Abstract
Insulin resistance, type 2 diabetes mellitus and associated hyperinsulinaemia can promote the development of a specific form of cardiomyopathy that is independent of coronary artery disease and hypertension. Termed diabetic cardiomyopathy, this form of cardiomyopathy is a major cause of morbidity and mortality in developed nations, and the prevalence of this condition is rising in parallel with increases in the incidence of obesity and type 2 diabetes mellitus. Of note, female patients seem to be particularly susceptible to the development of this complication of metabolic disease. The diabetic cardiomyopathy observed in insulin-resistant or hyperinsulinaemic states is characterized by impaired myocardial insulin signalling, mitochondrial dysfunction, endoplasmic reticulum stress, impaired calcium homeostasis, abnormal coronary microcirculation, activation of the sympathetic nervous system, activation of the renin–angiotensin–aldosterone system and maladaptive immune responses. These pathophysiological changes result in oxidative stress, fibrosis, hypertrophy, cardiac diastolic dysfunction and eventually systolic heart failure. This Review highlights a surge in diabetic cardiomyopathy research, summarizes current understanding of the molecular mechanisms underpinning this condition and explores potential preventive and therapeutic strategies.
Under physiological conditions, insulin stimulates the uptake of glucose into cardiac muscle, skeletal muscle, liver, adipose tissue and other metabolic tissues to maintain glucose homeostasis1,2. Reduced insulin signalling and/or insulin resistance, together with the associated diminution in glucose transport, promotes a compensatory increase in pancreatic production of insulin that results in hyperinsulinaemia3,4. Insulin resistance and hyperinsulinaemia are often associated with the cardiorenal metabolic syndrome, which comprises a constellation of cardiac, renal and metabolic disorders contributing to the early stages of cardiovascular and renal disease1-5.
The first clinical description of cardiomyopathy associ ated T2DM was published in 1972 and involved four patients with diabetes mellitus who had died of heart failure6. Anatomical dissection of their hearts revealed a form of cardiomyopathy characterized by abnormal myocardial structure but no evidence of coronary artery disease, hypertension or other known precipitating factors for heart failure1,2,7,8. The term ‘diabetic cardiomyo pathy’ was proposed to distinguish the pathophysiological changes observed among these patients from patients with other forms of cardiomyopathy2,9. Diabetic cardiomyopathy was defined as left ventricular dysfunction that occurs among patients with diabetes mellitus independent of recognized risk factors, such as coronary artery disease or hypertension10. Minimal criteria for diagnosis of diabetic cardiomyopathy include left ventricular diastolic dysfunction and/or reduced left ventricular ejection fraction, left ventricular hypertrophy and interstitial fibrosis1,4. Diabetic cardiomyopathy seems to progress through an initial subclinical period characterized by subtle structural and functional abnormalities (for example, diastolic relaxation) through to severe diastolic heart failure with normal ejection fraction followed by systolic dysfunction accompanied by heart failure with reduced ejection fraction7,10.
Research into the pathophysiology underpinning the progression of diabetic cardiomyopathy to heart failure has demonstrated the importance of systemic insulin resistance, impaired cardiac insulin signalling, mitochondrial dysfunction, endoplasmic reticulum stress, impaired calcium handling, abnormal coronary microcirculation, inappropriate neurohumoral activation and maladaptive immune responses3,10. However, the precise role of insulin resistance and hyperinsulinaemia in the pathogenesis of diabetic cardiomyopathy remains to be elucidated. Understanding the molecular and metabolic pathways underlying cardiac dysfunction in insulin resistance and hyperinsulinaemia will provide an improved understanding of the various cardiac abnormalities associated with diastolic dysfunction and its progression to systolic dysfunction and heart failure.
Predictive factors for heart failure
Type 2 diabetes mellitus
The prevalence of heart failure is estimated to be 22% among patients with type 2 diabetes mellitus (T2DM)11. These two conditions tend to coexist and the impact of each disorder on the other results in bidirectional effects in terms of causation and outcome12,13.
The Framingham Heart Study reported that 19% of patients with heart failure have T2DM and that the risk of heart failure increases by twofold to eightfold in the presence of T2DM7,12,14. Furthermore, a 1% increase in the levels of HbA1c is associated with an 8% increase in the risk of heart failure, independent of blood pressure, BMI, age and the presence of coronary artery disease, which suggests that the risk of heart failure is modulated by factors unique to T2DM, such as insulin resistance and hyperglycaemia7,12,14. Conversely, a 1% reduction of HbA1c levels is associated with a 16% reduced risk of developing heart failure and worsening outcomes12. Such a bidirectional interaction has, therefore, provided evidence to support the existence of diabetic cardiomyopathy as a unique clinical entity and suggests that the presence of diabetes mellitus might independently increase the risk of developing heart failure.
Insulin signalling
Cellular insulin signalling occurs through two key pathways (FIG. 1). The first pathway involves insulin receptor substrate 1 (IRS-1), which acts upstream of the phosphatidylinositol 3-kinase (PI3K)–protein kinase B (also known as AKT) signal transduction pathway to elicit predominantly metabolic responses. For example, PI3K is positioned to phosphorylate the lipid phosphatidylinositol 4,5-bisphosphate and induces the conversion of phosphatidylinositol 4,5-bisphosphate to phosphatidylin ositol 3,4,5-trisphosphate, which initiates a kinase cascade and further activates the AKT signalling pathway. Activation of AKT increases the glucose uptake in the heart by allowing the translocation of GLUT4 to the cell membrane of cardiac cells. PI3K–AKT can also activate endothelial nitric oxide synthase15. The resultant increase in bioavailable nitric oxide mediates coronary vasodilation, myocardial substrate flexibility and energy homeostasis16. The second pathway involves signal transduction via mitogen-activated protein kinase (MAPK)15, which contributes to growth and remodelling responses and the resultant myocardial hypertrophy, cardiac fibrosis, impaired myocardial–endothelial signalling and death of myocardial and endothelial cells17. Thus, insulin signalling in the heart constitutes a highly complex network with multiple feedback loops and crosstalk (FIG. 1).
Figure 1. Molecular mechanisms implicated in the development of diabetic cardiomyopathy.
Overfeeding, mobilization of free fatty acids and activation of the renin–angiotensin–aldosterone system can all cause mitochondrial dysfunction, endoplasmic reticulum stress and oxidative stress, which results in impairment of insulin signalling, abnormal Ca2+ handling, increases in intracellular Ca2+ and Ca2+ sensitization increase and cardiomyocyte death. Patients eventually develop cardiomyocyte stiffness and diabetic cardiomyopathy. [Ca2+]i, Ca2+ influx; IRS-1, insulin receptor substrate 1; mTOR, mechanistic target of rapamycin (mTOR); P, phosphorylation; PI3K, phosphatidylinositol 3-kinase; Redox, oxidation–reduction state; S6K1, S6 kinase 1; Ser, serine; Thr, threonine; Tyr, tyrosine.
Insulin-resistant states exhibit an imbalance in the metabolic and growth effects of insulin signalling, with the actions of the MAPK pathway predominating. A major converging point in insulin signal transduction seems to underlie this imbalance; namely, increased phosphorylation of serine of the critical insulin signalling factor IRS-1 leads to impaired PI3K engagement and stimulation of AKT15-17. As shown in FIG. 1, following activation by changes in oxidation–reduction state or nutrition-related factors, several serine kinases have the ability to attenuate engagement of IRS and PI3K via phosphoryl ation of serine residues on IRS-1 and/or IRS-2 (REF. 18). Overnutrition and excess renin–angiotensin–aldosterone system activation are increasingly recognized as having an interactive action on the stimulation of mechanistic target of rapamycin (mTOR)–S6 kinase 1 (S6K1) signalling, which, in turn, attenuates insulin metabolic signalling such as IRS-1 and IRS-2 and PI3K–AKT in cardiovascular tissues. In the past few years, work from our laboratory indicates that angiotensin II activates S6K1 in heart and vascular tissue and leads to diminished insulin metabolic signalling and biological consequences, such as impaired NO-mediated vascular relaxation and impaired myocardial glucose utilization and diastolic relaxation16-18. These findings support the notion that cardiac insulin resistance is a metabolic and functional disorder that is often associated with obesity and the development of diabetic cardiomyopathy (FIG. 1).
