1. Introduction
Heart failure (HF), particularly in the setting of preserved systolic function, disproportionately afflicts older individuals and results in significant morbidity, mortality, and health care costs. In the United States, among the Medicare population (age > 65 years), HF is among the leading cause of hospital admissions. Optimal care for older adults with HF requires knowledge of age-related physiologic changes, complex multi-organ, multi-dimensional syndromes, interdisciplinary teamwork and palliative/end of life care.
2. Epidemiology
There are currently estimated to be 5.7 million adults in the United States with HF, 92% of which are age 60 years or older, and more than half aged 80 years or older.[1] The elderly population shows a particular propensity for developing HF with preserved ejection fraction (HFPEF). Several large epidemiological studies have demonstrated that among patients with prevalent HF in the community, approximately 50% have a normal ejection fraction (EF), and that proportion increases with advancing age. There is also a female preponderance in HFPEF of 67% compared with 42% in men.[2] This well-known sex dominance of HFPEF in women has been validated in large, prospective population-based studies.[3] Thus, the profile of the typical older person with HF in the general population differs with that of the stereotypical HF patient. In contrast to younger HF patients, older patients are more likely to be women with comorbid conditions such as hypertension, diabetes mellitus (DM), obesity and atrial fibrillation, but, less likely to have coronary heart disease (Table 1).
Table 1. Differences in heart failure phenotype between young and older adult subjects.
| Older adults | Younger subjects | |
| Predominant heart failure | HFPEF | HFREF |
| Gender | Frequently female | More often male |
| Physical findings | Minimally displaced apical impulse, S4 | Laterally displaced maximal impulse, S3 |
| Pathophysiologic Mechanisms | Age related changes in cardiovascular structure and function, oxidative stress, vascular stiffness, skeletal muscle abnormalities | Ischemic heart disease, neurohormonal activation, LV remoldeling/dilation, |
| Potential targets of therapy | BP regulation, exercise training, peripheral targets | Enhance SV, neurohormonal blockade, decrease LV remodeling |
BP: blood pressure; HFPEF: heart failure with preserved left ventricular systolic function; HFREF: HF with reduced ejection fraction; LV: left ventricular; SV: stroke volume.
HF mortality increases with age, and is threefold higher in patients ages 65–74 years compared with those ages 25–54 years.[4] Further, even within the elderly population, mortality continues to increase strongly with age, and the 5-year mortality rate for elderly patients with HF, regardless of EF, approaches 50%.[5] Elderly patients who have been hospitalized with HF have extraordinary mortality rates, estimated at 10% at 10 days and 75% at 5 years.[6] Unlike HF with reduced ejection fraction (HFREF) where evidence-based treatment regimens have helped decrease mortality and hospitalizations, the percentage HFPEF hospitalizations have increased over the past 15 years from 38% to 54%, and mortality rates remain high.[7]
3. Age related changes in cardiovascular structure and function predisposes to HF
Older patients are predisposed to the development of HF because of age-related physiologic and pathologic changes. Cardiac aging is characterized by intrinsic changes at the cellular level (oxidative stress/mitochondrial damage), alterations in cardiovascular structure and function (ventricular-vascular stiffness) as well as peripheral abnormalities in the vasculature and skeletal muscle.
