Abstract
Background
Traumatic brain injury (TBI) often leads to mood and cognitive complications, impacting functional recovery. Understanding neurobiological alterations common in post-TBI depression (PTD) and cognition may identify novel biomarkers for TBI complications. Brain-derived neurotrophic factor (BDNF) is a likely target based on evidence of reduced BDNF signaling in experimental TBI and depression models and its role in learning and memory.
Objective
Evaluate BDNF as a biomarker for PTD, cognitive impairment, and functional cognition in a prospective cohort with severe TBI.
Methods
Participants with TBI (n=113) were evaluated for PTD (Patient Health Questionnaire-9), cognitive impairment (cognitive composite score) and functional cognition (Functional Independence Measure–Cognition, FIM-Cog). BDNF levels were measured in cerebrospinal fluid (CSF) and serum 0–6 days post-injury and in serum at 6 and 12 months post-injury.
Results
Serum BDNF was reduced after TBI versus controls at all time-points. Acute serum BDNF positively correlated with Memory composites (6 months: r=0.43, p=0.019, n=30; 12 months: r=0.53, p=0.005, n=26) and FIM-Memory scores (6 months: r=0.35, p=0.019, n=45; 12 months: r=0.38, p=0.018, n=38). Acute serum BDNF negatively correlated with 12 month PHQ-9 scores (r=−0.38, p=0.044, n=29). At 12 months, chronic serum BDNF tended to be lower in participants with PTD (p=0.07) and correlated with PHQ-9 scores (r=−0.41, p=0.019, n=32).
Conclusions
Acute BDNF associations with memory recovery may implicate hippocampal damage/degeneration. Comparatively, BDNF associations with PTD status were not as strong as associations with PTD severity. Further investigation may delineate longitudinal BDNF patterns, and BDNF responsive treatments, reflecting mood and cognitive recovery following TBI.
Keywords: Traumatic Brain Injury, Depression, Cognitive Impairment, BDNF, Biomarker, Rehabilomics
Introduction
Traumatic brain injury (TBI) is increasingly recognized as a chronic medical condition with accompanying mood and cognitive complications. Impaired cognition and negative mood can adversely affect quality of life and influence return to work or school following TBI1–4. Identifying sensitive TBI biomarkers for these two major complications may be useful for evaluating therapeutic needs and to measure responses to pharmacological and/or behavioral interventions.
Post-TBI depression (PTD) is the most common neurobehavioral complication following TBI. Individuals with TBI are 10 times more likely than the general population to experience a depressive episode during their first year of recovery (53%5 compared to 6%6 per 12 months in the general population). The identification of an early biomarker for PTD development would aid in screening and early intervention, by identifying those at greatest risk of depression and with the greatest need for high frequency tracking and follow-up. Additionally, a biomarker that is reflective of PTD symptoms may be useful in monitoring and improving treatment effectiveness, by informing dose or timing of interventions.
In non-brain injured populations, individuals with depression often have comorbid cognitive impairment, likely due to common underlying pathology7. While individuals with depression report a varying number of cognitive difficulties8, memory impairment is consistently problematic9. Similar to individuals with depression, individuals with TBI commonly exhibit significant memory, executive function, and attentional difficulties after their injury10–13. We recently reported that individuals with PTD have no additional cognitive deficits compared to non-depressed individuals with TBI14. Yet, in this same study, individuals with PTD had significantly greater functional cognitive limitations than those without PTD, suggesting that, for individuals with TBI, remittance of depressive symptoms may lead to improved functional cognitive15, which may be a result of overlapping biological pathways or co-occurring symptomology. Depression and cognitive dysfunction have known effects on multidimensional outcomes16–19 relevant to the Rehabilomics framework20, strongly supporting an analysis of biomarker relationships to both conditions.
One potential biomarker that may be relevant to the neurobiological substrates involved with both mood and cognitive dysfunction post-TBI is brain-derived neurotrophic factor (BDNF). BDNF, a neurotrophin involved in neuronal survival and synaptic plasticity, has been implicated in depression21, memory and learning22, and TBI pathology23–25. In the hippocampus, BDNF affects synaptogenesis and maintenance, particularly through long-term potentiation associated with activity-dependent secretion of BDNF26. BDNF is also reportedly an underlying substrate for persistent long-term memory storage27,28.
Reduced BDNF is known to be associated with depression, and serum BDNF levels are a consistent marker for depressive symptomology in neurologically intact populations29. Serum BDNF levels are decreased in untreated depression but increase with antidepressant treatment, indicating the viability for BDNF serum levels as a biomarker of depressive symptoms29–31. In TBI, serum BDNF is acutely decreased, correlating with injury severity32. Hippocampal BDNF is chronically decreased in experimental TBI25, and hippocampal BDNF expression has been linked to spatial memory in experimental TBI studies23. Importantly, therapies that increase brain BDNF expression, like environmental enrichment25 and exercise23,33,34, show promise for mood and cognitive recovery post-TBI. This body of work suggests that BDNF may be a viable biomarker for long-term complications like depression and memory impairments that impact TBI recovery.
In this study, we assessed BDNF as a viable biomarker for PTD, cognitive impairments, and functional cognitive limitations (with specific attention to memory) in the first year following TBI. BDNF serum levels have never been examined in clinical TBI beyond the first week or in relation to PTD or cognition. Thus, BDNF serum levels may be a novel biomarker reflecting these complications and may help elucidate convergent pathways to target for treatment and symptom monitoring in cognitive and depressive symptomology post-TBI.
Methods
Participants
Participants in this study, approved by the University of Pittsburgh’s Institutional Review Board, were recruited while receiving care at inpatient and/or outpatient clinics within the University of Pittsburgh Medical Center (UPMC). All participants sustained a non-penetrating traumatic brain injury (TBI), with evidence of intracranial injury on Computed Tomography (CT). Exclusion criteria included: cardiac arrest or documented prolonged hypoxia or hypotension prior to admission, or penetrating TBI. All participants survived for at least one year post-injury and were a subset of a larger study investigating biomarkers and genetic factors related to individual recovery following TBI.
Healthy adult controls were also recruited for comparison in biomarker analysis. Criteria for enrollment of controls included: (i) 18–70 years old; and (ii) no current or past history of brain injury, neurological disease, psychiatric disease, or bleeding disorder. Current depressive symptoms were not collected for this control group. All healthy control participants were Caucasian and ranged from 18–60 years old, with 40% women. Women were excluded if they were pregnant, were taking oral contraceptives or hormone replacement therapy, or had any history of reproductive or endocrine disorder.
Injury severity was described using the best GCS obtained within the first 24 hours post-injury. Demographic information, including age, sex, and education, was collected by chart review as well as through participant or caregiver interviews. Similarly, antidepressant use at 6 and 12 months was extracted from both participant interview and chart review. An individual was considered taking an antidepressant if any of the following medications were prescribed within 1 month of the 6 or 12 month assessment: fluoxetine, citalopram, sertraline, escitalopram, paroxetine, trazodone, duloxetine, venlafaxine, bupropion, and mirtazapine. A pre-injury history of mood disorders, including depression, bipolar disorder, and anxiety, was established by self-report and chart review.
Cognitive Assessment
Participants’ functional cognitive limitations were assessed with the FIM-Cog35,36 conducted via interview37 at both 6 and 12 months. FIM-Cog has five component scales: expression, comprehension, social interaction, problem solving, and memory. Each scale is rated from one to seven, with a 5 or lower indicative of need for caregiver assistance. The sum of these five components was considered the FIM-Cog Score.
