Abstract
Aromatase and estrogen receptor α (ER) are two key proteins for the proliferation of endocrine-responsive and – resistant breast cancers. Aromatase is an enzyme involved in the conversion of androgen (such as testosterone) to estrogen (such as 17β-estradiol). It is also a very effective therapeutic target for the treatment of endocrine-responsive breast cancer. Comparing endocrine-responsive and -resistant breast cancer, aromatase protein levels do not change significantly. Aromatase activity; however, can be increased via PI3K/Akt/IGFR signaling pathways in endocrine resistant cells. The activity of aromatase has been reported to be modulated by phosphorylation. The ER is an important steroid nuclear receptor in the proliferation of both endocrine-responsive and -resistant cells. Although the mutation or amplification of ER can cause endocrine resistance, it is not commonly found. Some point mutations and translocation events have been characterized and shown to promote estrogen-independent growth. Phosphorylation by cross-talk with growth factor pathways is one of the main mechanisms for ligand-independent activation of ER. Taken together, both ER and aromatase are important in ER-dependent breast cancer and the development of endocrine resistance.
Keywords: breast cancer, estrogen receptor α, aromatase, aromatase inhibitor resistance
1. Introduction
Breast cancers are mostly dependent on estrogens for proliferation and survival. Estrogens play an important role in hormone-receptor-positive breast cancer development, by binding to and activating the estrogen receptor (ER). Seventy-five percent of breast cancers are ER-positive and 65% are also progesterone (PgR) positive [1]. Receptor positive status is a stimulant for breast cancer development; thus, elimination of the production of estrogen from androgens and inhibition of the ER activation are important forms of hormonal therapy. Aromatase is an enzyme that converts androgens into estrogens, and aromatase inhibitors (AIs) can eliminate the production of estrogen. Furthermore, antiestrogens can bind to ER and antagonize its activity. Both of these strategies are successful in the clinic; however, some patients do not respond to the initial therapy, and a significant number of patients who respond will develop resistance to these therapies. A better understanding of the mechanisms of endocrine resistance will aid in the development of new therapeutic strategies to overcome the de novo and acquired resistance [2]. The structural and functional importance of ER and aromatase in endocrine-responsive and -resistant breast cancers will be discussed in more detail.
2. Estrogen Receptor
2.1 ER α and β Isoforms
The estrogen receptor exists in two isoforms: ERα and ERβ [3–5] with a 56% homology between the two isoforms [6]. Both ERs contain a DNA binding domain, a dimerization region, a ligand binding domain, and two transactivation domains—one located near the N-terminus (AF-1) and another near the C-terminus (AF-2). They share high sequence homology in the DNA binding region, but they are not redundant genes because they have different expression patterns and functions [7]. Recent data indicates that ERα is implicated in promoting growth and survival of breast epithelial cells, both cancerous and non-cancerous, while ERβ is involved in growth inhibitory properties [6, 8, 9]. The ERα is also able to form a heterodimer with ERβ, which has a similar binding affinity to DNA as the ERα homodimer, but a lower level of transcriptional activity [10]. Ligands such as estrogen (17β-estradiol/E2), tamoxifen and 4-hydroxytamoxifen (4-OHT), an activated derivative of tamoxifen, help to stabilize the ER binding to DNA; however, the antiestrogen ICI 182780 (referred to as ICI in this review and also known as fulvestrant) affects ERα and ERβ DNA binding differently. DNA binding capability of ERβ is less affected by ICI than that of ERα [11]. Another difference in the ERα and ERβ is in the ligand binding affinities, where estrogens bind to both isoforms with similar affinities [12]. The importance of ERα in breast cancer cell growth has been well studied and documented. On the other hand, the involvement of ERβ in estrogen signaling and breast cancer is not fully defined and remains controversial [13, 14]; thus, will not be extensively discussed here. For simplicity, ERα will be referred to as ER.
2.2 Estrogen Receptor Structure and Function
ER, a nuclear receptor, is mainly functional in the nucleus, where it activates transcription of ER-regulated genes, and its activity depends on binding of E2. ER is also found in the cytosol in an unliganded state, but enters the nucleus due to ligand-dependent and independent activation [6, 15–17]. Within the cytosol, ER is bound to chaperone proteins such as HSP90 and HSP70. Chaperones are essential for stability of proto-oncogenes and hormone receptors such as ER and PR [18, 19]. Upon E2 binding at the ligand binding domain (i.e., AF2) of ER, the receptor undergoes conformational changes. These changes include HSP dissociation from ER; ER dimerization; the receptor plus the bound hormone entering the nucleus; and the formation of a hydrophobic domain, exposing the two activating function (AF) sites to which co-activators (NCoAs) or co-repressors (NCoRs) bind [4–6, 18].
ER function can be broadly classified as genomic or non-genomic. In the genomic pathway, ER forms a dimer upon binding of E2 (Figure 1). The activated ER dimer then translocates into the nucleus and can bind the ERE in the promoter regions to initiate the “classical” transcriptional activation or repression. The ER can also interact with other transcription factors such as activator protein 1 (AP1) and specificity protein 1 (SP1) to bind DNA indirectly, and cause the activation or repression of target genes. This is also known as the “non-classical” or “ERE-independent” genomic action. A third genomic mechanism involves ligand-independent ER activation (at the AF1 domain) by phosphorylation via kinases in the growth factor receptor signaling pathways. With the aid of kinase signaling pathways, ER and its co-activators can be phosphorylated, independent of ligand, through the genomic or non-genomic mechanisms; thus, leading to endocrine resistance. These kinases include stress related kinases: p38 MAPK or JNK; p44/42 MAPK; PI3K/Akt; or p90rsk [20–22].
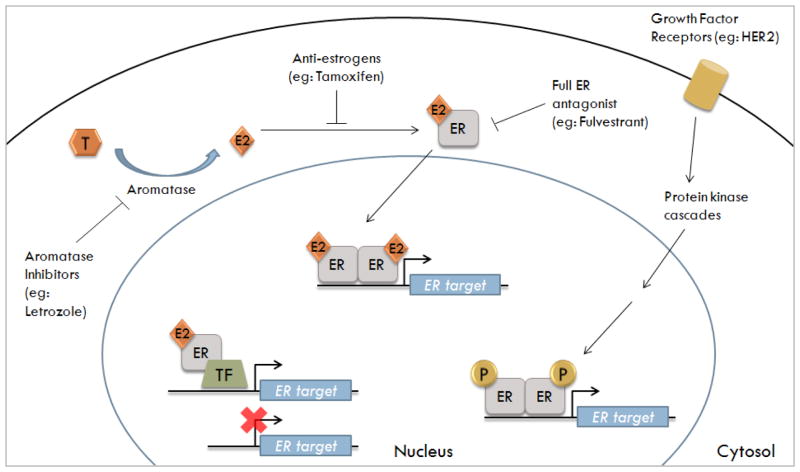
Figure 1. Three mechanisms of ER genomic signaling and the inhibition by antiestrogens and aromatase inhibitors.
Testosterone (T) is converted into estrogen (E2) by the enzyme aromatase. Normal breast cells synthesize E2 which has autocrine and paracrine functions. Breast cancer cells express higher levels of aromatase; thus, their E2 concentration is higher than normal breast cell. Furthermore, ER-positive breast cells require E2 for growth and utilize certain genomic signaling pathways to transcribe ER-regulated genes. These pathways include: classical genomic (E2-ER complex binds to the ERE); ERE-independent genomic (E2-ER complex binds to transcription factor-TF-binding sites); and non-classical genomic (ER is phosphorylated in absence of E2 via kinase cascades).
2.3 ER Phosphorylation
The C-terminus transactivation function 2 (AF2) of ER is activated by ligand binding of E2 [23] while the N-terminus transactivation domain (AF1) is activated by phosphorylation at several residues. Mostly post-translational modifications occur in the N-terminus, upon ligand binding, and upon ligand-independent growth factor signaling pathways [4, 24, 25]. Of the 14 serine residues in the N-terminus, several have been researched extensively due to their phosphorylation abilities. Serines 104, 106, 118, and 167 when mutated to alanine decrease ER transcription. Most notably, S167 is phosphorylated by PI3K/AKT [21] and S118 is phosphorylated by the Ras-MAPK signaling cascades [22], which are both important for the activation of ER and mediate ER binding to co-activators. MAPK activated S118 phosphorylation mediates SRC3 binding [26], and increases hypersensitivity to E2 [27]. S118 phosphorylation can lead to ligand-independent activation of ER; thus, leading to hormone-independent tumors [22, 28, 29]. In the clinical setting, many researchers have reported that S118 phosphorylation leads to good prognosis/response/relapse free survival (RFS) to hormonal therapy, specifically in letrozole, tamoxifen and AI/tamoxifen neoadjuvant therapy, respectively [30–32]. However, even though there are many conflicting outcomes, many report that S118 phosphorylation can be used as a predictive marker, especially in tamoxifen therapy [33–35]. Increased S118 phosphorylation has been found in AI-resistant breast cancer cells [36]. Moreover, AKT-stimulated S167 phosphorylation can also mediate ER binding to co-activator SRC3 in the presence of E2; thus, increase ER transcription [26, 28, 37]. One report correlated AKT phosphorylation of S167 to decreased survival of tamoxifen treated patients [38], while others report improved/prolonged survival of patients on endocrine therapy [39–41]. Furthermore, S305 phosphorylation has been also analyzed in clinical trials showing a negative RFS, poor clinical outcomes, and has been associated with aggressive tumors [42]. Clinical trial outcomes are summarized in Murphy et al. (2011) Table 5 [24].
2.4 ER Variants
In the non-genomic pathway, the ER is usually bound to the membrane proteins in regions known as lipid rafts. A recent study showed that a 36 kDa variant of ER is an important player in the non-genomic ER signaling, dimerizing with both ERα and ERβ [43]. Another ER variant 46 kDa can also be found on the cell membrane and plays a role in the endothelial nitric-oxide synthase (eNOS) pathway activation, causing the rapid release of nitric oxide [44]. These ERs interact with proteins of various kinase signaling pathways, such as PLC/PKC, Ras/Raf/MAPK, PI3K/AKT and cAMP/PKA [7]. G-protein coupled receptors have been reported to mediate signaling via the ER non-genomic pathway. For example: GPR30 has been identified to function as an ER [45, 46]; it is distributed between the cell and intracellular membranes [47, 48]; and it can activate EGFR [49]. These signaling events in the cytoplasm can crosstalk with the phosphorylation of other transcription factors; thus, indirectly regulating gene expression at the transcriptional level.
Computational search for the consensus ERE sequence has been attempted to identify potential ER binding sites, but the number of matches exceeds the actual binding sites found and resulted in many false positive predictions; thus, indicating that the presence of an ERE sequence does not dictate ER binding [50]. Due to the limited accuracy of computational predictions, the true ER binding sites need to be determined experimentally. There are other requirements for ER binding to the ERE, such as an open chromatin state or the presence of pioneer factors and co-regulators [51, 52]. ER can interact with other co-regulators and transcription factors such as AP1 and SP1 to activate or repress target genes via the “non-classical” pathway [53]. One such example is FOXA1, which is a pioneer factor that initiates binding to chromatin and recruits other factors. In addition, ER can interact with other transcription factors and act as a distal enhancer. In fact, a significant portion of ER binding sites have been found outside the promoter regions of the target genes [54]. Our genome-wide ER binding analysis in hormone resistant LTEDaro cells shows that ER is still able to bind to DNA in the absence of E2. There is also enrichment of ER binding sites close to transcriptional start sites, which is similar to the hormone responsive MCF-7aro cells treated with E2.