Pathophysiological mechanisms
Several factors have been proposed to explain the pathogenesis of diabetic cardiomyopathy. These factors include cardiac metabolic disturbances, subcellular signalling abnormalities, autonomic dysfunction, activation of the renin–angiotensin–aldosterone system, inflammation, oxidative stress and a maladaptive immune response. Collect ively, they contribute to the functional impairments associated with diabetic cardiomyopathy13,19 (FIG. 2).
Figure 2. The development and progression of diabetic cardiomyopathy.
Insulin resistance and hyperinsulinaemia increase systemic metabolic disorders, activate the SNS, activate RAAS, prompt oxidative stress, mitochondrial dysfunction and endoplasmic reticulum stress and impair calcium homeostasis. These effects result in cardiac fibrosis, hypertrophy, cardiomyocyte death, dysfunction of the coronary microcirculation and eventually heart failure. Furthermore, these pathophysiological changes in cardiomyocytes underlie the risk factors for insulin resistance and hyperinsulinaemia, which can result in a potentially vicious cycle. [Ca2+]i, Ca2+ influx; RAAS, renin–angiotensin–aldosterone system; SNS, sympathetic nervous system.
Systemic metabolic disorders
Alterations in substrate metabolism
Impaired myocardial substrate and energy metabolism has emerged as an important contributor to the development of diabetic cardiomyopathy and subsequent heart failure13,20.
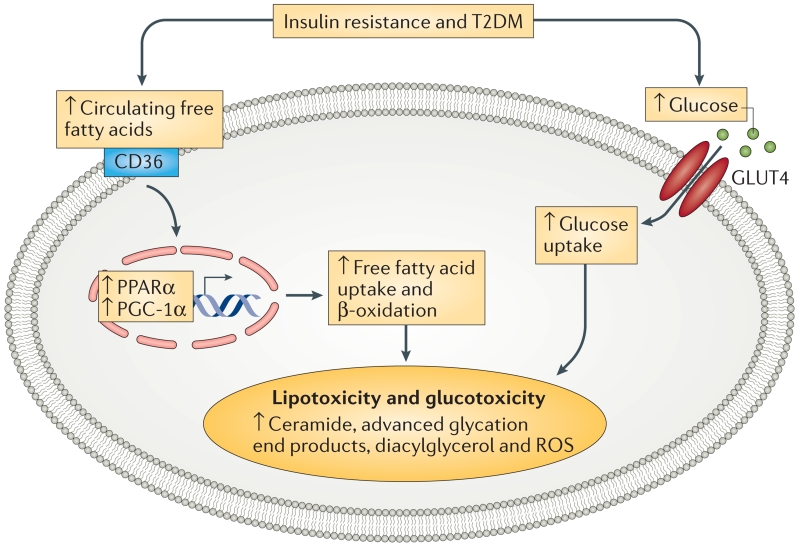
Under physiological conditions, the heart can use both fatty acids and glucose as energy substrates, thus providing metabolic flexibility. Uptake of fatty acids is mediated by the cluster of differentiation 36 (CD36) and fatty acid translocase (FAT), whereas glucose intake occurs through insulin-stimulated glucose transport mediated by glucose transporter 4 (GLUT4)13,15,21 (FIG. 3). Nutrients increase plasma levels of insulin and myocardial insulin signalling, which facilitates translocation of GLUT4 and CD36 to the myocyte sarcolemma to supply myocardial energy substrates13. By contrast, in states of insulin resistance and/or T2DM, CD36 becomes preferentially localized to the sarcolemma, whereas GLUT4 is internalized and returns to its intracellular location22,23 (FIG. 3).
Figure 3. Insulin resistance and T2DM leads to system metabolic disorders in the cardiomyocyte.
Under T2DM conditions, the expression of cardiac PPARα and PGC-1α is increased, which enhances the transcription of proteins that control free fatty acid uptake and oxidation. As glucose is a more efficient substrate than free fatty acids, a cardiac metabolic switch from glucose metabolism to free fatty acid oxidation decreases cardiac efficiency. This switch results in further metabolic disorders to cardiac dysfunction. CD36, cluster of differentiation 36; GLUT4, glucose transporter 4; T2DM, type 2 diabetes mellitus; PPARα; peroxisome proliferator activator receptor-α; PGC-1α, peroxisome proliferator-activated receptor gamma co-activator 1α; ROS, reactive oxygen species.
The reciprocal positioning of GLUT4 and CD36 influences the development of cardiac metabolic abnormalities characterized by metabolic inflexability22. Thus, the reduced glucose uptake as a result of systemic and cardiac insulin resistance facilitates a substrate shift toward increased free fatty acid oxidation in diabetes mellitus, resulting in reduced cardiac efficiency24,25. Excessive accumulation of fatty acids in cardiac tissue and the associated lipotoxicity impairs insulin signalling and reduces normal physiological autophagy, which leads to morphological and structural alterations, as well as impaired myocardial performance25. These abnormalities increase myocardial oxygen use and can reduce the efficiency of the function of muscle fibres in response to electrical stimuli (electrical–mechanical coupling)10,26.
During the advanced stage of diabetic cardiomyopathy, signal transduction molecules involved in regulating β-oxidation of fatty acids (for example, peroxisome proliferator activator receptor-α (PPARα) and peroxisome proliferator-activated receptor gamma co-activator 1α (PGC-1α)) are also decreased, thereby further dampening the metabolic efficiency of the myocardium19,27 (FIG. 3). Therefore, the metabolic switch from glucose metabolism to fatty acid β-oxidation in states of cardiac insulin resistance decreases functional efficiency of the cardiac tissue and thereby increases metabolic stress to the failing heart19.
Advanced glycation end products
Decreased glucose use and increased gluconeogenesis are both associated with the development of diabetic cardiomyopathy. Chronic hyperglycaemia impairs cardiac structure and function through generation of reactive oxygen species (ROS), which leads to DNA damage and inhibition of glyceraldehyde 3-phosphate dehydrogenase (G3PDH) activity and the formation of advanced glycation end products28 (FIG. 3). This diverse group of compounds is formed by nonenzymatic reactions between reducing sugars and the free amino groups of proteins, lipids and nucleic acids. Advanced glycation end products could have a pivotal role in the development and progression of diabetic cardiomyopathy by stimulating collagen expression and accumulation and by promoting collagen crosslinking, which leads to increased myocardial fibrosis and reduced compliance20.
Hyperlipidaemia
The combination of increased lipid synthesis in hepatocytes and increased lipolysis in adipo cytes results in raised levels of circulating free fatty acids and triglycerides in patients with insulin resistance and/or T2DM28. Lipid accumulation (lipotoxicity) can directly impede myocyte metabolism and contractility; it can also promote cardiomyocyte apoptosis via increased production of ROS and endoplasmic reticulum stress29.
Subcellular component abnormalities
Metabolic abnormalities involving mitochondria dysfunction, endoplasmic reticulum stress and impaired calcium handling contribute to the pathogenesis of diabetic cardiomyopathy13,25,30.
In conditions of excessive intake of carbohydrates and fat and associated insulin resistance, nutrient overflow into cells prompts electrons to transfer to oxygen without ATP production and also favours a state of increased ROS, which potentially leads to oxidative damage within mitochondria31. Therefore, in the presence of ROS generated from mitochondria, proteins, DNA and lipid membrane components are damaged and accumulation of ROS-mediated fibrosis promotes diastolic dysfunction that can progress to heart failure.
Excessive production of ROS also exerts deleterious effects on the endoplasmic reticulum by impairing protein folding and other post-translational modifications that occur in the rough endoplasmic reticulum32. Stress to the endoplasmic reticulum results in an adaptive response, termed the unfolded protein response, which results in increased proteasome degradation of incorrectly folded proteins30 (BOX 1). Conversely, endoplasmic reticulum stress also contributes to myocardial oxidative stress and the associated reduction in insulin signalling by activation of serine kinases dependent on the cellular oxidation-reduction state33,34 (BOX 1).
