Summary
Background
Despite WHO recommendations to offer pregnant women treatment with praziquantel, many nations continue to withhold treatment, awaiting data from controlled trials addressing safety and efficacy. The objectives of the study were to 1) assess whether treatment of pregnant women with schistosomiasis at 12–16 weeks gestation leads to improved maternal and newborn outcomes and 2) collect maternal and newborn safety data.
Methods
Women who were otherwise healthy and infected with S. japonicum (N=370) were enrolled and randomized 1:1 to receive either over-encapsulated praziquantel (60 mg/kg in split dose) or placebo. The following efficacy outcomes were ascertained: maternal hemoglobin, iron status, and gestational weight gain, birth weight (primary outcome), newborn hemoglobin and iron status. Safety data were collected including immediate reactogenicity, post dosing toxicology ascertained 24 hours after study agent administration, and maternal and newborn serious adverse events.
Findings
Most women harbored low intensity infections (90.9%). Treatment with praziquantel did not have a significant impact on birth weight (2.85 kg in both groups, Beta −0.002, [0.88, 0.083]) or the incidence of low birth weight (OR 1.319 [0.729, 2.387]. Lack of treatment success may be due to the lack of difference in measures of maternal inflammation at 32 weeks gestation. Treatment with praziquantel resulted in a higher likelihood of treatment success (OR 5.815, [3.52, 9.61], P < 0.0001). Treatment was well tolerated with reactogenicity rates similar to that observed in non-pregnant subjects. There were no significant differences in key safety outcomes including abortion, fetal death in utero and congenital anomalies.
Interpretation
Results from this study provide important data from a controlled trial in support of the expansion of treatment policies to include pregnant women as recommended by WHO.
Funding
The trial was funded by the United States National Institutes of Health, National Institute of Allergy and Infectious Diseases (U01AI066050).
Keywords: Schistosomiasis, praziquantel, pregnancy, birth weight
Introduction
Over 200 million individuals are infected with one of three species of schistosomes globally,1 including an estimated 40 million women of reproductive age. Schistosomiasis remains a significant cause of morbidity and mortality in low and middle income countries, despite the availability of effective pharmacologic therapy with praziquantel.2 Praziquantel was released in 1979, but was never studied in pregnant or lactating women and remains a United States Federal Drug Administration pregnancy Class B drug. Its Class B designation is based on numerous animal studies supporting its safety,3, 4 but a lack of well-controlled trials during human pregnancy. In 2002, a World Health Organization (WHO) report recommended that all schistosomiasis infected pregnant and lactating women be considered high-risk groups and be offered treatment with praziquantel individually or during treatment campaigns.5–7 This recommendation was reissued in 2006 as part of the WHO’s Guidelines for Preventative Chemotherapy for Helminthiasis,2 in which it was recommended that pregnant and lactating women be included in mass drug administration (MDA) campaigns. These recommendations were largely motivated by 1) the expected progression of both end-organ morbidity and anemia if women remained untreated during repeated cycles of pregnancy and lactation, 2) demonstrated safety in animal models of pregnancy, and 3) expected safety in humans based on inadvertent exposures during human pregnancy. Although some nations, particularly in Africa, have adopted this policy, many others, including The Philippines, have not, awaiting safety data from well-controlled trials in humans. Millions of women of reproductive age, therefore, are not treated for many years during repeated cycles of pregnancy and lactation.
In addition to the lack of safety data in humans, the specific impact of human schistosomiasis on pregnancy outcomes remains understudied. Schistosomiasis has been implicated as a contributor to undernutrition8–14 among non-pregnant subjects. In particular, it is thought that schistosomiasis culminates in undernutrition through effects on appetite15 (anorexia and symptomatology) and inflammation-mediated cachexia.16 Schistosomiasis also contributes to the global burden of anemia, largely through anemia of inflammation.10, 12, 17, 18 In addition, studies support the role of schistosomiasis in iron deficiency anemia at higher intensities of infection, as individuals experience occult blood loss in urine and stool.19–22
Given demonstrated morbidity among non-pregnant subjects, a few studies have sought to address the impact of schistosomiasis infection in human pregnancy.23, 24 One randomized, controlled trial conducted in Uganda, examined the impact of praziquantel given to pregnant women during the second or third trimester (mean gestational age 26.6 weeks).24 That study differed from this trial as women who were and were not infected with schistosomiasis were included in the randomized sample. That trial did not demonstrate a significant impact of praziquantel on maternal anemia or birth weight, even among the approximately 18% of women who were infected with S. mansoni. No studies have examined whether treatment earlier in gestation improves pregnancy outcomes, none have examined the impact of treatment for S. japonicum, and none have examined use of the higher dose of praziquantel recommended for S. japonicum.
The objectives of the study were to 1) assess whether treatment of pregnant women with schistosomiasis at 12–16 weeks gestation leads to improved maternal and newborn outcomes including birth weight (primary endpoint) and both maternal and newborn anemia and iron status and 2) collect safety data addressing immediate reactogenicity, adverse events during pregnancy, and adverse newborn outcomes such as congenital anomalies. We hypothesized that treatment of S. japonicum during pregnancy would lead to higher newborn birth weight by improving maternal appetite and nutritional status, higher maternal hemoglobin and bio-available iron through decreasing the risk for anemia of inflammation during pregnancy, and improved newborn iron stores through greater iron bio-availability to the developing fetus.
Methods
Study design and participants
This study was a Phase 2, double blind, placebo controlled trial addressing the effects of praziquantel given at 12–16 weeks gestation on maternal and newborn birth outcomes (efficacy) and initial safety and toxicology. The initial target sample size was 500 pregnant women, with a plan to enroll equal numbers of women with low intensity and moderate/high intensity infections with S. japonicum. The protocol was modified to omit this requirement when it was noted that the prevalence of moderate and high intensity infections was much lower than expected. It was later modified to decrease the target enrollment to N = 370 given a concern regarding study duration as the overall prevalence of S. japonicum infection was lower than originally anticipated. This approach was tenable as attrition rates were significantly lower than originally anticipated such that the primary outcome was still captured on more than the planned number of subjects (162 in each arm) needed to detect a difference in birth weight of 125 grams, with a type one error of 0.05, and a power of 0.80.
