Abstract
Accumulation and turnover of extracellular matrix is a hallmark of tissue injury, repair and remodeling in human diseases. Hyaluronan is a major component of the extracellular matrix and plays an important role in regulating tissue injury and repair, and controlling disease outcomes. The function of hyaluronan depends on its size, location, and interactions with binding partners. While fragmented hyaluronan stimulates the expression of an array of genes by a variety of cell types regulating inflammatory responses and tissue repair, cell surface hyaluronan provides protection against tissue damage from the environment and promotes regeneration and repair. The interactions of hyaluronan and its binding proteins participate in the pathogenesis of many human diseases. Thus, targeting hyaluronan and its interactions with cells and proteins may provide new approaches to developing therapeutics for inflammatory and fibrosing diseases. This review focuses on the role of hyaluronan in biological and pathological processes, and as a potential therapeutic target in human diseases.
Graphical abstract

1. Introduction
Hyaluronan (hyaluronic acid, HA) is a major component of extracellular matrix and is a non-sulfated glycosaminoglycan composed of repeating polymeric disaccharides D-glucuronic acid and N-acetyl-D-glucosamine linked by a glucuronidic β (1→3) bond. In humans, HA exist in all tissues and is abundant in the vitreous of the eye, the umbilical cord, synovial fluid, heart valves, skin, and skeletal tissues. HA can be produced by many cell types [1, 2], although mesenchymal cells are believed to be the predominant source of HA [3]. HA has multiple functions in normal biological states, such as space filling, hydration, lubrication of joints, and provision of a matrix through which cells can migrate [3]. HA is actively produced during tissue injury [4], regulating tissue repair and disease processes, such as activation of inflammatory cells to mount an innate response to injury [5] and regulation of behavior of epithelial cells [6–10] and fibroblasts [11, 12]. HA has been investigated in a wide range of biological and medical fields, and the research articles referring to HA have grown exponentially in recent years (Figure 1).
Figure 1. Scientific articles published referring to “hyaluronan” from 1940 to 2014.
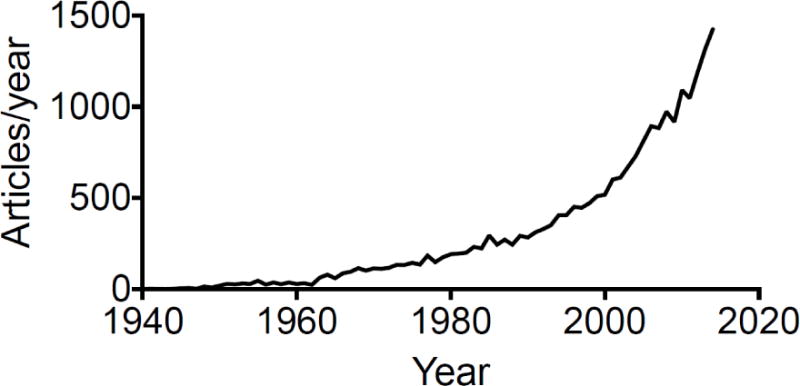
The number of articles published in each of the past years from 1940 to 2014 was identified by searching the PubMed database (http://www.ncbi.nlm.nih.gov/sites/entrez) using the search terms (hyaluronan OR hyaluronic acid) queried on August 8, 2015.
There are many excellent reviews on the roles of HA in different fields, such as in angiogenesis [4], reactive oxygen species [13], HA digestion [14], cancer [3, 15, 16], cancer therapeutics [17], cancer metastasis [18], chondrocytes [19], lung injury [20–22], wound healing [23], diabetes [24], leukocyte trafficking [25], and in immune regulation [5, 26, 27]. The current review will summarize the role of HA in biological and pathological conditions, and will emphasize the role of HA in human diseases.
1.1. HA synthases
HA is synthesized by membrane-bound synthases, and there are three mammalian hyaluronan synthases (HAS1-3) [28]. All three proteins are bona fide HA synthases. In vitro transfection experiments showed that HAS1 and HAS3 generated HA with broad size distributions (molecular masses of 2 × 105 to approximately 2 × 106 Da), whereas HAS2 generated HA with a broad but extremely large size (average molecular mass of > 2 × 106 Da) [29]. Subsequent studies suggested that all three HAS enzymes drive the biosynthesis and release of high molecular weight (HMW) HA (1 × 106 Da) [30]. Deletion of murine Has1 [31] or Has3 [12] or Has1-Has3 double knockout [32] did not reveal a significant phenotype under homeostatic conditions, whereas Has2 deletion generated an embryonic lethal phenotype due to impaired cardiac development [33]. With tissue injury, both Has1 [32] and Has3 deletions [12, 32] showed dysregulated tissue repair. Wound closure was significantly faster in Has1 and Has3 double null mice [32]. HAS2 protects skin fibroblasts against apoptosis induced by environmental stress such as UV exposure and serum starvation [34]. Furthermore, Has3 deficiency causes reduction in brain extracellular space leading to altered neuronal activity and seizures [35]. These studies generate important insights into the roles of hyaluronan in disease states.
1.2. HA degradating enzymes
Hyaluronidases (also called hyaluronoglucosaminidases) hydrolyze the hexosaminidic β(1–4) linkages between N-acetyl-D-glucosamine and D-glucuronic acid residues in HA and release disaccharide D-glucuronic acid-N-acetyl-D-glucosamine or HA fragments. In humans, there are six members of a gene family containing hyaluronidases identified: hyaluronidases 1 – 4, PH-20, and HYALP1 [14, 36]. Although Hyal1 deficient mice are viable, fertile and show no gross abnormalities, these mice do develop osteoarthritis [37]. HA fragments control dendritic cell migration from the skin, and HYAL1 expression activated migration and promoted loss of dendritic cells from the skin [38].
Hyal2 deficient mice displayed a significant increase in plasma HA and increased HA in the interstitial extracellular matrix of atrial cardiomyocytes [39, 40], suggesting that HYAL2 is essential for the breakdown of extracellular HA. Hyal2 deficient mice showed severe cardiopulmonary dysfunction [40]. Platelet-derived hyaluronidase 2 cleaves HA into fragments that trigger monocyte-mediated production of pro-inflammatory cytokines [41].
Hyal3 deficient mice showed a subtle change in the alveolar structure and extracellular matrix thickness in lung-tissues at 12–14 months-of-age, although there was no evidence of HA accumulation, suggesting that HYAL3 may not play a major role in constitutive HA degradation [42].
PH20 is elevated in demyelinating lesions and increased PH20 expression is sufficient to inhibit oligodendrocyte progenitor cell maturation and remyelination [43].
1.3. HA binding proteins
HA regulates an array of the biological and pathological processes, depending on its interacting proteins (Figure 2). Several cell surface receptors and secreted proteins have been suggested to be HA binding proteins (HABPs). CD44 is the major cell-surface HA binding protein [44]. It is widely expressed in almost every cell type in human and in mouse [45]. HA-CD44 interactions play an important role in development, inflammation, T cell recruitment and activation, and tumor growth and metastasis [46]. Although the genetic disruption of Cd44 in the mouse did not show much abnormality [47], the mice showed defective hematopoietic progenitor egress from bone marrow [47]. Under stress conditions, deficiency in CD44 results in some severe phenotypes. In a non-infectious lung injury mouse model, we showed that deficiency in CD44 resulted in exaggerated lung injury with increased HA accumulation, suggesting that CD44 is required to clearance of HA for the resolution of pulmonary inflammation [48]. Moreover, CD44 splicing variants have been shown to have a role in cancer metastasis [49, 50], and the strategies are developed to inhibit cancer metastasis with neutralizing antibodies against CD44 variants [51].
Figure 2. HA binding domains in HA binding proteins.

The locations of HA-binding domain Link modules were shown on the majority of HABPs. HA binding sequence, BX7B, was shown on HMMR. Protein CEMIP contains neither Link domain nor BX7B sequence, but four parallel beta-helix repeats, which are usually presented in the enzymes with polysaccharide substrates. CD44, STAB1, STAB2, SUSD5, LYVE1 and CEMIP are the single-pass transmembrane proteins, while others are secreted as extracellular matrix proteins. HMMR is a GPI-anchored protein. The proteins are drawn roughly to scale, except VCAN. Protein size was indicated with the number of total amino acids on last residue.
HA binds to HMMR (Hyaluronan-mediated motility receptor, or RHAMM, for receptor for hyaluronan-mediated motility expressed protein, also called CD168) [52]. HMMR is a functional receptor in many cell types, including endothelial cells [53]. HMMR-HA interactions play a role in Ras-dependent oncogenesis [54] and in tissue injury and repair [55]. Mice with deletion of HMMR gene were viable with normal appearance, but the deletion of the gene showed reduced formation of aggressive fibromatosis when crossed with mice that harbor a targeted mutation in the tumor suppressor APC gene [56]. RMMR deficient fibroblasts fail to repair wounds in an in vitro wound healing assay [57].
Lecticans (or hyalectans) are a family of HA-binding proteoglycans, including aggrecan, brevican, neurocan, and versican, mostly expressed in the brain. Lecticans interact with HA and tenascin-R to form a ternary complex [58]. HA binds to brevican, a brain-specific proteoglycan [59]. Brevican binds not only to HA [60] but also to chondroitin sulfate [61]. Neurocan is a nervous tissue-specific proteoglycan of the aggrecan family which interacts with neural cell adhesion molecules. Neurocan binds to HA as well as chondroitin sulfate in the brain [62]. Versican also binds to HA [63] and chondroitin sulfate [64]. Studies have shown that hyalectants have a role in neural development and brain injury [58].
HABP1 (hyaluronan-binding protein 1, also called C1QBP, for complement component 1, q subcomponent binding protein) binds to HA [65]. C1QBP is a multifunctional and multicompartmental protein involved in inflammatory regulation and mitochondria protein synthesis [66]. The extract sequence in HABP1 protein for HA binding is less clear.
HARE (hyaluronan receptor for endocytosis, also called stabilin-2 and FEEL-2) was identified in the abundant expression of 175- and approximately 300-kDa HARE species from sinusoidal endothelial cells of the liver, lymph node, and spleen [67]. HARE protein binds to HA and other sugar species [68] and acts as a systemic clearance receptor for heparin [69]. Deletion of stabilin-2 in mice resulted in a dramatic elevation in circulating HA levels without any overt phenotype [70].
LYVE1 (lymphatic vessel endothelial hyaluronan receptor 1, also called CRSBP-1 for cell surface retention sequence binding protein-1) was cloned as a lymph-specific HA receptor on the lymph vessel wall in human [71] and in mice [72]. Conditional ablation of LYVE-1+ cells showed defensive roles of lymphatic vessels in the small intestine and lymph nodes, but lymphatic vessels in the other organs were relatively intact [73].
SHAP (serum-derived hyaluronan-associated protein) was originally identified as a serum-derived HA-associated protein since it covalently binds to HA [74]. It was later found that SHAP is identical to inter-α (globulin) inhibitor (IαI) heavy chain 2 (ITIH2), which belongs to the inter-α-trypsin inhibitor (ITI) family of structurally related plasma serine protease inhibitors involved in extracellular matrix stabilization.
TNFIP6 (tumor necrosis factor α-induced protein 6, also called TSG-6 for TNFα-stimulated gene-6), contains a Link module binding to HA [75]. TNFIP6 has been shown to play a key role in ITI heavy chain transfer [76]. TNFIP6 amplifies HA synthesis by airway smooth muscle cells [77]. Knockout of Tnfip6 in mice with a BALB/c background did not change the onset of proteoglycan-induced arthritis, but progression and severity were significantly greater in Tnfip6-deficient mice when compared to wild type BALB/c mice [78]. An early and more extensive infiltration of the synovium with neutrophils was the most prominent histopathologic feature of proteoglycan-induced arthritis in Tnfip6-deficient mice [78].
CEMIP (cell-migration induced protein hyaluronan binding, aka, KIAA1199) was recently identified to encode a new HA binding protein. The protein lacks a typical HA binging motif, but contains four PbH1 (parallel beta-helix) repeats, suggesting the protein have hyalunidase activity catabolizing HMW HA to an intermediate-sized product in an endo-β-N-acetylglucosaminidase–dependent manner via the clathrin-coated pit pathway [79]. The CEMIP protein is also presented in endoplasmic reticulum having a role in cancer cell migration [80] and invasion [81]. Furthermore, KIAA1199 was overexpressed by synovial fibroblasts and synovial tissues from patients with osteoarthritis or rheumatoid arthritis [79], suggesting a role of HA catabolism in the dermis of the skin and arthritic synovium.
1.4. Biology of HA polymer size
HA plays different roles depending on its molecular weight [82–87] (Figure 3). In its native state, such as in normal synovial fluid, HA generally exists as a HMW polymer, usually in excess of 1000 kDa. The fragmentation of HA occurs during many pathological conditions, a possible consequence of dysregulated expression of HA synthases and HA degradation enzymes during tissue injury and inflammation. Mammalian hyaluronidases are hyaluronate-4-glycanohydrolases degrading HA into medium and small size fragments, but exhaustedly degrade HA to tetrasaccharides as the end product, while bacterial hyaluronidases can produce unsaturated disaccharides [88]. HA fragmentation can also be a result of the release of reactive oxygen species [89] and reactive nitrogen species such as peroxynitrite [90] during tissue injury. We have found that lower-molecular-weight (LMW) forms of HA (<500 kDa, many preparations in 100 – 250 kDa), but not the native form (>1000 kDa), induce inflammatory responses during tissue injury [5, 22]. The role of disaccharides generated during bacterial infection is unknown. Thus, HA fragments can be generated through multiple processes and are biologically active.
Figure 3. HA size.

Native HA exists usually in HMW form, having a role in normal development, biological processes and tissue protection. HMW HA can be degraded by hyaluronidases, ROS, and RNS to LMW HA and HA oligomers. The bacterial, not mammalian, hyaluronidases, produce disaccharides D-glucuronic acid and N-acetyl-D-glucosamine. The role of disaccharides in vivo is not clear.
Chemically modified LMW HA fragments retain their ability to regulate inflammatory responses. Selectively N-butyrylated LMW HA shows promise as an example of a novel semisynthetic anti-inflammatory molecule. Chemically modified N-acylated HA fragments modulate proinflammatory cytokine production by stimulated human macrophages [91]. Sulfated HA as a hyaluronidase inhibitor was showed as a potent inhibitor of prostate cancer [92]. Therefore, the modified HA preparations may have a broad use as therapeutics and as drug carriers.
