Abstract
Rationale
Scopolamine, a muscarinic antagonist, impairs learning and memory for many tasks, supporting an important role for the cholinergic system in these cognitive functions. The findings are most often interpreted to indicate that a decrease in postsynaptic muscarinic receptor activation mediates the memory impairments. However, scopolamine also results in increased release of acetylcholine in the brain as a result of blocking presynaptic muscarinic receptors.
Objectives
The present experiments assess whether scopolamine-induced increases in acetylcholine release may impair memory by overstimulating postsynaptic cholinergic nicotinic receptors, i.e., by reaching the high end of a nicotinic receptor activation inverted-U dose-response function.
Results
Rats tested in a spontaneous alternation task showed dose-dependent working memory deficits with systemic injections of mecamylamine and scopolamine. When an amnestic dose of scopolamine (0.15 mg/kg) was co-administered with a subamnestic dose of mecamylamine (0.25 mg/kg), this dose of mecamylamine significantly attenuated the scopolamine-induced memory impairments. We next assessed the levels of acetylcholine release in the hippocampus in the presence of scopolamine and mecamylamine. Mecamylamine injections resulted in decreased release of acetylcholine, while scopolamine administration caused a large increase in acetylcholine release.
Conclusions
These findings indicate that a nicotinic antagonist can attenuate impairments in memory produced by a muscarinic antagonist. The nicotinic antagonist may block excessive activation of nicotinic receptors postsynaptically or attenuate increases in acetylcholine release presynaptically. Either effect of a nicotinic antagonist—to decrease scopolamine-induced increases in acetylcholine output or to decrease post-synaptic acetylcholine receptor activation—may mediate the negative effects on memory of muscarinic antagonists.
Keywords: Cholinergic, In vivo microdialysis, Nicotinic, Muscarinic, Hippocampus, Spatial working memory, Aging, Alzheimer, Autoreceptor, Cholinesterase inhibitor
Introduction
Early studies of the role of brain acetylcholine systems in learning and memory showed that cholinergic antagonists impaired memory and agonists enhanced memory (e.g., Deutsch 1971; Drachman 1977). Acetylcholine receptor antagonists directed at either muscarinic or nicotinic receptors impair learning and memory for a wide range of tasks and species. Conversely, muscarinic agonists, nicotinic agonists, and cholinesterase inhibitors enhance learning and memory (Gold et al. 2013; Hasselmo 2006; Kutlu and Gould 2015; Morris et al. 2013; Pepeu and Giovannini 2010).
The general findings regarding acetylcholine functions in memory, obtained largely in rodents, were later coupled with evidence of loss of cholinergic functions in the brains of patients with Alzheimer's disease (Bartus et al. 1982; Coyle et al. 1983). The association of cholinergic dysfunction with dementia contributed importantly to a plethora of experiments using cholinergic receptor antagonists in rodents and other animals to model Alzheimer's disease by inducing memory loss with cholinergic receptor antagonists and by reversing memory loss and enhancing memory with cholinergic agonists.
Important to the present experiment is evidence that cholinergic agonists enhance memory at moderate doses but impair memory at higher doses, i.e., they affect memory in an inverted-U dose-response manner (e.g., Braida et al. 1996; MacLeod et al. 2006; Robbins et al. 1997; Terry et al. 1997); included in such reports is evidence that cholinesterase inhibitors also enhance learning and memory in an inverted-U dose-response manner (e.g., Braida et al. 1996; Stratton and Petrinovich 1963; Wanibuchi et al. 1994). One interpretation of the inverted-U effects of cholinergic agonists on memory is that while moderate increases in acetylcholine may enhance memory, high levels may result in cholinergic block thereby opposing the presumed benefits of activation of postsynaptic acetylcholine receptors.
A similar interpretation may also apply to the memory impairments produced by muscarinic receptor antagonists. There is substantial evidence that treatment with muscarinic antagonists results in large increases in acetylcholine release (Liu and Kato 1994; Mishima et al. 2000; Moor et al. 1995; Moore et al. 1996; Quirion et al. 1994; Stillman et al. 1996; Vannucchi et al. 1997), with decreases in release in the presence of muscarinic agonists or cholinesterase inhibitors (Bruno et al. 2006; Moor et al. 1995). Additionally, increases in cholinergic neuronal firing can be seen with application of scopolamine and decreases with muscarinic agonists (Givens and Olton 1995; Sakai et al. 1990). These results suggest that, in addition to postsynaptic actions, acetylcholine activates presynaptic autoreceptors (mostly M2 subtype) to limit the levels of acetylcholine released by the cholinergic neurons (Hasselmo and Sarter 2011; Hoss et al. 1990; Nordstrom and Bartfai 1980). In contrast, muscarinic receptor antagonists may block negative feedback mechanisms thereby decreasing the neurotransmitter feedback resulting in increased release of acetylcholine. An analogous interpretation does not apply well to nicotinic receptor antagonists. Unlike muscarinic autoreceptors, nicotinic autoreceptors have a positive feedback influence on acetylcholine release, such that activation of nicotinic autoreceptors increases acetylcholine release (Bruno et al. 2006; Wilkie et al. 1996; Wonnacott et al. 1996).
The inverted-U dose-response curves seen with cholinergic agonists suggest that an optimal level of acetylcholine release is necessary for neuronal processing during learning and memory activities. Cholinergic tone that is too low or too high is related to poor cognitive functions, depending on the task (Hasselmo and McGaughy 2004; Hasselmo and Sarter 2011). Thus, while scopolamine can have amnesic effects due to antagonism of postsynaptic muscarinic receptors, it is also possible that excessive acetylcholine release due to presynaptic muscarinic autoreceptor block may also be detrimental to memory formation. The present report tests one prediction of this view that a nicotinic antagonist might attenuate the impairing effects on memory of a muscarinic antagonist. This experiment used the nicotinic receptor antagonist, mecamylamine, to challenge the impairment in memory produced by the muscarinic receptor antagonist, scopolamine. In addition, we examined acetylcholine release during training in the presence and absence of either and both acetylcholine receptor antagonists. Specifically, we co-injected an amnestic dose of scopolamine and a low sub-amnestic dose of mecamylamine and assessed the effects on memory and on acetylcholine release. The rationale was that, if the increased release of acetylcholine is a significant contributor to memory impairments induced by scopolamine, the addition of mecamylamine would reduce these impairments by limiting the effects of excessive acetylcholine activation of nicotinic receptors.
