Abstract
To assess strategies for mitigating Pb and As transfer into leafy vegetables from contaminated garden soils, we conducted greenhouse experiments using two field-contaminated soils amended with materials expected to reduce metal phytoavailability. Lettuce and mustard greens grown on these soils were analysed by ICP-MS, showing that some Pb and As transfer into the vegetables occurred from both soils tested, but plant Pb concentrations were highly variable among treatment replicates. Soil-to-plant transfer was more efficient for As than for Pb. Contamination of the leaves by soil particles probably accounted for most of the vegetable Pb, since plant Pb concentrations were correlated to plant tissue concentrations of the immobile soil elements Al and Fe. This correlation was not observed for vegetable As concentrations, evidence that most of the soil-to-plant transfer for this toxic metal occurred by root uptake and translocation into the above-ground tissues. A follow-up greenhouse experiment with lettuce on one of the two contaminated soils revealed a lower and less variable foliar Pb concentration than observed in the first experiment, with evidence of less soil particle contamination of the crop. This reduced transfer of Pb to the crop appeared to be a physical effect attributable to the greater biomass causing reduced overall exposure of the above-ground tissues to the soil surface.
Attempts to reduce soil Pb and As solubility and plant uptake by amendment at practical rates with stabilizing materials including composts, peat, Ca phosphate, gypsum and Fe oxide, were generally unsuccessful. Only Fe oxide reduced soluble As in the soil, but this effect did not persist. Phosphate amendment rapidly increased soil As solubility but had no measurable effect on either soil Pb solubility or concentrations of Pb or As in the leafy vegetables. The ineffectiveness of these amendments in reducing Pb transfer into leafy vegetables is attributed in this study to the low initial Pb solubility of the studied soils and the fact that the primary mechanism of Pb transfer is physical contamination.
Keywords: lead, arsenic, leafy vegetables, soil remediation, metal stabilization, heavy metal uptake
Introduction
Lead and arsenic contamination of soils derive from past use of lead arsenate pesticides, leaded house paint, leaded gasoline and some types of pressure-treated wood. Because of the immobility of Pb in soils, historical Pb contamination in urban, industrial, and high-traffic areas persists today despite the phase out of leaded gasoline and paints beginning in the 1970's. Arsenic is more mobile than Pb in soils, but still persists for many decades in orchards where arsenate-based pesticides were applied (Schooley et al. 2008; Veneman et al. 1983). Consequently, Pb and As contamination of garden soils have been a concern because of potential transfer of these toxic metals into vegetable crops. Numerous published studies have reported Pb and As concentrations in edible crops grown on historically contaminated sites such as apple orchards (Kenyon et al. 1979; MacLean and Langille 1981). However, these older results may be biased by several factors that render them unreliable for present-day evaluation of potential risk. Firstly, older reported high Pb concentrations determined in vegetable crops grown in urban areas (Hibben et al. 1984; Preer et al. 1980a, 1980b) might have been strongly influenced by high aerial deposition of Pb in that era (Chamberlain 1983; Nali et al. 2009; Prasad and Nazareth 2000; Thornton and Jones 1984; Tjell et al. 1979). This hypothesis is supported by later studies showing vegetables grown in contaminated soils to be less severely contaminated at a time when the sources of aerial Pb deposition had been reduced by the phase-out of Pb in gasoline and other products (Hough et al. 2004; Jorhem et al. 2000). An additional limitation with the earlier studies is that analytical methods were typically insufficiently sensitive to correctly measure the low background levels of Pb and As in uncontaminated crops. This detection-limit problem with both flame atomic absorption (FAA) and ICP-optical emission spectrometry (ICP-OES) was recognized in the late 1970's (Capar et al 1978) and later discussed for the specific cases of Pb, Cd, and Hg measurement in samples (McBride 1998).
In summary, many older studies of Pb transfer from soils to crops have been compromised by poor analytical detection limits for Pb in plant tissues and a high probability of sample contamination by aerial deposition. Presently, random physical contamination of plants by both soil and airborne particles remains an important mechanism of Pb transfer to vegetables and other crops (Chamberlain, 1983; Finster et al. 2003; Nali et al. 2009; Prasad and Nazareth 2000) because of the fact that only minimal soil particle contamination of vegetables by high-Pb soil particles could greatly increase concentrations of Pb in the edible portion of the crop. The actual uptake of Pb into plant tops via roots (“physiological uptake”) is very low in the case of soils with near-neutral pH and high organic matter content. Therefore, it should not be surprising that it is difficult to predict vegetable Pb concentrations from simple soil properties such as total Pb or pH (Hough et al. 2004; Jorhem et al. 2000) when the usual dominant process of vegetable contamination is random and unpredictable. To assess the extent of soil particle contamination of vegetables, soil “indicator metals” such as titanium (Ti) or aluminum (Al) can be measured in the plant tissues (Cherney and Robinson 1983; Nali et al. 2009) . These indicator metals have the necessary properties of low solubility and minimal “physiological uptake” from non-acid soils into plants, and relatively high concentrations in soils.
In order to reduce the solubility, leachability, and bioavailability of Pb, As and other toxic metal in contaminated soils, various soil amendments, such as iron salts or iron oxides (Lee et al. 2009; Xenidis et al. 2010), peats and composts (Hashimoto et al. 2011; Kumpiene et al. 2007; Nwachukwu and Pulford 2009), gypsum (Hashimoto et al. 2011) and phosphate (Chrysochoou et al. 2007; Melamed et al. 2003; Sneddon et al. 2008), have been tested. However, there are questions about the effectiveness of these amendments. For example, the addition of gypsum or phosphate seems to mobilize As even as it stabilizes Pb in the soil. In addition, for urban soils with near-neutral or slightly alkaline pH, phosphate amendments may be ineffective in achieving the desired conversion of soil Pb into less bioavailable and more stable mineral forms such as pyromorphite (Chrysochoou et al. 2007).