1.5. HA as a signaling molecule
HA fragments are able to stimulate an array of cell types to regulate gene expression. The signaling pathway(s) of HA has been extensively investigated. In tumor cells, HA fragments may signal through CD44-dependent tyrosine kinase pathways to promote tumor cell growth [4, 93]. However, HA is able to stimulate chemokine production by macrophages in the absence of CD44, suggesting that the presence of CD44 is not required to mediate HA signaling [6]. The cumulative interpretation of these data is that the role of CD44 in regulating HA interactions depends on the cell type.
We found that HA fragment-induced chemokine and cytokine expression was completely abolished in TLR2−/−TLR4−/− peritoneal macrophages [6]. HA oligosaccharides induce the maturation of dendritic cells via TLR4 [94]. Priming of alloimmunity by HA-activated dendritic cells is dependent on signaling via TIRAP, a TLR adaptor downstream of TLR2 and TLR4 [95]. These studies demonstrated HA fragments signal through TLRs in inflammatory cells.
Studies also suggest CD44 may modulate other receptor signaling. We found that CD44 negatively regulated lipopolysaccharide-TLR signaling in mouse macrophages [96]. Engagement of CD44 by HA suppresses TLR4 signaling and the septic response to LPS [97]. CD44 acts as a co-receptor for several cell surface receptors including RTKs, G-protein coupled receptors, and LRP6 [98]. These studies point to a complex biology in HA-TLR4-CD44 signaling.
2. HA in biological processes
HA signaling involves a wide range of pathogenic process of diseases as well as providing a framework for ingrowth of blood vessels and fibroblasts, activating inflammatory cells to mount an immunological response [5] and regulating behavior of epithelial cells [7, 9] and fibroblasts [12](Li 2011). HA-stem cell interactions have been particularly explored in hematopoietic stem cells [99–101], mesenchymal stem cells [102–104], and adult multipotent progenitor cells, indicating that HA and its binding proteins may regulate tissue injury and repair processes through their interactions with a variety of stem cells. Elucidation of the role and mechanisms of HA in the stem cell niche is crucial in aiding the development of novel therapy for many diseases.
2.1. HA in development
HA, HA synthases, hyaluronidases, and HA binding proteins have been identified in many developmental processes, as well as in different organ development. HA and hyaluronidase activity were found in the chick embryonic heart [105] and kidney [106], suggesting a role for HA in the development of these organs. The direct demonstration of the role of HA in development came from a deletion study in mice. Targeted deletion of Has2 showed major abnormalities in heart and blood vessel development, resulting in an embryonic lethal phenotype [33, 107]. Has2-deficient embryos at embryonic day 9.5 completely lack endocardial cushions [33, 107], consistent with early observations that a high content of HA is present in normal human heart valves. These defects resemble the features in occurring versican knockouts [108]. These studies identified a role of HA in mediating epithelium to mesenchyme transition [33]. Moreover, HAS2 may be regulated through a Tbx 2-Bmp/TGFβ-Smad pathway in the development [109]. These reports suggested that HA and HA synthases play a critical role in the organ development.
HAS2 has a role in limb development. Overexpression of Has2 in the mesoderm of the chick limb bud in vivo results in the formation of shortened and severely malformed limbs that lack one or more skeletal elements [110]. Conditional inactivation of Has2 showed a role for HA in skeletal growth, patterning, chondrocyte maturation and joint formation in the developing limb [111]. Furthermore, sonic hedgehog signaling directly targets HAS2 in regulating phalangeal joint patterning [112].
HA has also been suggested in myogenesis and bone marrow hematopoiesis. Intrinsic HA synthesis is necessary for myoblasts to differentiate and form syncytial muscle cells, but the HA-dependent pericellular matrix is not sufficient to support differentiation alone [113]. By triple deletion of all three HA synthases, a report showed that HA expressed by the hematopoietic microenvironment is required for bone marrow hematopoiesis [114].
In mice, treated with exogenous HA at age 3 to 8 weeks increased intestinal and colonic epithelial proliferation, resulting in hyperplasia. Pep-1 (a peptide that blocks the binding of HA to its receptors) treatment markedly decreased intestinal and colonic length, crypt depth and villus height in the intestine, crypt depth in the colon, and epithelial proliferation in the intestine and colon, suggesting a role for HA in normal intestinal and colonic growth [115].
2.2. HA in growth and differentiation
HA promotes tumor cell growth through multiple signaling pathways. CD44 acts as a co-receptor for several cell surface receptors including Erb2/EGF receptor, G-protein coupled receptors, and Wnt signaling receptor LRP6 [98, 116]. Furthermore, HA strongly promotes anchorage-independent growth and the resistance of cancer cells to growth arrest and apoptosis under anchorage-independent conditions [3]. Therefore, strategies targeting HA and its signaling have being developed for patients with various cancers [3, 15–17].
HA and its binding proteins have been shown to have a role in fibroblast proliferation. HA facilitates TGF-β1-mediated fibroblast proliferation [117]. LMW HA or HA oligosaccharides stimulate fibroblast proliferation in various conditions [118, 119], suggesting a role for HA in tissue fibrosis.
HA fragments induce myofibroblastic differentiation [120, 121], endothelial cell differentiation [122], chondrogenic differentiation [123]. But, TNFIP6 inhibits osteoblastic differentiation of human mesenchymal stem cells (MSCs) induced by osteogenic differentiation [124], and HA inhibited the myocardiocyte differentiation [125]. These data suggest that HA and HA binding proteins regulate cell differentiation in a complex manner, which may be related to cell type and microenvironment.
2.3. HA in immune responses
HA and HA-binding proteins regulate inflammation, tissue injury, and repair through regulating inflammatory cell recruitment, release of inflammatory cytokines, and cell migration. Therefore, HA acts as an immune regulator in human diseases [5]. HA-CD44 interaction regulates T cell activation [126], Th1 generation [127], B-cell activation [128], and regulatory T cell functions [129]. A recent report suggested that the role of CD44 in adaptive regulatory T cells was through its interaction with Galectin-9 [130]. HA and CD44 modulate neutrophil adhesion and recruitment [131]. Endothelial CD44 rather than neutrophil CD44 mediates neutrophil migration [132]. Furthermore, HA is able to trigger inflammasome in response to injury [133].
While lymphocytes from CD44−/− mice preferentially homed to lymph nodes, their entry into the inflamed synovial joints was delayed when compared to wild type cells [134]. We showed that CD44 plays a role in HA fragment clearance during tissue injury [48]. CD44-deficient mice succumb to unremitting inflammation following noninfectious lung injury, characterized by impaired clearance of apoptotic neutrophils [48].
Moreover, HA fragments can influence dendritic cell maturation. For example, LMW HA fragments induce dendritic cell maturation and initiate alloimmunity [95]. Also, HA oligosaccharides are potent activators of dendritic cells [87]. The generation of small HA fragments, rather than the loss of large HA molecules, promotes DC migration and subsequent modification of allergic responses [38].
2.4. HA in senescence
HA and HA binding proteins participate in cellular senescence. Diminished versican deposition increases free HA fragments that interact with CD44 and increase phosphorylation of ERK1/2, leading to premature cellular senescence in embryonic fibroblasts [135].
The inhibition of HA synthesis by 4-methylumbelliferone induces senescence [136]. Targeting HAS2 by an miRNA caused cellular senescence, suggesting a role of HA in skin aging [137]. On the other side, HA concentrations in extracellular and pericellular matrices was greatly reduced in senescent mesenchymal stem cells, mainly due to the decreased expression of HA synthases [138]. An HA-based compound inhibited fibroblast senescence induced by oxidative stress in vitro [139]. Therefore, HA may participate in senescence in normal and pathological processes. Further investigation into the role of HA in senescence in disease states may shed light on the pathogenesis and help develop therapeutics for these diseases.
2.5. HA in apoptosis
We have reported that HA protected lung epithelial cells from apoptosis in bleomycin induced lung injury in mice [6]. HMW HA decreases UVB-induced apoptosis and inflammation in human epithelial corneal cells [140]. Has1/3 null skin fibroblasts, with higher levels of Has2 gene expression, are resistant to stress-induced apoptosis, suggesting that HAS2 protects skin fibroblasts against apoptosis induced by environmental stress [34]. For inflammatory cells, HA seems to induce apoptosis. HA induces apoptosis of activated T cells through CD44 [141]. LMW HA-TLR4 interaction induced inflammatory neutrophil apoptosis [142]. In vivo, 1600 kDa HA administration yielded significantly less smoke-induced neutrophil infiltration, lung edema, airway apoptosis and mucous plugging in rats, suggesting a potential beneficial therapy of HMW HA for smoke induced lung injury [143]. Moreover, the cross-linking of specific epitopes of the CD44 molecule rapidly induced neutrophil apoptosis in vitro and inhibited neutrophil-dependent renal injury in rats in vivo [144].
2.6. HA in cell migration and invasion
HA mediates inflammatory cell migration to regulate inflammatory responses and tissue injury. CD44 deficiency leads to enhanced neutrophil migration and lung injury in Escherichia coli pneumonia in mice [145]. TSG6 is a potent inhibitor of neutrophil migration in an in vivo model of acute inflammation [146]. Exogenous LMW HA or LMW HA generated by HYAL1 over-expressing promoted dendritic cell migration from the skin and subsequent modification of allergic responses at TLR4 dependent manner [38].
HA and HA binding proteins have a role in fibroblast migration as well as smooth muscle cell migration. Abnormal accumulation of HA matrix promotes fibroblast cell migration [11]. Specific sizes of HA oligosaccharides stimulate fibroblast migration and excisional wound repair [147]. Both CD44 [148] and HMMR [57] have been shown to have a role in fibroblast migration during tissue injury. Furthermore, myofibroblast migration can be regulated by HA [149]. These studies suggested a role for HA in tissue injury and fibrosis. Formation of HA- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells [150]. HMMR is also necessary for the migration of smooth muscle cells during wound repair [151].
CD44 and HA cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow [101]. HA promotes a CD44-dependent migration of the MSCs [152], suggesting a role for HA and CD44 in moving stem cells to a neighborhood in need of regeneration.
CD44 also regulates directional motility. The interaction between CD44 and the cytoskeletal linker protein ezrin is critical for CD44-dependent directional cell motility [153]. Fibroblast migration is mediated by CD44-dependent TGF-β activation [154], and the migration of CD44-deficient fibroblasts was increased in velocity, but was directionless [154].
HA regulates fibroblast invasion [148] ad tumor invasion [155, 156]. HA-mediated invasive cell motility is regulated by TGF-β [156, 157] and Src activation [157]. Furthermore, cellular heterogeneity profiling by HA probes reveals an invasive but slow-growing breast tumor subset [158]. We showed that fibroblasts isolated from transgenic mice overexpressing HAS2 had a greater capacity to invade matrix. Conditional deletion of HAS2 in mesenchymal cells abrogated the invasive fibroblast phenotype, impeded myofibroblast accumulation, and inhibited the development of lung fibrosis [159].
2.7. HA in stem cells
HA-stem cell interactions have been explored in hematopoietic stem cells [99–101], mesenchymal stem cells [103, 104], and adult multipotent progenitor cells, indicating that HA and its binding proteins may regulate tissue injury and repair processes through their interaction with a variety of stem cells.
During stem cell differentiation, HA synthesis was enhanced by 13- and 24-fold, most likely due to increased expression of HAS2 [160]. HA is required for generation of hematopoietic cells during differentiation of human embryonic stem cells [99, 161]. HAS2 knockdown results in suppression of human embryonic stem cell differentiation [162]. HA oligosaccharides increased epidermal cell stemness by modulation of integrin expression [163]. HA promotes a CD44-dependent migration of the mesenchymal stem cells [152].
CD44 has long been used as a marker for stem cells [102], including embryonic stem cells (ESCs), mesenchymal stem cells (MSCs), hematopoietic stem cells (HSCs), as well as cancer stem cells (CSCs) [164, 165]. In addition, CD44 may have a role in regulating stem cell functions. A recent review suggests that although CD44 is not a master stem cell gene, CD44 contributes to the generation of a stem cell niche and homing of stem cells in their niche [166]. HA is required for the regulation of the hematopoiesis-supportive function of bone marrow accessory cells and participates in hematopoietic niche assembly [167].
2.8. HA in angiogenesis
Partial degradation products of HA, between 4 and 25 disaccharides in length, induced an angiogenic response on the chick chorioallantoic membrane [82]. HA oligosaccharides enhance angiogenesis to promote excisional wound healing [119], while HMW HA is anti-angiogenic [168]. Both endothelial CD44 [169] and HMMR mediated angiogenesis [53]. A deficiency in stromal HA in Has2−/− mice attenuated tumor angiogenesis and lymphangiogenesis [31]. Given its role in tumor angiogenesis, targeting HA is proposed for tumor chemotherapy [17].
3. Hyaluronan in human diseases
3.1. Lung fibrosis
Idiopathic pulmonary fibrosis (IPF) is the most common form of interstitial lung diseases. The central hallmark of IPF is excessive fibroproliferation, a chronic, progressive disorder that results in impaired gas exchange and respiratory failure. IPF portends a poor prognosis, with a mean survival of 2–5 years from the time of diagnosis [170]. HA levels were higher in BAL fluid in patients with IPF than in healthy controls [171], and were also correlated with the severity of the disease [171]. Fibroblast clones derived from primary fibroblast cultures from the lung tissue of patients with pulmonary fibrosis secreted much greater amounts of HA and proteoglycan decorin than the clones from normal individuals [172]. Fibroblasts isolated from lungs of patients with IPF had increased HAS2 [159]. IPF fibroblasts also showed increased invasive capacity, an important fibroblast phenotype that is required for development of severe pulmonary fibrosis [159].
In vitro studies suggested a role of HA in fibrosis. HA facilitates transforming growth factor-beta1-mediated fibroblast proliferation [117], as TGF-β signaling plays a central role in fibrogenesis. HA orchestrates TGF-β 1-dependent maintenance of myofibroblast phenotype [173]. Age-related changes in pericellular HA organization leads to impaired dermal fibroblast to myofibroblast differentiation [174].