Aging is accompanied by numerous biologic changes including, but not limited to, oxidative stress, mitochondrial damage, beta-adrenoceptor (AR) desensitization and limitations in endothelium-dependent vasodilation.[8] Specifically, oxidative stress to the sarcoplasmic reticulum calcium/ATP (SERCA) pump has been shown to play a role in prolonged active diastolic relaxation.[9] It is important to note that normal aging is not associated with effects on heart rate, contractility or cardiac output or ejection fraction at rest. During normal vigorous physical activity in a young adult, cardiac output is augmented by increases in venous return with concomitant increased in end diastolic volume, contractility, heart rate and peripheral vasodilation.[10] In contrast, in healthy older persons, systolic and chronotropic reserve is blunted secondary to decreased beta-adrenergic signaling, impaired baroreceptor responsiveness, abnormal autonomic function and altered ventricular vascular coupling including altered diastolic stiffness, all of which significantly reduce the cardiovascular response to exercise in healthy older adults.[11]
Ventricular diastolic abnormalities have been the most emphasized in the pathophysiology of HFPEF. The term diastolic dysfunction refers to both active and passive relaxation of the ventricle. The former manifesting as prolongations in isovolumetric relaxation on echo Doppler and the time constant of relaxation, known as tau from invasive pressure analysis. An increased tau changes the pressure-volume relationship during early diastole and at fast heart rates can contribute to impair ventricular filling via a mechanism of incomplete relaxation.[12],[13] Passive relaxation of the ventricle is characterized by alterations in myocardial stiffness with resulting changes in ventricular capacitance. These age-related changes can hinder adequate filling of the ventricle especially during exertion and higher heart rates. Increased diastolic stiffness is accompanied by systolic stiffening manifested by an increase in end systolic elastance or chamber contractility (Ees) and is associated with a limited systolic reserve.
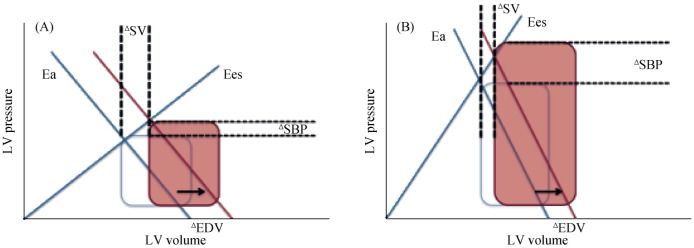
Ejection fraction is mathematically and physiologically an index of ventricular-vascular coupling that is expressed as a ratio of arterial elastance (Ea) to Ees. While the ratio of Ea/Ees (and thus EF) is maintained with normal aging, there is an increase in the Ea with aging that occurs because of central conduit artery stiffening which is accompanied by a concomitant increase in ventricular stiffness, which is indicative of enhanced chamber stiffness and contractility (Figure 1). This ‘high gain’ system is characterized by amplified blood pressure changes and blunted stroke volume augmentation for any alteration in preload or afterload. Acute afterload elevation in the setting of ventricular-arterial stiffening causes greater increase in blood pressure, which may then feedback to further impair relaxation.[14],[15] The pathophysiology of ventricular-vascular stiffness results in the hemodynamic consequences of HFPEF that cause congestion and acute pulmonary edema, exercise intolerance, ischemic risk and labile systemic pressures.[16]
Figure 1. Increased ventricular and arterial elastance in the elderly and its effect on blood pressure.
(A): Normal adults have a relatively low ratio of Ea to Ees. Isolated changes in preload EDV with normal elastance values leads to increases in SV and SBP; (B): Individuals with HFPEF have an increased ventricular and arterial eleastance. The same increase in EDV produces a larger increase in SBP with out a proportional augmentation in SV. This decreased elastance explains why older adults have labile SBPs in response to changes in preload especially during exercise. Ea: arterial elastance; EDV: end-diastolic volume; Ees: left ventricular elastance; HFPEF: heart failure with preserved left ventricular systolic function; LV: left ventricular; SV: stroke volume; SBP: systolic blood pressure.
Peripheral mechanisms also contribute significantly to the syndrome of HFPEF, most specifically exercise intolerance, which can be objectively measured by peak exercise oxygen uptake (peak VO2). Peak VO2 is a product of cardiac output (oxygen delivery) and arterial-venous oxygen content difference (A-VO2, oxygen extraction by skeletal muscles). Peak VO2 is 40% lower in HFPEF patients when compared to age and sex-matched controls. Severe exercise intolerance is the most common symptom in patients with chronic HFPEF and their reduced peak VO2 is driven primarily by reduced heart rate and impaired O2 utilization of skeletal muscles during peak exercise. This decline in A-V O2 is secondary to deconditioning, sarcopenia, increased adipose infiltration into skeletal muscle, impaired oxidative metabolism and impaired muscle blood flow.[17]–[22]
4. Diagnosing HFPEF
The diagnosis of HF is challenging in older adults, because older patients often present with symptoms that are nonspecific and are likely to have other co-morbidities that mimic symptoms of HF. The most common presentation of HF includes dyspnea on exertion (Table 2). Older adults may attribute their dyspnea on exertion to normal aging and respond to their early symptoms by restricting their physical activities, thus delaying clinical manifestation and diagnosis. Potential etiologies for the common symptoms of HF should be carefully examined with a detailed history and physical. Furthermore, it should be recognized that, particularly in the absence of an abnormal condition such as severe deconditioning or severe obesity, normal aging by itself does not cause exertional fatigue and dyspnea. These symptoms virtually always denote an underlying pathological process, even in the most advanced ages.
