Abstract
PURPOSE
Cobalamin C (cblC) deficiency impairs the biosynthesis of adenosyl- and methylcobalamin resulting in methylmalonic acidemia combined with hyperhomocysteinemia and hypomethioninemia. However, some patients with cblC deficiency are treated with medical foods, devoid of methionine and high in leucine content, that are formulated for patients with isolated propionate oxidative defects. We examined the effects of imbalanced branched-chain amino acid intake on growth outcomes in cblC patients.
METHODS
Dietary intake was correlated with biochemical, anthropometric, body composition measurements and other disease parameters in a cohort of 28 early-onset cblC patients.
RESULTS
Protein restricted diets were followed by 21% of the patients, while 32% received medical foods. Patients on protein-restricted diets had lower height-for-age Z-score (P=0.034), while patients consuming medical foods had lower head-circumference Z-scores (P=0.037), plasma methionine concentrations (P=0.001) and predicted methionine influx through the blood brain barrier Z-score (−1.29 vs. −0.0617, P=0.007). The combination of age of diagnosis, a history of seizures and the leucine/valine dietary intake ratio best predicted head circumference Z-score based on multiple regression modeling (R2= 0.945).
CONCLUSIONS
Patients with cblC deficiency treated with medical foods designed for isolated methylmalonic acidemia are at risk for iatrogenic methionine deficiency that could adversely affect brain growth and development.
TRIAL REGISTRATION
This clinical study is registered in www.clinicaltrials.gov with the ID: NCT00078078. Study URL: http://clinicaltrials.gov/ct2/show/NCT00078078
Keywords: Cobalamin C (cblC) deficiency, methylmalonic acidemia, medical foods, methionine, dietary guidelines
INTRODUCTION
Combined methylmalonic acidemia and hyperhomocysteinemia, cobalamin C (cblC) type, results from impaired biosynthesis of 5′-deoxyadenosyl- and methyl-cobalamin, cofactors for the methylmalonyl-CoA mutase and methionine synthase enzymes, respectively1–3. Although the biochemical phenotype of this disorder shares elevated methylmalonic acid concentrations with the classic mut-subtype of isolated methylmalonic acidemia (MMA), the propionate derived metabolic perturbations are of lesser magnitude, while the clinical findings and disease pathophysiology are distinct. CblC patients display significantly increased homocysteine and decreased methionine concentrations and the early onset patients present with failure to thrive, visual impairment due to macular degeneration/pigmentary retinopathy, hemolytic uremic syndrome and thromboembolic/ microangiopathic complications, cardiopulmonary findings, neurocognitive delay, seizures and other neurological manifestations4–8. Decreased methionine synthesis and secondary impairments of the remethylation cycle, methyltransfer reactions, homocysteine-thiolactone metabolism, and increased oxidative stress have been implicated in disease pathophysiology5,9–11. The mechanisms underlying the multisystemic manifestations of cblC deficiency remain unknown, in part because of the lack of viable animal models12.
Treatment remains particularly challenging in cblC patients because symptoms such as intrauterine growth retardation, microcephaly, mild craniofacial dysmorphism and congenital heart disease can develop in utero13–16. Moreover, despite early diagnosis by newborn screening, clinical manifestations, including the characteristic ocular and neurological complications, are at best, incompletely responsive to conventional therapies4,6,7,9. The mainstay of treatment involves high-dose intramuscular hydroxocobalamin injections that can ameliorate the biochemical perturbations, resulting in a decrease of homocysteine and methylmalonic acid concentrations and increase in methionine synthesis. Betaine supplementation also enhances remethylation of homocysteine to methionine via the betaine:homocysteine methyltransferase reaction. Folinic or folic acid and other supplements (carnitine, methylcobalamin, pyridoxine, methionine supplementation) have been used; yet longitudinal efficacy studies on disease-related clinical outcomes remain uncertain9.
Protein restriction can be considered to further decrease the propiogenic amino acid load (valine, isoleucine, threonine and methionine) and help lower methylmalonic acid production17. However, methylmalonic acid levels in cblC patients are typically orders of magnitude less than seen in isolated mut MMA patients, consistent with the observations that metabolic decompensations of the “intoxication” type seen in classic isolated MMA are very rare in cblC patients 18,19. Furthermore, protein restriction carries the risk of inducing iatrogenic methionine and methyl-donor (S-adenosylmethionine, SAM) deficiency, resulting in the impairment of multiple biological transmethylation reactions, such as creatine, phosphatidylcholine, and sphingomyelin biosynthesis, as well as DNA methylation9,20–22. This could adversely affect growth and neurodevelopmental outcomes, as evidenced by the severe neurological phenotypes variably seen in patients with other remethylation disorders, including 5′,10′-methylenetetrahydrofolate reductase deficiency (MTHFR), functional methionine synthase (cblE/G) deficiencies, or other intracellular cobalamin biosynthesis disorders (cblD, F, J)1,21,23,24.
Over the course of several years evaluating cblC patients via a natural history protocol, we observed a highly variable approach to the dietary management of cblC. Dietary practices ranged from following a completely unrestricted diet to prescribing strict protein restriction, supplemented by large quantities of medical foods. The surprisingly large number of patients with cblC that are managed with medical foods designed for isolated methylmalonic and propionic acidemia (MMA/PA) in our study and the published literature6 led us to critically reappraise the dietary management of cblC deficiency. We find that the use of medical foods in patients with cblC is associated with iatrogenic methionine and essential branched-chain amino acid deficiencies, impaired growth, and abnormal body composition.
METHODS
Study population
Patient studies were approved by the NHGRI Institutional Review Board and performed in compliance with the Helsinki Declaration (clinicaltrials.gov identifier: NCT00078078). Patients were enrolled between 2004 and 2014 from centers across the US and Canada. Long-term dietary management was dictated by each patient’s metabolic center. Study participants were without clinical symptoms or laboratory markers of metabolic instability during their NIH evaluations. The diagnosis of cblC deficiency was confirmed using cellular biochemistry (laboratory of Dr. David S. Rosenblatt, Division of Medical Genetics, McGill University, Canada) and/or through molecular genetic analysis of the MMACHC gene (GeneDx; Gaithersburg, MD)25.
Laboratory studies
The NIH Clinical Center laboratory performed routine laboratory investigations, including total homocysteine and vitamin B12 levels. Total homocysteine was determined by HPLC between 2004 –13 and by an enzymatic assay (Roche Diagnostics, Cobas 6000 analyzer) from 2013 to present. Plasma and urine methylmalonic acid and amino acid concentrations were determined by liquid chromatography–tandem mass spectrometry (LC-MS/MS, Mayo Medical Laboratories). Samples were obtained in the fasting state, or 2–4 hours after a meal, whenever possible.