In this study, we report a Pb and As uptake experiment for leafy vegetables grown on urban contaminated and orchard-contaminated soils in the greenhouse. We assess the extent to which metal transfer to the vegetables is due to physical contamination as opposed to root uptake. Additionally, we evaluate the effectiveness of soil amendments in altering solubility and chemical stability of Pb and As in soil and in reducing soil-to-plant metal transfer.
Methods
Collection of Contaminated Soils
Two surface soils (0-15 cm) with similarly high levels of lead contamination were collected from the site of an old apple orchard (G6) near the Cornell University campus and from a city garden in Ithaca, NY (Albany St.). These soils were chosen to represent two common types of Pb contamination, derived from lead arsenate in the orchard, and from leaded house paint and gasoline in the urban garden. The soils were well mixed and placed in large plastic containers for storage. Duplicate subsamples were taken from each container for the determination of total metals by microwave digestion and measurement using ICP-OES (EPA Method 3051-6010). Soil pH in water (1:1 by weight) and total organic matter by the Walkley-Black method were also determined. Relevant soil properties are given in Table 1.
Table 1.
Important properties of the contaminated soils used in Greenhouse experiment 1 study
| Soil | pH | Organic Matter (%) | Total Pb | Total As |
|---|---|---|---|---|
| Urban (Albany St) | 6.83 | 11.0 | 1135 ± 177 | 8.0 ± 0.8 |
| Orchard (G6) | 6.01 | 8.4 | 1287 ± 202 | 222 ± 33 |
Soil Amendments
Prior to putting the soils into pots for the greenhouse assay with leafy vegetables, a portion of each soil was either left unamended (control) or was amended with one of four different materials. The amendments were natural peat collected from a farm near Batavia, NY (69.4% organic matter based on weight loss on ignition at 430°C), soluble Ca phosphate (reagent-grade CaHPO4), iron oxide (fine-powdered reagent-grade Fe2O3 , identified by XRD as crystalline hematite with approximate particle size of 0.1 μm ), and gypsum (reagent-grade powdered CaSO4), added at 10% (for peat only) and 1% (for all other amendments) of the soil's moist weight. Since the water contents of the orchard and urban garden soils as collected were 33% and 34%, respectively, the amendments represented a 13% (for peat) and 1.3% addition (for the other amendments) to both soils’ dry weight. The choice of addition level was based on practical considerations (feasibility of soil amendment in terms of cost and ease of application) balanced against the need for sufficient material to potentially react with all or most of the Pb and As in the soil. For example, the phosphate addition of 1.3% supplied a 50 to 100-fold mole excess of phosphate over soil Pb, providing the large mole excess needed to drive the Pb phosphate precipitation reaction (Chrysochoou et al. 2007). Higher amendment rates than used here could risk undesirable secondary effects such as environmental contamination, large soil pH changes and induced micronutrient deficiencies (Boisson et al. 1999).
Greenhouse Experiment 1
The greenhouse trials were run using 4 replicate pots, with each pot containing one kg of original soil (orchard or urban soil) with the proper weight of amendment thoroughly mixed in. The pots were placed on a table in the greenhouse with supplemental lighting, and lettuce (lactuca sativa c.v. Black-seeded Simpson) and mustard seeds (brassica juncea) were planted in each pot to represent a “mesclun mix” of salad greens that is popular with gardeners. Mustard is a notable hyperaccumulator of heavy metals, although its ability to accumulate Pb is limited by the low solubility of Pb in soils (Epstein et al.,1999). Five seeds of both mustard and lettuce were planted into each pot and watered every other day. The plant tops (cut 1-2 cm above soil level) were harvested after only four and a half weeks growth to be consistent with gardeners’ preference for harvest at a tender stage. The harvested leaf and stem tissues were weighed to obtain a fresh-weight yield, then carefully washed under tap water to conform with practices followed by gardeners. The tissues were then placed in paper bags, dried in the oven at 62° C for 24 hours, and weighed again to determine dry weight. Finally, the plant tissues were ground in a mill with stainless steel blades and put in small labeled plastic bags. These samples were submitted to a certified laboratory (H2M Labs, Inc., Melville, NY) to be analysed for total Pb and As using ICP-MS. Subsamples of the same tissues were further analysed by ICP-OES for other elements of interest, particularly Fe, Al and S. Al and Fe are indicators of soil particle contamination of plant tissue (Cherney and Robinson 1983) in situations where soils are not strongly acid and plant uptake of these two metals via roots is relatively low. Tissue S is an indicator of the relative weight contribution of mustard and lettuce to the leafy green samples, as the S concentration in mustard leaves is much higher than that in lettuce.
All concentrations of metals in washed plant tissues are reported in units of mg/kg on a dry weight basis, with determined values being referred to as foliar or tissue total concentrations regardless of whether the metals were within plant tissues (physiological) or associated with soil particles physically entrained in the tissue samples and not removed by washing.
