Abstract
Background
Traumatic brain injury (TBI) is the most established environmental risk factor for Alzheimer’s disease (AD), but it is unclear if TBI is specifically associated with early-onset AD (EOAD).
Objective
To evaluate the relationship between TBI and EOAD (<65 years).
Methods
We identified 1,449 EOAD, 4,337 late-onset AD (LOAD), and corresponding EOAD-matched and LOAD-matched normal controls (NC) in the National Alzheimer’s Coordinating Center Uniform (NACC) database and compared the prevalence of any history of TBI as well as measures of cognition, function, behavior, and neuropathology. For validation, we determined TBI prevalence among 115 well-characterized clinic patients with EOAD.
Results
Part A: The prevalence of any TBI in the NACC-database EOAD participants (13.3%) was comparable to that observed in the clinic EOAD patients (13.9%) but significantly higher than in the NACC-database LOAD participants (7.7%; p < 0.0001) and trended to higher compared to EOAD-matched NC (11.1%; logistic regression p = 0.053). Part B: When we compared EOAD patients with documented non-acute and non-residually impairing TBI to EOAD without a documented history of prior TBI, those with TBI had significantly more disinhibition. Part C: Autopsies did not reveal differences in AD neuropathology based on a history of TBI.
Conclusions
These findings suggest, but do not establish, that TBI is a specific risk factor for EOAD and may lead to disinhibition, a feature that often results from the frontal effects of head injury. This study recommends further research on the effects of TBI in EOAD in larger numbers of participants.
Keywords: Aging, Alzheimer’s disease, concussion, dementia, epidemiology, head injury, memory loss, neurodegeneration, risk factors, traumatic brain injury
INTRODUCTION
Alzheimer’s disease (AD) is an increasing health problem worldwide, with the prevalence of the disorder projected to rise as people continue to live longer lives [1, 2]. Advancing age is the most important risk factor for AD, as most affected individuals are 65 years of age or older [1, 3]. Consequently, as therapeutic interventions have lagged in development, there has been increasing emphasis on identifying strategies for prevention in younger life, before the development of the disease. This emphasis on prevention increases the importance of identifying risk factors, other than aging, that can be the focus of intervention or modification.
After normal aging, a history of traumatic brain injury (TBI) is the most established environmental risk factor for dementia [4]. Studies have challenged this association [5–8], but the weight of the evidence appears to favor an increased risk for late-life AD after a single moderate-to-severe TBI involving loss of consciousness (LOC) in early or middle life [9–17]. Although there is limited support for a single mild TBI as a risk factor for dementia [18], repetitive mild TBIs can also result in chronic traumatic encephalopathy (CTE), a progressive neurodegenerative disorder with AD-like neurofibrillary tangle (NFT) formation [19–22]. Together, these studies strengthen the link between TBI and AD and the possibility that TBI promotes the deposition of AD neuropathology.
A lowering of the age of onset of AD and a consequent increased risk for early-onset AD (EOAD) may be evidence for the promotion of AD neuropathology by TBI. A number of studies have reported that a history of TBI lowers the age of onset of dementia [11, 17, 23–26], suggesting that TBI may be a particular risk factor for AD presenting at a relatively young age or for EOAD, defined as AD with onset <65 years of age [9, 27]. However, only a few studies have examined a history of TBI as a risk factor for EOAD, and their results have been contradictory [6, 28–30].
We aimed to investigate whether TBI is a specific risk factor for EOAD relative to the more typical late-onset AD (LOAD). This study could have clinical impact by renewing efforts to prevent head injuries and greater vigilance and cognitive evaluations of individuals <65 years of age who have had one or more TBIs. We investigated the effects of TBI in EOAD in a three part study from the large National Alzheimer’s Coordinating Center (NACC) database. Part A determined the prevalence of a history of TBI among NACC-database EOAD participants (age of onset <65), NACC-database LOAD participants (age of onset ≥65), and matched normal controls (NC), and, for validation, a cohort of carefully evaluated EOAD patients from our university-based clinic. Part B investigates whether a history of TBI altered the neurobehavioral symptoms of NACC-database EOAD participants, compared to EOAD participants without a history of TBI. Part C investigated whether a history of TBI resulted in qualitative differences in neuropathology among EOAD and LOAD participants in the NACC-database.
METHODS
The NACC-database compiles and maintains the Uniform Data Set (UDS) and Neuropathological database (NPD), which contain standardized clinical and pathologic data collected from participants evaluated at National Institute of Aging (NIA) funded Alzheimer’s Disease Centers (ADCs) throughout the United States [31]. The ADCs are funded, in part, for their established expertise in the accurate diagnosis of AD during life. We analyzed UDS and NPD data accumulated from September 2005 through the May 2014 data freeze. Detailed descriptions of the UDS and the NPD have previously been published [31] and are available at http://www.alz.washington.edu. This study was submitted and approved by the institutional review board at our institution. Furthermore, as part of the NACC-database, there was written informed consent from each participant and/or their authorized representative and approval by the Institutional Review Board at each participating ADC.