Dietary regimens and daily amino acid intake calculations
Diet analysis was performed using the patient’s medical foods (specialized formula) prescriptions and a detailed diet history obtained by a research dietitian. A three-day food record completed by the families was available in 16/28 patients.
Diet analyses and calculations were performed using Nutrition Data System for Research (NDS-R) software versions 2007–2012 developed by the Nutrition Coordinating Center (NCC), University of Minnesota, Minneapolis, MN 26. Medical food composition information was obtained from the respective manufacturers. Recommended dietary allowance (RDA) for protein and amino acids were based on the Dietary Reference Intakes for Protein and Amino acids (National Academies Press, NAP, 2005)27. Protein intake was recorded as complete protein from natural protein sources and incomplete/deficient protein equivalent (g/kg/day) intake in the form of specialized MMA/propionic acidemia medical foods, such as Propimex1/2 (Abbott Laboratories, Columbus, OH), XMTVI Analog, Maxamaid or Maxamum (Nutricia North America, Cedar Knolls, NJ) and MMA/PA express (Vitaflo USA, Alexandria, VA). Amounts of individual propiogenic amino acids (valine, isoleucine, methionine and threonine), as well as leucine were recorded for patients with detailed dietary records (N=13), while estimated deficient versus complete protein intake (g/kg/d) was documented in all patients.
All patients received intramuscular hydroxocobalamin therapy with different therapeutic dosages and intervals ranging between 0.01 to 0.61mg/kg/d. All patients received betaine at doses ranging from 31 to 338mg/kg/d, as well as folate or folinic acid supplementation, while a minority were prescribed aspirin, fish oil, DHA or other dietary supplements. This data are provided for each patient along with additional nutritional information in Supplemental Table 1.
Predicted cerebral amino acid influx calculations
Cerebral influx of substrates for the cationic (y+; lysine, arginine, ornithine) and large neutral amino acid transport systems (LAT1; glutamine, histidine, isoleucine, leucine, methionine, phenylalanine, threonine, tryptophan, tyrosine, and valine) was estimated as previously described for patient cohorts with 5,10-methylenetetrahydrofolate reductase-deficiency, maple syrup urine disease and glutaric acidemia21,28,29. These calculations were based on the principle that the cerebral uptake of each amino acid is influenced by ambient (plasma) concentrations of its competitors. Substrate competition is expressed by an apparent Km, called Kapp (μM), calculated for each amino acid according to the equation: Kapp=Km[1+Σ1→n(Ci/Ki)n]. Km is the classical Michaelis–Menten affinity constant for the single amino acid of interest, Ci is the plasma concentration (μM) for each of n competitors, and Ki is the classical affinity constant of that competitor (μM)30. The Kapp value was then used to calculate the brain influx (nanomoles per minute per gram of brain tissue) of each amino acid in the competing group, according to the equation: Influx = (Vmax)(C) / (Kapp + C), where Vmax and C are the maximal transport velocity (nmol/g/min) and plasma concentration (μM), respectively, of each amino acid. Estimated brain influx Z-score values were calculated using mean values observed in a control population of 52 children not affected by disorders of amino acid, folic acid or cobalamin metabolism from the Clinic for Special Children, Strasburg, PA.
Anthropometry, body composition and neurocognitive evaluations
Anthropometric measurements were expressed as age- and gender-specific Z-scores, using the epidemiological software package Epi Info™, Version 3.5.1., based on the CDC 2002 reference database (Centers for Disease Control and Prevention, Atlanta, GA, USA) for patient under 20yrs of age. Whole body composition in grams of fat or fat mass (FM), and fat free (or lean) body mass (FFM) were measured using dual energy X-ray absorptiometry (DXA, Hologic Delphi A; Hologic, Bedford, MA). For patients between 5–23y old a height adjusted Z-score was calculated using the Bone Mineral Density in Childhood Study online calculator: http://www.bmdcspublic.com/zscore.htm.
Data analysis
The results are presented as means (±SD). Significance was set at P<0.05. Statistical manipulations were performed using the IBM SPSS version 21.0 (Chicago, IL, USA), or GraphPad Prism version 6.0 software (San Diego, CA, USA). Independent Student’s t-test was used to compare values between patients that had or had not been receiving medical foods. Pearson’s correlation coefficient and multiple linear regression analyses were used to evaluate correlations between variables. Dummy variables were generated for dichotomous categorical variables such as sex, seizures or congenital microcephaly to be used in the regression model.
RESULTS
Anthropometric characteristics and body composition
We studied 28 patients with early onset cblC deficiency (18 male, 10 female; age range 2.03 – 27.04 years; 10.2 ± 7.61, mean ± SD). Age of diagnosis varied widely, from the first week (N=4) to 24 months (mean ± SD: 2.62 ± 4.4 months, median 1.5 month). 17/28 (60%) patients were homozygous for the common c.271dupA, p.R91KfsX14 mutation in the MMACHC gene, which is associated with a severe early onset phenotype (Table 1). Height, weight, BMI and head circumference (occipital-frontal circumference, OFC)-for-age Z-scores (mean ± SD) are presented (Table 1). Mean Z-score for height was −1.04 ± 1.33 (range 1.8 to −3.5), weight −0.66 ± 1.83 (range 3.00 to −6.8), BMI 0.13 ± 1.47 (range 2.6 to −4.4) and OFC −1.57 ± 1.23 (range 0.32 to −4.40, median −1.06). Height and OFC-Z-scores were <−2.00 in 6 and 7 patients, respectively. Of note, three of the patients had a history of congenital microcephaly, while 9/28 (32.14%) had a history of seizures, 2 of which had a diagnosis of infantile spasms (Table 1). Growth outcomes were not different between male and female subjects, or between patients homozygous for the c.271dupA mutation vs. others.
Table 1.