Greenhouse Experiment 2
In order to further explore the possible beneficial effects of compost amendments in reducing the bioavailability of Pb in metal-contaminated urban garden soils, a separate greenhouse experiment was carried out using 3 different composts applied at a single rate (10% on a dry weight basis). The composts were obtained from Cornell (largely food waste, biodegradable paper and animal bedding), and two commercial sources, a food-waste based (FW) and cow manure-based (CM) compost. Their elemental compositions were determined by acid digestion (EPA Method 3051) and measurement using ICP-emission. The composts were low in trace metals of concern, with total Pb being 2.7, 57 and 19 mg/kg in the Cornell, FW and CM products, respectively. Two soils were amended with these composts, the high-Pb urban soil (Albany St.) used in Greenhouse Experiment 1 (see Table 1) and a control soil (Arkport sandy loam) having very low total Pb (4.5 mg/kg) and organic matter content and a pH of 6.1. At the 10% addition level, the composts amendments had only minor effects on the relative Pb concentrations in the amended soils, with the largest effect occurring for the control soil with total Pb approximately doubled (from 4.5 to 10 mg/kg) by the FW compost. The Arkport soil served as a negative control to check on any plant contamination associated with cultivation in the greenhouse or plant sample processing, digestion and analysis.
This experiment followed the same basic procedures described in Experiment 1 with the exception that only lettuce (lactuca sativa c.v. Black-seeded Simpson) was planted and the growth period was longer. Seedling thinning was limited so that a closed canopy developed close to harvest, and the above-ground tissues were harvested after approximately 8 weeks, then water-washed, dried and ground in preparation for digestion. The lettuce grown in the Arkport control soils grew poorly at first, attributable to low inherent fertility of this sandy soil. It was necessary to add a small quantity of soluble complete-nutrient fertilizer in order to obtain sufficient yield for tissue analysis.
As in the first experiment, the washed lettuce tissues were analysed for total Pb and Al by ICP-MS (H2M Labs, Inc., Melville, NY).
Soil Chemical Tests
A lab study was conducted using the same soils and amendments at the same application rates as Greenhouse Experiment 1 in order to measure any chemical effects of the amendments on the soils over the 5-week period of the greenhouse trial. Five glass jars were filled with field-moist urban garden soil and five were filled with field-moist orchard (G6) soil (weight equivalent of 200 grams dry soil). The amendment materials were added to each jar at the appropriate rates, and the soils were thoroughly mixed. The jars were sealed with lids to prevent moisture loss. Soil was removed from the jars each week for the various chemical tests. Soil pH of a mixture of 10g soil and 10g distilled water was measured using a pH meter equipped with a glass electrode A Pb extraction test by the Modified Morgan method was also done weekly. This involved weighing 5 grams of each moist soil into Erlenmeyer flasks, adding 25mL of Modified Morgan's solution (1 M ammonium acetate, pH 4.8), and stirring the flasks on a rotary shaker for 15 minutes, after which each solution was filtered through Whatman #42 filter paper and stored in small plastic vials until analyzed for Pb. The solutions were analyzed for dissolved Pb at 217 nm using a Buck Scientific flame atomic absorption (FAA) spectrophotometer. After the amended soils had been aged in the glass jars for 5 months under field-moist conditions, the extraction test was repeated in triplicate on samples of the treated and control orchard (G6) soil in order to determine if the amendments had any long term effect on extractability of the Pb or As in the soil. Both Pb and As in the Modified Morgan extracts were analyzed by FAA at 217 and 193.7 nm, respectively.
Soil Water Sampling
The orchard soil (G6) was tested for water-soluble As and Pb by vacuum extraction of soil solutions 24 hours after being mixed with the various amendments. To do the water extractions, 340 g of each treated and control soil were placed in separate Buchner funnel cups lined with Whatman #42 filter papers, then wetted to field capacity with distilled water. After 24 hours of standing, water was drawn out of the soils by vacuum and collected in vials. There were three replicates for each soil treatment. The collected water samples were filtered again using sterile 0.2-micrometer Millipore filters to remove any fine soil particles. The water samples were placed in small vials, stabilized with a drop of trace-metal grade concentrated nitric acid and refrigerated until they could be analyzed for dissolved As and Pb by ICP-MS. Water was again collected in the same manner from these same soils after 1 week re-equilibration in order to analyze for additional elements by ICP-OES. After the amended soils had been aged in sealed glass jars for an additional 5 months under field-moist conditions, soil water was again collected from triplicate samples of the orchard soil as described above to assess any long-term soil reactions with the amendments that may have changed Pb or As solubility.
