Abstract
Rationale
Recent evidence suggests that imidazoline I2 receptor ligands are suitable for combination therapy with opioids. Quantitative analysis of I2 receptor ligands combined with non-opioid drugs is necessary for justification of alternative pain therapies.
Objective
This study systematically examined the anti-hyperalgesic and response rate-suppressing effects of selective I2 receptor ligands (2-BFI and phenyzoline) alone and in combination with acetaminophen.
Methods
Von Frey and Hargreaves tests were used to examine the anti-hyperalgesic effects of drugs in complete Freund’s adjuvant (CFA)-induced inflammatory pain in rats. Food-reinforced schedule-controlled responding was used to assess the rate-suppressing effects of study drugs. Dose-addition and isobolographic analyses were used to assess drug-drug interactions for all assays.
Results
2-BFI (3.2–17.8 mg/kg, i.p.), phenyzoline (17.8–100 mg/kg, i.p.), and acetaminophen (56–178 mg/kg, i.p.) all dose-dependently produced significant antinociceptive effects. When studied as combinations, 2-BFI and acetaminophen produced infra-additive to additive interactions while phenyzoline and acetaminophen produced additive to supra-additive interactions. The same drug combinations suppressed response rate in a supra-additive manner.
Conclusions
Quantitative analysis of the anti-hyperalgesic and response rate-suppressing effects suggests that I2 receptor ligands are not well suited to combination therapy with acetaminophen.
Keywords: Imidazoline I2 receptor, Acetaminophen, Complete Freund’s adjuvant, Dose-addition analysis, Antinociception, Rats
Introduction
Pain affects more Americans than diabetes, heart disease, and cancer combined. In addition to being the most common reason patients access the health care system, pain is also the leading cause of long-term disability (NIH 2013). Historically, pain pharmacotherapy has relied extensively on the prescription of opioids. However, the near doubling of opioid prescriptions due to pain symptoms between 2000 and 2010 contrasts the lack of prescription increases in other drug categories (Daubresse et al. 2013). This is problematic. For example, 151% and 85% increases in deaths for women and men, respectively, from 1999 to 2010 have resulted, at least in part, from this prescribing strategy (Mack et al. 2013). Therefore, the development of nonopioid pain management strategies is desperately needed. While monotherapies are effective in some cases, combining more than one drug in a treatment regimen, called combination therapy, has shown to be equally if not more effective under some pain circumstances. The goal of combination therapy is to enable the use of smaller doses of each drug in combination to achieve the same or better analgesic effects while circumventing unwanted side effects that may result from higher doses of a single drug (Gilron et al. 2013).
The imidazoline I2 receptor is a novel target for analgesics (Li et al. 2014; Li and Zhang 2011). It has been shown that I2 receptor ligands are effective as monotherapies in several animal models of chronic pain but also that they produce additive to synergistic effects when combined with opioids (Thorn et al. 2015; Thorn et al. 2011). While this may be a promising strategy, the current literature falls short on investigating the combinations of I2 receptor ligands with non-opioid drugs. In light of the problems with the dependence on opioids to treat pain stated above, acetaminophen (paracetamol) is a widely used, low-potency, effective, and inexpensive analgesic available without a prescription (Tauben 2015). While safe in relatively large doses (2–4 g daily), hepatotoxicity increases with age, alcohol use, and pre-existing liver conditions. The combination of acetaminophen with non-steroidal anti-inflammatory drugs (NSAIDs) can bolster analgesia but comes with increased risk of gastric and renal injury (Kumar et al. 2010; Ong et al. 2010). Thus, combining acetaminophen with other pain relievers may be a suitable management of chronic pain and circumvent these other health risks via reduced doses.
Since acetaminophen has no documented abuse liability, and because side effects are only present at high doses, we examined whether a favorable analgesic profile results from combining acetaminophen with I2 receptor ligands. This study used a quantitative analytical approach to examine the analgesic effect of the selective I2 receptor agonists 2-BFI and phenyzoline alone and in combination with acetaminophen using the von Frey filament and Hargreaves tests in rats with complete Freund’s adjuvant (CFA)-induced inflammatory pain. Isobolographic and dose-addition analyses were used to quantitatively examine the nature of the I2 receptor ligand-acetaminophen interactions in these chronic pain assays. Furthermore, we examined the effects of I2 receptor ligands alone and in combination with acetaminophen on food-maintained operant responding to examine the possibility of behavioral competition with the observed antinociceptive effects.
Methods
Subjects
Male Sprague-Dawley rats (n=62) (Harlan, Indianapolis, IN) approximately 10 weeks old at experiment onset were housed individually on a 12/12-h light/dark cycle (behavioral experiments were conducted during the light period) with free access to water except during testing sessions. All animals used in pain tests had free access to standard rodent chow in their home cages (n=6 per group; 9 groups). Animals used in operant responding studies were provided with restricted access to food after their daily sessions such that their body weights were maintained at 85% of their free-feeding counterparts. Animals were maintained and experiments were conducted in accordance with guidelines of the International Association for the Study of Pain (Zimmermann 1983) and were approved by the Institutional Animal Care and Use Committee, University at Buffalo, the State University of New York, and with the 2011 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources on Life Sciences, National Research Council, National Academy of Sciences, Washington, D.C.).
Induction of inflammatory pain
Inflammatory pain was induced by CFA inoculation as previously described (Li et al. 2014). Briefly, 0.1 mL of CFA (Sigma) containing approximately 0.05 mg Mycobacterium butyricum dissolved in paraffin oil was injected in the right foot pad (hind paw) of rats under isoflurane anesthesia (2% isoflurane mixed with 100% oxygen). The level of anesthesia was assessed by the loss of righting reflex.
Mechanical Hyperalgesia
Mechanical hyperalgesia was measured using the von Frey filament consisting of calibrated filaments (North Coast Medical, Morgan Hill, CA) (1.4–26 g). Rats (n=6 per group) were placed in elevated plastic chambers with a wire mesh floor (IITC Life Science Inc., Woodland Hills, CA, USA) immediately before the test. Filaments were applied perpendicularly to the medial plantar surface of the hind paw from below the mesh floor in an ascending order beginning with the lowest filament (1.4 g). A filament was applied until buckling occurred and maintained for approximately 2 s. Mechanical thresholds (expressed in % maximal possible effect [MPE]) correspond to the lowest force that elicited a behavioral response (withdrawal of the hind paw) in at least 2 out of 3 applications. Since the course of hyperalgesia does not seem to affect test results, all von Frey filament tests were conducted 24 h after CFA treatment. Tests were performed as cumulatively-dosed multiple cycle procedures, where measurements were taken immediately prior to drug administration, then 20 min after a dose treatment and immediately before the next drug administration. These cycles continued until near 100% MPE was achieved (corresponding to 26 g). When drugs were studied in combination, they were prepared in a mixture and administered as one injection. Experimenters were not blind to the treatments; however, they received extensive training with this procedure before the onset of this study to ensure accurate judgment of paw withdrawal responses and minimize experimenter bias.