Clinical, molecular and biochemical features of subjects with cblC deficiency.
| Age (yrs) |
Age at Dx (mo) |
MMACHC Variant 1 |
MMACHC Variant 2 |
Wt Z-score |
Ht Z-score |
OFC Z-score |
Complete Protein %RDA |
Deficient Protein (g/kg/d) |
Plasma Met (μmol/L) |
Predicted LAT1 Met influx Z-score |
|---|---|---|---|---|---|---|---|---|---|---|
| 2.03 | 4 | c.271dupA, p.R91KfsX14 | c.271dupA, p.R91KfsX14 | −1.2 | −0.2 | −0.47 | 118.9 | 0.20 | 19 | −0.36 |
| 2.04 | 0.3§ | c.271dupA, p.R91KfsX14 | c.271dupA, p.R91KfsX14 | 0.5 | −1.1 | −1.26 [##] | 61.0 | 1.42 | 2 | −2.89 |
| 2.18 | 0.5 | c.271dupA p.R91KfsX14 |
c.388_390delTAC p.Y130del |
3 | 1.8 | −0.72 | 0 | 41 | 1.69 | |
| 2.63 | 1.5 | c.331C>T, p.R111X | c.331C>T, p.R111X | −2.2 | −2.1 | −4.4 * | 133.4 | 1.50 | n/a | n/a |
| 2.67 | 0.1§ | c.271dupA, p.R91KfsX14 | c.609G>A, p.W203X | 0.8 | 0.2 | 0.32 | 369.7 | 0 | 21 | −0.83 |
| 3.14 | 0.5§ | c.271dupA, p.R91KfsX14 | c.271dupA, p.R91KfsX14 | 1.7 | −0.1 | −0.07 | 153.2 | 0 | 40 | 1.21 |
| 3.20 | 0.3§ | c.271dupA, p.R91KfsX14 | c.271dupA, p.R91KfsX14 | −1.2 | −1.5 | n/a | 100.4 | 0 | 27 | 0.29 |
| 3.25 | 0.25§ | c.619dupG, p.D207GfsX38 | c.440G>A, p.G147D | −0.16 | 0.55 | −0.88 | 315.9 | 0 | 37 | 1.53 |
| 3.76 | 0.8§ | c.271dupA, p.R91KfsX14 | c.331C>T, p.R111X | −2.48 | −2.1 | −3.75 [*##] | 56.1 [¶] | 0.45 | 9 | −1.68 |
| 4.18 | 1.1 | c.271dupA, p.R91KfsX14 | c.271dupA, p.R91KfsX14 | 0.3 | 0.4 | −1.69 | 271.3 | 0 | 35 | 0.42 |
| 4.49 | 0.6§ | c.471G>A, p.W157X | c.666C>A, p.Y222X | −0.9 | −0.9 | −0.97 | 263.5 | 0.61 | 17 | −0.93 |
| 5.25 | 4 | c.271dupA, p.R91KfsX14 | c.271dupA, p.R91KfsX14 | −0.9 | −1.4 | −2.83 [#] | 134.6 | 0 | 18 | −0.92 |
| 7.25 | 1.5 | c.3G>A, p.M1? | c.3G>A, p.M1? | 0.1 | −2.9 | −3.4 [#] | 56.2 | 0.54 | 15 | −1.84 |
| 8.90 | 0.1§ | c.271dupA, p.R91KfsX14 | c.271dupA, p.R91KfsX14 | −1.3 | −2.4 | −1.25 | 104.4 | 0 | 23 | 0.00 |
| 9.62 | 2.5 | c.271dupA, p.R91KfsX14 | c.271dupA, p.R91KfsX14 | −1.2 | −1.4 | −0.83 | 106.7 | 0 | n/a | n/a |
| 9.83 | 3 | c.271dupA, p.R91KfsX14 | c.331C>T, p.R111X | −0.6 | −1.3 | −0.99 | 118.9 | 0 | 20 | −0.50 |
| 10.85 | 1.75 | c.271dupA, p.R91KfsX14 | c.271dupA, p.R91KfsX14 | −1.9 | −3.5 | −2.94 [#] | 86.1 | 0 | 30 | 0.37 |
| 11.11 | 0.3 | c.271dupA, p.R91KfsX14 | c.271dupA, p.R91KfsX14 | −0.9 | −0.9 | −0.57 | 115.2 | 0 | 29 | −0.36 |
| 12.07 | 1.2 | c.271dupA, p.R91KfsX14 | c.271dupA, p.R91KfsX14 | 0.6 | −1.2 | −0.97 | 145.8 | 0 | 18 | −0.98 |
| 13.77 | 0.8 | c.271dupA, p.R91KfsX14 | c.271dupA, p.R91KfsX14 | 0.8 | 1 | −0.81 | 102.6 | 0 | 36 | 0.77 |
| 15.35 | 2 | c.271dupA, p.R91KfsX14 | c.388_390delTAC p.Y130del | −0.4 | −1.2 | −1.06 | 83.8 | 0 | 18 | −0.47 |
| 15.52 | 24 | c.271dupA, p.R91KfsX14 | c.271dupA, p.R91KfsX14 | −0.9 | n/a | −3.22 [#] | 74.9 | 0.60 | 11 | −1.62 |
| 19.38 | 5 | c.271dupA, p.R91KfsX14 | c.457C>T, p.R153X | −6.8 | −2.8 | −2.4 [#] | 104.1 | 0.34 | 19 | −0.06 |
| 20.33 | 1.5 | c.271dupA, p.R91KfsX14 | c.271dupA, p.R91KfsX14 | −1.8 | 90.7 | 0 | 12 | −1.23 | ||
| 20.79 | 1.8 | c.271dupA, p.R91KfsX14 | c.271dupA, p.R91KfsX14 | 128.8 | 0.28 | 24 | −0.94 | |||
| 22.08 | 5.5 | c.271dupA, p.R91KfsX14 | c.600G>A, p.W200X | [*#] | 132.1 | 0 | 20 | −0.76 | ||
| 23.04 | 3 | c.271dupA, p.R91KfsX14 | c.271dupA, p.R91KfsX14 | [#] | 129.6 | 0 | 23 | −0.49 | ||
| 24.10 | 2.25 | c.271dupA, p.R91KfsX14 | c.271dupA, p.R91KfsX14 | 79.3 | 0 | 17 | −0.85 |
Age at the time of patients’ evaluations and age of diagnosis, as well as the molecular genetic analysis of the MMACHC gene and major variables for the study are provided for each of the early onset cblC subjects (*) represent patients with congenital microcephaly; (#) patients with a history of seizures; (##) patients with infantile spasms; (¶) a patient requiring valine supplementation for persistently low plasma valine concentrations). Patients diagnosed by newborn screening are marked with (§) under age at diagnosis. Patients with the lowest methionine influx scores and head circumference Z-scores are in bold. n/a stands for data not available.