Previous studies in TBI38–42 have illustrated the use of composites or global test statistics to evaluate general cognitive performance and to improve study design consistency through aggregation of multiple tests43–45. Similar to previous studies14,41, cognitive impairment was measured at both 6 and 12 months post-injury using a cognitive composite score developed with a battery of 8 neuropsychological tests targeting 4 domains of cognition (attention, language fluency, memory, and executive function). Attention was measured using the Trails Making Test A46 and the combined score of the forward and backward digit span tests from the Wechsler Adult Intelligence Scale-R47. Memory was evaluated using the Rey-Osterreith Complex Figure Test48 and the Long Delay Free Recall Subsection of the California Verbal Learning Test49 (CVLT-II). Language Fluency domain scores were calculated using Controlled Oral Word Association50 Animals Subsection and the Delis-Kaplan Executive Function Systems Verbal Fluency Letter Fluency subsection. Lastly, executive function was measured using the Trails Making Test B46 and the Stroop Task51 Interference Sub-score. These tests were selected as representative measures for their associated domains. Raw scores from each test were converted into T-scores using appropriate metrics (i.e. education, age, sex, race) based on norms indicated by the test manufacturer. T-scores were averaged within each domain to create a domain sub-score. To calculate an overall cognitive composite score, participants had to complete at least one test in each of the four domains. Mean values across domain sub-scores were calculated for the overall cognitive composite score. Additionally, as T-scores have a mean of 50 and a standard deviation of 10, a t-score cut-off of 40 was used to delineate impaired (greater than 1 SD below the mean) vs. unimpaired performance in the TBI population. This cut-off is traditionally used in neuropsychological assessment to indicate the presence of mild cognitive impairment52.
Depressive Symptom Assessment
At 6 and 12 months, depressive symptoms were evaluated using the Patient Health Questionnaire-9 (PHQ9), a brief self-report symptom inventory based on the 9 DSM-IV diagnostic criteria for Major Depressive Disorder (MDD). The PHQ-9 asks participants to rate how often they have experienced symptoms of depression, on a scale between 0 (None) and 3 (Nearly Every Day), over a two-week period. Higher total scores (PHQ-9 Total) reflect greater number of and/or greater severity of depressive symptoms, with the maximum score being 27. Participants were grouped as “depressed” vs. “non-depressed” using the PHQ-9 questions as they map to DSM diagnostic criteria (previously described)53. For a categorization of depression (PTD), individuals responded positively to at least five symptom questions on the PHQ-9, with at least one pertaining to a cardinal symptom (anhedonia or depression). Compared to the Structured Clinical Interview for DSM Diagnosis (SCID)53, this method has been validated in populations with TBI showing a sensitivity of 93% and a specificity of 89%. Importantly, the PHQ-9 is reliably able to discriminate between chronic TBI symptoms and depression symptoms54.
BDNF Sample Cohort, Collection, and Processing
BDNF levels were measured in CSF and serum. When possible, CSF samples were collected via passive drainage up to twice daily for six days post-injury by an external ventricular drain (EVD) placed for clinical care. Serum was collected, via venipuncture, daily for the first six days and chronically at 6 months and 12 months post-injury via venipuncture. Acute CSF and serum samples were binned by day, and an average was calculated for each day post-injury for each participant.
CSF and serum samples were stored at −80° and were thawed immediately only for measurement. BDNF was assayed by an enzyme-linked immunosorbent assay (ELISA) kit (RayBiotech, Georgia, USA). Briefly, standards (of varying BDNF concentrations) and samples were pipetted onto a 96-well plate pre-coated with human BDNF antibody. Following shaking for 2.5 hours at room-temp, the plate was washed and then incubated with biotinylated BDNF antibody for 1 hour. HRP-conjugated streptavidin was then added for an incubation of 45 minutes. The addition of a tetramethylbenzidine substrate allows for a color reaction. Concentrations were calculated using mean absorbance of each sample at 460 nm, as it correlates to the amount of BDNF present in samples, as plotted on a per-assay standard curve. Samples were diluted within the range of the ELISA kit (no dilution for CSF, 1:250 for serum samples), with a sensitivity of 80 pg/ml, and a kit supplied intra-assay variation of <10%, and an inter-assay variation of <12%.
For biomarker analysis, participants (n=113) were assayed for BDNF levels in CSF or serum samples. BDNF levels have been shown to be moderated by racial background55. As the study population was 91.9% Caucasian, there was not enough power to detect racial differences between BDNF level associations and outcome in the current dataset. As a result, we limited BDNF level associations to Caucasians only, with the plan to collect further data that include additional racial groups. There were 97 participants with CSF samples (n=446) and 81 with acute serum samples (n=204). One acute serum sample measurement and 4 CSF sample measurements were removed as outliers (based on ±1.5*interquartile range). Chronic serum samples were collected at 6 and 12 months (±1 month) post-injury and averaged for each time-point for each participant. At 6 months, there were 54 participants with 112 samples. At 12 months, there were 36 participants with 36 samples. A subset of participants (n=44) had both acute and chronic samples.
For biomarker comparisons, healthy controls were recruited as a reference group (n=9). Control CSF was obtained via lumbar puncture for research purposes, and was not a part of a clinical work-up. Serum and CSF samples were obtained at ~7am to match samples from participants with TBI, avoiding possible confounding effects of diurnal variation.
Statistical Analysis
Analysis was conducted using Statistical Analysis Software (version 9.4; SAS Institute). Descriptive analysis included mean and standard deviation and/or median for continuous and ordinal variables such as age, GCS, and education. Frequencies were calculated for categorical variables such as sex and antidepressant use. Demographic and relevant clinical information was assessed for relationships with BDNF levels using Student’s t-tests or ANOVA to compare means. Non-parametric tests (Mann-Whitney and Kruskal-Wallis) were used when appropriate. Outliers were assessed using ±1.5 * interquartile range. Pearson’s or Spearman’s rho (r) correlations were used to assess relationships between two continuous variables.
Results
Specific cohort demographics are shown in Table 1. Overall, participants had a GCS (best in 24hrs) of 3–15 (mean GCS, 7.7 ± 2.8, median=7). Participants were aged 16–72 (mean age 33.1±13.3 years) and 17.7% of participants were women. In comparison, healthy controls were aged 18–60 (mean age 27.6±13.3 years) and 35.7% were women. At 6 months post-injury, 38.3% of participants with TBI had PTD, while 30.3% had PTD at 12 months. Participants with PTD tended to have a higher mean age compared to those without PTD (6 months, p=0.094; 12 months, p=0.098). At 6 months, women were more likely to be depressed than men (62.5% of women compared to 30.4% of men, p=0.018). Participants with premorbid mood disorders had higher PTD rates at both 6 (22.6 versus 5.6%, p=0.022) and 12 months (33.4 versus 8.3%, p=0.007) compared to those without premorbid mood disorders. At 6 months, 51.6% of participants with PTD were taking an antidepressant, compared to 26.4% of those with no PTD (p=0.021). There was no significant difference in antidepressant use between PTD groups at 12 months. Table 2 shows relationships between BDNF levels and demographic variables. Only sex was associated with BDNF levels at any time point. At 12 months, women had lower BDNF levels compared to men (p=0.009). Importantly, acute serum and CSF levels tended to be negatively correlated (r=−0.31, p=0.069, n=35).
Table 1.
