Abstract
Background
Because of their unique magnetic properties, Fe3O4 nanoparticles (Fe3O4-NPs) have extensive applications in various biomedical aspects. Investigation of the possible adverse aspects of these particles has lagged far behind their fast growing application.
Objectives
The current study aimed to evaluate the toxicity of Fe3O4-NPs in the liver of mice.
Materials and Methods
In the present clinical trial, 90 BALB/c mice were randomly divided in 15 groups. Five control groups were fed by usual water and food. Five placebo groups were gavaged with physiological serum in doses of 25, 50, 75, 150, and 300 micrograms per gram of body weight (μg/gr). Five experimental groups were gavaged with Fe3O4-NPs, in doses of 25, 50, 75, 150, and 300 μg/gr. This pattern was repeated every other day, for 3 days. Then, the levels of liver enzymes [alanine transaminase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP)] were compared between these groups. The histological alterations of livers were examined, as well. For statistical analysis, Kruskal-Wallis and Mann-Whitney, with type I Bonferroni correction, as post-hoc, have been used.
Results
The administration of 150 and 300 μg/gr doses of Fe3O4-NPs were associated with significant elevation in liver enzymes, compared to controls (P < 0.0001). Furthermore, the histopathological effects were observed in the liver tissue of these groups. However, in groups treated with lower doses of Fe3O4-NPs, no significant adverse effect was observed.
Conclusions
Based on our results, the administration of Fe3O4-NPs causes dose dependent adverse effects on liver.
Keywords: Ferric Oxide, Nanoparticles, Aspartate Aminotransferases, Alanine Transaminases, Alkaline Phosphatase, Hepatocytes, Liver
1. Background
Nanoparticles (NPs) have very specific chemical and physical characteristics in relation to their size, shape and high proportion of surface to volume. These characteristics have made them appropriate for use in multiple medical and biological applications, such as contrast media for magnetic resonance imaging (MRI) (1), immunoassays (2), hyperthermia (3), tissue repair (4), cellular therapy (4) and drug delivery (5). Nanoparticles, consisting of elements such as iron, nickel and cobalt, which exhibit magnetic properties, are called ‘‘magnetic NPs’’. Magnetic NPs offer several attractive possibilities, as they can be internally manipulated under the influence of an external magnetic field. The NPs have been shown to be taken directly into the bloodstream, following inhalation and ingestion exposure. Typically, magnetic NPs distribute to the liver (80-90%), spleen (5-8%) and bone marrow (1-2%) (6). As the liver is the most important detoxification organ, it is reached by the highest level of NPs concentration, over all the other tissues (7, 8). Therefore, the liver can be seen as a target organ to identify adverse effects of in vivo exposure to iron oxide NPs (IONPs).
Alanine transaminase (ALT) and aspartate aminotransferase (AST) are two of the most reliable markers of hepatocellular injury or necrosis. Their levels can be elevated in a variety of hepatic disorders. Of the two, ALT is thought to be more specific for hepatic injury, because it is present mainly in the cytosol of the liver and, in low concentrations, elsewhere. The AST has cytosolic and mitochondrial forms and is present in tissues of the liver, heart, skeletal muscle, kidneys, brain, pancreas, lungs and in white and red blood cells. Alkaline phosphatase (ALP), which is present in a number of tissues, including liver, bone, intestine and placenta, is measured to detect the liver disease or bone disorders. It is known that, following their exposure, NPs are primarily readily taken up by hepatocytes and Kupffer cells (specialized macrophages located in the liver) (9). Because of their unique properties, several of these NPs, such as IONPs, have been approved for clinical use. The application of IONPs has been practiced for nearly 40 years (10) and, in the last decade, they have received enormous attention in various fields of biotechnology and life sciences. The two promising forms of IONPs are magnetite (Fe3O4) and its oxidized form, maghemite (γ-Fe3O4), since their biocompatibility has already been proven (11).
2. Objectives
Although these nanoparticles are currently being widely used in modern technology, there is a serious lack of information concerning their impact on human health and environmental implications. Concerning the central role of liver in the clearance of NPs, we aimed to investigate the effect of Fe3O4-NPs on this organ, by measuring the changes in level of liver enzymes, as well as histopathological alteration of liver tissue (including hepatocytes and Kupffer cells) in mature male mice (BALB/c race).