Whole body composition analysis based on DXA imaging studies was available in 18 patients, 12 males and 6 females. Mean %fat mass was 31.56 ± 11.2 (mean ± SD), with 27.9 ± 8.86% in males and 38.11 ± 12.85% in the females (P=0.07). Mean bone mineral density-height-adjusted Z-score was −1.9 ± 1.55, with −1.67 ± 0.93 for males and −2.46 ± 2.77 for females (P=NS).
Correlations of growth outcomes with protein restriction and medical foods utilization
Six out of 28 (21%) of the patients received complete protein below the 85% of the recommended daily allowance for healthy children27. Nine out of the 28 patients (32.14%) received medical foods designed for MMA. Additional protein-free (N= 5) or elemental amino acid medical foods (N= 2) were used in a minority patients, while one patient required valine supplementation for persistently low valine plasma concentrations (Table 1).
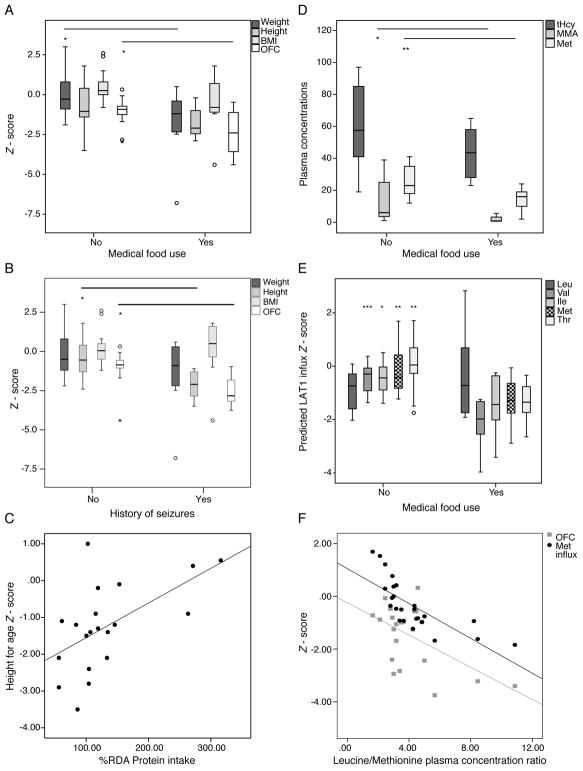
Comparing growth and biochemical parameters between patients following a protein-restricted diet or receiving medical foods versus those that were not, it became evident that protein restriction or chronic medical food use were associated with poorer growth parameters. Patients following a diet lower than 85% RDA for complete protein had a lower height – (−2.16 ± 1.04 vs. −0.72 ± 1.24, N= 5 vs. 17, P=0.030) and head circumference (OFC)-for age Z-scores (−2.3 ± 1.30 vs. −1.2 ± 1.11, P=0.062) and a higher % fat mass (hence, lower lean or skeletal muscle mass) by DXA analysis (43.1 ± 10.8 vs. 26.7 ± 7.3%, N=12 and 5, P=0.002). Similarly, a lower mean weight- (−1.7 ± 2.28 vs. −0.09 ± 1.3, P=0.038, N=15 and 8, respectively), height- (−1.72 ± 1.01 vs. −0.73 ± 1.37, P=0.075) and head circumference- for age Z-score (−2.48 ± 1.43 vs. −1.15 ± 0.88, P=0.037) was observed in the subset of patients consuming medical foods (Figure 1A). Patients with a history of seizures had a significantly lower height and OFC-Z-score than those without seizure history (P=0.004 and <0.001, respectively) (Figure 1B). Growth outcomes, height or OFC-Z-scores, in patients who carried no, one or two copies of the common c.271dupA mutation were not significantly different (One-way ANOVA, P=0.876 and 0.591, respectively), suggesting non-genic effects have a significant contribution to the variance observed. Similarly, no significant difference was observed in %RDA natural protein intake or the use of medical foods in g/kg/d between early onset cblC patients with different genotypes.
Figure 1.
A. A lower weight- (−1.7 ± 2.28 vs. −0.09 ± 1.3, P=0.038, N=15 and 8, respectively) and head circumference (−2.48 ± 1.43 vs. −1.15 ± 0.88, P=0.037) and a lower mean height- (−1.72 ± 1.01 vs. −0.73 ± 1.37, P=0.075) and BMI -for age Z-score (−0.60 ± 2.08 vs. 0.48 ± 0.99, P=NS) was observed in the group consuming medical foods as opposed to patients that did not at the time of evaluation.
B. Patients with a history of seizures had a significantly lower height and OFC-Z-score than those without seizure history (P=0.004 and <0.001, respectively)
C. Complete protein intake as %recommended daily allowance (RDA) correlated positively with height-for age Z-score (r=0.575, P=0.008, R2=0.31).
D. Patients consuming medical foods had slightly lower total homocysteine concentrations (48.7 ± 27.7 vs 59.8 ± 25,2, P=NS), significantly lower plasma methylmalonic acid (2.75 ± 3.27 vs. 13.9 ± 16.4, N=9 vs 18, P=0.012 and methionine levels (14.5 ± 6.92 vs. 25.8 ± 8.8, P= 0.004) as expected, and lower valine and isoleucine (P<0.001 and 0.026, respectively, data not shown), compared to patients not on medical foods.
E. Predicted influx through the blood-brain barrier transporter LAT1 for the propiogenic amino acids and leucine is provided as a Z-score from mean values derived from plasma amino acid concentrations in a control population of 52 children from the Clinic for Special Children. Patients on medical foods had mean predicted influx Z-scores lower than those not consuming special formulas (valine: −2.11 ± 0.92 vs. −0.47 ± 0.56, P=0.001; isoleucine: −1.41 ± 1.09 vs. −0.45 ± 0.56, P=0.045; methionine: −1.29 ± 0.90 vs. −0.61 ± 0.89, P=0.004; and threonine: −1.33 ± 0.72 vs. 0.06 ± 0.87, P=0.001).
F. The leucine/methionine plasma concentration ratio correlated negatively with the predicted cerebral methionine influx (r= −0.749, R2=0.541, P<0.001) as well as the head-circumference-for-age Z-scores (r= −0.553, R2=0.306, P=0.008).
Complete protein intake (%RDA) showed a positive correlation with the height for age Z-score (Figure 1C). Given that MMA/PA medical foods contain no propiogenic amino acid, but a normal to increased amount of leucine, ratios of leucine to methionine or valine dietary intake were significantly elevated in patients consuming medical foods (P=0.015 and 0.003 respectively). A higher leucine/valine dietary intake showed a negative correlation to the height-for-age (r=−0.673, P=0.033, R2=0.453) and OFC-for-age Z-scores (r=−0.840, P=0.001, R2=0.705).
