Abstract
Distressing symptoms such as hot flashes and sleep disturbances affect over 70% of women approaching menopause for an average of 4–7 years, and recent large cohort studies have shown that anxiety and stress are strongly associated with more severe and persistent hot flashes and can induce hot flashes. Although high estrogen doses alleviate symptoms, extended use increases health risks, and current non-hormonal therapies are marginally better than placebo. The lack of effective non-hormonal treatments is largely due to the limited understanding of the mechanisms that underlie menopausal symptoms. One mechanistic pathway that has not been explored is the wake-promoting orexin neuropeptide system. Orexin is exclusively synthesized in the estrogen receptor rich perifornical hypothalamic region, and has an emerging role in anxiety and thermoregulation. In female rodents, estrogens tonically inhibit expression of orexin, and estrogen replacement normalizes severely elevated central orexin levels in postmenopausal women. Using an ovariectomy menopause model, we demonstrated that an anxiogenic compound elicited exacerbated hot flash-associated increases in tail skin temperature (TST, that is blocked with estrogen), and cellular responses in orexin neurons and efferent targets. Furthermore, systemic administration of centrally active, selective orexin 1 or 2 and dual receptor antagonists attenuated or blocked TST responses, respectively. This included the reformulated Suvorexant, which was recently FDA-approved for treating insomnia. Collectively, our data support the hypothesis that dramatic loss of estrogen tone during menopausal states leads to a hyperactive orexin system that contributes to symptoms such as anxiety, insomnia, and more severe hot flashes. Additionally, orexin receptor antagonists may represent a novel non-hormonal therapy for treating menopausal symptoms, with minimal side effects.
Keywords: Orexin, Hypocretin, Hypothalamus, Hot flash, Estrogen, Menopause, Thermoregulation, Cutaneous, Serotonin, Norepinephrine
1. Introduction
Menopause occurs following loss of ovarian function during natural aging, and a simulated menopausal state follows some breast and ovarian cancer treatments [e.g., surgical oophorectomy, or estrogen inhibition therapies (Carpenter and Andrykowski, 1999; Gallicchio et al., 2006)]. Common symptoms during menopausal states include vasomotor symptoms (hot flashes and night sweats), anxiety and mood disruption, and sleep disturbances (Kronenberg, 1990). Hot flashes are the cardinal symptom, and 75% of postmenopausal women surveyed reported repeated hot flash episodes over an average of 4–7 years, but some over 10–13 years (Avis et al., 2015; Politi et al., 2008) following onset of menopause. Hot flash symptom presentations correlate well with objectively measurable, sympathetically-induced increases in cutaneous blood flow that raises skin temperature (Low et al., 2008).
Hormone replacement therapy [HRT, containing estradiol (E2) in combination with estrone (E1) and progesterone (P)] remains the most effective therapy for reducing hot flash symptomatology by ~75% treating in peri and post menopausal women[see systematic review (Maclennan et al., 2004)]. Yet, E2 replacement therapy (ERT) alone is as effective as HRT at moderate doses (1–2 mg) (Baerug et al., 1998; Greendale et al., 1998) which restore plasma estrogen levels to 85–100% of pre-menopausal levels, respectively (Waaseth et al., 2008).
Although ERT effectively alleviates hot flashes [even at lower doses of 0.5 mg (Joffe et al., 2014)], not all women are interested in taking ERT since initial use of E2 is associated with side effects such as uterine bleeding and breast tenderness (Maclennan et al., 2004), and long term ERT also increases the risk for estrogen positive breast cancer (Beral and Million Women Study, 2003; Bolland et al., 2015). Additionally, ERT is contraindicated for postmenopausal women with estrogen receptor positive breast cancer and hot flashes are the primary reason for noncompliance with estrogen inhibition therapies (Kemp et al., 2014), which increases mortality (Hershman et al., 2011). Collectively these side effects and identified risks may explain a significant decline in HRT prescription and compliance (Zbuk and Anand, 2012) and an increase interest in non-hormonal therapies. Unfortunately, the few existing non-hormonal therapies are much less effective than ERT (Nelson et al., 2006). For instance, ERT reduces hot flash symptomatology by by ~75% [see systematic review (Maclennan et al., 2004)], whereas clonidine or serotonin/norepinephrine reuptake inhibitors (SSRI/NRI) are ~46% and 30% effective, respectively and are only marginally better than placebo responses that typically range from 20 to 50% (Nelson et al., 2006). These therapies are also commonly associated with side effects such as headaches, nausea, decreased sexual function, and insomnia, which can reduce adherence. The lack of effective, non-hormonal treatments is largely due to the limited understanding of the mechanisms that underlie menopausal symptoms. Menopausal symptoms are clearly induced by estrogen withdrawal; yet, how loss of estrogens leads to menopausal symptoms is largely unknown (Miller and Li, 2004).
One mechanistic pathway that has not been explored is the orexin (OX and its two forms, OXA and OXB) neuropeptide system, which is unique in that OX-synthesizing neurons are restricted to the perifornical hypothalamic (PeF) region of rodents (Peyron et al., 1998) and humans (Thannickal et al., 2007). Estrogen receptors are expressed in the PeF (Laflamme et al., 1998), and stimulating the PeF in humans also produces symptoms associated with menopause [e.g., feelings of anxiety, racing heart, and hot flashes or chills (Wilent et al., 2011)]. Additionally, in female rats, E2 administration decreases OXA content within the hypothalamus as well as at postsynaptic target CNS sites (Russell et al., 2001). More importantly, menopausal women have 300% higher plasma OX levels compared to reproductive controls, which is restored following Prempac, comprised primarily of equine estrones, and norgestrel, a form of progestin (El-Sedeek et al., 2010)]. This dramatic increase in OX activity during menopause is likely contributing to disrupted sleep since OX’s most prominent role is to promote wakefulness (Sakurai, 2007), and higher OXA concentrations in cerebrospinal fluid have been demonstrated in individuals with poor sleep quality (Allen et al., 2002). But there is also emerging evidence that a hyperactive OX system contributes to anxiety states and panic-associated sympathetic activity in rodents (Johnson et al., 2012a, 2015, 2010), and that central OX levels are elevated in patients with increased anxiety symptoms (Johnson et al., 2010). These findings, combined with the building evidence that anxiety and stress are strongly associated with more severe and persistent hot flashes in large cohort studies (Avis et al., 2015), and that stressful stimuli can increase objective hot flashes (Swartzman et al., 1990) led to our current hypothesis that “the OX system plays a critical role in menopause-related symptoms such as hot flashes, anxiety, and sleep disruption”. We further posited that anxiety states and panicogenic-related stimuli may contribute to hot flash severity and frequency, and could help explain the presence of hot flashes in thermoneutral environments.
To test these hypotheses, we: (1) determined whether a pan-icogenic compound would elicit exacerbated tail skin temperature (TST: a correlate of a hot flash) responses in a surgical ovariec-tomy (OVEX) model of a menopausal state; (2) assessed cellular responses in OX neurons and efferent neurochemically identified targets that regulate arousal, anxiety, and thermoregulation; and (3) assessed orexin gene expression in the PeF, and determined if systemically treating OVEX rats with highly selective and brain-penetrant antagonists that target the two cognate OX receptors at optimal pharmacokinetic timelines would attenuate panicogenic drug induced TST responses.
2. Materials and methods
2.1. Animals and housing conditions
Adult female (225–250 g) Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) were housed individually in plastic cages in standard housing conditions (maintained at 22°C) for 5 days prior to experimental procedures. Rats had ad libitum access to food and water and were maintained on a 12:12 light/dark cycle (lights on at 07:00 h). All experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, Eighth Edition (Institute for Laboratory Animal Research, The National Academies Press, Washington, DC, 2011) and the guidelines of the IUPUI Institutional Animal Care and Use Committee.
2.2. Ovariectomy (OVEX) and sham OVEX surgeries
All surgical procedures were conducted under sterile conditions. Rats were anesthetized under isoflurane delivered through a nose cone (2–3% by volume MGX Research Machine, Vetamac, Rossvile, IN dissolved in medical air, Praxair, Indianapolis, IN). Animals were checked for corneal and paw-withdrawal reflexes to ensure adequate anesthesia before commencing surgical procedures. The skin was shaved on each side between the ribcage and hindlimbs and sterilized with iodine solution. A small (1–2 cm) incision was made to expose the lateral muscle wall, which was then opened and the ovaries were visualized. The ovaries are a prominent, spherical, reddish-pink structure that are readily seen from the incision site. In the even that the ovaries were not readily visible, a cotton swab was used to carefully move overlying tissue to expose them. For OVEX rats, after visualization, the ovary was gently pulled up and the fallopian tube was clamped 3–5 mm under the ovary with a hemostat. A length of suture was used to tie off the tube under the clamp, and the ovary was removed with a scalpel. The tissue was inspected for bleeding and re-tied if necessary. Silk sutures were used to close the muscle wall and the skin was closed with surgical staples. The entire procedure was then repeated on the other side. For sham operated control rats, after ovary visualization, the muscle wall and skin were closed as for OVEX rats and procedure repeated on the other side. Following surgery, all rats were given pain medication (buprenorphine, Indiana University School of Medicine Laboratory Animal Resources) and monitored until consciousness was restored. Animals then recovered for the next 12 days in the animal housing facilities, and were monitored daily.