Materials and methods
Animals
Male Sprague-Dawley rats (N=36; Harlan Laboratories, Indianapolis, IN), approximately 90–100 days old, were housed individually on a 12-h/12-h light-dark cycle (on 7:00 a.m.) with continuous access to food and water. All procedures described in this paper were approved by the University of Illinois Urbana-Champaign Institutional Animal Care and Use Committee in accordance with guidelines outlined in Guide for Care and Use of Laboratory Animals and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Spontaneous alternation testing
Each rat was handled for several minutes/day on each of the 5 days prior to testing. During behavioral testing, animals were placed on a 4-arm, plus-shaped maze (arms 45 cm long, 14 cm wide, 7.5 cm tall; center area 14×14 cm) constructed of opaque, black Plexiglas, as described previously (Newman et al. 2011). The maze was located in the center of the testing rooms on a table 76 cm above the floor surrounded by a rich assortment of extra-maze visual cues. Animals were tested between 9:00 a.m. and 12:00 p.m.
During the spontaneous alternation task, the rat was allotted 20 min with no interruption to explore the 4-arm maze. Each arm entry was recorded. An entry was defined as all four paws crossing an arm entrance. The entries were then evaluated to determine the number of successful alternations. A successful alternation consisted of the rat visiting all four possible arms across every five arms that the rat entered. The spontaneous alternation score was computed by dividing the number of successful alternations by the number of possible alternations times 100. With this measure, chance performance is 44 %.
All drugs were obtained from Sigma-Aldrich (St. Louis, MO, USA). A repeated testing design was used in which eight rats received subcutaneous injections (0.5 ml/kg) of mecamylamine hydrochloride (0.25, 0.5, 1.5, and 4.5 mg/kg) or saline 30 min prior to testing, and another group of eight rats received scopolamine hydrobromide (0.02, 0.05, and 0.15 mg/kg), 0.25 mg/kg mecamylamine+0.15 mg/kg scopolamine, 0.5 mg/kg mecamylamine+0.15 mg/kg scopolamine, or saline. Animals were given 48 h between testing sessions to allow drug effects to dissipate. In addition, the doses were given in a counterbalanced order to control for possible anterograde effects of injections on behavioral scores.
Acetylcholine measures testing drug×behavior interactions
Separate groups of rats were prepared surgically for microdialysis measures of acetylcholine release during spontaneous alternation testing. Under isofluorane (2–4 % v/v) anesthesia, rats received guide cannulae (CMA 12 Guide Cannula; CMA Microdialysis AB, Holliston, MA) for microdialysis probes approximately 10 days before behavioral testing. The guide cannulae were lowered through holes in the skull above the ventral hippocampus (coordinates 5.6 mm posterior to bregma, ±5.0 mm lateral and 3.8 mm ventral from the surface of the skull). The cannulae were anchored in place with dental cement affixed to four skull screws surrounding the cannulae. Dummy probes, which were flushed with the cannulae tips, were inserted into the cannulae until the start of microdialysis procedures. To avoid attrition during the dialysis sampling time of over 4 h total, we used bilateral probes and dialysis collections for all rats. In nine rats, one probe either gave poor recovery or was blocked. For these rats, dialysis was performed on the contralateral side. In all rats, only dialysis from a single side was used for data collection.
During all microdialysis procedures, the microdialysis probes (20 kD CMA 12 Elite Microdialysis Probe with a membrane length of 2 mm, CMA Microdialysis AD, Holliston, MA) were perfused continuously at a rate of 1.0 μl/min with artificial cerebrospinal fluid (aCSF 128 mM NaCl, 2.5 mM KCl, 1.3 mM CaCl2, 2.1 mM MgCl2, 0.9 mM NaH2PO4, 2.0 mM Na2HPO4, 1.0 mM dextrose, 200 nM neostigmine, pH=7.4). The inclusion of neostigmine protects acetylcholine in the dialysate from being hydrolyzed after collection from the extracellular fluid thereby increasing the measurable amount of acetylcholine in the dialysate (Chang et al. 2006; Himmelheber et al. 1998; Liu and Kato 1994; Noori et al. 2012).
Prior to insertion of microdialysis probes into the hippocampus, each probe was placed in a 100-nM acetylcholine solution for 10 min (10-μl dialysate sample) to assess probe efficiency. After, the 100 nM acetylcholine standards were collected, and the probes were inserted via the guide cannulae into the hippocampus. Rats were then placed into a holding cage with fresh bedding and allowed to rest for 1 h. The initial 1-h time permitted stabilization of the perfusion site; samples collected during this time were discarded. After this, dialysate samples were collected every 10 min into 0.5-μL microcentrifuge tubes. The first three samples were collected as a measure of baseline acetylcholine levels. After the baseline sampling period, an injection of saline, 0.15 mg/kg scopolamine, 0.25 mg/kg mecamylamine, or injections of both scopolamine and mecamylamine doses (Ns=5 for each dose) were given 30 min prior to spontaneous alternation testing. The rats were then tested behaviorally, while samples continued to be collected every 10 min for 20 min. Eight more samples were collected after injection. The samples were sealed and stored at –20 °C until they were assayed for acetylcholine content.
