Abstract
Objective
Ocular palatal tremor (OPT) typically develops after a breach in the Guillian‐Mollaret triangle. We herein describe a variant of this syndrome in which dystonia is also present, hence called, here, ocular palatal tremor plus dystonia.
Methods
We assessed eye‐head movements and dystonia in 6 patients with ocular palatal plus dystonia.
Results
Among 6 patients with OPT, 2 had focal dystonia, 3 had multifocal dystonia, and 1 had generalized dystonia. The dystonia affected the upper extremities and neck in 4 patients, the lower extremities in 3, and the face in 2. Three of four cervical dystonia patients had head tremor. Two patients also had speech involvement. Lack of correlation between eye and head oscillations suggested that head oscillations were not compensatory or secondary to the eye oscillations and vice versa.
Conclusions
We describe a novel variant of OPT with dystonia. We speculate that in such a variant, the dystonia possibly could be a result of abnormal cerebellar outflow in patients with a breach in the Guillain‐Mollaret triangle.
Keywords: cerebellum, oscillation, nystagmus, inferior olive, brainstem
The syndrome of ocular palatal tremor (OPT) is a unique manifestation subsequent to lesions involving the Guillian‐Mollaret triangle. The Guillian‐Mollaret triangle is comprised of the projection fibers from the deep cerebellar nuclei, which pass around the red nucleus to synapse with the contralateral inferior olive. Olivary fibers then project back to the deep cerebellar nuclei.1 Breach in the continuity of the Guillian‐Mollaret triangle results in pseudohypertrophy and spontaneous rhythmic discharges from the inferior olive.2, 3, 4, 5, 6 Such activity is the basis for the coarse, dysconjugate, and irregular eye oscillations associated with rhythmic palate movements that typify OPT.5, 7
Progressive ataxia palatal tremor syndrome is a variant of OPT where the eye and palate oscillations are accompanied by ataxia, but without a focal lesion compromising the integrity of the Guillian‐Mollaret triangle.8 Occasionally, patients with lesions of the Guillian‐Mollaret triangle with pseudohypertrophy of the inferior olive have pure eye nystagmus without involvement of the palate or vice versa.7
Here, we describe a series of patients with novel OPT variant where eye and palate oscillations were associated with various forms of dystonia. We refer to this variant as ocular palatal tremor plus dystonia (OPTD).
Methods
Six patients with OPTD were examined between July 2011 and June 2014. Three patients were diagnosed at Emory University, 2 at Case Western Reserve University, and 1 at the Cleveland Clinic. All subjects provided written informed consent before participating in the study. A neurological examination was videotaped for all 6 patients. Dystonia was quantitatively assessed with the motor component of the Burke‐Fahn‐Marsden Rating Scale (BFMRS).9 Eye and head movement were quantitatively assessed in 3 patients.
Results
All patients had pseudohypertrophy of the inferior olive associated with focal lesions interrupting the Guillian‐Mollaret triangle. Figure 1 depicts an example of inferior olive hypertrophy in T2‐weighted brain MRI. Table 1 summarizes the etiology of the lesions affecting the Guillian‐Mollaret triangle. Clinical examination in all patients revealed coarse, irregular, and dysconjugate oscillations of the eyes. Video 1 depicts an example from 1 patient. One patient had gaze position‐dependent torsional and vertical eye oscillations, 2 patients had eye oscillations along the horizontal and vertical axis, and in 2 patients oscillations were present in all three axes. Waveforms were coarse, irregular, and disconjugate. Consistent with the characteristics of OPT, eye oscillation frequency was 3.4 ± 1.6 Hz in the horizontal, 3.5 ± 2.3 Hz in the vertical, and 3.8 ± 1.7 Hz in the torsional axis. There was no evidence of change in characteristics of oscillations after removal of visual fixation, but they attenuated during ocular convergence. Palatal tremor was present in all but 1 patient.
Figure 1.
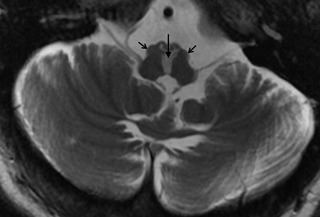
An example of T2‐weighted brain MRI showing bilateral inferior olive hypertrophy in a patient with OPTD. Open arrows show hypertrophied inferior olive, whereas hyperdense midline area shown by filled vertical arrow shows injury to input fibers to the inferior olive.