First, we identified NACC-database participants who were diagnosed with established criteria for “clinically probable AD dementia” at their ADC most recent UDS visit [32]. The ADCs carefully apply these criteria and, hence, these patients are representative of patients with clinically probable AD. In a NACC-database study, 438 of 526 patients with clinically probable AD met neuropathological criteria for AD, for a sensitive of the diagnosis of clinically probable AD dementia of 70.9%, specificity of 70.8%, and positive predictive value of 83.3% [33]. We defined EOAD as clinically probable AD with onset of cognitive decline <65 years of age and LOAD as clinically probable AD with onset of cognitive decline ≥65 years of age. Patients with familial or autosomal dominant AD from known genetic mutations, when evident in the record, were excluded. We then identified NC in the NACC-database comprised of participants with normal cognition on their initial visit. The NC were divided into younger (<65) and older (≥65) groups at the initial visit and matched with the corresponding younger (EOAD) or older (LOAD) participants on gender and years of education (within three years). This process yielded 1,449 EOAD participants with 1,449 EOAD-matched NC and 4,337 LOAD participants with 4,337 LOAD-matched NC (see Table 1).
Table 1.
Group characteristics of NACC-database participants
| EOAD | EOAD-matched NC | LOAD | LOAD-matched NC | |
|---|---|---|---|---|
| Number | 1,449 | 1,449 | 4,337 | 4,337 |
| Gender (% male) | 40.8% | 40.8% | 40.5% | 40.5% |
| Age of Initial Visit (years)* | 62.7 ± 6.8 | 58.8 ± 6.1 | 79.2 ± 6.3 | 75.0 ± 6.8 |
| Age of Onset (years) | 57.3 ± 5.7 | N/A | 74.5 ± 6.3 | N/A |
| Education (years) | 15.3 ± 3.0 | 15.4 ± 3.0 | 14.8 ± 3.3 | 15.0 ± 3.1 |
| White/Black/Asian/Other (%) | 85/10/2/2 | 82/13/3/2 | 83/15/1/1 | 82/15/2/1 |
Significant difference in Age of Initial Visit between: EOAD versus EOAD-matched controls: t = 16.25, df 2896, p < 0.0001. LOAD versus LOAD-matched controls: t = 29.84, df 8672, p < 0.0001. EOAD, early-onset Alzheimer’s disease; LOAD, late-onset Alzheimer’s disease; NC, normal controls.
Part A
We ascertained the prevalence of a history of Any TBI in the NACC-database EOAD and LOAD groups and their NC. Our initial analyses included Any history of one or more TBI surveyed in the NACC-UDS questions. In all groups, 75% or more had TBIs that were reported as remote with less than 5 min of LOC with most of the others suggested to have LOC less than 30 min without residual disability; however, remote and retrospective determination from patients and informants on exact TBI characteristics and LOC was not clearly reliable as the NACC-database was not designed for precise characterization of TBIs. Because of the problem of possible recall bias in the NACC-database retrospective dataset, we included a validation comparison group of 115 EOAD patients who were carefully evaluated in a university-based subspecialty clinic for early-onset dementias [34]. These patients had a uniform, physician-based assessment that specifically assayed and characterized a history of prior TBI; 88.7% of their TBIs were associated with LOC of <5 min) and 96.5% with LOC of <30 min.
Part B
We further evaluated the impact of prior TBI among the NACC-database EOAD participants. Within the NACC-database EOAD group, we compared cognitive, functional, and behavioral indices between participants with a clear and documented history of TBI and those with no history of TBI. In order to increase the reliability of the reported TBI for possible effects on EOAD, we surveyed the EOAD participants with TBI and included only those (n = 178) with documented non-acute (“remote” TBI >1 year per NACC-UDS terminology) TBI that did not leave residual impairments (“chronic dysfunction/disability” per NACC-UDS terminology). The 178 EOAD participants with documented TBI were comparable to the 1,234 without Any TBI in age of presentation (62.13 ± 6.77 versus 62.76 ± 6.84) and years of education (15.5 ± 3.03 versus 15.21 ± 3.03) but had a greater percentage of men (60% versus 37.8%, χ2 = 36.34; p < 0.001). Cognitive tests included Mini-Mental State Examination, logical memory (IA and IIA) of the revised Wechsler Memory Scale (WMS-R), digit spans (forward and backward) from the WMS-R, category fluency (animals and vegetables), the Boston Naming Test, the digit symbol subtest of the Wechsler Adult Intelligence Scale, and Trail-Making tests (A and B) [31, 35]. Functional scales included the Functional Activities Questionnaire and the Clinical Dementia Rating Sum of Boxes [36, 37]. Behavioral symptoms were evaluated with the Neuropsychiatric Inventory (NPI-Q), which was completed by caregivers or other informants [38].
Part C
Neuropathological data from the NACC-NPD was available on 241 (13.5%) EOAD and 626 (14.9%) LOAD participants. We evaluated for the presence of Consortium to Establish a Registry in AD (CERAD) criteria for definite or other AD [39], as well as for the presence of other neuropathological diagnoses.
Data analysis
Statistical analyses were performed using SPSS 22 for Windows (IBM, Armonk NY). Demographic variables were compared between the EOAD and LOAD groups using χ2 tests for nominal variables (gender, education, race), and unpaired t-tests for continuous variables (age, years of education). Distribution of data were tested with Shapiro-Wilk and Kolmogorov-Smirnov Tests. We subsequently used logistic regression to adjust for possible confounding effects due to differences in gender, age at initial visit, years of education, and race (percent White). The results of the cognitive, functional, and NPI assessments were non-normally distributed; hence they were analyzed with nonparametric tests, including their median and interquartile ratio (IQR) results, using Mann-Whitney U analyses. We used the false discovery rate (FDR) to correct for multiple comparisons.