Demographic description of study population.
| Total Population (n=113) |
6 Months | 12 Months | |||||
|---|---|---|---|---|---|---|---|
| None (n=54) |
PTD (n=31) |
p value | None (n=60) |
PTD (n=24) | p value | ||
| Age, mean±STD | 33.1±13.3 | 31.5±12.9 | 36.1±14.1 | 0.094 | 32.7±13.2 | 35.9±13.5 | 0.098 |
| GCS, median | 7 | 7 | 7.5 | 0.431 | 7 | 7.5 | 0.426 |
| Sex, # (%) Males | 93 (82.3) | 48 (88.9) | 21 (67.7) | 0.018 | 50 (83.3) | 16 (66.7) | 0.102 |
| Race, # (%) Caucasian | 106 (93.8) | 51 (94.4) | 29 (93.6) | 0.867 | 56 (93.3) | 21 (87.5) | 0.398 |
| Education, mean±STD | 12.8±1.7 | 12.9±1.9 | 12.4±1.5 | 0.261 | 12.9±1.7 | 12.1±1.5 | 0.137 |
| Premorbid Mood Disorders, # (%) | 3 (5.6) | 7 (22.6) | 0.022 | 5 (8.3) | 8 (33.4) | 0.007 | |
| Antidepressant Use, # (%) | 14 (26.4) | 16 (51.6) | 0.021 | 15 (25.4) | 10 (41.6) | 0.150 | |
STD, Standard Deviation; PTD, Post-TBI Depression; GCS, Glasgow Coma Scale
Table 2.
Demographic Associations with BDNF Levels.
| Acute CSF | Acute Serum | Chronic Serum | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | p | n | p | 6 Months | n | p | 12 Months | n | p | ||||
| Age, r | 0.059 | 57 | 0.659 | −0.089 | 49 | 0.543 | −0.168 | 48 | 0.252 | 0.026 | 35 | 0.880 | |
| GCS, r | −0.069 | 57 | 0.606 | −0.041 | 49 | 0.780 | 0.211 | 46 | 0.160 | 0.305 | 33 | 0.084 | |
| Sex, mean±STD | 0.203 | 0.253 | 0.187 | 0.009 | |||||||||
| Male | 0.17±0.07 | 46 | 153.5±41.1 | 43 | 210.2±73.9 | 41 | 201.9±80.2 | 29 | |||||
| Female | 0.18±0.06 | 11 | 136.0±24.1 | 6 | 178.3±44.8 | 7 | 124.5±40.7 | 6 | |||||
| Education, r | 0.144 | 52 | 0.309 | −0.250 | 42 | 0.110 | −0.009 | 47 | 0.947 | −0.065 | 35 | 0.711 | |
| Antidepressants, mean±STD | - | - | 0.111 | 0.415 | |||||||||
| No | 220.0±76.2 | 30 | 196.0±81.1 | 23 | |||||||||
| Yes | 182.4±38.4 | 11 | 188.0±81.0 | 10 | |||||||||
BDNF associations with PTD, Cognitive Impairment, and Cognitive Function
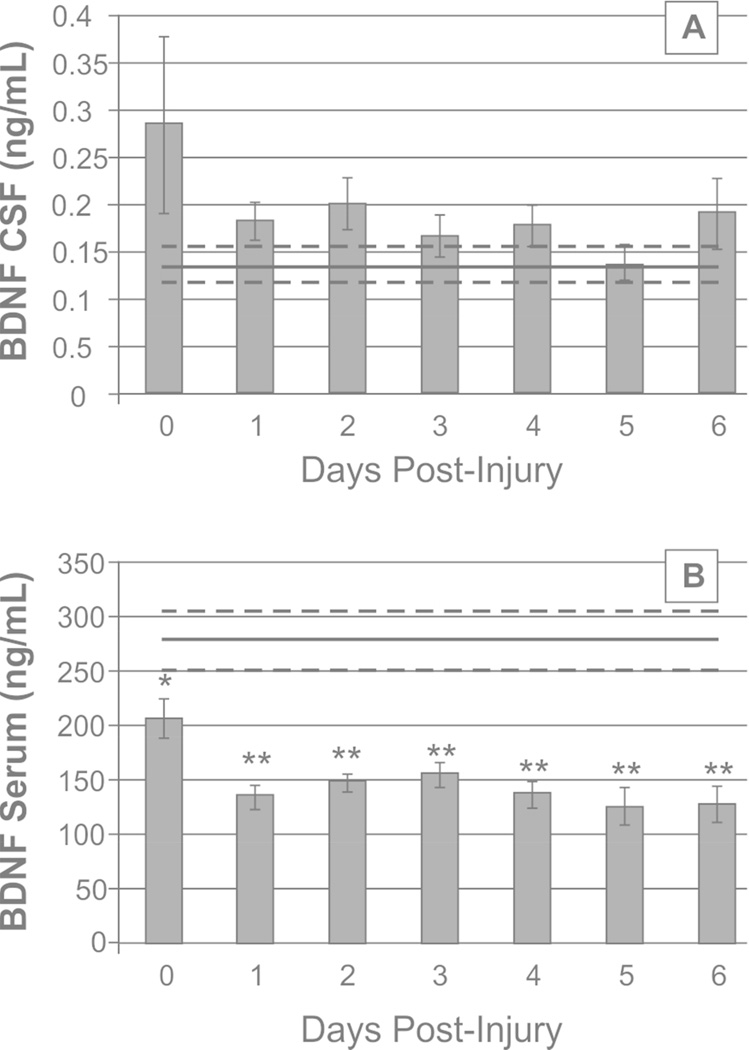
As shown in Figure 1A, daily mean CSF BDNF levels among TBI participants did not differ from healthy control levels. Conversely, daily mean serum BDNF levels in participants with TBI were consistently reduced compared to healthy control levels beginning day 0 (206.27±16.4ng/mL versus 277.86±28.1ng/mL for healthy controls, p=0.024) and remained below healthy controls for all days (all comparisons p<0.01, n=49, Figure 1B). Serum BDNF levels at 6 (205.57±10.2ng/mL, p=0.011) and 12 (188.67±13.5ng/mL, p=0.008) months were also below healthy control levels.
Figure 1.
Daily brain derived neurotrophic factor (BDNF) levels over the first 7 days post-injury, compared to healthy controls (mean in black line, standard error in light gray dashed lines). (A) Daily mean CSF BDNF levels do not differ significantly from control levels. (B) Daily mean serum BDNF levels fall below control levels at day 0 post-injury and remain reduced through day 6 (day 0, p<0.05, day 1–6, p<0.001).
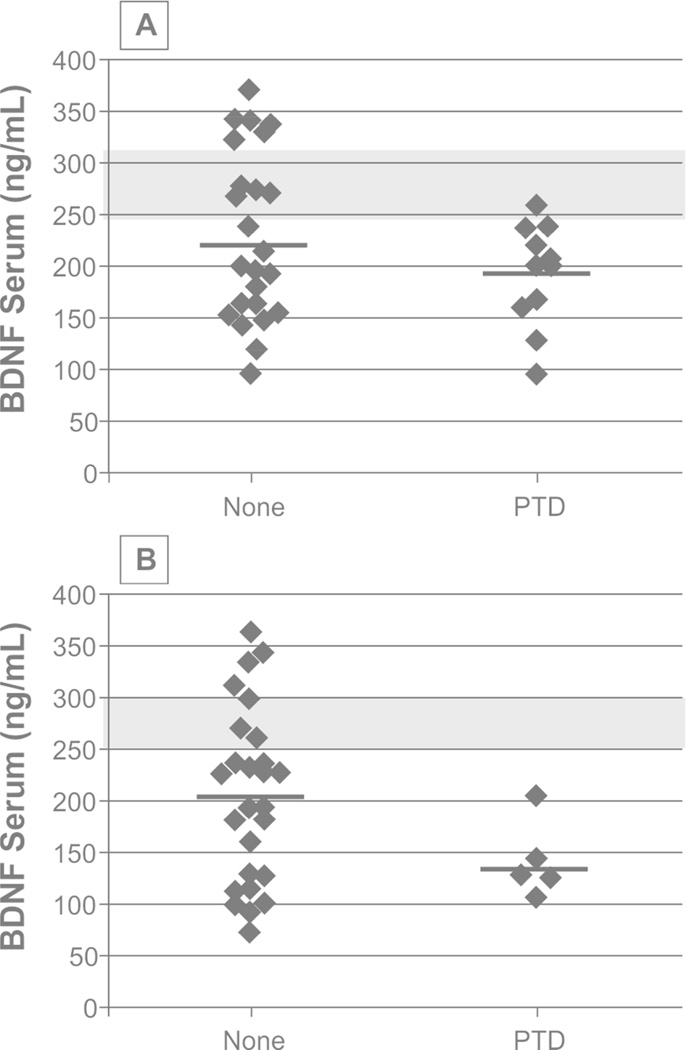
BDNF levels were examined for associations to PTD status and symptom severity. CSF BDNF levels were higher in participants with PTD at 6 months, though this association was not significant (p=0.089). Acute serum BDNF levels in participants with PTD tended to be reduced compared to those with no PTD at 6 months (p=0.074). Acute serum BDNF did not predict PTD status at 12 months. Figure 2 shows chronic serum BDNF levels by PTD status. At 6 months, there were no significant differences in chronic serum BDNF by PTD status (p=0.174). Yet, those with PTD had significantly lower serum BDNF compared to controls (p=0.012), while those without PTD did not differ significantly from controls (p=0.070). At 12 months, participants with PTD tended to have lower serum BDNF levels than those without PTD (p=0.066). Participants with and without PTD showed lower chronic serum BDNF levels compared to controls (p=0.037, 0.004, respectively). Table 3 shows associations between BDNF levels and PTD severity (PHQ-9 Total Score). Acute serum BDNF was negatively correlated with PHQ-9 scores at 12 months (r=−0.38, p=0.044, n=29) such that lower serum BDNF levels were associated with higher (worse) PHQ-9 scores. Similarly, chronic levels at 12 months were negatively correlated with PHQ-9 scores (r=−0.41, p=0.019, n=32).