Results
Greenhouse Experiment 1
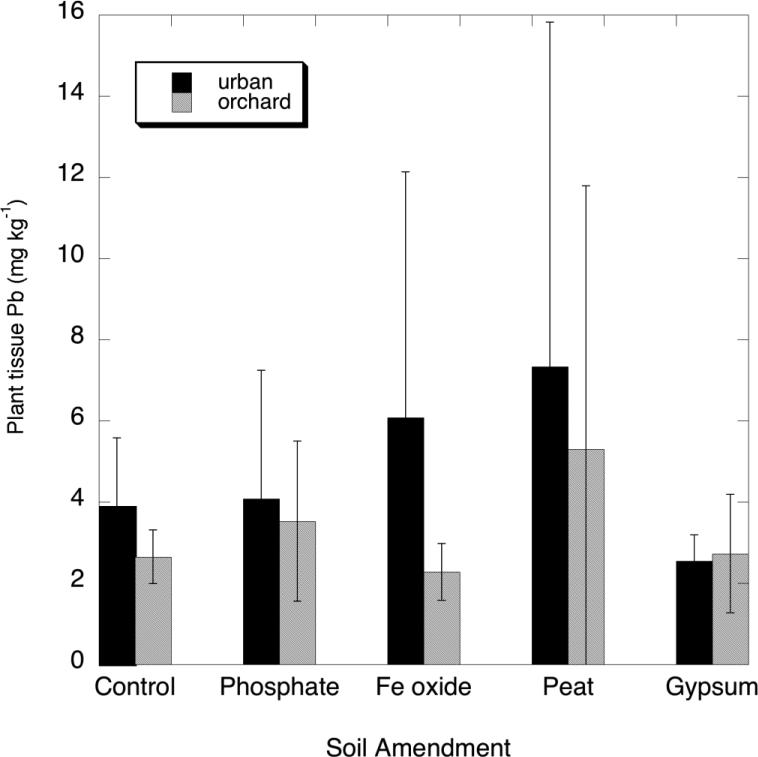
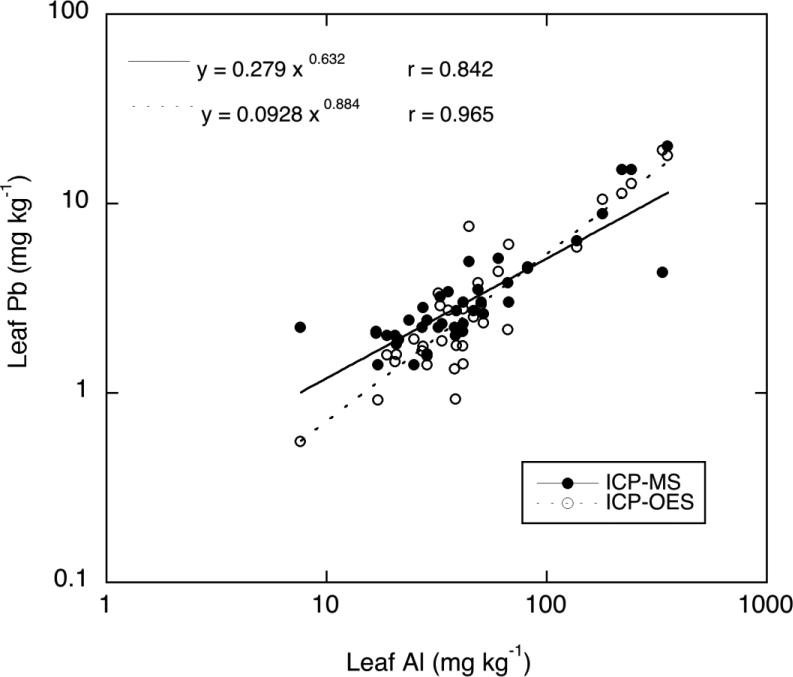
The Pb and As concentrations measured in the harvested mixed greens (“mesclun mix”) by ICP-MS are shown in Figures 1 and 2. The soil amendments had no significant effect on plant Pb or As concentrations resulting from the soil amendments, based on a comparison with the unamended controls. Plant tissue Pb concentrations tended to be relatively high for both soils regardless of amendment, but variability among replicates of individual treaments was quite high. This variability suggests that the elevated plant Pb levels were largely the result of physical contamination of leaves by soil particles rather than uptake via roots. To test this possibility, Al and Fe concentrations measured in all plant tissue samples (grown on both soils for all treatments) were correlated against Pb concentrations measured in the tissues by both ICP-MS and ICP-OES. Because the data were not normally distributed, the values were log-transformed to achieve a more nearly normal distribution, and a linear regression was done between the log values of Pb, Al and Fe. There were highly significant relationships to plant tissue Pb for both Al (r2 = 0.709 and 0.931 for ICP-MS and ICP-OES, respectively) and Fe (r2 = 0.529 and 0.891 for ICP-MS and ICP-OES, respectively). The log-transformed Pb-Al relationship is shown in Figure 3 for both ICP-MS and ICP-OES measurements of tissue Pb. The ICP-MS Pb measurements are often more reliable for low-Pb samples than ICP-OES because of the superior sensitivity (very low detection limit for Pb) of the former method. The ICP-OES measurements are included here only for further overall confirmation of the tissue Pb concentrations, but it is important to note that several plant samples having Pb concentrations below the detection limit of ICP-OES did in fact contain Pb in the 1-2 mg/kg range as determined by ICP-MS. Because of this limitation of ICP-OES, it is advisable that analysis of food crops for toxic metals such as Pb and Cd be done by ICP-MS where possible.
Fig. 1.
Measured concentrations of Pb (mg/kg, dry wt basis) in leafy greens grown on the urban (Albany) and orchard (G6) soils amended with phosphate, Fe oxide, peat and gypsum
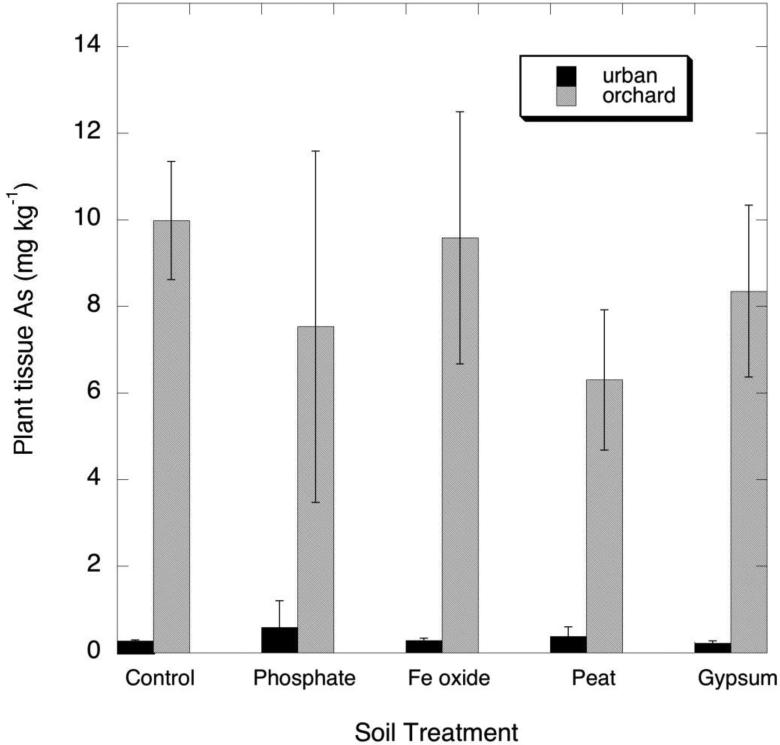
Fig. 2.