3. Materials and Methods
3.1. Preparation and Characterization of Magnetite Nanoparticles
The current clinical trial study was performed in Islamic Azad University, Department of Biology, Science and Research Branch, Iran, from March 2014 to September 2014. The Fe3O4-NPs (diameter 20-30 nm) were purchased from the company of Nano Pasargad Novin (Iran, Tehran). Twenty five grams of these particles were suspended in 10 mL of PBS (pH = 7.5) to create a stock solution and, at the time of experiment, were diluted to required doses. Ultra sonication was performed to disperse the coagulated particles, just before each administration, therefore excluding any disturbance of the heterogeneity of nanoparticles.
3.2. Experimental Animals
Ninety adult male mice of BALB/c strain, weighing 23 ± 2 grams were purchased from the Razi Vaccine and Serum Research Institute (Karaj, Iran). The laboratory animal facility was maintained under a 12 hours light/dark cycle, at a temperature of 22 ± 2°C, and a relative humidity of 20-50%. All methods performed in this study were in accordance with regulatory guidance on the care and use of experimental animals. Experimental animals were randomly divided into 15 groups, as follows: five control groups (comprising six mice in each group) fed by usual water and food. Five experimental groups (comprising six mice in each) were gavaged with Fe3O4-NPs, in doses of 25, 50, 75, 150 and 300 μg/gr. Five control groups that referred to placebo (comprising six mice in each) were gavaged with physiological serum, in doses of 25, 50, 75, 150 and 300 μg/gr. This pattern was repeated every other day, for 3 days.
3.3. Serum Biochemical Analysis
One day after 3 days of treatment with Fe3O4-NPs, the mice were anesthetized with ether. For biochemical analysis, the heart blood was collected and the serum was isolated by centrifugation (3000 rpm for 20 minutes). Then, concentrations of liver enzymes, including ALT, AST and ALP were determined using marketed reagent kits (Pars Azmoon, Tehran, Iran) and autoanalyzer system (Hitachi Ltd., Tokyo, Japan).
3.4. Histological Examinations
All mice fed with Fe3O4-NPs were then sacrificed by giving deep ether anesthesia and were operated to remove their liver. The tissue samples were fixed in buffered neutral 10% formaldehyde, decalcified in 5% nitric acid for 7 days, dehydrated stepwise through ascending series of alcohol solutions and, finally, degreased in xylene. The tissues were then embed in paraffin block, sliced at 5 μm, by microtome and stained using hematoxylin and eosin (HE) stains. Cells were observed under a CH-30 optical microscope (Olympus, Tokyo, Japan) and histological images were captured and analyzed. Then, the hepatocytes and Kupffer cells were counted per area.
3.5. Statistical Analysis
The data were analyzed using Kruskal-Wallis and Mann-Whitney with type I Bonferroni correction as post hoc. Continuous variables are presented as the mean ± SD and Median. The confidence level of current study is 95%. A P < 0.0001 was considered as statistically significant.
4. Results
4.1. Hepatic Histological Alterations
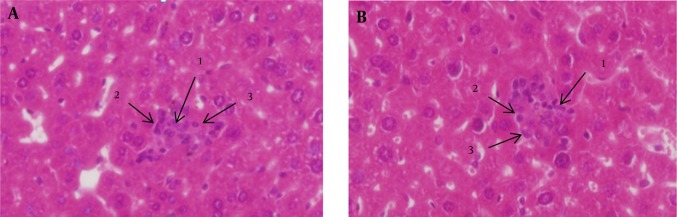
Hepatic tissue pieces that stained by HE were studied under light microscope, with magnification of 400×. Spotty necrosis of hepatocytes, with infiltration of lymphocytes and neutrophils in the area and a plethora of Kupffer cells were observed in mice treated with 150 and 300 μg/gr doses of Fe3O4-NPs (Figure 1). Furthermore, histopathological examination indicated the central venous congestion and hypertrophied hepatocytes in the livers of these two groups (Figure 2). Five percent of hepatocytes of mice treated with these doses showed regenerative activities. Three days administration of Fe3O4-NPs in doses of 150 and 300 μg/gr were also associated with a significant increase in the numbers of Kupffer cells (P < 0.0001), compared to unexposed controls. However, no significant increase in the number of these cells was determined in mice treated with 25, 50 and 75 μg/gr of Fe3O4-NPs (Table 1).