Thermal Hyperalgesia
Thermal hyperalgesia was measured using a plantar test apparatus (IITC Life Science Inc.), wherein the paw withdrawal latency (PWL) to a thermal stimulus was measured, as described previously (Hargreaves et al. 1988). The apparatus used a test unit containing a heat source that radiated a light beam. An adjustable angled mirror on the test unit was used to locate the correct targeting area on the paw. The beam source was set with an active intensity of 40%, an idle intensity of 10% and a cut-off time of 20 s. PWL comprised the time from the start of the beam light until the animal withdrew the paw from the heat stimulus (reaction time was measured to 0.01 s). An acrylic six-chamber container was used to separate the rats that were placed on the glass base. Measurements were taken in duplicate approximately 1 min apart, and the average was used for statistical analysis. Hargreaves’ tests were conducted 24 h after mechanical hyperalgesia tests, 48 h after CFA injection. Tests were performed using cumulatively-dosed multiple cycle procedures as described above. These cycles continued until 100% MPE was achieved (corresponding to 20 s) or until the highest dose studied in the mechanical hyperalgesia experiments were reached.
Schedule-controlled responding
Food-maintained operant responding experiments were conducted in commercially available chambers located within sound-attenuating, ventilated enclosures (Coulbourn Instruments Inc., Allentown, PA, USA). Chambers contained two levers; responses on the inactive (right) lever were recorded and had no programmed consequence. Data were collected using Graphic State 3.03 software and an interface (Coulbourn Instruments Inc.). Rats were trained to press lever for food under a multiple cycle procedure. Each cycle began with a 15 min pretreatment period, during which the chamber was dark and responses had no programmed consequences, followed by a 5 min response period during which a light above the active (left) lever was illuminated and rats could receive a maximum of 5 food pellets (45 mg dustless precision pellets; Bio Serv Inc., Frenchtown, NJ, USA) by responding on the active lever. Initially, a single response produced a food pellet; as performance improved the response requirement was progressively increased across days to a final fixed ratio (FR) of 10. The light was terminated after the delivery of 5 food pellets or after 5 min had elapsed, whichever occurred first. Daily sessions consisted of 6 cycles, and rats had to satisfy the following criteria for five days before testing began: the daily response rate averaged across all 6 cycles within a session did not vary by more than 20% (An et al. 2012). After the first test, all tests were preceded by at least 2 consecutive saline training sessions that satisfied the same criteria. During testing, rats received a drug or drug combination administration at the beginning of each inactive period in a cumulatively-dosed manner until the rate was reduced to less than 20% of the saline control rate or until the test reached the largest dose that could be safely studied (acetaminophen).
Data Analyses
Antihyperalgesic effects of the drugs and drug combinations studied were quantified for each animal as % MPE for each dose. The following formula was used to quantify % MPE: % MPE = (Post-drug value for a behavioral response (g or s) − Pre-drug value for a behavioral response)/(Pre-CFA value − Pre-drug value) × 100. To construct antihyperalgesic dose-effect curves, % MPEs were averaged within each group (±SEM) and plotted as a function of dose. Rate of operant responding is expressed as a percentage of the saline control response rate. For each cycle of a drug test, the control response rate for an individual rat was the average response rate of the corresponding cycle from the saline session immediately prior to the test. These percentages were averaged across eight rats (±SEM) and plotted as a function of dose. Log(ED50) (± 95% confidence limits [CLs]) values were determined from the %MPE for each animal within a particular group and averaged within the group to calculate the ED50 values for each drug for each behavioral assay. The mean MPEs (±SEM) were calculated from individual animals and repeated measures one-way analysis of variance (ANOVA) (dose × treatment) followed by post hoc Bonferroni’s test was used to determine statistical significance. P < 0.05 was considered statistically significant for all tests.
For the study that examined the interactions between 2-BFI or phenyzoline and acetaminophen, a fixed proportion dose-addition analysis method was used as described previously (An et al. 2012; Li et al. 2014; Li et al. 2011a; Li et al. 2011b). For this analysis, two drugs were combined in fixed proportions (1:1, 1:3, and 3:1) and administered using the cumulative dosing procedure. The actual doses of the drugs in the combination were determined by the potencies of the drugs. For example, for the ratio of 1:1 between 2-BFI and acetaminophen, equal proportions of the ED50 values of each drug were studied as a mixture (e.g., the combined 1/8×, 1/4×, 1/2×, 1×, and 2×ED50s of 2-BFI and acetaminophen were administered consecutively by cumulative dosing procedure to complete one dose-effect curve test). The dose-effect curve of the drug mixture was determined and the individual ED50 values of the two drugs in the mixture were calculated based upon the shared dose–response curves. Isobolograms were constructed to visually represent the nature of the drug interactions as additive, supra-additive or infra-additive. An isobologram plots equi-effective doses (e.g. ED50 ± 95% CLs) of one drug in the presence of a second drug. If the effects of the two drugs are additive, then the ED50 values (± 95% CLs) for the drug combination should overlap with the diagonal line between the ED50 values (± 95% CLs) for the two drugs alone (line of additivity). If the ED50 values (± 95% CLs) fall below the limits of the line of additivity, then the effects of the two drugs are considered supra-additive (i.e. in the presence of one drug, smaller than predicted doses of a second drug are needed to produce the same effect). If the ED50 values (± 95% CLs) fall above the limits of the line of additivity, then the effects of the two drugs are considered infra-additive (i.e. in the presence of one drug, larger than predicted doses of a second drug are needed to produce the same effect). If the 95% CLs of the measured ED50 values do not overlap with the boundaries of the additivity line, then the data are considered significantly different from simple additivity. Dose-addition analysis was performed as described previously (Tallarida 2000). Expected additive ED50 values (Zadd) were calculated from two individual drugs using Pharm Tools Pro version 1.1 software (The McCary Group, Inc., Elkins Park, PA, USA) representing the ED50 values of a composite dose-effect curve assuming simple additive interactions. Experimental ED50 values (Zmix) were determined from the 1:3, 1:1, and 3:1 combinations and were defined as the sum of the ED50 values of both drugs in the combination. If Zmix is significantly less than Zadd (the 95% CL does not overlap), the interaction is considered supra-additive. If Zmix is significantly greater than Zadd, the interaction is considered infra-additive. Dose ratios of Zmix/Zadd were also calculated to estimate the magnitude of the differences.
Drugs
2-BFI hydrochloride and phenyzoline oxalate were synthesized according to standard procedures (Ishihara and Togo 2007; Jarry C 1997). Acetaminophen was purchased from Sigma (Sigma, St. Louis, MO). When studied alone, 2-BFI and phenyzoline were dissolved in 0.9% saline. Acetaminophen and all combinations were dissolved in 20% DMSO in saline. All drugs were administered intraperitoneally in a volume of 1–2 ml/kg.
Results
Under control conditions, rats displayed a paw withdrawal threshold (PWT) of 24.2 ± 1.8 g for the von Frey test and a paw withdrawal latency (PWL) of approximately 19.6 ± 0.3 s for the Hargreaves test. Following CFA injection into the hindpaw, PWT decreased to 5.3±0.7 g and PWL decreased to 10.3±1.1 s, values similar to those described previously (Thorn et al., 2015; Li et al., 2014). For each group of rats (n=6 per group), the drug or drug combination used in the von Frey test was the same as was used in the Hargreaves test.