3. Aromatase
3.1 Aromatase and Aromatase Expression in the Breast
Aromatase is a key enzyme involved in the catalytic conversion adrenal androgens, such as testosterone and androstenedione, via three consecutive hydroxylation reactions, to aromatic estrogens (estradiol-E2 and estrone-E1, respectively) [55–57]. It belongs to the cytochrome p450 superfamily, which is characterized by the presence of a heme group. Aromatase is localized in the endoplasmic reticulum of estrogen-producing cells and it is a cytochrome P450 that requires NADPH-cytochrome P450 reductase (CPR) for catalysis. While the interaction between aromatase and CPR is essential for its catalytic activity, the structural basis of the electron transfer mechanisms between them is not completely understood. Our computer modeling studies have revealed that the FMN binding domain of CPR undergoes a structural rearrangement, allowing the proximal surface of aromatase to fit into the cleft between the FMN and FAD binding domains of CPR. K108, on the surface of aromatase, was found to be involved in its interaction with CPR [58].
In humans, there is only one aromatase protein, but its expression is tightly regulated. Aromatase protein is expressed mainly in the ovaries of premenopausal women; in the placenta of pregnant women; and in the adipose fibroblast cells of postmenopausal women. Furthermore, the specific cell type expressing aromatase in the breast has been demonstrated to be mesenchymal stromal (preadipocytes) and epithelial cells [59]. Compared to normal cells, some breast epithelial and stromal cancer cells show several fold greater aromatase mRNA and protein levels [60].
The human aromatase gene consists of 10 exons: untranslated exon Is (I.1, I.2, 2a, I.3, I.4, I.5, I.6, I.7, I.f, and PII) and translated exons II-X. The various exon Is are expressed in a tissue-specific manner and have a corresponding promoter located upstream which is regulated by different mechanisms [56]. Each 5′-exon I is spliced with the common 3′-coding exon II; therefore, producing the same translated aromatase protein. Studies conducted have shown exons I.3 and PII, which are cAMP/prostaglandin E2 (PGE2) regulated promoters, as the major exon Is responsible for aromatase expression in breast cancer tissues [56, 57, 61–64]. However, in normal breast stromal cells and adipose tissue, promoter I.4, which is a glucocorticoid regulated promoter, is the major promoter that drives aromatase expression [56, 57, 61]. Harada reported that exon I.4 switches to exon I.3 in tumor fibroblast with the addition of forskolin which mimics PGE2 [65]. Prostaglandins are a group of hormone-like lipids which are produced in many organs, and they act in paracrine and autocrine manner to affect their target cells. Furthermore, PGE2 synthesized in breast cancer cells induce the cAMP signaling pathway leading to a switch from transcriptional promoter I.4 to promoters II and I.3 [65–71]. Breast adipose tissues from breast cancer patients show an increase in aromatase expression through promoters II and I.3 [66], also high aromatase activity in the quadrant containing the tumor has been detected [66, 72, 73]. Therefore, elevated levels of aromatase expression lead to local synthesis of estrogen production in the breast tumor [65, 74–79].
3.2 Aromatase Deficiency in humans
Aromatase deficiency is an extremely rare disorder in humans. Only a small number of cases of women [80–82] and men [83–89] have been reported, majority of them are Caucasians. Affected women exhibit ambiguous genitalia at birth, elevated androgens and undetectable estrogens, primary amenorrhea, and failure of breast development at puberty. Affected men exhibit after puberty continuing linear growth, tall stature, unfused epiphyses, delayed bone age, eunuchoid skeletal proportions, genu valgum, decreased bone mineral density, overweight or obese, dyslipidemia, liver steatosis, insulin resistance, and impaired fertility.
A recent publication from our laboratory reports the identification of a man with clinical features of aromatase deficiency with novel heterozygous CYP19A1 mutations (Y81C and L451P) [90]. Three-dimensional modeling predicted that Y81C and L451P mutants disrupted the aromatase protein structure. Functional studies, on the basis of in vitro expression, showed that Y81C and L45P mutants significantly decreased the aromatase activity and its catalytic efficiency. Estrogen replacement in the patient increased bone mineral density, accelerated bone maturation, improved lipid profile and liver steatosis, and improved glucose levels but had no effect on insulin resistance.
3.3 Aromatase Inhibitors (AI)
Studies have confirmed the importance of aromatase in breast cancer proliferation leading to the development of AIs, which constituted a major breakthrough in breast cancer therapy (2). AIs [third generation which have great potency and specificity, and possess reduced toxicity: letrozole (LET), anastrozole (ANA), exemestane (EXE)] have been designed to combat ER-positive breast cancer. The structure of EXE (aromasin) mimics androgens; it can interact with ER and act as a weak agonist [91]; and it acts as a mechanism-based inhibitor that promotes aromatase degradation [92]. Due to its irreversible nature, EXE is a potent inhibitor with lengthy estrogen suppression [93]. On the other hand, non-steroidal inhibitors such as ANA (arimidex) and LET (femara) contain a triazole ring which binds non-covalently/reversibly to the heme iron group on the active site of the enzyme; thus, inhibiting the hydroxylation reactions in the steroidogenesis pathways. Research indicates that third generation aromatase inhibitors are highly effective without altering the other steroid biosynthesis pathways [94, 95].
The three-dimensional structure of androgen substrate-bound aromatase has been determined [96, 97]. Furthermore, extensive structure-function studies, through a combination of site-directed mutagenesis of the enzyme and inhibitor binding characterization with three AIs, have revealed that the AIs do not bind to the active site similarly. Our laboratory examined the aromatase–nonsteroidal inhibitor interaction by docking letrozole into the published aromatase structure using Glide Docking (Maestro, Schrödinger) software [58]. The distance between the heme of aromatase and the triazole functional group of letrozole in our model was 3.7 Å. Fifteen amino acids (I133, F134, F221, W224, A306, D309, T310, V313, V369, V370, V373, M374, L477, S478, and H480) were found within a 4 Å distance of the modeled letrozole structure. In our previous publications, we examined the roles of many of these active site residues through site-directed mutational studies [98–100]. We observed a decreased letrozole binding affinity to the D309A mutant. In contrast, the E302D and T310S mutants showed an increase in letrozole binding.
The published X-ray structure of aromatase indicates that the F221, W224 and M374 residues are located in the active site. Site-directed mutagenesis experiments have confirmed their importance in the binding of the androgen substrate as well as AIs, but these residues interact differently with steroidal inhibitors (exemestane) and non-steroidal inhibitors (letrozole and anastrozole). Furthermore, the residue W224 participates in the mechanism-based inhibition of exemestane, as time-dependent inhibition is eliminated with mutation on this residue. Therefore, W224, E302, D309 and S478 are important active site residues involved in the mechanism of exemestane’s inhibition of aromatase [58].
3.4 Antiestrogens and Clinical Trials with AIs
Antiestrogens are compounds that can antagonize the actions of estrogens in the treatment of ER-positive breast cancers. Tamoxifen (TAM) and fulvestrant (ICI 182780), the most widely used antiestrogens, have very different mechanisms of action (Figure 2). ICI is a selective estrogen receptor downregulator (SERD) that accelerates the degradation of ER. It is also a pure antiestrogen because it has no agonist activity [101]. On the other hand, TAM is a selective estrogen receptor modulator (SERM) that mainly acts as an ER antagonist to prevent the estrogen-mediated cell growth in breast cancer. However, in other tissues such as the bone and endometrium [102], or in breast cancer cells that have become resistant to TAM, the drug acts as a partial agonist of ER and may recruit coregulatory proteins to modulate some estrogen responsive genes [103–105].
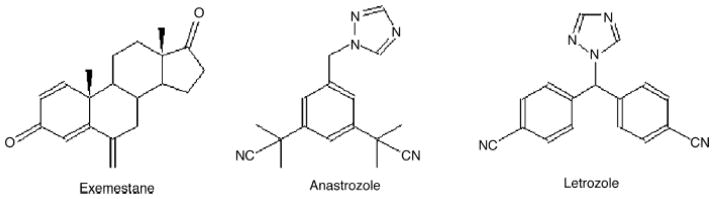
Figure 2. Structures of AIs.
From left to right: EXE, ANA, LET. EXE is a steroidal AI with a structure similar to E2. ANA and LET are non-steroidal AIs. LET has the highest potency out of the three AIs.
While many ER modulators have been developed, TAM is currently used for its high efficacy treatment of pre-menopausal women with ER-positive advanced breast cancer. Due to agonistic activities, which lower its efficacy, AIs have been developed to combat resistance to TAM therapy. The National Surgical Adjuvant Breast And Bowel Project (NSABP) results indicated that ER-positive pre- and post-menopausal women benefit from 5 years of adjuvant TAM therapy [106] with a reduction of 47% of new contralateral breast cancer [107]; however, an increase of endometrial cancer incidence [107, 108] and deep-vein thrombosis was reported [109]. Furthermore, the International Breast Cancer Prevention Study (IBIS)-I trial showed 20mg/day of TAM for 5 years led to reduced incidence of breast cancer [110], and the adverse thromboembolic effects reduced after cessation of treatment [111].
Since 2002, clinical trials have used AIs as first-line therapy to TAM (20mg/day) [112]. ANA at 1mg/day [113, 114] and LET at 2.5mg/day [115], and LET being the most potent AI that can inhibit >99% of aromatase activity [116]. EXE is used as adjuvant therapy for 2 years following 2–3 years of TAM therapy. Progression-free survival (PFS) and overall response rate (ORR) for EXE is greater than TAM [117]. Furthermore, data from the ANA, TAM, alone or in Combination (ATAC) clinical trial showed that AIs provide better disease-free survival (DFS) and prolonged time to regression (TTR) [118, 119]. The Breast International Group (BIG) 1–98 trial indicated that sequential treatment with TAM is not advantageous for DFS or breast cancer recurrence to LET therapy in early breast cancer cases [120, 121]. Also efficacy of switching from TAM, after 2–3 years, to ANA [122–125] or LET [126] has been shown to be beneficial.
Given the clear clinical benefits, AIs are gradually replacing TAM as the therapy of choice for eligible postmenopausal patients with hormone-dependent breast cancers. However, the use of AIs also exhibit adverse effects such as: loss of bone density, musculoskeletal pain and arthralgia [127]. Progression to disease following use of non-steroidal AIs has lead physicians to switch non-steroidal AIs to steroidal AI while achieving clinical benefit [128]. Lack of AI cross-resistance cannot be explained by drug efficacy as all AIs inhibit total body aromatase to a similar degree; however, the exact mechanism(s) responsible are not fully elucidated [129–131].