Intakes of other nutrients that can affect growth and cognitive function, including iron, zinc, and omega 3 fatty acids were also assessed (Supplemental Table 1). All subjects well surpassed the Recommended Dietary Allowance (RDA) for iron from dietary intake plus supplements. Three subjects had intake of zinc below the RDA, but none of these subjects had growth failure. Nine subjects had alpha-linolenic acid (ALA) below the Adequate Intake (AI). Two of the nine were taking docosahexaenoic (DHA) or fish oil supplements to compensate; the remaining seven had negligible DHA intake.
Biochemical indices and cerebral amino acid influx calculations
Since protein restriction and medical food supplementation are both employed to improve metabolic control, we looked at the effects of the dietary management on plasma metabolite levels. Patients who received medical foods had significantly lower plasma methylmalonic acid and methionine concentrations (Figure 1D), as well as lower valine and isoleucine (data not shown). Total homocysteine was not significantly different. The higher ratio of leucine over methionine, valine and isoleucine dietary intake resulted predictably also in elevated plasma leucine/methionine (P=0.05) and leucine/valine (P=0.002) plasma amino acid concentrations (similarly elevated ratios of tyrosine or phenylalanine etc. to any of the restricted amino acids were observed).
These skewed plasma amino acid ratios are predicted to alter amino acid transport across cells and physiological barriers, through competitive inhibition at shared transporters/transport systems, such as the leucine-preferring LNAA transporter type 1, LAT1 (SLC7A5). To investigate whether these skewed ratios would affect the influx of amino acids at the blood-brain-barrier (BBB) via the LAT1 transporter, we applied the cerebral influx model using plasma amino acid concentrations from our cblC patient cohort30. We document Z-scores for methionine influx ranging from 1.69 to −2.89, with 18/28 (64%) having a negative Z-score, and 5/27 (18%) a score <−1.00 (Table 1). The range of values for valine (0.37 to −3.97), isoleucine (0.51 to −3.42) and threonine (1.71 to −2.65) are also reported. These Z-scores were significantly lower in patients receiving medical foods compared to those that did not (Figure 1E), while methionine (and valine) BBB influx Z-scores was significantly lower in patients with a history of seizures (for methionine: −0.035 for no seizure history, N=16, vs −1.087 in patients with seizures, N=10, P=0.012). The leucine/methionine plasma concentration ratio correlated negatively with the predicted cerebral methionine influx (P<0.001) as well as the head-circumference-for-age Z-scores (P=0.008) (Figure 1F).
We present an illustrative case study of an infant, who presented with severe encephalopathy and hyperammonemia and was therefore treated with protein restriction and MMA/PA medical foods along with daily intramuscular hydroxocobalamin and adequate doses of betaine and carnitine. The infant showed a decline in head size and length centiles, from the 33rd centile to the 2nd for the head circumference and from the 60th to the 8th for length, with no significant improvement in growth till the age of 5 months. This trend was reversed upon increasing the relative ratio of natural/deficient protein intake and changing briefly to an MSUD formula (that contains minimal amounts of BCAA, but normal concentrations of methionine) (Supplemental Figure S1).
Stepwise multiple regression modeling showed that the combination of age of diagnosis (β coefficient= −0.295, P=0.031), history of seizures (β coefficient= −0.324, P=0.034), and leucine over valine intake ratio (β coefficient= −0.754, P<0.001), best predicted OFC Z-score, with a model R2 of 0.944. The addition of the dietary component (leucine/valine intake ratio) improves the R2 of the model from 0.4 to 0.944, suggesting that diet contributes significantly to the variance observed in the OFC-Z-score.
DISCUSSION
This study details the growth outcomes and dietary management of a large cohort of cblC patients evaluated as part of a dedicated, single center, natural history protocol (NCT00078078). The ability to enroll patients treated at a large number of metabolic clinics in North America afforded a broad representation of the varied therapeutic practices employed and the outcomes observed, and provided a large dataset for the survey of current management approaches.
Protein restriction in cblC deficiency is intended to lower the amino acid load into the propionate oxidation pathway that is partially blocked at the methylmalonyl-CoA mutase (MUT) step, due to impaired biosynthesis of the cofactor for MUT, 5′-deoxyadenosylcobalamin. Providing an appropriate dose of parenteral hydroxocobalamin usually leads to a significant decrease in plasma methylmalonic acid levels, which are generally 10–100 fold less than that observed in patients with mut deficiency. Despite the minimal effects on influencing metabolites in the face of OHcbl therapy, additional protein restriction and administration of propiogenic amino acid-free medical foods, containing minimal amounts of valine, isoleucine, threonine and importantly, methionine, were frequently employed to treat the patients. In the current study, we document that a number of cblC patients follow a diet restricted in protein (21%) and/or supplemented with medical foods designed for MMA/PA (32%) (Table 1). The use of MMA/PA medical foods, although not universally adopted, seems to have gained support in some metabolic centers, as evidenced by the fact that 76% of cblC patients identified by newborn screening were prescribed medical foods in one study6. Our data support the concept that protein restriction (and/or medical foods use) to lower the plasma methylmalonic acid concentration has no effect on total homocysteine levels, but, importantly, can lower methionine and essential BCAA concentrations.
Hypomethioninemia is considered a critical component of cblC disease pathophysiology and maximizing methionine synthesis, and the downstream provision of methyl groups, is the aim of the two major therapeutic modalities for the disorder, specifically high dose vitamin B12 (hydroxocobalamin) and betaine. Brain white matter demyelination and subacute combined degeneration have been observed in multiple case studies/series of cblC6,9,24,31,32, resembling findings in animal models with B12 deficiency. The animals symptoms and pathology could be mitigated by methionine supplementation33. Moreover, methionine supplementation was claimed to improve photoreceptor sensitivity (on electroretinogram) and retinal function in a single patient, but had no effect on the characteristic macular degeneration of the disease34. The pathophysiology of cblC deficiency is certainly complex, as it cannot be reversed or prevented simply by methionine supplementation or any currently available therapies5,7,9,23. Furthermore, decreased transmethylation products, such as choline, creatine or guanidinoacetate, have not been consistently aberrant in magnetic resonance spectroscopy (MRS) or serum/urine biochemical studies in cblC patients6,35, and a paradoxical increase in AdoMet has been noted, postulated to result from secondary inhibition of methylases through adenosylhomocysteine (AdoHcy) or other metabolites22,35.