Measured concentrations of As (mg/kg, dry wt basis) in leafy greens grown on the urban (Albany) and orchard (G6) soils amended with phosphate, Fe oxide, peat and gypsum
Fig. 3.
Logarithmic relationship between Pb and Al measured in the leafy greens grown on all amended and control soils. Plant Pb was measured by both ICP-MS and ICP-OES.
The strong correlation of vegetable Pb to Al and Fe is an indication that soil particle contamination of the leafy greens accounted for most of the Pb measured in the tissue. Specifically, the high Al (up to 350 mg/kg) in the plant samples is indicative of soil particulate rather than physiological metals (Rosborg et al. 2009), and Pb is a co-contaminant associated with these particles. Al is not taken up to a significant degree by plants except at quite low soil pH, so that physiological Al in leafy vegetables is typically no greater than 10-20 mg/kg (Pillay and Jonnalagadda 2007). Therefore, it can be inferred by extrapolation to low tissue Al in Figure 3 that Pb uptake into tops via roots may account for little more than about 1 mg/kg in the edible tissue. This represents a “true” physiological plant uptake factor (PUF) of 0.001 or less, consistent with numerous other studies that have shown very low Pb bioavailability and PUFs in non-acid soils.
The analyzed mixed greens included a somewhat variable proportion of mustard (brassica juncea), a known metal hyperaccumulator. However, it cannot be discerned from the data presented whether the mustard accounted for a larger proportion of Pb or As “uptake” than the lettuce. Some insight into this was possible using the total S analyses of the plant tissues and the fact that mustard has much higher foliar S concentrations than lettuce. For all the mixed leafy greens analyzed, the total S concentration showed a very wide range, from less than 2,000 mg/kg to nearly 10,000 mg/kg, indicating a highly variable proportion of mustard and lettuce in different samples. However, there was no correlation between tissue Pb and S (r2 = 0.002) or As and S (r2= 0.002) , an indication that there was no plant species effect on Pb or As transfer into the leafy greens.
As expected for a soil with high total As (see Table 1), the leafy greens grown on the orchard soil contained high As (as high as 10 mg/kg), whereas the low-As urban soil produced greens with As well below 1 mg/kg (Figure 2). Similar to the case for Pb uptake, the soil treatments had no statistically significant effect in either decreasing or increasing As in the greens. However, unlike Pb, the As concentrations in the leafy greens showed no tendency to correlate with tissue Al. The As PUFs for leafy greens, in the range of 0.03- 0.05, were much higher than those for Pb.
Greenhouse Experiment 2
Results of lettuce analysis for Pb and Al are reported in Table 2 for both the urban Pb-contaminated soil (Albany St.) and the low-Pb (Arkport) control soil. As expected, lettuce grown on the contaminated soil was consistently higher in Pb concentration (mean = 2.16 ± 0.46 mg/kg) than the lettuce grown on the uncontaminated soil (mean = 0.23 ± 0.07 mg/kg) (p < 0.0001). Nevertheless, given the very large difference in soil Pb between the control and contaminated soils (4.5 vs. 1140 mg/kg), it is evident that transfer of Pb from the contaminated non-acid high-organic matter soil into the lettuce leaves is very limited. None of the 3 compost amendments had any significant effect on Pb concentrations in the lettuce: this was the case for both the highly contaminated urban soil and the low-Pb control soil.
Table 2.
Concentrations of Pb and Al (mg/kg dry wt) in lettuce grown on contaminated and uncontaminated soils both unamended and amended with three different composts.
| Soil Amendment | Lettuce Pb (mg/kg) | Lettuce Al (mg/kg) |
|---|---|---|
| Urban Soil (Pb = 1140 mg/kg) | ||
| None | 2.25 ± 0.64 | 5.3 ± 0.2 |
| Cornell compost | 2.48 ± 0.51 | 4.6 ± 0.8 |
| FW compost | 1.98 ± 0.30 | 5.6 ± 1.4 |
| CM compost | 1.95 ± 0.26 | 5.5 ± 2.7 |
| Control Soil (Pb = 4.5 mg/kg) | ||
| None | 0.28 ± 0.11 | 54.5 ± 30.7* |
| Cornell compost | 0.17 ± 0.02 | 17.5 ± 6.6 |
| FW compost | 0.25 ± 0.05 | 19.3 ± 7.8 |
| CM compost | 0.21 ± 0.03 | 19.5 ± 3.4 |
Indicates significance at the 95% level of confidence
When compared to the results from Experiment 1, lettuce grown in the high-Pb urban soil contained lower Pb on average than the mixed greens grown on the same soil and the orchard soil. The main reason for this may be that the mixed greens in Experiment 1 were not grown to full maturity and had low stature and small biomass, with greater opportunity for contamination by contact with the soil surface. In support of this explanation, the much higher Al concentrations measured in a number of the leafy greens grown in Experiment 1 (see Figure 3) compared to Experiment 2 (see Table 2) indicated frequent more serious contamination by soil particles in Experiment 1 than in Experiment 2. In contrast to the tissue Pb and Al correlation observed in Experiment 1, lettuce tissue Pb in Experiment 2 did not significantly correlate with tissue Al, with the latter consistently averaging low concentrations (about 5 mg/kg) more typical of physiological Al than soil particle contamination. The results of Experiment 2 are consistent with plant uptake being the predominant pathway of Pb accumulation in this case. The plant uptake factor (PUF) for Pb is then estimated to be 0.0019, consistent with other estimates of Pb uptake where physical contamination of the crop is known to minimal (Prasad and Nazareth 2000; Samsoe-Petersen et al. 2002). It is likely that the properties of the urban contaminated soil, with near-neutral pH and high organic matter content, were responsible for limiting Pb solubility and plant uptake via roots.