Figure 2.
Serum brain derived neurotrophic factor (BDNF) levels during chronic recovery at 6 and 12 months were examined in participants with PTD and no PTD. At 6 months (A), there were no significant differences in serum BDNF by PTD status (p=0.174). At 12 months (B), participants with PTD had lower serum BDNF levels compared to participants with no PTD, though this was not significant (p=0.066). Levels in participants with PTD were reduced compared to control levels at 6 months and 12 months; similarly BDNF levels for participants with no PTD were reduced compared to controls (p<0.05 for all comparisons).
Table 3.
Bivariate Correlations between BDNF Levels and Mood and Cognitive Outcome.
| Acute CSF | Acute Serum | Chronic Serum | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spearman’s r |
p value |
n | Spearman’s r |
p value |
n | Spearman’s r |
p value |
n | ||
| 6 Months | ||||||||||
| PHQ-9 Total | 0.16 | 0.334 | 39 | −0.13 | 0.490 | 30 | −0.20 | 0.242 | 37 | |
| Cognitive Composites | ||||||||||
| Overall | 0.13 | 0.405 | 41 | 0.17 | 0.355 | 30 | 0.07 | 0.678 | 35 | |
| Memory | 0.11 | 0.490 | 42 | 0.43 | 0.019 | 30 | 0.04 | 0.803 | 35 | |
| Executive Function | 0.01 | 0.964 | 42 | −0.18 | 0.332 | 31 | 0.13 | 0.448 | 36 | |
| Attention | −0.09 | 0.574 | 44 | −0.07 | 0.712 | 33 | −0.10 | 0.520 | 40 | |
| Language Fluency | −0.09 | 0.589 | 42 | 0.10 | 0.599 | 30 | −0.01 | 0.953 | 35 | |
| Functional Independence Measure | ||||||||||
| Cognitive Total | −0.24 | 0.090 | 53 | 0.31 | 0.041 | 45 | 0.16 | 0.271 | 47 | |
| Memory | −0.24 | 0.080 | 53 | 0.35 | 0.019 | 45 | 0.13 | 0.369 | 47 | |
| Problem Solving | −0.33 | 0.015 | 53 | 0.33 | 0.029 | 45 | 0.11 | 0.463 | 47 | |
| Social Interaction | −0.22 | 0.116 | 53 | 0.30 | 0.044 | 45 | 0.05 | 0.738 | 47 | |
| Expression | −0.12 | 0.390 | 53 | 0.34 | 0.024 | 45 | 0.19 | 0.190 | 47 | |
| Comprehension | −0.06 | 0.648 | 53 | 0.20 | 0.186 | 45 | 0.27 | 0.068 | 47 | |
| 12 Months | ||||||||||
| PHQ-9 Total | 0.10 | 0.551 | 37 | −0.38 | 0.044 | 29 | −0.41 | 0.019 | 32 | |
| Cognitive Composites | ||||||||||
| Overall | 0.05 | 0.780 | 32 | 0.35 | 0.137 | 19 | 0.01 | 0.996 | 28 | |
| Memory | 0.17 | 0.339 | 34 | 0.53 | 0.005 | 26 | 0.11 | 0.580 | 28 | |
| Executive Function | 0.01 | 0.962 | 33 | −0.15 | 0.502 | 22 | −0.14 | 0.478 | 29 | |
| Attention | 0.07 | 0.716 | 32 | −0.13 | 0.583 | 19 | 0.12 | 0.530 | 32 | |
| Language Fluency | 0.10 | 0.584 | 34 | 0.18 | 0.372 | 26 | 0.00 | 0.984 | 29 | |
| Functional Independence Measure | ||||||||||
| Cognitive Total | 0.00 | 0.991 | 47 | 0.31 | 0.063 | 38 | 0.19 | 0.271 | 35 | |
| Memory | −0.15 | 0.327 | 47 | 0.38 | 0.018 | 38 | 0.20 | 0.251 | 34 | |
| Problem Solving | −0.05 | 0.762 | 47 | 0.30 | 0.068 | 38 | 0.04 | 0.805 | 34 | |
| Social Interaction | −0.02 | 0.893 | 47 | 0.25 | 0.133 | 38 | 0.08 | 0.672 | 34 | |
| Expression | 0.03 | 0.817 | 47 | 0.27 | 0.098 | 38 | 0.07 | 0.692 | 34 | |
| Comprehension | 0.24 | 0.101 | 47 | 0.18 | 0.282 | 38 | 0.32 | 0.062 | 34 | |
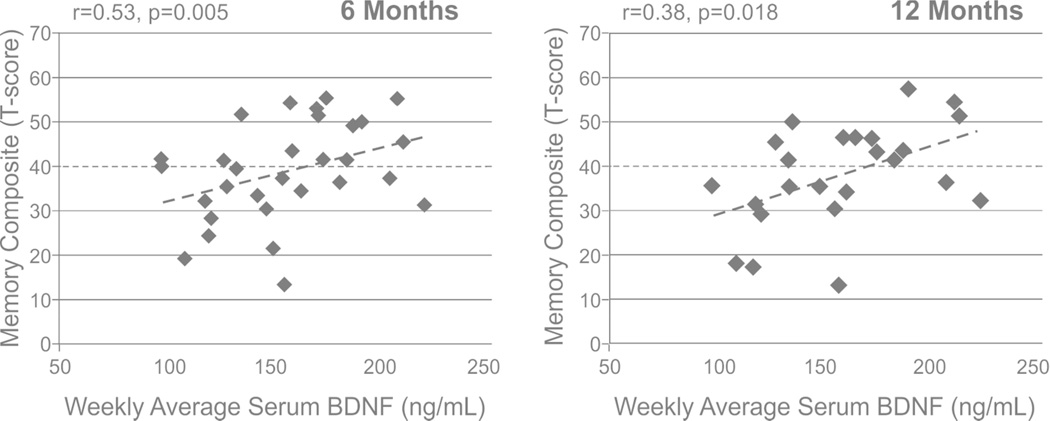
BDNF levels were examined for associations to cognitive impairments and functional cognitive limitations (Table 3). Acute serum BDNF levels were positively correlated with memory composite scores at 6 (r=0.53, p=0.005, n=30) and 12 months (r=0.38, p=0.018, n=26), Figure 3. Using a t-score cut off of 40 to designate impaired performance, acute BDNF levels were reduced in those individuals with a memory composite score<40 (impaired) compared to those with memory composites >40 at 6 months (146.9±8.4 vs 168.2±8.4, p=0.024) and 12 months (144.9±10.9 vs 167.2±7.5, p=0.027). CSF BDNF and chronic serum BDNF levels were not associated with any cognitive composite components. Acute serum BDNF levels were positively correlated with FIM-Cog at 6 months (r=0.31, p=0.041, n=45). All FIM-Cog component scales were positively associated with higher serum BDNF at 6 months (Table 3). CSF BDNF levels tended to show a negative correlation with FIM-Cog at 6 months (r=−0.24, p=0.090, n=53), with a significant association with FIM-Problem Solving (r=−0.33, p=0.015). At 12 months, acute serum BDNF levels were positively correlated to FIM-Memory (r=0.38, p=0.018); CSF BDNF did not show any significant correlations to 12 month FIM-Cog or FIM-Cog components. There were no significant relationships between chronic BDNF and functional cognition at either 6 or 12 months.