Box 1. Endoplasmic reticulum stress in diabetic cardiomyopathy.
Endoplasmic reticulum is an important post-translational modification site and is responsible for proper protein folding in eukaryotic cells. The presence of reactive oxygen species (ROS) can cause cellular damage through protein oxidation, improper protein folding, DNA damage and intrinsic action on mitochondria, as well as cell toxicity. Endoplasmic reticulum stress results in the unfolded protein response, which causes increased proteasomal degradation of improperly folded proteins. Endoplasmic reticulum stress also contributes to cardiac lipotoxicity by increasing lipogenesis through increased activation of the sterol regulatory element binding protein. Endoplasmic reticulum stress prompts membrane instability to release Ca2+ from sarcoplasmic reticulum stores into the cytosol and reduces activity of the sarcoplasmic reticulum calcium pump, which is responsible for Ca2+ sequestration during cardiomyocyte diastolic relaxation. The interaction of ROS, endoplasmic reticulum stress and abnormal calcium handing leads to the lengthened diastolic relaxation time seen in initial diastolic dysfunction and causes cardiomyocyte apoptosis and cell death as a result of the mitochondrial permeability transition pore response in the later stages of diabetic cardiomyopathy.
Oxidative stress and endoplasmic reticulum stress can induce abnormalities of calcium handling, which subsequently lead to diabetic cardiomyopathy and diastolic dysfunction. Long-chain acylcarnitines, ROS and abnormal membrane lipid content in the mitochondrial membrane, such as cardiolipin, can alter calcium handling by affecting various transporter proteins, which leads to impaired intracellular calcium uptake and delayed diastolic relaxation10,26,35. The interaction of ROS, endoplasmic reticulum stress and abnormal calcium handling promote subcellular component dysfunction and ultimately cause apoptosis, necrosis and autophagy2,36. Apoptosis is induced by pro apoptotic proteins through permeabilisation of the outer mitochondrial membrane in response to increased production of ROS and mitochondria dysfunction37,38. By contrast, necrosis occurs in response to opening of the mitochondrial permeability transition pore, which causes rapid influx of solutes and water into the mitochondrial matrix, collapse of the proton gradient and disruption of ATP synthesis39. Mitochondria are also closely associated with the endoplasmic reticulum and Ca2+ release from this organelle via inositol triphosphate; mitochondria can also promote apoptosis. Excess uptake of Ca2+ by the mitochondria leads to Ca2+ overload and opening of the mitochondrial permeability transition pore40.
Dysfunction of autophagy can contribute to the development of obesity-related cardiac dysfunction in individuals with insulin resistance or cardiorenal metabolic syndrome through mTOR-dependent signalling41. Overfeeding and obesity activate this pathway, which in turn inhibits activation of serine/threonine-protein kinase ULK1 via phosphorylation. This phosphorylation event disrupts the interaction between serine/threonine-protein kinase ULK1 and adenosine monophosphate-activated protein kinase (AMPK), thereby blocking autophagy and promoting development of cardiorenal metabolic syndrome5,41-43. Impairment of autophagy can be attributed to defects in autophagosome and lysosome fusion and results in both diastolic and systolic cardiac dysfunction as diabetic cardiomyopathy increases in severity41,44.
Impaired coronary microcirculation
Impaired coronary microcirculation is frequently observed in patients with insulin resistance, T2DM and diabetic cardiomyopathy32,36,45. This impairment is driven by reduced levels of bioavailable nitric oxide46. In coronary vascular smooth muscle cells, nitric oxide activates both guanylyl cyclase (the enzyme responsible for producing cyclic GMP) and kinases required for coron ary relaxation47. Under conditions of reduced insulin sensitivity, both reduced nitric oxide production and increased nitric oxide degradation occur. Nitric oxide usually induces vascular relaxation by reduction of the phosphorylated state of myosin light chain phosphatase and myosin light chain kinase sensitization or activation. Thus, both increases in vascular smooth muscle cell levels of intracellular Ca2+ and Ca2+ sensitization increase the ambient state of coronary constriction in states of diminished insulin signalling.
Insulin resistance and hyperinsulinaemia are associated with stiffness of both the large and small blood vessels48. The increased plasma levels of insulin that are characteristic of hyperinsulinaemia enhance the differentiation of vascular smooth muscle cells to an osteoblast-like phenotype, which could contribute to the observed increase in vascular stiffness49. Raised insulin levels might also promote vascular stiffness by increasing alkaline phosphatase activity, osteocalcin expression and the formation of mineralized nodules in vascular smooth muscle cells through increased levels of receptor activator of nuclear factor κB50. Thus, impaired endothelial cell and vascular smooth muscle cell function are associated with an increased risk of developing coronary artery disease in association with diabetic cardiomyopathy.
Inappropriate neurohumoral activation
A relationship exists between nervous system activation states and the development of diabetic cardiomyopathy51. Activation of the sympathetic nervous system increases β1-adrenergic expression and signalling, which promotes myocyte hypertrophy, interstitial fibrosis and reduced contractile function accompanied by increased myocyte apoptosis10,26. Activation of the parasympathetic nervous system is reduced, muscarinic receptor density and composition are altered and acetylcholinesterase activity is decreased in heart failure52. Either direct or indirect vagus nerve stimulation might have direct beneficial effects on cardiac remodelling and clinical outcomes52.
Increased activation of the renin–angiotensin–aldosterone system in states of insulin resistance or hyperinsulinaemia has an important role in the pathogenesis of various cardiovascular diseases, including coronary artery disease and heart failure5,28,53. Moreover, enhanced activation of angiotensin I and mineralocorticoid receptor signalling might promote insulin resistance through activation of the mTOR-S6K1 signal transduction pathway5,28,53 (FIG. 1). S6K1 is activated by angiotensin II type 1 receptor (AT1R) and mineralocorticoid receptor signalling in cardiovascular tissue leading to diminished insulin signalling and down-stream biological consequences, such as impaired vascular and cardiac relaxation mediated by nitric oxide16,17. Both AT1R and mineralocorticoid receptor signalling increase vascular oxidative stress, mainly through stimulation of NADPH oxidase activity5,28,53. Aldosterone signalling via the mineralocorticoid receptor also increases the expression and activity of the epithelial sodium channel in the vascular cell membrane and activates serum/glucocorticoid-regulated kinase 1 to reduce production of nitric oxide, promote vascular and cardiac stiffness and impair cardiac relaxation54. Both AT1R and mineralocorticoid receptor signalling in the myocardium enhance activation of growth signalling, as well as the profibrotic transforming growth factor β1 (TGF-β1)-SMAD pathway to regulate morphogenesis, proliferation, cellular differentiation, gene expression and tissue remodelling10,17. Thus, the interaction of insulin resistance with renin–angiotensin–aldosterone system activation contribute to the cardiac fibrosis and diastolic dysfunction that characterize the early stages of diabetic cardiomyopathy (FIG. 2).
Maladaptive immune responses
Diabetic cardiomyopathy can be promoted by alterations in the innate and adaptive immune systems55,56. Activation of proinflammatory T helper cells and macro phage polarization to classic (M1) or alternative (M2) phenotypes often occurs in states of obesity or insulin resistance17. In addition, chronic overfeeding results in immune responses that contribute to lowgrade inflammation within the white adipose tissue21. Increased levels of free fatty acid storage and visceral adipose tissue promotes systemic and cardiovascular inflammatory cytokine expression. Macrophage M1 polarization is a proinflammatory response and M2 polarization is an anti-inflammatory response in obesity and insulin-resistant states17,57. The M1 macrophages secrete inflammatory cytokines that cause reduced systemic and cardiac insulin signalling and promote the development of diabetic cardiomyopathy17. By contrast, M2 macrophages secrete IL-10 and macrophage mannose receptor 1, which reduce the development of cardiomyocyte hypertrophy and cardiac fibrosis17,58.