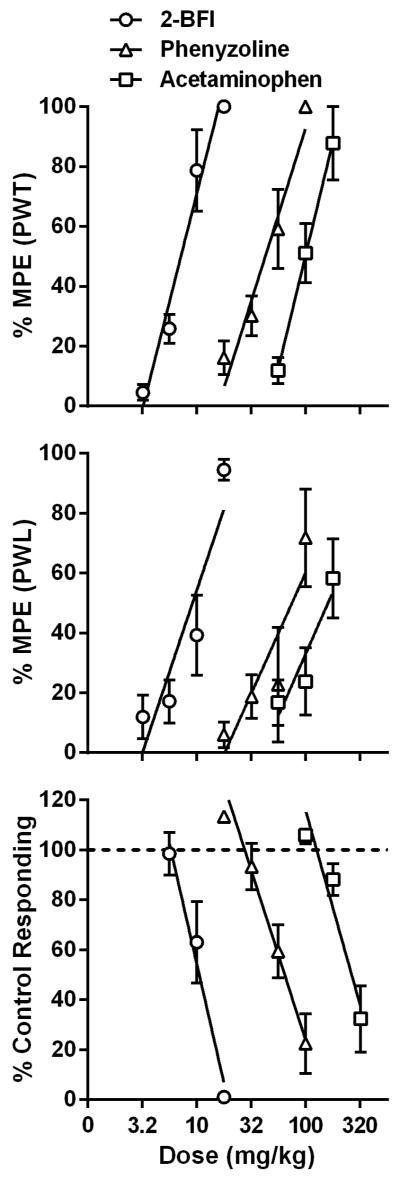
2-BFI dose-dependently increased the PWT and PWL (Fig. 1) (one-way ANOVA: F [4, 25] =50.19, p<0.0001 for PWT, F [4, 25] =30.61, p<0.0001 for PWL). Post hoc analyses indicated significant effects at 10–17.8 mg/kg 2-BFI for both PWT and PWL (P < 0.05). Phenyzoline dose-dependently increased the PWT and PWL (one-way ANOVA: F [4, 25] =46.68, p<0.0001 for PWT, F [4, 20] =4.755, p=0.0101 for PWL). Post hoc analyses indicated significant effects at 32–100 mg/kg phenyzoline for PWT and at 100 mg/kg phenyzoline for PWL (P < 0.05). Acetaminophen dose-dependently increased both the PWT and PWL (one-way ANOVA: F [3, 20] =31.99, p<0.0001 for PWT, F [3, 20] =7.832, p=0.0022 for PWL). Post hoc analyses indicated significant effects at 100–178 mg/kg acetaminophen for PWT and 178 mg/kg acetaminophen for PWL (P < 0.05). The dose-effect curves of these three drugs were presented as % MPE in Fig. 1, and the test of parallelism showed that the curves resulting from the von Frey and Hargreaves tests were not deviated from parallelism. The ED50 (95% CLs) values of the drugs for the von Frey filament and Hargreaves tests are listed in Table 1. The rank order of potency of these compounds in both assays was 2-BFI > phenyzoline > acetaminophen. The calculated potency ratio between acetaminophen and 2-BFI was 14.3 for the von Frey assay and 13 for the Hargreaves assay. The calculated potency ratio between acetaminophen and phenyzoline was 2.3 for the von Frey assay and 1.8 for the Hargreaves assay. In all of the following combination studies, these potency ratios were used to calculate the respective doses of 2-BFI and phenyzoline in the drug combination when doses of acetaminophen were given. For example, under a fixed ratio of 1:1, every 1 mg/kg 2-BFI administered in the von Frey assay was accompanied by 14.3 mg/kg acetaminophen.
Figure 1.
Percent maximal possible effects of acetaminophen and I2 receptor ligands on CFA-induced mechanical hyperalgesia (top), CFA-induced thermal hyperalgesia (middle), and schedule-controlled responding (bottom). Ordinates, percentage of maximal possible effects (top and middle) or percentage of control responding rate (bottom); Abscissa, drug doses (mg/kg).
Table 1.
ED50 values (95% CL) in mg/kg for individual drugs in the von Frey test, Hargreaves’ test, and on food-maintained schedule-controlled responding. For drug combinations, only the ED50 values of the I2 receptor ligands are presented.
| Treatment | von Frey | Hargreaves | Operant Responding | |
|---|---|---|---|---|
| 2-BFI | 7.1 (5.7, 8.8) | 10.0 (7.6, 13.2) | 9.9 (8.2, 11.8) | |
| Phenyzoline | 43.5 (33.7, 56.2) | 72.7 (53.4, 98.9) | 64.1 (51.1, 80.4) | |
| Acetaminophen | 100.8 (83.1, 122.3) | 131.9 (95.9, 181.3) | 261.7 (214.4, 319.3) | |
|
| ||||
| 2-BFI/Acetaminophen | von Frey ratios | Hargreaves ratios | ||
|
| ||||
| 1:3 | 2.1 (1.3, 3.1) | 4.2 (3.2, 5.7) | 2.8 (2.3, 3.4) | 1.6 (1.2, 2.2) |
