Abstract
Objective
To investigate the procedural outcomes and the long-term survival of patients undergoing transcatheter aortic valve implantation (TAVI) and compare study results of patients ≤ 80 years and patients > 80 years old.
Methods
A total of 240 patients treated with TAVI were divided into two groups according to age ≤ 80 years (n = 105; 43.8%) and > 80 years (n = 135; 56.2%). The baseline characteristics and the procedural outcomes were compared between these two groups of patients.
Results
With the exception of peripheral artery disease and hypercholesterolemia, which were more frequently observed in the older age group, baseline characteristics were comparable between groups. Complication rates did not differ significantly between patients ≤ 80 years and patients > 80 years. There were no differences in 30-day mortality rates between patients aged ≤ 80 years and patients > 80 years old (9.5% vs. 7.4%, respectively; P = 0.557). After a median follow-up of 28 months (interquartile range: 16–42 months), 50 (47.6%) patients aged ≤ 80 years died compared to 57 (42%) deaths in the group of patients > 80 years old (P = 0.404).
Conclusion
The results of the present single center study showed that age did not significantly impact the outcomes of TAVI.
Keywords: Age, Survival rate, Transcatheter aortic valve implantation
1. Introduction
Current evidence shows that transcatheter aortic valve implantation (TAVI) is a safe and feasible alternative treatment modality for patients with severe aortic stenosis and contraindications or at high risk for surgical intervention.[1]–[3] However, selection of patients is pivotal to ensure a high procedural success rate, minimize the risk of complications and improve the long-term survival of the patients. As the population becomes older, the prevalence of patients with severe aortic stenosis increases significantly.[4] There remains however concerns whether TAVI in very old patients is associated with increased procedural risks and if this treatment conveys any significant improvement in survival compared with “younger” patients. With regard to inoperable patients, TAVI has been demonstrated more cost-effective therapy compared to medical treatment, and in high-risk patients the cost-effectiveness of TAVI is less favorable compared to surgical aortic valve replacement.[5] Age is one of the variables included in currently used risk stratification engines. However, the evidence showing that TAVI is beneficial in terms of long-term survival across the several age subgroups is scarce.[6]–[9] Therefore, we evaluated the procedural outcomes and the long-term survival of patients undergoing TAVI and compared the results of patients ≤ 80 years and patients > 80 years old.
2. Methods
A total of 240 patients who underwent TAVI between November 2007 and March 2013, at the Leiden University Medical Centre (the Netherlands) were included in the present evaluation. All patients were diagnosed with symptomatic severe aortic valve stenosis and had high surgical risk or contraindications for surgery. A logistic European System for Cardiac Operative Risk Evaluation I (EuroSCORE I) > 20% defined high operative risk.[10] Patients who underwent valve-in-valve procedure were excluded.
Patients were clinically evaluated in a multidisciplinary team of clinical, imaging and interventional cardiologists, cardiothoracic surgeons and anesthesiologists, to assess operative risk, comorbidities,[11] frailty and procedural feasibility. In addition, transthoracic echocardiography, invasive coronary angiography, and multi-detector row computed tomography (unless contraindicated) were performed to assess aortic stenosis severity, left ventricular (LV) function, associated valvular heart disease, coronary artery disease and anatomy, dimensions of the aortic annulus and peripheral arteries. After TAVI, patients underwent clinical and echocardiographic evaluation at 1, 3, 6 and 12 months follow-up. Clinical and imaging data were prospectively collected in an electronic clinical patient file (EPD Vision version 11.3, Leiden, the Netherlands) and retrospectively analyzed. The institutional review board approved this retrospective analysis and waived the need of written patient informed consent.
TAVI was performed at the catheterization laboratory or a hybrid operating room under general anesthesia. A balloon expandable system [Edwards Sapien or Sapien XT valve (Edwards Lifesciences, Irvine, CA, USA)] or the self-expandable CoreValve System (Medtronic, Minneapolis, MN, USA) were the transcatheter valves implanted. Transfemoral access was the preferred approach in all patients. Transapical access was performed in patients with inappropriate peripheral artery anatomy. Both accesses were performed by surgical cut-down. Procedures were assisted by transesophageal echocardiography (iE33, Philips Medical Systems, Andover, MA, USA) and the hemodynamics of the transcatheter valve and the presence and grade of (para-) valvular aortic regurgitation were assessed immediately after implantation of the valve. Procedural success and complications were evaluated according to the Valve Academic Research Consortium (VARC) criteria;[12] mortality, myocardial infarction, stroke, bleeding, acute kidney injury, vascular complications, and prosthetic valve performance were recorded.