Another population of immune cells (T helper lymphocytes) have been identified in patients with diabetic cardiomyopathy59. A higher CD8+:CD4+ T-cell ratio is found in visceral adipose tissue of mice fed a high-fat diet than in lean mice60,61. Diet-induced insulin resistance also leads to a dramatic increase in type 1 T helper (TH1)-polarized cells, whereas the type 2 T helper (TH2)-polarized fraction is reduced by ~50%60. These cells can be subtyped according to their cytokine expression profile following activation61. Increased secretion of proinflammatory cytokines, chemokines and growth factors by T helper lymphocytes leads to increased cardiac fibrosis and impaired diastolic relaxation62. However, regulatory T cells usually attenuate the proinflammatory effects of T helper cells in the myocardium63. The mechanisms by which regulatory T cells protect against inflammation are thought to be mediated, at least in part, by directing cell-to-cell interactions, as well as through secretion of soluble anti-inflammatory cytokines, such as IL-10 and TGF-β64. Thus, systemic and local adaptive immune responses and inflammation might result in changes in myocardial structure and metabolism that can progress to diastolic dysfunction and eventually heart failure.
Progression of diabetic cardiomyopathy
Left ventricular filling of the normal healthy heart at rest is largely passive and is mostly accomplished (~90% of filling) before the next contraction of the left atrium. In healthy individuals, only ~10% of ventricular filling occurs as a result of left atrial contraction (active filling phase)65,66. Abnormalities in diastolic filling, like those that occur in association with obesity and T2DM, are characterized by metabolic and structural disturbances that over time lead to a decrease in ventricular wall compliance and impairment in the passive filling phase of the left ventricle. During disease progression this can be compensated for, in part, by a greater contribution of the left atrium to active filling. Noninvasive detection of diastolic dysfunction in the clinical setting is routinely accomplished with conventional echocardiography28,67. The progression of diabetic cardiomyopathy is characterized by three distinct stages with different pathophysiological features and clinical outcomes (TABLE 1).
Table 1. Progression of diabetic cardiomyopathy.
| Stage | Pathophysiological events |
Changes in structure and morphology |
Functional performance |
|---|---|---|---|
| Early | Hyperglycaemia; downregulation of GLUT4; increases in free fatty acid levels; insulin resistance; impairment of Ca2+ homeostasis; activated sympathetic nervous system |
Very small pathophysiological changes in myocytes; normal left ventricular mass and wall thickness |
Little or no LVDD |
| Advanced | Cardiomyocyte injury and death; fibrosis; activated RAAS; maladaptive inflammatory response |
Increased left ventricular mass, wall thickness and size |
Impairment of left ventricular diastolic function and slightly decreased ejection fraction |
| Late | Myocardial fibrosis; abnormal coronary microvascular; severe neurohumoral activation; inflammatory response |
Substantial increases in left ventricular mass, wall thickness and size |
Impairment of both diastolic and systolic function |
GLUT4, glucose transporter 4; LVDD, left ventricular diastolic dysfunction; RAAS, renin–angiotensin–aldosterone system.
Early stage
In the early stage of diabetic cardiomyopathy, metabolic disturbances, such as insulin resistance and hyperglycaemia, are not accompanied by substantial changes in myocardial structure and systolic function2,10.
However, impaired myocardial relaxation can be detected by echocardiography and MRI. Studies in rodents indicate that excessive dietary intake of refined carbohydrates and fats (the so-called Western diet) leads to systemic insulin resistance, impaired insulin signalling and diastolic relaxation without any evidence of systolic dysfunction17,68,69. This impaired diastolic relaxation was characterized as slow initial and peak filling rates as assessed by cine MRI17,68,69. These impairments were also evidenced by abnormal echocardiographic diastolic parameters, including impaired septal annular wall motion, abnormal myocardial performance index and a long period of isovolumic relaxation28,68.
Diabetic cardiomyopathy is initially characterized by increased cardiac stiffness and impaired relaxation with a reduction in early diastolic filling and an increase in atrial filling10 (TABLE 1). Impaired insulin signalling also causes a decrease in myocardial blood-flow reserve that can be detected by various imaging techniques70.
Advanced stage
In the advanced stage of diabetic cardiomyopathy, a number of changes at the cellular level (impaired autophagy of cells that have undergone death by apoptosis and/or necrosis, oxidative stress and maladaptive immune response) increase cardiac fibrosis, which results in substantial changes in diastolic function (initially) and systolic function (later in the process)26.
Late stage
In the late stage of diabetic cardiomyopathy, changes in metabolism, neurohumoral activation and development of myocardial fibrosis further impair coronary microcirculation, diastolic function and systolic function2,71 (TABLE 1). Numerous studies have reported that impaired myocardial insulin signalling leads to reduced activation of endothelial nitric oxide synthase and reduced levels of bioavailable nitric oxide10,17,68,69,72,73. The reduction in nitric oxide is further augmented by elevations in oxidative stress, which increase the destruction of this mol ecule. Increases in ROS and inflammation, together with decreases in bioavailable nitric oxide, promote inter stitial collagen deposition and crosslinking, which is associated with interstitial fibrosis and impaired myocardial relaxation. For example, activation of the profibrotic TGF-β1–SMAD signalling pathway increases myocardial collagen and fibronectin content and interstitial fibrosis in concert with impaired insulin signalling17,28. Structural abnormalities associated with diabetic cardiomyopathy include cardiomyocyte necrosis, progressive abolition of muscular fibrils, collagen formation in connective tissue, interstitial or/and perivascular fibrosis, hypertrophy, thickened and sclerotic small coronary vessels, basement membrane thickening, hyaline arteriolar sclerosis and capillary microaneurysms23,29.
Sexual dimorphism
Nondiabetic premenopausal women have a lower incidence of cardiovascular disease than age-matched men; however, the incidence of complications of cardiovascular disease is increased among women with obesity or diabetes mellitus74.
The Framingham Heart Study found sex-related differences in participants with worsening glucose intolerance, including the fact that left ventricular mass and wall thickness were greater in women than in men75. The levels of aldosterone were also higher in women than in men, and were positively associated with markers of concentric remodelling, such as left ventricular wall thickness74-76.
Feeding mice a Western diet for 8 weeks induced greater systemic and cardiac insulin resistance in female animals than in male animals74. Furthermore, female, but not male, mice went on to develop cardiac diastolic dysfunction in response to this dietary intevention74.
The observed dimorphic effects might be explained, in part, by loss of the beneficial actions of oestrogen, which protects against cardiovascular disease77. Thus, women seem to be prone to the development of diabetic cardiomyopathy associated with insulin resistance and/or hyperinsulinaemia.
Prevention of diabetic cardiomyopathy
Lifestyle interventions
Weight loss, regular physical activity and limitation of fat and total energy intake can positively modify metabolic abnormalities and improve systemic and tissue insulin resistance by increased postreceptor insulin signalling and insulin-mediated glucose transport, which seems to be related to enhanced signal transduction at the level of IRS proteins and PI3K8,28. Multifaceted lifestyle intervention, such as diet, exercise, nutrition education and lifestyle management, improved several risk factors associated with cardiovascular disease, including BMI and HbA1c in patients with, or at risk of, T2DM78. For example, long-term caloric restriction in patients with obesity and/or T2DM decreased myocardial triglyceride content and improved diastolic left ventricular dysfunction79. Following an exercise training program, an exercise-based lifestyle intervention prevented progression of diastolic left ventricular dysfunction80 and raised left ventricular ejection fraction by 2–5% in both the patients with obesity and those with T2DM81,82.