4. AI resistance
4.1 De Novo (Intrinsic) AI Resistance
De novo resistance refers to the lack of response to a drug upon initial treatment. Many potential mechanisms of endocrine resistance have been proposed, but there is no single explanation that can account for all cases. For example, lack of ER or PR expression is one way to explain de novo resistance, but the presence of ER and/or PR still fails to predict response to antiestrogens in some patients [132]. Altered expression of co-regulators may also explain the phenotype in some resistant cells [53]. Another study has shown that TAM resistance arises in about 30% of patients due to methylation of the ER promoter, leading to the loss of ER expression. Treatment with demethylating agents and histone deacetylase (HDAC) inhibitors reverses this transcriptional silencing and allow re-expression of ER which is responsive to TAM [133].
ER is expressed in about 75% of all breast cancers in postmenopausal women [134], but in clinical trials of LET, ANA, and TAM, a significant percentage of these patients had no clinical benefit, which means they exhibit de novo resistance [135]. For example, in breast cancer cells with high AIB1 and HER2 expression, TAM acts as a partial ER agonist and stimulates cell growth, which can be blocked by the EGFR inhibitor gefitinib [136]. Furthermore, over expression of HER2 or AKT in ER+ and aromatase+ MCF-7aro cells results in the resistance to the AI treatment [Cynthie paper]. In patients who do not exhibit de novo resistance, both TAM and AIs have proven to be very successful in controlling their cancer.
4.2 Acquired AI Resistance
However, a significant number of patients who initially respond will eventually acquire resistance to the drugs. Breast cancer cell lines that acquired resistance to AIs or TAM still expressed ER [137, 138]. This strongly suggests that the loss of ER is not the main mechanism of acquired resistance, and ER may still play an important role after resistance to anti-hormonal drugs has developed. This further demonstrates the need to understand endocrine resistance at the molecular level in order to devise new strategies to overcome this problem.
It has been reported that ER downregulation may play a role in endocrine resistance. The CUE domain–containing protein-2 (CUEDC2) protein was found to play a role in the downregulation of ER through the ubiquitin-proteasome pathway. This protein was found to have a strong inverse correlation with ER expression in a large breast cancer patient cohort. Patients with high CUEDC2 expression had a worse response to TAM treatment and also a higher chance of relapse. The role of CUEDC2 was further confirmed in cell line studies by overexpressing this protein in a breast cancer cell line, and the results show that CUEDC2 can inhibit the response to TAM treatment [139].
A closer look of the interactions between ER and the genome reveals an important role for pioneer factors and histone modifications, which both affect the accessibility of other transcription factors to the genome in the endocrine resistant phenotype. The NOTCH pathway was also found to be overactive in the endocrine resistant cells. One of the target genes of NOTCH, a transcription factor called PBX1, was shown to be required for growth in the endocrine therapy-resistant cells. Based on the NOTCH-PBX activity, a gene expression signature was found to have predictive value for the response to endocrine therapy [140].
Another proposed mechanism of AI resistance is the presence of other estrogenic compounds that are catalyzed by other enzymes besides aromatase. Sikora et al., reported that in breast cancer cells deprived of estrogen, the treatment of androgens caused the upregulation of steroidgenic enzymes that convert 5α-dihydrotestosterone into 5α-androstane-3β,17β-diol (3β Adiol), which acts as a weak estrogen to stimulate proliferation via the ER pathway[141]. Alternatively, estrogenic compounds such as synthetic estrogens, industrial pollutants, and phytoestrogens derived from exogenous sources may also contribute to AI resistance[142].
While several mechanisms have been proposed to explain the various phenomena observed in the AI-resistant cells, the key step is the activation of ER or change in ER behavior. However, the detailed mechanisms of how ER performs this function are just starting to be understood [143].
4.3 Models for AI resistance: Long Term Estrogen Deprived and Endocrine-Resistant Cell Lines
To study the mechanisms of endocrine resistance in breast cancer, a clearly defined cell culture model is needed. The MCF-7 cell line is well established for the study of hormone-dependent breast cancer. Wild-type MCF-7 cells do not express aromatase; therefore, we have developed an aromatase overexpressing MCF-7aro cell line to study the importance of aromatase in breast cancer [144]. There are two different types of AI resistance models, one induced by long term treatment of AI, and another induced by long term culture in the absence of estrogen (LTED). In the former case, the efficiency of AIs can be very high and thus the levels of estrogens are very low. In the latter case, the levels of estrogens are even lower and only traces of estrogen are present. In both cases, resistance is acquired through the adaptation to very low estrogen conditions, and the ER is still important for proliferation. In contrast, cells resistant to antiestrogens such as ICI do not require the ER for proliferation. For TAM resistance, the ER is also important, but the weak agonist activity of the TAM is still important for proliferation. Therefore, the AI resistance is different from TAM resistance because TAM requires the ER which is activated by ligand-independent mechanisms.
According to Jeng et al., the ability of the pure antiestrogen ICI to suppress LTED (Long Term Estrogen Deprived) cell growth indicates that in this AI resistance model, E2-independent cell growth is still dependent on ER [145]. In addition, Dowsett et al. reported that expression of ER is higher in LTED cells compared to the parental cell line [134]. They also demonstrated that ICI can inhibit LTED cell growth and the inhibition is reversed by addition of E2, suggesting that ER becomes hypersensitive to very low amounts of E2 [134]. This shows that LTED cell growth is fully or partially dependent on the ER-ERE pathway, and this model fits well with data from several groups.
Several other laboratories [29, 146–148] have independently derived LTED cell lines, which are created by culturing MCF-7 cells (without aromatase) in hormone-free media over a long period and used as endocrine-resistant models. In our lab, we derived the LTEDaro and four drug resistant cell lines from the MCF-7aro parental cell line: tamoxifen-resistant (TAMR), letrozole-resistant (LETR), anastrozole-resistant (ANAR), and exemestane-resistant (EXER) [36]. Furthermore, the LTLTCa is another letrozole-resistant cell line derived from MCF-7aro generated by the Brodie lab [149]. Microarray gene expression profiling of LTEDaro and AI-resistant lines in our lab showed very distinct gene expression profiles [36]. The LTEDaro and AI-resistant cells are able to proliferate with very low levels of E2 but the methods to generate them are different. LTEDaro cells are grown in hormone-free media, whereas the AI-resistant cells are grown in the presence of hormones but the production of E2 is inhibited by the various AIs. The studies from our laboratory indicate that the resistant mechanisms of these models are different, and LTEDaro represents a late stage of endocrine resistance [150].
5. Mutations and Modifications
5.1 ER Mutations and Amplification
ER-positive breast cancers have diverse clinical features. In order to understand the correlation with somatic alterations, whole genome sequencing was performed on tumor biopsies from patients in neoadjuvant AI treatment studies. The results show that eighteen genes were significantly mutated, and although phenotypes of ER-positive breast cancer can be associated with patterns of somatic mutations related to genes in tumor biology pathways, the frequency of such mutations are relatively rare [151].
Patient-derived xenograft (PDX) models have been created from patients with poor-prognosis and treatment-resistant tumors. These models were used to study the mutations in genomic sequence and its correlation with gene expression changes. There are several point mutations identified in the ESR1 (ER1) gene, as well as translocations that promote estrogen independent growth. This was confirmed in cell line studies expressing the ESR1/YAP1 chimeric protein or the ESR1-Y537S mutant. Both forms of ESR1 induced estrogen independence. The ER has been shown to undergo gene amplification in LTED cells [152].
5.2 Aromatase Gene Polymorphisms
Several aromatase gene polymorphisms have been reported to negatively or positively alter the expression of aromatase. Polymorphism of Val80 on exon 3 (rs700518) leads to a G to A base transition; thus, increasing the risk of AI-induced bone loss and enhanced bone resorption. When compared with healthy postmenopausal women, those with the A allele had lower bone mineral density (BMD) but no significant difference in estradiol levels compared to those with the G allele [153]. Furthermore, a polymorphism in the 3′UTR (rs10046), which leads to a T allele in the T/C base change, has been found to increase estrogen correlating to the tumor aromatase mRNA levels [154]. Homozygous carriers exhibit high BMD when treated with hormone replacement therapy [155]. Moreover, a SNP at exon I.2 (rs1062033) leading to a G to C base transversion exhibits an increase in aromatase expression; however, clinically, women with the CC allele exhibit low BMD [156]. Other polymorphisms such as in the 3′UTR (rs4646) effects disease-free survival with LET therapy [157, 158]; the (TTTA)n polymorphism reduces AI-induced arthralgia in the 1/8-repeat allele carriers [159]; and 5′region (rs6493497 and rs7176005) polymorphisms show differences in AI inhibition of aromatase activity [160].
5.3 Post-transcriptional and Post-translational Modifications of the Aromatase Enzyme
Estrogen (17β-estradiol), through c-Src kinase, phosphorylates tyrosine and increases aromatase enzymatic activity and its protein levels in an autocrine manner. Via site-directed mutagenesis and in vitro kinase assay, it was shown that tyrosine 361 to be the phosphorylation site of c-Src kinase, and inhibition of this kinase would lead to reversal of estrogen induced tyrosine phosphorylation [161, 162]. Furthermore, the PI3K/Akt pathway impairs tyrosine phosphatase (PTP1B) from dephosphorylating aromatase; thus, sustaining the activity of aromatase [163]. This regulation of aromatase activity by estrogen was also seen in ER-negative cell line (SKBR3), ectopically expressing ERα, and in a high aromatase expressing cell line (R2C). Estrogen Treatment with ER antagonists (TAM or ICI), abrogates estrogen’s influence on aromatase phosphorylation [161]. Moreover, since ERα and growth factor signaling pathways interact and estrogen has been shown to activate IGF-1R and EGFR, it was reported that estrogen upregulates aromatase expression via these interactions as well [162, 164]; while, Catalano et al. showed that ERα, EGFR, c-Src and aromatase form a complex to increase aromatase activity and its tyrosine phosphorylation [161]. Furthermore, phosphorylation/dephosphorylation is an important post-translational regulator of aromatase. For example: phosphorylation of aromatase at Ser118 contributes to enzymatic stability [165]. Also, growth factors are a major contributor of aromatase activity in ER+ breast cancer. IGF-1 activates IGF-1R, PI3K/Akt and MAPK signaling pathways which increase aromatase activity in breast cancer cells [166].
6. Conclusions
This comprehensive review covers the two important players in endocrine therapy: aromatase and ER. ER-positive breast cancer requires the aromatase enzyme for the conversion of androgen to estrogen/estradiol; thus, initiating the ER genomic signaling pathways. Moreover, the non-genomic ER signaling pathways utilize kinase signaling and ER co-regulators to activate ER independent of the ligand. Thus, the latest endocrine therapies involve AIs and ER antagonists to combat post and pre-menopausal ER-positive breast cancer, respectively. During treatment, either de novo or acquired resistance is seen with many patients. With de novo resistance, due to reduced influence of ER activity in these types of tumors, there is no response to a drug upon initial treatment. Furthermore, some patients acquire resistance after initially responding to a drug treatment while still maintaining ER expression. This strongly suggests that ER may still play an important role after acquiring resistance to anti-hormonal drugs has developed. Many reasons exist for resistance such as ER variants; ER/aromatase mutations; post-transcriptional/translational modifications of aromatase; or non-genomic ER signaling pathways leading to ER activation. In order to develop target specific drugs, research still needs to elucidate the exact mechanisms of endocrine resistance. Currently, TAM, a successful ER antagonist for pre- and post-menopausal women, and AIs, which show greater efficacy than TAM for post-menopausal women, are the best drugs on the market to combat this disease. Putting aside the issue of resistance to endocrine therapies, AIs and ER antagonists are still the best drugs to treat ER-positive breast cancers.