Table 1.
Pertinent clinical features of 6 patients with OPT plus dystonia
| Patient | Age/Gender | History | Nystagmus | Palate Tremor | Dystonia | Other Movement Disorders | Pharmacotherapy |
|---|---|---|---|---|---|---|---|
| 1 | 48/F | Resection of deep cerebellar mass | Torsional and vertical nystagmus on slight left gaze, nystagmus increases with further eccentric left gaze, no nystagmus on straight‐ahead gaze | Yes | Torticollis (mild), right hand (mild), both feet (moderate) | Lip tremor, speech tremor | Baclofen, clonazepam, BoTN |
| 2 | 46/M | Hemorrhagic stroke in pons and midbrain owing to unknown etiology | Coarse irregular dysconjugate eye oscillatiions in horizontal, and vertical directions | Yes | Left laterocollis (mild), right torticollis (mild), right hand | Head tremor | memantine |
| 3 | 26/M | Pliocytic astrocytoma status postradioablation, hemorrhage | Horizontal and vertical pendular nystagmus in straight‐ahead and eccentric gaze | No | Low face dystonia | Upper extremity ataxia | Leviteracetam (for history of seizure) |
| 4 | 42/F | Infarction of brainstem, left cerebellar hemisphere, superior vermis, left superior cerebellar peduncle, thalamus, and left occipital cortex owing to vertebral artery dissection | Irregular, coarse, disconjugate oscillations of both eyes in all three axes, more prominent nystagmus in vertical axis | Yes | Torticollis (mild), right‐hand dystonia (mild) | Both appendicular ataxia, mild head tremor, perioral twiches | memantine, gabapentin, clonazepam |
| 5 | 56/M | Pontine stroke | Prominent horizontal and mild torsional nystagmus in primary gaze; no change in eccentric gaze, enucleation of the left eye | Yes | Moderate‐to‐severe torticollis, mild retrocollis, mild shoulder elevation | Upper appendicular ataxia | BoTN |
| 6 | 48/M | Pontine hemorrhagic stroke after AVM rupture and fall | Horizontal, vertical, and torsional coars, irregular, disconjugate nystagmus | Yes | Moderate‐to‐severe torticollis, mild laterocollis, moderate bilateral hand dystonia, bilateral foot dystonia | memantine |
AVM, arteriovenous malformation.
Dystonia occurred in all 6 patients (Table 1). Dystonia had a variable distribution and wide range of severity (Table 1). Two patients had focal dystonia, 3 had multifocal dystonia, and 1 had generalized dystonia. The face was involved in 2 patients and 1 had blepharospasm. Speech was dysarthric in 2 patients, but it was difficult to characterize because of the apparent combination of ataxic and dystonic dyarthria. None of our patients had trunk dystonia. Appendicular and cervical dystonia were most common; both types were present in 4 patients. Upper extremities were involved in all 4 patients, whereas the lower extremity was also involved in 3. All 4 patients with cervical dystonia had torticollis, 3 also had laterocollis, and 2 had retrocollis. Three patients with cervical dystonia also had head tremor. Frequency of horizontal head oscillations was 4.0 ± 2.3 Hz, whereas vertical oscillations were 3.6 ± 2.4 Hz. Differences in frequency of eye and head oscillations supported for lack of temporal correlation between eye and head oscillations. These results suggested that head oscillations were not compensatory or causative for the eye oscillations. The results also suggested that eye oscillations were not compensatory physiological eye movements, such as vestibulo‐ocular reflex evoked by the head oscillations, but they were part of the phonemoenology of ocular palatal tremor plus dystonia. The BFMRS in our patients ranged from 6 to 90 (Table 2).
Table 2.