Soil Chemical Tests
Soil water was vacuum-extracted from the orchard soils (G6) 24 hours after thorough mixing with the various amendments, and again 5 months later in order to measure dissolved Pb and As in the soil solution by IC-MS. These results are summarized in Table 3. In addition, 2 weeks after soil equilibration with the amendments, water extracts were taken from subsamples by the same vacuum extraction method in order to conduct an ICP-OES determination of a wide range of elements in addition to Pb and As. The ICP-OES analyses showed high dissolved Ca (about 800 mg/L), S (about 580 mg/L) and Mg (about 60 mg/L) in the gypsum-amended soils compared to the unamended (control) soils, and about 2.7 times higher soluble P (about 3.7 mg/L) in the phosphate-amended soils compared to the controls. Other differences in soil water composition arising from the amendments were relatively minor.
Table 3.
Dissolved Pb and As in suction-extracted water from the orchard soil as measured by ICP-MS after 1 day and 150 days equilibration of the soil with amendments.
| Soil Amendment | Pb (μg/L) | As (μg/L) | ||
|---|---|---|---|---|
| 1 day | 150 days | 1 day | 150 days | |
| control | 20.9 ±3.2 | 49.3 ± 8.1 | 521 ± 17 | 482 ± 37 |
| peat | 23.0 ±14.3 | 54.0 ± 8.7 | 487 ± 23 | 445 ± 61 |
| Fe oxide | 23.1 ± 14.3 | 72.5 ± 2.1 | 348 ± 15* | 429 ± 23 |
| Ca phosphate | 18.8 ± 4.8 | 57.0 ± 24 | 1110 ± 50* | 625 ± 90* |
| Ca sulfate | 19.2 ± 5.0 | 48.3 ± 12 | 407 ± 23 | 332 ± 19* |
Signifies statistical significance (p < 0.05)
Most importantly, the soil amendments had no short-term effect on dissolved Pb in the orchard soil, which was consistently close to 20 μg/L regardless of treatment (Table 2). After 5 months, dissolved Pb had increased by a factor of 2 to 3 in the controls and all treatments, but again was not significantly altered by soil amendments. This indicates the unresponsiveness of the dissolved Pb level to adsorption or precipitation reactions expected to occur with the introduction of iron oxide, peat, sulfate, or soluble phosphate. A likely explanation is that virtually all the dissolved Pb in non-acid soils is expected to be complexed with soluble organic matter (Sauve et al.1998), and not free to react with the amendments.
The soil solution As concentration was significantly reduced in the short term by Fe oxide, a known effective adsorbent for arsenate, although this effect was not evident after 5 months equilibration (Table 3). Conversely, phosphate increased As solubility in the orchard soil over both the short and long term, in agreement with other studies showing that displacement of arsenate from surfaces by phosphate can mobilize As and enhance its bioavailability (Boisson et al. 1999; Peryea and Kammereck 1997).
Gypsum had no significant short-term influence on As solubility, although a longer-term reduction of As solubility by gypsum was observed (Table 3). The gypsum addition might have been expected to affect solubility of Pb and As by inducing precipitation of anglesite (PbSO4) or Ca arsenate, both relatively insoluble minerals (Bothe and Brown 1999; Davis et al. 1993), or by competition by Ca for Pb and sulfate for arsenate on surfaces. The observed results suggest that anglesite was not involved in the control of Pb solubility, but may indicate that Ca arsenate was involved in limiting As solubility.
The soil extractions with Modified Morgan's (MM) solution did not reveal any significant differences in the extractability of Pb as a result of the amendments to either of the two Pb-contaminated soils relative to the untreated soil over the 5-week testing period (data not shown). MM-extractable Pb was consistently higher for the orchard (G6) soil (average of 205 mg/kg, dry soil wt basis) compared to the urban (Albany St.) soil (average of 124 mg/kg, dry soil wt basis). This could be due at least in part to the lower organic matter content and pH of the orchard soil compared to the urban soil.
The Modified Morgan's extraction was repeated on the orchard (G6) soil after 5 months aging with the amendments in the moist state, and again showed in most cases no significant effect of amendment on extractable Pb or As compared to the unamended (control) soil. The control (unamended) orchard soil had 173 ± 12 and 5.2 ± 1.0 mg/kg (dry soil wt basis) of Pb and As, respectively, extracted by MM. The only significant treatment effects were for extractable Pb resulting from gypsum addition and for extractable As from phosphate addition. The higher extractable Pb (193 ± 11 mg/kg) from gypsum amendment may have been caused by Ca competition with Pb for soil exchange sites at the pH of the extraction (4.8). The higher extractable As (14.4 ± 3.1) resulting from the phosphate amendment presumably was caused by competition for sorption sites between phosphate and arsenate. The efficiency of As extraction by Modified Morgan's extraction was low, a possible result of the low pH of this extractant which would tend to favor arsenate adsorption to the soil.
In summary, none of the soil amendments showed long-term ability to reduce the labile fraction of soil Pb or As that is extracted by Modified Morgan solution.