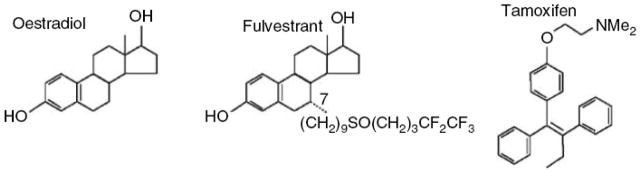
Figure 2.
Structures of E2 and Antiestrogens
From left to right: E2, ICI, and TAM. ICI is a full ER antagonist and causes the degradation of ER. TAM is a competitive inhibitor of E2 with partial agonist activity.
Highlights.
Aromatrase and estrogen receptor (ER) play important roles in endocrine-responsive and – resistant breast cancers.
Steroidal and non-steroidal aromatase inhibitors (AIs) bind to aromatase differently.
ER mutations have been identified and suggested to have roles in endocrine resistance.
Phosphorylation of ER can result in ligand-independent activation of the receptor.
The ligand-independent activation of ER is critical for the development of endocrine resistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Li CI, Daling JR, Malone KE. Incidence of invasive breast cancer by hormone receptor status from 1992 to 1998. J Clin Oncol. 2003;21(1):28–34. doi: 10.1200/JCO.2003.03.088. [DOI] [PubMed] [Google Scholar]
- 2.Wong C, Chen S. The development, application and limitations of breast cancer cell lines to study tamoxifen and aromatase inhibitor resistance. The Journal of steroid biochemistry and molecular biology. 2012;131(3–5):83–92. doi: 10.1016/j.jsbmb.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan VC, O’Malley BW. Selective estrogen-receptor modulators and antihormonal resistance in breast cancer. J Clin Oncol. 2007;25(36):5815–5824. doi: 10.1200/JCO.2007.11.3886. [DOI] [PubMed] [Google Scholar]
- 4.Lannigan DA. Estrogen receptor phosphorylation. Steroids. 2003;68(1):1–9. doi: 10.1016/s0039-128x(02)00110-1. [DOI] [PubMed] [Google Scholar]
- 5.Jordan VC, Brodie AM. Development and evolution of therapies targeted to the estrogen receptor for the treatment and prevention of breast cancer. Steroids. 2007;72(1):7–25. doi: 10.1016/j.steroids.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powles TJ. Anti-oestrogenic prevention of breast cancer--the make or break point. Nature reviews Cancer. 2002;2(10):787–794. doi: 10.1038/nrc908. [DOI] [PubMed] [Google Scholar]
- 7.Marino M, Galluzzo P, Ascenzi P. Estrogen signaling multiple pathways to impact gene transcription. Current genomics. 2006;7(8):497–508. doi: 10.2174/138920206779315737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swaby RF, Jordan VC. Low-dose estrogen therapy to reverse acquired antihormonal resistance in the treatment of breast cancer. Clinical breast cancer. 2008;8(2):124–133. doi: 10.3816/CBC.2008.n.012. [DOI] [PubMed] [Google Scholar]
- 9.Urruticoechea A. The oestrogen-dependent biology of breast cancer. Sensitivity and resistance to aromatase inhibitors revisited: a molecular perspective. Clin Transl Oncol. 2007;9(12):752–759. doi: 10.1007/s12094-007-0136-y. [DOI] [PubMed] [Google Scholar]
- 10.Cowley SM, Hoare S, Mosselman S, Parker MG. Estrogen receptors alpha and beta form heterodimers on DNA. The Journal of biological chemistry. 1997;272(32):19858–19862. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- 11.Pace P, Taylor J, Suntharalingam S, Coombes RC, Ali S. Human estrogen receptor beta binds DNA in a manner similar to and dimerizes with estrogen receptor alpha. The Journal of biological chemistry. 1997;272(41):25832–25838. doi: 10.1074/jbc.272.41.25832. [DOI] [PubMed] [Google Scholar]
- 12.Kuiper GG, Carlsson B, Grandien K, Enmark E, Haggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997;138(3):863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- 13.Murphy LC, Leygue E. The role of estrogen receptor-β in breast cancer. Seminars in reproductive medicine. 2012:05. doi: 10.1055/s-0031-1299592. [DOI] [PubMed] [Google Scholar]
- 14.Leung YK, Lee MT, Lam HM, Tarapore P, Ho SM. Estrogen receptor-beta and breast cancer: translating biology into clinical practice. Steroids. 2012;77(7):727–737. doi: 10.1016/j.steroids.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Molecular biology of the cell. 2005;16(1):231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evinger AJ, 3rd, Levin ER. Requirements for estrogen receptor alpha membrane localization and function. Steroids. 2005;70(5–7):361–363. doi: 10.1016/j.steroids.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 17.Marino M, Ascenzi P, Acconcia F. S-palmitoylation modulates estrogen receptor alpha localization and functions. Steroids. 2006;71(4):298–303. doi: 10.1016/j.steroids.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 18.Htun H, Holth LT, Walker D, Davie JR, Hager GL. Direct visualization of the human estrogen receptor alpha reveals a role for ligand in the nuclear distribution of the receptor. Molecular biology of the cell. 1999;10(2):471–486. doi: 10.1091/mbc.10.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pick E, Kluger Y, Giltnane JM, Moeder C, Camp RL, Rimm DL, Kluger HM. High HSP90 expression is associated with decreased survival in breast cancer. Cancer research. 2007;67(7):2932–2937. doi: 10.1158/0008-5472.CAN-06-4511. [DOI] [PubMed] [Google Scholar]
- 20.Bunone G, Briand PA, Miksicek RJ, Picard D. Activation of the unliganded estrogen receptor by EGF involves the MAP kinase pathway and direct phosphorylation. The EMBO journal. 1996;15(9):2174–2183. [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. The Journal of biological chemistry. 2001;276(13):9817–9824. doi: 10.1074/jbc.M010840200. [DOI] [PubMed] [Google Scholar]
- 22.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, Metzger D, Chambon P. Activation of the estrogen receptor through phosphorylation by mitogen-activated protein kinase. Science. 1995;270(5241):1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 23.Kumar V, Green S, Stack G, Berry M, Jin JR, Chambon P. Functional domains of the human estrogen receptor. Cell. 1987;51(6):941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- 24.Murphy LC, Seekallu SV, Watson PH. Clinical significance of estrogen receptor phosphorylation. Endocrine-related cancer. 2011;18(1):R1–14. doi: 10.1677/ERC-10-0070. [DOI] [PubMed] [Google Scholar]
- 25.Ward RD, Weigel NL. Steroid receptor phosphorylation: Assigning function to site-specific phosphorylation. BioFactors. 2009;35(6):528–536. doi: 10.1002/biof.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Likhite VS, Stossi F, Kim K, Katzenellenbogen BS, Katzenellenbogen JA. Kinase-specific phosphorylation of the estrogen receptor changes receptor interactions with ligand, deoxyribonucleic acid, and coregulators associated with alterations in estrogen and tamoxifen activity. Molecular endocrinology. 2006;20(12):3120–3132. doi: 10.1210/me.2006-0068. [DOI] [PubMed] [Google Scholar]
- 27.Vendrell JA, Bieche I, Desmetz C, Badia E, Tozlu S, Nguyen C, Nicolas JC, Lidereau R, Cohen PA. Molecular changes associated with the agonist activity of hydroxy-tamoxifen and the hyper-response to estradiol in hydroxy-tamoxifen-resistant breast cancer cell lines. Endocrine-related cancer. 2005;12(1):75–92. doi: 10.1677/erc.1.00899. [DOI] [PubMed] [Google Scholar]
- 28.Joel PB, Traish AM, Lannigan DA. Estradiol-induced phosphorylation of serine 118 in the estrogen receptor is independent of p42/p44 mitogen-activated protein kinase. The Journal of biological chemistry. 1998;273(21):13317–13323. doi: 10.1074/jbc.273.21.13317. [DOI] [PubMed] [Google Scholar]
- 29.Martin LA, Farmer I, Johnston SR, Ali S, Dowsett M. Elevated ERK1/ERK2/estrogen receptor cross-talk enhances estrogen-mediated signaling during long-term estrogen deprivation. Endocrine-related cancer. 2005;12(Suppl 1):S75–84. doi: 10.1677/erc.1.01023. [DOI] [PubMed] [Google Scholar]
- 30.Generali D, Buffa FM, Berruti A, Brizzi MP, Campo L, Bonardi S, Bersiga A, Allevi G, Milani M, Aguggini S, Papotti M, Dogliotti L, Bottini A, Harris AL, Fox SB. Phosphorylated ERalpha, HIF-1alpha, and MAPK signaling as predictors of primary endocrine treatment response and resistance in patients with breast cancer. J Clin Oncol. 2009;27(2):227–234. doi: 10.1200/JCO.2007.13.7083. [DOI] [PubMed] [Google Scholar]
- 31.Kok M, Holm-Wigerup C, Hauptmann M, Michalides R, Stal O, Linn S, Landberg G. Estrogen receptor-alpha phosphorylation at serine-118 and tamoxifen response in breast cancer. Journal of the National Cancer Institute. 2009;101(24):1725–1729. doi: 10.1093/jnci/djp412. [DOI] [PubMed] [Google Scholar]
- 32.Zoubir M, Mathieu MC, Mazouni C, Liedtke C, Corley L, Geha S, Bouaziz J, Spielmann M, Drusche F, Symmans WF, Delaloge S, Andre F. Modulation of ER phosphorylation on serine 118 by endocrine therapy: a new surrogate marker for efficacy. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2008;19(8):1402–1406. doi: 10.1093/annonc/mdn151. [DOI] [PubMed] [Google Scholar]
- 33.Bergqvist J, Elmberger G, Ohd J, Linderholm B, Bjohle J, Hellborg H, Nordgren H, Borg AL, Skoog L, Bergh J. Activated ERK1/2 and phosphorylated oestrogen receptor alpha are associated with improved breast cancer survival in women treated with tamoxifen. Eur J Cancer. 2006;42(8):1104–1112. doi: 10.1016/j.ejca.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 34.Murphy LC, Niu Y, Snell L, Watson P. Phospho-serine-118 estrogen receptor-alpha expression is associated with better disease outcome in women treated with tamoxifen. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10(17):5902–5906. doi: 10.1158/1078-0432.CCR-04-0191. [DOI] [PubMed] [Google Scholar]
- 35.Weitsman GE, Li L, Skliris GP, Davie JR, Ung K, Niu Y, Curtis-Snell L, Tomes L, Watson PH, Murphy LC. Estrogen receptor-alpha phosphorylated at Ser118 is present at the promoters of estrogen-regulated genes and is not altered due to HER-2 overexpression. Cancer research. 2006;66(20):10162–10170. doi: 10.1158/0008-5472.CAN-05-4111. [DOI] [PubMed] [Google Scholar]
- 36.Masri S, Phung S, Wang X, Wu X, Yuan YC, Wagman L, Chen S. Genome-wide analysis of aromatase inhibitor-resistant, tamoxifen-resistant, and long-term estrogen-deprived cells reveals a role for estrogen receptor. Cancer research. 2008;68(12):4910–4918. doi: 10.1158/0008-5472.CAN-08-0303. [DOI] [PubMed] [Google Scholar]