Total and subcategory values of the BFMRS from 6 patients with OPTD
| Patient No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Eye | 0 | 0 | 4.5 | 0 | 0 | 0 |
| Mouth | 0 | 0 | 1.5 | 0.5 | 0 | 2 |
| Speech/swallowing | 2 | 0 | 0 | 1 | 0 | 16 |
| Neck | 0 | 6 | 0 | 4 | 8 | 8 |
| Right arm | 12 | 0 | 0 | 6 | 0 | 16 |
| Left arm | 12 | 1 | 0 | 6 | 0 | 16 |
| Trunk | 0 | 0 | 0 | 0 | 0 | 0 |
| Right leg | 9 | 0 | 0 | 9 | 0 | 16 |
| Left leg | 9 | 0 | 0 | 9 | 0 | 16 |
| Total | 44 | 7 | 6 | 35.5 | 8 | 90 |
The burden of dystonia on activities of daily living was also variable when compared to other manifestations of OPTD, such as vertigo, diplopia, oscillopsia, and ataxia. The 3 patients, who were most concerned about dystonia, were the least cognizant of the eye movement deficits. In these cases, the primary reason for their clinic visit was dystonia; ocular and palatal tremor were incidental findings discovered during neurological examination. One patient was primarily affected by diplopia and vertigo. Although moderate, dystonia was least concerning to her. The subjective disability resulting from dystonia and diplopia appeared to be equal in 1 patient. One patient was confined to bed and was ventilator dependent owing to brainstem hemorrhage. Oscillopsia and generalized dystonia contributed significant burden to his overall morbidity. The lesions of the Guillian‐Mollaret triangle had acute onset in 3 patients, making it possible to estimate the latency of onset of dystonia in these patients. In 1 patient, dystonia started within 2 months after the lesion, in another dystonia occurred after 6 months, and the third had a latency of several years. Onset of dystonia in the first 2 patients paralleled that of eye oscillations.
Two of the three patients who primarily presented for the symptoms related to their dystonia were treated with botulinum toxin (BoTN) injections. Two of three patients who were most concerned for oscillopsia were treated with memantine, whereas 1 was treated with a combination of memantine, gabapentin, and clonazepam. These treatments had modest benefit for the oscillopsia.
Discussion
We describe a novel OPT variant that includes dystonia (OPTD) in 6 patients. All patients had typical lesions causing a breach in the Guillian‐Mollaret triangle and pseudohypertrphy of the inferior olive. The head oscillations were not compensatory or adaptive responses to suppress eye nystatgmus as observed in spasmus nutans or infantile nystagmus syndrome.10, 11, 12, 13 The eye oscillations were not physiological phenomenon, the vestibuloocular reflex, in response to head oscillations.14, 15, 16 None of the patients had structural lesions affecting basal ganglia on MRI scans. It is therefore possible that dystonia in OPTD patients is merely a coincidence. The alternate suggestion is that dystonia in OPTD could be related to alterations in cerebellar output.17, 18, 19, 20, 21 The controversial role of the cerebellum in the etiology of dystonia is a significant caveat to the cerebellar theory for dystonia in OPTD.22, 23 We will first outline the pathophysiology of OPT and then discuss the speculated mechanism for dystonia as a variant of OPT.
Elegant experiments have demonstrated that disruption of the Guillian‐Mollaret triangle leads to pseudohypertrophy and abnormal increase in somasomatic gap junctions in the inferior olive.2, 3, 4, 5, 6 Electrotronic coupling through these gap junctions leads to increased synchronous output from the inferior olive. Typical signs of OPT, such as eye and palate oscillations, develop as the inferior olive becomes enlarged.5, 24 A dual‐mechanism theory for OPT suggested that synchronized inferior olive oscillations are altered by maladaptive discharges from the cerebellum.7 The action potentials from the inferior olivary neurons travel into climbing fibers and give rise to complex spikes in Purkinje cells. The collaterals of climbing fibers also projects to the deep cerebellar nuclei and then also to the Purkinje neurons, this time by the granular cell layer. Hence, the synchronized oscillation signal from the inferior olive is received twice by the Purkinje neurons, once through the climbing fibers and then after a small delay through the parallel fiber and granular cell layer. Such redundant, randomized timed delayed signals to the Purkinje neurons cause maladaptive learning.7 This phenomenon induces an independent cerebellar signal that distorts the output of inferior olive as it passes through the cerebellum.7 We speculate that distorted cerebellar output in patients with OPT might cause dystonia, hence the variant OPTD.17, 18, 19, 20, 21, 23
The syndrome of OPT was reported in the form of several studies and case reports in the past. To our knowledge, this is the first description of dystonia in OPT patients. Two of the previous large studies on OPT by one of the authors for this study (A.G.S.) included several cases of OPT patients—most from two of the nation's leading eye movement clinics and laboratories.7, 25 Only 1 of these 21 patients had dystonia. Therefore, the reason for lack of report of dystonia in the previous literature with OPT could be an extremely rare incidence of OPTD. It is also possible that dystonia had a delayed onset and was considered a “separate” entity in the previous literature. The 4 of 6 patients were observed in Emory Movement Disorders Clinic for dystonia; hence, there was a clear selection bias. Nevertheless, the aim of this study was not to delineate the epidemiology of OPTD, but to describe it as a new syndromic entity co‐occurring with OPT.