Figure 3.
Serum brain derived neurotrophic factor (BDNF) levels during the first week post-injury were investigated for predictive associations with memory impairment at 6 and 12 months post-injury. At 6 months (A), acute serum BDNF levels predicted performance on memory composite (r=0.53, p=0.005). At 12 months (B), acute serum BDNF levels predicted performance on memory composite (r=0.38, p=0.018). A dashed line denotes the T-score cut-off for impairment on the memory composite.
Discussion
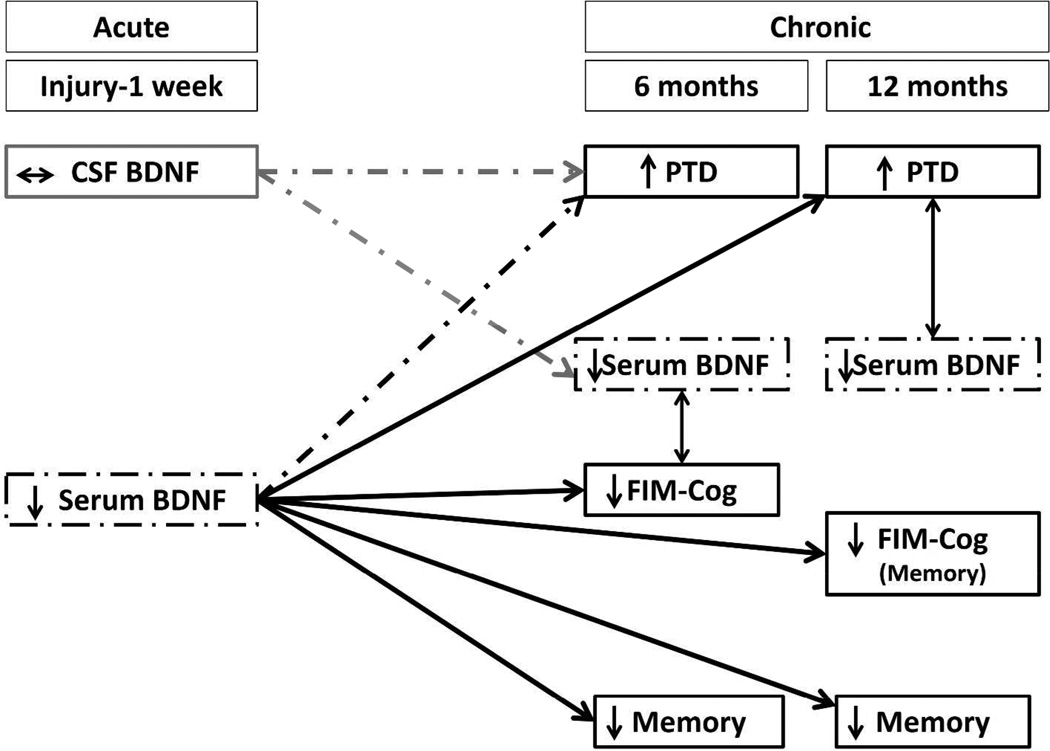
In this study we investigated BDNF levels as a common biomarker for PTD pathology, cognitive dysfunction, and functional cognitive impairment post-TBI. As PTD and cognitive difficulties often co-occur post-injury, we aimed to evaluate BDNF as a biomarker representing common pathology underlying these complications. In this study, acute serum BDNF levels were associated with chronic memory impairments, global functional cognitive limitations, and depressive symptom severity. Chronic serum BDNF levels, however, were not associated with any cognitive outcomes, but tended be lower in individuals with PTD. Yet, while acute CSF levels tended to correlate with acute serum, they were not predictive of chronic outcome measures. These findings are summarized in Figure 4.
Figure 4.
The following schematic summarizes the main findings regarding associations between brain derived neurotrophic factor (BDNF) levels and depression or cognition following traumatic brain injury (TBI). Dashed lines and boxes depict trends in the data while solid lines and boxes represent significant findings. These data are shown across a timeline from the date of injury through the 1st week (acute) and then 6 and 12 months post-injury (chronic). Memory refers to cognitive composites of neuropsychological tests examining memory. PTD, post-TBI depression. FIM, functional independence measure.
This work has two potentially important implications: first, acute serum BDNF may be a viable predictive biomarker for both mood and cognitive complications within the first year post-TBI, indicating a possible treatment window in the acute phase that could have implications for long-term mood and cognitive recovery; second, that chronic serum BDNF may be reflective of PTD severity, suggesting a potential biomarker for tracking treatment response and effectiveness in real-time. While future studies will need to evaluate this work in larger cohorts, the findings presented here suggests BDNF may be a viable treatment target with dynamic utility across recovery.
Serum BDNF levels were low immediately after injury, consistent with previous studies32,56. Also, our own previous work demonstrates low serum BDNF levels as a mortality marker following TBI, suggesting immediate reductions in serum BDNF levels are indicative of greater TBI related pathology. Kalish (2010) demonstrated immediate reductions in serum BDNF, but this finding was associated with GCS32. In our current study, there is no relationship between GCS and acute serum BDNF. However, our population included participants with moderate to severe injury, while the Kalish (2010) study included mild TBI32. We also report that serum BDNF levels remain reduced chronically, adding evidence to the emerging concept of TBI as a chronic condition with long-term pathophysiological alterations.
In uninjured populations, BDNF serum levels are consistently reduced among individuals experiencing depressive symptoms57. Under uninjured conditions, serum BDNF levels likely reflect CNS functioning, as BDNF is primarily synthesized in the brain and secreted in an activity dependent manner58. Several studies suggest serum increases in BDNF are due to brain level changes59,60. Serum BDNF is likely reflective of BDNF expression in the hippocampus, where tissue BDNF levels are decreased in correlation with stress and depression61. Hippocampal BDNF signaling is also implicated in mechanisms of antidepressant treatment62. In rat models, intracerebral BDNF infusions have antidepressant effects, while decreased BDNF signaling results in decreased hippocampal neurogenesis63.
Reduced BDNF may be indicative of a depressive state following neurological insult. Following acute stroke, there were relative reductions in acute serum BDNF for those with depressive symptoms that did not occur in those without depression within the same timeframe64. Similarly, our chronic BDNF levels at 12 months correlated with depressive symptom severity within the corresponding timeframe. However, there was limited discriminatory ability of BDNF levels at chronic time-points, possibly due to issues with sample size and variability in biomarker values. Additionally, there may also be a “floor effect” following TBI, where BDNF levels are already significantly lower than controls, impairing our ability to discriminate PTD status.