- 37.Riggins RB, Schrecengost RS, Guerrero MS, Bouton AH. Pathways to tamoxifen resistance. Cancer letters. 2007;256(1):1–24. doi: 10.1016/j.canlet.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirkegaard T, Witton CJ, McGlynn LM, Tovey SM, Dunne B, Lyon A, Bartlett JM. AKT activation predicts outcome in breast cancer patients treated with tamoxifen. The Journal of pathology. 2005;207(2):139–146. doi: 10.1002/path.1829. [DOI] [PubMed] [Google Scholar]
- 39.Jiang J, Sarwar N, Peston D, Kulinskaya E, Shousha S, Coombes RC, Ali S. Phosphorylation of estrogen receptor-alpha at Ser167 is indicative of longer disease-free and overall survival in breast cancer patients. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(19):5769–5776. doi: 10.1158/1078-0432.CCR-07-0822. [DOI] [PubMed] [Google Scholar]
- 40.Yamashita H, Nishio M, Kobayashi S, Ando Y, Sugiura H, Zhang Z, Hamaguchi M, Mita K, Fujii Y, Iwase H. Phosphorylation of estrogen receptor alpha serine 167 is predictive of response to endocrine therapy and increases postrelapse survival in metastatic breast cancer. Breast cancer research : BCR. 2005;7(5):R753–764. doi: 10.1186/bcr1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamashita H, Nishio M, Toyama T, Sugiura H, Kondo N, Kobayashi S, Fujii Y, Iwase H. Low phosphorylation of estrogen receptor alpha (ERalpha) serine 118 and high phosphorylation of ERalpha serine 167 improve survival in ER-positive breast cancer. Endocrine-related cancer. 2008;15(3):755–763. doi: 10.1677/ERC-08-0078. [DOI] [PubMed] [Google Scholar]
- 42.Holm C, Kok M, Michalides R, Fles R, Koornstra RH, Wesseling J, Hauptmann M, Neefjes J, Peterse JL, Stal O, Landberg G, Linn SC. Phosphorylation of the oestrogen receptor alpha at serine 305 and prediction of tamoxifen resistance in breast cancer. The Journal of pathology. 2009;217(3):372–379. doi: 10.1002/path.2455. [DOI] [PubMed] [Google Scholar]
- 43.Chaudhri RA, Schwartz N, Elbaradie K, Schwartz Z, Boyan BD. Role of ERalpha36 in membrane-associated signaling by estrogen. Steroids. 2014;81:74–80. doi: 10.1016/j.steroids.2013.10.020. [DOI] [PubMed] [Google Scholar]
- 44.Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(8):4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Filardo EJ, Thomas P. Minireview: G protein-coupled estrogen receptor-1, GPER-1: its mechanism of action and role in female reproductive cancer, renal and vascular physiology. Endocrinology. 2012;153(7):2953–2962. doi: 10.1210/en.2012-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146(2):624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 47.Cheng SB, Graeber CT, Quinn JA, Filardo EJ. Retrograde transport of the transmembrane estrogen receptor, G-protein-coupled-receptor-30 (GPR30/GPER) from the plasma membrane towards the nucleus. Steroids. 2011;76(9):892–896. doi: 10.1016/j.steroids.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 48.Sanden C, Broselid S, Cornmark L, Andersson K, Daszkiewicz-Nilsson J, Martensson UE, Olde B, Leeb-Lundberg LM. G protein-coupled estrogen receptor 1/G protein-coupled receptor 30 localizes in the plasma membrane and traffics intracellularly on cytokeratin intermediate filaments. Molecular pharmacology. 2011;79(3):400–410. doi: 10.1124/mol.110.069500. [DOI] [PubMed] [Google Scholar]
- 49.Filardo EJ, Quinn JA, Sabo E. Association of the membrane estrogen receptor, GPR30, with breast tumor metastasis and transactivation of the epidermal growth factor receptor. Steroids. 2008;73(9–10):870–873. doi: 10.1016/j.steroids.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 50.Jin VX, Leu YW, Liyanarachchi S, Sun H, Fan M, Nephew KP, Huang TH, Davuluri RV. Identifying estrogen receptor alpha target genes using integrated computational genomics and chromatin immunoprecipitation microarray. Nucleic acids research. 2004;32(22):6627–6635. doi: 10.1093/nar/gkh1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manavathi B, Samanthapudi VS, Gajulapalli VN. Estrogen receptor coregulators and pioneer factors: the orchestrators of mammary gland cell fate and development. Frontiers in cell and developmental biology. 2014;2:34. doi: 10.3389/fcell.2014.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jozwik KM, Carroll JS. Pioneer factors in hormone-dependent cancers. Nature reviews. Cancer. 2012;12(6):381–385. doi: 10.1038/nrc3263. [DOI] [PubMed] [Google Scholar]
- 53.Normanno N, Di Maio M, De Maio E, De Luca A, de Matteis A, Giordano A, Perrone F NC-NBC Group. Mechanisms of endocrine resistance and novel therapeutic strategies in breast cancer. Endocrine-related cancer. 2005;12(4):721–747. doi: 10.1677/erc.1.00857. [DOI] [PubMed] [Google Scholar]
- 54.Lupien M, Eeckhoute J, Meyer CA, Wang Q, Zhang Y, Li W, Carroll JS, Liu XS, Brown M. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell. 2008;132(6):958–970. doi: 10.1016/j.cell.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Subramanian A, Salhab M, Mokbel K. Oestrogen producing enzymes and mammary carcinogenesis: a review. Breast cancer research and treatment. 2008;111(2):191–202. doi: 10.1007/s10549-007-9788-0. [DOI] [PubMed] [Google Scholar]
- 56.Chen S. Aromatase and Breast Cancer. Frontiers in Bioscience. 1998;3:922–933. doi: 10.2741/a333. [DOI] [PubMed] [Google Scholar]
- 57.Chen D, Reierstad S, Lu M, Lin Z, Ishikawa H, Bulun SE. Regulation of breast cancer-associated aromatase promoters. Cancer letters. 2009;273(1):15–27. doi: 10.1016/j.canlet.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 58.Hong Y, Rashid R, Chen S. Binding features of steroidal and nonsteroidal inhibitors. Steroids. 2011;76(8):802–806. doi: 10.1016/j.steroids.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miki Y, Suzuki T, Tazawa C, Yamaguchi Y, Kitada K, Honma S, Moriya T, Hirakawa H, Evans DB, Hayashi S, Ohuchi N, Sasano H. Aromatase localization in human breast cancer tissues: possible interactions between intratumoral stromal and parenchymal cells. Cancer research. 2007;67(8):3945–3954. doi: 10.1158/0008-5472.CAN-06-3105. [DOI] [PubMed] [Google Scholar]
- 60.Lu Q, Nakmura J, Savinov A, Yue W, Weisz J, Dabbs DJ, Wolz G, Brodie A. Expression of aromatase protein and messenger ribonucleic acid in tumor epithelial cells and evidence of functional significance of locally produced estrogen in human breast cancers. Endocrinology. 1996;137(7):3061–3068. doi: 10.1210/endo.137.7.8770932. [DOI] [PubMed] [Google Scholar]
- 61.Bulun SE, Sebastian S, Takayama K, Suzuki T, Sasano H, Shozu M. The human CYP19 (aromatase P450) gene: update on physiologic roles and genomic organization of promoters. The Journal of steroid biochemistry and molecular biology. 2003;86(3–5):219–224. doi: 10.1016/s0960-0760(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 62.Chen S, Zhou D, Okubo T, Kao YC, Yang C. Breast tumor aromatase: functional role and transcriptional regulation. Endocrine-related cancer. 1999;6(2):149–156. doi: 10.1677/erc.0.0060149. [DOI] [PubMed] [Google Scholar]
- 63.Zhao Y, Mendelson CR, Simpson ER. Characterization of the sequences of the human CYP19 (aromatase) gene that mediate regulation by glucocorticoids in adipose stromal cells and fetal hepatocytes. Molecular endocrinology. 1995;9(3):340–349. doi: 10.1210/mend.9.3.7776980. [DOI] [PubMed] [Google Scholar]
- 64.Zhao Y, Nichols JE, Bulun SE, Mendelson CR, Simpson ER. Aromatase P450 gene expression in human adipose tissue. Role of a Jak/STAT pathway in regulation of the adipose-specific promoter. The Journal of biological chemistry. 1995;270(27):16449–16457. doi: 10.1074/jbc.270.27.16449. [DOI] [PubMed] [Google Scholar]
- 65.Harada N. Aberrant expression of aromatase in breast cancer tissues. The Journal of steroid biochemistry and molecular biology. 1997;61(3–6):175–184. [PubMed] [Google Scholar]
- 66.Agarwal VR, Bulun SE, Leitch M, Rohrich R, Simpson ER. Use of alternative promoters to express the aromatase cytochrome P450 (CYP19) gene in breast adipose tissues of cancer-free and breast cancer patients. The Journal of clinical endocrinology and metabolism. 1996;81(11):3843–3849. doi: 10.1210/jcem.81.11.8923826. [DOI] [PubMed] [Google Scholar]
- 67.Irahara N, Miyoshi Y, Taguchi T, Tamaki Y, Noguchi S. Quantitative analysis of aromatase mRNA expression derived from various promoters (I.4, I.3, PII and I.7) and its association with expression of TNF-alpha, IL-6 and COX-2 mRNAs in human breast cancer. International journal of cancer. Journal international du cancer. 2006;118(8):1915–1921. doi: 10.1002/ijc.21562. [DOI] [PubMed] [Google Scholar]
- 68.Mahendroo MS, Mendelson CR, Simpson ER. Tissue-specific and hormonally controlled alternative promoters regulate aromatase cytochrome P450 gene expression in human adipose tissue. The Journal of biological chemistry. 1993;268(26):19463–19470. [PubMed] [Google Scholar]
- 69.Zhou D, Zhou C, Chen S. Gene regulation studies of aromatase expression in breast cancer and adipose stromal cells. The Journal of steroid biochemistry and molecular biology. 1997;61(3–6):273–280. [PubMed] [Google Scholar]
- 70.Pasqualini JR, Chetrite G, Blacker C, Feinstein MC, Delalonde L, Talbi M, Maloche C. Concentrations of estrone, estradiol, and estrone sulfate and evaluation of sulfatase and aromatase activities in pre- and postmenopausal breast cancer patients. The Journal of clinical endocrinology and metabolism. 1996;81(4):1460–1464. doi: 10.1210/jcem.81.4.8636351. [DOI] [PubMed] [Google Scholar]
- 71.Zhao Y, Agarwal VR, Mendelson CR, Simpson ER. Transcriptional regulation of CYP19 gene (aromatase) expression in adipose stromal cells in primary culture. The Journal of steroid biochemistry and molecular biology. 1997;61(3–6):203–210. doi: 10.1016/s0960-0760(97)80013-1. [DOI] [PubMed] [Google Scholar]