Metabolic modulators
Several drugs increase myocardial efficiency by promoting glucose metabolism and decreasing metabolism of free fatty acids14. Perhexiline is an inhibitor of carnitine 0-palmitoyltransferase 1 that increases maximal O2 consumption (VO2 max), left ventricular ejection fraction, resting and peak stress myocardial function and skeletal muscle energetics83. In patients with chronic heart failure, a multicentre double-blind randomized controlled trial showed that perhexiline improved left ventricular ejection fraction, resting and peak stress myocardial function, VO2 max and skeletal muscle energetics. Despite these benefits, the use of the perhexiline is declining due to reports of hepatotoxicity and peripheral neuropathy84. Trimetazidine is a competitive inhibitor of the terminal enzyme in β-oxidation that might be used to potentially improve myocardial metabolic or substrate flexibility and cardiac function in the setting of diabetic cardiomyopathy83. Trimetazidine might affect myocardial substrate use by inhibiting oxidative phosphorylation and shifting energy production from free fatty acids to glucose oxidation85. This drug might also contribute to the preservation of intracellular levels of phosphocreatine and ATP, reduce calcium overload and free radical-induced injury, inhibit cell apoptosis and improve endothelial function85. Resveratrol reduces blood levels of glucose and improves insulin sensitivity and cardiac function in patients with heart failure86. Although resveratrol is associated with improved glycaemia, triglyceride levels and heart rate, resveratrol is not associated with prevention of total mortality, cancer or cardiovascular events87. Ranolazine, a potent late Na+ current inhibitor, might normalize altered cardiomyocyte intracellular Ca2+ concentration due to the close relationship between Na+ and Ca2+ handling by the Na+/Ca2+ exchanger88. Ranolazine improves measures of haemodynamics but cardiac relaxation parameters did not improve88. A single treatment with ranolazine is probably not sufficient to affect cardiac structure and function88.
Targeting mitochondrial oxidative stress
Given the important role of oxidative stress in the development of diabetic cardiomyopathy, targeting excess myocardial production of ROS with novel antioxidants might offer a promising approach for preventing diabetic cardiomyopathy. For example, the Szeto–Schiller peptide SS31 is a positively charged free-radical scavenger that can accumulate to high levels in the mitochondria and prevent cardiac hypertrophy, fibrosis and diastolic dysfunction89-91. SS31 prevents cardiolipin from converting cytochrome c into a peroxidase while protecting cardiolipin electron carrying function by interacting with cardiolipin92. SS31 also protects the structure of mitochondrial cristae and promotes oxidative phosphorylation92. Thus, SS31 represents a new class of compounds that can recharge the cellular powerhouse and restore bioenergetics. Coenzyme Q10 has also been reported to improve cardiac function in patients with diabetes mellitus who have heart failure90,91,93. A randomized controlled trial showed that long-term coenzyme Q10 treatment in patients with chronic heart failure is safe, improves symptoms and reduces cardiovascular death by 43% and all-cause mortality by 42%94.
Antidiabetic medications
Thiazolidinediones are insulin sensitizers that improve myocardial glucose uptake and contractile dysfunction by activation of PPARγ95,96. However, thiazolidinedione therapy can induce chronic symptoms that resemble heart failure by increasing sodium and water reabsorption in kidney collecting tubules, as well as vascular permeability, which leads to systemic oedema97. Metformin has been reported to improve the clinical outcome and reduce mortality in spite of the increased risk of lactic acidosis in overweight patients with diabetes mellitus and heart failure98. This drug stimulates GLUT4 translocation and glucose uptake in cardiac muscle and in insulin-resistant cardiomyocytes by activation of AMPK98,99. Glucagon-like peptide 1 stimulates insulin secretion, improves vasodilation mediated by nitric oxide and promotes glucose use in the myocardium89,95,100. Dipeptidyl peptidase 4 inhibitors can prevent cardiac hypertrophy and cardiac diastolic dysfunction by inhibition of oxidative stress and fibrosis in mouse models of obesity and insulin resistance69. However, no clinical studies have yet documented a beneficial effect of glucagon-like peptide 1 or dipeptidyl peptidase 4 agonists on cardiac function or clinical outcomes. Empagliflozin, an inhibitor of sodium-glucose cotransporter 2, is a new antihyperglycaemic agent and has been reported to reduce HbA1c levels in patients with T2DM by controlling blood pressure, weight, visceral adiposity, hyperinsulinaemia, arterial stiffness, albuminuria, circulating uric acid levels and oxidative stress101. In an EMPA-REG OUTCOME trial involving patients with T2DM, empagliflozin with standard therapy (such as a healthy diet plan, exercise and antidiabetic therapy) resulted in a lower rate of the primary composite cardiovascular outcome and of death than adding placebo to standard therapy, but an increased rate of genital infection was observed102.
Other therapies
Blockade of β-adrenergic responses, the renin–angiotensin-aldosterone system and xanthine dehydrogenase/oxidase can attenuate oxidative stress, inflammation and insulin resistance to improve cardiac function28. Cell-based therapy and genetic correction of abnormalities are also potential strategies to prevent the development of diabetic cardiomyopathy or to treat the early stage of this condition103,104. For example, gene delivery of nerve growth factor preserves left ventricular systolic and diastolic function, microvessel density and cardiac perfusion105. Systemic administration of the Pim-1 gene improves left ventricular diastolic function (E/A ratio) and prevents cardiac fibrosis, apoptosis and development of heart failure106. Furthermore, six miRNAs (miR-34b, miR-34c, miR-199b, miR-210, miR-223 and miR-650) might be involved in the pathogenesis of the failing myocardium in patients with diabetes mellitus107. Thus, miRNAs have become an active area of investigation to determine their potential contribution to heart disease in patients with T2DM. Therapies based on miRNAs might be useful in patients with diabetes mellitus who have cardiac pathology107. These studies have led to enthusiasm and anticipation for the development of further gene-based therapies. Pharmaceutical companies have invested in gene-based therapies to target heart failure in T2DM. Further understanding of the mechanisms responsible for the onset of the functional and structural complications in the diabetic heart will undoubtedly aid the development of more precise therapeutics for the treatment of diabetic cardiomyopathy.
Conclusions
We integrated abnormalities in cardiomyocytes, which are characterized by abnormalities of metabolism, substrate flexibility, inflammation, oxidative stress and endoplasmic reticulum stress, with resulting tissue fibrosis and reduced vascular and myocardial compliance, to understand the pathogenesis of diabetic cardiomyopathy and heart failure. Insulin resistance and hyperinsulinaemia are independently associated with the development of diabetic cardiomyopathy and cardiac diastolic dysfunction. Patients with obesity, insulin resistance and hyperinsulinaemia are at high risk of developing diabetic cardiomyopathy and heart failure. Further research is needed to understand the precise mechanisms involved in the development and course of diabetic cardiomyopathy to facilitate the discovery of clinically effective targets for preventing this condition and its progression to heart failure.
Key points.
Reported rises in the prevalence of diabetic cardiomyopathy among developed nations have occurred in parallel with increased rates of obesity, insulin resistance and hyperinsulinaemia
Insulin resistance and/or hyperinsulinaemia seem to underpin the development of diabetic cardiomyopathy, which is initially characterized by diastolic dysfunction in the absence of coronary artery disease and hypertension
Pathophysiological mechanisms include impaired insulin signalling, cardiac mitochondrial dysfunction, endoplasmic reticulum stress, impaired autophagy, impaired myocardial calcium handling, abnormal coronary microcirculation, inappropriate neurohumoral activation and maladaptive immune responses
Insulin resistance, or hyperinsulinaemia, independently predisposes to the development of diabetic cardiomyopathy and targeting insulin resistance or hyperinsulinaemia could be a potential therapeutic strategy to prevent the development of diabetic cardiomyopathy
Acknowledgements
The authors thank Brenda Hunter (Diabetes and Cardiovascular Center, University of Missouri School of Medicine, USA) for editorial assistance. The authors’ research was supported by the NIH (grants R01 HL73101-01A and R01 HL107910-01) for J.R.S and the Veterans Affairs Merit System (grant 0018) for J.R.S.
Footnotes
Author contributions
All authors contributed to all aspects of the manuscript.
Competing interests statement
The authors declare no competing interests.