High-performance liquid chromatography
Microdialysis samples were assayed for acetylcholine using high-performance liquid chromatography with electrochemical detection (Bioanalytical Systems Inc. (BASi), West Lafayette, IN, USA). During the analysis of the samples, a LC-10AD VP Shimadzu Liquid Chromatograph pump (Shimadzu Scientific Instruments, Columbia, MD, USA) continued to pump the fresh mobile phase (50 mM NaH2PO4, 0.5 mM EDTA, 0.005 % ProClin, pH=8.5) at a rate of 0.14 ml/min. Five-microliter samples were manually injected into the system via an injection port using a 10-μL loop (Rheodyne, Rohnert Park, CA, USA). Choline was first removed using a choline oxidase immobilized enzyme reactor (BASi UniJet P/N MF-8901). Samples were then separated using an ion-exchange microbore analytical column (BASi UniJet P/N MF-8904, 530 mm×1 mm) followed by a microbore ACh/choline immobilized enzyme reactor containing acetylcholinesterase and choline oxidase (BASi UniJet P/N MF-8903, 50 mm×1 mm). Electrochemical detection was performed by a 3-mm glassy fiber working electrode (BASi P/N MF-1095) coated with a redox polymer film containing horseradish peroxidase and an auxiliary electrode with a radial flow electrochemical thin-layer cell and a 13-mm thin-layer gasket (BASi P/N MF-1091). The working electrode held a 100 mV potential relative to an Ag/AgCl reference electrode (BASi P/N MF-2078). Assays were completed within 12.5 min. The detection limit of this system was approximately 5 fmol. Acetylcholine standards of 12.5, 25, 50, 100, 200, and 1000 nM were injected in triplicate to assure consistent readings and a linear curve. The acetylcholine peaks appeared at 7.8 min into the chromatograph. The height of the peak was used and compared to the acetylcholine standard curve to get the concentration of the extracellular acetylcholine in each sample. Microdialysis samples taken from the 100-nM acetylcholine standard were used to adjust readings for probe detection limitations. Acetylcholine standards (100 nM) were assayed after every seven or eight samples to ensure that there was no shift in detection throughout the assay.
Histology
Immediately after the last microdialysis sample was taken, rats received an overdose of sodium pentobarbital and were perfused transcardially 0.9 % NaCl saline followed by 4 % paraformaldehyde. Brains were removed and stored in 4 % paraformaldehyde for 48 h before being transferred to 20 % glycerol in phosphate-buffered solution (PBS) for another 48 h or until the brain sank. A notch was made in the right hemisphere. Brains were then frozen and 40-μm slices were collected with a cryostat targeting the ventral hippocampus. The collected sections were stained with cresyl violet and assessed for accurate microdialysis cannulae placement.
Statistical analysis
Statistics were performed using SPSS v20 (IBM, Armonk, NY, USA). The effects of scopolamine and mecamylamine on working memory were assessed using repeated measures ANOVAs. Bonferroni corrected planned multiple comparisons were done to compare scopolamine or mecamylamine doses to saline. Differences in acetylcholine release were analyzed through univariate or mixed factors ANOVAs with mecamylamine and scopolamine as between-subjects factors and time (10-min samples) as a within-subjects factor.
Results
Spatial working memory
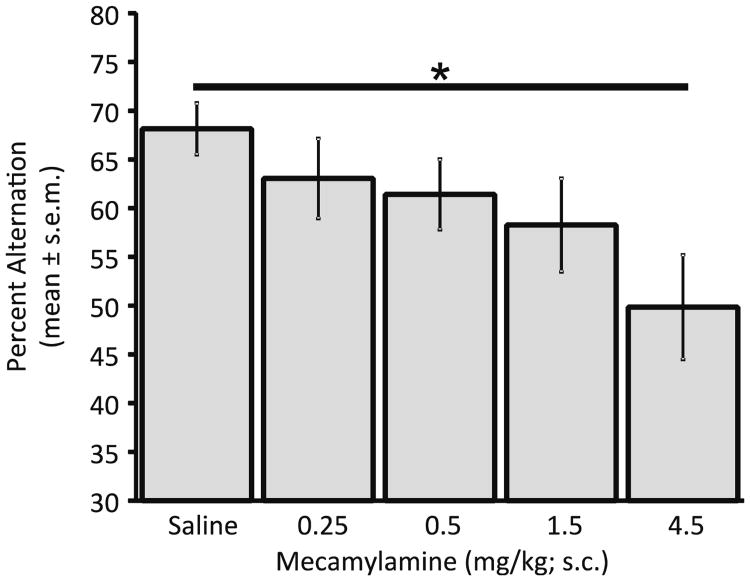
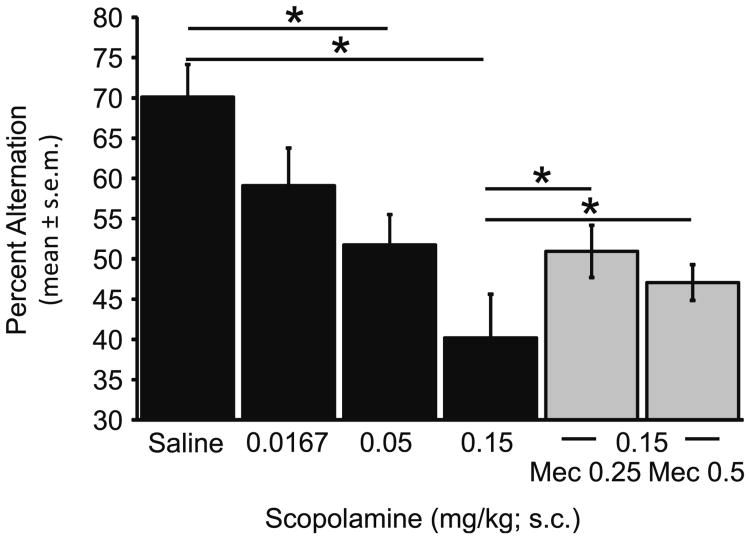
Rats showed dose-dependent decreases in the spontaneous alternation scores after mecamylamine (Fig. 1) or scopolamine (Fig. 2; mecamylamine: F4,28=4.75, p<0.01; saline vs. Mec 4.5 mg/kg: t7=4.83, p<0.005; scopolamine: F3,21= 11.71, p<0.001; saline vs. scop 0.05 mg/kg: t7=2.88, p<0.05, saline vs. scop 0.15 mg/kg: t7=6.18, p<0.001). Thus, both drugs impaired alternation scores at the high doses tested. When 0.25 mg/kg or 0.50 mg/kg doses of mecamylamine, which did not individually impair alternation scores, were paired with a 0.15 mg/kg dose of scopolamine, there was a partial reversal of the scopolamine-induced impairment (Fig. 2, right bars). Both doses of mecamylamine resulted in increases in spontaneous alternation scores as compared to those seen in the scopolamine alone condition (scop 0.15 mg/kg vs. scop 0.15/Mec 0.25: t7=3.17, p<0.05; 0.15 mg/kg scopolamine vs. scop 0.15/mec 0.5: t7=2.61, p<0.05).