Figure 1. Histopathology of Liver in Mice Treated With 300 μg/gr Doses of Magnetite Nanoparticles.
A, Histopathology of liver in mice treated with 300 μg/gr doses of Fe3O4-NPs; B, 1) Spotty necrosis, 2) Neutrophil and 3) Lymphocyte; Abbreviations: Fe3O4-NPs, Magnetite Nanoparticles.
Figure 2. Venous Congestion of Liver in Mice Treated With 300 μg/gr Doses of Magnetite Nanoparticles.
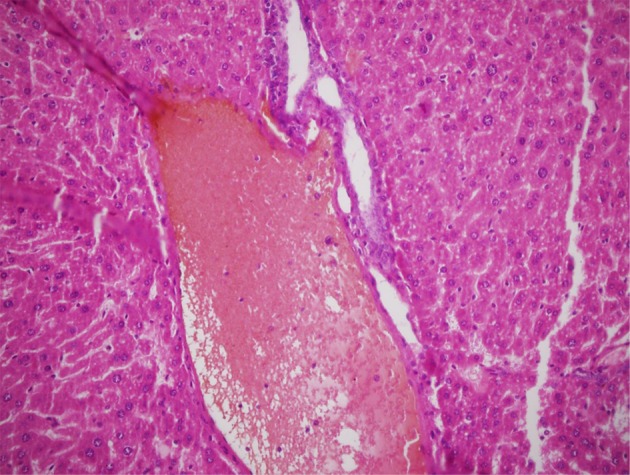
Table 1. Data Analysis Using Kruskal-Wallis Methoda.
| Groups | Intact Control | Placebo Control | 25 μg/gr dose | 50 μg/gr dose | 75 μg/gr dose | 150 μg/gr doseb | 300 μg/gr doseb | P value |
|---|---|---|---|---|---|---|---|---|
| Venous Cavities, n | 58.2500 ± 1.40955 | 58.0000 ± 1.37649 | 58.2500 ± 0.85070 | 59.0000 ± 0.91766 | 59.2500 ± 1.06992 | 43.1000 ± 1.88903 | 37.350 ± 1.18210 | < 0.0001 |
| Hepatic Vein Width, μm | 11.9500 ± 1.09904 | 12.2000 ± 0.83351 | 12.3500 ± 0.98809 | 13.0500 ± 1.09904 | 13.1500 ± 1.18210 | 18.2500 ± 1.06992 | 26.4500 ± 0.82558 | < 0.0001 |
| hepatocytes, n | 316.85 ± 18.49687 | 308.95 ± 14.92605 | 317.4500 ± 14.89428 | 324.8000 ± 14.48629 | 331.5000 ± 9.52283 | 234.1500 ± 3.43779 | 211.2500 ± 2.17340 | < 0.0001 |
| Kupffer cells, n | 31.5000 ± 1.84961 | 30.7500 ± 1.55174 | 31.5000 ± 1.53897 | 32.3500 ± 1.46089 | 33.5000 ± 1.14708 | 61.9000 ± 4.15363 | 71.050 ± 2.06410 | < 0.0001 |
aEvaluating the effect of different magnetite nanoparticles (Fe3O4-NPs) doses on various histological factors of liver indicated that administration of Fe3O4-NPs in 150 and 300 μg/gr doses was associated with significant histopathological changes.
bSignificant difference (P < 0.0001).
In comparison with unexposed controls, consumption of Fe3O4-NPs in both 150 and 300 μg/gr doses lead to significant decrease in the number of hepatocytes compared with unexposed controls (P < 0.0001). However, no significant changes were observed in the number of hepatocytes of mice treated with 25 and 50 μg/gr Fe3O4-NPs (Table 1).