A commercially available ultrasound system (Vivid 7, E9, General Electric Horten, Norway) was used for pre- and post-TAVI transthoracic echocardiography (TTE). The pre-procedural evaluation included the assessment of the valve morphology at the parasternal short-axis view, and the left ventricular outflow tract diameter was measured at the parasternal long-axis view.[13] The peak and mean transaortic pressure gradients were assessed in the apical long-axis or 5-chamber views and the aortic valve area was calculated with the continuity equation.[13] Aortic stenosis was considered severe if the aortic valve area was < 1.0 cm2 and/or the transaortic mean gradient was ≥ 40 mmHg or peak jet velocity > 4 m/s.[14] LV end-diastolic and end-systolic volumes were calculated by the method of Simpson and the LV ejection fraction was derived.[15] After TAVI, the presence of para-valvular aortic regurgitation was evaluated with color Doppler echocardiography as previously described, and graded according to the VARC-2 criteria.[16]
Follow-up was performed in our institution (Leiden University Medical Centre, the Netherlands) during the first year according to the clinical care track. After this period, patients were controlled on a yearly basis in our institution or at the referral hospital. The primary endpoint was all-cause mortality. Survival follow-up data were retrieved from municipality registries. Secondary endpoints included procedure related complications, defined as procedural mortality, stroke, vascular injury, major bleeding, renal failure, a repeat procedure, atrio-ventricular block and pacemaker implantation.[16]
A package of SPSS software version 20 (IBM Corp, Armonk, NY, USA) was used for statistical analyses. According to the Kolmogorov-Smirnov test and visual inspection of the histograms, continuous variables were categorized as normally distributed and were presented as mean ± SD or as non-normally distributed and were presented as median and inter-quartile range. The categorical variables were presented as number and frequency. Continuous variables were compared with the unpaired Student's t-test if they were normally distributed, or otherwise, the Mann-Whitney test. Categorical variables were compared with the χ2 test or Fisher's exact test, as indicated. The Kaplan Meier method was used to estimate the cumulative mortality. The Log-rank test was used to compare the two age groups. A two-sided P < 0.05 was considered statistically significant.
3. Results
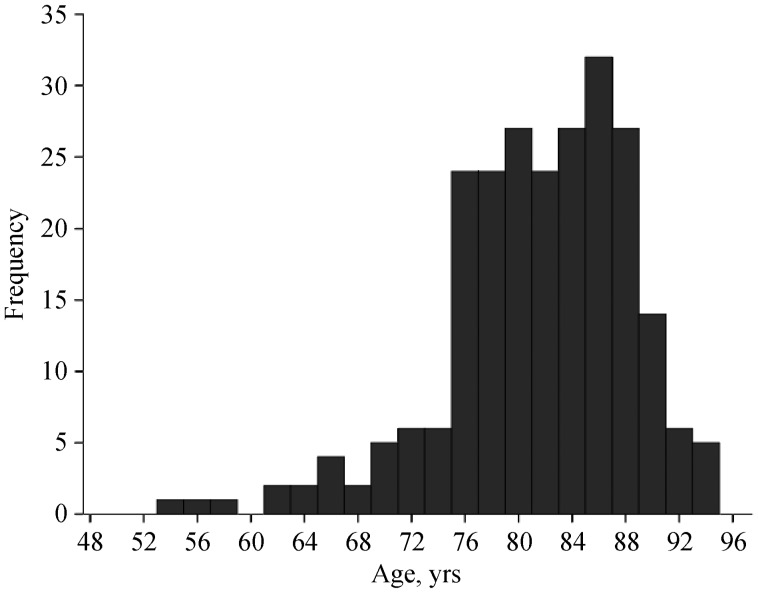
Table 1 summarizes the clinical and echocardiographic characteristics of the overall population and two subgroups according to age (≤ 80 years old versus > 80 years old). Of the 240 patients, 105 (43.8%) were included in the age group of ≤ 80 years and the remaining 135 were older than 80 years. Mean age was 81 ± 7 years. Age distribution is shown in Figure 1. Patients aged > 80 years were more frequently women and had significantly less frequent hypercholesterolemia and peripheral vascular disease than their younger counterparts. The remaining clinical characteristics were not significantly different between the groups. Logistic EuroSCORE I trended higher in the group of patients aged > 80 years (24.6% vs. 21.2%, P = 0.072).