| 1:1 | 5.7 (4.7, 7.7) | 6.9 (5.3, 9.1) | 3.7 (2.8, 5.0) | 2.7 (2.2, 3.4) |
| 3:1 | 8.8 (6.6, 11.7) | 10.8 (6.5, 17.9) | 3.0 (2.4, 3.8) | 1.7 (1.1, 2.8) |
|
| ||||
| Phenyzoline/acetaminophen | ||||
|
| ||||
| 1:3 | 7.4 (4.7, 11.7) | 22.0 (12.4, 38.9) | 8.0 (6.6, 9.7) | 5.9 (4.6, 7.5) |
| 1:1 | 20.8 (11.1, 39.1) | 25.0 (7.9, 78.7) | 10.0 (8.1, 12.3) | 7.3 (4.4, 12.3) |
| 3:1 | 18.6 (12.4, 27.9) | 50.7 (40.2, 63.9) | 11.3 (8.5, 15.1) | 18.7 (13.6, 25.5) |
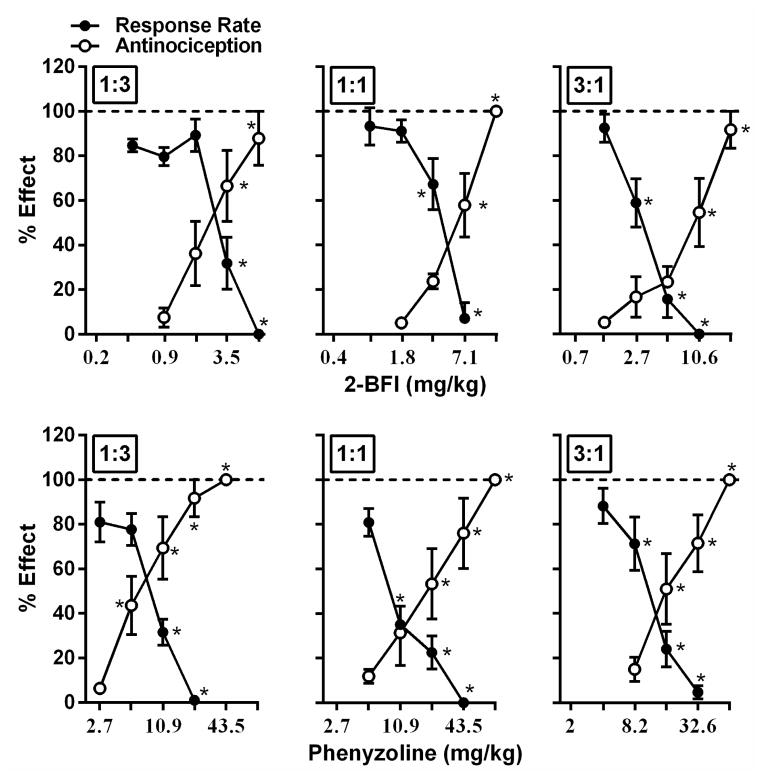
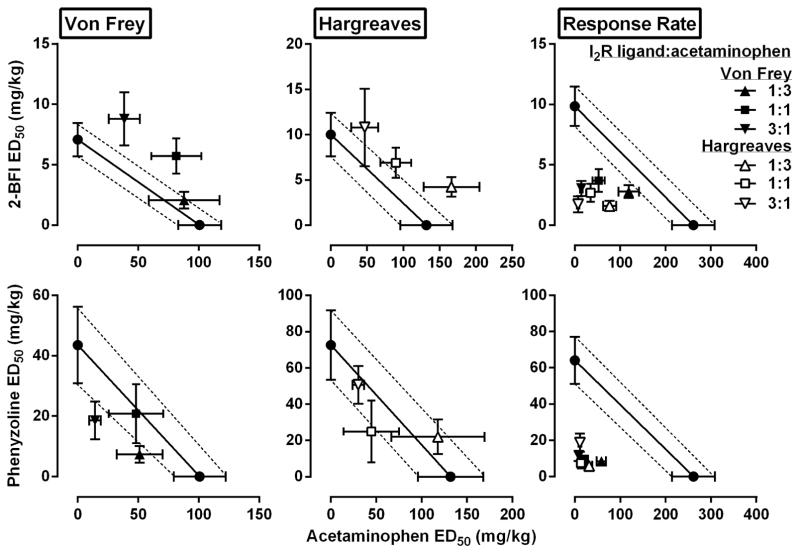
Using this approach, we found that all fixed ratio combinations of 2-BFI and acetaminophen dose-dependently increased the PWT (Fig. 2) (one-way ANOVA: F[4, 25]=19.54, p<0.0001 for 1:3, F[4, 25]=46.05, p<0.0001 for 1:1, F[5, 30]=21.93, p<0.0001 for 3:1) and PWL (Fig. 3) (one-way ANOVA: F[4, 25]=10.84, p<0.0001 for 1:3, F[4, 25]=10.55, p<0.0001 for 1:1, F[4, 25]=10.84, p<0.0001 for 3:1). Post hoc analyses revealed significant effects for PWT at 3.5 mg/kg or higher doses of 2-BFI in the 1:3 combination, at 7.1 mg/kg or higher doses of 2-BFI in the 1:1 combination, and at 10.6 mg/kg or higher doses of 2-BFI in the 3:1 combination (p<0.05). Post hoc analyses revealed significant effects for PWL at 2.5 mg/kg or higher doses of 2-BFI in the 1:3 combination, at 10.0 mg/kg or higher doses of 2-BFI in the 1:1 combination, and at 15.0 mg/kg or higher doses of 2-BFI in the 3:1 combination (p<0.05). The ED50s (95% CLs) of 2-BFI in the von Frey and Hargreaves tests are listed in Table 1. Isobolographic analyses indicated that the ED50 values (±(95% CLs)) of the 2-BFI-acetaminophen mixtures in 1:1 and 3:1 ratios fell above the line of additivity in the von Frey assay, as did the ED50 of the 1:3 mixture in the Hargreaves assay, which indicates infra-additive interactions for these mixtures (Fig. 4). The remaining mixtures, 1:3 in the von Frey assay and 1:1 and 3:1 in the Hargreaves assay, produced ED50s that fell within the CLs of the line of additivity, suggesting simple additive interactions in these combinations. Dose-addition analyses results confirmed these findings (Table 2).
Figure 2.
Effects of 2-BFI/acetaminophen (top) and phenyzoline/acetaminophen (bottom) combinations (left, 1:3; middle, 1:1; right, 3:1) against mechanical hyperalgesia (open circles) and on rate of food maintained responding (filled circles) in rats responding under a fixed ratio 10 schedule of food presentation. Ordinate, percentage of maximal possible effect for mechanical anti-hyperalgesia (open circles) or percentage of control responding rate (filled circles); Abscissa, dose of drug (mg/kg). *P<0.05 as compared to vehicle mechanical hyperalgesia or vehicle responding rate. Note that all the doses labelled on abscissa were the actual doses of I2 receptor ligands in the drug mixture. Doses of acetaminophen were not labelled.
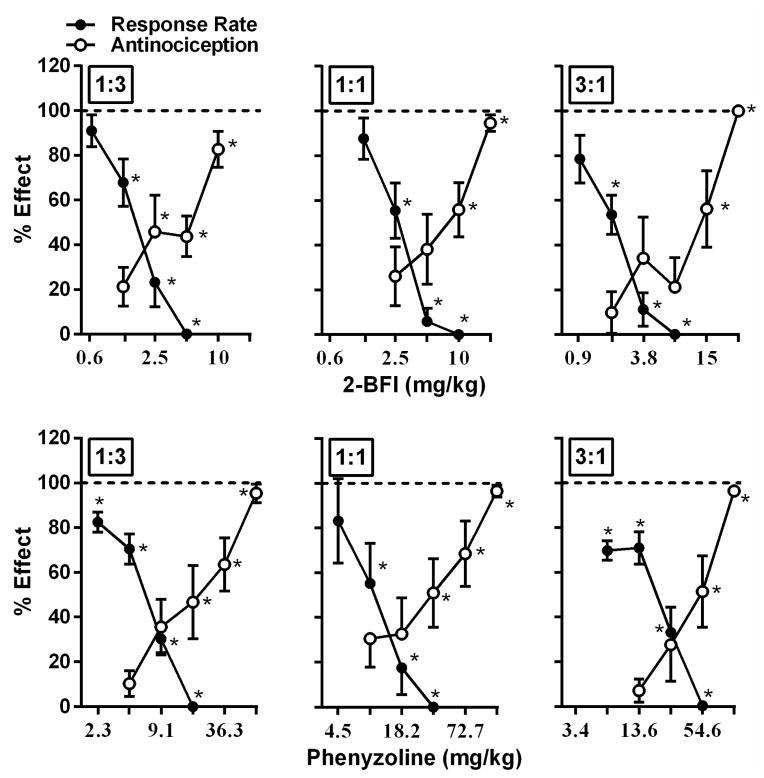
Figure 3.