Fig. 1.
Mecamylamine effects on spatial working memory. At higher doses, mecamylamine significantly impaired spontaneous alternation scores (*p<0.05)
Fig. 2.
Scopolamine and scopolamine+mecamylamine effects on spatial working memory (*p<0.05)
With the exception of the 4.5 mg/kg mecamylamine group, there was no significant effect of treatment on the number of arm entries (range=29.8±4.7 to 35.4±3.7). The group that received the high 4.5 mg/kg dose of mecamylamine had significantly fewer arm entries (20.5±2.9) than did the saline group (35.4±3.7) (t7=4.21, p<0.05). There were no other drug effects on arm entries (p>0.1).
Acetylcholine microdialysis
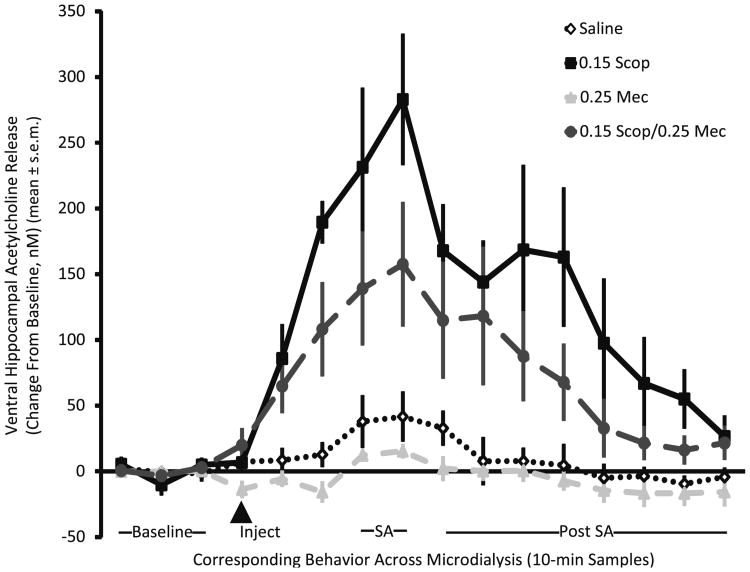
As shown in Fig. 3, scopolamine injections increased acetylcholine output in the samples taken throughout microdialysis (scopolamine F1,16=27.06, p<0.001). Conversely, mecamylamine injections resulted in a slight but significant decrease in acetylcholine output (F1,16=4.66, p<0.05). There was no mecamylamine×scopolamine interaction suggesting that when the drugs were combined, the scopolamine enhancement of acetylcholine release was attenuated by mecamylamine to a similar degree as when mecamylamine was given alone (p>0.3).
Fig. 3.
Acetylcholine release after saline, scopolamine, mecamylamine, or scopolaomine+mecamylamine injections. Saline (diamond), 0.15 scopolamine (square), 0.25 mecamylamine (triangle), and 0.15 scopolamine+0.25 mecamylamine (circle)
Significant increases in acetylcholine release were evident during spontaneous alternation testing. The increase in acetylcholine release after scopolamine continued into behavioral testing, complicating determining whether the increases during alternation testing were a result of the testing per se or were a continuation of the rise in acetylcholine release after scopolamine treatment. Of note, acetylcholine output increased during alternation testing in rats both in the groups that did and did not have scopolamine prior to testing (without scopolamine, t9=3.47, p<0.01; with scopolamine, t9=2.28, p<0.05). These analyses indicate that behavioral testing increased acetylcholine release as seen previously (Ragozzino et al. 1996, 1998).
All animals included in the study had accurate ventral hippocampal microdialysis probe placements (Fig. 4).
Fig. 4. Ventral hippocampus microdialysis placement.
The behavioral results obtained in rats tested during microdialysis procedures were generally consistent with those reported with unimplanted rats in Fig. 2. However, these rats were more variable, and alternation scores were lower in both the saline and low-dose mecamylamine rats, reducing the effect size of the scopolamine vs. control impairment, which here was not significantly different but exhibited a trend in the direction of scopolamine impairment in the microdialysis-tested rats (p<0.2). As before, the combination mecamylamine+scopolamine group did not differ from that of controls (p>0.7).
Discussion
At high doses, mecamylamine and scopolamine both decreased spontaneous alternation scores, reflecting the ability of both nicotinic and muscarinic receptor antagonists to impair working memory (Figs. 1 and 2). These results support previous findings that also showed that blocking nicotinic or muscarinic receptors can impair working memory (Hasselmo 2006; Kutlu and Gould 2015; Morris et al. 2013; Pepeu and Giovannini 2010), though there is also a report that a low dose of mecamylamine (0.125 mg/kg) can itself enhance learning in rats (Levin and Caldwell 2006). Of particular interest, the memory impairment induced by the muscarinic receptor antagonist was partially attenuated by the concomitant administration of the nicotinic receptor antagonist, which was administered at a dose that did not itself impair memory.
These findings are consistent with the possibility that scopolamine results in increase of acetylcholine to supraphysiological levels that may themselves be amnestic. The neurochemical results confirm the large increase in acetylcholine output in the hippocampus after scopolamine injections reported by others (Mishima et al. 2000; Moor et al. 1995; Quirion et al. 1994; Vannucchi et al. 1997). The increased output of acetylcholine after administration of scopolamine is consistent with the view that the drug acts at presynaptic muscarinic autoreceptors that would otherwise limit the release of acetylcholine, resulting in high acetylcholine levels that can possibly interfere with memory processing. Thus, impairments of memory by muscarinic antagonists may reflect actions at presynaptic muscarinic receptors. The high acetylcholine levels may interfere with memory by reaching the top of an inverted-U relationship between acetylcholine and memory (e.g., MacLeod et al. 2006; Robbins et al. 1997), perhaps by attaining cholinergic block.