References
- 1.Isfort M, Stevens SC, Schaffer S, Jong CJ, Wold LE. Metabolic dysfunction in diabetic cardiomyopathy. Heart Fail. Rev. 2014;19:35–48. doi: 10.1007/s10741-013-9377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adeghate E, Singh J. Structural changes in the myocardium during diabetes-induced cardiomyopathy. Heart Fail. Rev. 2014;19:15–23. doi: 10.1007/s10741-013-9388-5. [DOI] [PubMed] [Google Scholar]
- 3.Dhalla NS, Takeda N, Rodriguez-Leyva D, Elimban V. Mechanisms of subcellular remodeling in heart failure due to diabetes. Heart Fail. Rev. 2014;19:87–99. doi: 10.1007/s10741-013-9385-8. [DOI] [PubMed] [Google Scholar]
- 4.Velez M, Kohli S, Sabbah HN. Animal models of insulin resistance and heart failure. Heart Fail. Rev. 2014;19:1–13. doi: 10.1007/s10741-013-9387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia G, Aroor AR, Martinez-Lemus LA, Sowers JR. Overnutrition, mTOR signaling, and cardiovascular diseases. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307:R1198–R1206. doi: 10.1152/ajpregu.00262.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubler S, et al. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am. J. Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 7.Maisch B, Alter P, Pankuweit S. Diabetic cardiomyopathy — fact or fiction? Herz. 2011;36:102–115. doi: 10.1007/s00059-011-3429-4. [DOI] [PubMed] [Google Scholar]
- 8.Voulgari C, Papadogiannis D, Tentolouris N. Diabetic cardiomyopathy: from the pathophysiology of the cardiac myocytes to current diagnosis and management strategies. Vasc. Health Risk Manag. 2010;6:883–903. doi: 10.2147/VHRM.S11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Factor SM, Minase T, Sonnenblick EH. Clinical and morphological features of human hypertensive-diabetic cardiomyopathy. Am. Heart J. 1980;99:446–458. doi: 10.1016/0002-8703(80)90379-8. [DOI] [PubMed] [Google Scholar]
- 10.Falcao-Pires I, Leite-Moreira AF. Diabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatment. Heart Fail. Rev. 2012;17:325–344. doi: 10.1007/s10741-011-9257-z. [DOI] [PubMed] [Google Scholar]
- 11.Roberts AW, Clark AL, Witte KK. Review article: left ventricular dysfunction and heart failure in metabolic syndrome and diabetes without overt coronary artery disease — do we need to screen our patients? Diab. Vasc. Dis. Res. 2009;6:153–163. doi: 10.1177/1479164109338774. [DOI] [PubMed] [Google Scholar]
- 12.Wong AK, AlZadjali MA, Choy AM, Lang CC. Insulin resistance: a potential new target for therapy in patients with heart failure. Cardiovasc. Ther. 2008;26:203–213. doi: 10.1111/j.1755-5922.2008.00053.x. [DOI] [PubMed] [Google Scholar]
- 13.Aroor AR, Mandavia CH, Sowers JR. Insulin resistance and heart failure: molecular mechanisms. Heart Fail. Clin. 2012;8:609–617. doi: 10.1016/j.hfc.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Witteles RM, Fowler MB. Insulin-resistant cardiomyopathy clinical evidence, mechanisms, and treatment options. J. Am. Coll. Cardiol. 2008;51:93–102. doi: 10.1016/j.jacc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 15.Dhalla NS, Pierce GN, Innes IR, Beamish RE. Pathogenesis of cardiac dysfunction in diabetes mellitus. Can. J. Cardiol. 1985;1:263–281. [PubMed] [Google Scholar]
- 16.Kim JA, Jang HJ, Martinez-Lemus LA, Sowers JR. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin-stimulated endothelial nitric oxide synthase and vasodilation. Am. J. Physiol. Endocrinol. Metab. 2012;302:E201–E208. doi: 10.1152/ajpendo.00497.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia G, et al. Uric acid promotes left ventricular diastolic dysfunction in mice fed a Western diet. Hypertension. 2015;65:531–539. doi: 10.1161/HYPERTENSIONAHA.114.04737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ. Res. 2008;102:401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandavia CH, Aroor AR, Demarco VG, Sowers JR. Molecular and metabolic mechanisms of cardiac dysfunction in diabetes. Life Sci. 2013;92:601–608. doi: 10.1016/j.lfs.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regan TJ. Congestive heart failure in the diabetic. Annu. Rev. Med. 1983;34:161–168. doi: 10.1146/annurev.me.34.020183.001113. [DOI] [PubMed] [Google Scholar]
- 21.Schaffer SW. Cardiomyopathy associated with noninsulin-dependent diabetes. Mol. Cell. Biochem. 1991;107:1–20. doi: 10.1007/BF02424571. [DOI] [PubMed] [Google Scholar]
- 22.Battiprolu PK, et al. Diabetic cardiomyopathy and metabolic remodeling of the heart. Life Sci. 2013;92:609–615. doi: 10.1016/j.lfs.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mytas DZ, et al. Diabetic myocardial disease: pathophysiology, early diagnosis and therapeutic options. J. Diabetes Complications. 2009;23:273–282. doi: 10.1016/j.jdiacomp.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 24.Harmancey R, et al. Insulin resistance improves metabolic and contractile efficiency in stressed rat heart. FASEB J. 2012;26:3118–3126. doi: 10.1096/fj.12-208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mandavia CH, Pulakat L, DeMarco V, Sowers JR. Over-nutrition and metabolic cardiomyopathy. Metabolism. 2012;61:1205–1210. doi: 10.1016/j.metabol.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr. Rev. 2004;25:543–567. doi: 10.1210/er.2003-0012. [DOI] [PubMed] [Google Scholar]
- 27.Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660–671. doi: 10.1007/s00125-014-3171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeMarco VG, Aroor AR, Sowers JR. The pathophysiology of hypertension in patients with obesity. Nat. Rev. Endocrinol. 2014;10:364–376. doi: 10.1038/nrendo.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Song Y, Wang Q, Kralik PM, Epstein PN. Causes and characteristics of diabetic cardiomyopathy. Rev. Diabet. Stud. 2006;3:108–117. doi: 10.1900/RDS.2006.3.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dhalla NS, Liu X, Panagia V, Takeda N. Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc. Res. 1998;40:239–247. doi: 10.1016/s0008-6363(98)00186-2. [DOI] [PubMed] [Google Scholar]
- 31.Liu J, et al. Targeting mitochondrial biogenesis for preventing and treating insulin resistance in diabetes and obesity: hope from natural mitochondrial nutrients. Adv. Drug Deliv. Rev. 2009;61:1343–1352. doi: 10.1016/j.addr.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Factor SM, et al. Coronary microvascular abnormalities in the hypertensive-diabetic rat. A primary cause of cardiomyopathy? Am. J. Pathol. 1984;116:9–20. [PMC free article] [PubMed] [Google Scholar]
- 33.Hotamisligil GS. Inflammation and endoplasmic reticulum stress in obesity and diabetes. Int. J. Obes. (Lond.) 2008;32:S52–S54. doi: 10.1038/ijo.2008.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henstridge DC, Whitham M, Febbraio MA. Chaperoning to the metabolic party: the emerging therapeutic role of heat-shock proteins in obesity and type 2 diabetes. Mol. Metab. 2014;3:781–793. doi: 10.1016/j.molmet.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jain SS, et al. High-fat diet-induced mitochondrial biogenesis is regulated by mitochondrial-derived reactive oxygen species activation of CaMKII. Diabetes. 2014;63:1907–1913. doi: 10.2337/db13-0816. [DOI] [PubMed] [Google Scholar]
- 36.Adameova A, Dhalla NS. Role of microangiopathy in diabetic cardiomyopathy. Heart Fail. Rev. 2014;19:25–33. doi: 10.1007/s10741-013-9378-7. [DOI] [PubMed] [Google Scholar]
- 37.Kubli DA, Gustafsson AB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ. Res. 2012;111:1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sano R, Reed JC. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta. 2013;1833:3460–3470. doi: 10.1016/j.bbamcr.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mei Y, Thompson MD, Cohen RA, Tong X. Endoplasmic reticulum stress and related pathological processes. J. Pharmacol. Biomed. Anal. 2013;1:1000107. [PMC free article] [PubMed] [Google Scholar]