The study was conducted in approximately 50 baranguays (villages) serviced by six Municipal Health Centers (MHCs), in a schistosomiasis endemic region of northeastern Leyte, The Philippines. At this study site, soil-transmitted helminths (STHs), but not malaria, are endemic and the prevalence of HIV is <0.1%.25
Informed consent for screening procedures was conducted by one of 12 midwives servicing the six MHCs who were members of the study staff. Initial eligibility screening included a urine pregnancy test and three stool samples collected on different days for the quantification of S. japonicum and STH eggs using the Kato-Katz method. Kato-Katz was performed by trained medical technologists at the study laboratory in Palo. Two Kato-Katz slides were examined for each sample within 30 minutes for hookworm and up to 24 hours later for other helminth infections including schistosomiasis. Each stool sample was quantified in duplicate and mean determined. Intensity of infection was determined as the mean of the three samples. S. japonicum infection intensity was categorized as low (0–99 eggs per gram (epg) of stool, moderate (100–399 epg of stool) or high (400 + epg of stool). The second phase of screening was conducted at Remedios Trinidad Romualdez (RTR) Hospital in Tacloban, Leyte. The study physician performed a history and physical examination and a trans-abdominal ultrasound to assess fetal viability and estimate gestational age. In addition, a blood sample was obtained prior to administration of the study agent to measure serum chemistries and a complete blood count (CBC). Weight, height and other anthropometric measures were made including thigh skin fold thickness as described.26, 27
Women were eligible for the trial if they provided informed consent and were infected with S. japonicum, age 18 or older, otherwise healthy as determined by physician history, physical examination and laboratory studies, and pregnant at 12–16 weeks gestation with a live, singleton, intrauterine fetus. Pregnancies with estimated gestation less than 12 weeks were scheduled to return, as were women with an acute medical condition that could potentially be addressed prior to 12–16 weeks gestation.
All women were offered treatment for schistosomiasis and soil-transmitted helminths at the conclusion of lactation as it was the Philippines Department of Health policy to defer treatment until cessation of breast feeding. Infants who had a medical disorder or malnutrition diagnosed during the newborn period were referred for care. The study was separately approved by both the Rhode Island Hospital Institutional Review Board in Providence, RI and the Ethics Review Board of the Research Institute of Tropical Medicine in Manila, The Philippines. This trial was registered with ClinicalTrials.gov, number NCT00486863.
Randomization and masking
Women who met eligibility criteria were randomly assigned (1:1) to receive either over-encapsulated praziquantel (30 mg/kg×2) or over-encapsulated placebo (dextrose), as a split dose over three hours in a double blind fashion. The study agent was compounded by The Rhode Island Hospital Pharmacy, using praziquantel tablets obtained from Bayer’s United States distributor, Shering Plough. Dextrose and gelatin capsules were provided by Gallipot. Both provided certificates of analysis for these compounds prior to study initiation. Two capsule doses were made which were differentiated by color, containing 300 or 150 mg to allow for optimal dosing by weight. Statisticians at the EMMES Corporation, the Statistical and Data Coordinating Center for the study, randomly allocated study numbers in blocks of six. Study drug was administered at RTR Hospital.
Procedures
Following each of the split doses, subjects were actively assessed for adverse drug reactions and then observed in the hospital for 24 hours. Prior to discharge, a repeat blood sample was drawn for serum chemistries and CBC. In addition, after approximately N=100 subjects were recruited, the protocol was amended to include two additional blood samples collected at varying times post-dosing for population pharmacokinetics, the results of which will be reported elsewhere. Approximately 10–14 days after discharge, subjects were visited at their home by study staff to elicit any symptoms experienced since hospital discharge.
Subjects had two scheduled follow up visits prior to delivery at 22 +/− 2 weeks and 32 +/− 2 weeks gestation, both at RTR Hospital. Stool was collected at the 22 week visit for assessment of parasitologic cure. In addition, women were seen by the study obstetrician and a detailed history and physical examination were performed, including measures of nutritional status as described above. At the 32 week visit, the history, physical exam, and nutritional measures were repeated, a blood sample was collected for CBC as well as analytes capturing iron (ferritin, serum transferrin receptor (sTfR)) and inflammatory (C-reactive protein (CRP), hepcidin, TNF-α, IFN-λ, IL-6, and IL-1) markers. Serum samples were aliquoted and stored at −80°C. Bioactive serum hepcidin (DRG® Hepcidin 25 bioactive ELISA (EIA-5258)) was measured according to manufacturer's instructions. Other analytes were quantified using a multiplex bead-based platform (Bio-Rad, Hercules, CA) as described previously.8 A urine sample was collected to screen for pre-eclampsia and urinary tract infections. Women were scheduled for additional visits as needed based on obstetrician-identified diagnoses and were also asked to come to RTR for unscheduled visits for other concerns that arose.
Mothers gave birth in one of the six MHCs or were referred to RTR Hospital if indicated. Gestational age at birth was assessed by a modified Dubowitz scoring system developed by Ballard.28 For deliveries that occurred at home based on maternal preference or inability to get to an MHC, mothers contacted the study midwife who visited the mother within 48 hours of delivery.
Both mother and newborn returned to RTR hospital at 2–6 days of life for follow-up. The study pediatrician performed a history and physical examination and a heel stick blood sample was obtained for newborn screening, CBC, and the aforementioned iron and inflammatory analytes. Study close out occurred at RTR hospital at 28 days of life, at which point the pediatrician again performed a history and physical examination and referred the newborn for any identified concerns.
Outcomes
The primary outcome was birth weight. Newborns were weighed within 48 hours of delivery on a Tanita model BD-585 portable scale (Arlington Heights, MD) to 10 grams. Birth weights taken 24–48h hours after delivery were corrected by a factor of +2%, to obtain the estimated weight at birth. Secondary outcomes included low birth weight, defined as weight < 2.5 kg. Small for gestational age (SGA), used as an indicator for possible intra-uterine growth restriction (IUGR), was defined based on gestational age determined at the 12–16 week ultrasound. SGA was defined as gestational age adjusted birthweight that was <10th percentile based on the multi-racial Williams curves.29 Other secondary efficacy outcomes included change in maternal nutritional status, maternal anemia (hemoglobin < 11.0 g/dL), hemoglobin, and iron status at 32 weeks gestation, newborn hemoglobin and iron status measured on a heel stick blood sample at 2–6 days of life, prematurity defined as gestation < 37 weeks, and parasitologic “cure” as defined by a >90% reduction in S. japonicum egg count from screening to 22 weeks gestation.