RESULTS
Part A
There were no significant differences in group characteristics except in older age of initial visit for the EOAD and LOAD participants compared to their respective NC (see Table 1). The prevalence of a history of Any TBI in the NACC-database EOAD group was 13.3% (see Table 2). This was significantly higher than the prevalence of a history of Any TBI in the NACC-database LOAD group (7.7%; χ2 = 45.43, p < 0.0001), but comparable to the prevalence of a history of Any TBI in the EOAD patients evaluated in the early-onset dementia subspecialty clinic (13.9%; n.s.). The prevalence of a history of Any TBI trended toward significantly higher in the NACC-database EOAD group compared to EOAD-matched NC (11.1%; χ2 = 3.64, p = 0.056). The prevalence of a history of Any TBI was not significantly different in the NACC-database LOAD group compared to LOAD-matched NC (8.7%; χ2 = 2.73, p = 0.100).
Table 2.
Prevalence of TBI in NACC-database groups
| EOAD | EOAD-matched NC | LOAD | LOAD-matched NC | |
|---|---|---|---|---|
| No previous TBI | 1234 (85.2%) | 1279 (88.3%) | 3954 (91.2%) | 3931 (90.6%) |
| Any TBI* | 193 (13.3%) | 161 (11.1%) | 335 (7.7%) | 379 (8.7%) |
| Insufficient information on TBI | 22 (1.5%) | 9 (0.6%) | 48 (1.1%) | 27 (0.6%) |
Trend toward significance for EOAD versus EOAD-matched controls: χ2 = 3.64, p = 0.056. TBI, traumatic brain injury; EOAD, early-onset Alzheimer’s disease; LOAD, late-onset Alzheimer’s disease; NC, normal controls.
Logistic regression adjusted for possible confounding effects due to differences in gender, age, years of education, and race (% White): 1) When comparing NACC-database EOAD and LOAD groups, a history of Any TBI was significantly more common in the EOAD group (β 0.56; p < 0.001; OR 1.75, 95% CI 1.47–2.07) and in men (β0.82; p < 0.001; OR 2.28, 95% CI 1.92–2.70); 2) When comparing NACC-database EOAD and the EOAD-matched NC, a history of Any TBI still trended to significance (β 0.22; p = 0.053; OR 1.28, 95% CI 1.00–1.57) despite the group differences in age (see Table 1); 3) When comparing NACC-database LOAD and the LOAD-matched NC, a history of Any TBI was not significant (β–0.12; p = 0.132; OR 0.90, 95% CI 0.76–1.04). Other variables (gender, age, years of education, race) were not significant other than as noted
Part B
Among EOAD participants, we evaluated the impact of TBI on cognitive, functional, and behavioral performance by comparing EOAD participants with documented TBI to those without a history of Any TBI. After FDR adjustment for multiple corrections, there were no significant differences between groups on cognitive or functional scores, except for paradoxically better logical memory IA scores among those with TBI compared to those without a history of Any TBI (see Table 4). However, the significant difference in logical memory IA scores did not survive logistic regression. Age differences accounted for the significant difference in logical memory IA scores between groups (β –0.12; p = 0.011; OR 0.89, 95% CI 0.81–0.97).
Table 4.
Median and interquartile range of functional measures for NACC-database EOAD participants
| EOAD with History of TBI | EOAD without History of TBI | Mann-Whitney U | p-value | |
|---|---|---|---|---|
| Total FAQ Score | 19 (14) | 19 (15) | 44748.5 | n.s. |
| CDR Sum of Boxes Score | 5 (3) | 5 (5) | 109508 | n.s. |
Non-normally distributed. Medians and Interquartile Range (IQR). TBI, traumatic brain injury; EOAD, early-onset Alzheimer’s disease; FAQ, functional activities questionnaire; CDR, Clinical Dementia Rating; n.s., non-significant.
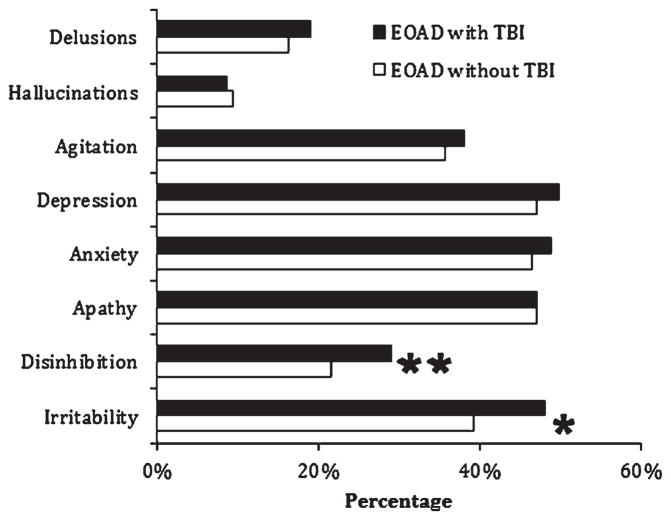
On the NPI-Q behavioral subscales, EOAD participants with documented TBI, when compared to those without a history of Any TBI (see Fig. 1), had a significantly higher prevalence of disinhibition even after FDR correction for multiple comparisons (29.0% versus 21.6% respectively; χ2 = 6.19, p = 0.011). These findings remained significant after logistic regression (β 0.36; p = 0.027; OR 1.50, 95% CI 1.04–1.96). Disinhibition was also significantly more likely among males (β–0.27; p = 0.022; OR 0.76, 95% CI 0.60–0.96), but was unaffected by age, years of education, or race.