Severity of dystonia was mild in some patients with OPTD. Such a mild form of dystonia can be expected in an OPT variant called progressive ataxia and palatal tremor.8 We did not consider the diagnosis of progressive ataxia and palatal tremor in any of our patients because all of them had a primary (acquired) lesion causing a breach in the Guillian‐Molaret triangle, hence explaining the basis for the conventional form of OPT. As a consequence, further investigations for the etiologies of progressive ataxia and palatal tremor were not justified.
In conclusion, we describe a novel variant of OPT that includes dystonia (OPTD). We speculate that the dystonia in OPTD is more than just an epiphenomenon. It can be explained through the contemporary concept of abnormal cerebellar output as a cause of OPT and dystonia. Future experiments and animal models are needed to support our hypothetical scheme describing the pathophysiology of OPTD. Future studies will also delineate the epidemiology and incidence of OPTD.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
A.G.S.: 1A, 1B, 1C, 2A, 2B, 3A, 3B
F.F.G.: 1B, 1C, 2A, 2B, 3B
M.R.L.: 1B, 1C, 2A, 2B, 3B
H.A.J.: 1B, 1C, 2A, 2B, 3B
A.F.: 1B, 1C, 2A, 2B, 3B
S.A.F.: 1B, 1C, 2A, 2B, 3B
Disclosures
Funding Sources and Conflicts of Interest: This work was supported by a Dystonia Medical Research Foundation Clinical Fellowship grant (to A.G.S.), a Knights Templar Eye Foundation grant (to F.F.G.), a Research to Prevent Blindness grant (to F.F.G.), a Blind Children's Center grant (to F.F.G.), the Dystonia Coalition NIH NS065701 (to H.A.J.), and the Sartain Lanier Family Foundation (to S.A.F.). The authors thank Dr. John Leigh for collegial support and help with technical equipment. Dr. Leigh was supported by NIH EY06717.
Financial Disclosures for previous 12 months: The authors declare that there are no disclosures to report.
Supporting information
A video accompanying this article is available in the supporting information here.
Video 1. The video clip depicts an example of OPTD in 1 patient who had a surgical lesion of the Guillian‐Molaret triangle. The video depicts torsional nystagmus, palatal tremor, and prominent foot dystonia.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1. Guillain G, Mollaret P. Both syncronous and myoclonus rhythms of Larynx‐pharynx‐eye‐diaphragm. Rev Neurol 1931;2:545–566. [Google Scholar]
- 2. Koeppen AH, Barron KD, Dentinger MP. Olivary hypertrophy: histochemical demonstration of hydrolytic enzymes. Neurology 1980;30:471–480. [DOI] [PubMed] [Google Scholar]
- 3. Sperling MR, Herrmann C Jr. Syndrome of palatal myoclonus and progressive ataxia: two cases with magnetic resonance imaging. Neurology 1985;35:1212–1214. [DOI] [PubMed] [Google Scholar]
- 4. Birbamer G, Gerstenbrand F, Aichner F, et al. MR‐imaging of post‐traumatic olivary hypertrophy. Funct Neurol 1994;9:183–187. [PubMed] [Google Scholar]
- 5. Deuschl G, Toro C, Valls‐Sole J, Zeffiro T, Zee DS, Hallett M. Symptomatic and essential palatal tremor. 1. Clinical, physiological and MRI analysis. Brain 1994;4:775–788. [DOI] [PubMed] [Google Scholar]
- 6. Goyal M, Versnick E, Tuite P, et al. Hypertrophic olivary degeneration: metaanalysis of the temporal evolution of MR findings. AJNR Am J Neuroradiol 2000;21:1073–1077. [PMC free article] [PubMed] [Google Scholar]
- 7. Shaikh AG, Hong S, Liao K, et al. Oculopalatal tremor explained by a model of inferior olivary hypertrophy and cerebellar plasticity. Brain 2010;133:923–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Samuel M, Torun N, Tuite PJ, Sharpe JA, Lang AE. Progressive ataxia and palatal tremor (PAPT): clinical and MRI assessment with review of palatal tremors. Brain 2004;127:1252–1268. [DOI] [PubMed] [Google Scholar]