Safety outcomes included toxicity to maternal bone marrow, kidney, and liver as measured by laboratory parameters collected just before, and 24 hours after dosing, maternal seizures, immediate toxicity to the fetus as assessed by abortion (fetal demise before 20 weeks gestation), live birth rate, and newborn congenital anomalies.
Assessment of potential confounders and modifying covariates
Household socio-economic status was captured using a questionnaire developed previously for use in this population as described.30 A summary score reflected numeric weights assigned to SES questionnaire items as described by Filmer,31 using the FACTOR procedure in SAS 9.3 (Cary, NC). Summary SES scores were categorized by quartile and compared by treatment group.
The following other pre-specified covariates were evaluated as potential confounders: maternal age, maternal weight and height at enrollment, smoking status at enrollment (yes/no), reported alcohol intake at enrollment (1–2, 3–5, 6–8, 9–10 and more than 10 glasses of alcohol consumed per week), newborn sex, reported compliance with iron supplementation, intensity of infection with S. japonicum at enrollment (low, medium, high as described above) and treated as an ordinal variable in analyses, obstetrical history (defined as having one or more occurrences of a miscarriage, abortion or stillbirth) treated as a dichotomous variable in analyses, and intensity of infection with geohelminths at enrollment defined using World Health Organization (WHO) criteria.
Statistical Analysis
Outcomes and potential confounders were compared between praziquanel and placebo groups at enrollment using Chi-square and Wilcoxon two-sample or t-tests. Potential confounders were considered for inclusion in final models if associated with treatment at the alpha level of 0.10. Of note, none met this criteria such that outcomes are presented by treatment group without adjustment for potential confounders.
Primary and secondary efficacy analyses were performed using hierarchical linear models to account for potential clustering of observations within villages and municipalities. Models of each outcome included a main effect for treatment and random intercepts for villages and health centers. Intraclass correlations were estimated from the covariance parameters for each random effect to evaluate the proportion of the total variance attributable to village or health center. Covariance parameter estimates were evaluated for significance with a Wald test. Random effects whose intercept variance estimates were not significantly different from zero at the alpha level of 0.05 were removed from the final model. Standard model diagnostics were used to evaluate model assumptions of linearity, normality and constant variance of residuals. Outcomes were log transformed if necessary to avoid violation of regression assumptions. Analyses were performed using the MIXED procedure for continuous outcomes and NLMIXED procedure for binary outcomes in SAS 9.3. Primary efficacy models additionally evaluated the interaction of infection intensity with S. japonicum (epg) at enrollment with treatment allocation, to determine whether baseline intensity of infection modified the relationship between treatment and birth weight.
Analyses were performed based on the intention to treat population, from which there were no deviations in product received.
Role of the funding source
The study funder (United States National Institutes of Health, National Institute of Allergy and Infectious Diseases) supported protocol development in collaboration with the investigators and engaged a data management and statistical analyses partner, EMMES Corporation, responsible for design of data bases, summary reports for Data Safety and Monitoring Board meetings, and final statistical analyses for this report. The funder had no role in generation of hypotheses, data collection, data analysis, data interpretation, or writing of the report. The authors, with the exception of Dr. Watson, did not have access to study data until all databases were locked and the analyses reported in the manuscript were complete. The corresponding author had access to the locked data set and had final responsibility for the decision to submit for publication.
Results
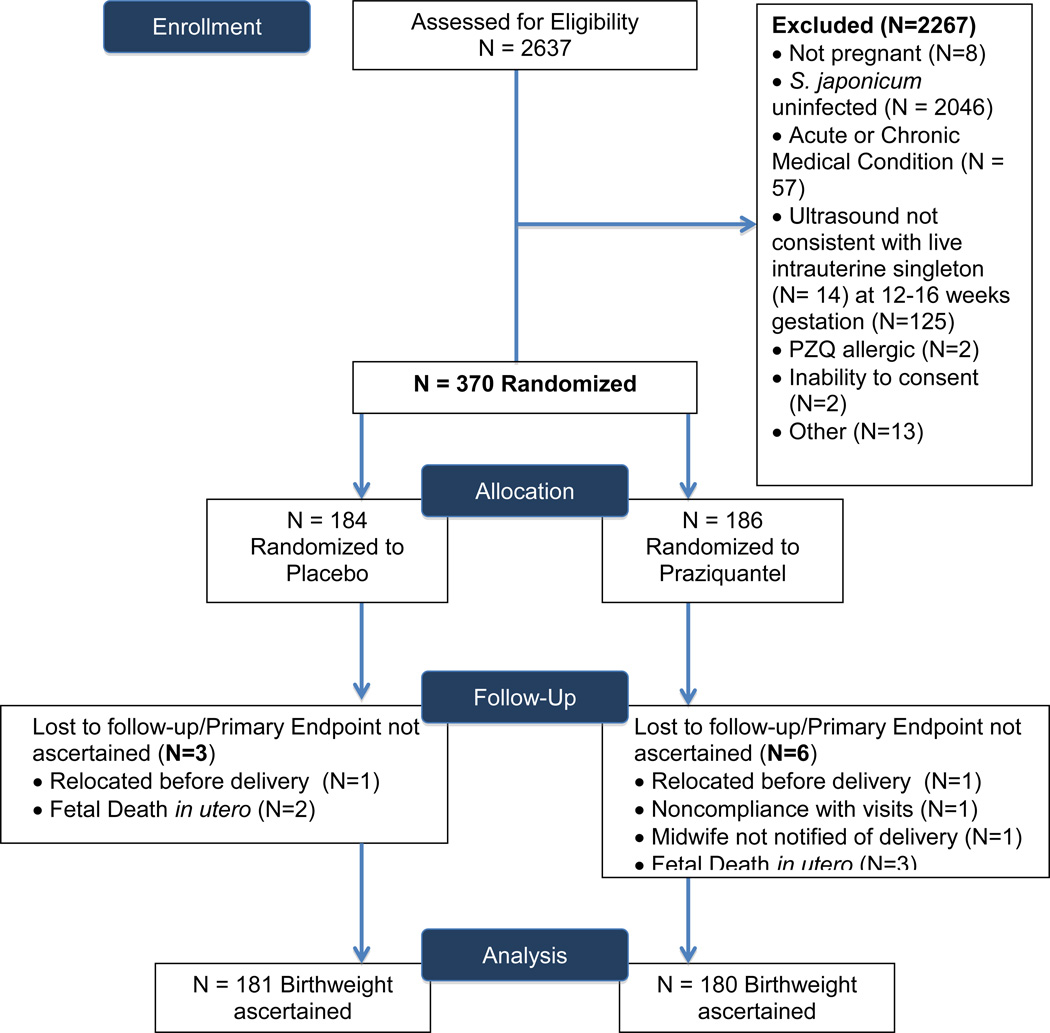
Of 2637 women screened for eligibility, the primary reasons for exclusion were 1) not infected with S. japonicum (N=2046) and 2) ultrasound not consistent with viable intrauterine, singleton pregnancy at 12–16 weeks gestation (N=139). Of 370 pregnant women enrolled and randomized, there were five fetal deaths in utero. Birth weight was ascertained for 361 of 365 live births (Figure 1).