Elevated HA levels have been reported in animal models of pulmonary fibrosis. There was a transient histological accumulation of HA in the alveolar interstitium, corresponding to increases in HA levels in BAL fluid and lung tissue extracts in bleomycin-induced alveolitis in rats [175]. HA was found not only accumulate in mouse lungs after bleomycin injury, but also fragmented in the injured lung [159, 176]. We showed that over expression Has2 under control of β-SMA promoter resulted in worsened lung fibrosis after bleomycin injury indicated a direct association between HA accumulation and fibrosis [159]. We further showed that conditional knockout Has2 in Col1α2 expressing cells or FSP-1 expressing cells reduced bleomycin induced lung fibrosis in mice [159], demonstrating a causative role for mesenchymal Has2 in the pathogenesis of pulmonary fibrosis.
An anti-fibrotic effect of hyaluronidases was reported in the recent studies. Hyaluronidase-loaded PLGA microparticles reduced neutrophil recruitment and collagen deposition after bleomycin treatment [177]. Intranasal therapy with immobilized and testicular hyaluronidases prevented the deposition of collagen in the parenchyma of bleomycin-treated lungs [178]. Hyaluronidase attenuation of bleomycin-induced lung fibrosis might be due to promoting a population of mesenchymal stem cell-like cells in the bleomycin injured lung [178]. Further study is needed to dissect the role of hyaluronidase in pulmonary fibrosis and the potential molecular mechanisms.
3.2. Asthma
The characteristics of human asthma are chronic inflammation and airway remodeling. HA is an important mediator in asthma. A genomewide association study identified HAS2, the major HA synthesis gene as the strongest statistical significance associated with asthma for adult asthma in Japanese population [179]. The level of HA in lavage fluid from patients with allergic asthma was increased [180]. HA levels were also closely correlated with the severity of asthma, and with the pulmonary function of the patients [181].
The molecular mechanism of HA accumulation and its biology function in the disease is complicated. LMM HA functioned through a CD44-mediated mechanism to elicit chemotaxis of B lymphocytes. HA stimulates B lymphocyte chemotaxis and cytokine production ex vivo in a murine model of fungal allergic asthma [182]. We found that fibroblasts isolated from human asthmatic lung had elevated HAS2 expression, without significant changes in Hyal1 and hyal2 expression levels [183]. Asthmatic fibroblasts produced increased amount and LMW HA compare to that of the fibroblasts from health lungs [183]. One of the reasons for increased HA levels in asthma is an increase in TGF-β [184] and IL-13 levels [185], which promote airway smooth muscle cells to secret glycosaminoglycans such as HA.
Asthmatic airway epithelial cells differentially regulate fibroblast expression of extracellular matrix components. Lung fibroblasts co-cultured with alveolar epithelial cells from asthmatic patients showed differential expression of the ECM constituents COL1A1 and COL3A1 and HAS2 [186], supporting a role for altered ECM production in asthmatic airway remodeling, regulated by unbalanced alveolar epithelial cell signaling.
3.3. Chronic Obstructive Pulmonary Disease and Emphysema
Chronic obstructive pulmonary disease (COPD) is characterized by infiltration of inflammatory cells, alveolar wall destruction, and airway remodeling. HA levels were significantly higher in patients with COPD than in those from controls [187–189]. Some reports showed that HA levels were decreased in COPD [190] and in COPD with alpha-1 antiprotease deficiency [191]. The decrease of HA was associated with a significant reduction in the expression of HAS1 and HAS2 and a significant increase of hyaluronidase-1 [190]. Lower HA levels possible happen in some COPD/emphysema where inflammation is not predominant.
HA plays multiple roles in the progression of COPD. LMW HA promotes lung inflammation. Cigarette smoke exposure leads to enhanced deposition of mostly LMW HA in alveolar and bronchial walls by altering the expression of HA modulating enzymes [192]. Both collagen and HA was increased in mouse models of COPD induced by exposure to cigarette smoke, chronic intratacheal lipopolysaccharide instillation, or over expression TNF-α in SPC-expressing cells in mice [193]. LMW HA contributes to persistent inflammation in the lung [183]. Systemic administration of Hyaruonidase to rats caused alveolar distension and larger functional residual capacity [194].
HA protects elastic fibers by its hydrating effect, and erosolized HA may be an effective means of preventing pulmonary emphysema and perhaps other lung diseases that involve elastic fiber injury [195]. Aerosolized HA limited airspace enlargement in a mouse model of cigarette-induced emphysema [196] and smoke-induced elastic fiber injury [197]. The effect of HA on inflammation appears to be related to its molecular size, with larger polysaccharide chains having anti-inflammatory activity and smaller ones having proinflammatory properties. The breakdown of inhaled HA into smaller fragments could possibly induce an inflammatory reaction in the lung that counteracts any beneficial effect [198]. Consequently, the proposed therapeutic use of HA will require the development of treatment strategies aimed at minimizing its pro-inflammatory activity [198]. Increased studies of biological functions of HA during the past years provided better understanding of the roles in human diseases, and to develop HA as a therapeutic reagent is in need.
3.4. HA in allograft rejection and BOS
Lung transplantation is a therapeutic option for patients with end stage lung diseases, but long-term survival remains very poor due to chronic allograft rejection. Bronchiolitis obliterans syndrome (BOS), a fibrotic process resulting in progressive narrowing of bronchiolar lumens and airflow obstruction, is a manifestation of chronic allograft rejection. HA as an immune regulator plays an important role in lung allograft. Lung transplant patients with acute rejection had significantly higher levels of HA in BAL compared to the patients with no rejection [199]. HA localized within areas of intraluminal small airways fibrosis in BOS lung tissue [200]. The transcripts of HA synthases were significantly elevated in BOS versus normal lung tissues [200]. HA concentrations were increased in lavage fluid and plasma of recipients with BOS [95, 200].
Elevated HA level was also observed in murine skin transplant rejection model. [95]. In experiment cardiac allograft rejection rat model, HA concentration in serum significantly higher in the rejecting group than that in the non-rejecting group [201].
Treatment with various HA preparations showed contradictory results. LMW HA abrogated tolerance in murine orthotopic lung recipients [200], and prolonged renal and cardiac allograft survival in experimental animals [202, 203], while HMW HA attenuated basal allograft inflammation [200]. Hyaluronidase treatment reduced HA contents of the graft and ameliorated rejection-induced edema in a heterotopic heart transplantation model in rats [204]. More studies are in need to better understand the role of HA in allograft and develop therapeutic strategies.
3.5. Pneumonia
Serum HA in patients with chronic interstitial pneumonia was significantly greater when compared with healthy controls [205]. In mouse models, CD44 deficiency mice showed increased neutrophil accumulation in the lung and edema formation in Escherichia coli-induced pneumonia [145], but no difference was observed between these genotypes in Streptococcus pneumoniae-induced pneumonia [145]. CD44 deficiency is associated with increased bacterial clearance but enhanced lung inflammation in Klebsiella pneumonia-induced pneumonia [206].
As HA accumulates, HA fragmentation occurs during infections. HA is extremely sensitive to ROS-mediated degradation [89]. In vitro, oxidative fragmentation of HA resulted in neutrophil chemotaxis and extracellular superoxide dismutase prevented this effect [207]. CD44 plays a key role to clear HA in the lung to resolve lung inflammation [48]. HA fragment promotes inflammation by down-regulating the anti-inflammatory adenosine A2a receptor through CD44 [207]. Mice with CD44 deficiency showed increased HA accumulation in the lungs and increased inflammatory chemokine and cytokine levels with both bleomycin and LPS induced lung injury [48, 96].
3.6. Hepatitis and liver fibrosis
Elevated serum HA levels have been found in patients with chronic viral hepatitis and cirrhosis, and often correlate with the disease progress [208]. A recent study performed a systematic review showing that serum HA can be used as a noninvasive biomarker to assess the presence of liver fibrosis, and to monitor disease progression [209]. Serum HA in combination with other measurements form a score system [210] or an algorithm model [211] may be used as a clinic tool to monitor liver fibrosis. However, serum HA alone may not be regarded as reliable marker for clinical decisions [212], because of the heterogeneous nature of liver fibrosis.
In mouse models, LMW HA induces NF-κB-dependent resistance against TNF-α mediated liver injury [213]. CD44-deficient mice exhibited suppressed liver inflammation during the early phase after CCl4 injection due to reduced inflammatory cell infiltration and cytokine production, but showed severe liver inflammation with increased numbers of apoptotic hepatocytes at the late phase [214]. Therefore, HA and CD44 may have multiple function depending the progress of the disease.
3.7. Heart diseases
HA is expressed in all aortic layers. In atherosclerosis, HA associates with leukocytes and vascular smooth muscle cells, and is involved in vascular remodeling [215]. HAS1 and HAS2 are upregulated in response to prostaglandins in human vascular smooth muscle cells [216]. Overproduction of HA in the genetic background of the ApoE-deficient mouse strain promoted atherosclerosis development in the aorta, suggesting that accumulation of HA accelerates the progression of atherosclerosis [217]. However, systemic inhibition of HA synthesis by 4-MU interferes with the protective function of the endothelial glycocalyx, thereby facilitating leukocyte adhesion, subsequent inflammation, and progression of atherosclerosis [218]. Therefore, an HA synthase inhibitor that avoids adverse effects such as impacting the endothelial glycocalyx may be needed for blockage of HA synthase pathways in patients with atherosclerosis.
CD44 promotes atherosclerosis by mediating inflammatory cell recruitment and vascular cell activation [219]. Furthermore, CD44 expressed on both bone marrow-derived and non-bone marrow-derived cells promotes atherosclerosis in ApoE-deficient mice through regulating macrophage and T cell recruitment; leukocyte-endothelial cell adhesion and transendothelial migration; and HA-dependent migration of vascular smooth muscle cells [220].
3.8. Diabetes
Increased HA levels were seen in diabetic patients [221] and in the glomeruli from diabetic rats [222]. Serum HA levels correlate with poor blood glucose control and diabetic angiopathy and could be used as a marker of diabetic angiopathy [221]. However, others found a decrease of HA in skin in diabetic patients [223] or rats with chronic diabetes mellitus [224]. This discrepancy may be due to the measurement of HA during different stages of the disease, inflammatory status and treatments.
HA plays an active role in the development of diabetes. HA production in response to a raised glucose environment in diabetes can contribute to mesangial hypercellularity [225]. HA increases vascular smooth muscle cell expression of PAI-1, altering the balance between proteolysis and its inhibition in vessels of patients with type 2 diabetes, thereby contributing to the acceleration of macroangiopathy [226].
In addition, HA binding proteins were also increased in patients with diabetes and in experimental diabetic animal models. Expression-based genome-wide association study (GWAS) links the receptor CD44 in adipose tissue with type 2 diabetes [227]. Injection of anti-CD44 monoclonal antibody or administration of hyaluronidase induced appreciable resistance to insulin-dependent diabetes mellitus in mice [228], suggesting that the CD44-HA interaction is involved in the development of the disease.
3.9. Kidney diseases
Spatially restricted HA production by Has2 drives epithelial tubulogenesis suggesting a role for HA in kidney development [229]. In disease state, marked accumulation of HA was demonstrated in developing and sclerosing crescents, in association with local infiltration of T cells and monocyte/macrophages in the rats [230].
Serum HA was increased in lupus nephritis patients [231] as well as dermatomyositis patients [232]. Serum HA levels were significantly increased in patients with renal insufficiency and with end-stage renal failure, and were correlated well with the degree of impaired renal function [233]. Patients with deteriorated clinical conditions often had greater HA levels, suggesting that HA may be a biochemical marker of patients in these conditions [234], and also a risk predictor of poor survival in dialysis [235]. Fibroblasts derived from active lesions of nephrogenic fibrosing dermopathy synthesize elevated levels of HA when compared with normal controls [236].
CD44 is dramatically up-regulated in a rat model of crescentic anti-glomerular basement membrane disease, suggesting a role for the CD44-HA interaction in leukocyte recruitment and renal fibrosis [237]. Administration of anti-CD44 to mice reduced the influx of neutrophils into the postischemic tissue with associated renal function preservation [132]. Furthermore, sulfated HA as a selectin-blocking agent inhibits intraglomerular infiltration of macrophages and prevents the progression of experimental crescentic glomerulonephritis [238].
3.10. Intestinal inflammation
During homeostasis, endogenous HA regulates normal intestinal and colonic growth [115]. In mice, treated with exogenous HA at age 3 to 8 weeks increased intestinal and colonic epithelial proliferation, resulting in hyperplasia [115]. Pep-1 treatment markedly decreased intestinal and colonic length, crypt depth and villus height in the intestine, crypt depth in the colon, and epithelial proliferation in the intestine and colon [115]. It is not known if the effect was dependent on the receptor CD44 or TLR4.
It is believed that inflammatory bowel disease (IBD) such as Crohn’s disease and ulcerative colitis results from exaggerated activation of the mucosal immune system by the flora found within the intestinal lumen [239]. Cytokines, especially Th1 cytokines, have been suggested to have a key role in intestinal inflammation [239]. HA accumulates in the colon in the nonvascular space of patients with chronic intestinal inflammation, such as Crohn’s disease and ulcerative colitis [240]. HA deposition within blood vessels of the colon was observed well before any significant inflammatory infiltration and likely promoted leukocyte infiltration in the dextran sulfate sodium (DSS)-induced experimental mouse model of colitis [241]. Indeed, mononuclear leukocytes bind to HA on human intestinal mucosal smooth muscle cells via CD44 after virus infection or treatment with poly(I:C) [242], indicating a role of HA in leukocyte retention and recruitment during intestinal inflammation. STAT1 plays a role in HA-mediated leukocyte adhesion, since both tissue damage and HA deposition were attenuated in STAT1-null mice compared with that in wild-type control mice in the DSS-induced colitis mouse model [243]. In line with the deteriorative role of HA in intestinal injury, Has3 null mice showed significant reduction in HA deposition in the colon tissue and thus protected from DSS-induced colitis [244]. The role of HA in intestinal inflammation may function through its receptor CD44. In a TNF-driven mouse model of chronic ileitis, soluble HA levels and expression of Has1 were increased coincided with increased expression of CD44 on CD4+ T cells, and deficiency in CD44 resulted in attenuated the severity of ileitis of the mice [245].
Interestingly, reports revealed that platelet-derived HYAL2 is able to cleave HA into fragments that stimulate mononuclear leukocytes in the immediate microenvironment to produce proinflammatory cytokines [41]. However, a recent study showed that patients with IBD had lower platelet HYAL2 levels and activity than healthy controls [246]. Platelet activation caused the translocation of HYAL2 from α-granules to platelet surfaces where it exerts its catalytic activity [246].