The data from this study suggest acute serum BDNF levels are highly associated with memory impairment post-TBI. Other studies have demonstrated similar relationships between serum BDNF levels and cognitive impairment65. Specifically, in patient populations (schizophrenia, bipolar disorder, mild cognitive impairment) and healthy controls66, there are relationships between BDNF levels and memory67. Animal studies using conditional BDNF knock-out mice show impaired hippocampal-dependent cognition and behavior68. Thus, injury induced acute reductions in serum BDNF may be indicative of the degree of direct damage to the hippocampus. Similarly, levels may also be indicative of an acute injury state that is conducive to neuronal death and atrophy that would predict later memory performance. Low BDNF signaling in the hippocampus may diminish synaptic plasticity and neurogenesis, negatively affecting these chronic recovery endpoints. Future studies are needed to examine the relationship between acute BDNF levels and chronic hippocampal volume, as this may aid interpretation of these findings. As experimental TBI models show that hippocampal BDNF expression is correlated with cognitive recovery23, it will be critical to understand if/how early interventions targeting BDNF might improve chronic memory problems. Participants with lower BDNF levels acutely do tend to have lower levels chronically. One recent study demonstrated detrimental effects of long-term BDNF reductions on age-related decline in animal models69. Thus, understanding the trajectory of BDNF profiles, and their relationship to memory performance, could guide treatment and intervention strategies and improve longterm outcomes. Acute BDNF serum levels did not correlate with any other measures of cognitive performance, but were significantly associated with multiple components of functional cognition. This finding may be due to the importance of memory in other aspects of functional cognition70.
BDNF CSF levels did not show consistent associations with mood or cognitive recovery. However, higher CSF levels tended to be associated with worse outcomes on the FIM-Cog subcomponents. This trend is consistent with our previous work56 showing high levels of CSF were associated with greater mortality post-TBI. However, it is important to understand interactions between CSF and serum BDNF levels. In fact, we show a trend for a negative correlation between CSF and serum BDNF (consistent with previous work56). While it has been suggested that BDNF can cross the blood brain barrier (BBB) in both directions under normal conditions71,72, BBB disruption following TBI could allow for increased BDNF transit into the brain, especially during the initial days after TBI71,73. This increased transit from blood into CSF could reflect a possible protective process, as platelets dump BDNF in response to vascular injury74. Therefore, lower BDNF levels in serum acutely may be suggestive of more extensive injury, and subsequent expenditure of systemic stores of BDNF into the CNS, correlating with later development of chronic conditions.
In line with personalized medicine and Rehabilomics20, it is important to consider several demographic, medical, and genetic factors that may influence BDNF levels. Some studies suggest BDNF levels vary by BDNF genetic variation75. Future studies in larger cohorts are needed to determine the effect of BDNF variation on chronic BDNF levels after TBI. It will also be important to evaluate serum BDNF post-TBI in relation to therapies that stimulate BDNF signaling (e.g. exercise, SSRIs). In uninjured populations, serum BDNF levels are decreased in untreated depression but increase with antidepressant treatment29–31. However, this study was not designed to evaluate the utility of BDNF as a biomarker of PTD remittance following antidepressant use. A limitation in this study is the inclusion of individuals on antidepressants, however, BDNF levels are thought to be more indicative of depressive symptomology and remittance than antidepressant use76. Additionally, many individuals who are on antidepressants are still depressed; the opposing effects of antidepressant use and depression may reduce associations with BDNF levels. Future studies in PTD will need to examine these relationships in carefully designed studies where relationships between BDNF levels, antidepressant type/dose, and depressive symptoms are examined.
It is also possible that this study was underpowered to detect some associations between BDNF and TBI-related outcomes given possible effects of demographics like age and sex on BDNF levels77. In this study, age and sex were not consistently associated with BDNF levels, though BDNF levels were significantly lower in women at 12 months post-injury. Future studies are needed to examine the impact of demographic variables on BDNF levels specifically following TBI and in concert with TBI outcomes.
There are other important limitations to consider concerning this study and BDNF evaluations. Similarly, it will be important to understand the relationship between BDNF levels in participants with PTD, with and without cognitive impairment. A general limitation is the assumption that serum BDNF levels represent brain levels, as there is a substantial peripheral production of BDNF in vascular endothelial cells74 and stored in platelets78. Literature suggests platelet release is not altered in depression57, but it is unclear if platelet release could be altered in PTD or TBI, or how peripheral stores of BDNF might influence our findings. As a reference group for biomarker levels, the sample size is small and cannot account for potential genetic or other sources of variability. Controls were not screened for depressive symptoms. It is possible that some healthy controls could have depressive symptoms or other disorders that they did not report at the time of their interview. However, if that is the case in controls, then our results comparing individuals with TBI to controls would be biased toward the null.
The work presented here provides preliminary evidence of potential utility for BDNF as a predictive biomarker for cognitive recovery following TBI. Larger studies are needed to evaluate BDNF utility in discriminating PTD status, however our data indicate that BDNF may be associated with depressive symptoms post-TBI. Examining BDNF levels in stratified cohorts with and without PTD and cognitive impairment may elucidate the specificity of BDNF as a biomarker post-TBI. It will be important to understand, mechanistically, utilizing animal models, what the resulting BDNF levels in this study indicate about underlying TBI pathology and possible treatment development. Future studies are likely needed to evaluate temporal BDNF trajectories over time and across recovery, as PTD and cognitive impairments develop and/or resolve, for a better understanding of BDNF effects on TBI recovery.
Acknowledgments
Research/Grant Support:
This research was supported by NIH R01 HD048162; NIDRR H133A120087; DODW81XWH-071-0701; CDC R49 CCR 323155, University of Pittsburgh Women’s Studies Faculty Research Fund.
Footnotes
Conflict of Interest Statement:
The authors declare no conflicts of interest.