Fig. 1.
Prevalence of neuropsychiatric symptoms among NACC-database early-onset Alzheimer’s disease (EOAD) participants: Eight main behavioral measures of the Neuropsychiatric Inventory. *Significance to p = 0.01 level for disinhibition (29.0% versus 21.6%; χ2 = 6.19) and for irritability (48.1% versus 39.3%; χ2 = 6.40). **Only disinhibition remained significant after application of FDR cut-off for multiple comparisons. TBI, traumatic brain injury.
Part C
Neuropathological data were available for 241 EOAD participants (29 with TBI and 212 without TBI) and 815 LOAD participants (79 with TBI and 736 without TBI). Overall, the vast majority (92.4%) of the EOAD and LOAD participants had some form of AD on neuropathology (94.2% for EOAD versus 91.9% for LOAD; n.s.). The remaining exhibited a range of other neuropathologies (see Table 5). The EOAD participants were significantly more likely to have a diagnosis of definite AD on neuropathology (77% versus 71%) than the LOAD participants (χ2 = 37.96, p < 0.0001). There were no significant differences in the prevalence of definite or any AD between participants with and without a history of Any TBI, either within or across the EOAD and LOAD groups.
Table 5.
Available data for neuropathology in NACC-database EOAD and LOAD participants
| Clinical Diagnosis | No. | CERAD Neuropathological Criteria
|
Other Non-AD Neuropathology | |
|---|---|---|---|---|
| Definite AD | All AD Criteria* | |||
| EOAD with Any TBI | 29 | 23 (79.3%) | 24 (82.8%) | 5 (FTLD = 1, HS = 2, Other = 2) |
| EOAD without TBI | 212 | 195 (92%) | 201 (94.8%) | 11 (FTLD = 6, HS = 1, DLB = 2, PSP = 1; Other = 1) |
| LOAD with Any TBI | 79 | 54 (68.4%) | 70 (88.6%) | 9 (FTLD = 3, HS = 1, DLB = 1, VASC = 2; Other = 2) |
| LOAD without TBI | 736 | 536 (72.8%) | 679 (92.3%) | 57 (FTLD = 10, DLB = 10, HS = 9, VASC = 5; PSP = 4, NORM = 3, Other = 16) |
Definite + Probable + Possible AD; TBI, traumatic brain injury, EOAD, early-onset Alzheimer’s disease; LOAD, late-onset Alzheimer’s disease; CERAD, The Consortium to Establish a Registry for Alzheimer’s Disease; FTLD, frontotemporal lobar degeneration; HS, hippocampal sclerosis; DLB, dementia with Lewy bodies; PSP, progressive supranuclear palsy; VASC, vascular pathology; NORM, normal brain; Other, otherwise undefined.
DISCUSSION
In the NACC database, a history of TBI was significantly more common in EOAD than in LOAD and trended to significance (p = 0.056) when compared to EOAD-matched normal controls. Furthermore, among the EOAD participants, those with a history of documented TBI, compared to those without a history of TBI, had a higher prevalence of disinhibition. Disinhibition is a characteristic clinical sign of frontal lobe dysfunction that may be influenced by the neuropathological process of TBI. Autopsies of EOAD and LOAD participants did not show qualitative differences related to a history of TBI but did not exclude quantitative effects on the neuropathology of AD. Together, these findings suggest an effect of TBI in promoting the development of EOAD.
Although a number of reports have failed to document an association between TBI and increased rates of AD [5–8], the weight of the evidence suggests that moderate-to-severe TBI with LOC is a risk factor for dementia and probably for AD [40]. An early meta-analysis of 11 case control studies confirms the association of TBI with AD, but primarily in men [11, 41], and other studies show a greater risk for AD or dementia with increasing numbers and/or severity of moderate-to-severe TBIs [12, 14]. Among U.S. Veterans, a cohort study indicates that prior moderate-to-severe TBI have an increased risk of AD [14] and, among Veterans ≥55 years, there may be a 60% increase in the incidence of dementia after 9 years [26]. In another retrospective cohort study, those with moderate-to-severe TBI at ≥55 years or mild TBI at ≥65 years have an increased risk of developing dementia after 5–7 years [42]. One study with a mean follow-up of one year reports an increased risk of dementia after a single mild TBI [18], but other evidence for mild TBI precipitating subsequent AD or dementia is less clear. A recent meta-analysis of studies on mild TBI (defined as loss of consciousness of <30 min and post-traumatic amnesia of <24 h) finds no association with dementia [8]. Other cohort studies have also failed to find an association between lifetime history of any TBI and an increased risk of developing dementia or AD [6, 7, 25]. In sum, the relationship between TBI and AD remains somewhat controversial; though there is greater support for a relationship between moderate-to-severe TBI and subsequent dementia.
Assuming that TBI is a risk factor for AD, one mechanism may be to hasten the age of AD onset [9, 27], and a number of studies indicate an association between a history of TBI and lower age of dementia onset [11, 17, 23–26]. The early meta-analysis of TBI in AD reported an earlier age of AD onset in individuals below 70 years of age [11]. Two early studies with small sample sizes reported a younger mean age of AD onset if they had a history of TBI [23, 24], and a community based prospective study found a younger age of AD onset among individuals with prior TBI [17]. In a well-designed, population-based investigation of all documented episodes of TBI from 1935–1984 in Olmsted County, Minnesota, the observed time from TBI to AD was less than the expected time to onset of AD (median 10 versus 18 years, p = 0.015), and, when stratified by age of AD onset, the number of TBI cases with EOAD was more than twice that expected [25]. Furthermore, in the aforementioned study of U.S. veterans with 9 years of follow-up, a history of TBI lowered the age of dementia onset by 2.1 years [26].