In addition, HA interacts with TLR4 to protect intestinal epithelium. Endogenous HA expression was markedly increased in DSS-induced colitis and preserved the epithelium through TLR activation [247]. Furthermore, intraperitoneal administration of HA was able to reduce DSS-induced colitis [247] or radiation-induced apoptosis and increased crypt survival [248]. The roles that HA plays in regulating intestine inflammatory response and in protection of intestinal epithelium through different HA binding proteins and different signaling pathways. Moreover, HA may function differently at different cell compartments. Further studies are needed to dissect the mechanism that HA promote inflammation and protect intestine epithelium. Targeting HA may present a potential strategy in treatment of inflammatory intestinal diseases.
3.11. Neoplasia and metastasis
A body of work has demonstrated a role of HA, HA synthases, hyaluronidases, and HA binding proteins in cancer initiation and progression, metastasis, cancer therapeutics and chemoresistance. There are many excellent reviews on the topic [3, 15–18, 249]. An example of the role of HA in cancer can be seen in a recent report on naked mole rats. Naked mole-rat fibroblasts make extremely HMW HA (6–12 MDa) that accumulates abundantly in naked mole-rat tissues, conferring cancer resistance [250]. Absence of the CD44 gene prevents sarcoma metastasis [251]. The de-repression of CD44 resulting from inactivation of p53 can potentially aid the survival of immortalized, premalignant cells [252]. Therefore, therapeutics targeting HA and its binding proteins are in the development. For example, a humanized monoclonal antibody specific for CD44 is to target chronic lymphocytic leukemia [253].
4. HA as therapeutic
4.1. HA species as drugs
Native HA products such as Healon (Abbott) and Hylan GF-20 (Genzyme) were approved for eye surgery and osteoarthritis, respectively. In other areas, HA treatment showed a dramatic decrease of oral antibiotic need in a cystic fibrosis patient with asthma-like symptoms [254]. A multicenter, randomized, controlled clinical trial in Italy showed the addition of HA to hypertonic saline reduces the prevalence and severity of cough, throat irritation, and saltiness in the chronic treatment of cystic fibrosis patients [255]. Intravesical HA therapy shows long-term (4.9 years) efficacy, with a high rate of acute symptom remission, in bladder pain syndrome/interstitial cystitis [256].
Preclinically, HMW HA inhibited smoke inhalation-induced lung injury and improved survival [143] and sepsis-induced lung injury with mechanical ventilation in rats [257]. HMW (1,600 kDa) HA, not LMW (35 kDa) HA blocked both monocyte and neutrophil infiltration and decreased the lung injury [257]. Aerosolized HA limited airspace enlargement in a mouse model of cigarette-induced emphysema [196]. However, the proposed therapeutic use of HA will require the development of treatment strategies aimed at minimizing its proinflammatory activity [198].
LMW HA has been shown to prolong renal and cardiac allograft survival in experimental animals [202, 203]. Furthermore, hyaluronidase treatment reduced HA contents of the graft and ameliorated rejection-induced edema in a heterotopic heart transplantation model in rats [204]. These studies suggest that LMW HA may present a nontoxic and non-immunogenic immunotherapy.
Given the role of HA and HA binding proteins in cancer, either HA oligomers or soluble hyaluronan-binding proteins may antagonize HA-HABP interactions for cancer therapy [3]. HA oligosaccharides (6–18 disaccharides), but not large polymers, can kill many types of cancer cells by triggering apoptosis while leaving normal cells unaffected [258]. Moreover, when co-treated with HA oligosaccharides, chemoresistant cancer cells become drug-sensitive [258].
Chemically modified HA preparations have been investigated in their role in inflammatory responses. Selectively N-butyrylated LMW HA showed an anti-inflammatory effect in human macrophages [91]. Sulfated HA as a selectin-blocking agent inhibited intraglomerular infiltration of macrophages and prevented the progression of mesangial proliferative glomerulonephritis [259], as well as crescentic glomerulonephritis in rats [238]. In an animal model, sulfated HA as a hyaluronidase inhibitor was showed to block the proliferation, motility, and invasion of prostate cancer cells, acting as a potent inhibitor of prostate cancer [92]. Modifications of HA with sulfate groups influence the interaction with recombinant human BMP-4 [260]. The high-sulfated HA preparations exhibited the tightest interaction with BMP-4 [260] and with TGF-β1 [261]. The modified HA preparations may have a broad use as therapeutics and as drug carriers (Figure 4).
Figure 4. Targeting HA biology for human diseases.

Unmodified or modified HA species and hyaluronidase have a role in inflammation and cancer (in green), while blocking HA system can be used to develop therapeutics for human diseases (in blue).
4.2. HA as therapeutic carrier
HA has been investigated as a drug delivery agent for ophthalmic, nasal, pulmonary, parenteral, and dermal routes, as shown in a review [262]. Conjugation of chemodrugs to HA has been shown to have a beneficial effect. HA conjugation significantly increased drug uptake and extended the residence time of drugs in tumor cells in mice [263]. For example, conjugation of irinotecan to HA significantly improved the profile of in vivo tolerability for ovarian cancer [264]. Conjugation of paclitaxel improved therapeutic efficacy for peritoneal tumor carcinomatosis [265] and ovarian cancer [266]. An intralymphatic drug delivery method using an HA-cisplatin conjugate was proposed in breast cancer to preferentially treat at-risk regional lymph nodes and avoid systemic toxicities [267]. Furthermore, HA-based nanocarriers were investigated as drug vehicles for systemic targeting of CD44-overexpressed cancers [268, 269]. HA-coated superparamagnetic iron oxide nanoparticles conjugated anticancer drug doxorubicin was delivered at much higher levels and distributed wider in the tumor tissue than intravenously injected free doxorubicin, leading to significant reduction of tumor growth [270]. HA-Irinotecan was tested in a phase II trial to determine progression-free survival in 5-fluorouracil refractory patients with metastatic colorectal cancer [271].
In addition to cancer therapy, HA conjugation has been investigated in other fields. HA-TRAIL (tumor necrosis factor-related apoptosis-inducing ligang) conjugate was reported for targeted treatment of liver fibrosis in rats [272]. An HA-insulin complex was developed to test whether it could be used to treat diabetic patients by oral administration. Glucose-lowering activity was demonstrated after oral administration of the HA-insulin complex to diabetic rats [273]. The HA-insulin complex was active after oral administration and the complexed insulin significantly decreased blood glucose concentrations within 1 hour after oral administration in rats [274].
4.3. Targeting HA and HABPs
4.3.1. 4-methylumbelliferone
4-methylumbelliferone (4-MU, aka hymecromone) was showed to reduce the HA production of fibroblasts markedly [275], by depletion of cellular UDP-glucuronic acid and down-regulation of HAS2 and HAS3 [276]. 4-MU treatment has viewed as a potential therapeutic strategy in inflammation, autoimmunity, and cancer [277]. Unrelated its role in HA inhibition, 4-MU is used in clinic for bile duct motor disorders [278].
Several studies with inhibition of HA synthesis by treatment with 4-MU showed a beneficial effect in various tissue injury and inflammation. 4-MU suppresses staphylococcal enterotoxin B- [279] and LPS-induced lung inflammation [280]. 4-MU treatment in vivo protected against central nervous system autoimmunity and increased CXCL12 expression in the inflamed nervous system [281]. 4-MU was protective against renal ischaemia-reperfusion injury, and significantly attenuated inflammation [282]. Treatment with 4-MU dramatically decreased the severity of ollagen-induced arthritis in mice [283]. Therefore, development of therapeutics of 4-MU and its analogs can be used for these inflammatory conditions.
In addition, 4-MU inhibited hepatocellular carcinoma growth by decreasing IL-6 production and angiogenesis [284]. Tumors systemically treated with 4-MU showed the extensive areas of necrosis, inflammatory infiltrate and 2–3-fold reduced number of tumor satellites [285], suggesting inhibition of HA synthesis can be a means to treat certain cancers.
4-MU belongs to the family coumarin derivatives and is structurally simple, so that the inhibition of HA synthases may not be very specific. Therefore, modification of 4-MU or designing new class of inhibitors may provide better ways to treat conditions such as cancer and tissue remodelling.
4.3.2. Hyaluronidase and inhibitors
Hyaluronidase is commonly used as an adjuvant to local anesthesia in ophthalmic surgery [286]. Early studies have suggested hyaluronidase may reduce acute myocardial infarction in animals and in humans [287, 288]. However, a large trial did not find a beneficial effect of hyaluronidase on mortality or infarct size in patients with acute myocardial infarction [289]. Recently, immobilized hyaluronidases have been investigated. Pegylated hyaluronidase reduced bleomycin-induced lung fibrosis in mice [178]. Hyaluronidase-loaded poly(D,L-lactide-co-glycolide) (PLGA) microparticles reduced neutrophil recruitment and collagen deposition after bleomycin treatment in mice [177].
Hyaluronidase inhibitors have also been investigated and suggested having roles in treatment of human diseases [290]. Glycyrrhizin from liquorice root acts as a potent inhibitor of hyaluronidase in vitro and has anti-inflammatory effect [291]. Hyaluromycin isolated from sea squirt Streptomyces sp. was even more potent to inhibit hyaluronidase activity than glycyrrhizin [292]. Glycyrrhizin has been used to treat patients with chronic hepatitis C [293] as well as upper respiratory tract infections [294]. Inhibition of hyaluronidase activity with 6-O-Palmitoyl-L-ascorbic acid (VCPAL) promoted remyelination which may benefit patients with multiple sclerosis [43].
Many studies suggested an oncogenic role of hyaluronidases in many cancers. Therefore, various strategies with inhibition of hyaluronidases have been investigated. Sulfated HA as a hyaluronidase inhibitor was shown to block proliferation, motility, and invasion of prostate cancer cells [92]. On the other hand, digestion of HA by hyaluronidases may remodel the tumor environment and favor chemotherapeutics to access tumor cells. Combination of PEGylated human recombinant PH20 hyaluronidase (PEGPH20) with gemcitabine showed that PEGPH20 rapidly and sustainably depleted HA, and improved drug delivery in a mouse model of pancreatic cancer [295, 296].
4.3.3. Targeting HABPs and HA-HABP interactions
Using phage display, a 12-mer (GAHWQFNALTVR) peptide inhibitor, Pep-1, was identified to inhibit HA binding in vitro [297]. Systemic, local, or topical administration of Pep-1 was able to block skin-directed homing of inflammatory leukocytes and inhibited the expression of contact hypersensitivity responses in mice [297, 298]. Pep-1 efficiently inhibited dendritic cell-dependent cluster formation, IL-2 and IFN-γ production, and T cell proliferation [299]. Moreover, Pep-1 ameliorated naphthalene-induced lymphocytic bronchitis in a mouse model [300].
Single injection of Pep-1 significantly reduced the incidence of lung metastasis of melanoma cells and prolonged the survival of the tumor-bearing animals [301]. Furthermore, Pep-1 treatment maintained the effectiveness of IL-2 and prevented the metastasis of melanoma [302].
A 15-mer peptide with homology to HA binding sequences of HMMR blocks HA signaling and reduces inflammation and fibrogenesis in excisional skin wounds [303]. The peptide was also able to prevent nuclear accumulation of HA [158] and to inhibit prostate tumor cell migration toward to fragmented HA [304].
Some new peptide inhibitors such as A-6 peptide [305] and P6 peptide [306] have recently been reported. A6 is a capped eight amino acid peptide (Acetyl-KPSSPPEE-NH2) derived from human urokinase plasminogen activator (uPA). A6 binds to CD44 resulting in the inhibition of migration, invasion, and metastasis of tumor cells, and the modulation of CD44-mediated cell signaling [307]. P6 peptide containing FDAIAEIGNQLYLFKDGKYW sequence from human MMP-9 hemopexin domain specifically interacts with CD44, possibly interfering MMP9-CD44 interaction in chronic lymphocytic leukemia cells [306]. CD44 has been shown to provide a cell surface docking receptor for proteolytically active MMP-9, and localization of MMP-9 to cell surface is required for its ability to promote angiogenesis and tumor invasion [156].
Blocking CD44 with neutralizing antibodies has been investigated in many fields. Anti-CD44 monoclonal antibodies induced differentiation and apoptosis of human myeloid leukemia cells [308, 309]. Anti-CD44 monoclonal antibodies inhibited human melanoma growth and metastasis in mice in vivo [310], and disrupted intracerebral progression of rat glioblastoma in rats in vivo [311]. Blocking CD44v6 with humanized anti-CD44v6 monoclonal antibody U36 has been tested in patients with head and neck cancer [51].
It has been suggested that HA-binding proteins play a role in arthritis. CD44 is up-regulated on many synovial cell types in patients with rheumatoid arthritis, and the level of CD44 present in synovial tissue is correlated with the degree of synovial inflammation [312]. An anti-CD44 antibody abrogates tissue swelling and leukocyte infiltration in mice [313].
Consistent with the role of CD44 in mediating fibroblast invasion and subsequent tissue fibrosis, immunohistochemical analysis of lung tissue from patients who died from acute alveolar fibrosis after lung injury reveals CD44-expressing mesenchymal cells throughout newly formed fibrotic tissue [148]. Anti-CD44 antibody blocked fibroblast migration [148], and induced fibroblast apoptosis [314]. Study results from our lab showed that systemic administration of neutralizing CD44 antibody to mice reduced invasiveness of fibroblasts and attenuated lung fibrosis after bleomycin lung injury [159].
Given the role of HA and HABPs in allograft rejections, blocking CD44 with neutralizing antibodies has been investigated. Anti-CD44 monoclonal antibody down-modulated host reactivity in skin transplantation in rats [315], and inhibited heart transplant rejection in nude mice [316].
In order to move into clinic, fully human anti-CD44 antibodies are developed. A fully human IgG2 anti-CD44 neutralizing antibody (PF-03475952 developed by Pfizer) was reported for therapeutic applications in inflammatory diseases such as rheumatoid arthritis [317]. A humanized anti-CD44 neutralizing antibody (RG7356 developed by Roche) directly induced chronic lymphocytic leukemia cells to undergo caspase-dependent apoptosis [253]. Administration of RG7356 at 1 mg/kg to immune-deficient mice engrafted with human chronic lymphocytic leukemia cells resulted in complete clearance of engrafted leukemia cells [253]. These studies suggested that these humanized antibodies have therapeutic activities in patients with inflammatory diseases and cancer.