Table 1. Baseline characteristics.
| All patients (n = 240) | ≤ 80 years (n = 105) | > 80 years (n = 135) | P-value | |
| Age, yrs | 81 ± 6.9 | 74.8 ± 5.5 | 85.8 ± 3.1 | - |
| Female | 119 (49.6%) | 39 (37.1%) | 80 (59.3%) | 0.001 |
| Hypertension | 180 (75%) | 79 (75.2%) | 101 (74.8%) | 0.940 |
| Smoking | 105 (43.8%) | 53 (50.5%) | 52 (38.5%) | 0.064 |
| Diabetes | 70 (29.2%) | 30 (28.6%) | 40 (29.6%) | 0.858 |
| Hypercholesterolemia | 148 (61.7%) | 78 (74.3%) | 70 (51.9%) | < 0.001 |
| Ischemic heart disease | 152 (63.3%) | 70 (66.7%) | 82 (60.7%) | 0.345 |
| Peripheral artery disease | 119 (49.6%) | 60 (57.1%) | 59 (43.7%) | 0.039 |
| Chronic obstructive pulmonary disease | 74 (30.8%) | 36 (34.3%) | 38 (25.9%) | 0.307 |
| Creatinine clearance, mL/min per 1.73 m2(median: percentiles 25–7 5) | 53 ± 21.9(43–67.7) | 54 ± 27.6(43–8 2.5) | 52.0 ± 14.5(43–5 9) | 0.494 |
| Logistic EuroSCORE I | 23.2% ± 14.6% | 21.2% ± 14.8% | 24.6% ± 14.1% | 0.072 |
| NYHA Class 1–2 | 96 (40%) | 44 (41.9%) | 52 (38.5%) | 0.595 |
| NYHA Class 3–4 | 144 (60%) | 61 (58.1%) | 83 (61.5%) | 0.595 |
| Angina | 88 (36.7%) | 40 (38.1%) | 48 (35.6%) | 0.685 |
| Syncope | 40 (16.7%) | 14 (13.3%) | 26 (19.3%) | 0.222 |
| Sinus rhythm | 171 (71.3%) | 79 (75.2%) | 92 (68.1%) | 0.229 |
| Atrial fibrillation | 44 (18.3%) | 15 (14.3%) | 29 (21.5%) | 0.153 |
| Pacemaker | 25 (10.4%) | 11 (10.5%) | 14 (10.4%) | 0.979 |
| LV ejection fraction | 52.1% ± 14.5% | 50.1% ± 14.6% | 53.6% ± 14.3% | 0.064 |
| Mean transaortic pressure gradient, mmHg | 44.3 ± 19.3 | 41.7 ± 19.3 | 46.4 ± 19.1 | 0.062 |
| AVA, cm2 | 0.78 ± 0.8 | 0.76 ± 0.2 | 0.80 ± 1.0 | 0.673 |
| Pulmonary artery pressure, mmHg | 36.3 ±11.5 | 36.2 ± 11.6 | 36.3 ± 11.4 | 0.912 |
| Mitral regurgitation (moderate-severe) | 72 (30%) | 27 (25.7%) | 45 (33.3%) | 0.201 |
Data are presented as mean ± SD or n (%). Hypertension: history of high blood pressure and/or on antihypertensive treatment; hypercholesterolaemia: history of hypercholesterolemia and/or on statin therapy; diabetes (type 1 and 2) was defined as a fasting plasma glucose level C126 mg/dL, use of oral glucose lowering medication or insulin. AVA: aortic valve area; EuroSCORE I: logistic European System for Cardiac Operative Risk Evaluation I; LV: left ventricle; NYHA: New York Heart Association.
Figure 1. Age distribution.
The transfemoral access was used in 99 (41.3%) patients whereas the remaining patients underwent TAVI through a transapical access. The Edwards Sapien or Sapien XT was implanted in 222 patients. Eighteen patients received a CoreValve bioprosthesis. TAVI was successfully performed in all patients. Peri-procedural complications according to the VARC-2 criteria are described in Table 2. The incidence of periprocedural complications between the two age groups was not significantly different.