- 40.Yi CH, Vakifahmetoglu-Norberg H, Yuan J. Integration of apoptosis and metabolism. Cold Spring Harb. Symp. Quant. Biol. 2011;76:375–387. doi: 10.1101/sqb.2011.76.010777. [DOI] [PubMed] [Google Scholar]
- 41.Jia G, Sowers JR. Autophagy: a housekeeper in cardiorenal metabolic health and disease. Biochim. Biophys. Acta. 2015;1852:219–224. doi: 10.1016/j.bbadis.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wensley I, Salaveria K, Bulmer AC, Donner DG, du Toit EF. Myocardial structure, function and ischaemic tolerance in a rodent model of obesity with insulin resistance. Exp. Physiol. 2013;98:1552–1564. doi: 10.1113/expphysiol.2013.074948. [DOI] [PubMed] [Google Scholar]
- 43.Falskov B, et al. The effect of chronic heart failure and type 2 diabetes on insulin-stimulated endothelial function is similar and additive. Vasc. Health Risk Manag. 2011;7:771–776. doi: 10.2147/VHRM.S25724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie Z, et al. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011;60:1770–1778. doi: 10.2337/db10-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 46.Zhou X, et al. Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the Zucker obese rat. Hypertension. 2010;55:880–888. doi: 10.1161/HYPERTENSIONAHA.109.145136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayden MR, Habibi J, Joginpally T, Karuparthi PR, Sowers JR. Ultrastructure study of transgenic Ren2 rat aorta — part 1: endothelium and intima. CardioRenal Med. 2012;2:66–82. doi: 10.1159/000335565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blaha MJ, et al. The relationship between insulin resistance and incidence and progression of coronary artery calcification: the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetes Care. 2011;34:749–751. doi: 10.2337/dc10-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olesen P, Nguyen K, Wogensen L, Ledet T, Rasmussen LM. Calcification of human vascular smooth muscle cells: associations with osteoprotegerin expression and acceleration by high-dose insulin. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1058–H1064. doi: 10.1152/ajpheart.00047.2006. [DOI] [PubMed] [Google Scholar]
- 50.Yuan LQ, et al. RANKL is a downstream mediator for insulin-induced osteoblastic differentiation of vascular smooth muscle cells. PLoS ONE. 2011;6:e29037. doi: 10.1371/journal.pone.0029037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iyngkaran P, Anavekar N, Majoni W, Thomas MC. The role and management of sympathetic overactivity in cardiovascular and renal complications of diabetes. Diabetes Metab. 2013;39:290–298. doi: 10.1016/j.diabet.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 52.Olshansky B, Sabbah HN, Hauptman PJ, Colucci WS. Parasympathetic nervous system and heart failure: pathophysiology and potential implications for therapy. Circulation. 2008;118:863–871. doi: 10.1161/CIRCULATIONAHA.107.760405. [DOI] [PubMed] [Google Scholar]
- 53.Nistala R, Sowers JR. Hypertension: synergy of antihypertensives in elderly patients with CKD. Nat. Rev. Nephrol. 2013;9:13–15. doi: 10.1038/nrneph.2012.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tirosh A, Garg R, Adler GK. Mineralocorticoid receptor antagonists and the metabolic syndrome. Curr. Hypertens. Rep. 2010;12:252–257. doi: 10.1007/s11906-010-0126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McMaster WG, Kirabo A, Madhur MS, Harrison DG. Inflammation, immunity, and hypertensive end-organ damage. Circ. Res. 2015;116:1022–1033. doi: 10.1161/CIRCRESAHA.116.303697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hofmann U, Frantz S. Role of lymphocytes in myocardial injury, healing, and remodeling after myocardial infarction. Circ. Res. 2015;116:354–367. doi: 10.1161/CIRCRESAHA.116.304072. [DOI] [PubMed] [Google Scholar]
- 57.Mori J, et al. ANG II causes insulin resistance and induces cardiac metabolic switch and inefficiency: a critical role of PDK4. Am. J. Physiol. Heart Circ. Physiol. 2013;304:H1103–H1113. doi: 10.1152/ajpheart.00636.2012. [DOI] [PubMed] [Google Scholar]
- 58.Asrih M, et al. Role of mitogen-activated protein kinase pathways in multifactorial adverse cardiac remodeling associated with metabolic syndrome. Mediators Inflamm. 2013;2013:367245. doi: 10.1155/2013/367245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weirather J, et al. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ. Res. 2014;115:55–67. doi: 10.1161/CIRCRESAHA.115.303895. [DOI] [PubMed] [Google Scholar]
- 60.Sell H, Habich C, Eckel J. Adaptive immunity in obesity and insulin resistance. Nat. Rev. Endocrinol. 2012;8:709–716. doi: 10.1038/nrendo.2012.114. [DOI] [PubMed] [Google Scholar]
- 61.Ait-Oufella H, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat. Med. 2006;12:178–180. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 62.Yu Q, Vazquez R, Zabadi S, Watson RR, Larson DF. T-lymphocytes mediate left ventricular fibrillar collagen cross-linking and diastolic dysfunction in mice. Matrix Biol. 2010;29:511–518. doi: 10.1016/j.matbio.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao Y, Xu W, Xiong S. Adoptive transfer of regulatory T cells protects against Coxsackievirus B3-induced cardiac fibrosis. PLoS ONE. 2013;8:e74955. doi: 10.1371/journal.pone.0074955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.He S, Li M, Ma X, Lin J, Li D. CD4+CD25+ Foxp3+ regulatory T cells protect the proinflammatory activation of human umbilical vein endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2010;30:2621–2630. doi: 10.1161/ATVBAHA.110.210492. [DOI] [PubMed] [Google Scholar]
- 65.Erdei T, et al. Pathophysiological rationale and diagnostic targets for diastolic stress testing. Heart. 2015;101:1355–1360. doi: 10.1136/heartjnl-2014-307040. [DOI] [PubMed] [Google Scholar]
- 66.Dori G, Rudman M, Lichtenstein O, Schliamser JE. Ejection fraction in patients with heart failure and preserved ejection fraction is greater than that in controls — a mechanism facilitating left ventricular filling and maximizing cardiac output. Med. Hypotheses. 2012;79:384–387. doi: 10.1016/j.mehy.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 67.De Boeck BW, Cramer MJ, Oh JK, van der Aa RP, Jaarsma W. Spectral pulsed tissue Doppler imaging in diastole: a tool to increase our insight in and assessment of diastolic relaxation of the left ventricle. Am. Heart J. 2003;146:411–419. doi: 10.1016/S0002-8703(03)00322-3. [DOI] [PubMed] [Google Scholar]
- 68.Bostick B, et al. Mineralocorticoid receptor blockade prevents western diet-induced diastolic dysfunction in female mice. Am. J. Physiol. Heart Circ. Physiol. 2015;308:H1126–H1156. doi: 10.1152/ajpheart.00898.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bostick B, et al. Dipeptidyl peptidase inhibition prevents diastolic dysfunction and reduces myocardial fibrosis in a mouse model of Western diet induced obesity. Metabolism. 2014;63:1000–1011. doi: 10.1016/j.metabol.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ernande L, Derumeaux G. Diabetic cardiomyopathy: myth or reality? Arch. Cardiovasc. Dis. 2012;105:218–225. doi: 10.1016/j.acvd.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 71.Battiprolu PK, Gillette TG, Wang ZV, Lavandero S, Hill JA. Diabetic cardiomyopathy: mechanisms and therapeutic targets. Drug Discov. Today Dis. Mech. 2010;7:e135–e143. doi: 10.1016/j.ddmec.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.D’Souza A, et al. Chronic effects of mild hyperglycaemia on left ventricle transcriptional profile and structural remodelling in the spontaneously type 2 diabetic Goto-Kakizaki rat. Heart Fail. Rev. 2014;19:65–74. doi: 10.1007/s10741-013-9376-9. [DOI] [PubMed] [Google Scholar]