Table 2. Challenges in the clinical history of heart failure in older adults.
| Atypical symptoms |
Malaise, confusion, irritability, anorexia, sleep disturbance, decreased activity, abdominal complaints |
| Alternative explanations for symptoms |
Fluid retention: drug (NSAIDS), venous insufficiencyDyspnea: COPD, anemia, pneumoniaFatigue: anemia, hypothyroidism, obesity, deconditioning, depression |
| Minimize symptoms |
“I just can't get around, I'm 87” |
| Fewer exertional symptoms | Osteoarthritis, sarcopenia, loss of balance, poor vision |
COPD: chronic obstructive pulmonary disease; NSAIDS: Non-steroidal anti-inflammatory drugs.
Atypical manifestations of HF are more common in the elderly who may present with confusion, weakness, gastrointestinal disturbances, failure to thrive or disorientation. Corroboration with a family member, caregiver, or witness can be helpful in obtaining a reliably history in those with cognitive impairment or sensory dysfunction. Older adults may fail to perceive gradual but progressive declines in exercise tolerance, and clinicians can often attribute them to old age, thereby limiting identification of important and early decrements. Furthermore, the clinical signs and symptoms of HFREF and HFPEF are similar. While a dilated left ventricular (LV), third heart sound, or signs of right-sided HF are more frequent in HFREF; a forceful, minimally displaced apical impulse and a fourth heart sound suggest HFPEF. The ability to distinguish these two phenotypes requires further evaluation with diagnostic imaging.
Echocardiography is central to the clinical evaluation of the patient with HF. It provides an assessment of systolic function, and may diagnose other causes of HF, such as aortic stenosis, valvular regurgitation, pericardial disease, hypertrophic cardiomyopathy, and cardiac amyloidosis. Diastolic filling abnormalities are seen with normal aging in other disease states (e.g., hypertension, diabetes mellitus, renal insufficiency, obesity), so Doppler abnormalities may be present without the clinical circumstance of HFPEF. Combining Doppler mitral valve inflow with tissue Doppler imaging of the mitral valve annulus is recommended to enhance the accurate assessment of diastolic function. A high ratio of early mitral inflow velocity and early mitral valve annular tissue Doppler velocity (E/e') has been suggested to be the best index of elevated LV filling pressure and LV stiffness in HF.[23],[24]
The American College of Cardiology/American Heart Association (ACC/AHA) outlined diagnostic criteria for HFPEF in 2013 and in contrast to the European Society of Cardiology (ESC) did not include imaging requirements for diastolic dysfunction, specific structural parameters or B-type natriuretic peptide (BNP) levels. The diagnosis is based on a normal LVEF with typical symptoms and signs of HF and no valvular abnormalities or other factors to explain HF symptoms.[25] Myocardial performance has been shown to be decreased in HFPEF despite a normal ejection fraction, when using a mid-wall measure of ventricular function (mid-wall shortening fraction).[26] Another measure, analogous to EF, the myocardial contraction fraction (MCF), which is the ratio of stroke volume to myocardial volume, has been studied in the HFPEF population and has both diagnostic and prognostic applicability. MCF represents a volumetric index of myocardial shortening that is highly correlated with global longitudinal strain.[26],[27]
5. Treatment of HFPEF—goals of pharmacotherapy
HFPEF and HFREF share similarities in terms of clinical presentation, however, important differences exist in pathophysiology and response to therapy. Medications that have shown be beneficial in HFREF have not produced similar beneficial effects in the HFPEF population. Empiric targets of pharmacotherapy in HFPEF include controlling hypertension, reducing LV hypertrophy and fibrosis, preventing inappropriate tachycardia, and improving LV relaxation for adequate diastolic filling.
