182178199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Wilhelm I, Born J. Developmental differences in sleep’s role for implicit off-line learning: comparing children with adults. Journal of Cognitive Neuroscience. 2007;19(2):214–227. doi: 10.1162/jocn.2007.19.2.214. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Luria R. Reliability, validity, and clinical application of the visual analogue mood scale. Psychological Medicine. 1973;3(04):479–486. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cerebral Cortex. 1996;6(4):551–559. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Graves L, Pack A, Abel T. Sleep and memory: a molecular perspective. Trends in Neurosciences. 2001;24(4):237–243. doi: 10.1016/s0166-2236(00)01744-6. [DOI] [PubMed] [Google Scholar]
- Harrington SA. Relationships of objectively measured physical activity and sleep with BMI and academic outcomes in 8-year-old children. Applied Nursing Research. 2013;26(2):63–70. doi: 10.1016/j.apnr.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Haywood K, Getchell N. Lifespan Motor Development. 5th ed. Champaign, IL: Human Kinetics; 2009. [Google Scholar]
- Howard DV, Howard JH. When it does hurt to try: Adult age differences in the effects of instructions on implicit pattern learning. Psychonomic Bulletin & Review. 2001;8(4):798–805. doi: 10.3758/bf03196220. [DOI] [PubMed] [Google Scholar]
- Hultsch H, Todt D. Approaches to the mechanisms of song memorization and singing provide evidence for a procedural memory. Annals of the Brazilian Academy of Sciences. 2004;76(2):219–230. doi: 10.1590/s0001-37652004000200005. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P. Synaptic density in human frontal cortex — Developmental changes and effects of aging. Brain Research. 1979;163(2):195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Pascual-Leone A, Press DZ. Awareness modifies the skill-learning benefits of sleep. Current Biology. 2004;14(3):208–212. doi: 10.1016/j.cub.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: Reference values and generational trends. Pediatrics. 2003;111(2):302–307. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- Kurdziel L, Duclos K, Spencer RMC. Sleep spindles in midday naps enhance learning in preschool children. Proceedings of the National Academy of Sciences. 2013;110(43):17267–17272. doi: 10.1073/pnas.1306418110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado CC, Bentley AJ, Mitchell D. A pictorial sleepiness scale based on cartoon faces. Sleep. 2004;27(3):541–548. doi: 10.1093/sleep/27.3.541. [DOI] [PubMed] [Google Scholar]
- Montgomery-Downs HE, O’Brien LM, Gulliver TE, Gozal D. Polysomnographic characteristics in normal preschool and early school-aged children. Pediatrics. 2006;117(3):741–753. doi: 10.1542/peds.2005-1067. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cognitive Psychology. 1987;19(1):1–32. [Google Scholar]
- Pace-Schott EF, Spencer RMC. Age-related changes in consolidation of perceptual and muscle-based learning of motor skills. Frontiers in Aging Neuroscience. 2013;5(83):1–7. doi: 10.3389/fnagi.2013.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehn-Kristensen A, Göder R, Chirobeja S, Breßmann I, Ferstl R, Baving L. Sleep in children enhances preferentially emotional declarative but not procedural memories. Journal of Experimental Child Psychology. 2009;104(1):132–139. doi: 10.1016/j.jecp.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Gogtay N. Brain neuroplasticity in healthy, hyperactive and psychotic children: Insights from neuroimaging. Neuropsychopharmacology. 2008;33(1):181–197. doi: 10.1038/sj.npp.1301553. [DOI] [PubMed] [Google Scholar]
- Rauchs G, Bertran F, Guillery-Girard B, Desgranges B, Kerrouche N, Denise P, Eustache F. Consolidation of strictly episodic memories mainly requires rapid eye movement sleep. Sleep. 2004;27(3):395–401. doi: 10.1093/sleep/27.3.395. [DOI] [PubMed] [Google Scholar]
- Savion-Lemieux T, Bailey JA, Penhune VB. Developmental contributions to motor sequence learning. Experimental Brain Research. 2009;195(2):293–306. doi: 10.1007/s00221-009-1786-5. [DOI] [PubMed] [Google Scholar]
- Song S, Howard JH, Howard DV. Sleep does not benefit probabilistic motor sequence learning. Journal of Neuroscience. 2007;27(46):12475–12483. doi: 10.1523/JNEUROSCI.2062-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer RMC, Gouw AM, Ivry RB. Age-related decline of sleep-dependent consolidation. Learning & Memory. 2007;14(7):480–484. doi: 10.1101/lm.569407. [DOI] [PubMed] [Google Scholar]
- Spencer RMC, Sunm M, Ivry RB. Sleep-dependent consolidation of contextual learning. Current Biology. 2006;16(10):1001–1005. doi: 10.1016/j.cub.2006.03.094. [DOI] [PubMed] [Google Scholar]
- Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437(7063):1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Walker MP. Sleep-dependent memory consolidation and reconsolidation. Sleep Medicine. 2007;8(4):331–343. doi: 10.1016/j.sleep.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas KM, Nelson CA. Serial reaction time learning in preschool and school-age children. Journal of Experimental Child Psychology. 2001;79:364–387. doi: 10.1006/jecp.2000.2613. [DOI] [PubMed] [Google Scholar]
- Uematsu A, Matsui M, Tanaka C, Takahashi T, Noguchi K, Suzuki M, Nishijo H. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS ONE. 2012;7(10):1–10. doi: 10.1371/journal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35(1):205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44(1):121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R, Alsop D, Gaab N, Schlaug G. Sleep-dependent motor memory plasticity in the human brain. Neuroscience. 2005;133(4):911–917. doi: 10.1016/j.neuroscience.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Weissbluth M. Naps in children; 6 months-7 years. Sleep. 1995;18(2):82–87. doi: 10.1093/sleep/18.2.82. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Diekelmann S, Born J. Sleep in children improves memory performance on declarative but not procedural tasks. Learning & Memory. 2008;15(5):373–377. doi: 10.1101/lm.803708. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Metzkow-Mészàros M, Knapp S, Born J. Sleep-dependent consolidation of procedural motor memories in children and adults: the pre-sleep level of performance matters: Sleep-dependent consolidation of motor memories. Developmental Science. 2012;15(4):506–515. doi: 10.1111/j.1467-7687.2012.01146.x. [DOI] [PubMed] [Google Scholar]
- Wilhelm I, Rose M, Imhof KI, Rasch B, Büchel C, Born J. The sleeping child outplays the adult’s capacity to convert implicit into explicit knowledge. Nature Neuroscience. 2013;16(4):391–393. doi: 10.1038/nn.3343. [DOI] [PubMed] [Google Scholar]
- Wilson JK, Baran B, Pace-Schott EF, Ivry RB, Spencer RMC. Sleep modulates word-pair learning but not motor sequence learning in healthy older adults. Neurobiology of Aging. 2012;33(5):991–1000. doi: 10.1016/j.neurobiolaging.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak RH. Introduction to memory: Hermann Ebbinghaus (1885/1913) Classics in the History of Psychology. 1999 [Google Scholar]
- Yang G, Lai CSW, Cichon J, Ma L, Li W, Gan WB. Sleep promotes branch-specific formation of dendritic spines after learning. Science. 2014;344(6188):1173–1178. doi: 10.1126/science.1249098. [DOI] [PMC free article] [PubMed] [Google Scholar]