References
- 1.Fleming J, Tooth L, Hassell M, Chan W. Prediction of community integration and vocational outcome 2–5 years after traumatic brain injury rehabilitation in Australia. Brain Inj. BI. 1999;13:417–431. doi: 10.1080/026990599121476. [DOI] [PubMed] [Google Scholar]
- 2.Juengst S, Skidmore E, Arenth PM, Niyonkuru C, Raina KD. Unique contribution of fatigue to disability in community-dwelling adults with traumatic brain injury. Arch. Phys. Med. Rehabil. 2013;94:74–79. doi: 10.1016/j.apmr.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cifu DX, et al. Acute predictors of successful return to work 1 year after traumatic brain injury: a multicenter analysis. Arch. Phys. Med. Rehabil. 1997;78:125–131. doi: 10.1016/s0003-9993(97)90252-5. [DOI] [PubMed] [Google Scholar]
- 4.Yasuda S, Wehman P, Targett P, Cifu D, West M. Return to work for persons with traumatic brain injury. Am. J. Phys. Med. Rehabil. Assoc. Acad. Physiatr. 2001;80:852–864. doi: 10.1097/00002060-200111000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Bombardier CH, et al. Rates of Major Depressive Disorder and Clinical Outcomes Following Traumatic Brain Injury. JAMA. 2010;303:1938–1945. doi: 10.1001/jama.2010.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, Severity, and Comorbidity of 12-Month DSM-IV Disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin RL, Heller W, Mohanty A, Herrington JD, Miller GA. Cognitive deficits in depression and functional specificity of regional brain activity. Cogn. Ther. Res. 2007;31:211–233. [Google Scholar]
- 8.Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu. Rev. Clin. Psychol. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burt DB, Zembar MJ, Niederehe G. Depression and memory impairment: a meta-analysis of the association, its pattern, and specificity. Psychol. Bull. 1995;117:285–305. doi: 10.1037/0033-2909.117.2.285. [DOI] [PubMed] [Google Scholar]
- 10.Vakil E. The effect of moderate to severe traumatic brain injury (TBI) on different aspects of memory: a selective review. J. Clin. Exp. Neuropsychol. 2005;27:977–1021. doi: 10.1080/13803390490919245. [DOI] [PubMed] [Google Scholar]
- 11.Brooks J, Fos LA, Greve KW, Hammond JS. Assessment of executive function in patients with mild traumatic brain injury. J. Trauma. 1999;46:159–163. doi: 10.1097/00005373-199901000-00027. [DOI] [PubMed] [Google Scholar]
- 12.Niogi SN, et al. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008;131:3209–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- 13.Perlstein WM, Larson MJ, Dotson VM, Kelly KG. Temporal dissociation of components of cognitive control dysfunction in severe TBI: ERPs and the cued-Stroop task. Neuropsychologia. 2006;44:260–274. doi: 10.1016/j.neuropsychologia.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Failla MD, Juengst SB, Graham KM, Arenth PM, Wagner AK. Effects of Depression and Antidepressant Use on Cognitive Deficits and Functional Cognition following Severe Traumatic Brain Injury. doi: 10.1097/HTR.0000000000000214. (In Submission). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fann JR, Uomoto JM, Katon WJ. Cognitive Improvement With Treatment of Depression Following Mild Traumatic Brain Injury. Psychosomatics. 2001;42:48–54. doi: 10.1176/appi.psy.42.1.48. [DOI] [PubMed] [Google Scholar]
- 16.Goverover Y, Chiaravalloti N. The impact of self-awareness and depression on subjective reports of memory, quality-of-life and satisfaction with life following TBI. Brain Inj. BI. 2013 doi: 10.3109/02699052.2013.860474. [DOI] [PubMed] [Google Scholar]
- 17.Hibbard MR, et al. Relationship between depression and psychosocial functioning after traumatic brain injury. Arch. Phys. Med. Rehabil. 2004;85:S43–S53. doi: 10.1016/j.apmr.2003.08.116. [DOI] [PubMed] [Google Scholar]
- 18.Satz P, et al. Depression, cognition, and functional correlates of recovery outcome after traumatic brain injury. Brain Inj. BI. 1998;12:537–553. doi: 10.1080/026990598122313. [DOI] [PubMed] [Google Scholar]
- 19.Larsson J, Björkdahl A, Esbjörnsson E, Sunnerhagen KS. Factors affecting participation after traumatic brain injury. J. Rehabil. Med. Off. J. UEMS Eur. Board Phys. Rehabil. Med. 2013;45:765–770. doi: 10.2340/16501977-1184. [DOI] [PubMed] [Google Scholar]
- 20.Wagner AK. TBI translational rehabilitation research in the 21st Century: exploring a Rehabilomics research model. Eur. J. Phys. Rehabil. Med. 2010;46:549–556. [PubMed] [Google Scholar]
- 21.Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat. Neurosci. 2007;10:1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- 22.Alonso M, et al. BDNF-triggered events in the rat hippocampus are required for both short- and long-term memory formation. Hippocampus. 2002;12:551–560. doi: 10.1002/hipo.10035. [DOI] [PubMed] [Google Scholar]
- 23.Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Failla MD, et al. Variation in the BDNF Gene Interacts With Age to Predict Mortality in a Prospective, Longitudinal Cohort with Severe TBI. Neurorehabil. Neural Repair. 2014 doi: 10.1177/1545968314542617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X, et al. Gender and environmental effects on regional brain-derived neurotrophic factor expression after experimental traumatic brain injury. Neuroscience. 2005;135:11–17. doi: 10.1016/j.neuroscience.2005.05.041. [DOI] [PubMed] [Google Scholar]
- 26.Kovalchuk Y, Hanse E, Kafitz KW, Konnerth A. Postsynaptic Induction of BDNF-Mediated Long-Term Potentiation. Science. 2002;295:1729–1734. doi: 10.1126/science.1067766. [DOI] [PubMed] [Google Scholar]
- 27.Bekinschtein P, Cammarota M, Izquierdo I, Medina JH. Reviews: BDNF and Memory Formation and Storage. The Neuroscientist. 2008;14:147–156. doi: 10.1177/1073858407305850. [DOI] [PubMed] [Google Scholar]
- 28.Bekinschtein P, et al. BDNF is essential to promote persistence of long-term memory storage. Proc. Natl. Acad. Sci. 2008;105:2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sen S, Duman R, Sanacora G. Serum Brain-Derived Neurotrophic Factor, Depression, and Antidepressant Medications: Meta-Analyses and Implications. Biol. Psychiatry. 2008;64:527–532. doi: 10.1016/j.biopsych.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karege F, et al. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109:143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto K. Brain-derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psychiatry Clin. Neurosci. 2010;64:341–357. doi: 10.1111/j.1440-1819.2010.02113.x. [DOI] [PubMed] [Google Scholar]
- 32.Kalish H, Phillips TM. Analysis of neurotrophins in human serum by immunoaffinity capillary electrophoresis (ICE) following traumatic head injury. J. Chromatogr. B Analyt. Technol. Biomed. Life. Sci. 2010;878:194–200. doi: 10.1016/j.jchromb.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoffman JM, et al. A randomized controlled trial of exercise to improve mood after traumatic brain injury. PM R. 2010;2:911–919. doi: 10.1016/j.pmrj.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Wise EK, Hoffman JM, Powell JM, Bombardier CH, Bell KR. Benefits of exercise maintenance after traumatic brain injury. Arch. Phys. Med. Rehabil. 2012;93:1319–1323. doi: 10.1016/j.apmr.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Dodds TA, Martin DP, Stolov WC, Deyo RA. A validation of the functional independence measurement and its performance among rehabilitation inpatients. Arch. Phys. Med. Rehabil. 1993;74:531–536. doi: 10.1016/0003-9993(93)90119-u. [DOI] [PubMed] [Google Scholar]
- 36.Linacre JM, Heinemann AW, Wright BD, Granger CV, Hamilton BB. The structure and stability of the Functional Independence Measure. Arch. Phys. Med. Rehabil. 1994;75:127–132. [PubMed] [Google Scholar]
- 37.Smith PM, et al. Intermodal agreement of follow-up telephone functional assessment using the Functional Independence Measure in patients with stroke. Arch. Phys. Med. Rehabil. 1996;77:431–435. doi: 10.1016/s0003-9993(96)90029-5. [DOI] [PubMed] [Google Scholar]
- 38.Hart T, Whyte J, Kim J, Vaccaro M. Executive function and self-awareness of ‘real-world’ behavior and attention deficits following traumatic brain injury. J. Head Trauma Rehabil. 2005;20:333–347. doi: 10.1097/00001199-200507000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Hartikainen KM, et al. Persistent symptoms in mild to moderate traumatic brain injury associated with executive dysfunction. J. Clin. Exp. Neuropsychol. 2010;32:767–774. doi: 10.1080/13803390903521000. [DOI] [PubMed] [Google Scholar]