Adequate control of systolic hypertension is an important strategy for management and prevention of HFPEF in older persons. Caution is warranted since excessive reduction in stroke volume and blood pressure could potentially precipitate syncope or a fall in older adults in whom orthostatic intolerance from ventricular vascular stiffening is more problematic. Preferred initial therapy for blood pressure (BP) control in HFPEF includes using a thiazide diuretic with angiotensin-converting enzyme (ACE) inhibitors or angiotensin-receptor blockers (ARBs); beta-adrenergic antagonists as second line therapies for BP reduction.
ACE inhibitors and ARBs are routinely used and thought to be beneficial through interference with neuroendocrine activation, which is central to the HF state, but have not been shown to decrease mortality in HFPEF.[28],[29] These agents reduce LV hypertrophy, fibrosis, and improve LV relaxation and aortic distensibility, significantly improving functional class, exercise duration, diastolic filling, and LV mass in several small studies.[30]–[33] Beta-blockers similarly reduce blood pressure and promote regression of LV hypertrophy. The negative chronotropic properties of beta-blockers with the concomitant increase in the diastolic filling period are often employed clinically as a justification for their use. However, concern regarding beta blocker use is raised because chronotropic incompetence is highly frequent in older adults with HFPEF and the addition of such agents could further blunt the heart rate response with exercise, impair diastolic LV relaxation, and contribute to exertional intolerance.[34] Aldosterone antagonists were shown in the TOPCAT trial not to significantly reduce the incidence of the composite outcome of death from cardiovascular causes, aborted cardiac arrest, or hospitalization. However, spironolactone did have a positive effect on HF hospitalizations which when coupled with significant heterogeneity in this outcome by region suggests that spironolactone may be of value in the management of HFPEF.[35]
6. Managing end stage HF
HF is a terminal illness in older patients with advanced symptoms with a one-year mortality rate around 50% (25%–50%).[36] Device therapies such as biventricular pacemakers, left ventricular assist devices and implantable cardioverter defibrillators for advanced HF have not been well studied in older adults (> 75 years of age) and thus the benefits in this population are not well defined. Accordingly, the decision process for these interventions requires a comprehensive approach directed not only at physiologic benefits and potential clinical risks, but attention to psychosocial and ethical issues as well as comorbidities and life expectancy.
The proper management includes discussing end of life planning and palliative approaches to prevent readmissions and set realistic expectations. This includes engaging in frank discussions with elderly patients afflicted by significant comorbidities and addressing the individual's health care goals, values, and priorities, including quality versus quantity of life, preferences regarding sudden cardiac death versus death from progressive HF or other causes, and avoidance of invasive procedures, in order to make the best shared decisions. A full discussion of prognosis includes not only the risks of death, but also the potential burdens of worsening symptoms, limited functional capacity, lost of independence, reduced social functioning, decreased quality of life, and increased caregiver commitment. Particular attention should be paid to evaluating frailty in these patients and addressing advance directives. These discussions should involve close family members, friends and caregivers when making clinical assessments as to appoint surrogate decision makers who best understand the patient's wishes.
7. Opportunities for future research and clinical pearls
Future research opportunities in the area of HFPEF and HF in the elderly have the potential to improve our current outlook on management. By focusing on the multi-factorial pathophysiology of the aging cardiovascular system and other organ systems and their contributions to adverse outcomes in older HF patients, we may shift attention from a singular focus on cardiac function to include peripheral abnormalities such as vascular and skeletal muscle dysfunction. Recognizing the success achieved in the treatment of HFREF by addressing neurohormonal and renal mechanisms, new therapies for HFPEF may be achieved by a similar shift to include contributing factors outside the heart.