2.3. Assessments of tail and core body temperature
For assessments of TST, a thermistor (cat. no. 5SRTC-TT-K-30-36; Omega Engineering, Stamford, CT) was secured 1 cm from the tail base to the ventral side using 3 M Durapore® tape. The thermistor was attached to a T-type pod and connected to a Powerlab data acquisition system (LabChart software, ADInstruments, Colorado Springs, CO) for continuous monitoring of temperature. Infrared thermal images were acquired using a FLIR T440 thermal imaging camera (FLIR Systems, Boston, MA). For assessments of core body temperature (CBT), a radiotelemetry probe with an internal thermistor was implanted into the abdominal cavity (model HD-S11, Data Sciences International, St. Paul, MN) under isoflurane. The probe was held in place with sutures into the muscle wall (during the ovariectomy surgery). Probes were interfaced with DataQuest ART software (DSI) for continuous recording of temperature.
2.3.1. Experiment 1—subthreshold panicogenic drug effect on TST of OVEX rats+/−estrogen
Female rats were anesthetized under isoflurane, and the ovaries were visualized (for sham controls) and excised bilaterally for OVEX rats. Twelve days post surgeries, OVEX rats received daily subcutaneous (s.c.) injections of either sesame oil vehicle (0.2 ml, sham-OVEX received this as well) or 17-β estradiol (0.25 mg/kg in 10% DMSO/90% sesame oil; Sigma–Aldrich, St. Louis MO) for 5 days prior to testing. Once a baseline was established for TST, rats were injected intraperitoneally (i.p.) with either vehicle or a low dose of the panicogenic drug FG-7142 (3 mg/kg, dissolved in 10% DMSO/90% ultrapure H2O). FG-7142 is a partial inverse agonist at the GABAA receptor, which decreases central GABA-mediated inhibition. We have also previously shown that systemically injecting males with a higher dose of FG-7142 (7.5 mg/kg) increases panic-associated behavioral and cardioexcitatory responses and cellular responses in anxiety/panic brain circuitry (e.g., amygdala and orexin neurons in PeF) (Johnson et al., 2012c). A systemic OX1 receptor antagonist also attenuates FG-7142-induced panic-associated behavioral and cardioexcitatory responses and cellular responses in the anxiety/panic circuitry(Johnson et al., 2012c). The experiment utilized a crossover design such that all rats received either vehicle or panicogenic drug with 48 h between treatments.
2.3.2. Experiment 2—subthreshold panicogenic drug effect on TST and CBT in OVEX rats
Female rats were anesthetized under isoflurane and the ovaries were excised bilaterally and allowed 12 days’ recovery. Radiotelemetry probes that contained an internal thermistor were also implanted into the abdominal cavity. Probes were interfaced with DataQuest ART software (DSI) for continuous recording of temperature. Once a baseline for TST and CBT was established, rats were injected intraperitoneally (i.p.) with either vehicle or FG-7142 (3 mg/kg) while monitoring TST and CBT simultaneously. The experiment utilized a crossover design such that all rats received either vehicle or FG-7142 with 48 h between treatments.
2.3.3. Experiments 3–4—neurochemical system cellular responses in OVEX rats treated with a panicogenic drug
Female rats were anesthetized under isoflurane, and the ovaries were visualized (for sham controls) and excised bilaterally for OVEX rats. Following 12 days, OVEX and sham-OVEX rats were injected with either vehicle or a low dose of FG-7142 (1 mg/kg, i.p.). Ninety minutes post-injection, rats were anesthetized with isoflurane and transcardially perfused with 4% paraformaldehyde in 0.1 M sodium phosphate buffer (PB). Brains were removed, post-fixed in the same fixative for 12h, rinsed twice in PB (12 h), and placed in 0.1 M PB containing 30% sucrose for 12 h. Brains were blocked using a standard adult rat brain matrix (model RBM-4000C, ASI Instruments, Warren, MI) and frozen using liquid isopentane cooled by liquid nitrogen. Serial coronal sections (30 μm) were cut using a Leica freezing microtome and were immediately placed in cryoprotectant consisting of 27% ethylene glycol and 16% glycerol in 0.05 M PB to yield 6 alternative sets of sections. Sections were stored at −20&#°C until immunohistochemical processing. All solutions used had pH level of 7.4.
We used immunohistochemical staining for c-Fos, in the presence or absence of other neurochemical markers, to identify specific brain regions involved in responses to the anxiogenic drug. Immunostaining of nuclear c-Fos expression is a useful method of identifying functional cellular responses to anxiety-related stimuli (Johnson et al., 2012a). Four of the six alternate sets of 30 μm coronal brain sections were immunostained, one set each for c-Fos (full brain), or c-Fos (day 1) then on day 2 the tissue was immunostained with the cytoplasmically expressed OXA (hypothalamus); tyrosine hydroxylase (TH, brainstem), or tryptophan hydroxylase (TPH, brainstem). Immunostaining of OXA, TH, and TPH were done to identify orexin, noradrenergic, and serotonergic neurons, respectively. Specifically, free-floating sections were washed in 0.05 MPBS for 30 min, then incubated in 1% H2O2 in PBS for 20 min. Sections were then washed 10 min in PBS and 20 min in PBS with 0.3% Triton X-100 (PBST). Sections were then incubated 12–16 h in PBST with the primary antibody solution at room temperature [i.e., day 1: c-Fos (rabbit anti-c-Fos polyclonal antibody, cat. no. sc-52, Santa Cruz Biotech., Santa Cruz, CA; diluted 1:10,000); day 2: OXA (rabbit anti-OXA-polyclonal, affinity-purified antibody, cat. no. H-003-30, Phoenix Pharmaceuticals, Burlingame, CA; diluted 1:8000); TH (rabbit anti-TH polyclonal affinity-purified antibody cat. no. AB152, Millipore, Billerica, MA; diluted 1:800); or TPH (sheep anti-TPH polyclonal affinity-purified antibody, cat. no. 9260–2505, AbD Serotec; diluted 1:1000 for midbrain/pons and 1:2000 for medulla)]. After a 30 min wash in PBST, sections were incubated 2 h in the appropriate secondary antibody: biotinylated goat anti-rabbit IgG (c-Fos, OXA, TH; cat no. BA-1000, Vector Laboratories, Burlingame, CA; diluted 1:200), biotinylated rabbit anti-sheep IgG (TPH; cat. no BA-6000; Vector Laboratories, 1:200 dilution). Sections were washed again for 30 min in PBST then incubated 1.5 h in an avidin-biotin complex provided in a standard Vector Elite kit (cat no. PK-6100, Vector Laboratories; diluted 1:500). Substrates for chromagen reactions were SG (c-Fos; SK-4700, Vector Laboratories) or 0.01% 3,3′-diaminobenzidine tetrahydrochloride (DAB; OXA, TH; TPH) (D-5637, Sigma, St. Louis MO, USA) in PBS containing 0.003% H2O2, pH 7.4. Substrate reactions were run for ~10–15 min. All sections were mounted in 0.1% gelatin dissolved in ultrapure water on glass slides, dried overnight, dehydrated and mounted with coverslips using DPX mounting medium (BDH Laboratory Supplies, Poole, UK). All washes and incubations were done in 12 well polystyrene plates with low frequency shaking on an orbital shaker.
2.3.4. Experiment 5—orexin, glutamate, GABA gene expression in the PeF of OVEX rats
Twelve days after sham/OVEX surgery, rats were anaesthetized and brains removed and flash frozen in isopentane precooled with dry ice then sored at −80°C until ready for processing. The PeF region was bilaterally dissected from two consecutive 300 μm coronal sections and tissue was processed for absolute qRT-PCR for Orexin mRNA using methods previously described (Johnson et al., 2010), or for glutamate and GABA-related genes using the custom-designed TaqMan Low Density Array (TLDA) as previously described (Truitt et al., 2015). Orexin gene expression analysis was normalized to beta-actin as previously described (Johnson et al., 2010). The glutamate and GABA-related gene expression panel was normalized using geNorm approach as previously described in detail (Truitt et al., 2015).