5. Conclusion and outlook
HA, HA synthases and degradating enzymes, and HABPs play an important role in many biological and pathological processes. The function of hyaluronan depends on its size, location, and interactions with its binding patterns. Dysregulated HA activities involve in multiple human disease processes. Significant progress has been made in dissecting the role of HA in human diseases and in designing and investigating strategies of targeting HA system for human diseases. High molecular weight HA and chemically modified HA preparations, HA inhibitor, HA blocking peptides, and hyaluronidases have been tested as therapeutics on inflammation, fibrosis, and cancers. Especially the humanized antibodies have showed therapeutic activities in patients with inflammatory diseases and cancer. Therefore, understanding the roles of HA and underlying mechanisms in the normal and disease settings may provide a new way to develop therapeutics for these diseases.
Table 1.
Genetically modified mice of HA metabolic enzymes
| Gene | Genotype | Phenotype | References |
|---|---|---|---|
| Has1 | Has1−/− | Viable, no obvious abnormity | [31] |
| Has2 | Has2−/− | Heart defect leading to embyonic lethality | [33] |
| Col1α2-Cre;Has2flox/flox | Embryonic lethal | [159] | |
| FSP-1–Cre+;Has2flox/flox | Inhibited lung fibrosis in bleomycin model | [159] | |
| ASMA-HAS2+ | Promoted atherosclerosis | [217] | |
| ASMA-HAS2+ | Accumulated HA, increased lung fibrosis and mortality after bleomycin | [159] | |
| Has3 | Has3−/− | Viable, no obvious abnormity, normal lifespan | [12] |
| Reduction in brain extracellular space leading to seizures | [35] | ||
| Has1, Has3 | Has1−/−;Has3−/− | Viable, no obvious abnormity | [32] |
| Enhanced wound repair | [32] | ||
| Has1, Has2, Has3 | Prx1-Cre;Has2flox/flox; Has1−/−;Has3−/− | Decreased hematopoietic activity in long term bone marrow cultures | [114] |
| Hyal1 | Hyal1−/− | Viable, fertile and show no gross abnormalities | [37] |
| Accumulation of cytoplasmic HA, and osteoarthritis | [37] | ||
| Krt14-CreERT;Hyal1tg | Decreased dendritic cells and suppressed contact hypersensitivity | [38] | |
| Hyal2 | Hyal2−/− | An increase in plasma hyaluronidase activity and plasma HA | [39, 40] |
| Severe cardiopulmonary dysfunction | [40] | ||
| Hyal3 | Hyal3−/− | A subtle change in the alveolar structure and extracellular matrix thickness in lung-tissues at 12–14 months-of-age | [42] |
| No evidence of HA accumulation | [42] |
Table 2.
Genetically modified mice of major HA-binding proteins
| Gene | Genotype | Phenotype | References |
|---|---|---|---|
| CD44 | Cd44−/− | No obvious developmental defects; altered tissue distribution of myeloid progenitors; highly tumorigenic of CD44-deficient fibroblasts | [47] |
| Exaggerated lung injury; failed to clear HA | [48] | ||
| Enhanced hepatitis | [318] | ||
| Reduced atherosclerosis | [219] | ||
| Increased resistance to collagen-induced arthritis | [319] | ||
| Reduction in ischemic infarct | [320] | ||
| Enhanced lung injury in E. coli pneumonia | [145] | ||
| Exaggerated acute pulmonary inflammation | [96] | ||
| Ameliorated insulin resistance and adipose tissue inflammation | [227] | ||
| HMMR (RHAMM) | Hmmr−/− | Viable, normal lifespan; fewer litters | [56] |
| Attenuated the formation of aggressive fibromatosis | [56] | ||
| Defective skin wound repair | [57] | ||
| Aberrant mitotic spindles and increased multinucleated cells | [321] | ||
| Decreased macrophage chemotaxis | [322] | ||
| Prevented constrictive artery wall remodeling | [323] | ||
| Reduced LMW HA-mediated fibrosarcoma cell adhesion | [324] | ||
| Versican | hdf | Defect in right cardiac chamber and endocardial cushion formation leading to lethality | [325] |
| Defect in limb chondrogenesis | [326] | ||
| Versican V2 | VcanV2−/− | Viable and fertile, have a normal life span; defect in extracellular matrix assembly in the central nervous system | [327] |
| Prx1-Cre;Vcanflox/flox | Viable and fertile; distorted digits | [328] | |
| VcanV2−/− | Viable and fertile, have a normal life span; defect in extracellular matrix assembly in the central nervous system | [327] | |
| Aggrecan | Cloe | Develop normally with no skeletal deformities | [329] |
| Jaffa | Protected against cartilage erosion and may potentiate cartilage repair. | [330] | |
| HARE (Stab2) | Stab2−/− | No obvious physical or behavioral abnormalities | [331] |
| Stab2−/− | The metastasis of B16F10 melanoma cells to the lungs was markedly suppressed | [70] | |
| Stab1−/−Stab2−/− | Developed severe glomerular fibrosis and mild perisinusoidal hepatic fibrosis | [331] | |
| LYVE1 | Lyve1−/− | Normal; increased interstitial-lymphatic flow and lack of typical irregularly-shaped lumens | [332] |
| Lyve1-Cre;iDTR | Lymphatic vessel defect in the intestine and lymph nodes. | [73] | |
| Tnfip6 (TSG-6) | Tnfip6−/− | Impaired cumulus mucification and female sterility | [333] |
| Enhanced neutrophil extravasation and rapid progression of proteoglycan-induced arthritis | [78] | ||
| Severe arthritis | [334] | ||
| Attenuated inflammation in asthma | [335] |
Notes:
hdf mouse line is a transgenic mouse with official name VcanTg(Hoxa1)1. The transgene consisted of 3.5kb of the Hoxa1 regulatory region gene with a lacZ reporter sequence. The transgene was inserted into intron 7 of Vcan gene on chromosome 13 and disrupted the two chondroitin sulfate binding domains of VCAN. The insertion of the transgene caused a recessive lethal mutation, displaying cardiac defect in homozygous mice.
Cloe is an aggrecan knock-in mouse line, in which MMP cleavage site of ACAN was mutated, leading to resistant to proteolysis by MMPs.
Jaffa is an aggrecan knockin mouse line, in which aggrecanase cleavage site of ACAN was mutated, leading to resistant to proteolysis by aggrecanases.
Acknowledgments
The authors would like to acknowledge the funding support from the National Institutes of Health (R01-HL 060539, R01-AI052201, R01 HL122068, and P01 HL108793), and support from California Institute for Regenerative Medicine (CIRM, RB5-07302).
Abbreviations
- HA
hyaluronan or hyaluronic acid
- HAS
hyaluronan synthase
- HYAL
hyaluronidase
- HMW
high molecule weight
- LMW
low molecule weight
- HMMR
hyaluronan-mediated motility receptor
- HARE
hyaluronan receptor for endocytosis
- LYVE1
lymphatic vessel endothelial hyaluronan receptor 1
- TNFIP6
tumor necrosis factor α-induced protein 6
- CEMIP
cell-migration induced protein, hyaluronan binding
- IPF
idiopathic pulmonary fibrosis
- COPD
chronic obstructive pulmonary disease
- BOS
bronchiolitis obliterans syndrome
- 4-MU
4-methylumbelliferone
- IBD
inflammatory bowl disease
- DSS
dextran sulfate sodium
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Chemical compounds studied in this article
Sodium hyaluronate (PubChem CID: 3084049)
Hyaluronic Acid (PubChem CID: 453618)
4-methylumbelliferone (PubChem CID: 5280567)
Glycyrrhizin (PubChem CID: 3495)
References
- 1.Lee JY, Spicer AP. Hyaluronan: a multifunctional, megaDalton, stealth molecule. Curr Opin Cell Biol. 2000;12:581–586. doi: 10.1016/s0955-0674(00)00135-6. [DOI] [PubMed] [Google Scholar]
- 2.Toole BP. Hyaluronan is not just a goo! J Clin Invest. 2000;106:335–336. doi: 10.1172/JCI10706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 4.Slevin M, Krupinski J, Gaffney J, Matou S, West D, Delisser H, Savani RC, Kumar S. Hyaluronan-mediated angiogenesis in vascular disease: uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007;26:58–68. doi: 10.1016/j.matbio.2006.08.261. [DOI] [PubMed] [Google Scholar]
- 5.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91:221–264. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 7.Jameson JM, Cauvi G, Sharp LL, Witherden DA, Havran WL. {gamma}{delta} T cell-induced hyaluronan production by epithelial cells regulates inflammation. J Exp Med. 2005;201:1269–1279. doi: 10.1084/jem.20042057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck-Schimmer B, Oertli B, Pasch T, Wuthrich RP. Hyaluronan induces monocyte chemoattractant protein-1 expression in renal tubular epithelial cells. J Am Soc Nephrol. 1998;9:2283–2290. doi: 10.1681/ASN.V9122283. [DOI] [PubMed] [Google Scholar]
- 9.Zoltan-Jones A, Huang L, Ghatak S, Toole BP. Elevated hyaluronan production induces mesenchymal and transformed properties in epithelial cells. J Biol Chem. 2003;278:45801–45810. doi: 10.1074/jbc.M308168200. [DOI] [PubMed] [Google Scholar]
- 10.Jiang D, Liang J, Li Y, Noble PW. The role of Toll-like receptors in noninfectious lung injury. Cell Res. 2006;16:693–701. doi: 10.1038/sj.cr.7310085. [DOI] [PubMed] [Google Scholar]
- 11.Itano N, Atsumi F, Sawai T, Yamada Y, Miyaishi O, Senga T, Hamaguchi M, Kimata K. Abnormal accumulation of hyaluronan matrix diminishes contact inhibition of cell growth and promotes cell migration. Proc Natl Acad Sci U S A. 2002;99:3609–3614. doi: 10.1073/pnas.052026799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai KJ, Spicer AP, Mascarenhas MM, Yu L, Ochoa CD, Garg HG, Quinn DA. The role of hyaluronan synthase 3 in ventilator-induced lung injury. Am J Respir Crit Care Med. 2005;172:92–98. doi: 10.1164/rccm.200405-652OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soltes L, Mendichi R, Kogan G, Schiller J, Stankovska M, Arnhold J. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules. 2006;7:659–668. doi: 10.1021/bm050867v. [DOI] [PubMed] [Google Scholar]
- 14.Stern R, Kogan G, Jedrzejas MJ, Soltes L. The many ways to cleave hyaluronan. Biotechnol Adv. 2007;25:537–557. doi: 10.1016/j.biotechadv.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Heldin P, Basu K, Kozlova I, Porsch H. HAS2 and CD44 in breast tumorigenesis. Adv Cancer Res. 2014;123:211–229. doi: 10.1016/B978-0-12-800092-2.00008-3. [DOI] [PubMed] [Google Scholar]
- 16.Schwertfeger KL, Cowman MK, Telmer PG, Turley EA, McCarthy JB. Hyaluronan Inflammation, and Breast Cancer Progression. Frontiers in immunology. 2015;6:236. doi: 10.3389/fimmu.2015.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lokeshwar VB, Mirza S, Jordan A. Targeting hyaluronic acid family for cancer chemoprevention and therapy. Adv Cancer Res. 2014;123:35–65. doi: 10.1016/B978-0-12-800092-2.00002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seton-Rogers S. Metastasis: multitasking hyaluronic acid. Nat Rev Cancer. 2012;12:228. doi: 10.1038/nrc3254. [DOI] [PubMed] [Google Scholar]
- 19.Knudson CB, Knudson W. Hyaluronan and CD44: modulators of chondrocyte metabolism. Clin Orthop Relat Res. 2004:S152–162. [PubMed] [Google Scholar]
- 20.Turino GM, Cantor JO. Hyaluronan in respiratory injury and repair. Am J Respir Crit Care Med. 2003;167:1169–1175. doi: 10.1164/rccm.200205-449PP. [DOI] [PubMed] [Google Scholar]
- 21.Noble PW. Hyaluronan and its catabolic products in tissue injury and repair. Matrix Biol. 2002;21:25–29. doi: 10.1016/s0945-053x(01)00184-6. [DOI] [PubMed] [Google Scholar]
- 22.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–461. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 23.Aya KL, Stern R. Hyaluronan in wound healing: rediscovering a major player. Wound Repair Regen. 2014;22:579–593. doi: 10.1111/wrr.12214. [DOI] [PubMed] [Google Scholar]
- 24.Moretto P, Karousou E, Viola M, Caon I, D’Angelo ML, De Luca G, Passi A, Vigetti D. Regulation of hyaluronan synthesis in vascular diseases and diabetes. J Diabetes Res. 2015;2015:167283. doi: 10.1155/2015/167283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald B, Kubes P. Interactions between CD44 and Hyaluronan in Leukocyte Trafficking. Frontiers in immunology. 2015;6:68. doi: 10.3389/fimmu.2015.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Termeer C, Sleeman JP, Simon JC. Hyaluronan–magic glue for the regulation of the immune response? Trends Immunol. 2003;24:112–114. doi: 10.1016/s1471-4906(03)00029-2. [DOI] [PubMed] [Google Scholar]
- 27.Jackson DG. Immunological functions of hyaluronan and its receptors in the lymphatics. Immunol Rev. 2009;230:216–231. doi: 10.1111/j.1600-065X.2009.00803.x. [DOI] [PubMed] [Google Scholar]
- 28.McDonald JA, Camenisch TD. Hyaluronan: genetic insights into the complex biology of a simple polysaccharide. Glycoconj J. 2002;19:331–339. doi: 10.1023/A:1025369004783. [DOI] [PubMed] [Google Scholar]
- 29.Itano N, Sawai T, Yoshida M, Lenas P, Yamada Y, Imagawa M, Shinomura T, Hamaguchi M, Yoshida Y, Ohnuki Y, Miyauchi S, Spicer AP, McDonald JA, Kimata K. Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J Biol Chem. 1999;274:25085–25092. doi: 10.1074/jbc.274.35.25085. [DOI] [PubMed] [Google Scholar]