The Dietary Approaches to Stop Hypertension, Salt Restricted Diet (DASH/SRD) features a diet high in fruits, vegetables, low-fat products, whole grains, poultry, fish and nuts with a restriction in red meat and sugar containing foods/drinks. This diet has been studied in animal models of HFPEF and shown initial promising success in the hypertensive HFPEF population. The hypothesis for its use is based upon the “salt-sensitive hypertensive” phenotype of this cohort. These individuals have blood pressure changes that are directly related to sodium intake and have been linked to oxidative stress and cardiac and vascular remodeling. Studies have shown that HFPEF patients who follow the DASH/SRD have a significant improvement in blood pressure, ventricular diastolic function, ventricular-vascular coupling as well as a reduction of oxidative stress.[37]–[39] These findings offer a new perspective in treating hypertensive HFPEF patients and further supports investigation in dietary and lifestyle modifications in clarifying the pathophysiologic link between hypertension and cardiovascular damage.
Exercise intolerance is the primary symptom in chronic HFPEF and a strong determinant of quality of life (QOL).[40] A patient's decrement in exercise tolerance is an independent predictor of survival and thus a key therapeutic target. As described earlier, this can be objectively measured as peak VO2 defined as a product of cardiac output (oxygen delivery) and A-VO2. Studies have assessed left ventricular cardiac function as well as peripheral arterial and skeletal muscle function and their relative contributions to improvements in peak VO2 in subjects with HFPEF. One study showed that exercise training was associated with an increase in three separate measures of exercise capacity (peak VO2, power output and exercise time).[41] Exercise training improves exercise capacity, QOL, NYHA class and reduces hospitalizations, which is predominately mediated by increases in A-VO2 differences.[21],[42]
The high rate of recurrent HF hospitalizations has been in part attributed to the post hospitalization syndrome, in which perturbations in sleep wake cycles, poor oral intake, exposure to challenging situations and deconditioning induced by bed rest or inactivity can contribute to altered mental state and decreased physical function which are key drivers of recurrent admissions soon after discharge.[43] To address one key aspect of this process, the REHAB-HF (A Trial of Rehabilitation Therapy in Older Acute Heart Failure Patients) is a multicenter, randomized, attention-controlled, single-blind trial designed to examine the hypothesis that, in addition to standard care, a novel, tailored, progressive, multi-domain rehabilitation intervention administered to older patients with acute decompensated HF with a normal or reduced EF, beginning early during hospitalization and continuing for 12 weeks will improve physical function and key clinical outcomes, including the rate of re-hospitalization.
Health care providers are trained to focus on the diagnosis, treatment and prevention of disease. This “disease centered model” inadvertently leads to under-treatment, overtreatment, or mistreatment.[44] The concept of goal directed care focuses on the individual and ability to identify their preferences and individualized goals. Current health care approaches have led to care that is often fragmented and can contribute to discrepancies between the outcomes that the caregivers and patients are interested in achieving and those that are being addressed by health care providers. For example, 20% of patients aged greater than 70 years with HF are prescribed at least 10 medications. The likelihood of an adverse event is 10% with the addition of each medication, which translates into a 100% chance of adverse drug reactions.[45] Accordingly, it is critical to evaluate individuals in light of their preferences and transition to goal-directed care when the treatment becomes burdensome. The advantages of this approach include simplifying decision making by focusing on outcomes that span conditions, articulating which health states are important to the patient and creates a common ground for goals of care and desired health states.