Figure 1. Trial Profile.
Trial profile capturing initial screening for eligibility at municipal health centers, stage two hospital based screening, randomization, and final disposition of study subjects for whom the primary outcome, birth weight, was ascertained.
The randomized cohort included 334 women, most with low intensity infection (90.3%). Table 1 presents baseline socio-demographic, obstetrical, nutritional, and clinical covariates by treatment allocation. These variables were equally distributed between treatment groups.
Table 1.
Baseline characteristics of study sample by treatment allocation
| Covariate | Placebo (N=184) | Praziquantel (N=186) |
|---|---|---|
| Age ψ | 25.9 (6.26) | 26.2 (6.60) |
| SES quartile | ||
| 1st quartile | 21 (11%) | 26 (14%) |
| 2nd quartile | 67 (36%) | 73 (39%) |
| 3rd quartile | 46 (25%) | 46 (25%) |
| 4th quartile | 50 (27%) | 41 (22%) |
| Current Smoking Status | ||
| No | 183 (99%) | 185 (99%) |
| Yes | 1 (1%) | 1 (1%) |
| Current Alcohol Consumption | ||
| No | 48 (26%) | 39 (21%) |
| Yes | 136 (74%) | 147 (79%) |
| Height (cm)ψ | 147.1 (5.8) | 147.8 (5.2) |
| Weight (Kg)ψ | 47.3 (7.5) | 47.8 (7.0) |
| S. japonicum epg at screening* | 10.00, 26.67 | 10.00, 26.67 |
| S. japonicum epg at screeningψ | 40.4 (95.3) | 32.7 (58.3) |
|
A. lumbricoides epg at screening* |
475.0, 7923 | 803.3, 8460 |
| Prevalence of A. lubricoides infection at screening |
112/184 (61%) | 115/186 (62%) |
| Hookworm epg at screening * | 0.00, 23.33 | 0.00, 23.33 |
| Prevalence of hookworm infection at screening |
61/184 (33%) | 72/186 (39%) |
| T. trichiura epg at screening * | 83.33, 290.0 | 101.7, 290.0 |
| Prevalence of T. trichiura infection at screening |
146/184 (79%) | 153/186 (82%) |
| History of any obstetrical complication |
28(15.22%) | 31(16.67%) |
| Number of prior pregnancies | ||
| 1–5 | 151 (82%) | 141 (76%) |
| 6–10 | 32 (17%) | 45 (24%) |
| 11+ | 1 (1%) | 0 (0%) |
Mean (SD).
Parasitologic results prior to treatment as determined by Kato-Katz and reported as eggs per gram of stool. Median, interquartile range.
Treatment with praziquantel did not have a significant impact on the primary outcome, birth weight (2.85 kg in both praziquantel and placebo groups, P = 0.99) or secondary efficacy outcomes including prevalence of low birth weight and SGA newborns. (Table 2) The estimated association of treatment with birth weight (kg) from the mixed effects model was Beta = −0.002 ([95% CI −.088, 0.083]; P = 0.962). In mixed models of outcomes other than birth weight, covariance parameter estimates for the village and municipality random effects were not significantly different from zero, indicating that reported bivariate comparisons in Table 2 are sufficient for these data.
Table 2.
Unadjusted primary and secondary efficacy outcomes by treatment allocation
| Covariate | Placebo (N=184) | Praziquantel (N=186) | P value |
|---|---|---|---|
| Newborn Outcomes | |||
| Birthweight (Kg)Ψ | 2.85 (0.39) | 2.85 (0.44) | 0.988 |
| Low Birth Weight (< 2.5 kg)Φ | 23 (12.71%) | 29 (16.11%) | 0.357 |
| Small for Gestational Age Φ | 43 (23.76%) | 48 (26.67%) | 0.82 |
| Live Birth Rate Φ | 181/183 (99%) | 181/183 (99%) | >0.99 |
| Newborn Hemoglobin Ψ | 17.33, 2.48 | 17.50, 2.33 | 0.521 |
| Newborn serum transferrin receptor (ng/ml) (heel stick)* |
0.000, 3.680 | 0.000, 0.000 | 0.068 |
| Newborn ferritin (ng/ml) (heel stick)* |
313.7, 190.9 | 289.4, 193.7 | 0.818 |
| Newborn serum transferrin receptor: ferritin ratio (heel stick) * |
0.000, 0.010 | 0.000, 0.000 | 0.070 |
| Maternal Outcomes | |||
| Maternal hemoglobin at 32 weeks gestation (g/dL)Ψ |
11.04 (1.30) | 11.05 (1.18) | 0.923 |
| Change in maternal hemoglobin (g/dL) Ψ |
−0.42 (1.39) | −0.44 (1.38) | 0.926 |
| Maternal ferritin (ng/ml) at 32 weeks gestation * |
10.52, 19.22 | 14.71, 25.99 | 0.049 |
| Maternal serum transferrin receptor: ferritin ratio at 32 weeks gestation* |
0.00, 27.11 | 0.33, 13.26 | 0.993 |
| Maternal hepcidin (ng/ml) * | 2.58 (5.06) | 3.38 (4.32) | 0.439 |
| Maternal weight gain from enrollment to 32 weeks gestation (kg/week) Ψ |
0.33 (0.13) | 0.32 (0.13) | 0.704 |
| Mean change in maternal thigh skinfold thickness from enrollment to 32 weeks gestation (mm/week)Ψ |
0.08 (0.09) | 0.08 (0.09) | 0.517 |
| Treatment success (>90% reduction in egg count from screening to 22 weeks gestation)Φ |
92/184 (50.0%) | 157/184 (85.3%) | < 0.0001 |
| Cure rate (defined as percentage with 0 epg at 22 weeks gestation)Φ |
85/184 (46.2%) | 154/184 (83.7%) | <0.0001 |
| Percent change in S. japonicum (epg) from screening to 22 weeks gestationΨ |
128.74, 1241.04 | −53.11, 306.71 | 0.054 |
Mean (Standard deviation), T-test
Median, interquartile range, Wilcoxon 2 sample.