Table 2. Procedural characteristics and periprocedural complications.
| All patients (n = 240) | ≤ 80 years (n = 105) | > 80 years (n = 135) | P-value | |
| Access | ||||
| Transfemoral | 99 (41.3%) | 39 (37.1%) | 60 (44.4%) | 0.254 |
| Transapical | 141 (58.8%) | 66 (62.9%) | 75 (55.6%) | 0.254 |
| Edwards sapien (XT) | 222 (92.5%) | 99 (94.3%) | 123 (91.1%) | 0.354 |
| CoreValve | 18 (7.5%) | 6 (5.7%) | 12 (8.9%) | 0.354 |
| Vascular injury | 23 (9.6%) | 10 (9.5%) | 13 (9.6%) | 0.978 |
| Major | 13 (5.4%) | 7 (6.7%) | 6 (4.4%) | 0.451 |
| Minor | 12 (5.0%) | 4 (3.8%) | 8 (5.9%) | 0.455 |
| Bleeding | 19 (7.9%) | 8 (7.6%) | 11 (8.1%) | 0.880 |
| Major | 4 (1.7%) | 2 (1.9%) | 2 (1.5%) | 0.799 |
| Minor | 15 (6.3%) | 6 (5.7%) | 9 (6.7%) | 0.762 |
| Stroke | 8 (3.3%) | 1 (1%) | 7 (5.2%) | 0.070 |
| Major | 5 (2.1%) | 1 (1%) | 4 (3%) | 0.279 |
| Minor | 3 (1.3%) | 0 | 3 (2.2%) | 0.124 |
| All-cause death (in hospital) | 16 (6.7%) | 9 (8.6%) | 7 (5.2%) | 0.297 |
| Non cardiac complications | 30 (12.5%) | 17 (16.2%) | 13 (9.6%) | 0.127 |
| Valve migration | 1 (0.4%) | 0 (0%) | 1 (0.7%) | 0.377 |
| Acute kidney injury | 11 (4.6%) | 5 (4.8%) | 6 (4.4%) | 0.907 |
| AR ≥ 3 | 12 (5%) | 5 (4.8%) | 7 (5.2%) | 0.881 |
| AV block | 13 (5.4%) | 6 (5.7%) | 7 (5.2%) | 0.857 |
| Cardiac tamponade | 8 (3.3%) | 2 (1.9%) | 6 (4.4%) | 0.277 |
| Myocardial infarction | 0 | 0 | 0 |
Data are presented as n (%). AR: aortic regurgitation; AV: atrial-ventricular.
Moderate to severe aortic valve regurgitation (valvular or paravalvular) was observed in 5% of the total population and an emergency valve-in-valve implantation was performed in four (1.7%) patients to reduce the grade of aortic regurgitation. Subsequently, valve-in-valve implantation procedures were performed in four (1.7%) additional patients: two patients presented with recurrent, symptomatic, moderate, paravalvular regurgitation within one week, and one patient at three months follow-up after the initial TAVI, and one patient presented with symptomatic aortic stenosis due to degenerative trans-catheter valve prosthesis four years after the initial procedure. The non-cardiac complications were mainly infectious disorders such as pneumonia and post-procedural fever without positive cultures.
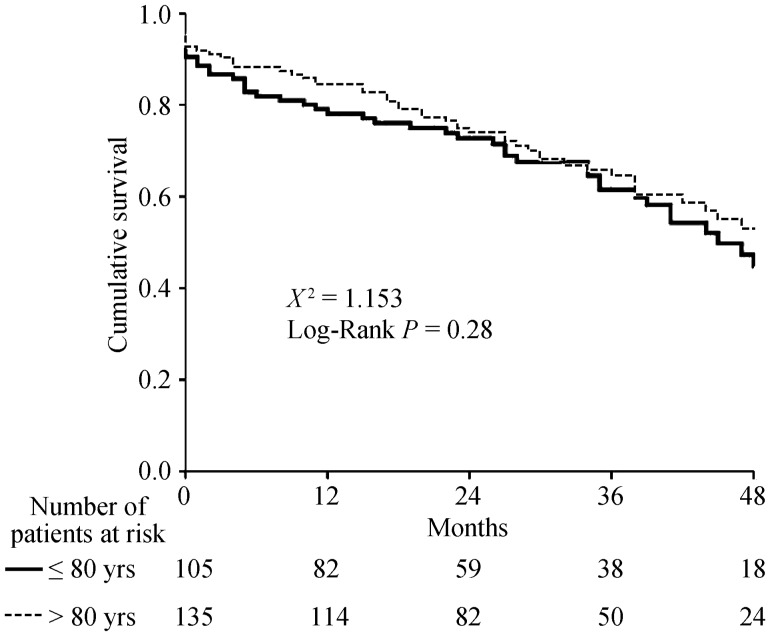
There were no differences in the 30-day all-cause mortality rates between patients aged ≤ 80 years and patients > 80 years old (9.5% vs. 7.4%, respectively; P = 0.557) (Table 3). During a median follow-up of 28 months (interquartile range: 16–42 months), 50 (47.6%) patients aged ≤ 80 years died compared with 57 (42%) deaths in the group of patients > 80 years old (P = 0.404) (Figure 2). The cumulative 1- and 2-year all-cause mortality rates among patients aged ≤ 80 years were 20.9% and 26.7% respectively compared with 15.6% and 24.4% in patients > 80 years old.