The number of venous cavities and the width of hepatic vein showed a significant increase in mice treated with Fe3O4-NPs in 150 and 300 μg/gr doses, compared to untreated groups (Table 1).
4.2. Hepatic Enzymes Alterations
The serum level of ALT in the groups treated with 150 and 300 μg/gr doses of Fe3O4-NPs were shown to be significantly higher, compared to unexposed controls (P < 0.0001). Administration of Fe3O4-NPs in three other concentrations (25, 50 and 75 μg/mg) had not significant effect on ALT level, in comparison with unexposed controls (Table 2).
Table 2. Data Analysis Using Kruskal-Wallis Method for Evaluating Serum Concentration of Alanine Transaminase, Aspartate Aminotransferase, and Alkaline Phosphatase in Control Groups and Groups Treated With Different Doses of Magnetite Nanoparticlesa.
| Groups | Intact Control | Placebo Control | 25 μg/gr dose | 50 μg/gr dose | 75 μg/gr dose | 150 μg/gr doseb | 300 μg/gr doseb | P value |
|---|---|---|---|---|---|---|---|---|
| ALT, U/lit | 34.8500 ± 4.93404 | 42.0500 ± 2.91051 | 35.7000 ± 4.94283 | 35.1500 ± 2.73909 | 38.4000 ± 3.05045 | 48.0000 ± 3.92026 | 69.3500 ± 1.42441 | < 0.0001 |
| AST, U/lit | 85.9000 ± 6.95020 | 80.0000 ± 6.19847 | 79.8500 ± 5.44131 | 93.4500 ± 4.29780 | 74.3000 ± 2.86724 | 195.2000 ± 38.94477 | 217.9500 ± 6.79377 | < 0.0001 |
| ALP, U/lit | 76.4500 ± 4.59376 | 77.8500 ± 6.27673 | 75.8000 ± 4.66341 | 77.1500 ± 3.40704 | 74.8500 ± 3.63137 | 128.9000 ± 13.67633 | 146.3500 ± 10.58934 | < 0.0001 |
Abbreviations: ALP, Alkaline Phosphatase; ALT, Alanine Transaminase; AST, Aspartate Aminotransferase.
aBALB/c mice treated with 150 and 300 μg/gr doses of Fe3O4-NPs showed significant difference compared with groups that received lower doses (25, 50, 75 μg/gr) and controls.
bSignificant difference (P < 0.0001).
In addition, the levels of both AST and ALP enzymes showed a significant increase in mice treated with 150 and 300 μg/gr Fe3O4-NPs, compared to unexposed controls (P < 0.0001). However, no significant difference was observed in the level of these enzymes, when comparing the groups of mice treated with 25, 50 and 75 μg/mg doses of these NPs with the control groups (Table 2).
5. Discussion
The unique features of NPs, including their specific chemical and physical characteristics in size, shape and high proportion of surface to volume, have made them attractive for use in a variety of medical and biological settings. However, to date, there are few studies directly or indirectly evaluating the toxic effects of these nanomaterials and, presently, there is a lack of clear guidelines to quantify these effects. Among them, Fe3O4-NPs, due to their specific magnetic, optical, electrical and chemical properties, are considered for a wide range of therapeutical and biological applications, such as materials for magnetic hyperthermia, as contrast agents in magnetic resonance imaging (12), for cell labeling or for targeted drug delivery (13). The hazards of these particles, as well as their benefits, should be explored urgently to determine a safe dose at which the risks and benefits of Fe3O4-NPs application are equivalent. Therefore, the aim of the current study was to determine potential adverse effects of Fe3O4-NPs, by evaluating the changes in the level of liver enzymes (AST, ALT and ALP) and histological properties of liver tissue, after exposure to different doses of these NPs. Our results indicated that administration of Fe3O4-NPs in 150 and 300 μg/gr doses leads to histopathological effects on liver, including necrosis of hepatocytes, plethora of Kupffer cells, congestion of central venous circulation and hypertrophy of hepatocytes. A significant increase in Kupffer cells and significant decrease in hepatocytes count per unit area were observed in these groups, as well. Meanwhile, evaluating the alteration in hepatic enzymes showed a significant increase in the levels of AST, ALT and ALP, which are common markers for hepatic toxicity. However, administration of lower doses of these nanoparticles (25, 50 and 75 μg/gr) was not associated with significant adverse histological effects on liver and no significant alterations in liver enzymes were detected.