Despite the unresolved underlying pathophysiology of cblC deficiency, and the lack of evidence in favor of additional methionine supplementation, a basic therapeutic goal remains to avoid essential amino acid deficiencies, including methionine, especially during periods of brain development. Brain methionine concentrations depend on uptake through the circulation, as well as from de novo methionine synthesis36. Given that MMA/PA medical foods contain no methionine, they may exacerbate methionine deficiency, given the already impaired de novo methionine synthesis that is a feature of cblC deficiency. Therefore, there is no theoretical rationale or experimental support for the practice of administering medical foods to patients with cblC deficiency or any disorder that impairs the remethylation of homocysteine. Furthermore, MMA/PA medical foods contain normal to increased amounts of leucine, which, when administered out of proportion to the other BCAA, carries the risk for inducing iatrogenic amino acids deficiencies, likely due to KIC-induced branched-chain ketoacid dehydrogenase kinase inhibition and increased valine and isoleucine oxidation rates37. Moreover, BCAA imbalances exert a multitude of effects in different organ systems, as detailed in the previous report (Part 1 – Isolated MMA), and summarized in extensive reviews on branched chain amino acid metabolism28,37,38. The complexity of leucine effects and the tight coupling with isoleucine and valine metabolism have been overlooked in the design of MMA/PA medical foods, perhaps because leucine does not generate propionyl-CoA and is therefore considered “safe” in patients with MMA and PA. However, in the case of cblC deficiency, the imbalanced intake of leucine and other large neutral amino acids relative to methionine is predicted to result in competitive inhibition for the uptake of dietary methionine at the blood-brain-barrier, and further accentuate methionine deficiency in the brain/CNS of the patients. Using the plasma amino acid concentrations observed in our cblC patient cohort as the dataset for the predictive equations employed to study MSUD, we indeed document a lower calculated influx for methionine into the CNS in patients on medical foods as compared to those not consuming special formulas (Figure 1E). Moreover, the leucine/methionine ratio correlated negatively with the head circumference Z-scores (Figure 1F). While the cerebral amino acid influx model only takes into account two of the multiple transport systems at different physiological barriers, such as the GI and blood-brain barrier, the principle has been employed with success in other disorders21,28,29. Manipulations of brain amino acid influx have gained experimental support in animal studies in glutaric aciduria type 1, where lysine brain uptake through the y+ transport system (SLC7A1–3) was modified with homoarginine or arginine supplementation, leading to lower lysine concentrations in brain homogenates, as well as improved disease-related outcomes39,40. Moreover, modulation of brain methionine and S-adenosylmethionine (SAM) concentrations with dietary methionine modifications has been demonstrated in animal models36, while increased methionine synthesis mediated via betaine supplementation was shown to increase CSF methionine and SAM concentrations, reduce cellular Hcy-thiolactone formation and improve brain growth in a cohort of patients with MTHFR deficiency21. Although LAT1 and y+ are discussed here from a proof of concept perspective, there are multiple additional transport systems that control amino acid flux between the GI, kidney, systemic circulation and brain compartment. For example, B0AT1 (SLC 6A19) and ATB0+ (SLC6A14) are transporters for large aliphatic amino acids, including leucine, valine, isoleucine and methionine, in kidney, GI tract and the blood brain barrier, respectively41–43. The complex interactions amongst these various systems and how metabolic foods deficient in selected amino acids may interfere with amino acid flux between different organs remains largely unexplored.
A delay in symptomatic diagnosis, a history of seizures and a high relative leucine to valine dietary intake were the main predictors of a lower head circumference Z-score in our multiple regression modeling. Although assigning a cause or effect-relationship between the different variables is not possible with the cross sectional approach used here, the strong correlation observed between dietary parameters and plasma methionine levels, together with the considerations regarding the leucine competition for transport of methionine across the BBB, suggests that these medical foods could contribute to a poor neurocognitive outcome and provide a strong theoretical argument against the use of medical foods with high leucine and absent methionine in patients with cblC deficiency. If protein restriction is deemed necessary, for example at presentation or during a rare metabolic crisis with hyperammonemia, medical foods designed for MSUD (like Ketonex/Abbott Nutrition, MSUD Analog/Nutricia) that contain no BCAA (no leucine, valine or isoleucine), but have a regular amount of methionine, provide a safer choice for managing cblC patients. Further studies need to be designed to estimate the effects of methionine or other methyl-donor supplementation on disease outcome.
In addition to the protein restriction considerations outlined in detail in this work other aspects of the dietary needs in this population remain largely understudied, such as essential fatty acid intake. Alpha-linolenic acid (ALA) and decosahexaenoic acid (DHA) intake were low to negligible in 9/16 (56%) of the patients, where sufficient data were available. Erythrocyte phospholipid and plasma content of DHA and AA may be lower in inborn errors of metabolism secondary to reduced intake on restricted diets44 and/or interference with conversion of ALA to DHA and AA secondary to toxic metabolites45. Six out of seven subjects with inadequate ALA and DHA intake followed a restricted diet related to prescribed protein restriction or had poor intake for other reasons. It is well recognized that omega-3-fatty acids are important for optimal cognitive function. Furthermore, omega-3-fatty-acid deficiency can also affect platelet aggregation, an important consideration in patients with hyper-homocystinemia prone to thromboembolic complications46,47. Monitoring of their dietary intake and plasma concentrations should be incorporated in the management of cblC patients.
Our study emphasizes the need to develop disease-specific guidelines under the American College for Medical Genetics (ACMG) ACT sheet and algorithms for the follow-up of increased propionylcarnitine (C3) detected by newborn screening to more clearly distinguish isolated MMA from early cobalamin biosynthetic defects because the dietary management should be distinct. Additionally, the studies presented here highlight the paucity of experimental and clinical trial support underlying dietary management practices commonly used to treat patients with varied forms of methymalonic acidemia, and underscore the community need for a more rigorous scientific and clinical study of medical foods in the treatment of patients with IEMs.
Supplementary Material
Acknowledgments
We thank all patients and their families for their participation in our natural history protocol and donation of blood/tissues for our studies; referring physicians, nurses and dietitians for their help with patient’s evaluations; Isa Bernardini and Roxanne Fischer for processing patient samples; the nurses, research dietitians of the NIH Clinical Research Center and clinical fellows of the NHGRI genetics fellowship program for their help with patient care and dedication to clinical research. I.M, J.L.S. and C.P.V were supported by the Intramural Research Program of the National Human Genome Research Institute, Bethesda, MD. J.M. was supported by the NIH Clinical Center.