2.3.5. Experiments 6–7—effects of an OX1R antagonist (SB-334867) or estradiol on anxiety-like behaviors in OVEX rats
2.3.5.1. Experiment 6
Following 12 days, OVEX and sham-OVEX rats were injected i.p. with a control vehicle [0.2 ml/100 g volume dimethyl sulfoxide (DMSO)] or a 30 mg/kg dose of the single OX1R antagonist (SORA1) SB334867 (cat. no. 1960, Tocris Bioscience, Bristol, UK, in 0.2 ml/100 g volume DMSO) which has selectivity of 50X for the OX1 receptor compared to the OX2 receptor; occupies 100% of central OX1Rs within 30 min post systemic injection (Bonaventure et al., 2015); and attenuates FG-7142 (7.5 mg/kg, i.p.) induced anxiety-like behavior and panic-associated cardioex-citatory responses without inducing somnolence (Johnson et al., 2012a). Therefore, we tested rats in an accepted test of anxiety-associated behaviors (i.e., open field test which was videorecorded and scored by Anymaze software, Stoelting, Woods Dale, IL) 60 min following injections. The experiment utilized a crossover design such that all rats received either vehicle or the SORA1 with 48 h between treatments.
2.3.5.2. Experiment 7
Following 12 days, OVEX rats received daily subcutaneous injections of either sesame oil vehicle (0.2 ml, sham-OVEX received this as well) or 17-β estradiol (0.25 mg/kg) for 5 days prior to testing in the open field.
2.3.6. Experiment 8–12—effects of an OX1R (SB-334867 or Compound 56), OX2R (TCS OX2 29 or JNJ10397049) or dual OXR antagonist (DORA-12) on TST responses to an panicogenic drug in OVEX rats
Following 12 days, all OVEX rats were pretreated systemically with highly selective and centrally active OXR antagonists (details below) prior to receiving an injection of the panicogenic drug FG-7142 (3 mg/kg, i.p.). These experiments utilized a crossover design such that all rats received either vehicle or an OXR antagonist with 48 h between treatments. Five days prior to experiments, rats were handled for 5 min and trained with either i.p or s.c injections or mock gavages. Tail skin temperature was assessed as previously described.
2.3.6.1. Experiment 8—dual orexin receptor antagonist (DORA; DORA-12)
On the experimental day, rats received an oral gavage of a control vehicle (0.2 ml/100 g volume 20% vitamin E/TPGS) or 30 mg/kg of a dual OXR antagonist, DORA-12 (Merck & Co.) with balanced potency for OX1R and OX2R; good brain exposure; 47% oral bioavailability; and a favorable brain to plasma ratio of 0.4–0.6. This compound is a close structural analog of suvorexant, and the 30 mg/kg p.o. dose of DORA-12 being used here achieves a plasma Cmax of 2.02 μM with CSF exposure of 66 nM and ex vivo occupancy of 97%, and has been shown to promote sleep in rats (Gotter et al., 2013).
2.3.6.2. Experiments 9—single orexin-1 receptor antagonist (SORA1; SB-334867)
On the experimental day, rats received an i.p. injection of a control vehicle (0.2 ml/100 g volume DMSO) or a 30 mg/kg dose of SB-334867 i.p.
2.3.6.3. Experiment 10—single orexin-1 receptor antagonist (SORA1; Compound 56)
All rats were injected s.c. with a control vehicle (0.2 ml/100 g volume DMSO) or the highly selective SORA1, Compound 56 (10 mg/kg), dissolved in 30% SBE-β-cyclodextrin/70% ddH2O supplied byJanssen Research & Development, LLC, La Jolla, CA, which has 44X selectivity for the OX1R compared to the OX2R, and the 10 mg/kg systemic dose occupies ~90% of central OX1Rs within 30 min (Bonaventure et al., 2015). While SB334867 has been shown to have off-target affinities for non-OXRs, in a binding assay panel of 50 receptors, ion channels, and transporters, Compound 56 did not exhibit a significant affinity to anything other than the OX1R (Bonaventure et al., 2015).
2.3.6.4. Experiments 11—single orexin 2 receptor antagonist (SORA2; JNJ10397049)
All rats were injected s.c. with a control vehicle (0.2 ml of 10% pharmasolve, 5% solutol, and 85% dextrose in water) or a dose of JnJ10397049 (10 or 30 mg/kg, Tocris Bioscience, Bristol, UK, in 0.2 ml) which has selectivity of 630X for the OX2R compared to the OX1R and the 30 mg/kg systemic dose occupies ~80% of central OX2Rs within 30 min (Dugovic et al., 2009).
2.3.6.5. Experiment 12—single orexin 2 receptor antagonist (SORA2, TCS OX229)
All rats were injected i.p. with a control vehicle (0.9% saline) or TCS OX2 29 (30 mg/kg, Tocris Bioscience, in 0.2 ml), a SORA2 that displays >250-fold selectivity for OX2Rs over OX1Rs (Bonaventure et al., 2015).
2.4. Statistical analyses
Analyses of behavior (SORA1), and TST responses were analyzed using an ANOVA with drug treatments and/or surgical treatments as main factors (i.e., independent variables) and time as a repeated measure. In the presence of a significant drug effect or drug × time interaction, an ANOVA was run at each time point in combination with a Fisher’s LSD test post hoc test to detect within subject differences. Analyses of OXA gene expression and behavior (estrogen) were performed with an independent two-tailed t-test or a non-parametric Mann-Whitney Rank Sum Test U if unequal variance was detected with a Shapiro-Wilk normality test p< 0.05. Expression of each glutamate/GABA-related gene was calculated relative to the sham treatment group using the delta Ct method. These values were then converted to log10 (log10(ΔΔCt)) for statistical analyses with an ANOVA and Fisher’s LSD for post hoc testing. Analyses of cell counts was analyzed with an ANOVA with drug treatment and surgical treatments as main factors and a Tukey’s post hoc test. Levene’s Test of Equality of Error Variance was also done to determine equal variances in the groups to determine if nonparametric testing was needed. The alpha level was set at 0.05 in all cases. All statistical analyses were carried out using SPSS 21.0 (SPSS Inc., Chicago, IL, USA), and all graphs were generated using SigmaPlot 12.0 (SPSS Inc.) and an illustration program (CorelDraw X5, Ontario, Canada).
3. Results
3.1. Experiment 1: OVEX rats have exacerbated TST responses to a low dose of a panicogenic drug that is blocked by estrogen replacement
Compared to sham-OVEX controls, systemically injecting female OVEX rats with a low dose of the panicogenic drug FG-7142 (n = 7,5,5) produced a marked 6–7°C increase in TST, which was blocked with estrogen replacement (Fig. 1a-representative thermal imaging; Fig. 1b-treatment × time interaction F(55,770) =3.4, p< 0.001).
Fig. 1.
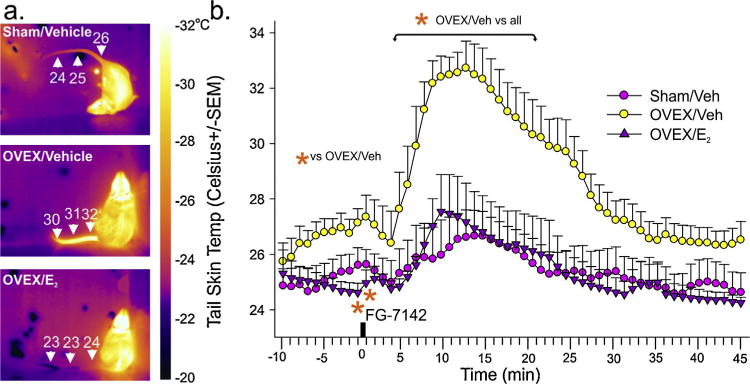
Rats with surgical menopause are vulnerable to displaying hot-flash associated cutaneous vasomotor responses to a low dose of a panicogenic drug.
Effects of a low dose of FG-7142 (3 mg/kg) on tail skin temp of female sham-OVEX rats given daily 0.2 ml s.c. injections of sesame oil control vehicle (n = 7), or in OVEX rats given daily s.c. injections of control vehicle (n = 5) or 0.25 mg/kg 17-β estradiol (n = 5). (a) Thermal images of rats 10 min after FG-7142 injection from each treatment group with scale bar to right. (b) Line graph with error bars (SEM) represents the mean tail skin temp prior to and following a 3 mg/kg i.p. injection of FG-7142 in each treatment group assessed with a thermistor at base of tail. *Denotes significance with a one-way ANOVA with drug as an independent variable and time as a repeated measures p < 0.05, and significant differences between groups using a Fisher’s LSD post hoc test protected by an ANOVA at each time point, p< 0.05.
3.2. Experiment 2: FG-7142 induced increases in TST precedes a decrease in core body temperature (CBT) decrease in OVEX rats
Compared to vehicle treated OVEX controls, FG-7142 treated OVEX rats had a dramatic increase in TST within 3 min (F(55,825) = 18.7, p< 0.0001, n = 8,9, see thermal imaging of tail in Fig. 2a, and TST response on bottom line graphs in Fig. 2b) which was followed by a decrease in CBT that occurred 11 min post injection (F(55,825) = 11.2, p < 0.001, n = 8,9, see CBT response on top line graphs in Fig. 2b).
Fig. 2.