Our findings suggest that TBI may be a more specific risk factor for EOAD, as compared to LOAD. While the vast majority of cases of AD are 65 years of age or older, a smaller cohort of 4–5% are younger than 65 [1], and these EOAD cases, which are generally sporadic and not related to presenilin mutations or apolipoprotein ε4 allele [40, 43], can be phenotypically distinct from LOAD [44]. Few other studies have specifically examined a history of TBI as a risk factor for EOAD, and the results have been somewhat contradictory [28, 29]. In a recent investigation of over 800,000 Swedish men conscripted for military service and followed for 33 years, “young onset dementia,” but not specifically AD, is linked to a history of TBI, with greater risk seen with more frequent or severe TBI [28]. The current investigation has the advantage of using the NACC-database, which is one of the largest and most comprehensive databases of its kind, with a specific focus on individuals diagnosed with AD [31].
A previous investigation by Sayed and colleagues using the NACC database failed to find a relationship between TBI and an earlier age of dementia onset [30]; however, they compared dementia participants with and without a history of TBI, rather than comparing prevalence of TBI among those with EOAD compared to LOAD. They also found an increased risk for dementia among participants with dementia associated with TBI with chronic deficit/dysfunction and concluded that these participants may differ from AD because of higher rates of neuropsychiatric symptoms. Similarly, we also found that EOAD participants with determined TBI had more disinhibition than those without TBI.
There are several possible mechanistic explanations for the association between TBI and EOAD, including a reduction of cognitive reserve, diffuse axonal injury facilitation of tau deposition, frontotemporal contusions, and, most specifically, the promotion of AD-related neurodegeneration [29]. Severe TBI increases the likelihood of AD-related neuropathology changes [43]. Widespread NFTs and amyloid-β plaques are present in up to a third of patients surviving a year or more after a single TBI, even in those who are relatively young, thus supporting the role of TBI in accelerating the age of onset of AD [9]. Amyloid-β plaques can emerge hours after a single TBI, but they are usually diffuse and not fibrillar as in AD [45], and recede several months after the injury. CTE, an AD-like neurodegenerative process with extensive tauimmunoreactive NFTs, has been linked to repetitive mild TBI among boxers, other athletes, veterans, and others [46–48]. In CTE, NFTs initially deposit perivascularly and in superficial cortical layers, especially in the frontal lobes [46, 48], and CTE may manifest with early clinical “frontal” symptoms such as disinhibition, irritability, and mood disturbances, and even suicidal behavior [48, 49]. These neurobehavioral changes are similar to the disinhibition and other behavioral changes present in our EOAD participants and other demented individuals who have a history of TBI [50, 51].
A number of important issues may limit the interpretation of our results. First, we can not completely exclude the possibility that younger age in general accounts for a higher prevalence of prior TBI among the NACC-database EOAD participants; we report only a trend to significance (p = 0.05) when they are compared to normal controls. Moreover, male gender, as in prior studies [11], significantly accounts for much of the difference in TBI between EOAD and LOAD participants. Second, the NACC-database does not define the nature and characteristics of the TBI, including time course, the presence of multiple TBIs, and clearly reliable LOC and severity information. Although the NACC-database does not sufficiently and reliably characterize the TBIs, it is reasonable to presume, based on similar clinic patients and the inclusion criteria for Part B, that most TBIs are mild. A third concern regarding the NACC-database is the use of retrospective ascertainment for prior TBI, a methodology fraught with recall bias, with the possibility that informants for AD participants more readily recall a previous TBI than for the normal controls. Our inclusion of a “validation” group of well-studied EOAD clinic patients, who reported a similar prevalence of prior TBI as the NACC-database EOAD participants, mitigated, but did not eliminate, the potential effects of recall bias. Finally, the inclusion of clinically probable AD does not guarantee the presence of AD on neuropathology [33].
Despite the reservations, our data suggest, but do not prove, that TBI is associated with EOAD and recommends further research on how TBI may interact with other risk factors to accelerate the onset of AD [25]. Our findings also suggest that a prior history of TBI may promote the neuropsychiatric presentation of EOAD, such as disinhibition. The results emphasize the importance of assessing TBI history and evaluating neuropsychiatric symptoms in patients who present with AD at an early age. From a public health standpoint, our findings underscore the value of public health efforts in reducing the incidence of TBI, a potentially preventable risk factor for AD, and also recommend following-up this study with further research employing larger numbers of participants in clarifying the role of TBI in accelerating the age of onset for AD.
Table 3.