Most interpretations of scopolamine-induced impairments of learning and memory attribute the amnestic effects to interference with postsynaptic muscarinic receptors necessary for the cognitive processes. In contrast, the present results suggest that an increase in acetylcholine release may contribute to the amnestic properties of scopolamine, and presumably other muscarinic receptor antagonists, by achieving levels of acetylcholine that reach the upper impairing range of an inverted-U function. A nicotinic receptor antagonist reduces scopolamine-induced memory impairments, suggesting further that the overstimulation of ACh release may impair learning and memory via nicotinic receptors. However, the neurochemical results also open the possibility that mecamylamine may act not only by blocking overexcitation of postsynaptic receptors per se but also indirectly by inactivating feed-forward presynaptic nicotinic receptors. The results also show that the nicotinic receptor antagonist not only reduces the high levels of acetylcholine release after scopolamine but also significantly, if modestly, reduces acetylcholine release during memory testing, a result similar to those obtained before (Tani et al. 1998). Thus, the mitigating effects on memory of the low mecamylamine dose may be due to mecamylamine attenuating the effect of acetylcholine release on postsynaptic nicotinic receptors or to mecamylamine decreasing acetylcholine release through presynaptic nicotinic autoreceptors, or both. Together, the results of the present experiments support the hypothesis that either too much or too little acetylcholine can contribute to disruptions in mnemonic processing (Deutsch 1971; Hasselmo and McGaughy 2004; Hasselmo and Sarter 2011).
The amnesia produced by high levels of neurotransmitters has been seen before with many examples of inverted-U dose-response curves with drugs that increase neurotransmitter, hormone, and other neurobiological functions, showing enhancement of memory at moderate doses and impairment of memory at high doses (Baldi and Bucherelli 2005; Calabrese 2008; Gold 2006; Gold and Korol 2012; Koob 1991; Mattson 2008).
In addition to the reduced memory impairments in rats that received scopolamine plus mecamylamine, scopolamine-induced memory impairments can also be attenuated by other substances including glucose, nicotinic and muscarinic receptor agonists, and cholinesterase inhibitors (e.g., Bejar et al. 1999; Higgins et al. 2002; Nakahara et al. 1990; Ragozzino et al. 1994; Snyder et al. 2005; Stone et al. 1988, 1991). In the presence of muscarinic antagonists, both M1 and M2 selective agonists can improve memory deficits, with the M1 agonists potentially reversing some of the postsynaptic effects of scopolamine and the M2 potentially reducing the amount of acetylcholine release (Chambon et al. 2012; Nakahara et al. 1990; Stone et al. 1991; Wanibuchi et al. 1994).
Administration of cholinesterase inhibitors enhances learning, memory, and attention processes and reverses deficits in an inverted-U dose-response manner (Bejar et al. 1999; Braida et al. 1996; Deutsch 1971; Robbins et al. 1997; Stratton and Petrinovich 1963; Yoshida and Suzuki 1993). Cholinesterase inhibitors can also attenuate scopolamine-induced memory impairments (Bejar et al. 1999; Braida et al. 1996; Higgins et al. 2002; Snyder et al. 2005). If scopolamine impairs memory by increasing acetylcholine release, this effect of cholinesterase inhibitors to blunt the actions of scopolamine appears counterintuitive, as it would seem that this would further increase acetylcholine in the synapse. However, microdialysis studies have shown that if the concentration of cholinesterase inhibitors in the dialysate is high, the amount of acetylcholine in the perfusate decreases (Liu and Kato 1994; Noori et al. 2012). Therefore, depending on the concentrations used, it is possible that cholinesterase inhibitors in combination with scopolamine can lead to a decreased release of acetylcholine. The muscarinic autoreceptor activation with the addition of cholinesterase inhibitors could also account for the memory impairments of cholinesterase inhibitors at higher doses in several species and training conditions, effects seen in early and recent reports (e.g., Stratton and Petrinovich 1963; Bohdanecký and Jarvik 1967; Hamburg 1967; Deutsch 1971; Bejar et al. 1999; Pan et al. 2006) and for the inverted-U dose-response curve in the effective amelioration of the scopolamine deficit (Braida et al. 1996; Flood et al. 1983; Wanibuchi et al. 1994). Furthermore, some findings suggest that scopolamine increases the activity of the enzyme, leading to scopolamine potentially decreasing the efficacy of cholinesterase inhibitors (Kato 1972; Tota et al. 2012).
These results could have important implications for the treatment of dementia in aging and Alzheimer's disease. Previous work has shown in addition to cholinergic transmission loss in aging and Alzheimer's disease that there is also a reduction in muscarinic receptors with some studies finding a specific loss of M2 receptors (Araujo et al. 2011; Banuelos et al. 2013; Decossas et al. 2005; Grothe et al. 2012; Mash et al. 1985; Mesulam 2013). Aged individuals and patients with Alzheimer's disease also show greater cognitive impairment with lower doses of scopolamine (Huff et al. 1988). This could be an indication that the cholinergic system in aged and Alzheimer patients does not regulate acetylcholine release as well. Treatment with acetylcholinesterase inhibitors has also been shown to significantly decrease the number of muscarin-ic autoreceptors (Araujo et al. 2011) potentiating the unregulated release of acetylcholine. Aged animals also show increased sensitivity of nicotinic autoreceptors, which might lead to increased release of acetylcholine at the remaining cholinergic neurons (Grilly et al. 2000; Wu et al. 2003).
Thus, the interpretation of the drug effects on acetylcholine functions in learning and memory is complicated by multiple controls over acetylcholine release together with evidence that too much or too little acetylcholine can cause mnemonic impairments. Changes that lead to the loss of the control of acetylcholine release, such as with the administration of scopolamine or during aging and Alzheimer's disease, contribute to memory impairments. While there has been a focus on methods to increase acetylcholine release to combat dementia, the current data suggest that this could lead to mnemonic problems as well. The findings shown here suggest that overactivation of nicotinic receptors may lead to mnemonic impairments, and high levels of acetylcholine can be detrimental to memory. If cholinesterase inhibitors attenuate the release of acetylcholine through activity on M2 autoreceptors, this may lead to lower release when the cholinergic system is activated, such as during times of learning and memory. Therefore, cholinesterase inhibitors may actually decrease acetylcholine release in a system already compromised during aging and Alzheimer's disease.