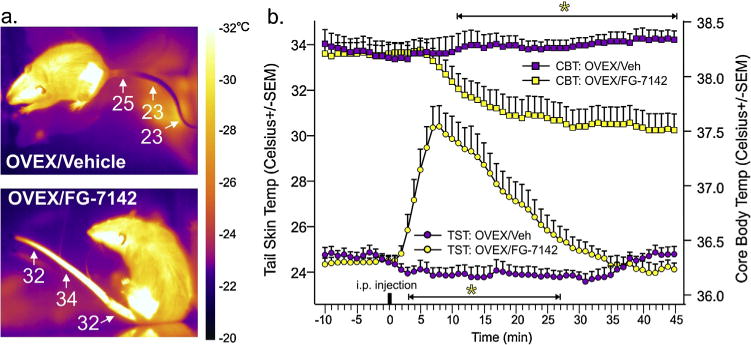
Panicogenic drug-induced cutaneous vasomotor responses precede a decrease in core body temperature in rats with surgical menopause.
Effects of a low dose of FG-7142 (3 mg/kg) on tail skin temp and core body temperature of female OVEX rats. (a) Representative thermal images of OVEX rats 10 min after vehicle or FG-7142 injection from each treatment group with scale bar to right. (b) Line graph with error bars (SEM) represents the mean tail skin temp and core body temperature of OVEX rats prior to and following systemic injections of either vehicle (n = 8) or 3 mg/kg FG-7142 (n = 9). *Denotes significance with a one-way ANOVA with drug as an independent variable and time as a repeated measures p<0.05, and significant differences between groups using a Fisher’s LSD post hoc test protected by an ANOVA at each time point, p< 0.05.
3.3. Experiment 3: neural circuits of OVEX rats that display hyperactive responses following a low dose of a panicogenic drug
Among the brain regions implicated in menopause-related symptoms (see Fig. 3a for convergent evidence for brain region involvement and Fig. 3b for coronal illustration of brain regions assessed), the following brain regions of OVEX rats showed exacerbated cellular c-Fos responses to the low dose of a panicogenic drug FG-7142 (i.e., OVEX × FG-7142 interaction, Fig. 3c): (1) PeF [n = 7,7,7,7, F(1,24) = 4.5, p = 0.044]; (2) central amygdala [CeA, n = 7,7,6,7 (area damaged unilaterally on one rat), F(1,23) = 16.1, 0.001]; (3) dorsolateral region of dorsal raphe nucleus [DRVL, n = 6,6,7,7, (area damaged unilaterally on two rats), F(1,22) = 6.6, p = 0.018]; (4) locus ceruleus [LC, n = 7,7,7,7, F(1,24) = 6.1, p = 0.021]; (5) raphe pallidus [RPa, n = 7,7,7,7, F(1,24) = 7.4, p = 0.012]; (6) rostroventrolateral medulla [RVLM, n = 7,7,7,7, F(1,24) = 4.4, p = 0.048]. Cellular responses were not significantly different in the periventricular hypothalamic nucleus (PVN), medial preoptic hypothalamic area (mPOA), basolateral, lateral, or medial amygdala (BLA, LA, MeA), bed nucleus of the stria terminalis (BNST), dorsome-dial or dorsolateral periaqueductal gray (dmPAG, dlPAG), ventral or dorsal part of the dorsal raphe nucleus (DRV or DRD), median raphe nucleus (MRn), or raphe magnus (RMg).
Fig. 3.
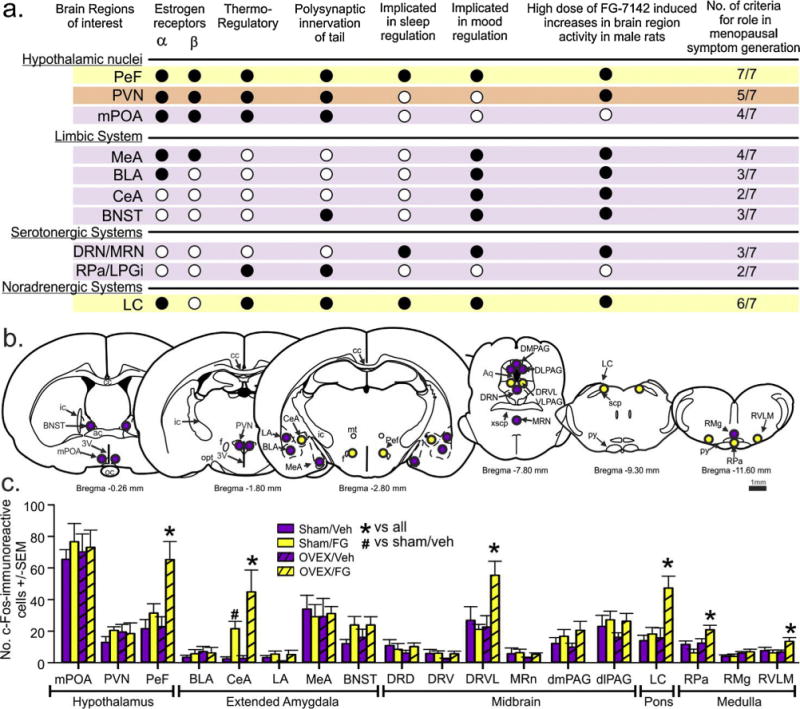
Rats with surgical menopause display hyperactive cellular responses following a low dose of a panicogenic drug in neural circuits heavily implicated in menopausal symptoms. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
(a) Table with convergent evidence of neurochemical circuit involvement in hot flash-associated cutaneous vasomotor responses. Columns respectively represent brain regions and additive criteria to strengthen the likelihood that a particular brain region will be impacted by loss of estrogen, but also involved in menopause-related symptoms. Closed circles means the region meets the criterion, open circles mean the region does not meet the criterion. Yellow shaded rows meeting most criterion, with orange and purple shaded rows meeting less. See results section for anatomical abbreviation definitions. Effects of vehicle or a dose of FG-7142 (that evokes hot flashes in OVEX, but not sham rats) on cellular responses (c-Fos immunoreactive, ir) in brain regions from OVEX and sham-OVEX rats from table 1 (n = 6,6,7,7). (b) illustrates coronal brain sections of a rat at different rostrocaudal levels, with yellow circles indicating brain regions where OVEX rats showed hyperactive responses to FG-7142 compared to sham-OVEX rats. Purple circles indicate areas that were counted but had no significant differences between groups. Bars in(c) represent mean number of single c-Fos-ir cells + -SEM. *Denotes significant differences between groups using aTukey’s HSD post hoc test protected by 2 way ANOVA, p < 0.05.
3.4. Experiment 4: neurochemical circuits of OVEX rats that display hyperactive responses following a low dose of a panicogenic drug
Neurochemical phenotyping of c-Fos responses in OXA-ir neurons in the PeF and adjacent lateral hypothalamus (LH) revealed that OX neurons in the PeF, but not LH had exacerbated responses to FG-7142 in OVEX rats (n = 6,6,7,7) [PeF: OVEX × drug interaction, F(1,22) = 4.1, p = 0.055; OVEX effect, F(1,22) = 4.1, p = 0.055; FG-7142 effect F(1,22) = 4.5, p = 0.046; LH data not shown: OVEX × drug interaction, F(1,22) = 1.1, p = 0.306; OVEX effect, F(1,22) = 2.0, p = 0.169; FG-7142 effect F(1,22) = 0.5, p = 0.482] (Fig. 4a). This difference in the number of c-Fos-ir OXA positive neurons was unlikely due to differences in numbers of OXA-ir neurons counted since there was no difference in the PeF [see gray bars in Fig. 4a: PeF: OVEX × drug interaction, F(1,22) = 0.9, p = 0.770; OVEX effect, F(1,22) = 0.3, p = 0.601; FG-7142 effect F(1,22) = 0.9, p = 0.770; LH: OVEX × drug interaction, F(1,22) = 1.3, p = 0.258; OVEX effect, F(1,22) = 3.2, p = 0.086; FG-7142 effect F(1,22)< 0.01, p = 0.997].
Fig. 4.
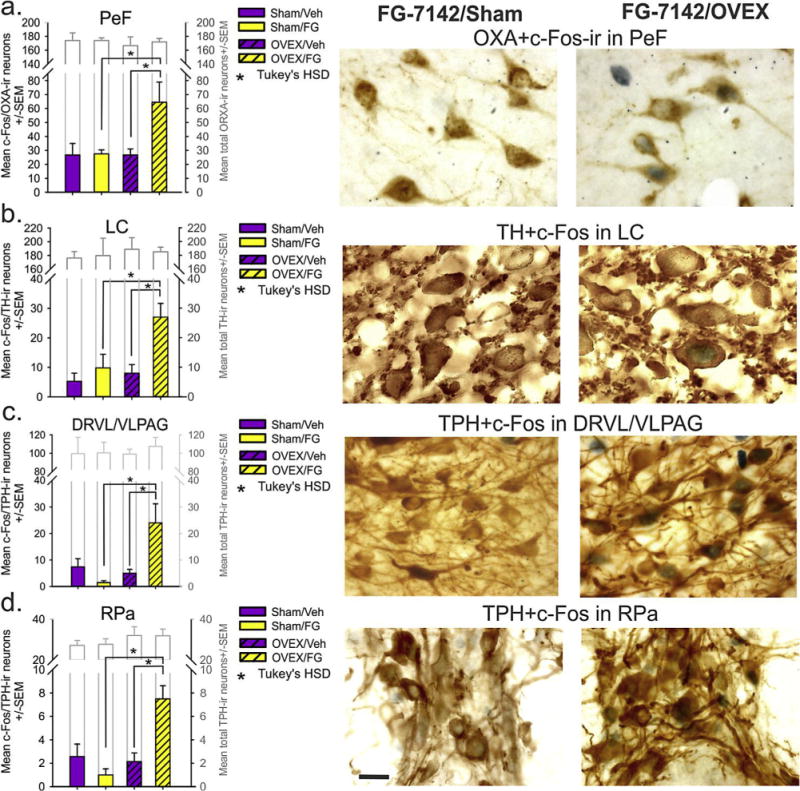
Neurochemical circuits of rats with surgical menopause that display hyperactive responses following a low dose of a panicogenic drug.