- 73.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 74.Manrique C, et al. Obesity and insulin resistance induce early development of diastolic dysfunction in young female mice fed a Western diet. Endocrinology. 2013;154:3632–3642. doi: 10.1210/en.2013-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rutter MK, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Circulation. 2003;107:448–454. doi: 10.1161/01.cir.0000045671.62860.98. [DOI] [PubMed] [Google Scholar]
- 76.Mihailidou AS, Ashton AW. Cardiac effects of aldosterone: does gender matter? Steroids. 2014;91:32–37. doi: 10.1016/j.steroids.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 77.Barrett Mueller K, et al. Estrogen receptor inhibits mineralocorticoid receptor transcriptional regulatory function. Endocrinology. 2014;155:4461–4472. doi: 10.1210/en.2014-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen L, et al. Effect of lifestyle intervention in patients with type 2 diabetes: a meta-analysis. Metabolism. 2015;64:338–347. doi: 10.1016/j.metabol.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 79.Hammer S, et al. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J. Am. Coll. Cardiol. 2008;52:1006–1012. doi: 10.1016/j.jacc.2008.04.068. [DOI] [PubMed] [Google Scholar]
- 80.Hare JL, et al. Application of an exercise intervention on the evolution of diastolic dysfunction in patients with diabetes mellitus: efficacy and effectiveness. Circ. Heart Fail. 2011;4:441–449. doi: 10.1161/CIRCHEARTFAILURE.110.959312. [DOI] [PubMed] [Google Scholar]
- 81.Schrauwen-Hinderling VB, et al. Improved ejection fraction after exercise training in obesity is accompanied by reduced cardiac lipid content. J. Clin. Endocrinol. Metab. 2010;95:1932–1938. doi: 10.1210/jc.2009-2076. [DOI] [PubMed] [Google Scholar]
- 82.Schrauwen-Hinderling VB, et al. Cardiac lipid content is unresponsive to a physical activity training intervention in type 2 diabetic patients, despite improved ejection fraction. Cardiovasc. Diabetol. 2011;10:47. doi: 10.1186/1475-2840-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nickel A, Loffler J, Maack C. Myocardial energetics in heart failure. Basic Res. Cardiol. 2013;108:358. doi: 10.1007/s00395-013-0358-9. [DOI] [PubMed] [Google Scholar]
- 84.Senanayake EL, et al. Multicentre double-blind randomized controlled trial of perhexiline as a metabolic modulator to augment myocardial protection in patients with left ventricular hypertrophy undergoing cardiac surgery. Eur. J. Cardiothorac. Surg. 2015;48:354–362. doi: 10.1093/ejcts/ezu452. [DOI] [PubMed] [Google Scholar]
- 85.Gao D, Ning N, Niu X, Hao G, Meng Z. Trimetazidine: a meta-analysis of randomised controlled trials in heart failure. Heart. 2011;97:278–286. doi: 10.1136/hrt.2010.208751. [DOI] [PubMed] [Google Scholar]
- 86.Sulaiman M, et al. Resveratrol, an activator of SIRT1, upregulates sarcoplasmic calcium ATPase and improves cardiac function in diabetic cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2010;298:H833–H843. doi: 10.1152/ajpheart.00418.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rabassa M, Zamora-Ros R, Urpi-Sarda M, Andres-Lacueva C. Resveratrol metabolite profiling in clinical nutrition research — from diet to uncovering disease risk biomarkers: epidemiological evidence. Ann. NY Acad. Sci. 2015;1348:107–115. doi: 10.1111/nyas.12851. [DOI] [PubMed] [Google Scholar]
- 88.Maier LS, et al. RAnoLazIne for the treatment of Diastolic Heart Failure in patients with preserved ejection fraction: the RALI-DHF proof-of-concept study. JACC Heart Fail. 2013;1:115–122. doi: 10.1016/j.jchf.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 89.Doehner W, Frenneaux M, Anker SD. Metabolic impairment in heart failure: the myocardial and systemic perspective. J. Am. Coll. Cardiol. 2014;64:1388–1400. doi: 10.1016/j.jacc.2014.04.083. [DOI] [PubMed] [Google Scholar]
- 90.Dhalla NS, Temsah RM, Netticadan T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000;18:655–673. doi: 10.1097/00004872-200018060-00002. [DOI] [PubMed] [Google Scholar]
- 91.Xu YJ, Tappia PS, Neki NS, Dhalla NS. Prevention of diabetes-induced cardiovascular complications upon treatment with antioxidants. Heart Fail. Rev. 2014;19:113–121. doi: 10.1007/s10741-013-9379-6. [DOI] [PubMed] [Google Scholar]
- 92.Szeto HH. First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br. J. Pharmacol. 2014;171:2029–2050. doi: 10.1111/bph.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huynh K, et al. Coenzyme Q10 attenuates diastolic dysfunction, cardiomyocyte hypertrophy and cardiac fibrosis in the db/db mouse model of type 2 diabetes. Diabetologia. 2012;55:1544–1553. doi: 10.1007/s00125-012-2495-3. [DOI] [PubMed] [Google Scholar]
- 94.Mortensen SA, et al. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: results from Q-SYMBIO: a randomized double-blind trial. JACC Heart Fail. 2014;2:641–649. doi: 10.1016/j.jchf.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 95.Mamas MA, et al. Impaired glucose tolerance and insulin resistance in heart failure: underrecognized and undertreated? J. Card. Fail. 2010;16:761–768. doi: 10.1016/j.cardfail.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 96.Sacca L. Heart failure as a multiple hormonal deficiency syndrome. Circ. Heart Fail. 2009;2:151–156. doi: 10.1161/CIRCHEARTFAILURE.108.821892. [DOI] [PubMed] [Google Scholar]
- 97.Sacca L, Napoli R. Insulin resistance in chronic heart failure: a difficult bull to take by the horns. Nutr. Metab. Cardiovasc. Dis. 2009;19:303–305. doi: 10.1016/j.numecd.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 98.von Bibra H, St John Sutton M. Impact of diabetes on postinfarction heart failure and left ventricular remodeling. Curr. Heart Fail. Rep. 2011;8:242–251. doi: 10.1007/s11897-011-0070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wong AK, et al. The effect of metformin on insulin resistance and exercise parameters in patients with heart failure. Eur. J. Heart Fail. 2012;14:1303–1310. doi: 10.1093/eurjhf/hfs106. [DOI] [PubMed] [Google Scholar]
- 100.Kolwicz SC, Jr., Purohit S, Tian R. Cardiac metabolism and its interactions with contraction, growth, and survival of cardiomyocytes. Circ. Res. 2013;113:603–616. doi: 10.1161/CIRCRESAHA.113.302095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Inzucchi SE, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab. Vasc. Dis. Res. 2015;12:90–100. doi: 10.1177/1479164114559852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zinman B, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015 doi: 10.1056/NEJMc1600827. http://dx.doi.org/10.1056/NEJMoa1504720. [DOI] [PubMed]
- 103.Huynh K, Bernardo BC, McMullen JR, Ritchie RH. Diabetic cardiomyopathy: mechanisms and new treatment strategies targeting antioxidant signaling pathways. Pharmacol. Ther. 2014;142:375–415. doi: 10.1016/j.pharmthera.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 104.Drawnel FM, et al. Disease modeling and phenotypic drug screening for diabetic cardiomyopathy using human induced pluripotent stem cells. Cell Rep. 2014;9:810–821. doi: 10.1016/j.celrep.2014.09.055. [DOI] [PubMed] [Google Scholar]
- 105.Meloni M, et al. Nerve growth factor gene therapy using adeno-associated viral vectors prevents cardiomyopathy in type 1 diabetic mice. Diabetes. 2012;61:229–240. doi: 10.2337/db11-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Katare R, et al. Intravenous gene therapy with PIM-1 via a cardiotropic viral vector halts the progression of diabetic cardiomyopathy through promotion of prosurvival signaling. Circ. Res. 2011;108:1238–1251. doi: 10.1161/CIRCRESAHA.110.239111. [DOI] [PubMed] [Google Scholar]
- 107.Greco S, et al. MicroRNA dysregulation in diabetic ischemic heart failure patients. Diabetes. 2012;61:1633–1641. doi: 10.2337/db11-0952. [DOI] [PMC free article] [PubMed] [Google Scholar]