Abstract
This paper describes the synthesis and properties of a new type of magnetic nanoparticle (MNP) for use in the hyperthermia treatment of tumors. These particles consist of 2–4 nm crystals of gamma-Fe2O3 gathered in 20–40 nm aggregates with a coating of carboxymethyl-dextran, producing a zetasize of 110–120 nm. Despite their very low saturation magnetization (1.5–6.5 emu/g), the specific absorption rate (SAR) of the nanoparticles is 22–200 W/g at applied alternating magnetic field (AMF) with strengths of 100–500 Oe at a frequency of 160 kHz.
Keywords: Magnetic nanoparticles, hyperthermia treatment, specific absorption rate
1. Introduction
Magnetic hyperthermia, which is sometimes called thermotherapy, operates on the principle that magnetic nanoparticles (MNPs) produce heat when subjected to an alternating magnetic field (AMF) of suitable frequency and amplitude. This effect may cause, for example, the temperature inside a tumor to rise to therapeutic levels if the nanoparticles are injected into a tumor. MNP injected directly into a tumor and heated with an AMF have been shown to destroy cancer cells.1 Such magnetic hyperthermia treatments can also enhance the effects of subsequent radiation therapy or chemotherapy.2 With nanoparticles localized in the tumor, magnetic hyperthermia can provide treatment of the tumor while producing minimal damage in the surrounding healthy tissue. A key issue with MNPs is that they must have a high specific absorption rate (SAR) so that not only is the dose of nanoparticles required for hyperthermia treatment minimized, but also so that lower values of the product of magnetic field strength and frequency are used. Having a large SAR is also a key parameter in decreasing the size of tumor which can be treated.3
The heating effect of these MNPs is associated with number of phenomena, including the well-known magnetic hysteresis. This is demonstrated in the form of a hysteresis loop that results from placing the nanoparticles in a magnetic field which reverses direction over time. The area of the loop represents thermal energy that may be absorbed from the field and dissipated into the environment. The SAR due to heating in a magnetic field can be defined as:
where C is the specific heat capacity of the media, and ΔT is the temperature change, over a time interval Δt.
The Stoner–Wohlfarth model is sometimes used to approximate the effects of magnetization reversal in single domain particles.4 The size of nanoparticles influences the number of magnetic domains. Where the larger particles have multiple domains, the model frequently used is that of a Rayleigh loop.3 The most widely used nanoparticles for hyperthermia applications are composed of iron oxide.5–8 These must be biocompatible and stable against further oxidation. Iron and cobalt particles may have higher SAR values, but problems may exist with respect to toxicity and stability.9,10 The relatively lower SAR values of currently available iron oxide nanoparticles at l require their use in larger quantities. This is problematic in the sense that cells have a limited uptake capacity. The use of magnetic fields with higher amplitude is generally undesirable or practically unattainable due to eddy current heating.
Widely known and used methods of synthesizing MNPs are based on: (a) mechanical dispersion11; (b) precipitation of iron oxides,12 (c) thermal decomposition,13 (d) microemulsion14 and (e) flame spray synthesis.15 The resulting nanoparticles are typically decorated further with stabilizers or other types of functional molecules. In the present work, we have developed MNPs with a high SAR that are stable in biological fluids and can be used for hyperthermia in a high-frequency AMF 160 kHz but at relatively low field strengths of 100–300 Oe.
2. Experimental Methods
2.1. Materials
Commercially available ferric chloride (FeCl3 · 6H2O), ferrous sulfate (FeSO4 · 7H2O), 25 wt.% ammonium hydroxide solution, NaNO3, NaOH and Europium (III) chloride hexahydrate were purchased from VWR. Carboxymethyl-dextran (CM-dextran) 40 kDa was purchased from TdB Consultancy AB. All reactants were used as received without further purification.