Acknowledgments
Special thanks to Jamie Richards, Disha Goswami, Emily Pajerski, Huzefa Chinwala, Laura Manning, Sydney Muchnik, Heather Lin, and Fiona Weingartner for their valuable participation on this project.
Supported by NSF IOS 13-18490, the Alzheimer's Association, and the Syracuse University Center for Aging and Policy Studies (NIA P30 AG034464). L.A.N. was a postdoctoral trainee on NICHD Training Grant HD00733.
References
- Araujo JA, Nobrega JN, Raymond R, Milgram NW. Aged dogs demonstrate both increased sensitivity to scopolamine impairment and decreased muscarinic receptor density. Pharmacol Biochem Behav. 2011;98:203–209. doi: 10.1016/j.pbb.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Baldi E, Bucherelli C. The inverted “U-shaped” dose-effect relationships in learning and memory: modulation of arousal and consolidation. Nonlinearity in Biology, Toxicology, Medicine 3: nonlin-003. 2005 doi: 10.2201/nonlin.003.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuelos C, LaSarge CL, McQuail JA, Hartman JJ, Gilbert RJ, Ormerod BK, Bizon JL. Age-related changes in rostral basal forebrain cholinergic and GABAergic projection neurons: relationship with spatial impairment. Neurobiol Aging. 2013;34:845–862. doi: 10.1016/j.neurobiolaging.2012.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Bejar C, Wang RH, Weinstock M. Effect of rivastigmine on scopolamine-induced memory impairment in rats. Eur J Pharmacol. 1999;383:231–240. doi: 10.1016/s0014-2999(99)00643-3. [DOI] [PubMed] [Google Scholar]
- Bohdanecký Z, Jarvik ME. Impairment of one-trial passive avoidance learning in mice by scopolamine, scopolamine methylbromide, and physostigmine. Int J Neuropharmacol. 1967;6:217–222. doi: 10.1016/0028-3908(67)90008-1. [DOI] [PubMed] [Google Scholar]
- Braida D, Paladini E, Griffini P, Lamperti M, Maggi A, Sala M. An inverted U-shaped curve for heptylphysostigmine on radial maze performance inrats: comparison with other cholinesterase inhibitors. Eur J Pharmacol. 1996;302:13–20. doi: 10.1016/0014-2999(96)00072-6. [DOI] [PubMed] [Google Scholar]
- Bruno JP, Gash C, Martin B, Zmarowski A, Pomerleau F, Burmeister J, Huettl P, Gerhardt GA. Second-by-second measurement of acetylcholine release in prefrontal cortex. Eur J Neurosci. 2006;24:2749–2757. doi: 10.1111/j.1460-9568.2006.05176.x. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Converging concepts: adaptive response, preconditioning, and the Yerkes-Dodson Law are manifestations of hormesis. Ageing Res Rev. 2008;7:8–20. doi: 10.1016/j.arr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Chambon C, Jatzke C, Wegener N, Gravius A, Danysz W. Using cholinergic M1 receptor positive allosteric modulators to improve memory via enhancement of brain cholinergic communication. Eur J Pharmacol. 2012;697:73–80. doi: 10.1016/j.ejphar.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Chang Q, Savage LM, Gold PE. Microdialysis measures of functional increases in ACh release in the hippocampus with and without inclusion of acetylcholinesterase inhibitors in the perfusate. J Neurochem. 2006;97:697–706. doi: 10.1111/j.1471-4159.2006.03765.x. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Price DL, DeLong MR. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- Decossas M, Doudnikoff E, Bloch B, Bernard V. Aging and sub-cellular localization of m2 muscarinic autoreceptor in basalocortical neurons in vivo. Neurobiol Aging. 2005;26:1061–1072. doi: 10.1016/j.neurobiolaging.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Deutsch JA. The cholinergic synapse and the site of memory. Science. 1971;174:788–794. doi: 10.1126/science.174.4011.788. [DOI] [PubMed] [Google Scholar]
- Drachman DA. Memory and cognitive function in man: does the cholinergic system have a specific role? Neurology. 1977;27:783–790. doi: 10.1212/wnl.27.8.783. [DOI] [PubMed] [Google Scholar]
- Flood JF, Smith GE, Cherkin A. Memory retention: potentiation of cholinergic drug combinations in mice. Neurobiol Aging. 1983;4:37–43. doi: 10.1016/0197-4580(83)90052-0. [DOI] [PubMed] [Google Scholar]
- Givens B, Olton DS. Bidirectional modulation of scopolamine-induced working memory impairments by muscarinic activation of the medial septal area. Neurobiol Learn Mem. 1995;63:269–276. doi: 10.1006/nlme.1995.1031. [DOI] [PubMed] [Google Scholar]
- Gold PE. The many faces of amnesia. Learn Mem. 2006;13:506–514. doi: 10.1101/lm.277406. [DOI] [PubMed] [Google Scholar]
- Gold PE, Korol DL. Making memories matter. Front Integr Neurosci. 2012;6:116. doi: 10.3389/fnint.2012.00116.Epub. special issue: the impact of emotion on cognition – dissociating between enhancing and impairing effects. F. Dolcos, L. Wang, and M. Mather, hosts. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PE, Newman LA, Scavuzzo CJ, Korol DL. Modulation of multiple memory systems: from neurotransmitters to metabolic substrates. Hippocampus. 2013;23:1053–1065. doi: 10.1002/hipo.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilly DM, Simon BB, Levin ED. Nicotine enhances stimulus detection performance of middle- and old-aged rats: a longitudinal study. Pharmacol Biochem Behav. 2000;65:665–670. doi: 10.1016/s0091-3057(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Grothe M, Heinsen H, Teipel SJ. Atrophy of the cholinergic basal forebrain over the adult age range and in early stages of Alzheimer's disease. Biol Psychiatry. 2012;71:805–813. doi: 10.1016/j.biopsych.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburg MD. Retrograde amnesia produced by intraperitoneal injection of physostigmine. Science. 1967;156:973–974. doi: 10.1126/science.156.3777.973. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16:710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Prog Brain Res. 2004;145:207–231. doi: 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Enderlin M, Fimbel R, Haman M, Grottick AJ, Soriano M, Richards JG, Kemp JA, Gill R. Donepezil reverses a mnemonic deficit produced by scopolamine but not by perforant path lesion or transient cerebral ischaemia. Eur J Neurosci. 2002;15:1827–1840. doi: 10.1046/j.1460-9568.2002.02018.x. [DOI] [PubMed] [Google Scholar]