- 72.Bulun SE, Sharda G, Rink J, Sharma S, Simpson ER. Distribution of aromatase P450 transcripts and adipose fibroblasts in the human breast. The Journal of clinical endocrinology and metabolism. 1996;81(3):1273–1277. doi: 10.1210/jcem.81.3.8772611. [DOI] [PubMed] [Google Scholar]
- 73.O’Neill JS, Elton RA, Miller WR. Aromatase activity in adipose tissue from breast quadrants: a link with tumour site. British medical journal. 1988;296(6624):741–743. doi: 10.1136/bmj.296.6624.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.What Role Do Hormones Play in Breast Cancer Treatment? Treatment and Side Effects. 2008 [Google Scholar]
- 75.Bulun SE, Price TM, Aitken J, Mahendroo MS, Simpson ER. A link between breast cancer and local estrogen biosynthesis suggested by quantification of breast adipose tissue aromatase cytochrome P450 transcripts using competitive polymerase chain reaction after reverse transcription. The Journal of clinical endocrinology and metabolism. 1993;77(6):1622–1628. doi: 10.1210/jcem.77.6.8117355. [DOI] [PubMed] [Google Scholar]
- 76.James VH, McNeill JM, Lai LC, Newton CJ, Ghilchik MW, Reed MJ. Aromatase activity in normal breast and breast tumor tissues: in vivo and in vitro studies. Steroids. 1987;50(1–3):269–279. doi: 10.1016/0039-128x(83)90077-6. [DOI] [PubMed] [Google Scholar]
- 77.Miller WR, O’Neill J. The importance of local synthesis of estrogen within the breast. Steroids. 1987;50(4–6):537–548. doi: 10.1016/0039-128x(87)90037-7. [DOI] [PubMed] [Google Scholar]
- 78.Zhou C, Zhou D, Esteban J, Murai J, Siiteri PK, Wilczynski S, Chen S. Aromatase gene expression and its exon I usage in human breast tumors. Detection of aromatase messenger RNA by reverse transcription-polymerase chain reaction. The Journal of steroid biochemistry and molecular biology. 1996;59(2):163–171. doi: 10.1016/s0960-0760(96)00100-8. [DOI] [PubMed] [Google Scholar]
- 79.Miller WR. Aromatase activity in breast tissue. The Journal of steroid biochemistry and molecular biology. 1991;39(5B):783–790. doi: 10.1016/0960-0760(91)90026-2. [DOI] [PubMed] [Google Scholar]
- 80.Bouchoucha N, Samara-Boustani D, Pandey AV, Bony-Trifunovic H, Hofer G, Aigrain Y, Polak M, Fluck CE. Characterization of a novel CYP19A1 (aromatase) R192H mutation causing virilization of a 46, XX newborn, undervirilization of the 46, XY brother, but no virilization of the mother during pregnancies. Molecular and cellular endocrinology. 2014;390(1–2):8–17. doi: 10.1016/j.mce.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 81.Hauri-Hohl A, Meyer-Boni M, Lang-Muritano M, Hauri-Hohl M, Schoenle EJ, Biason-Lauber A. Aromatase deficiency owing to a functional variant in the placenta promoter and a novel missense mutation in the CYP19A1 gene. Clinical endocrinology. 2011;75(1):39–43. doi: 10.1111/j.1365-2265.2011.04012.x. [DOI] [PubMed] [Google Scholar]
- 82.Zirilli L, Rochira V, Diazzi C, Caffagni G, Carani C. Human models of aromatase deficiency. The Journal of steroid biochemistry and molecular biology. 2008;109(3–5):212–218. doi: 10.1016/j.jsbmb.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 83.Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpson ER. Effect of testosterone and estradiol in a man with aromatase deficiency. The New England journal of medicine. 1997;337(2):91–95. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 84.Deladoey J, Fluck C, Bex M, Yoshimura N, Harada N, Mullis PE. Aromatase deficiency caused by a novel P450arom gene mutation: impact of absent estrogen production on serum gonadotropin concentration in a boy. The Journal of clinical endocrinology and metabolism. 1999;84(11):4050–4054. doi: 10.1210/jcem.84.11.6135. [DOI] [PubMed] [Google Scholar]
- 85.Herrmann BL, Saller B, Janssen OE, Gocke P, Bockisch A, Sperling H, Mann K, Broecker M. Impact of estrogen replacement therapy in a male with congenital aromatase deficiency caused by a novel mutation in the CYP19 gene. The Journal of clinical endocrinology and metabolism. 2002;87(12):5476–5484. doi: 10.1210/jc.2002-020498. [DOI] [PubMed] [Google Scholar]
- 86.Lanfranco F, Zirilli L, Baldi M, Pignatti E, Corneli G, Ghigo E, Aimaretti G, Carani C, Rochira V. A novel mutation in the human aromatase gene: insights on the relationship among serum estradiol, longitudinal growth and bone mineral density in an adult man under estrogen replacement treatment. Bone. 2008;43(3):628–635. doi: 10.1016/j.bone.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 87.Maffei L, Murata Y, Rochira V, Tubert G, Aranda C, Vazquez M, Clyne CD, Davis S, Simpson ER, Carani C. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. The Journal of clinical endocrinology and metabolism. 2004;89(1):61–70. doi: 10.1210/jc.2003-030313. [DOI] [PubMed] [Google Scholar]
- 88.Maffei L, Rochira V, Zirilli L, Antunez P, Aranda C, Fabre B, Simone ML, Pignatti E, Simpson ER, Houssami S, Clyne CD, Carani C. A novel compound heterozygous mutation of the aromatase gene in an adult man: reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clinical endocrinology. 2007;67(2):218–224. doi: 10.1111/j.1365-2265.2007.02864.x. [DOI] [PubMed] [Google Scholar]
- 89.Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. The Journal of clinical endocrinology and metabolism. 1995;80(12):3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 90.Chen Z, Wang O, Nie M, Elison K, Zhou D, Li M, Jiang Y, Xia W, Meng X, Chen S, Xing X. Aromatase deficiency in a Chinese adult man caused by novel compound heterozygous CYP19A1 mutations: effects of estrogen replacement therapy on the bone, lipid, liver and glucose metabolism. Molecular and cellular endocrinology. 2015;399:32–42. doi: 10.1016/j.mce.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Masri S, Lui K, Phung S, Ye J, Zhou D, Wang X, Chen S. Characterization of the weak estrogen receptor alpha agonistic activity of exemestane. Breast cancer research and treatment. 2009;116(3):461–470. doi: 10.1007/s10549-008-0151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang X, Chen S. Aromatase destabilizer: novel action of exemestane, a food and drug administration-approved aromatase inhibitor. Cancer research. 2006;66(21):10281–10286. doi: 10.1158/0008-5472.CAN-06-2134. [DOI] [PubMed] [Google Scholar]
- 93.Evans TR, Di Salle E, Ornati G, Lassus M, Benedetti MS, Pianezzola E, Coombes RC. Phase I and endocrine study of exemestane (FCE 24304), a new aromatase inhibitor, in postmenopausal women. Cancer research. 1992;52(21):5933–5939. [PubMed] [Google Scholar]
- 94.Brueggemeier RW, Hackett JC, Diaz-Cruz ES. Aromatase inhibitors in the treatment of breast cancer. Endocrine reviews. 2005;26(3):331–345. doi: 10.1210/er.2004-0015. [DOI] [PubMed] [Google Scholar]
- 95.Masri SAL, Somlo G, Paz B, Chen S. Hormonal aspects of breast cancer development, progression and therapy. In: Ben-Baruch A, editor. Breast Cancer: From Pathogenesis to Potential Therapeutic Modalities. Research Signpost/Transworld Research Network; Kerala, India: 2009. pp. 177–210. [Google Scholar]
- 96.Ghosh D, Griswold J, Erman M, Pangborn W. X-ray structure of human aromatase reveals an androgen-specific active site. The Journal of steroid biochemistry and molecular biology. 2010;118(4–5):197–202. doi: 10.1016/j.jsbmb.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghosh D, Griswold J, Erman M, Pangborn W. Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature. 2009;457(7226):219–223. doi: 10.1038/nature07614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hong Y, Li H, Yuan YC, Chen S. Molecular characterization of aromatase. Annals of the New York Academy of Sciences. 2009;1155:112–120. doi: 10.1111/j.1749-6632.2009.03703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kao YC, Cam LL, Laughton CA, Zhou D, Chen S. Binding characteristics of seven inhibitors of human aromatase: a site-directed mutagenesis study. Cancer research. 1996;56(15):3451–3460. [PubMed] [Google Scholar]
- 100.Kao YC, Korzekwa KR, Laughton CA, Chen S. Evaluation of the mechanism of aromatase cytochrome P450. A site-directed mutagenesis study. European journal of biochemistry/FEBS. 2001;268(2):243–251. doi: 10.1046/j.1432-1033.2001.01886.x. [DOI] [PubMed] [Google Scholar]
- 101.Osborne CK, Wakeling A, Nicholson RI. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. British journal of cancer. 2004;90(Suppl 1):S2–6. doi: 10.1038/sj.bjc.6601629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cuzick J, Powles T, Veronesi U, Forbes J, Edwards R, Ashley S, Boyle P. Overview of the main outcomes in breast-cancer prevention trials. Lancet. 2003;361(9354):296–300. doi: 10.1016/S0140-6736(03)12342-2. [DOI] [PubMed] [Google Scholar]
- 103.Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engstrom O, Ohman L, Greene GL, Gustafsson JA, Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389(6652):753–758. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- 104.Tora L, White J, Brou C, Tasset D, Webster N, Scheer E, Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989;59(3):477–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- 105.Fleming FJ, Hill AD, McDermott EW, O’Higgins NJ, Young LS. Differential recruitment of coregulator proteins steroid receptor coactivator-1 and silencing mediator for retinoid and thyroid receptors to the estrogen receptor-estrogen response element by beta-estradiol and 4-hydroxytamoxifen in human breast cancer. The Journal of clinical endocrinology and metabolism. 2004;89(1):375–383. doi: 10.1210/jc.2003-031048. [DOI] [PubMed] [Google Scholar]
- 106.Stewart HJ, Prescott RJ, Forrest AP. Scottish adjuvant tamoxifen trial: a randomized study updated to 15 years. Journal of the National Cancer Institute. 2001;93(6):456–462. doi: 10.1093/jnci/93.6.456. [DOI] [PubMed] [Google Scholar]