Footnotes
The authors would like to dedicate this manuscript to the memory of Dr. Harvey S. Mudd, who inspired us to study the role of methionine, methylation and transsulfuration cycles in human physiology and disease. His stimulating discussions contributed significantly to our understanding of the dietary effects in the management of cblC patients.
Disclosures: All authors have no conflicts of interest to declare.
Author contributions:
I.M. designed the study, performed data collection and statistical analyses and wrote the paper.
J.G.M. collected and analyzed dietary data and edited the paper.
J.L.S. coordinated the clinical research and edited the paper.
N.C.C. collected data and edited the paper.
E.M. contributed clinical data and edited the paper.
K.S. and H.M. contributed data on control subjects and equations for the calculations of the expected amino acid influx through the blood brain barrier large neutral amino acid transporter, LAT1.
C.P.V. designed the clinical research studies and wrote the paper.
References
- 1.Rosenblatt DS, Fenton WA. Inherited disorders of folate and cobalamin transport and metabolism. In: Scriver CR, et al., editors. The Metabolic and Molecular Bases of Inherited Disease. Mc-Graw Hill; New York: 2001. pp. 3897–3933. [Google Scholar]
- 2.Levy HL, Mudd SH, Schulman JD, Dreyfus PM, Abeles RH. A derangement in B12 metabolism associated with homocystinemia, cystathioninemia, hypomethioninemia and methylmalonic aciduria. Am J Med. 1970;48:390–7. doi: 10.1016/0002-9343(70)90070-7. [DOI] [PubMed] [Google Scholar]
- 3.Mudd SH, Levy HL, Abeles RH, Jennedy JP., Jr A derangement in B 12 metabolism leading to homocystinemia, cystathioninemia and methylmalonic aciduria. Biochem Biophys Res Commun. 1969;35:121–6. doi: 10.1016/0006-291x(69)90491-4. [DOI] [PubMed] [Google Scholar]
- 4.Carrillo-Carrasco N, Chandler RJ, Venditti CP. Combined methylmalonic acidemia and homocystinuria, cblC type. I. Clinical presentations, diagnosis and management. J Inherit Metab Dis. 2012;35:91–102. doi: 10.1007/s10545-011-9364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carrillo-Carrasco N, Venditti CP. Combined methylmalonic acidemia and homocystinuria, cblC type. II. Complications, pathophysiology, and outcomes. J Inherit Metab Dis. 2012;35:103–14. doi: 10.1007/s10545-011-9365-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisfeld-Adams JD, et al. Neurologic and neurodevelopmental phenotypes in young children with early-treated combined methylmalonic acidemia and homocystinuria, cobalamin C type. Mol Genet Metab. 2013;110:241–7. doi: 10.1016/j.ymgme.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Fischer S, et al. Clinical presentation and outcome in a series of 88 patients with the cblC defect. J Inherit Metab Dis. 2014;37:831–40. doi: 10.1007/s10545-014-9687-6. [DOI] [PubMed] [Google Scholar]
- 8.Gizicki R, et al. Long-term visual outcome of methylmalonic aciduria and homocystinuria, cobalamin C type. Ophthalmology. 2014;121:381–6. doi: 10.1016/j.ophtha.2013.08.034. [DOI] [PubMed] [Google Scholar]
- 9.Smith SE, Kinney HC, Swoboda KJ, Levy HL. Subacute combined degeneration of the spinal cord in cblC disorder despite treatment with B12. Mol Genet Metab. 2006;88:138–45. doi: 10.1016/j.ymgme.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Mc Guire PJ, Parikh A, Diaz GA. Profiling of oxidative stress in patients with inborn errors of metabolism. Mol Genet Metab. 2009;98:173–80. doi: 10.1016/j.ymgme.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pastore A, et al. Glutathione metabolism in cobalamin deficiency type C (cblC) J Inherit Metab Dis. 2014;37:125–9. doi: 10.1007/s10545-013-9605-3. [DOI] [PubMed] [Google Scholar]
- 12.Moreno-Garcia MA, Pupavac M, Rosenblatt DS, Tremblay ML, Jerome-Majewska LA. The Mmachc gene is required for pre-implantation embryogenesis in the mouse. Mol Genet Metab. 2014;112:198–204. doi: 10.1016/j.ymgme.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Tanpaiboon P, et al. Noncompaction of the ventricular myocardium and hydrops fetalis in cobalamin C disease. JIMD Rep. 2013;10:33–8. doi: 10.1007/8904_2012_197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Profitlich LE, Kirmse B, Wasserstein MP, Diaz GA, Srivastava S. High prevalence of structural heart disease in children with cblC-type methylmalonic aciduria and homocystinuria. Mol Genet Metab. 2009;98:344–8. doi: 10.1016/j.ymgme.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Cerone R, Schiaffino MC, Caruso U, Lupino S, Gatti R. Minor facial anomalies in combined methylmalonic aciduria and homocystinuria due to a defect in cobalamin metabolism. J Inherit Metab Dis. 1999;22:247–50. doi: 10.1023/a:1005521702298. [DOI] [PubMed] [Google Scholar]
- 16.Andersson HC, Marble M, Shapira E. Long-term outcome in treated combined methylmalonic acidemia and homocystinemia. Genet Med. 1999;1:146–50. doi: 10.1097/00125817-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Weisfeld-Adams JD, et al. Newborn screening and early biochemical follow-up in combined methylmalonic aciduria and homocystinuria, cblC type, and utility of methionine as a secondary screening analyte. Mol Genet Metab. 2010;99:116–23. doi: 10.1016/j.ymgme.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinelli D, et al. Cobalamin C defect presenting as severe neonatal hyperammonemia. Eur J Pediatr. 2011;170:887–90. doi: 10.1007/s00431-010-1371-8. [DOI] [PubMed] [Google Scholar]
- 19.Harding CO, et al. Potential for misdiagnosis due to lack of metabolic derangement in combined methylmalonic aciduria/hyperhomocysteinemia (cblC) in the neonate. J Perinatol. 2003;23:384–6. doi: 10.1038/sj.jp.7210955. [DOI] [PubMed] [Google Scholar]
- 20.Mudd SH, et al. Methyl balance and transmethylation fluxes in humans. Am J Clin Nutr. 2007;85:19–25. doi: 10.1093/ajcn/85.1.19. [DOI] [PubMed] [Google Scholar]