Effects of vehicle or FG-7142 injection (that evokes hot flashes in OVEX, but not sham rats) on cellular responses (c-Fos immunoreactive, ir) in brain regions from OVEX and sham-OVEX rats from table 1 (n = 6,6,7,7). Bars in (a) represent c-Fos + OX double labelled neurons in the PeF/DMN and LH. Bars in (b) represent c-Fos + tyrosine hydroxylase (TH) in noradrenergic neurons in the locus ceruleus (LC). Bars in (c and d) represent c-Fos + tryptophan hydroxylase (TPH) in serotonergic neurons in the ventrolateral region of the dorsal raphe nucleus (DRVL/VLPAG), and in the raphe pallidus (RPa), respectively. *Denotes significant differences between groups using Tukey’s HSD post hoc test p< 0.05, protected by a 2 way ANOVA. Photographs in (a–d) represent high magnification brain sections illustrating dark nuclear immunostaining of c-Fos +-brown cytoplasmic immunostaining of OX-ir neurons in the PeF, TH-ir neurons in the LC, and TPH-ir neurons in the DRVL/VLPAG and RPa from sham and OVEX rats treated with FG-7142, respectively. Scalebar in d = 20 μm.
Neurochemical phenotyping of c-Fos responses of tyrosine hydroxylase-ir (TH)-noradrenergic neurons in the LC revealed that these neurons had exacerbated responses to FG-7142 in OVEX rats [Fig. 4b, OVEX × drug interaction, F(1,22) = 3.8, p = 0.064; OVEX effect, F(1,22) = 7.2, p = 0.014; FG-7142 effect F(1,22) = 10.1, p = 0.004]. Neurochemical phenotyping of c-Fos responses TPH-ir serotonergic neurons in the DRVL and RPa revealed that these neurons had exacerbated responses to FG-7142 in OVEX rats [Fig. 4c, n = 6,6,7,7, OVEX × drug interaction, F(1,23) = 9.4, p = 0.006 (area damaged unilaterally on two rats); Fig. 4d, n = 7,6,7,6, OVEX × drug interaction, F(1,22) = 14.7, p = 0.001 (area damaged on two rats)]. No difference was noted in the total number of TH or TPH-ir neurons counted between groups (see gray bars in Fig. 4c and d).
3.5. Experiment 5: orexin, glutamate, GABA gene expression in the PeF of OVEX rats
Gene expression analyses in the PeF region (see Fig. 3a for convergent evidence of PeF region involvement) revealed that compared to sham-OVEX controls, OVEX rats had 200% higher expression of prepro-orexin (n = 7,6, see Fig. 5a for illustration of area micropunched; Fig. 5b, t(14) = −2.1, p = 0.029). A TaqMan Low Density Array (TLDA) custom designed for GABA and glutamate-related genes revealed that compared to sham-OVEX rats, OVEX rats had had a 1.81 fold increased gene expression of GAD1 (aka GAD67, p = 0.040) and decreased expression of Grm4 (−1.83 fold; p = 0.003) and Grin2a (−1.63 fold; p = 0.024) (see volcano plot in Fig. 5c).
Fig. 5.
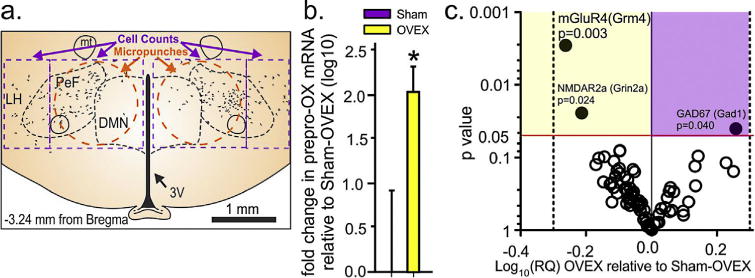
Surgical menopause enhances baseline hypothalamic OX/glutamate gene expression. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
(a) Illustration of a coronal section of rat hypothalamus with the PeF/DMNand LH with representative distribution of OX neurons (black dots) and areas where micropunches were done (orange dashed circles) in a 300 μm fresh frozen tissue section for gene expression data in (b and c) and where cell counts were done (purple dashed lines) in Figs. 2 C and 3). (b) Indicates expression of prepro-OX mRNA in sham and OVEX rats. *Indicates p < 0.05 unpaired t-test. (c) A volcano plot of hypothalamic mRNA levels of OVEX rats relative to sham rats, determined by a custom designed TaqMan Low Density Array (TLDA). Plotted on the y-axis are p values from Fisher’s LSD test (each gene) and ±fold difference (Log10) on the x-axis. Genes with p< 0.05 are indicated by solid circles and all others are open circles. Horizontal red line is p = 0.05 and vertical dotted lines represent a ±2 fold change in expression (0.0301 on log10 scale).
3.6. Experiments 6–7: systemicpretreatment with a SORA1 or estrogen is anxiolytic in OVEX rats
Compared to sham-OVEX controls, OVEX rats spent less time exploring the center region of an open field test (indicating an anxiety-like behavioral response, Suppl. Fig. 1a). Furthermore, pretreating OVEX rats with a SORA1 blocked the OVEX-induced anxiety-associated behavior [SORA1 effect, F(1,35) = 7.1, p = 0.012, n = 10,10,9,10, an outlier detected and removed from OVEX/vehicle group, Grubb’s test z = 2.3, p<0.05]. In a second experiment, compared to OVEX rats treated with vehicle, OVEX rats with estrogen replacement spent more time exploring the center of the open field [Suppl. Fig. 1b, n = 9,7, failed Shapiro–Wilk normality test p<0.05, Mann–Whitney Rank Sum Test U= 5.0, p = 0.006].
3.7. Experiments 8–12: systemic pretreatment with an OX1R, OX2R, or dual OX antagonist attenuates TST responses to a panicogenic drug in OVEX rats
Here the highest dose of DORA-12 blocked the TST response to the panicogenic drug FG-7142 (Fig. 6a); drug × time interaction, F(108,1458) = 1.4, p = 0.006, n = 9,10,10]. In the SORA1 experiments, both SB334867 and Compound 56 attenuated the TST response to the panicogenic drug FG-7142 (Fig. 6b); no drug × time interaction F(55,715) = 1.1, p = 0.247, but there was a significant drug effect, F(1,13) = 7.6, p = 0.016, n = 5,10; Fig. 6c, drug × time interaction F(54,648) = 2.2, p< 0.001, F(1,13) = 7.6, p = 0.016, n = 7,7]. Initially, we administered a SORA2 (TCS OX2 29, 5 and 10mg/kg, i.p.) which, compared to vehicle group, did not alter FG-7142 induced TST responses in OVEX rats (data not shown, drug × time interaction F(130,2535) = 0.5, p = 1.00, drug effect F(2,39) = 0.5, p = 0.600, n = 14,14,14). We later learned that this compound only achieves about ~50% OX2R occupancy and quickly dissociates (P. Bonaventure, personal communication). In a subsequent experiment using a brain penetrant SORA2 called JnJ10397049, which achieves greater (~75%) and sustained (8 h) receptor occupancy an attenuation of the TST response to the panicogenic drug FG-7142 (3mg/kg, i.p.) was observed at both doses [Fig. 6d; drug × time interaction F(100,1950) = 37.4, p< 0.001, n = 14,14,14].
Fig. 6.