Table 3. Cumulative all-cause mortality according to transcatheter aortic valve implantation access.
| 30 days |
1-yr |
2-yrs |
Total follow up |
|||||||||
| ≤ 80 yrs | > 80 yrs | P-value | ≤ 80 yrs | > 80 yrs | P-value | ≤ 80 yrs | > 80 yrs | P-value | ≤ 80 yrs | > 80 yrs | P-value | |
| Transfemoral | 4 (10.3%) | 5 (8.3%) | 0.746 | 7 (17.9%) | 9 (15%) | 0.704 | 31 (20.5%) | 46 (23.3%) | 0.840 | 16 (41%) | 23 (38.3%) | 0.591 |
| Transapical | 6 (9.1%) | 5 (6.7%) | 0.594 | 15 (22.7%) | 12 (16%) | 0.309 | 46 (30.3%) | 56 (25.3%) | 0.467 | 34 (51.5%) | 34 (45.3%) | 0.452 |
| Total | 10 (9.5%) | 10 (7.4%) | 0.557 | 22 (20.9%) | 21 (15.6%) | 0.276 | 77 (26.7%) | 102 (24.4%) | 0.592 | 50 (47.6%) | 57 (42.2%) | 0.283 |
Data are presented as n (%).
Figure 2. Kaplan Meier survival curve.
4. Discussion
The present evaluation showed that TAVI is associated with comparable short- and mid-term outcomes in patients aged ≤ 80 years and patients older than 80 years.
Data from randomized controlled trials comparing TAVI versus surgical aortic valve replacement or medical treatment demonstrated that age was not an independent determinant of 1-year all-cause mortality.[1],[17],[18] In the Placement of Aortic Transcatheter Valves (PARTNER) trial cohort B, which showed that TAVI presented better outcomes than medical treatment in patients with severe aortic stenosis and contraindications for surgical replacement, in which 46% of included patients were aged > 85 years and showed similar benefits from TAVI than patients ≤ 85 years old.[17] Similarly, the trials that have compared TAVI versus surgical aortic valve replacement in patients with high operative risk included 47% of patients aged > 85 years and showed that both therapeutic arms provided similar outcomes.[1],[18] In addition, data from single- or multi-center registries have also shown that age does not significantly impact on the mid- and long-term outcomes of patients undergoing TAVI.[19]–[22] For example, in the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry including 7,710 patients undergoing TAVI (80% high-risk patients, 20% inoperable patients), the median age was 84 (interquartile range 78–88 years) and the reported 30-day mortality was 7.6%.[21] Similarly, the FRANCE-2 registry including 3,195 patients treated by TAVI (mean age 82.7 ± 7.2 years) showed a 30-day mortality rate of 9.7%,[20] while in the Sentinel Registry, including 4,571 patients (mean age 81.4 ± 7.1 years), the in-hospital mortality rate was 7.4%.[19] However, in the Sentinel registry, each 8-year increase in age was significantly associated with in-hospital mortality (OR: 1.18; 95% CI: 1.01–1.37, P = 0.04).[19] Notably, the percentage of patients aged > 80 years was 62.4%. These data are comparable to the data provided by the present study where 57% of patients were > 80 years old. The lack of significant differences in logistic EuroSCORE indicates that the younger group of patients had significantly more comorbidities to justify the high operative risk. These may have influenced the short- and long-term outcomes of these patients leading to similar survival rates between the groups. Pilgrim, et al.[22] recently showed that in high-risk patients the independent determinants of 5-year mortality were body mass index ≤ 20 kg/m2, diabetes, peripheral artery disease, atrial fibrillation and pulmonary hypertension, whereas age was not associated. Therefore, these data suggest that the associated co-morbidities may have a larger impact on outcomes by TAVI than age.