Effects of 2-BFI/acetaminophen (top) and phenyzoline/acetaminophen (bottom) combinations (left, 1:3; middle, 1:1; right, 3:1) against thermal hyperalgesia (open circles) and on rate of food maintained responding (filled circles) in rats responding under a fixed ratio 10 schedule of food presentation. Ordinate, percentage of maximal possible effect for thermal anti-hyperalgesia (open circles) or percentage of control responding rate (filled circles); Abscissa, dose of drug (mg/kg). *P<0.05 as compared to vehicle thermal hyperalgesia or vehicle responding rate. See Figure 2 for other details.
Figure 4.
Effects of 2-BFI or phenyzoline alone and in combination with acetaminophen on CFA-induced mechanical hyperalgesia, thermal hyperalgesia, and responding rate. Top, left: isobologram for 2-BFI/acetaminophen mixture for mechanical hyperalgesia; Top, middle: isobologram for 2-BFI/acetaminophen mixture for thermal hyperalgesia; Top, right: isobologram for 2-BFI/acetaminophen mixture for responding rate. Ordinate, ED50 value (95% CL) of 2-BFI (mg/kg); Abscissa, ED50 value (95% CL) of acetaminophen (mg/kg). Bottom, left: isobologram for phenyzoline/acetaminophen mixture for mechanical hyperalgesia; Bottom, middle: isobologram for phenyzoline/acetaminophen mixture for thermal hyperalgesia; Bottom, right: isobologram for phenyzoline/acetaminophen mixture for responding rate. Ordinate, ED50 value (95% CL) of phenyzoline (mg/kg); Abscissa, ED50 value (95% CL) of acetaminophen (mg/kg).
Table 2.
Expected additive ED50 values (Zadd), actual (experimentally determined) ED50 values (Zmix), and the ratio of expected/actual ED50 values for drug mixtures for antinociception and response rate in rats.
| Combination relative dose ratios | Zadd | Zmix | Ratio Zadd/Zmix |
|---|---|---|---|
| von Frey | |||
| 2-BFI/acetaminophen | |||
| 1:3 | 73.9 (72.8–75.0) | 90.1 (60.1–135.0) | 0.82 |
| 1:1 | 51.6 (49.5–53.8) | 82.1 (61.7–109.1)b | 0.63 |
| 3:1 | 29.4 (29.2–30.6) | 46.7 (31.1–70.2)b | 0.63 |
| Phenyzoline/acetaminophen | |||
| 1:3 | 82.5 (79.4–85.6) | 58.6 (37.0–92.67) | 1.41 |
| 1:1 | 68.9 (66.4–71.6) | 69.0 (36.7–129.7) | 1.00 |
| 3:1 | 55.5 (53.4–57.6) | 32.9 (21.9–49.5)a | 1.68 |
|
| |||
| Hargreaves | |||
| 2-BFI/acetaminophen | |||
| 1:3 | 121.1 (107.2–135.1) | 170.6 (131.4–221.5)b | 0.71 |
| 1:1 | 84.3 (74.5–94.1) | 97.1 (74.1–127.2) | 0.87 |
| 3:1 | 46.8 (41.3–52.3) | 57.6 (34.8–95.3) | 0.81 |
| Phenyzoline/acetaminophen | |||
| 1:3 | 145.9 (120.6–171.3) | 140.0 (79.1–247.9) | 1.04 |
| 1:1 | 128.5 (106.5–150.4) | 69.7 (22.1–219.5) | 1.84 |
| 3:1 | 111.3 (92.5–130.0) | 80.9 (64.2–102.0) | 1.38 |
|
| |||
| Response Rate - Mixtures in von Frey | |||
| 2-BFI/acetaminophen | |||
| 1:3 | 203.3 (191.5–215.0) | 121.8 (98.8–150.3)a | 1.67 |
| 1:1 | 138.2 (130.1–146.2) | 56.6 (42.2–75.8)a | 2.44 |
| 3:1 | 74.8 (70.4–79.1) | 17.5 (13.9–22.0)a | 4.27 |
| Phenyzoline/acetaminophen | |||
| 1:3 | 216.8 (205.3–228.3) | 63.8 (52.7–77.4)a | 3.40 |
| 1:1 | 165.8 (156.9–174.6) | 33.1 (26.9–40.7)a | 5.01 |
| 3:1 | 114.5 (107.9–120.6) | 20.1 (15.1–26.8)a | 5.70 |
|
| |||
| Response Rate – Mixtures in Hargreaves | |||
| 2-BFI/acetaminophen | |||
| 1:3 | 203.3 (191.5–215.0) | 78.8 (63.9–97.2)a | 2.58 |
| 1:1 | 138.2 (130.1–146.2) | 37.9 (30.4–47.3)a | 3.64 |
| 3:1 | 74.8 (70.5–79.1) | 9.2 (5.7–15.1)a | 8.10 |
| Phenyzoline/acetaminophen | |||
| 1:3 | 216.8 (205.3–228.3) | 37.5 (29.2–48.1)a | 5.79 |
| 1:1 | 165.8 (156.9–174.6) | 20.4 (12.2–34.3)a | 8.12 |
| 3:1 | 114.5 (107.9–120.6) | 29.8 (21.8–40.7)a | 3.84 |
Note:
Indicates Zmix confidence limits do not overlap with Zadd confidence limits. a Zmix lower than Zadd (supra-additivity). b Zmix higher than Zadd (infra-additivity).
All fixed ratio combinations of phenyzoline and acetaminophen also dose-dependently increased the PWT (Fig. 2) (one-way ANOVA: F[5, 30]=34.42, p<0.0001 for 1:3, F[5, 30]=16.51, p<0.0001 for 1:1, F[4, 25]=24.09, p<0.0001 for 3:1) and PWL (Fig. 3) (one-way ANOVA: F[5, 30]=20.15, p<0.0001 for 1:3, F[5, 30]=13.81, p<0.0001 for 1:1, F[4, 25]=15.77, p<0.0001 for 3:1). Post hoc analyses revealed significant effects for PWT at 5.4 mg/kg phenyzoline or higher in the 1:3 combination, at 21.8 mg/kg phenyzoline or higher in the 1:1 combination, and at 16.3 mg/kg phenyzoline or higher in the 3:1 combination (p<0.05). Post hoc analyses revealed statistically significant effects on thermal hyperalgesia at 18.2 mg/kg phenyzoline or higher in the 1:3 combination, at 36.3 mg/kg phenyzoline or higher in the 1:1 combination, and at 54.6 mg/kg phenyzoline or higher in the 3:1 combination (p<0.05). The ED50s (95% CLs) of phenyzoline in the von Frey and Hargreaves tests are listed in Table 1. Isobolographic analyses indicated that the ED50s (±95% CLs) of all phenyzoline-acetaminophen mixtures in both assays fell within the CLs of the line of additivity, suggesting simple additive interactions between phenyzoline and acetaminophen (Fig. 4). These results were confirmed with dose-addition analyses (Table 2).