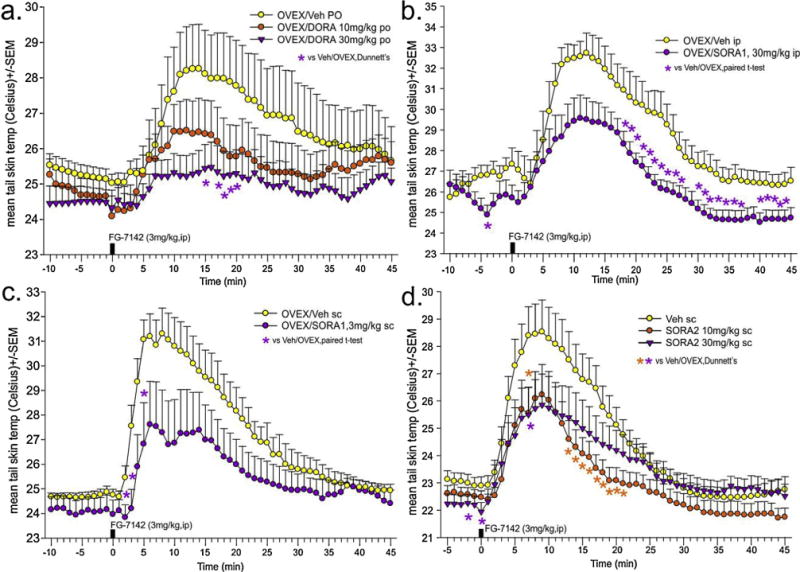
Systemically treating ovariectomized rats with centrally active orexin 1 and/or 2 receptor antagonists attenuates hot flash associated cutaneous vasomotor responses to a low dose of a panicogenic compound.
(a) Effects of FG-7142 (3 mg/kg) on tail skin temp of female OVEX rats orally pre-treated with vehicle or a dual OXR antagonist (DORA-12. 10 and 30mg/kg p.o.); (b and c) Effects of FG-7142 (3 mg/kg) on tail skin temp of female OVEX, or sham-OVEX rats systemically pre-treated with vehicle or an OX1R antagonist (b: SB334867 30 mg/kg i.p.; c: compound 56,3 mg/kg s.c); (d) Effects of FG-7142 (3 mg/kg) on tail skin temp of female OVEX, or sham-OVEX rats systemically pre-treated with vehicle or an OX2R antagonist (JnJ-10397049,10 and 30 mg/kg s.c). *Denotes significant differences between groups using a two-tailed Dunnett’s post hoc test against vehicle protected by a one-way ANOVA with drug as main factor and time as repeated measures, and a Fisher’s LSD post hoc test protected by a one-way ANOVA at each time point, p< 0.05. All OX receptor antagonist doses, routes, and treatment timelines were done based on published pharmacokinetic information (e.g., brain penetrance, OX1R and OX2R occupancy in the brain, and Cmax in plasma and brain).
4. Discussion
Here we first ovariectomized female rats to model a surgical menopausal state, which increased baseline anxiety-associated behaviors, and when challenged with a low dose of the panicogenic compound FG-7142, OVEX rats had an exacerbated hot flash-associated TST responses >7°C that preceded a decrease in CBT (suggesting a heat dissipation response), thus providing face validity. Furthermore, ER, which also attenuated anxiety-associated behaviors, almost blocked the TST responses. Thus, predictive validity is provided since ER effectively treats hot flashes and FG-7142 is known to cause hot flashes and/or chills in humans, and also elicits anxiety (Dorow et al, 1983). These data also suggest that there is a loss of GABAergic inhibitory signaling following loss of ovarian function. Consistent with this hypothesis is that in one clinical study the benzodiazepine oxazepam was effective in reducing hot flashes in a cohort of premenopausal women who underwent oophorectomy (Erkkola et al, 1973). Collectively, these clinical studies and our preclinical studies may explain part of the effectiveness of these treatments for hot flashes. Finally, since this method of inducing hot flash-associated TST responses also produces strong anxiety at higher doses, it suggests that some stress-related stimuli and or existing anxiety states could contribute to hot flash severity and prevalence.
The link between anxiety and stress with hot flashes has been controversial. For instance, although a recent systematic review concluded that anxiety symptoms were low during the menopause transition, they noted that the majority of studies utilized brief and largely non-validated measures of anxiety symptoms (Bryant et al., 2011). Bryant and colleagues further stated that studies that utilized accepted diagnostic assessments did observe an increase in anxiety in postmenopausal women [see (Cagnacci et al, 1997; Pimenta et al., 2012)] that is alleviated with ERT + P (Cagnacci et al., 1997). Furthermore, greater presence of anxiety predicts more frequent and/or severe hot flashes (Freeman et al., 2005), and is also positively correlated with hot flash severity (Gibson et al., 2011). Additionally, women with premenstrual syndrome, which includes high levels of anxiety, are more likely to have hot flashes (Freeman et al, 2004) and hot flashes are common symptom associated with panic attacks that occur in severe anxiety disorders (DSM-V). Perhaps more compelling is that in a recent large (n = 3302) multiracial/multiethnic longitudinal investigation [the Study of Women’s Health Across the Nation], greater perceived stress, anxiety symptoms, depressive symptoms, and decreased socioeconomic status were associated with more persistent and frequent vasomotor symptoms (Avis et al., 2015). Thus, these data are supportive of the hypothesis that anxiety and stress, which are associated with more severe and persistent hot flashes, may also contribute to hot flash severity and prevalence. This hypothesis is also consistent with a hypothetical review that observed similarities between hot flashes and severe anxiety/panic attacks that included: symptom presentation; neurochemical circuitry; pharmacotherapy; and psychological treatments (Hanisch et al, 2009) and also suggests that neurochemical systems implicated in generating anxiety-associated emotional, behavioral, and physiological responses may represent novel targets for treating symptoms associated with menopause.
Our subsequent experiments sought to determine if the hypothalamic OX system might represent one such novel therapeutic target. In a series of experiments, we demonstrated that rats with a surgical menopausal state have a two-fold increase in prepro-OX mRNA expression within the PeF region, and exacerbated OX neuronal responses to a subthreshold panicogenic drug that excites this system (FG-7142) (Johnson et al., 2012a). We further demonstrated that efferent targets of OX neurons heavily implicated in hot flash etiology (Freedman et al, 1990; Stearns et al, 2005) also showed exacerbated cellular responses. These included limbic structures such as the amygdala, as well as noradrenergic neurons in the locus ceruleus, and serotonergic neurons in the dorsal raphe nucleus and raphe pallidus. Artificially increasing central OX activity in rodents with intracerebroventricular injections of OXs disrupts sleep (Espana et al., 2002); increases anxiety-associated behaviors (Suzuki et al., 2005); induces thermoregulatory changes (Yoshimichi et al, 2001); elicits cardio-excitation (Samson et al., 1999), and stimulates corticosteroid release (Brunton and Russell, 2003), which is related to greater hot flash symptomatology in menopausal women (Woods et al., 2006)]. These data are consistent with preclinical data showing that estrogen inhibits OX expression in rodents (Russell et al, 2001), and clinical data showing OX activity is dramatically elevated following menopause (El-Sedeek et al, 2010). Thus, a hyperactive OX system seen here in our surgically-induced menopausal state in rodents and mirrored in postmenopausal women (El-Sedeek et al., 2010) could be contributing to multiple core symptoms that occur during and/or following menopause. An outstanding question for future studies, would be determining if the interactions observed between orexin and estrogen are indirect or direct (e.g., determining if the prepro-orexin gene has an estrogen response element, or whether expression of ERα and/or ERβ are colocalized with orexin neurons).
In a final series of experiments, we systemically treated OVEX rats with highly selective and brain-penetrant OXR antagonists at optimal pharmacokinetic timepoints to determine if antagonizing this system would decrease hot-flash associated increases in TST. There are two forms of OXs, OXA and OXB, and two known receptors. The OX1R has greater affinity for OXA than OXB by an order of magnitude, while the OX2R has similar affinity for both OXA and OXB (Sakurai, 2007). Both receptors are G-protein coupled, and are either co-located or selectively located in specific brain areas (Marcus et al, 2001), suggesting differentiated roles. Here we show that a dual OXR antagonist, DORA-12 (a reformulated version of Suvorexant) blocked the TST response elicited by FG-7142, whereas antagonizing either receptor alone partly attenuated hot flash-associated TST responses. This suggests that these drugs may provide a novel non-hormonal treatment strategy for hot flashes in menopausal women. Interestingly, Suvorexant was recently FDA-approved with an indication for insomnia. Sleep problems, broadly defined, are a known problem at midlife and at the menopause (Burleson et al, 2010), and many have posited that hot flashes during sleep contribute to nighttime awakenings/sleep problems. Therefore, it seems reasonable to postulate that targeting either symptom may lead to improvement in the other. The sleep-promoting properties of DORAs are primarily through antagonism of OX2Rs which are exclusive to histaminergic neurons in the tuberomammillary nucleus, which is a brain region that plays a critical role in wake promotion (Huang et al., 2001; Marcus et al., 2001), but also plays a role in regulating thermogenic activity (Yasuda et al., 2004) which could partially explain the ability of the OX2R antagonist to attenuate TST responses. The anxiolytic effects of DORAs may be preferentially through antagonism of OX1Rs (Bonaventure et al., 2015; Johnson et al., 2012a, 2015a, 2010), but also potentially OX2Rs (Li et al., 2010). Anatomically, OX1Rs appear to be more selectively expressed in panic and anxiety-associated neural circuits such as the bed nucleus of the stria terminalis, amygdala, cingulate cortex and exclusively in noradrenergic neurons in the locus ceruleus [(Marcus et al., 2001), see review (Johnson et al., 2012b)], which also play a role in sympathetic mobilization. Yet, anxiolytic effects of ORX2R antagonism have been demonstrated in the paraventricular thalamus (Li et al., 2010). Thus, DORAs may alleviate sympathetically-mediated hot flashes and or hot flashes resulting from pre-existing anxiety states or in responses to some stress-related stimuli.