- 107.G. Early Breast Cancer Trialists’ Collaborative, Tamoxifen for early breast cancer. The Cochrane database of systematic reviews. 2001;(1):CD000486. doi: 10.1002/14651858.CD000486. [DOI] [PubMed] [Google Scholar]
- 108.Lavie O, Barnett-Griness O, Narod SA, Rennert G. The risk of developing uterine sarcoma after tamoxifen use. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2008;18(2):352–356. doi: 10.1111/j.1525-1438.2007.01025.x. [DOI] [PubMed] [Google Scholar]
- 109.Baum M. Has tamoxifen had its day? Breast cancer research : BCR. 2002;4(6):213–217. doi: 10.1186/bcr536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cuzick J, Forbes J, Edwards R, Baum M, Cawthorn S, Coates A, Hamed A, Howell A, Powles T I investigators. First results from the International Breast Cancer Intervention Study (IBIS-I): a randomised prevention trial. Lancet. 2002;360(9336):817–824. doi: 10.1016/s0140-6736(02)09962-2. [DOI] [PubMed] [Google Scholar]
- 111.Cuzick J, Forbes JF, Sestak I, Cawthorn S, Hamed H, Holli K, Howell A International Breast Cancer Intervention Study, II. Long-term results of tamoxifen prophylaxis for breast cancer--96-month follow-up of the randomized IBIS-I trial. Journal of the National Cancer Institute. 2007;99(4):272–282. doi: 10.1093/jnci/djk049. [DOI] [PubMed] [Google Scholar]
- 112.Gltick S. Changing the gold standard in adjuvant therapy for breast cancer: from tamoxifen to aromatase inhibition. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2005;59(Suppl 2):S321–322. [PubMed] [Google Scholar]
- 113.Bonneterre J, Thurlimann B, Robertson JF, Krzakowski M, Mauriac L, Koralewski P, Vergote I, Webster A, Steinberg M, von Euler M. Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol. 2000;18(22):3748–3757. doi: 10.1200/JCO.2000.18.22.3748. [DOI] [PubMed] [Google Scholar]
- 114.Nabholtz JM, Buzdar A, Pollak M, Harwin W, Burton G, Mangalik A, Steinberg M, Webster A, von Euler M. Anastrozole is superior to tamoxifen as first-line therapy for advanced breast cancer in postmenopausal women: results of a North American multicenter randomized trial. Arimidex Study Group. J Clin Oncol. 2000;18(22):3758–3767. doi: 10.1200/JCO.2000.18.22.3758. [DOI] [PubMed] [Google Scholar]
- 115.Mouridsen H, Gershanovich M, Sun Y, Perez-Carrion R, Boni C, Monnier A, Apffelstaedt J, Smith R, Sleeboom HP, Janicke F, Pluzanska A, Dank M, Becquart D, Bapsy PP, Salminen E, Snyder R, Lassus M, Verbeek JA, Staffler B, Chaudri-Ross HA, Dugan M. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J Clin Oncol. 2001;19(10):2596–2606. doi: 10.1200/JCO.2001.19.10.2596. [DOI] [PubMed] [Google Scholar]
- 116.Dowsett M, Jones A, Johnston SR, Jacobs S, Trunet P, Smith IE. In vivo measurement of aromatase inhibition by letrozole (CGS 20267) in postmenopausal patients with breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 1995;1(12):1511–1515. [PubMed] [Google Scholar]
- 117.Paridaens RJ, Dirix LY, Beex LV, Nooij M, Cameron DA, Cufer T, Piccart MJ, Bogaerts J, Therasse P. Phase III study comparing exemestane with tamoxifen as first-line hormonal treatment of metastatic breast cancer in postmenopausal women: the European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J Clin Oncol. 2008;26(30):4883–4890. doi: 10.1200/JCO.2007.14.4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS, Group AT. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365(9453):60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 119.Cuzick J, Sestak I, Baum M, Buzdar A, Howell A, Dowsett M, Forbes JF AL investigators. Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 10-year analysis of the ATAC trial. The Lancet. Oncology. 2010;11(12):1135–1141. doi: 10.1016/S1470-2045(10)70257-6. [DOI] [PubMed] [Google Scholar]
- 120.Doughty JC. A review of the BIG results: the Breast International Group 1–98 trial analyses. Breast. 2008;17(Suppl 1):S9–S14. doi: 10.1016/S0960-9776(08)70003-1. [DOI] [PubMed] [Google Scholar]
- 121.Group BIGC, Mouridsen H, Giobbie-Hurder A, Goldhirsch A, Thurlimann B, Paridaens R, Smith I, Mauriac L, Forbes J, Price KN, Regan MM, Gelber RD, Coates AS. Letrozole therapy alone or in sequence with tamoxifen in women with breast cancer. The New England journal of medicine. 2009;361(8):766–776. doi: 10.1056/NEJMoa0810818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Boccardo F, Rubagotti A, Guglielmini P, Fini A, Paladini G, Mesiti M, Rinaldini M, Scali S, Porpiglia M, Benedetto C, Restuccia N, Buzzi F, Franchi R, Massidda B, Distante V, Amadori D, Sismondi P. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer. Updated results of the Italian tamoxifen anastrozole (ITA) trial. Annals of oncology : official journal of the European Society for Medical Oncology/ESMO. 2006;17(Suppl 7):vii10–14. doi: 10.1093/annonc/mdl941. [DOI] [PubMed] [Google Scholar]
- 123.Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C, Hilfrich J, Kwasny W, Menzel C, Samonigg H, Seifert M, Gademann G, Kaufmann M, Wolfgang J, Abcsg G. the Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet. 2005;366(9484):455–462. doi: 10.1016/S0140-6736(05)67059-6. [DOI] [PubMed] [Google Scholar]
- 124.Jonat W, Gnant M, Boccardo F, Kaufmann M, Rubagotti A, Zuna I, Greenwood M, Jakesz R. Effectiveness of switching from adjuvant tamoxifen to anastrozole in postmenopausal women with hormone-sensitive early-stage breast cancer: a meta-analysis. The Lancet. Oncology. 2006;7(12):991–996. doi: 10.1016/S1470-2045(06)70948-2. [DOI] [PubMed] [Google Scholar]
- 125.Kaufmann M, Jonat W, Hilfrich J, Eidtmann H, Gademann G, Zuna I, von Minckwitz G. Improved overall survival in postmenopausal women with early breast cancer after anastrozole initiated after treatment with tamoxifen compared with continued tamoxifen: the ARNO 95 Study. J Clin Oncol. 2007;25(19):2664–2670. doi: 10.1200/JCO.2006.08.8054. [DOI] [PubMed] [Google Scholar]
- 126.Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T, Jones SE, Alvarez I, Bertelli G, Ortmann O, Coates AS, Bajetta E, Dodwell D, Coleman RE, Fallowfield LJ, Mickiewicz E, Andersen J, Lonning PE, Cocconi G, Stewart A, Stuart N, Snowdon CF, Carpentieri M, Massimini G, Bliss JM, van de Velde C S Intergroup Exemestane. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. The New England journal of medicine. 2004;350(11):1081–1092. doi: 10.1056/NEJMoa040331. [DOI] [PubMed] [Google Scholar]
- 127.Fabian CJ. The what, why and how of aromatase inhibitors: hormonal agents for treatment and prevention of breast cancer. International journal of clinical practice. 2007;61(12):2051–2063. doi: 10.1111/j.1742-1241.2007.01587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Miller WR, Bartlett J, Brodie AM, Brueggemeier RW, di Salle E, Lonning PE, Llombart A, Maass N, Maudelonde T, Sasano H, Goss PE. Aromatase inhibitors: are there differences between steroidal and nonsteroidal aromatase inhibitors and do they matter? The oncologist. 2008;13(8):829–837. doi: 10.1634/theoncologist.2008-0055. [DOI] [PubMed] [Google Scholar]
- 129.Lonning PE. Cross-resistance to different aromatase inhibitors in breast cancer treatment. Endocrine-related cancer. 1999;6(2):251–257. doi: 10.1677/erc.0.0060251. [DOI] [PubMed] [Google Scholar]
- 130.Lonning PE. Lack of complete cross-resistance between different aromatase inhibitors; a real finding in search for an explanation? Eur J Cancer. 2009;45(4):527–535. doi: 10.1016/j.ejca.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 131.Van Asten K, Neven P, Lintermans A, Wildiers H, Paridaens R. Aromatase inhibitors in the breast cancer clinic: focus on exemestane. Endocrine-related cancer. 2014;21(1):R31–49. doi: 10.1530/ERC-13-0269. [DOI] [PubMed] [Google Scholar]
- 132.Clarke R, Liu MC, Bouker KB, Gu Z, Lee RY, Zhu Y, Skaar TC, Gomez B, O’Brien K, Wang Y, Hilakivi-Clarke LA. Antiestrogen resistance in breast cancer and the role of estrogen receptor signaling. Oncogene. 2003;22(47):7316–7339. doi: 10.1038/sj.onc.1206937. [DOI] [PubMed] [Google Scholar]
- 133.Sharma D, Saxena NK, Davidson NE, Vertino PM. Restoration of tamoxifen sensitivity in estrogen receptor-negative breast cancer cells: tamoxifen-bound reactivated ER recruits distinctive corepressor complexes. Cancer research. 2006;66(12):6370–6378. doi: 10.1158/0008-5472.CAN-06-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dowsett M, Martin LA, Smith I, Johnston S. Mechanisms of resistance to aromatase inhibitors. The Journal of steroid biochemistry and molecular biology. 2005;95(1–5):167–172. doi: 10.1016/j.jsbmb.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 135.Anderson H, Bulun S, Smith I, Dowsett M. Predictors of response to aromatase inhibitors. The Journal of steroid biochemistry and molecular biology. 2007;106(1–5):49–54. doi: 10.1016/j.jsbmb.2007.05.024. [DOI] [PubMed] [Google Scholar]
- 136.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. Journal of the National Cancer Institute. 2004;96(12):926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 137.Schafer JM, Bentrem DJ, Takei H, Gajdos C, Badve S, Jordan VC. A mechanism of drug resistance to tamoxifen in breast cancer. The Journal of steroid biochemistry and molecular biology. 2002;83(1–5):75–83. doi: 10.1016/s0960-0760(02)00251-0. [DOI] [PubMed] [Google Scholar]
- 138.Long BJ, Jelovac D, Thiantanawat A, Brodie AM. The effect of second-line antiestrogen therapy on breast tumor growth after first-line treatment with the aromatase inhibitor letrozole: long-term studies using the intratumoral aromatase postmenopausal breast cancer model. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8(7):2378–2388. [PubMed] [Google Scholar]
- 139.Pan X, Zhou T, Tai YH, Wang C, Zhao J, Cao Y, Chen Y, Zhang PJ, Yu M, Zhen C, Mu R, Bai ZF, Li HY, Li AL, Liang B, Jian Z, Zhang WN, Man JH, Gao YF, Gong WL, Wei LX, Zhang XM. Elevated expression of CUEDC2 protein confers endocrine resistance in breast cancer. Nature medicine. 2011;17(6):708–714. doi: 10.1038/nm.2369. [DOI] [PubMed] [Google Scholar]