For comparison of the heating properties, BNF–starch MNPs were obtained from Micromond Partikeltechnologie GmbH.16
2.2. Synthesis of nanoparticles
MNPs with organic chain material embedded in their structure were obtained using the following steps. A solution containing 10 wt.% iron salts with a Fe(II):Fe(III) molar ratio of 10:1 was added with vigorous stirring to a 15 wt.% CM-dextran solution in DI water held at 40°C. The resulting solution was added to an 8.5% ammonia solution (a pH > 10 was maintained) in order to precipitate iron oxides and hydroxides. The resulting mixture was transferred to a three-neck flask in a sand bath while continuing the mechanical stirring. The temperature of the mixture was then increased to 70°C, and NaOH was added to maintain an alkaline solution, while NaNO3 was introduced (molar ratio of Fe(II): NaNO3 = 5: 1) to promote oxidation of the Fe(II) to Fe(III). The temperature was further increased to 100°C at a rate of 10°C/h, and the mixture was centrifuged at 5000 rpm for 15 min to remove any large aggregates.
The resulting nanoparticles were purified by sedimenting them using a centrifuge operated at 20,000 rpm for 45 min and re-suspending them in water by using an ultrasonic bath for 15–30 min. This procedure was repeated five times. The nanoparticles were subsequently sterilized by adding 0.1 M NaOH to them for 60 min, followed by washing with an endotoxin-free sterile phosphate buffer (1X) and endotoxin-free sterile DI water using a Spectrumlab™ system. The nano-particle containing solution was concentrated to the desired level either simultaneously with the sterilization step or afterward by evaporation at room temperature.
Doping the resulting iron-based nanoparticles with a small amount of a rare metal (such as 1% of Eu) can significantly increase the accuracy of the nanoparticle tracking compared to the commonly used Fe ion analysis. Thus, in some cases, Europium in the form of a water soluble salt was added along with the iron salts to produce 1 wt.% Eu in the resulting nanoparticles.
2.3. Nanoparticle characterization
Transmission electron micrographs of the nano-particles were taken using a FEI Technai F20ST field emission gun transmission electron microscope (TEM) operated at 200 kV. 500 particles from three different locations on a grid were used to produce frequency versus particle size histograms.
Iron and Europium elemental analyzes were performed on an Agilent 7500 cx after dissolving the sample in concentrated HCl.
The Zetasize was measured using a Dynamic light scattering Zetasizer (Malvern Instruments).
The quasi-static magnetic properties of the nanoparticles were determined (saturation magnetization, Ms; remanence magnetization, Mr; and coercivity, Hc) from hysteresis loop measurements using a Lakeshore model 7300 vibrating sample magnetometer (VSM).
Heating measurements were performed using a home-built system, consisting of 14-turns hollow copper coil (internal diameter 32 mm, length 12 cm) which was powered by a TIG 10/300 generator and cooled by running water kept at 20°C by a chiller (Tek-Temp Instruments, Croydon, PA). A 0.7 mm diameter fiber optic probe (FISO Inc, Quebec, Canada), accurate to 0.1°C was used for the temperature measurements. A fiber optic temperature probe was positioned in the sample close to the middle of the copper coil to measure the temperature in the sample. Experiments were performed at 100–600 Oe at frequency 160 KHz. The sample was placed in the middle of the coil. The temperature was recorded electronically at one-second intervals throughout the experimental period and monitored using a real-time temperature monitoring system. The SAR was calculated based on the temperature rise recorded.
3. Results and Discussion
TEM examination showed that the Dartmouth nanoparticles consisted of 2–5 nm crystals in 20–40 nm aggregates, while Micromod BNF-starch MNPs have rhomboidal single crystals with length of facet 12 nm (Fig. 1). MNPs size distributions are shown in Fig. 2.
Fig. 1.
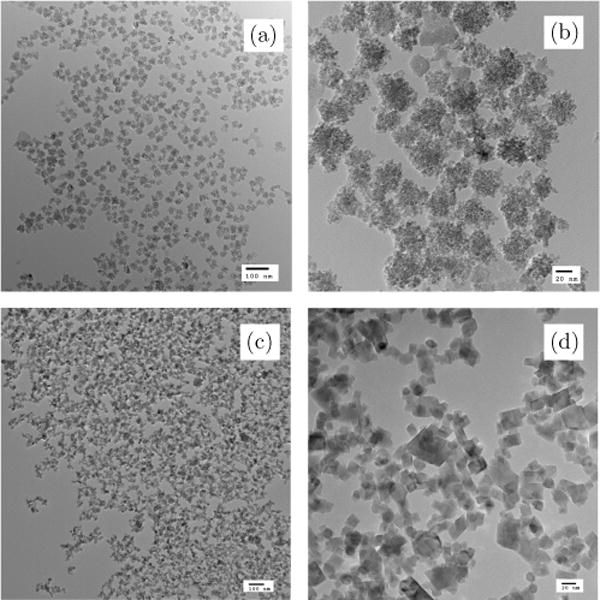
TEM images of: (a) and (b) Dartmouth MNP, (c) and (d) Micromod MNP.
Fig. 2.