5. Conclusion
Collectively, our previous and current data support the hypothesis that estrogen tonically inhibits the orexin arousal system, and dramatic loss of estrogen tone during menopausal states leads to a hyperactive orexin system that contributes to the cardinal menopausal symptoms such as recurrent hot flashes, insomnia, and anxiety. Additionally, our data suggests that OXR antagonists, possibly in combination with lower doses of ERT, may represent a novel non-hormonal therapy for treating all of these symptoms, with minimal side effects.
Supplementary Material
Acknowledgments
The following work was funded by a K01AG044466 from the National Institute of Aging; a Young Investigator’s KL-2 Scholars Award (RR025760); an Indiana CTSI Project Development Team Pilot Grant (RR025761) from the Indiana CTSI (UL1 RR025761 to AS), an Indiana University Simon Cancer Center Basic Science Pilot Funding Grant (23-87597) to PLJ; and an IUPUI University Fellowship to LMF. The Compound 56 and DORA-12 were respectively obtained from Janssen Research and Development, LLC, and Merck & Co.
Funding:
The funding sources provided guidance in experimental design, but played no role in the execution of experiments, analyses, interpretation, writing of report, or decision on where to submit the article.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.psyneuen.2015.12.011.
Footnotes
Conflict of interest
Within the last 3 years PLJ received a research grant from Janssen Research and Development, LLC to for screen orexin antagonists for anxiolytic properties in male rats. Within the last 3 years AS received research grants from Johnson &Johnson and Eli Lilly & Co., for conducting preclinical studies that are unrelated to the present paper. PLJ and AS also have a patent filed for the use of orexin receptor antagonists in the treatment of anxiety and hot flashes.
Contributors
Lauren M. Federici, Andrei I. Molosh, Stephanie D. Fitz, William A. Truitt, Janet Carpenter, Anantha Shekhar, and Philip L. Johnson.
References
- Allen RP, Mignot E, Ripley B, Nishino S, Earley CJ. Increased CSF hypocretin-1 (orexin-A) in restless legs syndrome. Neurology. 2002;59:639–641. doi: 10.1212/wnl.59.4.639. [DOI] [PubMed] [Google Scholar]
- Avis NE, Crawford SL, Greendale G, Bromberger JT, Everson-Rose SA, Gold EB, Hess R, Joffe H, Kravitz HM, Tepper PG, Thurston RC, Study of Women’s Health Across the Nation Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015 doi: 10.1001/jamainternmed.2014.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baerug U, Winge T, Nordland G, Faber-Swensson E, Heldaas K, Norling B, Larsen S, Arce JC. Do combinations of 1 mg estradiol and low doses of NETA effectively control menopausal symptoms? Climacteric. 1998;1:219–228. doi: 10.3109/13697139809085544. [DOI] [PubMed] [Google Scholar]
- Beral V, Million Women Study, C Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- Bolland MJ, Grey A, Gamble GD, Reid IR. Concordance of results from randomized and observational analyses within the same study: a re-analysis of the women’s health initiative limited-access dataset. PLoS One. 2015;10:e0139975. doi: 10.1371/journal.pone.0139975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaventure P, Yun S, Johnson PL, Shekhar A, Fitz SD, Shireman B, Lebold TP, Nepomuceno D, Lord B, Wennerholm M, Shelton J, Carruthers N, Lovenberg TW, Dugovic C. A selective orexin-1 receptor antagonist attenuates stress induced hyperarousal without hypnotic effects. J Pharmacol Exp Ther. 2015 doi: 10.1124/jpet.114.220392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. Hypothalamic-pituitary-adrenal responses to centrally administered orexin-A are suppressed in pregnant rats. J Neuroendocrinol. 2003;15:633–637. doi: 10.1046/j.1365-2826.2003.01045.x. [DOI] [PubMed] [Google Scholar]
- Bryant C, Judd FK, Hickey M. Anxiety during the menopausal transition: a systematic review. J Affect Disord. 2011 doi: 10.1016/j.jad.2011.06.055. [DOI] [PubMed] [Google Scholar]
- Burleson MH, Todd M, Trevathan WR. Daily vasomotor symptoms, sleep problems, and mood: using daily data to evaluate the domino hypothesis in middle-aged women. Menopause. 2010;17:87–95. doi: 10.1097/gme.0b013e3181b20b2d. [DOI] [PubMed] [Google Scholar]
- Cagnacci A, Volpe A, Arangino S, Malmusi S, Draetta FP, Matteo ML, Maschio E, Vacca AMB, Melis GB. Depression and anxiety in climacteric women: role of hormone replacement therapy. Menopause. 1997;4:206–211. [Google Scholar]
- Carpenter JS, Andrykowski MA. Menopausal symptoms in breast cancer survivors. Oncol Nurs Forum. 1999;26:1311–1317. [PubMed] [Google Scholar]
- Dorow R, Horowski R, Paschelke G, Amin M. Severe anxiety induced by FG 7142, a beta-carboline ligand for benzodiazepine receptors. Lancet. 1983;2:98–99. doi: 10.1016/s0140-6736(83)90076-4. [DOI] [PubMed] [Google Scholar]
- Dugovic C, Shelton JE, Aluisio LE, Fraser IC, Jiang X, Sutton SW, Bonaventure P, Yun S, Li X, Lord B, Dvorak CA, Carruthers NI, Lovenberg TW. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009;330:142–151. doi: 10.1124/jpet.109.152009. [DOI] [PubMed] [Google Scholar]
- El-Sedeek MS, Korish AA, Deef MM. Plasma orexin-A levels in postmenopausal women: possible interaction with estrogen and correlation with cardiovascular risk status. BJOG. 2010 doi: 10.1111/j.1471-0528.2009.02474.x. [DOI] [PubMed] [Google Scholar]
- Erkkola R, Iisalo E, Punnonen R. The effect of propranolol and oxazepam on some vegetative menopausal symptoms. Ann Clin Res. 1973;5:208–213. [PubMed] [Google Scholar]
- Espana RA, Plahn S, Berridge CW. Circadian-dependent and circadian-independent behavioral actions of hypocretin/orexin. Brain Res. 2002;943:224–236. doi: 10.1016/s0006-8993(02)02653-7. [DOI] [PubMed] [Google Scholar]
- Freedman RR, Woodward S, Sabharwal SC. Alpha 2-adrenergic mechanism in menopausal hot flushes. Obstet Gynecol. 1990;76:573–578. [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Rinaudo PJ, Sheng L. Premenstrual syndrome as a predictor of menopausal symptoms. Obstet Gynecol. 2004;103:960–966. doi: 10.1097/01.AOG.0000124804.81095.7f. [DOI] [PubMed] [Google Scholar]
- Freeman EW, Sammel MD, Lin H, Gracia CR, Kapoor S, Ferdousi T. The role of anxiety and hormonal changes in menopausal hot flashes. Menopause. 2005;12:258–266. doi: 10.1097/01.gme.0000142440.49698.b7. [DOI] [PubMed] [Google Scholar]
- Gallicchio L, Whiteman MK, Tomic D, Miller KP, Langenberg P, Flaws JA. Type of menopause, patterns of hormone therapy use, and hot flashes. Fertil Steril. 2006;85:1432–1440. doi: 10.1016/j.fertnstert.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Gibson CJ, Thurston RC, Bromberger JT, Kamarck T, Matthews KA. Negative affect and vasomotor symptoms in the Study of Women’s Health Across the Nation Daily Hormone Study. Menopause. 2011;18:1270–1277. doi: 10.1097/gme.0b013e3182230e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotter AL, Winrow CJ, Brunner J, Garson SL, Fox SV, Binns J, Harrell CM, Cui D, Yee KL, Stiteler M, Stevens J, Savitz A, Tannenbaum PL, Tye SJ, McDonald T, Yao L, Kuduk SD, Uslaner J, Coleman PJ, Renger JJ. The duration of sleep promoting efficacy by dual orexin receptor antagonists is dependent upon receptor occupancy threshold. BMC Neurosci. 2013;14:90. doi: 10.1186/1471-2202-14-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greendale GA, Reboussin BA, Hogan P, Barnabei VM, Shumaker S, Johnson S, Barrett-Connor E. Symptom relief and side effects of postmenopausal hormones: results from the postmenopausal estrogen/progestin interventions trial. Obstet Gynecol. 1998;92:982–988. doi: 10.1016/s0029-7844(98)00305-6. [DOI] [PubMed] [Google Scholar]
- Hanisch LJ, Palmer SC, Marcus SC, Hantsoo L, Vaughn DJ, Coyne JC. Comparison of objective and patient-reported hot flash measures in men with prostate cancer. J Support Oncol. 2009;7:131–135. [PubMed] [Google Scholar]
- Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, Kwan M, Gomez SL, Neugut AI. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZL, Qu WM, Li WD, Mochizuki T, Eguchi N, Watanabe T, Urade Y, Hayaishi O. Arousal effect of orexin A depends on activation of the histaminergic system. Proc Natl Acad Sci U S A. 2001;98:9965–9970. doi: 10.1073/pnas.181330998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe H, Guthrie KA, LaCroix AZ, Reed SD, Ensrud KE, Manson JE, Newton KM, Freeman EW, Anderson GL, Larson JC, Hunt J, Shifren J, Rexrode KM, Caan B, Sternfeld B, Carpenter JS, Cohen L. Low-dose estradiol and the serotonin-norepinephrine reuptake inhibitor venlafaxine for vasomotor symptoms: a randomized clinical trial. JAMA Intern Med. 2014;174:1058–1066. doi: 10.1001/jamainternmed.2014.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Truitt W, Fitz SD, Minick PE, Dietrich A, Sanghani S, Traskman-Bendz L, Goddard AW, Brundin L, Shekhar A. A key role for orexin in panic anxiety. Nat Med. 2010;16:111–115. doi: 10.1038/nm.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Samuels BC, Fitz SD, Federici LM, Hammes N, Early MC, Truitt W, Lowry CA, Shekhar A. Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiol Behav. 2012a Dec;107(5):733–742. doi: 10.1016/j.physbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Molosh A, Fitz SD, Truitt WA, Shekhar A. Orexin, stress, and anxiety/panic states. Prog Brain Res. 2012b;198:133–161. doi: 10.1016/B978-0-444-59489-1.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Samuels BC, Fitz SD, Federici LM, Hammes N, Early MC, Truitt W, Lowry CA, Shekhar A. Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiol Behav. 2012c;107:733–742. doi: 10.1016/j.physbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PL, Federici LM, Fitz Renger SDJJ, Shireman Winrow BCJ, Bonaventure P, Shekhar A. Orexin 1 and 2 receptor involvement in CO2-induced panic-associated behavior and autonomic responses. Depress Anxiety. 2015 Sep;32(9):671–683. doi: 10.1002/da.22403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp A, Preen DB, Saunders C, Boyle F, Bulsara M, Malacova E, Roughead EE. Early discontinuation of endocrine therapy for breast cancer: who is at risk in clinical practice? SpringerPlus. 2014;3:282. doi: 10.1186/2193-1801-3-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg F. Hot flashes: epidemiology and physiology. Ann N Y Acad Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. discussion 123–133. [DOI] [PubMed] [Google Scholar]
- Laflamme N, Nappi RE, Drolet G, Labrie C, Rivest S. Expression and neuropeptidergic characterization of estrogen receptors (ERalpha and ERbeta) throughout the rat brain: anatomical evidence of distinct roles of each subtype. J Neurobiol. 1998;36:357–378. doi: 10.1002/(sici)1097-4695(19980905)36:3<357::aid-neu5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Li Y, Li S, Wei C, Wang H, Sui N, Kirouac GJ. Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology (Berl) 2010;212:251–265. doi: 10.1007/s00213-010-1948-y. [DOI] [PubMed] [Google Scholar]
- Low DA, Davis SL, Keller DM, Shibasaki M, Crandall CG. Cutaneous and hemodynamic responses during hot flashes in symptomatic postmenopausal women. Menopause. 2008;15:290–295. doi: 10.1097/gme.0b013e3180ca7cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclennan AH, Broadbent JL, Lester S, Moore V. Oral oestrogen and combined oestrogen/progestogen therapy versus placebo for hot flushes. Cochrane Database Syst Rev. 2004:CD002978. doi: 10.1002/14651858.CD002978.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Miller HG, Li RM. Measuring hot flashes: summary of a National Institutes of Health workshop. Mayo Clin Proc. 2004;79:777–781. doi: 10.4065/79.6.777. [DOI] [PubMed] [Google Scholar]
- Nelson HD, Vesco KK, Haney E, Fu R, Nedrow A, Miller J, Nicolaidis C, Walker M, Humphrey L. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057–2071. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta F, Leal I, Maroco J, Ramos C. Menopausal symptoms: do life events predict severity of symptoms in peri- and post-menopause? Maturitas. 2012 doi: 10.1016/j.maturitas.2012.04.006. [DOI] [PubMed] [Google Scholar]
- Politi MC, Schleinitz MD, Col NF. Revisiting the duration of vasomotor symptoms of menopause: a meta-analysis. J Gen Intern Med. 2008;23:1507–1513. doi: 10.1007/s11606-008-0655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SH, Small CJ, Kennedy AR, Stanley SA, Seth A, Murphy KG, Taheri S, Ghatei MA, Bloom SR. Orexin A interactions in the hypothalamo-pituitary gonadal axis. Endocrinology. 2001;142:5294–5302. doi: 10.1210/endo.142.12.8558. [DOI] [PubMed] [Google Scholar]
- Sakurai T. The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness. Nat Rev Neurosci. 2007;8:171–181. doi: 10.1038/nrn2092. [DOI] [PubMed] [Google Scholar]
- Samson WK, Gosnell B, Chang JK, Resch ZT, Murphy TC. Cardiovascular regulatory actions of the hypocretins in brain. Brain Res. 1999;831:248–253. doi: 10.1016/s0006-8993(99)01457-2. [DOI] [PubMed] [Google Scholar]
- Stearns V, Slack R, Greep N, Henry-Tilman R, Osborne M, Bunnell C, Ullmer L, Gallagher A, Cullen J, Gehan E, Hayes DF, Isaacs C. Paroxetine is an effective treatment for hot flashes: results from a prospective randomized clinical trial. J Clin Oncol. 2005;23:6919–6930. doi: 10.1200/JCO.2005.10.081. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Beuckmann CT, Shikata K, Ogura H, Sawai T. Orexin-A (hypocretin-1) is possibly involved in generation of anxiety-like behavior. Brain Res. 2005;1044:116–121. doi: 10.1016/j.brainres.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Swartzman LC, Edelberg R, Kemmann E. Impact of stress on objectively recorded menopausal hot flushes and on flush report bias. Health Psychol. 1990;9:529–545. doi: 10.1037//0278-6133.9.5.529. [DOI] [PubMed] [Google Scholar]
- Thannickal TC, Lai YY, Siegel JM. Hypocretin (orexin) cell loss in Parkinson’s disease. Brain. 2007;130:1586–1595. doi: 10.1093/brain/awm097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truitt WA, Hauser SR, Deehan GA, Jr, Toalston JE, Wilden JA, Bell RL, McBride WJ, Rodd ZA. Ethanol and nicotine interaction within the posterior ventral tegmental area in male and female alcohol-preferring rats: evidence of synergy and differential gene activation in the nucleus accumbens shell. Psychopharmacology (Berl) 2015;232:639–649. doi: 10.1007/s00213-014-3702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waaseth M, Bakken K, Dumeaux V, Olsen KS, Rylander C, Figenschau Y, Lund E. Hormone replacement therapy use and plasma levels of sex hormones in the Norwegian Women and Cancer postgenome cohort—a cross-sectional analysis. BMC Womens Health. 2008;8:1. doi: 10.1186/1472-6874-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilent WB, Oh MY, Buetefisch C, Bailes JE, Cantella D, Angle C, Whiting DM. Mapping of microstimulation evoked responses and unit activity patterns in the lateral hypothalamic area recorded in awake humans. Technical note J Neurosurg. 2011;115:295–300. doi: 10.3171/2011.3.JNS101574. [DOI] [PubMed] [Google Scholar]
- Woods NF, Carr MC, Tao EY, Taylor HJ, Mitchell ES. Increased urinary cortisol levels during the menopausal transition. Menopause. 2006;13:212–221. doi: 10.1097/01.gme.0000198490.57242.2e. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Masaki T, Sakata T, Yoshimatsu H. Hypothalamic neuronal histamine regulates sympathetic nerve activity and expression of uncoupling protein 1 mRNA in brown adipose tissue in rats. Neuroscience. 2004;125:535–540. doi: 10.1016/j.neuroscience.2003.11.039. [DOI] [PubMed] [Google Scholar]
- Yoshimichi G, Yoshimatsu H, Masaki T, Sakata T. Orexin-A regulates body temperature in coordination with arousal status. Exp Biol Med (Maywood) 2001;226:468–476. doi: 10.1177/153537020122600513. [DOI] [PubMed] [Google Scholar]
- Zbuk K, Anand SS. Declining incidence of breast cancer after decreased use of hormone-replacement therapy: magnitude and time lags in different countries. J Epidemiol Community Health. 2012;66:1–7. doi: 10.1136/jech.2008.083774. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