- 140.Magnani L, Stoeck A, Zhang X, Lanczky A, Mirabella AC, Wang TL, Gyorffy B, Lupien M. Genome-wide reprogramming of the chromatin landscape underlies endocrine therapy resistance in breast cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(16):E1490–1499. doi: 10.1073/pnas.1219992110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sikora MJ, Cordero KE, Larios JM, Johnson MD, Lippman ME, Rae JM. The androgen metabolite 5alpha-androstane-3beta,17beta-diol (3betaAdiol) induces breast cancer growth via estrogen receptor: implications for aromatase inhibitor resistance. Breast cancer research and treatment. 2009;115(2):289–296. doi: 10.1007/s10549-008-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Miller WR, Larionov AA. Understanding the mechanisms of aromatase inhibitor resistance. Breast cancer research : BCR. 2012;14(1):201. doi: 10.1186/bcr2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Carroll JS, Meyer CA, Song J, Li W, Geistlinger TR, Eeckhoute J, Brodsky AS, Keeton EK, Fertuck KC, Hall GF, Wang Q, Bekiranov S, Sementchenko V, Fox EA, Silver PA, Gingeras TR, Liu XS, Brown M. Genome-wide analysis of estrogen receptor binding sites. Nature genetics. 2006;38(11):1289–1297. doi: 10.1038/ng1901. [DOI] [PubMed] [Google Scholar]
- 144.Sun XZ, Zhou D, Chen S. Autocrine and paracrine actions of breast tumor aromatase. A three-dimensional cell culture study involving aromatase transfected MCF-7 and T-47D cells. The Journal of steroid biochemistry and molecular biology. 1997;63(1–3):29–36. doi: 10.1016/s0960-0760(97)00068-x. [DOI] [PubMed] [Google Scholar]
- 145.Jeng MH, Shupnik MA, Bender TP, Westin EH, Bandyopadhyay D, Kumar R, Masamura S, Santen RJ. Estrogen receptor expression and function in long-term estrogen-deprived human breast cancer cells. Endocrinology. 1998;139(10):4164–4174. doi: 10.1210/endo.139.10.6229. [DOI] [PubMed] [Google Scholar]
- 146.Santen RJ, Song RX, Zhang Z, Kumar R, Jeng MH, Masamura S, Lawrence J, Jr, MacMahon LP, Yue W, Berstein L. Adaptive hypersensitivity to estrogen: mechanisms and clinical relevance to aromatase inhibitor therapy in breast cancer treatment. The Journal of steroid biochemistry and molecular biology. 2005;95(1–5):155–165. doi: 10.1016/j.jsbmb.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 147.Nicholson RI, Staka C, Boyns F, Hutcheson IR, Gee JM. Growth factor-driven mechanisms associated with resistance to estrogen deprivation in breast cancer: new opportunities for therapy. Endocrine-related cancer. 2004;11(4):623–641. doi: 10.1677/erc.1.00778. [DOI] [PubMed] [Google Scholar]
- 148.Sabnis GJ, Jelovac D, Long B, Brodie A. The role of growth factor receptor pathways in human breast cancer cells adapted to long-term estrogen deprivation. Cancer research. 2005;65(9):3903–3910. doi: 10.1158/0008-5472.CAN-04-4092. [DOI] [PubMed] [Google Scholar]
- 149.Jelovac D, Sabnis G, Long BJ, Macedo L, Goloubeva OG, Brodie AM. Activation of mitogen-activated protein kinase in xenografts and cells during prolonged treatment with aromatase inhibitor letrozole. Cancer research. 2005;65(12):5380–5389. doi: 10.1158/0008-5472.CAN-04-4502. [DOI] [PubMed] [Google Scholar]
- 150.Masri S, Phung S, Wang X, Chen S. Molecular characterization of aromatase inhibitor-resistant, tamoxifen-resistant and LTEDaro cell lines. The Journal of steroid biochemistry and molecular biology. 2010;118(4–5):277–282. doi: 10.1016/j.jsbmb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ellis MJ, Ding L, Shen D, Luo J, Suman VJ, Wallis JW, Van Tine BA, Hoog J, Goiffon RJ, Goldstein TC, Ng S, Lin L, Crowder R, Snider J, Ballman K, Weber J, Chen K, Koboldt DC, Kandoth C, Schierding WS, McMichael JF, Miller CA, Lu C, Harris CC, McLellan MD, Wendl MC, DeSchryver K, Allred DC, Esserman L, Unzeitig G, Margenthaler J, Babiera GV, Marcom PK, Guenther JM, Leitch M, Hunt K, Olson J, Tao Y, Maher CA, Fulton LL, Fulton RS, Harrison M, Oberkfell B, Du F, Demeter R, Vickery TL, Elhammali A, Piwnica-Worms H, McDonald S, Watson M, Dooling DJ, Ota D, Chang LW, Bose R, Ley TJ, Piwnica-Worms D, Stuart JM, Wilson RK, Mardis ER. Whole-genome analysis informs breast cancer response to aromatase inhibition. Nature. 2012;486(7403):353–360. doi: 10.1038/nature11143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li S, Shen D, Shao J, Crowder R, Liu W, Prat A, He X, Liu S, Hoog J, Lu C, Ding L, Griffith OL, Miller C, Larson D, Fulton RS, Harrison M, Mooney T, McMichael JF, Luo J, Tao Y, Goncalves R, Schlosberg C, Hiken JF, Saied L, Sanchez C, Giuntoli T, Bumb C, Cooper C, Kitchens RT, Lin A, Phommaly C, Davies SR, Zhang J, Kavuri MS, McEachern D, Dong YY, Ma C, Pluard T, Naughton M, Bose R, Suresh R, McDowell R, Michel L, Aft R, Gillanders W, DeSchryver K, Wilson RK, Wang S, Mills GB, Gonzalez-Angulo A, Edwards JR, Maher C, Perou CM, Mardis ER, Ellis MJ. Endocrine-therapy-resistant ESR1 variants revealed by genomic characterization of breast-cancer-derived xenografts. Cell reports. 2013;4(6):1116–1130. doi: 10.1016/j.celrep.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Napoli N, Rastelli A, Ma C, Yarramaneni J, Vattikutti S, Moskowitz G, Giri T, Mueller C, Kulkarny V, Qualls C, Ellis M, Armamento-Villareal R. Genetic polymorphism at Val80 (rs700518) of the CYP19A1 gene is associated with aromatase inhibitor associated bone loss in women with ER + breast cancer. Bone. 2013;55(2):309–314. doi: 10.1016/j.bone.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kristensen VN, Harada N, Yoshimura N, Haraldsen E, Lonning PE, Erikstein B, Karesen R, Kristensen T, Borresen-Dale AL. Genetic variants of CYP19 (aromatase) and breast cancer risk. Oncogene. 2000;19(10):1329–1333. doi: 10.1038/sj.onc.1203425. [DOI] [PubMed] [Google Scholar]
- 155.Tofteng CL, Kindmark A, Brandstrom H, Abrahamsen B, Petersen S, Stiger F, Stilgren LS, Jensen JE, Vestergaard P, Langdahl BL, Mosekilde L, Danish S. Osteoporosis Prevention, Polymorphisms in the CYP19 and AR genes--relation to bone mass and longitudinal bone changes in postmenopausal women with or without hormone replacement therapy: The Danish Osteoporosis Prevention Study. Calcified tissue international. 2004;74(1):25–34. doi: 10.1007/s00223-002-2158-3. [DOI] [PubMed] [Google Scholar]
- 156.Riancho JA, Sanudo C, Valero C, Pipaon C, Olmos JM, Mijares V, Fernandez-Luna JL, Zarrabeitia MT. Association of the aromatase gene alleles with BMD: epidemiological and functional evidence. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009;24(10):1709–1718. doi: 10.1359/jbmr.090404. [DOI] [PubMed] [Google Scholar]
- 157.Colomer R, Monzo M, Tusquets I, Rifa J, Baena JM, Barnadas A, Calvo L, Carabantes F, Crespo C, Munoz M, Llombart A, Plazaola A, Artells R, Gilabert M, Lloveras B, Alba E. A single-nucleotide polymorphism in the aromatase gene is associated with the efficacy of the aromatase inhibitor letrozole in advanced breast carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14(3):811–816. doi: 10.1158/1078-0432.CCR-07-1923. [DOI] [PubMed] [Google Scholar]
- 158.Garcia-Casado Z, Guerrero-Zotano A, Llombart-Cussac A, Calatrava A, Fernandez-Serra A, Ruiz-Simon A, Gavila J, Climent MA, Almenar S, Cervera-Deval J, Campos J, Albaladejo CV, Llombart-Bosch A, Guillem V, Lopez-Guerrero JA. A polymorphism at the 3′-UTR region of the aromatase gene defines a subgroup of postmenopausal breast cancer patients with poor response to neoadjuvant letrozole. BMC cancer. 2010;10:36. doi: 10.1186/1471-2407-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mao JJ, Su HI, Feng R, Donelson ML, Aplenc R, Rebbeck TR, Stanczyk F, DeMichele A. Association of functional polymorphisms in CYP19A1 with aromatase inhibitor associated arthralgia in breast cancer survivors. Breast cancer research : BCR. 2011;13(1):R8. doi: 10.1186/bcr2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wang L, Ellsworth KA, Moon I, Pelleymounter LL, Eckloff BW, Martin YN, Fridley BL, Jenkins GD, Batzler A, Suman VJ, Ravi S, Dixon JM, Miller WR, Wieben ED, Buzdar A, Weinshilboum RM, Ingle JN. Functional genetic polymorphisms in the aromatase gene CYP19 vary the response of breast cancer patients to neoadjuvant therapy with aromatase inhibitors. Cancer research. 2010;70(1):319–328. doi: 10.1158/0008-5472.CAN-09-3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Catalano S, Barone I, Giordano C, Rizza P, Qi H, Gu G, Malivindi R, Bonofiglio D, Ando S. Rapid estradiol/ERalpha signaling enhances aromatase enzymatic activity in breast cancer cells. Molecular endocrinology. 2009;23(10):1634–1645. doi: 10.1210/me.2009-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer cell. 2004;6(3):209–214. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 163.Barone I, Giordano C, Malivindi R, Lanzino M, Rizza P, Casaburi I, Bonofiglio D, Catalano S, Ando S. Estrogens and PTP1B function in a novel pathway to regulate aromatase enzymatic activity in breast cancer cells. Endocrinology. 2012;153(11):5157–5166. doi: 10.1210/en.2012-1561. [DOI] [PubMed] [Google Scholar]
- 164.Kinoshita Y, Chen S. Induction of aromatase (CYP19) expression in breast cancer cells through a nongenomic action of estrogen receptor alpha. Cancer research. 2003;63(13):3546–3555. [PubMed] [Google Scholar]
- 165.Miller TW, Shin I, Kagawa N, Evans DB, Waterman MR, Arteaga CL. Aromatase is phosphorylated in situ at serine-118. The Journal of steroid biochemistry and molecular biology. 2008;112(1–3):95–101. doi: 10.1016/j.jsbmb.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Su B, Wong C, Hong Y, Chen S. Growth factor signaling enhances aromatase activity of breast cancer cells via post-transcriptional mechanisms. The Journal of steroid biochemistry and molecular biology. 2011;123(3–5):101–108. doi: 10.1016/j.jsbmb.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]