Number (%) Chi-Square
With respect to maternal nutritional status, treatment with praziquantel did not impact maternal weight gain or change in thigh circumference (Table 2). At 32 weeks gestation, the group that received praziquantel had significantly higher ferritin, however, there was no significant difference in maternal hemoglobin at 32 weeks gestation. There was no significant difference between groups in hepcidin, the principal regulator of systemic iron homeostasis. (Table 2) Of note, there were no significant differences between groups with respect to inflammatory markers such as CRP, TNF-alpha, and IL-6. In both groups, all women (100%) reported taking pre-natal vitamins with iron as directed.
Treatment was effective at reducing maternal intensity of infection as evidenced by a significantly higher proportion of women with a >90% reduction in egg (85.3 versus 50.0%, P < 0.0001). Praziquantel had a significant impact on cure rate as defined by the percentage of women who were not infected with S. japonicum at 22 weeks gestation (83.7% versus 46.2%, P < 0.0001). Praziquantel treatment had no effect on the prevalence or intensity of the STH infections, in particular hookworm. There was also a difference between groups in the percent change in S. japonicum (epg) from screening to 22 weeks gestation, though this did not reach statistical significance (−53.11 versus 128.7; P = 0.054).
With respect to other secondary efficacy outcomes for newborns, maternal treatment did not influence newborn hemoglobin level or ferritin. (Table 2) Non-significant differences were noted for newborn sTfR and sTfR:ferritin ratios; newborns of mothers treated with praziquantel had slightly lower levels for both bio-markers, potentially indicating less cellular iron thirst and greater total body iron, respectively, among newborns of treated versus untreated mothers. (Table 2)
There were no significant changes in serum chemistries or hematologic parameters in mothers before and 24 hours after dosing, with the following exceptions. There was a significant difference in the change in white blood cell and lymphocyte counts with women in the placebo group experiencing a greater increase from pre- to post-treatment. (Table 3) Women in the praziquantel group experienced a significantly higher change in creatinine from pre to post-dosing compared to the placebo group (0.07 versus 0.01 mg/dL, respectively; P = 0.004), however, these were all mild (grade I) elevations.
Table 3.
Laboratory and clinical safety endpoints by treatment allocation
| Outcome | Placebo (N=184) |
Praziquantel (N=186) |
P value |
|---|---|---|---|
| Change in maternal laboratory parameters (follow up measure minus initial pre-dosing) | |||
| Creatinine change (mg/dL)Ψ | 0.01, 0.17 | 0.07, 0.22 | 0.004 |
| BUN change (mg/dL)Ψ | 1.22, 6.13 | 1.71, 5.25 | 0.410 |
| ALT change (U/L)Ψ | −1.63, 10.50 | −0.39, 8.42 | 0.211 |
| AST change (U/L) Ψ | −0.96, 9.87 | −0.63, 9.15 | 0.738 |
| Biliribun change (mg/dL) Ψ | −0.10, 0.31 | −0.08, 0.33 | 0.605 |
| WBC change Ψ | 1.25, 1.39 | 0.78, 1.85 | 0.006 |
| Granulocyte count changeΨ | 0.68, 1.39 | 0.38, 1.54 | 0.062 |
| Lymphocyte count change Ψ | 0.49, 0.42 | 0.33, 0.51 | 0.001 |
| Hemoglobin (g/dL) changeΨ | −0.23, 0.74 | −0.22, 0.78 | 0.921 |
| Platelets (1000/uL) change Ψ | 9.81, 50.73 | 2.90, 40.74 | 0.150 |
| Clinical Endpoints | |||
| Serious adverse events (maternal) | 16 (8.70%) | 18 (9.68%) | 0.74 |
| Serious adverse events associated with treatment (maternal) |
0 (0%) | 0 (0%) | 1.00 |
| Maternal seizure within 24 hours of treatment |
0 (0%) | 0 (0%) | 1.00 |
| Any severe adverse reaction within 24 hours of treatment |
2 (1.09%) | 5 (2.69%) | 0.45 |
| Serious adverse events (infant)+ | 18 (9.94%) | 27 (14.92%) | 0.15 |
| Serious adverse events associated with treatment (infant) |
0 (0%) | 0 (0%) | 1.00 |
| Abortion (fetal loss between treatment and 20 weeks gestation) |
0 | 0 | 1.00 |
| Fetal death in utero Φ | 1 (0.54%) | 2 (1.08%) | 1.00 |
| Newborn not healthy Φ++ | 12 (6.56%) | 12 (6.56%) | 1.00 |
| Congenital Anomaly+ | 2 (1.10%) | 3 (1.66%) | 1.00 |
Mean (Standard deviation), T-test. Change measures calculated as follow up measure - initial measure.
Number (%) Chi-Square or Fisher’s exact test
Among 362 live births.
Among 366 evaluated deliveries
Overall 18 subjects in the praziquanel group and 16 in the placebo group experienced a serious adverse event (SAE) with a wide range of event classifications that were largely expected during pregnancy in a high-risk population. None occurred in the immediate post-treatment period. There were no significant differences in the rate of fetal death in utero, the live birth rate or the number of congenital anomalies. (Table 3) Congenital anomalies included two newborns (one in each treatment group) with talipes equinovarus (club feet) and three infants with cleft lip/palate (two in praziquantel group and 1 in the placebo group).