When treated with saline, the food-maintained operant responding group exhibited very stable rate of responding across the 6 cycles under a FR 10 schedule of food presentation (in responses/s ± SEM): 0.66 ± 0.05, 0.67 ± 0.04, 0.67 ± 0.08, 0.65 ± 0.07, 0.64 ± 0.07, 0.64 ± 0.06. Studied individually, 2-BFI, phenyzoline, and acetaminophen all produced dose-dependent suppression of the rate of responding (Fig. 1) (one-way ANOVA: F [3, 28] =22.56, p<0.0001 for 2-BFI, F[4, 35] =15.85, p<0.0001 for phenyzoline, F[4, 35]=16.68, p<0.0001 for acetaminophen). Post hoc analyses revealed significant effects for17.8 mg/kg 2-BFI, 56 and 100 mg/kg phenyzoline, and 320 mg/kg acetaminophen (p<0.05). To examine the response rate-suppressing effects of combinations of acetaminophen and 2-BFI or phenyzoline, we tested the same fixed ratio dose combinations as were used for the von Frey and Hargreaves tests. Relative dose combinations of 2-BFI and acetaminophen used for von Frey tests produced dose-dependent suppression of response rate under fixed ratios of 1:3, 1:1, and 3:1 (Fig. 2) (one-way ANOVA: F[5, 35]=36.24, p<0.0001 for 1:3, F[4, 28]=42.34, p<0.0001 for 1:1, F[4, 28]=46.59, p<0.0001 for 3:1) as did dose combinations used for Hargreaves tests (Fig. 3) (one-way ANOVA: F[5, 35]=43.69, p<0.0001 for 1:3, F[4, 28]=47.13, p<0.0001 for 1:1, F[4, 28]=39.04, p<0.0001 for 3:1). Post hoc analyses of doses used in the von Frey test revealed significant suppression at 3.5 mg/kg 2-BFI or higher in 1:3 and 1:1 combinations, and at 2.7 mg/kg 2-BFI or higher in the 3:1 combination. Post hoc analyses of doses used in the Hargreaves test revealed significant suppression at 1.3 mg/kg or higher in the 1:3 combination, at 2.5 mg/kg 2-BFI or higher in the 1:1 combination, and at 1.9 mg/kg 2-BFI or higher in the 3:1 combination (p<0.05). The ED50s (95% CLs) for these effects are listed in Table 1. Isobolographic analysis revealed that the ED50 (± 95% CLs) values of the 2-BFI-acetaminophen mixtures in all ratios fell below the line of additivity, indicating supra-additive interactions between 2-BFI and acetaminophen (Fig. 4). Dose-addition analyses confirmed these results (Table 2). Similarly, dose-dependent response rate suppression was produced by all phenyzoline-acetaminophen dose combinations in the von Frey test (Fig. 2) (one-way ANOVA: F[4, 28]=39.53, p<0.0001 for 1:3, F[4, 28]=39.18, p<0.0001 for 1:1, F[4, 28]=39.06, p<0.0001 for 3:1) and the Hargreaves test (Fig. 3) (one-way ANOVA: F[4, 28]=88.82, p<0.0001 for 1:3, F[4, 28]=32.27, p<0.0001 for 1:1, F[4, 28]=41.44, p<0.0001 for 3:1). Post hoc analyses of doses used in the von Frey test revealed significant suppression at 10.9 mg/kg phenyzoline or higher in the 1:3 combination, at 10.9 mg/kg phenyzoline or higher in the 1:1 combination, and at 8.2 mg/kg phenyzoline or higher in the 3:1 combination. Post hoc analyses of doses used in the Hargreaves test revealed significant suppression at 2.3 mg/kg phenyzoline or higher in the 1:3 combination, at 9.1 mg/kg phenyzoline or higher in the 1:1 combination, and at 6.8 mg/kg phenyzoline or higher in the 3:1 combination (p<0.05). The ED50s (95% CLs) of the rate-suppressing effects of phenyzoline in the drug mixtures are listed in Table 1. Isobolographic analysis revealed that the ED50 (± 95% CLs) of the phenyzoline-acetaminophen mixtures in all ratios fell below the line of additivity, indicating supra-additive interactions between phenyzoline and acetaminophen (Fig. 4). Dose-addition analysis confirmed these results (Table 2).
Discussion
This study found that 2-BFI and phenyzoline, two selective imidazoline I2 receptor ligands, produced significant antinociceptive effects on both mechanical and thermal hyperalgesia in a commonly used rat model of chronic inflammatory pain. Combinations of 2-BFI with the commonly used pain reliever acetaminophen produced generally infra-additive interactions on antinociception, whereas combinations of phenyzoline and acetaminophen produced generally additive interactions on antinociception. However, both I2 receptor ligands produced synergistic interactions for the suppression of food-maintained operant responding. While previous studies demonstrated favorable interactive profiles between I2 receptor ligands and opioids, these results suggest that the antinociceptive effects of I2 receptor ligands are not enhanced by the combination treatment with acetaminophen, and that the combination of I2 receptor ligands and acetaminophen does not seem to be a beneficial strategy.
CFA-induced hyperalgesia is a well-characterized and commonly used animal model of chronic inflammatory pain. CFA injection into the hindpaw of a rat induces mechanical and thermal hyperalgesia that persist for weeks (Nagakura et al. 2003).. Independently, 2-BFI, phenyzoline, and acetaminophen all dose-dependently produced anti-hyperalgesic effects in the von Frey and Hargreaves tests (Fig. 1), which is consistent with previous investigations from our lab (Li et al. 2014; Thorn et al. 2015). The finding that acetaminophen was effective in treating inflammatory hyperalgesia also agrees with earlier reports (Bianchi et al. 2007; Tomić et al. 2013). The three compounds were comparable in effectiveness, but differed in potency.
Previous studies combining I2 receptor ligands with other analgesics have focused on opioids; these combinations seem to be favorable overall, producing additive to synergistic interactions which may depend on the particular I2 receptor ligand, dosing protocol, route of administration, or pain model (Ferrari et al. 2011; Lanza et al. 2014; Li et al. 2014; Li et al. 2011b; Thorn et al. 2011). However, little effort has been afforded to studying I2 receptor ligands with non-opioid pain medications. Thus, this study was designed to use the CFA-induced pain model to examine the effects of two I2 receptor ligands alone and in combination with acetaminophen, a commonly used analgesic. To do so, we used dose-addition analysis, a powerful and systematical method of examining pharmacological interactions (Tallarida 2000). It is clear that 2-BFI produced additive to infra-additive interactions with acetaminophen for decreasing either mechanical or thermal hyperalgesia, and that these effects are apparent across a wide range of drug proportions, from 25% 2-BFI (1:3 ratio) to 75% 2-BFI (3:1 ratio). In contrast, phenyzoline produced additive to supra-additive interactions with acetaminophen across a similarly broad range of proportions. In a separate assay, we observed that both 2-BFI and phenyzoline produced supra-additive interactions with acetaminophen for decreasing the rate of operant responding for food reward, which is considered a measure of non-specific behavioral suppression. Notably, in many cases the least effective doses of the drug mixture that significantly suppressed the response rate were smaller than the least effective doses of the same drug combination for producing antinociception. This is particularly obvious for Hargreaves’ test (Fig. 3). This indicates that the observed effects for elevated paw withdrawal in the von Frey and Hargreaves tests may be partially due to generalized behavioral suppression. Overall, these findings suggest that I2 receptor ligands are not well-suited to combination therapy with acetaminophen, as other adverse behavioral effects may negatively impact the analgesic effects.