- 21.Strauss KA, et al. Prevention of brain disease from severe 5,10-methylenetetrahydrofolate reductase deficiency. Mol Genet Metab. 2007;91:165–75. doi: 10.1016/j.ymgme.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Bodamer OA, et al. Creatine metabolism in combined methylmalonic aciduria and homocystinuria. Annals of neurology. 2005;57:557–60. doi: 10.1002/ana.20419. [DOI] [PubMed] [Google Scholar]
- 23.Schiff M, et al. Isolated remethylation disorders: do our treatments benefit patients? J Inherit Metab Dis. 2011;34:137–45. doi: 10.1007/s10545-010-9120-8. [DOI] [PubMed] [Google Scholar]
- 24.Surtees R, Leonard J, Austin S. Association of demyelination with deficiency of cerebrospinal-fluid S-adenosylmethionine in inborn errors of methyl-transfer pathway. Lancet. 1991;338:1550–4. doi: 10.1016/0140-6736(91)92373-a. [DOI] [PubMed] [Google Scholar]
- 25.Lerner-Ellis JP, et al. Spectrum of mutations in MMACHC, allelic expression, and evidence for genotype-phenotype correlations. Hum Mutat. 2009;30:1072–81. doi: 10.1002/humu.21001. [DOI] [PubMed] [Google Scholar]
- 26.Schakel SF. Maintaining a nutrient database in a changing marketplace: Keeping pace with changing food products - A research perspective. J Food Compos Anal. 2001:315–322. [Google Scholar]
- 27.National Research Council, N. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids (Macronutrients) Washington, DC: 2005. [DOI] [PubMed] [Google Scholar]
- 28.Strauss KA, et al. Classical maple syrup urine disease and brain development: principles of management and formula design. Mol Genet Metab. 2010;99:333–45. doi: 10.1016/j.ymgme.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strauss KA, et al. Safety, efficacy and physiological actions of a lysine-free, arginine-rich formula to treat glutaryl-CoA dehydrogenase deficiency: Focus on cerebral amino acid influx. Mol Genet Metab. 2011 doi: 10.1016/j.ymgme.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Smith QR, Stoll JS. In: Blood-brain barrier amino acid transport. Pardridge WM, editor. Cambridge University Press; Cambridge: 1998. pp. 188–197. [Google Scholar]
- 31.Enns GM, et al. Progressive neurological deterioration and MRI changes in cblC methylmalonic acidaemia treated with hydroxocobalamin. J Inherit Metab Dis. 1999;22:599–607. doi: 10.1023/a:1005517727451. [DOI] [PubMed] [Google Scholar]
- 32.Rossi A, et al. Early-onset combined methylmalonic aciduria and homocystinuria: neuroradiologic findings. AJNR Am J Neuroradiol. 2001;22:554–63. [PMC free article] [PubMed] [Google Scholar]
- 33.Scott JM, Dinn JJ, Wilson P, Weir DG. Pathogenesis of subacute combined degeneration: a result of methyl group deficiency. Lancet. 1981;2:334–7. doi: 10.1016/s0140-6736(81)90649-8. [DOI] [PubMed] [Google Scholar]
- 34.Tsina EK, Marsden DL, Hansen RM, Fulton AB. Maculopathy and retinal degeneration in cobalamin C methylmalonic aciduria and homocystinuria. Arch Ophthalmol. 2005;123:1143–6. doi: 10.1001/archopht.123.8.1143. [DOI] [PubMed] [Google Scholar]
- 35.Debray FG, et al. Reduced brain choline in homocystinuria due to remethylation defects. Neurology. 2008;71:44–9. doi: 10.1212/01.wnl.0000316391.40236.c3. [DOI] [PubMed] [Google Scholar]
- 36.Rubin RA, Ordonez LA, Wurtman RJ. Physiological dependence of brain methionine and S-adenosylmethionine concentrations on serum amino acid pattern. J Neurochem. 1974;23:227–31. doi: 10.1111/j.1471-4159.1974.tb06938.x. [DOI] [PubMed] [Google Scholar]
- 37.Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–54. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- 38.Manoli I, Venditti C. Branched chain amino acid disorders. In: Lee B, Scaglia F, editors. Inborn Errors of Metabolism. From neonatal screening to metabolic pathways. Oxford University Press; New York, NY: 2015. [Google Scholar]
- 39.Zinnanti WJ, et al. Mechanism of age-dependent susceptibility and novel treatment strategy in glutaric acidemia type I. J Clin Invest. 2007;117:3258–70. doi: 10.1172/JCI31617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sauer SW, et al. Therapeutic modulation of cerebral L-lysine metabolism in a mouse model for glutaric aciduria type I. Brain. 2011;134:157–70. doi: 10.1093/brain/awq269. [DOI] [PubMed] [Google Scholar]
- 41.Broer S. The SLC6 orphans are forming a family of amino acid transporters. Neurochem Int. 2006;48:559–67. doi: 10.1016/j.neuint.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 42.Sloan JL, Mager S. Cloning and functional expression of a human Na(+) and Cl(−)dependent neutral and cationic amino acid transporter B(0+) J Biol Chem. 1999;274:23740–5. doi: 10.1074/jbc.274.34.23740. [DOI] [PubMed] [Google Scholar]
- 43.Michalec K, et al. Protein kinase C restricts transport of carnitine by amino acid transporter ATB(0,+) apically localized in the blood-brain barrier. Arch Biochem Biophys. 2014;554:28–35. doi: 10.1016/j.abb.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 44.Acosta PB, et al. Intake and blood levels of fatty acids in treated patients with phenylketonuria. J Pediatr Gastroenterol Nutr. 2001;33:253–9. doi: 10.1097/00005176-200109000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Gil-Campos M, Sanjurjo Crespo P. Omega 3 fatty acids and inborn errors of metabolism. Br J Nutr. 2012;107 (Suppl 2):S129–36. doi: 10.1017/S0007114512001523. [DOI] [PubMed] [Google Scholar]
- 46.Riediger ND, Othman RA, Suh M, Moghadasian MH. A systemic review of the roles of n-3 fatty acids in health and disease. J Am Diet Assoc. 2009;109:668–79. doi: 10.1016/j.jada.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 47.Din JN, et al. Dietary intervention with oil rich fish reduces platelet-monocyte aggregation in man. Atherosclerosis. 2008;197:290–6. doi: 10.1016/j.atherosclerosis.2007.04.047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.