Median and interquartile range of cognitive test scores for NACC-database EOAD participants
| EOAD with History of TBI | EOAD without History of TBI | Mann-Whitney U | p-value | |
|---|---|---|---|---|
| Mini-Mental State Examination | 21 (9) | 21 (9) | 95734 | n.s. |
| Logical Memory IA | 4 (6) | 3 (IQR) | 67839 | p < 0.001* |
| Logical Memory IIA | 1 (4) | 0 (3) | 70200 | p < 0.05 |
| Digit Span - Forward | 6 (1.75) | 6 (2) | 86793 | n.s. |
| Digit Span - Backward | 3 (2) | 3 (2) | 85314 | n.s. |
| “Animal” Category Fluency | 11 (8) | 10 (8) | 82655 | n.s. |
| “Vegetable” Category Fluency | 6 (5) | 6 (7) | 85608 | n.s. |
| Boston Naming Test | 24 (8) | 23 (10) | 77974 | n.s. |
| WAIS-R Digit Symbol Subtest | 28 (25) | 23 (25.75) | 49680 | n.s. |
| Trail Making A Test‡ | 54 (69) | 58 (78.25) | 62826 | n.s. |
| Trail Making B Test‡ | 214.5 (205.25) | 240.5 (184) | 32366 | n.s. |
Non-normally distributed. Medians and Interquartile Range (IQR).
Still significant after False Discovery Rate correction for multiple comparisons but no longer significant after further accounting for the age differences between the two groups.
Excludes those who did not complete Trail Making A Test in ≤150 s and Trail Making B Test in ≤300 s. TBI, traumatic brain injury; EOAD, early-onset Alzheimer’s disease; WAIS-R, Wechsler Adult Intelligence Scale; n.s. = non-significant.
Acknowledgments
This research was funded by The Medical Student Training in Aging Research Program, the National Institute on Aging (T35AG026736), the John A. Hartford Foundation, the Lillian R. Gleitsman Foundation, and NIA Grant U01 AG016976.
The authors thank Michelle Mather, Kate Heller, and Dr. Stephen Hawes for their contributions. The NACC database is funded by NIA/NIH Grant U01 AG016976. NACC data are contributed by the NIA funded ADCs: P30 AG019610 (PI Eric Reiman, MD), P30 AG013846 (PI Neil Kowall, MD), P50 AG008702 (PI Scott Small, MD), P50 AG025688 (PI Allan Levey, MD, PhD), P30 AG010133 (PI Andrew Saykin, PsyD), P50 AG005146 (PI Marilyn Albert, PhD), P50 AG005134 (PI Bradley Hyman, MD, PhD), P50 AG016574 (PI Ronald Petersen, MD, PhD), P50 AG005138 (PI Mary Sano, PhD), P30 AG008051 (PI Steven Ferris, PhD), P30 AG013854 (PI M. Marsel Mesulam, MD), P30 AG008017 (PI Jeffrey Kaye, MD), P30 AG010161 (PI David Bennett, MD), P30 AG010129 (PI Charles DeCarli, MD), P50 AG016573 (PI Frank LaFerla, PhD), P50 AG016570 (PI David Teplow, PhD), P50 AG005131 (PI Douglas Galasko, MD), P50 AG023501 (PI Bruce Miller, MD), P30 AG035982 (PI Russell Swerdlow, MD), P30 AG028383 (PI Linda Van Eldik, PhD),P30AG010124(PIJohnTrojanowski,MD,PhD), P50 AG005133 (PI Oscar Lopez, MD), P50 AG005142 (PI Helena Chui, MD), P30 AG012300 (PI Roger Rosenberg,MD),P50AG005136(PIThomasMontine, MD, PhD), P50 AG033514 (PI Sanjay Asthana, MD, FRCP), and P50 AG005681 (PI John Morris, MD).
Footnotes
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/14-3207r2).
References
- 1.Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013;9:63–75. e62. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Corrada MM, Brookmeyer R, Berlau D, Paganini-Hill A, Kawas CH. Prevalence of dementia after age 90: Results from the 90+ study. Neurology. 2008;71:337–343. doi: 10.1212/01.wnl.0000310773.65918.cd. [DOI] [PubMed] [Google Scholar]
- 4.Sivanandam TM, Thakur MK. Traumatic brain injury: A risk factor for Alzheimer’s disease. Neurosci Biobehav Rev. 2012;36:1376–1381. doi: 10.1016/j.neubiorev.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Mehta KM, Ott A, Kalmijn S, Slooter AJ, van Duijn CM, Hofman A, Breteler MM. Head trauma and risk of dementia and Alzheimer’s disease: The Rotterdam Study. Neurology. 1999;53:1959–1962. doi: 10.1212/wnl.53.9.1959. [DOI] [PubMed] [Google Scholar]
- 6.Dams-O’Connor K, Gibbons LE, Bowen JD, McCurry SM, Larson EB, Crane PK. Risk for late-life re-injury, dementia and death among individuals with traumatic brain injury: A population-based study. J Neurol Neurosurg Psychiatry. 2013;84:177–182. doi: 10.1136/jnnp-2012-303938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helmes E, Ostbye T, Steenhuis RE. Incremental contribution of reported previous head injury to the prediction of diagnosis and cognitive functioning in older adults. Brain Inj. 2011;25:338–347. doi: 10.3109/02699052.2011.556104. [DOI] [PubMed] [Google Scholar]
- 8.Godbolt AK, Cancelliere C, Hincapie CA, Marras C, Boyle E, Kristman VL, Coronado VG, Cassidy JD. Systematic review of the risk of dementia and chronic cognitive impairment after mild traumatic brain injury: Results of the International Collaboration on Mild Traumatic Brain Injury Prognosis. Arch Phys Med Rehabil. 2014;95:S245–S256. doi: 10.1016/j.apmr.2013.06.036. [DOI] [PubMed] [Google Scholar]