Conclusions
This study, designed to assess the effectiveness of soil amendments to reduce Pb and As transfer into leafy green vegetables from historically contaminated soils, reveals that transfer into leafy greens from soil is more efficient for As than for Pb. Furthermore, Pb transfer to above-ground plant tissues is largely due to soil particle contamination, whereas As in the tops is mainly taken up via roots. This conclusion is based on several observations: the high degree of variability among replicates in Pb transfer from contaminated soils in situations where above-ground tissue is more likely to have contact with soil, the lack of correlation between Pb and As uptake from a soil contaminated by both metals, and the fact that high Pb (but not As) concentration in washed leafy greens was associated with elevated tissue Al and Fe, two soil “indicator” elements.
Attempts to reduce Pb solubility, extractability, and plant uptake by amendment with potential Pb-stabilizing materials, including composts, soluble Ca phosphate, peat and Fe oxide, were not successful. The failure of phosphate to reduce Pb solubility despite published reports to the contrary is a likely result of the low initial solubility of Pb in these non-acid soils containing significant levels of organic matter. Reported successes with phosphate have invariably occurred with soils where low pH already existed or was achieved by amendments, whereas in near-neutral soils with low Pb solubility, Ca phosphate addition had little effect on Pb solubility (Ma et al. 2007). In contrast to the non-response of dissolved Pb in soil to amendments, dissolved As in the soil was reduced by Fe oxide and increased by Ca phosphate, a result consistent with other remediation studies. Nevertheless, these short-term changes in soil As solubility did not significantly change the levels of As measured in the harvested green vegetables.
Compost amendments to the high-Pb urban soil failed to reduce Pb transfer into the crop, but this is a likely result of the pre-existing low Pb solubility and low phytoavailability in this soil caused by its inherent high organic matter content and near-neutral pH. Low phytoavailability of Pb in this urban soil was evidenced by the relatively low Pb concentration in lettuce grown on this soil (about 2 mg/kg). The low Al concentration ( < 10 mg/kg) in lettuce harvested from this soil indicated minimal soil particle contamination of the washed crop, accounting for the consistently low tissue Pb concentrations.
Acknowledgements
This research was supported by the National Institute of Environmental Health Sciences Grant Number 1R21ES017921-01. We wish to thank Dr. Joshua Cheng at Brooklyn College for the soil water analyses by ICP-MS.
Contributor Information
Murray B. McBride, Department of Crop and Soil Sciences, Cornell University, Bradfield Hall, Ithaca, NY 14853
Tobi Simon, Cornell University student.
Geoffrey Tam, Cornell University student.
Sarah Wharton, Cornell University former student.
References
- Boisson J, Ruttens A, Mench AM, Vangronsveld J. Evaluation of hydroxyapatite as a metal immobilizing soil additive for the remediation of polluted soils. Part 1. Influence of hydroxyapatite on metal exchangeability in soil, plant growth and plant metal accumulation. Environmental Pollution. 1999;104:225–233. [Google Scholar]
- Bothe JV, Brown PW. Arsenic immobilization by calcium arsenate formation. Environmental Science and Technology. 1999;33:3806–3811. [Google Scholar]
- Capar SG, Tanner JT, Friedman MH, Boyer KW. Multielement analysis of animal feed, animal wastes, and sewage sludge. Environmental Science and Technology. 1978;12:785–790. [Google Scholar]
- Chamberlain AC. Fallout of lead and uptake by crops. Atmospheric Environment. 1983;17:693–706. [Google Scholar]
- Cherney JH, Robinson DL. A comparison of plant digestion methods for identifying soil contamination of plant tissue by Ti analysis. Agronomy Journal. 1983;75:145–147. [Google Scholar]
- Chrysochoou M, Dermatas D, Grubb DG. Phosphate application to firing range soils for Pb immobilization: The unclear role of phosphate. Journal of Hazardous Materials. 2007;144:1–14. doi: 10.1016/j.jhazmat.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Davis A, Drexler JW, Ruby MV, Nicholson A. Micromineralogy of mine wastes in relation to lead bioavailability, Butte, Montana. Environmental Science and Technology. 1993;27:1415–1425. [Google Scholar]
- Epstein AL, Gussman CD, Blaylock MJ, Yermiyahu U, Huang JW, Kapulnik Y, Orser CS. EDTA and Pb-EDTA accumulation in Brassica juncea grown in Pb-amended soil. Plant and Soil. 1999;208:87–94. [Google Scholar]
- Finster ME, Gray KA, Binns HJ. Lead levels of edibles grown in contaminated residential soils: a field survey. Science of the Total Environment. 2003;320:245–257. doi: 10.1016/j.scitotenv.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Yamaguchi N, Takaoka M, Shiota K. EXAFS speciation and phytoavailability of Pb in a contaminated soil amended with compost and gypsum. Environmental Science and Technology. 2011;409:1001–1007. doi: 10.1016/j.scitotenv.2010.11.018. [DOI] [PubMed] [Google Scholar]
- Hibben CR, Hagar SS, Mazza CP. Comparison of cadmium and lead content of vegetable crops grown in urban and suburban gardens. Environmental Pollution (Series B) 1984;7:71–80. [Google Scholar]