Different available studies have investigated toxicity of IONPs (14, 15). However, the reported results are not consistent with one another. Kim et al. (16) suggested that IONPs do not cause apparent toxicity to mice, in vivo. In contrast, multiple other studies have reported severe adverse effects, associated with exposure to these NPs (17-19). For example, in their study Zhu et al. (17) indicated that administration of Fe3O4-NPs induce lung injury, increase microvascular permeability and cause the lysis of lung airways epithelial cells, in mice. The Fe3O4-NPs were also shown to enter the central nervous system, induce severe oxidative stress and damage nerve cells in mice (18). A study conducted by Babadi et al. (20) indicated that exposure to 150 μg/kg body weight Fe3O4-NPs for 15 days leads to a significant increase in AST, ALT and ALP levels. Factors that can cause these conflicting reports include the physicochemical properties of the NPs, such as size, shape and solubility. In addition, the experimental model, exposure times and concentrations may cause different results (14). Different available studies explore the possible underlying toxicity mechanisms of IONPs. Several reports have suggested contribution of excessive reactive oxygen species (ROS) to most intracellular and in vivo toxicities from IONPs. Another reported mechanism of IONPs induced toxicity is iron accumulation in tissue/organ, due to their required high concentration in biomedical application.
The results of our study indicated a dose dependent toxicity of Fe3O4-NPs. These experiments helped to determine a recommended tentative low-risk dose of Fe3O4-NPs for in vivo systemic delivery. We indicated that, even though 150 and 300 μg/gr doses showed adverse effects on liver, the administration of Fe3O4-NPs in doses of 25, 50 and 75 μg/gr can be regarded as safe. However, the number of samples for this study was not high and the duration of expositor was short. Longer treatment periods should be tested with a larger number of animals to confirm that the mentioned doses are safe.
Acknowledgments
The authors declare their gratitude towards the kind personnel of the library of Royan institute and islamic azad university, department of biology, sciences and research branch, Iran, Tehran.
Footnotes
Authors’ Contribution:Study concept and design: Kazem Parivar, Fatemeh Malekvand Fard, Mahsa Motavaf; acquisition of data: Fatemeh Malekvand Fard, Mahdieh Bayat; analysis and interpretation of data: Kazem Parivar, Seyed Moayed Alavian; drafting of the manuscript: Mahsa Motavaf, Mahdieh Bayat; critical revision of the manuscript for important intellectual content: Mahsa Motavaf, Seyed Moayed Alavian, Fatemeh Malekvand Fard.
References
- 1.Oghabian MA, Gharehaghaji N, Amirmohseni S, Khoei S, Guiti M. Detection sensitivity of lymph nodes of various sizes using USPIO nanoparticles in magnetic resonance imaging. Nanomedicine. 2010;6(3):496–9. doi: 10.1016/j.nano.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Tang D, Cui Y, Chen G. Nanoparticle-based immunoassays in the biomedical field. Analyst. 2013;138(4):981–90. doi: 10.1039/c2an36500f. [DOI] [PubMed] [Google Scholar]
- 3.Silva AC, Oliveira TR, Mamani JB, Malheiros SM, Malavolta L, Pavon LF, et al. Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. Int J Nanomedicine. 2011;6:591–603. doi: 10.2147/IJN.S14737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu C, Mu L, Roes I, Miranda-Nieves D, Nahrendorf M, Ankrum JA, et al. Nanoparticle-based monitoring of cell therapy. Nanotechnology. 2011;22(49):494001. doi: 10.1088/0957-4484/22/49/494001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang ZY, Wang L, Zhang J, Li YT, Zhang DS. A study on the preparation and characterization of plasmid DNA and drug-containing magnetic nanoliposomes for the treatment of tumors. Int J Nanomedicine. 2011;6:871–5. doi: 10.2147/IJN.S16485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Priprem A. Cytotoxicity Studies of Superparamagnetic Iron Oxide Nanoparticles in Macrophage and Liver Cells. American Nanotech J. 2010;1(2):78–85. doi: 10.3844/ajnsp.2010.78.85. [DOI] [Google Scholar]