Previous reports have ruled out I2 receptor ligands having classical anti-inflammatory effects, such as decreasing CFA-induced paw edema (Li et al. 2014); however their exact mechanism of action is still unclear. In vivo electrophysiological recordings in rat spinal cord showed that I2 ligands decrease nociceptive transmission in dorsal horn neurons and inhibit C-fiber evoked responses (Diaz et al. 1997), suggesting a direct action on CNS excitability. Past documentation of I2 receptor existing as a binding site on monoamine oxidase (MAO) (Anderson et al. 2006; Tesson et al. 1995) to inhibit MAO function may support this hypothesis and account for other I2 receptor-mediated behavioral effects. However, more recently described I2 receptor-mediated effects on central glial activity (Li et al. 2012; Zhu et al. 2015) suggest that the role of the I2 receptors in mediating neurological effects (e.g., analgesia) may be more complicated. For example, our recent unpublished observations suggest a prominent role of I2 receptor agonists in suppressing activated microglial activity in the spinal cord in rats with chronic inflammatory pain. Despite being one of the most widely used non-prescription analgesics, the mechanism of action of acetaminophen is not fully understood. Acetaminophen has been reported to open the transient receptor potential A1 ion channel on sensory neurons in the dorsal horn (Andersson et al. 2011), and also to inhibit cyclooxygenase (COX) 2 or 3 (Chandrasekharan et al. 2002; Ruud et al. 2013). Therefore, acetaminophen appears to be centrally-active analgesic rather than an anti-inflammatory agent, despite its wide use in the treatment of inflammatory pain in humans. Because of the unclear and apparently non-overlapping mechanisms of these drug classes, a mechanistic explanation of the observed interaction profiles is difficult to put forth. Nonetheless, the lack of beneficial interactions against pain as seen in this study does not support further pursuits of these drug combinations.
Recent characterization of several selective I2 receptor ligands has made it clear that these ligands have important differences. For example, phenyzoline fully substitutes for CR4056 in a drug discrimination procedure whereas 2-BFI only partially substitutes for CR4056 (Qiu et al. 2014). Similarly, CR4056 and phenyzoline both display supra-additive interactions with opioids (Ferrari et al. 2011; Thorn et al. 2015) while 2-BFI only displays interactions of an additive nature (Li et al. 2014). I2 receptor heterogeneity may account for the observed differing interactive profiles of I2 receptor ligands. Radioligand binding studies using [3H] 2-BFI or [3H] idazoxan (Escriba et al. 1999; Regunathan and Reis 1996) suggest multiple subtypes of I2 receptors where certain subtypes of I2 receptors are composed of different proteins than other subtypes. Therefore, the functional and behavioral differences observed between I2 receptor ligands in the current and previous studies may be explained by their preferential activation of certain I2 receptor subtypes or their components within. When paired with other analgesics, the subset of I2 receptors differentially activated by phenyzoline may allow for more favorable interactions than the subset activated by 2-BFI. Supra-additive effects on response rate suppression for both 2-BFI and phenyzoline in the current study make it difficult to conclude whether this trend holds true for acetaminophen. Further investigation is needed to confirm whether the multiple I2 receptor subtype hypothesis can indeed explain the disparity among I2 receptor ligands.
In summary, this study found that selective imidazoline I2 receptor ligands and acetaminophen have significant effects against mechanical and thermal hyperalgesia in a rat model of chronic inflammatory pain. 2-BFI produced additive to infra-additive antinociceptive interactions with acetaminophen while phenyzoline produced additive to supra-additive antinociceptive interactions with acetaminophen. However, all combinations caused supra-additive interactions for suppression of operant responding. As these effects were found across a broad range of proportions, combinations of I2 receptor ligands with acetaminophen may represent a less favorable pain treatment option due to the potential enhancement of non-analgesic effects of these drugs.
Acknowledgments
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (Award no. R01DA034806) and by a grant from National Natural Science Foundation of China (81373390).
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- An XF, Zhang Y, Winter JC, Li JX. Effects of imidazoline I(2) receptor agonists and morphine on schedule-controlled responding in rats. Pharmacol Biochem Behav. 2012;101:354–359. doi: 10.1016/j.pbb.2012.01.024. [DOI] [PubMed] [Google Scholar]
- Anderson NJ, Seif I, Nutt DJ, Hudson AL, Robinson ES. Autoradiographical distribution of imidazoline binding sites in monoamine oxidase A deficient mice. J Neurochem. 2006;96:1551–1559. doi: 10.1111/j.1471-4159.2006.03662.x. [DOI] [PubMed] [Google Scholar]
- Andersson DA, Gentry C, Alenmyr L, Killander D, Lewis SE, Andersson A, Bucher B, Galzi J-L, Sterner O, Bevan S. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid 9-tetrahydrocannabiorcol. Nature communications. 2011;2:551. doi: 10.1038/ncomms1559. [DOI] [PubMed] [Google Scholar]
- Bianchi M, Martucci C, Ferrario P, Franchi S, Sacerdote P. Increased tumor necrosis factor-alpha and prostaglandin E2 concentrations in the cerebrospinal fluid of rats with inflammatory hyperalgesia: the effects of analgesic drugs. Anesth Analg. 2007;104:949–954. doi: 10.1213/01.ane.0000258060.89380.27. [DOI] [PubMed] [Google Scholar]
- Chandrasekharan NV, Dai H, Roos KLT, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: Cloning, structure, and expression. Proceedings of the National Academy of Sciences. 2002;99:13926–13931. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubresse M, Chang HY, Yu Y, Viswanathan S, Shah ND, Stafford RS, Kruszewski SP, Alexander GC. Ambulatory diagnosis and treatment of nonmalignant pain in the United States, 2000–2010. Med Care. 2013;51:870–878. doi: 10.1097/MLR.0b013e3182a95d86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz A, Mayet S, Dickenson AH. BU-224 produces spinal antinociception as an agonist at imidazoline I2 receptors. European Journal of Pharmacology. 1997;333:9–15. doi: 10.1016/s0014-2999(97)01118-7. [DOI] [PubMed] [Google Scholar]