With respect to immediate reactogenicity, 323 subjects, 169 of whom received praziquanel, reported at least one side effect of treatment that was of mild or greater severity. Reactions graded as severe occurred in five subjects in the praziquanel group and two in the placebo and included headache, fever and malaise. All severe reactions were attributed to study product administration and resolved during the 14-day reactogenicity period.
Discussion
This is the first clinical trial to investigate the efficacy and safety of treating pregnant women with S. japonicum infection, at the higher recommended dose of praziquantel (60 mg/kg) recommended for Asian Schistosomiasis. The study was a phase two trial designed to assess impact on pregnancy outcomes and collect initial toxicology data for both mother and newborn. Treatment did not have a significant impact on the primary outcome, birthweight, or the rate of low birth weight newborns. Treatment may have led to improved iron status in both the mother at 32 weeks gestation and newborn. Importantly, praziquantel was well-tolerated with rates of immediate reactogenicity similar in this cohort of pregnant women to those observed in non-pregnant subjects.13 In addition, there were no significant differences in the rates of serious adverse events among mothers or newborns, comparing the treated versus untreated groups.
This trial differs from another important, four arm RCT conducted in an S. mansoni endemic region of Uganda. In that study women were randomized to receive either albendazole + praziquantel, albendazole alone, praziquantel alone, or placebo. All women were randomized to one of the four groups, whether or not they harbored specific baseline infections with STH and/or S. mansoni.24 The mean gestational age at enrollment was 26.6 weeks. Neither the Ugandan study, which treated women later in gestation, nor our trial, which randomized women early in the second trimester, demonstrated a significant impact on birth weight. In the Ugandan study, this was true even when analyses were restricted to women found to be infected with S. mansoni. In both studies, of the women who were infected, most had low intensity infections. In the present study, interactions between baseline intensity of infection and treatment effect on birth weight were also not significant, suggesting that there were not differences in treatment efficacy among women with moderate or high intensity infections. This should be interpreted with caution, however, due to the small number of women with moderate and high intensity infections enrolled (9.7% at baseline).
Other explanations for lack of impact on birth weight include the fact that treatment did not significantly impact maternal weight gain, a hypothesized potential mechanism. This may be due, in part, to a lack of effect on maternal inflammation even by 32 weeks gestation. In previous observational studies conducted by our group, women infected with S. japonicum had significantly higher TNF-α levels at 32 weeks gestation (mean 2.11 pg/ml) than uninfected women (mean 0.34 pg/ml).32 In the trial, these values at 32 weeks gestation were 1.6 pg/ml and 1.3 pg/ml for the placebo and praziquantel group, respectively, suggesting that treatment did not resolve inflammation. Another hypothesized mechanism through which praziquantel could lead to improved birth weight was through effects on placental health. Observational studies from our group suggested that infection with S. japonicum culminates in a pro-inflammatory signature at the maternal-fetal interface and this is associated with lower birth weight.32 Further, schistosome antigens elicit a pro-inflammatory immune response from primary human trophoblast cells from healthy placentas.33 It is possible, however, that praziquantel given during gestation does not sufficiently disrupt the delivery of immunogenic schistosome antigens to the placenta, given treatment results in a period of increased circulating antigens with a prolonged inflammatory immune response.8, 34 It remains possible that schistosome infections adversely affect placental health, but treatment after the first trimester may not mitigate this risk.
In addition, neither our study nor the Ugandan trial demonstrated a significant impact on maternal hemoglobin or prevalence of maternal anemia. In studies conducted by our group among non-pregnant subjects, we have demonstrated a significant time lag after treatment before improvements in hemoglobin are noted, with no significant difference noted three months following therapy, but a significant increase six months following treatment.8 We did find that women treated with praziquantel had significantly higher ferritin levels at 32 weeks gestation. In addition, there was a trend toward increased newborn iron endowment as captured by sTfR:ferritin ratio, a marker for total body iron, and sTfR, a marker of cellular iron thirst among their newborns. It is likely that treatment began to modify the risk of maternal anemia of inflammation, which would be expected to increase iron absorption from the gut and increase the pool of maternal bioavailable iron before an impact on maternal hemoglobin could be observed.35
With respect to safety outcomes, both the Ugandan and our study did not observe significant differences in the rate of congenital anomalies or other serious adverse events comparing the praziquantel and placebo groups. Our study additionally captured abortion, defined as fetal loss occurring before 20 weeks gestation, which in this case would have been within 4–8 weeks of praziquantel dosing. There were no differences between groups in either abortion or rates of fetal death in utero at any point in gestation. Taken together, these studies contribute key toxicology data regarding the use of praziquantel during human pregnancy, supporting its safety at different timepoints during pregnancy after 12 weeks gestation.
In WHO guidelines from 2002 and 2006, it was recommended that all schistosomiasis infected pregnant and lactating women be considered high-risk groups and be offered treatment with praziquantel individually or during treatment campaigns.5–7 These recommendations were based on safety from animal studies, as well as inadvertent exposures during human pregnancy, rather than data from controlled trials in humans. As such, the recommendations did not provide conclusive data regarding safety and, due in part to this constraint, many schistosomiasis endemic regions continue to exclude pregnant and lactation women from both MDA and individualized treatment programs. Results from this study, together with the Ugandan study, should provide necessary reassurance that praziquantel given after 12 weeks gestation is safe, effectively treats schistosomiasis, and may improve both maternal and newborn iron status.