References
- 1.Go A, Mozaffarian D, Roger V, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.deFilippi C, Christenson R, Gottdiener J, et al. Dynamic cardiovascular risk assessment in elderly people. The role of repeated N-terminal pro-B-type natriuretic peptide testing. J Am Coll Cardiol. 2010;55:441–450. doi: 10.1016/j.jacc.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottdiener J, Arnold A, Aurigemma G, et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 4.Mosterd A, Hoes A, de Bruyne M, et al. Prevalence of heart failure and left ventricular dysfunction in the general population; The Rotterdam Study. Eur Heart J. 1999;20:447–455. [PubMed] [Google Scholar]
- 5.Gottdiener J, McClelland R, Marshall R, et al. Outcome of congestive heart failure in elderly persons: influence of left ventricular systolic function. The Cardiovascular Health Study. Ann Intern Med. 2002;137:631–639. doi: 10.7326/0003-4819-137-8-200210150-00006. [DOI] [PubMed] [Google Scholar]
- 6.Croft J, Giles W, Pollard R, et al. Heart failure survival among older adults in the United States: a poor prognosis for an emerging epidemic in the Medicare population. Arch Intern Med. 1999;159:505–510. doi: 10.1001/archinte.159.5.505. [DOI] [PubMed] [Google Scholar]
- 7.Lam C, Donal E, Kraigher-Krainer E, et al. Epidemiology and clinical course of heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:18–28. doi: 10.1093/eurjhf/hfq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeSouza C, Shapiro L, Clevenger C, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 9.Lancel S, Qin F, Lennon S, et al. Oxidative posttranslational modifications mediate decreased SERCA activity and myocyte dysfunction in Galphaq-overexpressing mice. Circ Res. 2010;107:228–232. doi: 10.1161/CIRCRESAHA.110.217570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nonogi H, Hess O, Ritter M, et al. Diastolic properties of the normal left ventricle during supine exercise. Br Heart J. 1988;60:30–38. doi: 10.1136/hrt.60.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chattopadhyay S, Alamgir MF, Nikitin NP, et al. Lack of diastolic reserve in patients with heart failure and normal ejection fraction. Circ Heart Fail. 2010;3:35–43. doi: 10.1161/CIRCHEARTFAILURE.108.824888. [DOI] [PubMed] [Google Scholar]
- 12.Hay I, Rich J, Ferber P, et al. Role of impaired myocardial relaxation in the production of elevated left ventricular filling pressure. Am J Physiol Heart Circ Physiol. 2005;288:1203–1208. doi: 10.1152/ajpheart.00681.2004. [DOI] [PubMed] [Google Scholar]
- 13.Zile M, Baicu C, Gaasch W. Diastolic heart failure—abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350:1953–1959. doi: 10.1056/NEJMoa032566. [DOI] [PubMed] [Google Scholar]
- 14.Gillebert T, Leite-Moreira A, De Hert S. Load dependent diastolic dysfunction in heart failure. Heart Fail Rev. 2000;5:345–355. doi: 10.1023/a:1026563313952. [DOI] [PubMed] [Google Scholar]
- 15.Borlaug B, Melenovsky V, Redfield M, et al. Impact of arterial load and loading sequence on left ventricular tissue velocities in humans. J Am Coll Cardiol. 2007;50:1570–1577. doi: 10.1016/j.jacc.2007.07.032. [DOI] [PubMed] [Google Scholar]
- 16.Gandhi S, Powers J, Nomeir A, et al. The pathogenesis of acute pulmonary edema associated with hypertension. N Engl J Med. 2001;344:17–22. doi: 10.1056/NEJM200101043440103. [DOI] [PubMed] [Google Scholar]
- 17.Bhella PS, Prasad A, Heinicke K, et al. Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. Eur J Heart Fail. 2011;13:1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitzman D, Higginbotham M, Cobb F, et al. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17:1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 19.Haykowsky M, Brubaker P, John J, et al. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abudiab M, Redfield M, Melenovsky V, et al. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail. 2013;15:776–785. doi: 10.1093/eurjhf/hft026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haykowsky M, Kitzman D. Exercise physiology in heart failure and preserved ejection fraction. Heart Fail Clin. 2014;10:445–452. doi: 10.1016/j.hfc.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kitzman D, Nicklas B, Kraus W, et al. Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Physiol Heart Circ Physiol. 2014;306:1364–1370. doi: 10.1152/ajpheart.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasner M, Westermann D, Steendijk P, et al. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: a comparative Doppler-conductance catheterization study. Circulation. 2007;116:637–647. doi: 10.1161/CIRCULATIONAHA.106.661983. [DOI] [PubMed] [Google Scholar]