A sub-study of the FRANCE-2 registry including 2,254 patients aged > 80 years showed in patients within the age group between 80–84 years old, that the prevalence of diabetes, prior myocardial infarction, prior cardiac surgery, chronic obstructive pulmonary disease and reduced LVEF was significantly higher than in the groups of patients aged 85–89 years and > 90 years.[9] In addition, the transapical access, frequently used in patients with increased morbidity and mortality risks, was more frequently used in younger patients (80–84 years old) than in the other two groups (85–89 years and > 90 years) (20.5% vs. 14.7 % and 11.6%, respectively; P < 0.001).[9] Upon multivariate analysis, female sex, higher logistic EuroSCORE, New York Heart Association functional class III-IV, renal failure and transapical access were independently associated with one-year mortality. However, age was not associated with outcome.
The present study has additional value by showing similar short- and mid-term outcomes of patients treated by TAVI independent of age. Along with previous studies, the present study reinforces the concept of age as no longer a valid parameter to select patients for TAVI. Age is one of the variables included in currently used risk scores (Logistic EuroSCORE I and II and the STS-PROM score).[10],[23] However, several studies have demonstrated the modest discrimination and calibration properties of current risk scores,[24],[25] and several engines have been proposed to better identify the patients who will benefit from TAVI.[24],[26] While the engine derived from the FRANCE-2 registry included the parameter age ≥ 90 years, [26] the TAVI2-SCORe included a cut-off value of > 85 years.[24] The Harrel's C-statistic (measure of discrimination property) for the FRANCE-2 derived score was 0.59,[26] while the TAVI2-SCORe had a C-statistic of 0.71.[24] The number of patients aged > 85 years in the present study was 27.9%.
In conclusion, the results of the present single center study showed that age is not associated with outcomes after TAVI. The impact of developments in valvular technology and long term experience based on procedural outcomes has not been considered.
Acknowledgments
The Department of Cardiology received research grants from Biotronik, Medtronic and Boston Scientific, Germany. Delgado V received speaking fees from Abbott Vascular. Kamperidis V received a European Society of Cardiology training grant, a European Association of Cardiovascular Imaging research grant, a Hellenic Cardiological Society training grant and a Hellenic Foundation of Cardiology research grant.
References
- 1.Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med. 2014;370:1790–1798. doi: 10.1056/NEJMoa1400590. [DOI] [PubMed] [Google Scholar]
- 2.Kodali SK, Williams MR, Smith CR, et al. Two-year outcomes after transcatheter or surgical aortic-valve replacement. N Engl J Med. 2012;366:1686–1695. doi: 10.1056/NEJMoa1200384. [DOI] [PubMed] [Google Scholar]
- 3.Makkar RR, Fontana GP, Jilaihawi H, et al. Transcatheter aortic-valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–1704. doi: 10.1056/NEJMoa1202277. [DOI] [PubMed] [Google Scholar]
- 4.Iung B, Vahanian A. Epidemiology of acquired valvular heart disease. Can J Cardiol. 2014:962–970. doi: 10.1016/j.cjca.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Boothroyd LJ, Spaziano M, Guertin JR, et al. Transcatheter aortic valve implantation: recommendations for practice based on a multidisciplinary review including cost-effectiveness and ethical and organizational issues. Can J Cardiol. 2013;29:718–726. doi: 10.1016/j.cjca.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Havakuk O, Finkelstein A, Steinvil A, et al. Comparison of outcomes in patients ≤ 85 versus >85 years of age undergoing transcatheter aortic-valve implantation. Am J Cardiol. 2014;113:138–141. doi: 10.1016/j.amjcard.2013.09.044. [DOI] [PubMed] [Google Scholar]