- Himmelheber AM, Fadel J, Sarter M, Bruno JP. Effects of local cholinesterase inhibition on acetylcholine release assessed simultaneously in prefrontal and frontoparietal cortex. Neuroscience. 1998;86:949–957. doi: 10.1016/s0306-4522(98)00097-9. [DOI] [PubMed] [Google Scholar]
- Hoss W, Messer WS, Jr, Monsma FJ, Jr, Miller MD, Ellerbrock BR, Scranton T, Ghodsi-Hovsepian S, Price MA, Balan S, Mazloum Z, Bohnett M. Biochemical and behavioral evidence for muscarinic autoreceptors in the CNS. Brain Res. 1990;517:195–201. doi: 10.1016/0006-8993(90)91026-d. [DOI] [PubMed] [Google Scholar]
- Huff FJ, Mickel SF, Corkin S, Growdon JH. Cognitive functions affected by scopolamine in Alzheimer's disease and normal aging. Drug Dev Res. 1988;12:271–278. [Google Scholar]
- Kato G. Acetylcholinesterase. II. A study by nuclear magnetic resonance of the acceleration of acetylcholinesterase by atropine and inhibition by eserine. Mol Pharmacol. 1972;8:582–588. [PubMed] [Google Scholar]
- Koob GF. Arousal, stress and inverted-U shaped curves: implications for cognitive function. In: Lister RG, Weingartner HJ, editors. Perspectives on Cognitive Neuroscience. Oxford University Press; London: 1991. pp. 300–313. [Google Scholar]
- Kutlu MG, Gould TJ. The Neurobiology and Genetics of Nicotine and Tobacco. Springer International Publishing; 2015. Nicotinic receptors, memory, and hippocampus; pp. 137–163. [DOI] [PubMed] [Google Scholar]
- Levin ED, Caldwell DP. Low-dose mecamylamine improves learning of rats in the radial-arm maze repeated acquisition procedure. Neurobiol Learn Mem. 2006;86:117–122. doi: 10.1016/j.nlm.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Liu JK, Kato T. Effect of physostigmine on relative acetylcholine output induced by systemic treatment with scopolamine in in vivo microdialysis of rat frontal cortex. Neurochem Int. 1994;24:589–596. doi: 10.1016/0197-0186(94)90012-4. [DOI] [PubMed] [Google Scholar]
- MacLeod JE, Potter AS, Simoni MK, Bucci DJ. Nicotine administration enhances conditioned inhibition in rats. Eur J Pharmacol. 2006;551:76–79. doi: 10.1016/j.ejphar.2006.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mash DC, Flynn DD, Potter LT. Loss of M2 muscarine receptors in the cerebral cortex in alzheimer's disease and experimental cholinergic denervation. Science. 1985;228:1115–1117. doi: 10.1126/science.3992249. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer's disease. J Comp Neurol. 2013;521:4124–4144. doi: 10.1002/cne.23415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima K, Iwasaki K, Tsukikawa H, Matsumoto Y, Egashira N, Abe K, Egawa T, Fujiwara M. The scopolamine-induced impairment of spatial cognition parallels the acetylcholine release in the ventral hippocampus in rats. Jpn J Pharmacol. 2000;84:163–173. doi: 10.1254/jjp.84.163. [DOI] [PubMed] [Google Scholar]
- Moor E, Deboer P, Auth F, Westerink BHC. Characterisation of muscarinic autoreceptors in the septo-hippocampal system of the rat: a microdialysis study. Eur J Pharmacol. 1995;294:155–161. doi: 10.1016/0014-2999(95)00522-6. [DOI] [PubMed] [Google Scholar]
- Moore H, Stuckman S, Sarter M, Bruno JP. Potassium, but not atropine-stimulated cortical acetylcholine efflux, is reduced in aged rats. Neurobiol Aging. 1996;17:565–571. doi: 10.1016/0197-4580(96)00075-9. [DOI] [PubMed] [Google Scholar]
- Morris KA, Li S, Bui DD, Gold PE. Glucose attenuates impairments in memory and CREB activation produced by an α4β2 but not an α7 nicotinic receptor antagonist. Neuropharmacology. 2013;67:233–242. doi: 10.1016/j.neuropharm.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahara N, Fujise N, Kawanishi G, Mizobe F. Central muscarinic activities of an M1-selective agonist: preferential effect on reversal of amnesia. Brain Res. 1990;507:172–175. doi: 10.1016/0006-8993(90)90541-i. [DOI] [PubMed] [Google Scholar]
- Newman LA, Korol DL, Gold PE. Lactate produced by glycogenolysis in astrocytes regulates memory. PLoS One. 2011;6:e28427. doi: 10.1371/journal.pone.0028427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noori HR, Fliegel S, Brand I, Spanagel R. The impact of acetylcholinesterase inhibitors on the extracellular acetylcholine concentrations in the adult rat brain: a meta-analysis. Synapse. 2012;66:893–901. doi: 10.1002/syn.21581. [DOI] [PubMed] [Google Scholar]
- Nordstrom Ö, Bartfai T. Muscarinic autoreceptor regulates acetyl-choline release in rat hippocampus: in vitro evidence. Acta Physiol Scand. 1980;108:347–353. doi: 10.1111/j.1748-1716.1980.tb06543.x. [DOI] [PubMed] [Google Scholar]