- 40.Wagner AK, et al. Persistent hypogonadism influences estradiol synthesis, cognition and outcome in males after severe TBI. Brain Inj. BI. 2012 doi: 10.3109/02699052.2012.667594. [DOI] [PubMed] [Google Scholar]
- 41.Failla MD, et al. Posttraumatic Brain Injury Cognitive Performance Is Moderated by Variation Within ANKK1 and DRD2 Genes. J. Head Trauma Rehabil. 2015 doi: 10.1097/HTR.0000000000000118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zafonte RD, et al. Effect of citicoline on functional and cognitive status among patients with traumatic brain injury: Citicoline Brain Injury Treatment Trial (COBRIT) JAMA. 2012;308:1993–2000. doi: 10.1001/jama.2012.13256. [DOI] [PubMed] [Google Scholar]
- 43.Van Veelen NMJ, et al. Short term neurocognitive effects of treatment with ziprasidone and olanzapine in recent onset schizophrenia. Schizophr. Res. 2010;120:191–198. doi: 10.1016/j.schres.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Dawson JD, Uc EY, Anderson SW, Johnson AM, Rizzo M. Neuropsychological predictors of driving errors in older adults. J. Am. Geriatr. Soc. 2010;58:1090–1096. doi: 10.1111/j.1532-5415.2010.02872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green RE, et al. Examining moderators of cognitive recovery trajectories after moderate to severe traumatic brain injury. Arch. Phys. Med. Rehabil. 2008;89:S16–S24. doi: 10.1016/j.apmr.2008.09.551. [DOI] [PubMed] [Google Scholar]
- 46.Reitan R, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Neuropsychology Press; 1985. [Google Scholar]
- 47.Larrabee GJ, Curtiss G. Construct validity of various verbal and visual memory tests. J. Clin. Exp. Neuropsychol. 1995;17:536–547. doi: 10.1080/01688639508405144. [DOI] [PubMed] [Google Scholar]
- 48.Osterrieth P. The Complex Figure Copy Test. Arch. Psychol. 1944;30:206–356. [Google Scholar]
- 49.Delis DC, et al. The California Verbal Learning Test. The Psychological Corporation; 2000. [Google Scholar]
- 50.Borkowski J, Benton A, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–140. [Google Scholar]
- 51.Stroop JR. Studies of interference in serial verbal reactions. J. Exp. Psychol. Exp. Psychol. 1935;18:643–662. [Google Scholar]
- 52.Lezak M, Howieson D, Bigler E, Tranel D, et al. Neuropsychological Assessment. Oxford University Press; 2012. [Google Scholar]
- 53.Fann JR, et al. Validity of the Patient Health Questionnaire-9 in assessing depression following traumatic brain injury. J. Head Trauma Rehabil. 2005;20:501–511. doi: 10.1097/00001199-200511000-00003. [DOI] [PubMed] [Google Scholar]
- 54.Cook KF, et al. Do Somatic and Cognitive Symptoms of Traumatic Brain Injury Confound Depression Screening? Arch. Phys. Med. Rehabil. 2011;92:818–823. doi: 10.1016/j.apmr.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nettiksimmons J, et al. The Associations between Serum Brain-Derived Neurotrophic Factor, Potential Confounders, and Cognitive Decline: A Longitudinal Study. PLoS ONE. 2014;9:e91339. doi: 10.1371/journal.pone.0091339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Failla MD, Conley YP, Wagner AK. Brain-Derived Neurotrophic Factor (BDNF) in Traumatic Brain Injury-Related Mortality: Interrelationships Between Genetics and Acute Systemic and Central Nervous System BDNF Profiles. Neurorehabil. Neural Repair. 2015 doi: 10.1177/1545968315586465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karege F, et al. Low Brain-Derived Neurotrophic Factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol. Psychiatry. 2005;57:1068–1072. doi: 10.1016/j.biopsych.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 58.Chen Z-Y, et al. Variant Brain-Derived Neurotrophic Factor (BDNF) (Met66) Alters the Intracellular Trafficking and Activity-Dependent Secretion of Wild-Type BDNF in Neurosecretory Cells and Cortical Neurons. J Neurosci. 2004;24:4401–4411. doi: 10.1523/JNEUROSCI.0348-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dawood T, et al. Reduced overflow of BDNF from the brain is linked with suicide risk in depressive illness. Mol. Psychiatry. 2007;12:981–983. doi: 10.1038/sj.mp.4002059. [DOI] [PubMed] [Google Scholar]
- 60.Rasmussen P, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009;94:1062–1069. doi: 10.1113/expphysiol.2009.048512. [DOI] [PubMed] [Google Scholar]
- 61.Pittenger C, Duman RS. Stress, Depression, and Neuroplasticity: A Convergence of Mechanisms. Neuropsychopharmacology. 2007;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 62.D’Sa C, Duman RS. Antidepressants and neuroplasticity. Bipolar Disord. 2002;4:183–194. doi: 10.1034/j.1399-5618.2002.01203.x. [DOI] [PubMed] [Google Scholar]
- 63.Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacol. Biochem. Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 64.Yang L, et al. Low serum BDNF may indicate the development of PSD in patients with acute ischemic stroke. Int. J. Geriatr. Psychiatry. 2010 doi: 10.1002/gps.2552. [DOI] [PubMed] [Google Scholar]
- 65.Griffin ÉW, et al. Aerobic exercise improves hippocampal function and increases BDNF in the serum of young adult males. Physiol. Behav. 2011;104:934–941. doi: 10.1016/j.physbeh.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 66.Gunstad J, et al. Serum brain-derived neurotrophic factor is associated with cognitive function in healthy older adults. J. Geriatr. Psychiatry Neurol. 2008;21:166–170. doi: 10.1177/0891988708316860. [DOI] [PubMed] [Google Scholar]
- 67.Dias VV, et al. Cognitive function and serum levels of brain-derived neurotrophic factor in patients with bipolar disorder. Bipolar Disord. 2009;11:663–671. doi: 10.1111/j.1399-5618.2009.00733.x. [DOI] [PubMed] [Google Scholar]
- 68.Bath KG, Lee FS. Variant BDNF (Val66Met) impact on brain structure and function. Cogn. Affect. Behav. Neurosci. 2006;6:79–85. doi: 10.3758/cabn.6.1.79. [DOI] [PubMed] [Google Scholar]
- 69.Petzold A, Psotta L, Brigadski T, Endres T, Lessmann V. Chronic BDNF deficiency leads to an age-dependent impairment in spatial learning. Neurobiol. Learn. Mem. 2015;120:52–60. doi: 10.1016/j.nlm.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 70.Lewis FD, Horn GJ. Traumatic brain injury: analysis of functional deficits and posthospital rehabilitation outcomes. J. Spec. Oper. Med. Peer Rev. J. SOF Med. Prof. 2013;13:56–61. doi: 10.55460/ATYP-5WSB. [DOI] [PubMed] [Google Scholar]
- 71.Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ. Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology. 1998;37:1553–1561. doi: 10.1016/s0028-3908(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 72.Poduslo JF, Curran GL. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Brain Res. Mol. Brain Res. 1996;36:280–286. doi: 10.1016/0169-328x(95)00250-v. [DOI] [PubMed] [Google Scholar]
- 73.Başkaya MK, Rao AM, Doğan A, Donaldson D, Dempsey RJ. The biphasic opening of the blood-brain barrier in the cortex and hippocampus after traumatic brain injury in rats. Neurosci. Lett. 1997;226:33–36. doi: 10.1016/s0304-3940(97)00239-5. [DOI] [PubMed] [Google Scholar]
- 74.Nakahashi T, et al. Vascular endothelial cells synthesize and secrete brain-derived neurotrophic factor. FEBS Lett. 2000;470:113–117. doi: 10.1016/s0014-5793(00)01302-8. [DOI] [PubMed] [Google Scholar]
- 75.Terracciano A, et al. Genetics of serum BDNF: Meta-analysis of the Val66Met and genome-wide association study. World J. Biol. Psychiatry Off. J. World Fed. Soc. Biol. Psychiatry. 2013;14 doi: 10.3109/15622975.2011.616533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Piccinni A, et al. Plasma and serum brain-derived neurotrophic factor (BDNF) in depressed patients during 1 year of antidepressant treatments. J. Affect. Disord. 2008;105:279–283. doi: 10.1016/j.jad.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 77.Lommatzsch M, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 78.Gass P, Hellweg R. Peripheral brain-derived neurotrophic factor (BDNF) as a biomarker for affective disorders? Int. J. Neuropsychopharmacol. Off. Sci. J. Coll. Int. Neuropsychopharmacol. CINP. 2010;13:1–4. doi: 10.1017/S1461145709991039. [DOI] [PubMed] [Google Scholar]