- 9. Burke RE, Fahn S, Marsden CD, Bressman SB, Moskowitz C, Friedman J. Validity and reliability of a rating scale for the primary torsion dystonias. Neurology 1985;35:73–77. [DOI] [PubMed] [Google Scholar]
- 10. Carl JR, Optican LM, Chu FC, Zee DS. Head shaking and vestibulo‐ocular reflex in congenital nystagmus. Invest Ophthalmol Vis Sci 1985;26:1043–1050. [PubMed] [Google Scholar]
- 11. Gresty M, Halmagyi GM, Leech J. The relationship between head and eye movement in congenital nystagmus with head shaking: objective recordings of a single case. Br J Ophthalmol 1978;62:533–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gresty MA, Barratt HJ, Page NG, Ell JJ. Assessment of vestibulo‐ocular reflexes in congenital nystagmus. Ann Neurol 1985;17:129–136. [DOI] [PubMed] [Google Scholar]
- 13. Gresty MA, Ell JJ. Spasmus nutans or congenital nystagmus? Classification according to objective criteria. Br J Ophthalmol 1981;65:510–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kaski D, Saifee TA, Buckwell D, Bronstein AM. Ocular tremor in Parkinson's disease is due to head oscillation. Mov Disord 2013;28:534–537. [DOI] [PubMed] [Google Scholar]
- 15. Leigh RJ, Martinez‐Conde S. Tremor of the eyes, or of the head, in Parkinson's disease? Mov Disord 2013;28:691–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shaikh AG, Reich S, Zee DS. Pseudonystagmus – clinical features and quantitative characteristics. Nat Rev Neurol 2010;6:519–523. [DOI] [PubMed] [Google Scholar]
- 17. Neychev VK, Gross RE, Lehericy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis 2011;42:185–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pizoli CE, Jinnah HA, Billingsley ML, Hess EJ. Abnormal cerebellar signaling induces dystonia in mice. J Neurosci 2002;22:7825–7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prudente CN, Pardo CA, Xiao J, et al. Neuropathology of cervical dystonia. Exp Neurol 2013;241:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Raike RS, Pizoli CE, Weisz C, van den Maagdenberg AM, Jinnah HA, Hess EJ. Limited regional cerebellar dysfunction induces focal dystonia in mice. Neurobiol Dis 2012;49C:200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zoons E, Tijssen MA. Pathologic changes in the brain in cervical dystonia pre‐ and post‐mortem – a commentary with a special focus on the cerebellum. Exp Neurol 2013;247:130–133. [DOI] [PubMed] [Google Scholar]
- 22. Sadnicka A, Hoffland BS, Bhatia KP, van de Warrenburg BP, Edwards MJ. The cerebellum in dystonia – help or hindrance? Clin Neurophysiol 2012;123:65–70. [DOI] [PubMed] [Google Scholar]
- 23. Lehericy S, Tijssen MA, Vidailhet M, Kaji R, Meunier S. The anatomical basis of dystonia: current view using neuroimaging. Mov Disord 2013;28:944–957. [DOI] [PubMed] [Google Scholar]
- 24. Kim JS, Moon SY, Choi KD, Kim JH, Sharpe JA. Patterns of ocular oscillation in oculopalatal tremor: imaging correlations. Neurology 2007;68:1128–1135. [DOI] [PubMed] [Google Scholar]
- 25. Shaikh AG, Thurtell MJ, Optican LM, Leigh RJ. Pharmacological tests of hypotheses for acquired pendular nystagmus. Ann N Y Acad Sci 2011;1233:320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A video accompanying this article is available in the supporting information here.
Video 1. The video clip depicts an example of OPTD in 1 patient who had a surgical lesion of the Guillian‐Molaret triangle. The video depicts torsional nystagmus, palatal tremor, and prominent foot dystonia.