- 9.Johnson VE, Stewart W, Smith DH. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol. 2012;22:142–149. doi: 10.1111/j.1750-3639.2011.00513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graves AB, White E, Koepsell TD, Reifler BV, van Belle G, Larson EB, Raskind M. The association between head trauma and Alzheimer’s disease. Am J Epidemiol. 1990;131:491–501. doi: 10.1093/oxfordjournals.aje.a115523. [DOI] [PubMed] [Google Scholar]
- 11.Mortimer JA, van Duijn CM, Chandra V, Fratiglioni L, Graves AB, Heyman A, Jorm AF, Kokmen E, Kondo K, Rocca WA, Shalat SL, Soininen H, Hofman A for the Eurodem Risk Factors Research Group. Head trauma as a risk factor for Alzheimer’s disease: A collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int J Epidemiol. 1991;20(Suppl 2):S28–S35. doi: 10.1093/ije/20.supplement_2.s28. [DOI] [PubMed] [Google Scholar]
- 12.Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L, Chui H, Green RC, Sadovnick AD, Duara R, DeCarli C, Johnson K, Go RC, Growdon JH, Haines JL, Kukull WA, Farrer LA. Head injury and the risk of AD in the MIRAGE study. Neurology. 2000;54:1316–1323. doi: 10.1212/wnl.54.6.1316. [DOI] [PubMed] [Google Scholar]
- 13.Molgaard CA, Stanford EP, Morton DJ, Ryden LA, Schubert KR, Golbeck AL. Epidemiology of head trauma and neurocognitive impairment in a multi-ethnic population. Neuroepidemiology. 1990;9:233–242. doi: 10.1159/000110778. [DOI] [PubMed] [Google Scholar]
- 14.Plassman BL, Havlik RJ, Steffens DC, Helms MJ, Newman TN, Drosdick D, Phillips C, Gau BA, Welsh-Bohmer KA, Burke JR, Guralnik JM, Breitner JC. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology. 2000;55:1158–1166. doi: 10.1212/wnl.55.8.1158. [DOI] [PubMed] [Google Scholar]
- 15.O’Meara ES, Kukull WA, Sheppard L, Bowen JD, McCormick WC, Teri L, Pfanschmidt M, Thompson JD, Schellenberg GD, Larson EB. Head injury and risk of Alzheimer’s disease by apolipoprotein E genotype. Am J Epidemiol. 1997;146:373–384. doi: 10.1093/oxfordjournals.aje.a009290. [DOI] [PubMed] [Google Scholar]
- 16.Salib E, Hillier V. Head injury and the risk of Alzheimer’s disease: A case control study. Int J Geriatr Psychiatry. 1997;12:363–368. doi: 10.1002/(sici)1099-1166(199703)12:3<363::aid-gps515>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 17.Schofield PW, Tang M, Marder K, Bell K, Dooneief G, Chun M, Sano M, Stern Y, Mayeux R. Alzheimer’s disease after remote head injury: An incidence study. J Neurol Neurosurg Psychiatry. 1997;62:119–124. doi: 10.1136/jnnp.62.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee YK, Hou SW, Lee CC, Hsu CY, Huang YS, Su YC. Increased risk of dementia in patients with mild traumatic brain injury: A nationwide cohort study. PLoS One. 2013;8:e62422. doi: 10.1371/journal.pone.0062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeKosky ST, Blennow K, Ikonomovic MD, Gandy S. Acute and chronic traumatic encephalopathies: Pathogenesis and biomarkers. Nat Rev Neurol. 2013;9:192–200. doi: 10.1038/nrneurol.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshiyama Y, Uryu K, Higuchi M, Longhi L, Hoover R, Fujimoto S, McIntosh T, Lee VM, Trojanowski JQ. Enhanced neurofibrillary tangle formation, cerebral atrophy, and cognitive deficits induced by repetitive mild brain injury in a transgenic tauopathy mouse model. J Neurotrauma. 2005;22:1134–1141. doi: 10.1089/neu.2005.22.1134. [DOI] [PubMed] [Google Scholar]
- 21.Smith DH, Johnson VE, Stewart W. Chronic neuropathologies of single and repetitive TBI: Substrates of dementia? Nat Rev Neurol. 2013;9:211–221. doi: 10.1038/nrneurol.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt ML, Zhukareva V, Newell KL, Lee VM, Trojanowski JQ. Tau isoform profile and phosphorylation state in dementia pugilistica recapitulate Alzheimer’s disease. Acta Neuropathol. 2001;101:518–524. doi: 10.1007/s004010000330. [DOI] [PubMed] [Google Scholar]
- 23.Sullivan P, Petitti D, Barbaccia J. Head trauma and age of onset of dementia of the Alzheimer type. JAMA. 1987;257:2289–2290. doi: 10.1001/jama.1987.03390170045014. [DOI] [PubMed] [Google Scholar]
- 24.Gedye A, Beattie BL, Tuokko H, Horton A, Korsarek E. Severe head injury hastens age of onset of Alzheimer’s disease. J Am Geriatr Soc. 1989;37:970–973. doi: 10.1111/j.1532-5415.1989.tb07283.x. [DOI] [PubMed] [Google Scholar]
- 25.Nemetz PN, Leibson C, Naessens JM, Beard M, Kokmen E, Annegers JF, Kurland LT. Traumatic brain injury and time to onset of Alzheimer’s disease: A population-based study. Am J Epidemiol. 1999;149:32–40. doi: 10.1093/oxfordjournals.aje.a009724. [DOI] [PubMed] [Google Scholar]
- 26.Barnes DE, Kaup A, Kirby KA, Byers AL, Diaz-Arrastia R, Yaffe K. Traumatic brain injury and risk of dementia in older veterans. Neurology. 2014;83:312–319. doi: 10.1212/WNL.0000000000000616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu XC, Tan L, Wang HF, Jiang T, Cao L, Wang C, Wang J, Tan CC, Meng XF, Yu JT. Rate of early onset Alzheimer’s disease: A systematic review and meta-analysis. Ann Transl Med. 2015;3:38. doi: 10.3978/j.issn.2305-5839.2015.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nordstrom P, Michaelsson K, Gustafson Y, Nordstrom A. Traumatic brain injury and young onset dementia: A nationwide cohort study. Ann Neurol. 2014;75:374–381. doi: 10.1002/ana.24101. [DOI] [PubMed] [Google Scholar]