- 30.Spicer AP, Tien JY. Hyaluronan and morphogenesis. Birth Defects Res C Embryo Today. 2004;72:89–108. doi: 10.1002/bdrc.20006. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi N, Miyoshi S, Mikami T, Koyama H, Kitazawa M, Takeoka M, Sano K, Amano J, Isogai Z, Niida S, Oguri K, Okayama M, McDonald JA, Kimata K, Taniguchi S, Itano N. Hyaluronan deficiency in tumor stroma impairs macrophage trafficking and tumor neovascularization. Cancer Res. 2010;70:7073–7083. doi: 10.1158/0008-5472.CAN-09-4687. [DOI] [PubMed] [Google Scholar]
- 32.Mack JA, Feldman RJ, Itano N, Kimata K, Lauer M, Hascall VC, Maytin EV. Enhanced inflammation and accelerated wound closure following tetraphorbol ester application or full-thickness wounding in mice lacking hyaluronan synthases Has1 and Has3. J Invest Dermatol. 2012;132:198–207. doi: 10.1038/jid.2011.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Lauer ME, Anand S, Mack JA, Maytin EV. Hyaluronan synthase 2 protects skin fibroblasts against apoptosis induced by environmental stress. J Biol Chem. 2014;289:32253–32265. doi: 10.1074/jbc.M114.578377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arranz AM, Perkins KL, Irie F, Lewis DP, Hrabe J, Xiao F, Itano N, Kimata K, Hrabetova S, Yamaguchi Y. Hyaluronan deficiency due to Has3 knockout causes altered neuronal activity and seizures via reduction in brain extracellular space. J Neurosci. 2014;34:6164–6176. doi: 10.1523/JNEUROSCI.3458-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur J Cell Biol. 2006;85:699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Martin DC, Atmuri V, Hemming RJ, Farley J, Mort JS, Byers S, Hombach-Klonisch S, Csoka AB, Stern R, Triggs-Raine BL. A mouse model of human mucopolysaccharidosis IX exhibits osteoarthritis. Hum Mol Genet. 2008;17:1904–1915. doi: 10.1093/hmg/ddn088. [DOI] [PubMed] [Google Scholar]
- 38.Muto J, Morioka Y, Yamasaki K, Kim M, Garcia A, Carlin AF, Varki A, Gallo RL. Hyaluronan digestion controls DC migration from the skin. J Clin Invest. 2014;124:1309–1319. doi: 10.1172/JCI67947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jadin L, Wu X, Ding H, Frost GI, Onclinx C, Triggs-Raine B, Flamion B. Skeletal and hematological anomalies in HYAL2-deficient mice: a second type of mucopolysaccharidosis IX? FASEB J. 2008;22:4316–4326. doi: 10.1096/fj.08-111997. [DOI] [PubMed] [Google Scholar]
- 40.Chowdhury B, Hemming R, Hombach-Klonisch S, Flamion B, Triggs-Raine B. Murine hyaluronidase 2 deficiency results in extracellular hyaluronan accumulation and severe cardiopulmonary dysfunction. J Biol Chem. 2013;288:520–528. doi: 10.1074/jbc.M112.393629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de la Motte C, Nigro J, Vasanji A, Rho H, Kessler S, Bandyopadhyay S, Danese S, Fiocchi C, Stern R. Platelet-derived hyaluronidase 2 cleaves hyaluronan into fragments that trigger monocyte-mediated production of proinflammatory cytokines. Am J Pathol. 2009;174:2254–2264. doi: 10.2353/ajpath.2009.080831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atmuri V, Martin DC, Hemming R, Gutsol A, Byers S, Sahebjam S, Thliveris JA, Mort JS, Carmona E, Anderson JE, Dakshinamurti S, Triggs-Raine B. Hyaluronidase 3 (HYAL3) knockout mice do not display evidence of hyaluronan accumulation. Matrix Biol. 2008;27:653–660. doi: 10.1016/j.matbio.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 43.Preston M, Gong X, Su W, Matsumoto SG, Banine F, Winkler C, Foster S, Xing R, Struve J, Dean J, Baggenstoss B, Weigel PH, Montine TJ, Back SA, Sherman LS. Digestion products of the PH20 hyaluronidase inhibit remyelination. Ann Neurol. 2013;73:266–280. doi: 10.1002/ana.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 45.Sherman L, Sleeman J, Herrlich P, Ponta H. Hyaluronate receptors: key players in growth, differentiation, migration and tumor progression. Curr Opin Cell Biol. 1994;6:726–733. doi: 10.1016/0955-0674(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 46.Lesley J, Hyman R, Kincade PW. CD44 and its interaction with extracellular matrix. Adv Immunol. 1993;54:271–335. doi: 10.1016/s0065-2776(08)60537-4. [DOI] [PubMed] [Google Scholar]
- 47.Schmits R, Filmus J, Gerwin N, Senaldi G, Kiefer F, Kundig T, Wakeham A, Shahinian A, Catzavelos C, Rak J, Furlonger C, Zakarian A, Simard JJ, Ohashi PS, Paige CJ, Gutierrez-Ramos JC, Mak TW. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood. 1997;90:2217–2233. [PubMed] [Google Scholar]
- 48.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science. 2002;296:155–158. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- 49.Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- 50.Arch R, Wirth K, Hofmann M, Ponta H, Matzku S, Herrlich P, Zoller M. Participation in normal immune responses of a metastasis-inducing splice variant of CD44. Science. 1992;257:682–685. doi: 10.1126/science.1496383. [DOI] [PubMed] [Google Scholar]
- 51.Borjesson PK, Postema EJ, Roos JC, Colnot DR, Marres HA, van Schie MH, Stehle G, de Bree R, Snow GB, Oyen WJ, van Dongen GA. Phase I therapy study with (186)Re-labeled humanized monoclonal antibody BIWA 4 (bivatuzumab) in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2003;9:3961S–3972S. [PubMed] [Google Scholar]
- 52.Hardwick C, Hoare K, Owens R, Hohn HP, Hook M, Moore D, Cripps V, Austen L, Nance DM, Turley EA. Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility. J Cell Biol. 1992;117:1343–1350. doi: 10.1083/jcb.117.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Savani RC, Cao G, Pooler PM, Zaman A, Zhou Z, DeLisser HM. Differential involvement of the hyaluronan (HA) receptors CD44 and receptor for HA-mediated motility in endothelial cell function and angiogenesis. J Biol Chem. 2001;276:36770–36778. doi: 10.1074/jbc.M102273200. [DOI] [PubMed] [Google Scholar]
- 54.Hall CL, Yang B, Yang X, Zhang S, Turley M, Samuel S, Lange LA, Wang C, Curpen GD, Savani RC, Greenberg AH, Turley EA. Overexpression of the hyaluronan receptor RHAMM is transforming and is also required for H-ras transformation. Cell. 1995;82:19–26. doi: 10.1016/0092-8674(95)90048-9. [DOI] [PubMed] [Google Scholar]
- 55.Zaman A, Cui Z, Foley JP, Zhao H, Grimm PC, Delisser HM, Savani RC. Expression and role of the hyaluronan receptor RHAMM in inflammation after bleomycin injury. Am J Respir Cell Mol Biol. 2005;33:447–454. doi: 10.1165/rcmb.2004-0333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tolg C, Poon R, Fodde R, Turley EA, Alman BA. Genetic deletion of receptor for hyaluronan-mediated motility (Rhamm) attenuates the formation of aggressive fibromatosis (desmoid tumor) Oncogene. 2003;22:6873–6882. doi: 10.1038/sj.onc.1206811. [DOI] [PubMed] [Google Scholar]
- 57.Tolg C, Hamilton SR, Nakrieko KA, Kooshesh F, Walton P, McCarthy JB, Bissell MJ, Turley EA. Rhamm−/− fibroblasts are defective in CD44-mediated ERK1,2 motogenic signaling, leading to defective skin wound repair. J Cell Biol. 2006;175:1017–1028. doi: 10.1083/jcb.200511027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamaguchi Y. Lecticans: organizers of the brain extracellular matrix. Cell Mol Life Sci. 2000;57:276–289. doi: 10.1007/PL00000690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jaworski DM, Kelly GM, Hockfield S. Intracranial injury acutely induces the expression of the secreted isoform of the CNS-specific hyaluronan-binding protein BEHAB/brevican. Exp Neurol. 1999;157:327–337. doi: 10.1006/exnr.1999.7062. [DOI] [PubMed] [Google Scholar]
- 60.Jaworski DM, Kelly GM, Piepmeier JM, Hockfield S. BEHAB (brain enriched hyaluronan binding) is expressed in surgical samples of glioma and in intracranial grafts of invasive glioma cell lines. Cancer Res. 1996;56:2293–2298. [PubMed] [Google Scholar]
- 61.Yamada H, Fredette B, Shitara K, Hagihara K, Miura R, Ranscht B, Stallcup WB, Yamaguchi Y. The brain chondroitin sulfate proteoglycan brevican associates with astrocytes ensheathing cerebellar glomeruli and inhibits neurite outgrowth from granule neurons. J Neurosci. 1997;17:7784–7795. doi: 10.1523/JNEUROSCI.17-20-07784.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Deepa SS, Carulli D, Galtrey C, Rhodes K, Fukuda J, Mikami T, Sugahara K, Fawcett JW. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J Biol Chem. 2006;281:17789–17800. doi: 10.1074/jbc.M600544200. [DOI] [PubMed] [Google Scholar]
- 63.LeBaron RG, Zimmermann DR, Ruoslahti E. Hyaluronate binding properties of versican. J Biol Chem. 1992;267:10003–10010. [PubMed] [Google Scholar]
- 64.Zimmermann DR, Ruoslahti E. Multiple domains of the large fibroblast proteoglycan, versican. EMBO J. 1989;8:2975–2981. doi: 10.1002/j.1460-2075.1989.tb08447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deb TB, Datta K. Molecular cloning of human fibroblast hyaluronic acid-binding protein confirms its identity with P-32, a protein co-purified with splicing factor SF2. Hyaluronic acid-binding protein as P-32 protein, co-purified with splicing factor SF2. J Biol Chem. 1996;271:2206–2212. doi: 10.1074/jbc.271.4.2206. [DOI] [PubMed] [Google Scholar]
- 66.Yagi M, Uchiumi T, Takazaki S, Okuno B, Nomura M, Yoshida S, Kanki T, Kang D. p32/gC1qR is indispensable for fetal development and mitochondrial translation: importance of its RNA-binding ability. Nucleic Acids Res. 2012;40:9717–9737. doi: 10.1093/nar/gks774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou B, Oka JA, Singh A, Weigel PH. Purification and subunit characterization of the rat liver endocytic hyaluronan receptor. J Biol Chem. 1999;274:33831–33834. doi: 10.1074/jbc.274.48.33831. [DOI] [PubMed] [Google Scholar]
- 68.Harris EN, Kyosseva SV, Weigel JA, Weigel PH. Expression, processing, and glycosaminoglycan binding activity of the recombinant human 315-kDa hyaluronic acid receptor for endocytosis (HARE) J Biol Chem. 2007;282:2785–2797. doi: 10.1074/jbc.M607787200. [DOI] [PubMed] [Google Scholar]
- 69.Harris EN, Weigel JA, Weigel PH. The human hyaluronan receptor for endocytosis (HARE/Stabilin-2) is a systemic clearance receptor for heparin. J Biol Chem. 2008;283:17341–17350. doi: 10.1074/jbc.M710360200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirose Y, Saijou E, Sugano Y, Takeshita F, Nishimura S, Nonaka H, Chen YR, Sekine K, Kido T, Nakamura T, Kato S, Kanke T, Nakamura K, Nagai R, Ochiya T, Miyajima A. Inhibition of Stabilin-2 elevates circulating hyaluronic acid levels and prevents tumor metastasis. Proc Natl Acad Sci U S A. 2012;109:4263–4268. doi: 10.1073/pnas.1117560109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Prevo R, Banerji S, Ferguson DJ, Clasper S, Jackson DG. Mouse LYVE-1 is an endocytic receptor for hyaluronan in lymphatic endothelium. J Biol Chem. 2001;276:19420–19430. doi: 10.1074/jbc.M011004200. [DOI] [PubMed] [Google Scholar]
- 73.Jang JY, Koh YJ, Lee SH, Lee J, Kim KH, Kim D, Koh GY, Yoo OJ. Conditional ablation of LYVE-1+ cells unveils defensive roles of lymphatic vessels in intestine and lymph nodes. Blood. 2013;122:2151–2161. doi: 10.1182/blood-2013-01-478941. [DOI] [PubMed] [Google Scholar]
- 74.Huang L, Yoneda M, Kimata K. A serum-derived hyaluronan-associated protein (SHAP) is the heavy chain of the inter alpha-trypsin inhibitor. J Biol Chem. 1993;268:26725–26730. [PubMed] [Google Scholar]
- 75.Lee TH, Wisniewski HG, Vilcek J. A novel secretory tumor necrosis factor-inducible protein (TSG-6) is a member of the family of hyaluronate binding proteins, closely related to the adhesion receptor CD44. J Cell Biol. 1992;116:545–557. doi: 10.1083/jcb.116.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Milner CM, Day AJ. TSG-6: a multifunctional protein associated with inflammation. J Cell Sci. 2003;116:1863–1873. doi: 10.1242/jcs.00407. [DOI] [PubMed] [Google Scholar]
- 77.Lauer ME, Cheng G, Swaidani S, Aronica MA, Weigel PH, Hascall VC. Tumor necrosis factor-stimulated gene-6 (TSG-6) amplifies hyaluronan synthesis by airway smooth muscle cells. J Biol Chem. 2013;288:423–431. doi: 10.1074/jbc.M112.389882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Szanto S, Bardos T, Gal I, Glant TT, Mikecz K. Enhanced neutrophil extravasation and rapid progression of proteoglycan-induced arthritis in TSG-6-knockout mice. Arthritis Rheum. 2004;50:3012–3022. doi: 10.1002/art.20655. [DOI] [PubMed] [Google Scholar]