- 7.Baratli Y, Charles AL, Wolff V, Ben Tahar L, Smiri L, Bouitbir J, et al. Age modulates Fe3O4 nanoparticles liver toxicity: dose-dependent decrease in mitochondrial respiratory chain complexes activities and coupling in middle-aged as compared to young rats. Biomed Res Int. 2014;2014:474081. doi: 10.1155/2014/474081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol In Vitro. 2005;19(7):975–83. doi: 10.1016/j.tiv.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 9.Sadauskas E, Wallin H, Stoltenberg M, Vogel U, Doering P, Larsen A, et al. Kupffer cells are central in the removal of nanoparticles from the organism. Part Fibre Toxicol. 2007;4:10. doi: 10.1186/1743-8977-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilchrist RK, Medal R, Shorey WD, Hanselman RC, Parrott JC, Taylor CB. Selective inductive heating of lymph nodes. Ann Surg. 1957;146(4):596–606. doi: 10.1097/00000658-195710000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng J, Huang T, Zheng YF. Microstructure, mechanical property, biodegradation behavior, and biocompatibility of biodegradable Fe-Fe2O3 composites. J Biomed Mater Res A. 2014;102(7):2277–87. doi: 10.1002/jbm.a.34882. [DOI] [PubMed] [Google Scholar]
- 12.Starmans LW, Moonen RP, Aussems-Custers E, Daemen MJ, Strijkers GJ, Nicolay K, et al. Evaluation of iron oxide nanoparticle micelles for magnetic particle imaging (MPI) of thrombosis. PLoS One. 2015;10(3):e0119257. doi: 10.1371/journal.pone.0119257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta S, Tripathi M. A review of TiO2 nanoparticles. Chinese Sci Bull. 2011;56(16):1639–57. doi: 10.1007/s11434-011-4476-1. [DOI] [Google Scholar]
- 14.Sadeghi L, Tanwir F, Yousefi Babadi V. In vitro toxicity of iron oxide nanoparticle: oxidative damages on Hep G2 cells. Experimental and toxicologic pathology. J Gesell Toxikol Pathol. 2015;67(2):197–203. doi: 10.1016/j.etp.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Chamorro S, Gutierrez L, Vaquero MP, Verdoy D, Salas G, Luengo Y, et al. Safety assessment of chronic oral exposure to iron oxide nanoparticles. Nanotechnology. 2015;26(20):205101. doi: 10.1088/0957-4484/26/20/205101. [DOI] [PubMed] [Google Scholar]
- 16.Kim JS, Yoon TJ, Yu KN, Kim BG, Park SJ, Kim HW, et al. Toxicity and tissue distribution of magnetic nanoparticles in mice. Toxicol Sci. 2006;89(1):338–47. doi: 10.1093/toxsci/kfj027. [DOI] [PubMed] [Google Scholar]
- 17.Zhu MT, Feng WY, Wang B, Wang TC, Gu YQ, Wang M, et al. Comparative study of pulmonary responses to nano- and submicron-sized ferric oxide in rats. Toxicology. 2008;247(2-3):102–11. doi: 10.1016/j.tox.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Wang B, Feng W, Zhu M, Wang Y, Wang M, Gu Y, et al. Neurotoxicity of low-dose repeatedly intranasal instillation of nano- and submicron-sized ferric oxide particles in mice. Nanopart Res J. 2008;11(1):41–53. doi: 10.1007/s11051-008-9452-6. [DOI] [Google Scholar]
- 19.Noori A, Amiri G. Effect of magnetic iron oxide nanoparticle on pregnancy and testicular development of mice. Clinic Bioch J. 2011;44(13):S324. doi: 10.1016/j.clinbiochem.2011.08.796. [DOI] [Google Scholar]
- 20.Babadi VY. Evaluation of iron oxide nanoparticles effects on tissue and enzymes of liver in rats. J Pharm Biomed Sci. 2012;23(23):1–4. [Google Scholar]