- Escriba PV, Ozaita A, Garcia-Sevilla JA. Pharmacologic characterization of imidazoline receptor proteins identified by immunologic techniques and other methods. Ann N Y Acad Sci. 1999;881:8–25. doi: 10.1111/j.1749-6632.1999.tb09336.x. [DOI] [PubMed] [Google Scholar]
- Ferrari F, Fiorentino S, Mennuni L, Garofalo P, Letari O, Mandelli S, Giordani A, Lanza M, Caselli G. Analgesic efficacy of CR4056, a novel imidazoline-2 receptor ligand, in rat models of inflammatory and neuropathic pain. J Pain Res. 2011;4:111–125. doi: 10.2147/JPR.S18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilron I, Jensen TS, Dickenson AH. Combination pharmacotherapy for management of chronic pain: from bench to bedside. Lancet Neurol. 2013;12:1084–1095. doi: 10.1016/S1474-4422(13)70193-5. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Ishihara M, Togo H. Direct oxidative conversion of aldehydes and alcohols to 2-imidazolines and 2-oxazolines using molecular iodine. Tetrahedron. 2007;63:1474–1480. [Google Scholar]
- Jarry CFI, Bosc J, Renard P, Scalbert E, Guardiola B. 5-(Arloxymethyl) oxazoline. US Patent. 1997;5:686, 477. Adir eCompagnie. [Google Scholar]
- Kumar G, Hota D, Nahar Saikia U, Pandhi P. Evaluation of analgesic efficacy, gastrotoxicity and nephrotoxicity of fixed-dose combinations of nonselective, preferential and selective cyclooxygenase inhibitors with paracetamol in rats. Exp Toxicol Pathol. 2010;62:653–662. doi: 10.1016/j.etp.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Lanza M, Ferrari F, Menghetti I, Tremolada D, Caselli G. Modulation of imidazoline I2 binding sites by CR4056 relieves postoperative hyperalgesia in male and female rats. Br J Pharmacol. 2014;171:3693–3701. doi: 10.1111/bph.12728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Zhang ZX, Liu YF, Xu HQ, Hou ST, Zheng RY. 2-BFI ameliorates EAE-induced mouse spinal cord damage: effective therapeutic time window and possible mechanisms. Brain Res. 2012;1483:13–19. doi: 10.1016/j.brainres.2012.09.016. [DOI] [PubMed] [Google Scholar]
- Li J-X, Thorn DA, Qiu Y, Peng B-W, Zhang Y. Antihyperalgesic effects of imidazoline I(2) receptor ligands in rat models of inflammatory and neuropathic pain. British Journal of Pharmacology. 2014;171:1580–1590. doi: 10.1111/bph.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Crocker C, Koek W, Rice KC, France CP. Effects of serotonin (5-HT)1A and 5-HT2A receptor agonists on schedule-controlled responding in rats: drug combination studies. Psychopharmacology (Berl) 2011a;213:489–497. doi: 10.1007/s00213-010-2136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JX, Zhang Y. Imidazoline I2 receptors: target for new analgesics? Eur J Pharmacol. 2011;658:49–56. doi: 10.1016/j.ejphar.2011.02.038. [DOI] [PubMed] [Google Scholar]
- Li JX, Zhang Y, Winter JC. Morphine-induced antinociception in the rat: supra-additive interactions with imidazoline I(2) receptor ligands. Eur J Pharmacol. 2011b;669:59–65. doi: 10.1016/j.ejphar.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Mack K, Jones C, Paulozzi L. Morbidity and Mortality Weekly Report (MMWR) National Center for Injury Prevention and Control, CDC; 2013. Vital Signs: Overdoses of Prescription Opioid Pain Relievers and Other Drugs Among Women — United States, 1999–2010. [Google Scholar]
- Nagakura Y, Okada M, Kohara A, Kiso T, Toya T, Iwai A, Wanibuchi F, Yamaguchi T. Allodynia and hyperalgesia in adjuvant-induced arthritic rats: time course of progression and efficacy of analgesics. J Pharmacol Exp Ther. 2003;306:490–497. doi: 10.1124/jpet.103.050781. [DOI] [PubMed] [Google Scholar]
- NIH. Pain Management Research Portfolio Online Reporting Tools. NIH; 2013. [Google Scholar]
- Ong CK, Seymour RA, Lirk P, Merry AF. Combining paracetamol (acetaminophen) with nonsteroidal antiinflammatory drugs: a qualitative systematic review of analgesic efficacy for acute postoperative pain. Anesth Analg. 2010;110:1170–1179. doi: 10.1213/ANE.0b013e3181cf9281. [DOI] [PubMed] [Google Scholar]
- Qiu Y, He XH, Zhang Y, Li JX. Discriminative stimulus effects of the novel imidazoline I(2) receptor ligand CR4056 in rats. Scientific Reports. 2014;4:6605. doi: 10.1038/srep06605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regunathan S, Reis DJ. Imidazoline receptors and their endogenous ligands. Annu Rev Pharmacol Toxicol. 1996;36:511–544. doi: 10.1146/annurev.pa.36.040196.002455. [DOI] [PubMed] [Google Scholar]
- Ruud LE, Wilhelms DB, Eskilsson A, Vasilache AM, Elander L, Engblom D, Blomqvist A. Acetaminophen reduces lipopolysaccharide-induced fever by inhibiting cyclooxygenase-2. Neuropharmacology. 2013;71:124–129. doi: 10.1016/j.neuropharm.2013.03.012. [DOI] [PubMed] [Google Scholar]
- Tallarida R. The composite additive curve. In: Tallarida R, editor. Drug synergism and dose-effect data analysis. Chapman & Hall/CRC; Boca Raton: 2000. pp. 77–89. [Google Scholar]
- Tauben D. Nonopioid medications for pain. Phys Med Rehabil Clin N Am. 2015;26:219–248. doi: 10.1016/j.pmr.2015.01.005. [DOI] [PubMed] [Google Scholar]
- Tesson F, Limon-Boulez I, Urban P, Puype M, Vandekerckhove J, Coupry I, Pompon D, Parini A. Localization of I2-imidazoline binding sites on monoamine oxidases. J Biol Chem. 1995;270:9856–9861. doi: 10.1074/jbc.270.17.9856. [DOI] [PubMed] [Google Scholar]
- Thorn DA, Siemian JN, Zhang Y, Li JX. Anti-hyperalgesic effects of imidazoline I receptor ligands in a rat model of inflammatory pain: interactions with oxycodone. Psychopharmacology (Berl) 2015 doi: 10.1007/s00213-015-3983-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn DA, Zhang Y, Peng BW, Winter JC, Li JX. Effects of imidazoline I(2) receptor ligands on morphine- and tramadol-induced antinociception in rats. Eur J Pharmacol. 2011;670:435–440. doi: 10.1016/j.ejphar.2011.09.173. [DOI] [PubMed] [Google Scholar]
- Tomić MA, Micov AM, Stepanović-Petrović RM. Levetiracetam Interacts Synergistically With Nonsteroidal Analgesics and Caffeine to Produce Antihyperalgesia in Rats. The Journal of Pain. 2013;14:1371–1382. doi: 10.1016/j.jpain.2013.06.003. [DOI] [PubMed] [Google Scholar]
- Zhu YB, Xia NG, Zhang YT, Wang XS, Liang SS, Yin WY, Xu HQ, Hou ST, Zheng RY. Brain protection conferred by long-term administration of 2-(2-benzofuranyl)-2-imidazoline against experimental autoimmune encephalomyelitis. Neurochem Res. 2015;40:572–578. doi: 10.1007/s11064-014-1502-0. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]