MNP size distribution: (a) Dartmouth MNP and (b) Micromod MNP.
The aggregate size was found to be within 7.5% for different batches. Average sizes of aggregates after purification by centrifugation method were 27 nm.
As one can see in Fig. 3, Z-sizes of Micromod and Dartmouth MNPs are the same (110–120 nm). It means that in terms of the MNP–cell interaction particles will behave similar.
Fig. 3.

Z-size distributions: red line — Dartmouth MNP, green line — Micromod BNF starch MNPs.
The resulting material is stable against settling for one year and under a permanent magnetic field, i.e., application of a Ni-B permanent magnet (7 T) for seven days did not produce any increase in agglomeration (Fig. 4). These facts prove high efficiency of biopolymer matrix in stabilization of MNPs.
Fig. 4.

Bright field TEM image for MNP: (a) initial sample, (b) after 7 days treatment under strong (7T) permanent magnetic field.
Elemental analysis showed that the weight percentage of CM-dextran-40 embedded in the particles of MNP@CM-dex-40 was 28 wt.% of solids. Any excess organic chain material was largely removed by purification via centrifuging. Purification via centrifuging does not change either the MNP:organic chain material ratio or the Z-size (Fig. 5).
Fig. 5.

Z-size of nanoparticles after different numbers of centrifugations: I — initial sample, IC — after removing large aggregates using centrifugation (5000 rpm for 15 min), ICx2–8 — after 2–8 times high-speed centrifugation at 20 000 rpm for 45 min and further ultrasound re-suspension.
Other metals can be incorporated into the MNPs either for tracking the nanoparticles; e.g., Eu, or for increasing their SAR, e.g., Co,17 where Co replaces Fe(II) in the ferrite structure. The added Europium is “locked” in the crystalline structure and does not leak even after numerous washings (Fig. 6). Eu in the nanoparticles provides a unique opportunity to track the nanoparticles. There is no natural Eu in either animals or humans and nanoparticle location identification becomes very accurate compared to analysis of the nanoparticles based on the iron content (since the body contains large amounts of iron) nanoparticles. A small amount of Eu in the structure does not have an influence on any of the nanoparticle parameters such as single crystal or aggregates size, Z-size, magnetic properties or SAR.
Fig. 6.

Fraction of Eu compared to the total amount of metal in the nanoparticles as a function of the number of purifications.
The magnetization curves show super-paramagnetic behavior with very low saturation magnetization for both fractions (Fig. 7). The low saturation magnetization is caused by the small size of the single crystals, as expected from the literature.18
Fig. 7.

Magnetization versus magnetic field strength for Dartmouth MNP.
The MNP produces therapeutically significant amount of heat (temperature raise over 45°C) at all tested AFM field strengths (Fig. 8).
Fig. 8.

Temperature versus time for MNP (16.1 wt.% of solids) at different field strengths at a frequency of 160 kHz.
As shown in Fig. 9, the new nanoparticles have a much higher SAR at low magnetic field strengths, compared to commercial nanoparticles. This ability to have a large SAR at low field strength is important since high field strengths can produce damage to tissue through eddy current heating. In other words, having a very high SAR at high field strength (at high frequency) is not practically useful for magnetic hyperthermia treatment.
Fig. 9.