Study limitations include the low number of women who harbored moderate or high intensity infections, who may have been more likely to experience schistosomiasis-associated morbidity and benefit from treatment. This limits our ability to understand the impact of treatment in the context of higher intensity infections. We were also surprised to find that 46.2% of women in the placebo group were “cured” by 22 weeks gestation. It is unlikely that this is due to treatment outside of the study given it was the strict policy of the Philippines Department of Health to withhold treatment from pregnant women and almost all treatment occurs during MDA campaigns. Further, all women in both groups provided three stools at follow up for evaluation. It is more likely that many women in the placebo group with light infections at baseline may have been missed at 22 weeks gestation, contributing to an apparently high rate of “cure.” In addition, by design, our study sample consisted of otherwise healthy, adult women, limiting generalizability of findings somewhat. In addition, in many parts of the world, MDA is the current approach to schistosomiasis control, whereas women in this study had to be infected with S. japonicum to participate. In addition, given the use of single dosing in MDA campaigns as opposed to the split dose employed, reactogenicity in practice may be higher than observed in this trial. Finally, in the context of inflammation, ferritin is elevated, thus complicating its interpretation as a measure of stored iron. Therefore, it is possible that treated women had higher levels of ferritin at 32 weeks gestation due to prolonged inflammation following treatment. This is unlikely given the expectation that acute phase responses such as elevated ferritin would resolve 4–5 months after treatment and is further evidenced by a lack of difference in other acute phase proteins such as CRP.
Results from this trial provide important data from a controlled trial in support of the expansion of treatment policies to include pregnant women as recommended by WHO. Though this study was not powered to detect differences in rates of rare outcomes such as congenital anomalies, these results, together with results from the Ugandan study, post-marketing surveillance data including experience treating pregnant women in MDA campaigns in some African nations, and data from animal studies support ending the exclusion of pregnant women from MDA campaigns and individualized treatment. Though we did not observe an impact on birth weight, it remains possible, based on animal models, as well as human observational and mechanistic studies, that schistosomiasis adversely affects pregnancy outcomes, however treatment during gestation is too late to be impactful. If this is the case, including pregnant women in MDA campaigns will increase the likelihood that they enter subsequent pregnancies free of schistosomiaisis infection.
Acknowledgements
We thank the women and their newborns who participated in this study. We thank the administrative staff and leadership of Remedios Trinidad Romualdez Hospital and Dr. Josephine Y. Sequito for conducting the ultrasound studies. We thank the midwives, municipal health officers, data managers, medical technologists, and field staff for their diligence and energy. We thank program staff (Dr. Malla Rao) and our Medical Monitor (Dr. Mirjana Nesin) at the National Institutes of Health/National Institute of Allergy and Infectious Diseases/Division of Microbiology and Infectious Diseases for their support throughout the study. We thank the members of the Data Safety and Monitoring Board for their engagement and oversight, in particular W. Ripley Ballou (Chair) and Charles Harding King, MD (Co-Chair).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
RMO, LPA, VT, AI, PIB, JDK and JFF participated in the study planning and design. RMO, LPA, VT, PIB, JLL, GGE, EBA, DBM, and AI participated in data collection. RMO participated in data interpretation. NW conducted data analyses. JFF and EAM participated in literature review and primary manuscript writing. JDK executed laboratory assays.
Declarations of interest
We declare that we have no conflicts of interest
References
- 1.Schistosomiasis: number of people treated in 2009. Releve epidemiologique hebdomadaire / Section d'hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record / Health Section of the Secretariat of the League of Nations. 2011;86(9):73–80. [PubMed] [Google Scholar]
- 2.World Health Organization. Preventive chemotherapy in human helminthiasis. Coordinated use of anthelminthic drugs in control interventions. Geneva: 2006. [Google Scholar]
- 3.Frohberg H. The toxicological profile of praziquantel in comparison to other anthelminthic drugs. Acta Leidensia. 1989;57(2):201–215. [PubMed] [Google Scholar]
- 4.Ni YC, Shao BR, Zhan CQ, Xu YQ, Ha SH, Jiao PY. Mutagenic and teratogenic effects of anti-schistosomal praziquantel. Chinese medical journal. 1982;95(7):494–498. [PubMed] [Google Scholar]
- 5.Olds GR. Administration of Praziquantel to pregnant and lactating women. Acta Trop. 2003;86(2–3):185–195. doi: 10.1016/s0001-706x(03)00033-0. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Report of the WHO Informal Consultation on the use of Praziquantel during Pregnancy Lactation and Albendazole/Mebendazole in Children under 24 months. Geneva: 2002. [Google Scholar]
- 7.Allen HE, Crompton DW, de Silva N, LoVerde PT, Olds GR. New policies for using anthelmintics in high risk groups. Trends in parasitology. 2002;18(9):381–382. doi: 10.1016/s1471-4922(02)02386-3. [DOI] [PubMed] [Google Scholar]
- 8.Coutinho HM, Acosta LP, McGarvey ST, et al. Nutritional status improves after treatment of schistosoma japonicum-infected children and adolescents. The Journal of nutrition. 2006;136(1):183–188. doi: 10.1093/jn/136.1.183. [DOI] [PubMed] [Google Scholar]
- 9.Coutinho HM, McGarvey ST, Acosta LP, et al. Nutritional status and serum cytokine profiles in children, adolescents, and young adults with Schistosoma japonicum-associated hepatic fibrosis, in Leyte, Philippines. The Journal of infectious diseases. 2005;192(3):528–536. doi: 10.1086/430929. [DOI] [PubMed] [Google Scholar]
- 10.Friedman JF, Kanzaria HK, Acosta LP, et al. Relationship between Schistosoma japonicum and nutritional status among children and young adults in Leyte, the Philippines. The American journal of tropical medicine and hygiene. 2005;72(5):527–533. [PubMed] [Google Scholar]