- 29.Gardner RC, Yaffe K. Traumatic brain injury may increase risk of young onset dementia. Ann Neurol. 2014;75:339–341. doi: 10.1002/ana.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sayed N, Culver C, Dams-O’Connor K, Hammond F, Diaz-Arrastia R. Clinical phenotype of dementia after traumatic brain injury. J Neurotrauma. 2013;30:1117–1122. doi: 10.1089/neu.2012.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris JC, Weintraub S, Chui HC, Cummings J, Decarli C, Ferris S, Foster NL, Galasko D, Graff-Radford N, Peskind ER, Beekly D, Ramos EM, Kukull WA. The Uniform Data Set (UDS): Clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer Dis Assoc Disord. 2006;20:210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 32.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 33.Beach TG, Monsell SE, Phillips LE, Kukull W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J Neuropathol Exp Neurol. 2012;71:266–273. doi: 10.1097/NEN.0b013e31824b211b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendez MF, Lee AS, Joshi A, Shapira JS. Nonamnestic presentations of early-onset Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2012;27:413–420. doi: 10.1177/1533317512454711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lezak MD. Neuropsychological assessment. Oxford University Press; Oxford; New York: 2012. [Google Scholar]
- 36.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 37.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 38.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, Lopez OL, DeKosky ST. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 39.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brown-lee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 40.Van Den Heuvel C, Thornton E, Vink R. Traumatic brain injury and Alzheimer’s disease: A review. Prog Brain Res. 2007;161:303–316. doi: 10.1016/S0079-6123(06)61021-2. [DOI] [PubMed] [Google Scholar]
- 41.Fleminger S, Oliver DL, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer’s disease: The evidence 10 years on; a partial replication. J Neurol Neurosurg Psychiatry. 2003;74:857–862. doi: 10.1136/jnnp.74.7.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: The role of age and severity. JAMA Neurol. 2014;71:1490–1497. doi: 10.1001/jamaneurol.2014.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jellinger KA, Paulus W, Wrocklage C, Litvan I. Traumatic brain injury as a risk factor for Alzheimer disease. Comparison of two retrospective autopsy cohorts with evaluation of ApoE genotype. BMC Neurol. 2001;1:3. doi: 10.1186/1471-2377-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mendez MF. Early-onset Alzheimer’s disease: Non-amnestic subtypes and type 2 AD. Arch Med Res. 2012;43:677–685. doi: 10.1016/j.arcmed.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson VE, Stewart W, Smith DH. Traumatic brain injury and amyloid-beta pathology: A link to Alzheimer’s disease? Nat Rev Neurosci. 2010;11:361–370. doi: 10.1038/nrn2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, Santini VE, Lee HS, Kubilus CA, Stern RA. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–735. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCrory P, Meeuwisse WH, Kutcher JS, Jordan BD, Gardner A. What is the evidence for chronic concussion-related changes in retired athletes: Behavioural, pathological and clinical outcomes? Br J Sports Med. 2013;47:327–330. doi: 10.1136/bjsports-2013-092248. [DOI] [PubMed] [Google Scholar]
- 48.McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH, Lee HS, Wojtowicz SM, Hall G, Baugh CM, Riley DO, Kubilus CA, Cormier KA, Jacobs MA, Martin BR, Abraham CR, Ikezu T, Reichard RR, Wolozin BL, Budson AE, Goldstein LE, Kowall NW, Cantu RC. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stern RA, Daneshvar DH, Baugh CM, Seichepine DR, Mon-tenigro PH, Riley DO, Fritts NG, Stamm JM, Robbins CA, McHale L, Simkin I, Stein TD, Alvarez VE, Gold-stein LE, Budson AE, Kowall NW, Nowinski CJ, Cantu RC, McKee AC. Clinical presentation of chronic traumatic encephalopathy. Neurology. 2013;81:1122–1129. doi: 10.1212/WNL.0b013e3182a55f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dams-O’Connor K, Spielman L, Hammond FM, Sayed N, Culver C, Diaz-Arrastia R. An exploration of clinical dementia phenotypes among individuals with and without traumatic brain injury. NeuroRehabilitation. 2013;32:199–209. doi: 10.3233/NRE-130838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rao V, Rosenberg P, Miles QS, Patadia D, Treiber K, Bertrand M, Norton M, Steinberg M, Tschanz J, Lyketsos C. Neuropsychiatric symptoms in dementia patients with and without a history of traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2010;22:166–172. doi: 10.1176/appi.neuropsych.22.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]