SARs for (1) Dartmouth MNP (green line), (2) JHU (blue line), (3) Micromod BNF measured in our lab (red circles), (4) Micromod BNF measured at JHU (red line). The SAR values for JHU and BNF (red line) are given for comparison.
The resulting material is a stable colloidal solution, which can be dried out to form a powder. The magnetic nanopowder may be re-suspended in water or other liquid carrier to obtain the desired concentration. The concentration of colloid solutions may be up to 50% w/w.
4. Conclusion
A new method of production of magnetic nano-composites with high SAR values has been developed. Particle synthesis includes precipitation of iron oxides and hydroxides in the presence of organic chain materials (e.g., carbohydrates or their derivatives) followed by hydrothermal treatment. The ratio of Fe(II):Fe(III) may vary, but usually is greater than 1:2. These MNP consist of small (2–4 nm) magnetic crystals in 20–40 nm aggregates. They produce therapeutically significant amounts of heat at AMFs of as low as 50–300 Oe at a frequency of 160 kHz.
Acknowledgments
This work was supported in part by the Dartmouth Center for Cancer Nanotechnology Excellence funded by NCI grant U54CA151662-01. The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing official policies, either expressed or implied of the NCI or the U.S. Government.
References
- 1.Roizin-Towle L, Pirro JP. Int J Radiat Oncol Biol Phys. 1991;20:751. doi: 10.1016/0360-3016(91)90018-y. [DOI] [PubMed] [Google Scholar]
- 2.Sardari D, Verga N. Cancer treatment with hyperthermia. In: Özdemir Ö, editor. Current Cancer Treatment – Novel Beyond Conventional Approaches. 2011. Chapter 21, InTech. [Google Scholar]
- 3.Hergt R, Dutz S, Muller R, Zeisberger M. J Phys: Condens Matter. 2006;18:S2919. [Google Scholar]
- 4.Stoner EC, Wohlfarth EP. Philos Trans R Soc A Phys Math Eng Sci. 1948;240:599. [Google Scholar]
- 5.Thiesen B, Jordan A. Int J Hyperthermia. 2008;24:467. doi: 10.1080/02656730802104757. [DOI] [PubMed] [Google Scholar]
- 6.Giustini AJ, et al. NanoLife. 2010;12:17. [Google Scholar]
- 7.Huang HS, Hainfeld JF. Int J Nanomed. 2013;8:2521. doi: 10.2147/IJN.S43770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johannsen M, Thiesen B, Wust P, Jordan A. Int J Hyperthermia. 2010;26:790. doi: 10.3109/02656731003745740. [DOI] [PubMed] [Google Scholar]
- 9.U.S. Department of Health and Human Services Public Health Service Agency for Toxic Substances and Disease Registry. Toxicological Profile for Cobalt. 2004 [Google Scholar]
- 10.Kekalo K, Koo K, Zeitchick E, Baker I. Microemulsion synthesis of core/shell Fe/Fe oxide nanoparticles: properties, tolerance to saline solution and APTMES coating. MRS Proc. 2012;1416 doi: 10.1557/opl.2012.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papell SS. Low viscosity magnetic fluid obtained by the colloidal suspension of magnetite particles. 3215572 USA, 1965. H01F/1/10/ US19630315096. Patent. 1965
- 12.Rosenazweig RI. Ferrofluid composition and process of making same. 3917538 USA, Applicant Ferrofluidics Corp. US19730324414 17. Patent. 1975
- 13.Bonnemann H, Brand RA, Brijoux W, Hofstadt HW, Frerichs M, Kempter V, Maus-Friedrichs W, Matoussevitch N, Nagabhushana KS, Voigts F, Caps V. Appl Organometal Chem. 2005;19:790. [Google Scholar]
- 14.Zhang G, Liao Y, Baker I. Mater Sci Eng C. 2010;30:92. doi: 10.1016/j.msec.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buyukhatipoglu K, Clyne AM. J Nanopart Res. 2012;12:1495. [Google Scholar]
- 16.Ivkov R, Gruettner C, Teller J, Westphal F. Patent WO 2005/013897 A2. Applicant: Triton Biosystems Inc; 2006
- 17.Kekalo KA, Kashevsky BE, Agabekov VE, Kashevsky SB, Prokhorov IV, Zhavnerko GK. J Magn Magn Mater. 2009;321:1514. [Google Scholar]
- 18.Lu HM, Zheng WT, Jiang Q. J Phys D: Appl Phys. 2007;40:320. [Google Scholar]