- 24.Ommen S, Nishimura R, Appleton C, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 25.Yancy C, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 26.King D, El-Khoury Coffin L, Maurer M. Myocardial contraction fraction: a volumetric index of myocardial shortening by freehand three-dimensional echocardiography. J Am Coll Cardiol. 2002;40:325–329. doi: 10.1016/s0735-1097(02)01944-7. [DOI] [PubMed] [Google Scholar]
- 27.Tendler A, Helmke S, Teruya S, et al. The myocardial contraction fraction is superior to ejection fraction in predicting survival in patients with AL cardiac amyloidosis. Amyloid. 2015;22:61–66. doi: 10.3109/13506129.2014.994202. [DOI] [PubMed] [Google Scholar]
- 28.Yusuf S, Pfeffer M, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-preserved trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 29.Cleland J, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 30.Groban L, Pailes N, Bennett C, et al. Growth hormone replacement attenuates diastolic dysfunction and cardiac angiotensin II expression in senescent rats. J Gerontol A Biol Sci Med Sci. 2006;61:28–35. doi: 10.1093/gerona/61.1.28. [DOI] [PubMed] [Google Scholar]
- 31.Groban L, Yamaleyeva L, Westwood B, et al. Progressive diastolic dysfunction in the female mRen(2). Lewis rat: influence of salt and ovarian hormones. J Gerontol A Biol Sci Med Sci. 2008;63:3–11. doi: 10.1093/gerona/63.1.3. [DOI] [PubMed] [Google Scholar]
- 32.Lasocki S, Iglarz M, Seince P, et al. Involvement of reninangiotensin system in pressure-flow relationship: role of angiotensin-converting enzyme gene polymorphism. Anesthesiology. 2002;96:271–275. doi: 10.1097/00000542-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Little W, Wesley-Farrington D, Hoyle J, et al. Effect of candesartan and verapamil on exercise tolerance in diastolic dysfunction. J Cardiovasc Pharmacol. 2004;43:288–293. doi: 10.1097/00005344-200402000-00019. [DOI] [PubMed] [Google Scholar]
- 34.Upadhya B, Taffet G, Cheng C, et al. Heart failure with preserved ejection fraction in the elderly: scope of the problem. J Mol Cell Cardiol. 2015;83:73–87. doi: 10.1016/j.yjmcc.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pfeffer M, Claggett B, Assmann S, et al. Regional variation in patients and outcomes in the Treatment of Preserved Cardiac Function Heart Failure With an Aldosterone Antagonist (TOPCAT) trial. Circulation. 2015;131:34–42. doi: 10.1161/CIRCULATIONAHA.114.013255. [DOI] [PubMed] [Google Scholar]
- 36.Roger V. Epidemiology of heart failure. Circ Res. 2013;113:646–659. doi: 10.1161/CIRCRESAHA.113.300268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Solaiman Y, Jesri A, Zhao Y, et al. Low-Sodium DASH reduces oxidative stress and improves vascular function in saltsensitive humans. J Hum Hypertens. 2009;23:826–835. doi: 10.1038/jhh.2009.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hummel S, Seymour E, Brook R, et al. Low-sodium dietary approaches to stop hypertension diet reduces blood pressure, arterial stiffness, and oxidative stress in hypertensive heart failure with preserved ejection fraction. Hypertension. 2012;60:1200–1206. doi: 10.1161/HYPERTENSIONAHA.112.202705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hummel S, Seymour E, Brook R, et al. Low-sodium DASH diet improves diastolic function and ventricular-arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ Heart Fail. 2013;6:1165–1171. doi: 10.1161/CIRCHEARTFAILURE.113.000481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen S, Chang J, Tsai Y, et al. Ratio of transmitral E-wave velocity to early diastole mitral annulus velocity with cardiovascular and renal outcomes in chronic kidney disease. Nephron Clin Pract. 2013;123:52–60. doi: 10.1159/000351513. [DOI] [PubMed] [Google Scholar]
- 41.Kitzman D, Brubaker P, Morgan T, et al. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pandey A, Parashar A, Kumbhani D, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta analysis of randomized control trials. Circ Heart Fail. 2015;8:33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krumholz H. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med. 2013;368:100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tinetti M, Fried T. The end of the disease era. Am J Med. 2004;116:179–185. doi: 10.1016/j.amjmed.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 45.Gandhi T, Weingart S, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med. 2003;348:1556–1564. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]