- 7.Pilgrim T, Kalesan B, Wenaweser P, et al. Predictors of clinical outcomes in patients with severe aortic stenosis undergoing TAVI: a multistate analysis. Circ Cardiovasc Interv. 2012;5:856–861. doi: 10.1161/CIRCINTERVENTIONS.112.974899. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto M, Meguro K, Mouillet G, et al. Comparison of effectiveness and safety of transcatheter aortic valve implantation in patients aged ≥ 90 years versus < 90 years. Am J Cardiol. 2012;110:1156–1163. doi: 10.1016/j.amjcard.2012.05.058. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto M, Mouillet G, Meguro K, et al. Clinical results of transcatheter aortic valve implantation in octogenarians and nonagenarians: insights from the FRANCE-2 registry. Ann Thorac Surg. 2014;97:29–36. doi: 10.1016/j.athoracsur.2013.07.100. [DOI] [PubMed] [Google Scholar]
- 10.Nashef SA, Roques F, Michel P, et al. European system for cardiac operative risk evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16:9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 11.Wendt D, Osswald BR, Kayser K, et al. Society of Thoracic Surgeons score is superior to the EuroSCORE determining mortality in high risk patients undergoing isolated aortic valve replacement. Ann Thorac Surg. 2009;88:468–474. doi: 10.1016/j.athoracsur.2009.04.059. [DOI] [PubMed] [Google Scholar]
- 12.Leon MB, Piazza N, Nikolsky E, et al. Standardized endpoint definitions for transcatheter aortic valve implantation clinical trials: a consensus report from the valve academic research consortium. Eur Heart J. 2011;32:205–217. doi: 10.1093/eurheartj/ehq406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumgartner H, Hung J, Bermejo J, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr. 2009;10:1–25. doi: 10.1093/ejechocard/jen303. [DOI] [PubMed] [Google Scholar]
- 14.Vahanian A, Alfieri O, Andreotti F, et al. Guidelines on the management of valvular heart disease (version 2012) Eur Heart J. 2012;33:2451–2496. doi: 10.1093/eurheartj/ehs109. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 16.Kappetein AP, Head SJ, Genereux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document (VARC-2) Eur J Cardiothorac Surg. 2012;42:S45–S60. doi: 10.1093/ejcts/ezs533. [DOI] [PubMed] [Google Scholar]
- 17.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 18.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364:2187–2198. doi: 10.1056/NEJMoa1103510. [DOI] [PubMed] [Google Scholar]
- 19.Mario C, Eltchaninoff H, Moat N, et al. The 2011–2012 pilot European Sentinel Registry of Transcatheter Aortic Valve Implantation: in-hospital results in 4,571 patients. EuroIntervention. 2013;8:1362–1371. doi: 10.4244/EIJV8I12A209. [DOI] [PubMed] [Google Scholar]
- 20.Gilard M, Eltchaninoff H, Iung B, et al. Registry of transcatheter aortic-valve implantation in high-risk patients. N Engl J Med. 2012;366:1705–1715. doi: 10.1056/NEJMoa1114705. [DOI] [PubMed] [Google Scholar]
- 21.Mack MJ, Brennan JM, Brindis R, et al. Outcomes following transcatheter aortic valve replacement in the United States. JAMA. 2013;310:2069–2077. doi: 10.1001/jama.2013.282043. [DOI] [PubMed] [Google Scholar]
- 22.Pilgrim T, Englberger L, Rothenbuhler M, et al. Long-term outcome of elderly patients with severe aortic stenosis as a function of treatment modality. Heart. 2015;101:30–36. doi: 10.1136/heartjnl-2014-306106. [DOI] [PubMed] [Google Scholar]
- 23.Edwards FH, Clark RE, Schwartz M. Coronary artery bypass grafting: the Society of Thoracic Surgeons National Database experience. Ann Thorac Surg. 1994;57:12–19. doi: 10.1016/0003-4975(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 24.Debonnaire P, Fusini L, Wolterbeek R, et al. Value of the “TAVI2-SCORe” versus surgical risk scores for prediction of one year mortality in 511 patients who underwent transcatheter aortic valve implantation. Am J Cardiol. 2015;115:234–242. doi: 10.1016/j.amjcard.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 25.Sedaghat A, Sinning JM, Vasa-Nicotera M, et al. The revised EuroSCORE II for the prediction of mortality in patients undergoing transcatheter aortic valve implantation. Clin Res Cardiol. 2013;102:821–829. doi: 10.1007/s00392-013-0596-8. [DOI] [PubMed] [Google Scholar]
- 26.Iung B, Laouenan C, Himbert D, et al. Predictive factors of early mortality after transcatheter aortic valve implantation: individual risk assessment using a simple score. Heart. 2014;100:1016–1023. doi: 10.1136/heartjnl-2013-305314. [DOI] [PubMed] [Google Scholar]