- 79.Yoshida H, Nagaoka A, Kusaka-Kikushima A, Tobiishi M, Kawabata K, Sayo T, Sakai S, Sugiyama Y, Enomoto H, Okada Y, Inoue S. KIAA1199, a deafness gene of unknown function, is a new hyaluronan binding protein involved in hyaluronan depolymerization. Proc Natl Acad Sci U S A. 2013;110:5612–5617. doi: 10.1073/pnas.1215432110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Evensen NA, Kuscu C, Nguyen HL, Zarrabi K, Dufour A, Kadam P, Hu YJ, Pulkoski-Gross A, Bahou WF, Zucker S, Cao J. Unraveling the role of KIAA1199, a novel endoplasmic reticulum protein, in cancer cell migration. J Natl Cancer Inst. 2013;105:1402–1416. doi: 10.1093/jnci/djt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shostak K, Zhang X, Hubert P, Goktuna SI, Jiang Z, Klevernic I, Hildebrand J, Roncarati P, Hennuy B, Ladang A, Somja J, Gothot A, Close P, Delvenne P, Chariot A. NF-kappaB-induced KIAA1199 promotes survival through EGFR signalling. Nat Commun. 2014;5:5232. doi: 10.1038/ncomms6232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228:1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- 83.Horton MR, McKee CM, Bao C, Liao F, Farber JM, Hodge-DuFour J, Pure E, Oliver BL, Wright TM, Noble PW. Hyaluronan fragments synergize with interferon-gamma to induce the C-X-C chemokines mig and interferon-inducible protein-10 in mouse macrophages. J Biol Chem. 1998;273:35088–35094. doi: 10.1074/jbc.273.52.35088. [DOI] [PubMed] [Google Scholar]
- 84.McKee CM, Lowenstein CJ, Horton MR, Wu J, Bao C, Chin BY, Choi AM, Noble PW. Hyaluronan fragments induce nitric-oxide synthase in murine macrophages through a nuclear factor kappaB-dependent mechanism. J Biol Chem. 1997;272:8013–8018. doi: 10.1074/jbc.272.12.8013. [DOI] [PubMed] [Google Scholar]
- 85.Noble PW, McKee CM, Cowman M, Shin HS. Hyaluronan fragments activate an NF-kappa B/I-kappa B alpha autoregulatory loop in murine macrophages. J Exp Med. 1996;183:2373–2378. doi: 10.1084/jem.183.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest. 1996;98:2403–2413. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Termeer CC, Hennies J, Voith U, Ahrens T, Weiss JM, Prehm P, Simon JC. Oligosaccharides of hyaluronan are potent activators of dendritic cells. J Immunol. 2000;165:1863–1870. doi: 10.4049/jimmunol.165.4.1863. [DOI] [PubMed] [Google Scholar]
- 88.Hynes WL, Walton SL. Hyaluronidases of Gram-positive bacteria. FEMS Microbiol Lett. 2000;183:201–207. doi: 10.1111/j.1574-6968.2000.tb08958.x. [DOI] [PubMed] [Google Scholar]
- 89.Agren UM, Tammi RH, Tammi MI. Reactive oxygen species contribute to epidermal hyaluronan catabolism in human skin organ culture. Free Radic Biol Med. 1997;23:996–1001. doi: 10.1016/s0891-5849(97)00098-1. [DOI] [PubMed] [Google Scholar]
- 90.Li M, Rosenfeld L, Vilar RE, Cowman MK. Degradation of hyaluronan by peroxynitrite. Arch Biochem Biophys. 1997;341:245–250. doi: 10.1006/abbi.1997.9970. [DOI] [PubMed] [Google Scholar]
- 91.Babasola O, Rees-Milton KJ, Bebe S, Wang J, Anastassiades TP. Chemically modified N-acylated hyaluronan fragments modulate proinflammatory cytokine production by stimulated human macrophages. J Biol Chem. 2014;289:24779–24791. doi: 10.1074/jbc.M113.515783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Benitez A, Yates TJ, Lopez LE, Cerwinka WH, Bakkar A, Lokeshwar VB. Targeting hyaluronidase for cancer therapy: antitumor activity of sulfated hyaluronic acid in prostate cancer cells. Cancer Res. 2011;71:4085–4095. doi: 10.1158/0008-5472.CAN-10-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bourguignon LY, Gilad E, Brightman A, Diedrich F, Singleton P. Hyaluronan-CD44 interaction with leukemia-associated RhoGEF and epidermal growth factor receptor promotes Rho/Ras co-activation, phospholipase C epsilon-Ca2+ signaling, and cytoskeleton modification in head and neck squamous cell carcinoma cells. J Biol Chem. 2006;281:14026–14040. doi: 10.1074/jbc.M507734200. [DOI] [PubMed] [Google Scholar]
- 94.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tesar BM, Jiang D, Liang J, Palmer SM, Noble PW, Goldstein DR. The role of hyaluronan degradation products as innate alloimmune agonists. Am J Transplant. 2006;6:2622–2635. doi: 10.1111/j.1600-6143.2006.01537.x. [DOI] [PubMed] [Google Scholar]
- 96.Liang J, Jiang D, Griffith J, Yu S, Fan J, Zhao X, Bucala R, Noble PW. CD44 is a negative regulator of acute pulmonary inflammation and lipopolysaccharide-TLR signaling in mouse macrophages. J Immunol. 2007;178:2469–2475. doi: 10.4049/jimmunol.178.4.2469. [DOI] [PubMed] [Google Scholar]
- 97.Muto J, Yamasaki K, Taylor KR, Gallo RL. Engagement of CD44 by hyaluronan suppresses TLR4 signaling and the septic response to LPS. Mol Immunol. 2009;47:449–456. doi: 10.1016/j.molimm.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Orian-Rousseau V. CD44 Acts as a Signaling Platform Controlling Tumor Progression and Metastasis. Frontiers in immunology. 2015;6:154. doi: 10.3389/fimmu.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Khaldoyanidi S, Moll J, Karakhanova S, Herrlich P, Ponta H. Hyaluronate-enhanced hematopoiesis: two different receptors trigger the release of interleukin-1beta and interleukin-6 from bone marrow macrophages. Blood. 1999;94:940–949. [PubMed] [Google Scholar]
- 100.Nilsson SK, Haylock DN, Johnston HM, Occhiodoro T, Brown TJ, Simmons PJ. Hyaluronan is synthesized by primitive hemopoietic cells, participates in their lodgment at the endosteum following transplantation, and is involved in the regulation of their proliferation and differentiation in vitro. Blood. 2003;101:856–862. doi: 10.1182/blood-2002-05-1344. [DOI] [PubMed] [Google Scholar]
- 101.Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, Kollet O, Hershkoviz R, Alon R, Hardan I, Ben-Hur H, Naor D, Nagler A, Lapidot T. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- 102.Zohar R, Sodek J, McCulloch CA. Characterization of stromal progenitor cells enriched by flow cytometry. Blood. 1997;90:3471–3481. [PubMed] [Google Scholar]
- 103.Calabro A, Oken MM, Hascall VC, Masellis AM. Characterization of hyaluronan synthase expression and hyaluronan synthesis in bone marrow mesenchymal progenitor cells: predominant expression of HAS1 mRNA and up-regulated hyaluronan synthesis in bone marrow cells derived from multiple myeloma patients. Blood. 2002;100:2578–2585. doi: 10.1182/blood-2002-01-0030. [DOI] [PubMed] [Google Scholar]
- 104.Zhu H, Mitsuhashi N, Klein A, Barsky LW, Weinberg K, Barr ML, Demetriou A, Wu GD. The role of the hyaluronan receptor CD44 in mesenchymal stem cell migration in the extracellular matrix. Stem Cells. 2006;24:928–935. doi: 10.1634/stemcells.2005-0186. [DOI] [PubMed] [Google Scholar]
- 105.Orkin RW, Toole BP. Hyaluronidase activity and hyaluronate content of the developing chick embryo heart. Dev Biol. 1978;66:308–320. doi: 10.1016/0012-1606(78)90240-3. [DOI] [PubMed] [Google Scholar]
- 106.Belsky E, Toole BP. Hyaluronate and hyaluronidase in the developing chick embryo kidney. Cell Differ. 1983;12:61–66. doi: 10.1016/0045-6039(83)90056-8. [DOI] [PubMed] [Google Scholar]
- 107.Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat Med. 2002;8:850–855. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- 108.Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol. 1998;202:56–66. doi: 10.1006/dbio.1998.9001. [DOI] [PubMed] [Google Scholar]
- 109.Shirai M, Imanaka-Yoshida K, Schneider MD, Schwartz RJ, Morisaki T. T-box 2, a mediator of Bmp-Smad signaling, induced hyaluronan synthase 2 and Tgf{beta}2 expression and endocardial cushion formation. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0900635106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li Y, Toole BP, Dealy CN, Kosher RA. fHyaluronan in limb morphogenesis. Dev Biol. 2007;305:411–420. doi: 10.1016/j.ydbio.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Matsumoto K, Li Y, Jakuba C, Sugiyama Y, Sayo T, Okuno M, Dealy CN, Toole BP, Takeda J, Yamaguchi Y, Kosher RA. Conditional inactivation of Has2 reveals a crucial role for hyaluronan in skeletal growth, patterning, chondrocyte maturation and joint formation in the developing limb. Development. 2009;136:2825–2835. doi: 10.1242/dev.038505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Liu J, Li Q, Kuehn MR, Litingtung Y, Vokes SA, Chiang C. Sonic hedgehog signaling directly targets Hyaluronic Acid Synthase 2, an essential regulator of phalangeal joint patterning. Dev Biol. 2013;375:160–171. doi: 10.1016/j.ydbio.2012.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hunt LC, Gorman C, Kintakas C, McCulloch DR, Mackie EJ, White JD. Hyaluronan synthesis and myogenesis: a requirement for hyaluronan synthesis during myogenic differentiation independent of pericellular matrix formation. J Biol Chem. 2013;288:13006–13021. doi: 10.1074/jbc.M113.453209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goncharova V, Serobyan N, Iizuka S, Schraufstatter I, de Ridder A, Povaliy T, Wacker V, Itano N, Kimata K, Orlovskaja IA, Yamaguchi Y, Khaldoyanidi S. Hyaluronan expressed by the hematopoietic microenvironment is required for bone marrow hematopoiesis. J Biol Chem. 2012;287:25419–25433. doi: 10.1074/jbc.M112.376699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Riehl TE, Ee X, Stenson WF. Hyaluronic acid regulates normal intestinal and colonic growth in mice. Am J Physiol Gastrointest Liver Physiol. 2012;303:G377–388. doi: 10.1152/ajpgi.00034.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bourguignon LY, Zhu H, Zhou B, Diedrich F, Singleton PA, Hung MC. Hyaluronan promotes CD44v3-Vav2 interaction with Grb2-p185(HER2) and induces Rac1 and Ras signaling during ovarian tumor cell migration and growth. J Biol Chem. 2001;276:48679–48692. doi: 10.1074/jbc.M106759200. [DOI] [PubMed] [Google Scholar]
- 117.Meran S, Thomas DW, Stephens P, Enoch S, Martin J, Steadman R, Phillips AO. Hyaluronan facilitates transforming growth factor-beta1-mediated fibroblast proliferation. J Biol Chem. 2008;283:6530–6545. doi: 10.1074/jbc.M704819200. [DOI] [PubMed] [Google Scholar]
- 118.Kozlova I, Ruusala A, Voytyuk O, Skandalis SS, Heldin P. IQGAP1 regulates hyaluronan-mediated fibroblast motility and proliferation. Cell Signal. 2012;24:1856–1862. doi: 10.1016/j.cellsig.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 119.Gao F, Liu Y, He Y, Yang C, Wang Y, Shi X, Wei G. Hyaluronan oligosaccharides promote excisional wound healing through enhanced angiogenesis. Matrix Biol. 2010;29:107–116. doi: 10.1016/j.matbio.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 120.Jenkins RH, Thomas GJ, Williams JD, Steadman R. Myofibroblastic differentiation leads to hyaluronan accumulation through reduced hyaluronan turnover. J Biol Chem. 2004;279:41453–41460. doi: 10.1074/jbc.M401678200. [DOI] [PubMed] [Google Scholar]
- 121.Webber J, Jenkins RH, Meran S, Phillips A, Steadman R. Modulation of TGFbeta1-dependent myofibroblast differentiation by hyaluronan. Am J Pathol. 2009;175:148–160. doi: 10.2353/ajpath.2009.080837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Takahashi Y, Li L, Kamiryo M, Asteriou T, Moustakas A, Yamashita H, Heldin P. Hyaluronan fragments induce endothelial cell differentiation in a CD44- and CXCL1/GRO1-dependent manner. J Biol Chem. 2005;280:24195–24204. doi: 10.1074/jbc.M411913200. [DOI] [PubMed] [Google Scholar]
- 123.Kavalkovich KW, Boynton RE, Murphy JM, Barry F. Chondrogenic differentiation of human mesenchymal stem cells within an alginate layer culture system. In Vitro Cell Dev Biol Anim. 2002;38:457–466. doi: 10.1290/1071-2690(2002)038<0457:cdohms>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 124.Tsukahara S, Ikeda R, Goto S, Yoshida K, Mitsumori R, Sakamoto Y, Tajima A, Yokoyama T, Toh S, Furukawa K, Inoue I. Tumour necrosis factor alpha-stimulated gene-6 inhibits osteoblastic differentiation of human mesenchymal stem cells induced by osteogenic differentiation medium and BMP-2. Biochem J. 2006;398:595–603. doi: 10.1042/BJ20060027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Iocono JA, Bisignani GJ, Krummel TM, Ehrlich HP. Inhibiting the differentiation of myocardiocytes by hyaluronic acid. J Surg Res. 1998;76:111–116. doi: 10.1006/jsre.1998.5305. [DOI] [PubMed] [Google Scholar]
- 126.Ariel A, Lider O, Brill A, Cahalon L, Savion N, Varon D, Hershkoviz R. Induction of interactions between CD44 and hyaluronic acid by a short exposure of human T cells to diverse pro-inflammatory mediators. Immunology. 2000;100:345–351. doi: 10.1046/j.1365-2567.2000.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baaten BJ, Li CR, Deiro MF, Lin MM, Linton PJ, Bradley LM. CD44 regulates survival and memory development in Th1 cells. Immunity. 2010;32:104–115. doi: 10.1016/j.immuni.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rafi A, Nagarkatti M, Nagarkatti PS. Hyaluronate-CD44 interactions can induce murine B-cell activation. Blood. 1997;89:2901–2908. [PubMed] [Google Scholar]
- 129.Firan M, Dhillon S, Estess P, Siegelman MH. Suppressor activity and potency among regulatory T cells is discriminated by functionally active CD44. Blood. 2006;107:619–627. doi: 10.1182/blood-2005-06-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu C, Thalhamer T, Franca RF, Xiao S, Wang C, Hotta C, Zhu C, Hirashima M, Anderson AC, Kuchroo VK. Galectin-9-CD44 interaction enhances stability and function of adaptive regulatory T cells. Immunity. 2014;41:270–282. doi: 10.1016/j.immuni.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Khan AI, Kerfoot SM, Heit B, Liu L, Andonegui G, Ruffell B, Johnson P, Kubes P. Role of CD44 and hyaluronan in neutrophil recruitment. J Immunol. 2004;173:7594–7601. doi: 10.4049/jimmunol.173.12.7594. [DOI] [PubMed] [Google Scholar]