- Hough RL, Breward N, Young SD, Crout NMJ, Tye AM, Moir AM, Thornton I. Assessing potential risk of heavy metal exposure from consumption of home-produced vegetables by urban populations. Environmental Health Perspectives. 2004;112:215–221. doi: 10.1289/ehp.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorhem L, Engman J, Lindestrom L, Schroder T. Uptake of lead by vegetables grown in contaminated soil. Communications in Soil Science and Plant Analysis. 2000;31:2403–2411. [Google Scholar]
- Kenyon DJ, Elfving DC, Pakkala IS, Bache CA, Lisk DJ. Residues of Lead and arsenic in crops cultured on old orchard soils. Bulletin of Environment Contamination and Toxicology. 1979;22:221–223. doi: 10.1007/BF02026933. [DOI] [PubMed] [Google Scholar]
- Kumpiene J, Lagerkvist A, Maurice C. Stabilization of Pb- and Cu-contaminated soil using coal fly ash and peat. Environmental Pollution. 2007;145:365–73. doi: 10.1016/j.envpol.2006.01.037. [DOI] [PubMed] [Google Scholar]
- Lee SH, Lee JS, Choi YJ, Kim JG. In situ stabilization of cadmium-, lead-, and zinc-contaminated soil using various amendments. Chemosphere. 2009;77:1069–075. doi: 10.1016/j.chemosphere.2009.08.056. [DOI] [PubMed] [Google Scholar]
- Ma LQ, Hardison DW, Jr., Harris WG, Cao X, Zhou Q. Effect of soil property and soil amendment on weathering of abraded metallic Pb in shooting ranges. Water, Air, & Soil Pollution. 2007;178:297–307. [Google Scholar]
- MacLean KS, Langille WM. Arsenic in orchard and potato soils and plant tissue. Plant and Soil. 1981;61:413–418. [Google Scholar]
- McBride MB. Growing food crops on sludge-amended soils: problems with the U.S. Environmental Protection Agency method of estimating toxic metal transfer. Environmental Toxicology and Chemistry. 1998;17:2274–2281. [Google Scholar]
- Melamed R, Cao XD, Chen M, Ma LQ. Field assessment of lead immobilization in a contaminated soil after phosphate application. Science of the Total Environment. 2003;305:117–127. doi: 10.1016/S0048-9697(02)00469-2. [DOI] [PubMed] [Google Scholar]
- Nali C, Balducci E, Frati L, Paoli L, Loppi S, Lorenzini G. Lettuce plants as bioaccumulators of trace elements in a community of central Italy. Environmental Monitoring and Assessment. 2009;149:143–149. doi: 10.1007/s10661-008-0189-6. [DOI] [PubMed] [Google Scholar]
- Nwachukwu OI, Pulford ID. Soil metal immobilization and ryegrass uptake of lead, copper and zinc as affected by application of organic materials as soil amendments in a short-term greenhouse trial. Soil Use Management. 2009;25:159–167. [Google Scholar]
- Peryea FJ, Kammereck R. Phosphate-enhanced movement of arsenic out of lead arsenate-contaminated topsoil and through uncontaminated subsoil. Water, Air, & Soil Pollution. 1997;93:243–254. [Google Scholar]
- Pillay V, Jonnalagadda SB. Elemental uptake by edible herbs and lettuce (Latuca sativa). Journal of Environmental Science and Health Part B. Pesticides, Food Contaminants, and Agricultural Wastes. 2007;42:423–428. doi: 10.1080/03601230701316416. [DOI] [PubMed] [Google Scholar]
- Prasad LR, Nazareth B. Contamination of allotment soil with lead: managing potential risks to health. Journal of Public Health Medicine. 2000;22:525–530. doi: 10.1093/pubmed/22.4.525. [DOI] [PubMed] [Google Scholar]
- Preer JR, Sekhon HS, Stephens BR, Collins MS. Factors affecting heavy metal content of garden vegetables. Environmental Pollution (Series B) 1980a;1:95–104. [Google Scholar]
- Preer JR, Sekhon HS, Weeks J, Jr., Stephens BR. Heavy metals in garden soil and vegetables in Washington, D.C. Trace Substances in Environmental Health-XIV. 1980b:95–104. [Google Scholar]
- Rosborg I, Gerhardsson L, Nihlgard B. Mineral element concentrations in vegetables cultivated in acidic compared to alkaline areas of south Sweden. Air, Soil Water Research. 2009;2:15–29. [Google Scholar]
- Samsoe-Petersen L, Larsen EH, Larsen PB, Bruun P. Uptake of trace elements and PAHs by fruit and vegetables from contaminated soils. Environmental Science and Technology. 2002;36:3057–3063. doi: 10.1021/es015691t. [DOI] [PubMed] [Google Scholar]
- Sauve S, McBride MB, Hendershot W. Soil solution speciation of lead (II): Effects of organic matter and pH. Soil Science Society of America Journal. 1998;62:618–621. [Google Scholar]
- Schooley T, Weaver MJ, Mullins D, Eick M. The history of lead arsenate use in apple production: comparison of its impact in Virginia with other states. Journal of Pesticide Safety Education. 2008;10:22–53. [Google Scholar]
- Sneddon IR, Orueetxebarria M, Hodson ME, Schofield PF, Valsami-Jones E. Field trial using bone meal amendments to remediate mine waste derived soil contaminated with zinc, lead and cadmium. Applied Geochemistry. 2008;23:2414–2424. [Google Scholar]
- Thornton I, Jones TH. Sources of lead and associated metals in vegetables grown in British urban soils: uptake from the soil versus air deposition. Trace Substances in Environmental Health. 1984;18:303–310. [Google Scholar]
- Tjell JC, Hovmand MF, Mosbaek H. Atmospheric lead pollution of grass grown in a background area in Denmark. Nature. 1979;280:425–426. [Google Scholar]
- Veneman PLM, Murray JR, Baker JH. Spatial distribution of pesticide residues in a former apple orchard. Journal of Environmental Quality. 1983;12:101–14. [Google Scholar]
- Xenidis A, Stouraiti C, Papassiopi N. Stabilization of Pb and As in soils by applying combined treatment with phosphates and ferrous iron. Journal of Hazardous Materials. 2010;177:929–937. doi: 10.1016/j.jhazmat.2010.01.006. [DOI] [PubMed] [Google Scholar]