- 11.McGarvey ST, Aligui G, Daniel BL, Peters P, Olveda R, Olds GR. Child growth and schistosomiasis japonica in northeastern Leyte, the Philippines: cross-sectional results. The American journal of tropical medicine and hygiene. 1992;46(5):571–581. doi: 10.4269/ajtmh.1992.46.571. [DOI] [PubMed] [Google Scholar]
- 12.McGarvey ST, Aligui G, Graham KK, Peters P, Olds GR, Olveda R. Schistosomiasis japonica and childhood nutritional status in northeastern Leyte, the Philippines: a randomized trial of praziquantel versus placebo. The American journal of tropical medicine and hygiene. 1996;54(5):498–502. doi: 10.4269/ajtmh.1996.54.498. [DOI] [PubMed] [Google Scholar]
- 13.Olds GR, King C, Hewlett J, et al. Double-blind placebo-controlled study of concurrent administration of albendazole and praziquantel in schoolchildren with schistosomiasis and geohelminths. The Journal of infectious diseases. 1999;179(4):996–1003. doi: 10.1086/314686. [DOI] [PubMed] [Google Scholar]
- 14.McGarvey ST, Wu G, Zhang S, et al. Child growth, nutritional status, and schistosomiasis japonica in Jiangxi, People's Republic of China. The American journal of tropical medicine and hygiene. 1993;48(4):547–553. doi: 10.4269/ajtmh.1993.48.547. [DOI] [PubMed] [Google Scholar]
- 15.Latham MC, Stephenson LS, Kurz KM, Kinoti SN. Metrifonate or praziquantel treatment improves physical fitness and appetite of Kenyan schoolboys with Schistosoma haematobium and hookworm infections. The American journal of tropical medicine and hygiene. 1990;43(2):170–179. doi: 10.4269/ajtmh.1990.43.170. [DOI] [PubMed] [Google Scholar]
- 16.Coutinho HM, Leenstra T, Acosta LP, et al. Pro-inflammatory cytokines and C-reactive protein are associated with undernutrition in the context of Schistosoma japonicum infection. The American journal of tropical medicine and hygiene. 2006;75(4):720–726. [PubMed] [Google Scholar]
- 17.Leenstra T, Acosta LP, Langdon GC, et al. Schistosomiasis japonica, anemia, and iron status in children, adolescents, and young adults in Leyte, Philippines 1. The American journal of clinical nutrition. 2006;83(2):371–379. doi: 10.1093/ajcn/83.2.371. [DOI] [PubMed] [Google Scholar]
- 18.Leenstra T, Coutinho HM, Acosta LP, et al. Schistosoma japonicum reinfection after praziquantel treatment causes anemia associated with inflammation. Infection and immunity. 2006;74(11):6398–6407. doi: 10.1128/IAI.00757-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanzaria HK, Acosta LP, Langdon GC, et al. Schistosoma japonicum and occult blood loss in endemic villages in Leyte, the Philippines. The American journal of tropical medicine and hygiene. 2005;72(2):115–118. [PubMed] [Google Scholar]
- 20.Bustinduy AL, Sousa-Figueiredo JC, Adriko M, et al. Fecal occult blood and fecal calprotectin as point-of-care markers of intestinal morbidity in Ugandan children with Schistosoma mansoni infection. PLoS neglected tropical diseases. 2013;7(11):e2542. doi: 10.1371/journal.pntd.0002542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ndamba J, Makaza N, Kaondera KC, Munjoma M. Morbidity due to Schistosoma mansoni among sugar-cane cutters in Zimbabwe. International journal of epidemiology. 1991;20(3):787–795. doi: 10.1093/ije/20.3.787. [DOI] [PubMed] [Google Scholar]
- 22.Betson M, Sousa-Figueiredo JC, Kabatereine NB, Stothard JR. Use of fecal occult blood tests as epidemiologic indicators of morbidity associated with intestinal schistosomiasis during preventive chemotherapy in young children. The American journal of tropical medicine and hygiene. 2012;87(4):694–700. doi: 10.4269/ajtmh.2012.12-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qunhua L, Jiawen Z, Bozhao L, et al. Investigation of association between female genital tract diseases and Schistosomiasis japonica infection. Acta Trop. 2000;77(2):179–183. doi: 10.1016/s0001-706x(00)00129-7. [DOI] [PubMed] [Google Scholar]
- 24.Ndibazza J, Muhangi L, Akishule D, et al. Effects of deworming during pregnancy on maternal and perinatal outcomes in Entebbe, Uganda: a randomized controlled trial. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;50(4):531–540. doi: 10.1086/649924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.United States Agency for International Development (USAID) USAID ED USAID from the American People. 2005. Health Profile: Philippines. HIV/AIDS. [Google Scholar]
- 26.World Health Organization. Maternal anthropometry and pregnancy outcomes. A WHO Collaborative Study. Bull World Health Organ. 1995;73(Suppl):1–98. [PMC free article] [PubMed] [Google Scholar]
- 27.Villar J, Cogswell M, Kestler E, Castillo P, Menendez R, Repke JT. Effect of fat and fatfree mass deposition during pregnancy on birth weight. Am J Obstet Gynecol. 1992;167(5):1344–1352. doi: 10.1016/s0002-9378(11)91714-1. [DOI] [PubMed] [Google Scholar]
- 28.Ballard JL, Novak KK, Driver M. A simplified score for assessment of fetal maturation of newly born infants. The Journal of pediatrics. 1979;95(5 Pt 1):769–774. doi: 10.1016/s0022-3476(79)80734-9. [DOI] [PubMed] [Google Scholar]
- 29.Williams RL, Creasy RK, Cunningham GC, Hawes WE, Norris FD, Tashiro M. Fetal growth and perinatal viability in California. Obstet Gynecol. 1982;59(5):624–632. [PubMed] [Google Scholar]
- 30.Ezeamama AE, Friedman JF, Acosta LP, et al. Helminth infection and cognitive impairment among Filipino children. The American journal of tropical medicine and hygiene. 2005;72(5):540–548. [PMC free article] [PubMed] [Google Scholar]
- 31.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data--or tears: an application to educational enrollments in states of India. Demography. 2001;38(1):115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 32.Kurtis JD, Higashi A, Wu HW, et al. Maternal Schistosomiasis japonica is associated with maternal, placental, and fetal inflammation. Infection and immunity. 2011;79(3):1254–1261. doi: 10.1128/IAI.01072-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonald EA, Kurtis JD, Acosta L, et al. Schistosome egg antigens elicit a proinflammatory response by trophoblast cells of the human placenta. Infection and immunity. 2013;81(3):704–712. doi: 10.1128/IAI.01149-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassan MM, Medhat A, Makhlouf MM, et al. Detection of circulating antigens in patients with active Schistosoma haematobium infection. The American journal of tropical medicine and hygiene. 1998;59(2):295–301. doi: 10.4269/ajtmh.1998.59.295. [DOI] [PubMed] [Google Scholar]
- 35.Lewis S, Bain B, Bates I. Practical Hematology, Dacie and Lewis. 10th Edition ed. Churchill, Livingstone: Elsevier; 2006. [Google Scholar]