- Pan SY, Han YF, Yu ZL, Yang R, Dong H, Ko KM. Evaluation of acute tacrine treatment on passive-avoidance response, open-field behavior, and toxicity in 17-and 30-day-old mice. Pharmacol Biochem Behav. 2006;85:50–56. doi: 10.1016/j.pbb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Pepeu G, Giovannini MG. Cholinesterase inhibitors and memory. Chem Biol Interact. 2010;187:403–408. doi: 10.1016/j.cbi.2009.11.018. [DOI] [PubMed] [Google Scholar]
- Quirion R, Richard J, Wilson A. Muscarinic and nicotinic modulation of cortical acetylcholine release monitored by in vivo micro-dialysis in freely moving adult rats. Synapse. 1994;17:92–100. doi: 10.1002/syn.890170205. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Arankowsky-Sandoval G, Gold PE. Glucose attenuates the effect of combined muscarinic-nicotinic receptor blockade on spontaneous alternation. Eur J Pharmacol. 1994;256:31–36. doi: 10.1016/0014-2999(94)90612-2. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Unick KE, Gold PE. Hippocampal acetylcholine release during memory testing in rats: augmentation by glucose. Proc Natl Acad Sci U S A. 1996;93:4693–4698. doi: 10.1073/pnas.93.10.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Pal SN, Unick K, Stefani MR, Gold PE. Modulation of hippocampal acetylcholine release and spontaneous alternation scores by intrahippocampal glucose injections. J Neurosci. 1998;18:1595–1601. doi: 10.1523/JNEUROSCI.18-04-01595.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, McAlonan G, Muir JL, Everitt BJ. Cognitive enhancers in theory and practice: studies of the cholinergic hypothesis of cognitive deficits in Alzheimer's disease. Behav Brain Res. 1997;83:15–23. doi: 10.1016/s0166-4328(97)86040-8. [DOI] [PubMed] [Google Scholar]
- Sakai K, El Mansari M, Jouvet M. Inhibition of carbachol micro-injections of presumptive cholinergic PGO-on neurons in freely moving cats. Brain Res. 1990;527:213–223. doi: 10.1016/0006-8993(90)91140-c. [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Bednar MM, Cromer JR, Maruff P. Reversal of scopolamine-induced deficits with a single dose of donepezil, an acetylcholinesterase inhibitor. Alzheimers Dement. 2005;1:126–135. doi: 10.1016/j.jalz.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Stillman MJ, Shukitt-Hale B, Galli RL, Levy A, Lieberman HR. Effects of M2 antagonists on in vivo hippocampal acetylcholine levels. Brain Res Bull. 1996;41:221–226. doi: 10.1016/s0361-9230(96)00180-3. [DOI] [PubMed] [Google Scholar]
- Stone WS, Croul CE, Gold PE. Attenuation of scopolamine-induced amnesia in mice. Psychopharmacology. 1988;96:417–420. doi: 10.1007/BF00216073. [DOI] [PubMed] [Google Scholar]
- Stone WS, Walser B, Gold SD, Gold PE. Scopolamine- and morphine-induced impairments of spontaneous alternation performance in mice: reversal with glucose and with cholinergic and adrenergic agonists. Behav Neurosci. 1991;105:264–271. doi: 10.1037//0735-7044.105.2.264. [DOI] [PubMed] [Google Scholar]
- Stratton LO, Petrinovich L. Post-trial injections of an anticholinesterase drug and maze learning in two strains of rats. Psychopharmacologia. 1963;5:47–54. doi: 10.1007/BF00405574. [DOI] [PubMed] [Google Scholar]
- Tani Y, Saito K, Imoto M, Ohno T. Pharmacological characterization of nicotinic receptor-mediated acetylcholine release in rat brain–an in vivo microdialysis study. Eur J Pharmacol. 1998;351:181–188. doi: 10.1016/s0014-2999(98)00314-8. [DOI] [PubMed] [Google Scholar]
- Terry AV, Buccafusco JJ, Decker MW. Cholinergic channel activator, ABT-418, enhances delayed-response accuracy in rats. Drug Dev Res. 1997;40:304–312. [Google Scholar]
- Tota S, Nath C, Najmi AK, Shukla R, Hanif K. Inhibition of central angiotensin converting enzyme ameliorates scopolamine induced memory impairment in mice: role of cholinergic neurotransmission, cerebral blood flow and brain energy metabolism. Behav Brain Res. 2012;232:66–76. doi: 10.1016/j.bbr.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Vannucchi MG, Scali C, Kopf SR, Pepeu G, Casamenti F. Selective muscarinic antagonists differentially affect in vivo acetylcholine release and memory performances of young and aged rats. Neuroscience. 1997;79:837–846. doi: 10.1016/s0306-4522(97)00091-2. [DOI] [PubMed] [Google Scholar]
- Wanibuchi F, Nishida T, Yamashita H, Hidaka K, Koshiya K, Tsukamoto S, Usuda S. Characterization of a novel muscarinic receptor agonist, YM796: comparison with cholinesterase inhibitors in in vivo pharmacological studies. Eur J Pharmacol. 1994;265:151–158. doi: 10.1016/0014-2999(94)90425-1. [DOI] [PubMed] [Google Scholar]
- Wilkie GI, Hutson P, Sullivan JP, Wonnacott S. Pharmacological characterization of a nicotinic autoreceptor in rat hippocampal synaptosomes. Neurochem Res. 1996;21:1141–1148. doi: 10.1007/BF02532425. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Soliakov L, Wilkie G, Redfern P, Marshall D. Presynaptic nicotinic acetylcholine receptors in the brain. Drug Dev Res. 1996;38:149–159. [Google Scholar]
- Wu YJ, Harp P, Yan XR, Pope CN. Nicotinic autoreceptor function in rat brain during maturation and aging: possible differential sensitivity to organophosphorus anticholinesterases. Chem Biol Interact. 2003;142:255–268. doi: 10.1016/s0009-2797(02)00121-7. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Suzuki N. Antiamnesic and cholinomimetic side-effects of the cholinesterase inhibitors, physostigmine, tacrine and NIK-247 in rats. Eur J Pharmacol. 1993;250:117–124. doi: 10.1016/0014-2999(93)90628-u. [DOI] [PubMed] [Google Scholar]