- 132.Rouschop KM, Roelofs JJ, Claessen N, da Costa Martins P, Zwaginga JJ, Pals ST, Weening JJ, Florquin S. Protection against renal ischemia reperfusion injury by CD44 disruption. J Am Soc Nephrol. 2005;16:2034–2043. doi: 10.1681/ASN.2005010054. [DOI] [PubMed] [Google Scholar]
- 133.Yamasaki K, Muto J, Taylor KR, Cogen AL, Audish D, Bertin J, Grant EP, Coyle AJ, Misaghi A, Hoffman HM, Gallo RL. NLRP3/cryopyrin is necessary for interleukin-1beta (IL-1beta) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J Biol Chem. 2009;284:12762–12771. doi: 10.1074/jbc.M806084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stoop R, Gal I, Glant TT, McNeish JD, Mikecz K. Trafficking of CD44-deficient murine lymphocytes under normal and inflammatory conditions. Eur J Immunol. 2002;32:2532–2542. doi: 10.1002/1521-4141(200209)32:9<2532::AID-IMMU2532>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 135.Suwan K, Choocheep K, Hatano S, Kongtawelert P, Kimata K, Watanabe H. Versican/PG-M Assembles Hyaluronan into Extracellular Matrix and Inhibits CD44-mediated Signaling toward Premature Senescence in Embryonic Fibroblasts. J Biol Chem. 2009;284:8596–8604. doi: 10.1074/jbc.M806927200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lompardia SL, Papademetrio DL, Mascaro M, Alvarez EM, Hajos SE. Human leukemic cell lines synthesize hyaluronan to avoid senescence and resist chemotherapy. Glycobiology. 2013;23:1463–1476. doi: 10.1093/glycob/cwt074. [DOI] [PubMed] [Google Scholar]
- 137.Rock K, Tigges J, Sass S, Schutze A, Florea AM, Fender AC, Theis FJ, Krutmann J, Boege F, Fritsche E, Reifenberger G, Fischer JW. miR-23a-3p causes cellular senescence by targeting hyaluronan synthase 2: possible implication for skin aging. J Invest Dermatol. 2015;135:369–377. doi: 10.1038/jid.2014.422. [DOI] [PubMed] [Google Scholar]
- 138.Jung EM, Kwon O, Kwon KS, Cho YS, Rhee SK, Min JK, Oh DB. Evidences for correlation between the reduced VCAM-1 expression and hyaluronan synthesis during cellular senescence of human mesenchymal stem cells. Biochem Biophys Res Commun. 2011;404:463–469. doi: 10.1016/j.bbrc.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 139.Cirillo N, Vicidomini A, McCullough M, Gambardella A, Hassona Y, Prime SS, Colella G. A hyaluronic acid-based compound inhibits fibroblast senescence induced by oxidative stress in vitro and prevents oral mucositis in vivo. J Cell Physiol. 2015;230:1421–1429. doi: 10.1002/jcp.24908. [DOI] [PubMed] [Google Scholar]
- 140.Pauloin T, Dutot M, Joly F, Warnet JM, Rat P. High molecular weight hyaluronan decreases UVB-induced apoptosis and inflammation in human epithelial corneal cells. Mol Vis. 2009;15:577–583. [PMC free article] [PubMed] [Google Scholar]
- 141.Ruffell B, Johnson P. Hyaluronan induces cell death in activated T cells through CD44. J Immunol. 2008;181:7044–7054. doi: 10.4049/jimmunol.181.10.7044. [DOI] [PubMed] [Google Scholar]
- 142.Leu SW, Shi L, Xu C, Zhao Y, Liu B, Li Y, Shiedlin A, Xiang C, Shen H, Quinn DA, Hales CA, Zhao H. TLR4 through IFN-beta promotes low molecular mass hyaluronan-induced neutrophil apoptosis. J Immunol. 2011;186:556–562. doi: 10.4049/jimmunol.1001630. [DOI] [PubMed] [Google Scholar]
- 143.Huang PM, Syrkina O, Yu L, Dedaj R, Zhao H, Shiedlin A, Liu YY, Garg H, Quinn DA, Hales CA. High MW hyaluronan inhibits smoke inhalation-induced lung injury and improves survival. Respirology. 2010;15:1131–1139. doi: 10.1111/j.1440-1843.2010.01829.x. [DOI] [PubMed] [Google Scholar]
- 144.Takazoe K, Tesch GH, Hill PA, Hurst LA, Jun Z, Lan HY, Atkins RC, Nikolic-Paterson DJ. CD44-mediated neutrophil apoptosis in the rat. Kidney Int. 2000;58:1920–1930. doi: 10.1111/j.1523-1755.2000.00364.x. [DOI] [PubMed] [Google Scholar]
- 145.Wang Q, Teder P, Judd NP, Noble PW, Doerschuk CM. CD44 deficiency leads to enhanced neutrophil migration and lung injury in Escherichia coli pneumonia in mice. Am J Pathol. 2002;161:2219–2228. doi: 10.1016/S0002-9440(10)64498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wisniewski HG, Hua JC, Poppers DM, Naime D, Vilcek J, Cronstein BN. TNF/IL-1-inducible protein TSG-6 potentiates plasmin inhibition by inter-alpha-inhibitor and exerts a strong anti-inflammatory effect in vivo. J Immunol. 1996;156:1609–1615. [PubMed] [Google Scholar]
- 147.Tolg C, Telmer P, Turley E. Specific sizes of hyaluronan oligosaccharides stimulate fibroblast migration and excisional wound repair. PloS one. 2014;9:e88479. doi: 10.1371/journal.pone.0088479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Svee K, White J, Vaillant P, Jessurun J, Roongta U, Krumwiede M, Johnson D, Henke C. Acute lung injury fibroblast migration and invasion of a fibrin matrix is mediated by CD44. J Clin Invest. 1996;98:1713–1727. doi: 10.1172/JCI118970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Evanko SP, Potter-Perigo S, Petty LJ, Workman GA, Wight TN. Hyaluronan Controls the Deposition of Fibronectin and Collagen and Modulates TGF-beta1 Induction of Lung Myofibroblasts. Matrix Biol. 2015;42:74–92. doi: 10.1016/j.matbio.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1004–1013. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- 151.Savani RC, Wang C, Yang B, Zhang S, Kinsella MG, Wight TN, Stern R, Nance DM, Turley EA. Migration of bovine aortic smooth muscle cells after wounding injury. The role of hyaluronan and RHAMM. J Clin Invest. 1995;95:1158–1168. doi: 10.1172/JCI117764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Herrera MB, Bussolati B, Bruno S, Morando L, Mauriello-Romanazzi G, Sanavio F, Stamenkovic I, Biancone L, Camussi G. Exogenous mesenchymal stem cells localize to the kidney by means of CD44 following acute tubular injury. Kidney Int. 2007;72:430–441. doi: 10.1038/sj.ki.5002334. [DOI] [PubMed] [Google Scholar]
- 153.Legg JW, Lewis CA, Parsons M, Ng T, Isacke CM. A novel PKC-regulated mechanism controls CD44 ezrin association and directional cell motility. Nat Cell Biol. 2002;4:399–407. doi: 10.1038/ncb797. [DOI] [PubMed] [Google Scholar]
- 154.Acharya PS, Majumdar S, Jacob M, Hayden J, Mrass P, Weninger W, Assoian RK, Pure E. Fibroblast migration is mediated by CD44-dependent TGF beta activation. J Cell Sci. 2008;121:1393–1402. doi: 10.1242/jcs.021683. [DOI] [PubMed] [Google Scholar]
- 155.Henke CA, Roongta U, Mickelson DJ, Knutson JR, McCarthy JB. CD44-related chondroitin sulfate proteoglycan, a cell surface receptor implicated with tumor cell invasion, mediates endothelial cell migration on fibrinogen and invasion into a fibrin matrix. J Clin Invest. 1996;97:2541–2552. doi: 10.1172/JCI118702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 157.Allison P, Espiritu D, Barnett JV, Camenisch TD. Type III TGFbeta receptor and Src direct hyaluronan-mediated invasive cell motility. Cell Signal. 2015;27:453–459. doi: 10.1016/j.cellsig.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Veiseh M, Kwon DH, Borowsky AD, Tolg C, Leong HS, Lewis JD, Turley EA, Bissell MJ. Cellular heterogeneity profiling by hyaluronan probes reveals an invasive but slow-growing breast tumor subset. Proc Natl Acad Sci U S A. 2014;111:E1731–1739. doi: 10.1073/pnas.1402383111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med. 2011;208:1459–1471. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nairn AV, Kinoshita-Toyoda A, Toyoda H, Xie J, Harris K, Dalton S, Kulik M, Pierce JM, Toida T, Moremen KW, Linhardt RJ. Glycomics of proteoglycan biosynthesis in murine embryonic stem cell differentiation. J Proteome Res. 2007;6:4374–4387. doi: 10.1021/pr070446f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Schraufstatter IU, Serobyan N, Loring J, Khaldoyanidi SK. Hyaluronan is required for generation of hematopoietic cells during differentiation of human embryonic stem cells. J Stem Cells. 2010;5:9–21. [PMC free article] [PubMed] [Google Scholar]
- 162.Choudhary M, Zhang X, Stojkovic P, Hyslop L, Anyfantis G, Herbert M, Murdoch AP, Stojkovic M, Lako M. Putative role of hyaluronan and its related genes, HAS2 and RHAMM, in human early preimplantation embryogenesis and embryonic stem cell characterization. Stem Cells. 2007;25:3045–3057. doi: 10.1634/stemcells.2007-0296. [DOI] [PubMed] [Google Scholar]
- 163.Choi HR, Kang YA, Na JI, Huh SY, Huh CH, Kim KH, Park KC. Oligosaccharides of hyaluronic acid increased epidermal cell stemness by modulation of integrin expression. J Cosmet Dermatol. 2012;11:290–296. doi: 10.1111/jocd.12009. [DOI] [PubMed] [Google Scholar]
- 164.Calloni R, Cordero EA, Henriques JA, Bonatto D. Reviewing and updating the major molecular markers for stem cells. Stem Cells Dev. 2013;22:1455–1476. doi: 10.1089/scd.2012.0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zoller M. CD44, Hyaluronan, the Hematopoietic Stem Cell, and Leukemia-Initiating Cells. Frontiers in immunology. 2015;6:235. doi: 10.3389/fimmu.2015.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Matrosova VY, Orlovskaya IA, Serobyan N, Khaldoyanidi SK. Hyaluronic acid facilitates the recovery of hematopoiesis following 5-fluorouracil administration. Stem Cells. 2004;22:544–555. doi: 10.1634/stemcells.22-4-544. [DOI] [PubMed] [Google Scholar]
- 168.Deed R, Rooney P, Kumar P, Norton JD, Smith J, Freemont AJ, Kumar S. Early-response gene signalling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells. Inhibition by non-angiogenic, high-molecular-weight hyaluronan. Int J Cancer. 1997;71:251–256. doi: 10.1002/(sici)1097-0215(19970410)71:2<251::aid-ijc21>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 169.Cao G, Savani RC, Fehrenbach M, Lyons C, Zhang L, Coukos G, Delisser HM. Involvement of endothelial CD44 during in vivo angiogenesis. Am J Pathol. 2006;169:325–336. doi: 10.2353/ajpath.2006.060206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Noble PW. Idiopathic pulmonary fibrosis: natural history and prognosis. Clin Chest Med. 2006;27:S11–16. v. doi: 10.1016/j.ccm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 171.Bjermer L, Lundgren R, Hallgren R. Hyaluronan and type III procollagen peptide concentrations in bronchoalveolar lavage fluid in idiopathic pulmonary fibrosis. Thorax. 1989;44:126–131. doi: 10.1136/thx.44.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Westergren-Thorsson G, Sime P, Jordana M, Gauldie J, Sarnstrand B, Malmstrom A. Lung fibroblast clones from normal and fibrotic subjects differ in hyaluronan and decorin production and rate of proliferation. Int J Biochem Cell Biol. 2004;36:1573–1584. doi: 10.1016/j.biocel.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 173.Webber J, Meran S, Steadman R, Phillips A. Hyaluronan orchestrates transforming growth factor-beta1-dependent maintenance of myofibroblast phenotype. J Biol Chem. 2009;284:9083–9092. doi: 10.1074/jbc.M806989200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Simpson RM, Meran S, Thomas D, Stephens P, Bowen T, Steadman R, Phillips A. Age-related changes in pericellular hyaluronan organization leads to impaired dermal fibroblast to myofibroblast differentiation. Am J Pathol. 2009;175:1915–1928. doi: 10.2353/ajpath.2009.090045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Nettelbladt O, Tengblad A, Hallgren R. High-dose corticosteroids during bleomycin-induced alveolitis in the rat do not suppress the accumulation of hyaluronan (hyaluronic acid) in lung tissue. Eur Respir J. 1990;3:421–428. [PubMed] [Google Scholar]
- 176.Teder P, Heldin P. Mechanism of impaired local hyaluronan turnover in bleomycin-induced lung injury in rat. Am J Respir Cell Mol Biol. 1997;17:376–385. doi: 10.1165/ajrcmb.17.3.2698. [DOI] [PubMed] [Google Scholar]
- 177.da Silva Bitencourt C, Gelfuso GM, Pereira PA, de Assis PA, Tefe-Silva C, Ramos SG, Arantes EC, Faccioli LH. Hyaluronidase-loaded PLGA microparticles as a new strategy for the treatment of pulmonary fibrosis. Tissue engineering.Part A. 2015;21:246–256. doi: 10.1089/ten.TEA.2013.0403. [DOI] [PubMed] [Google Scholar]
- 178.Skurikhin EG, Pershina OV, Reztsova AM, Ermakova NN, Khmelevskaya ES, Krupin VA, Stepanova IE, Artamonov AV, Bekarev AA, Madonov PG, Dygai AM. Modulation of bleomycin-induced lung fibrosis by pegylated hyaluronidase and dopamine receptor antagonist in mice. PloS one. 2015;10:e0125065. doi: 10.1371/journal.pone.0125065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yatagai Y, Sakamoto T, Yamada H, Masuko H, Kaneko Y, Iijima H, Naito T, Noguchi E, Hirota T, Tamari M, Konno S, Nishimura M, Hizawa N. Genomewide association study identifies HAS2 as a novel susceptibility gene for adult asthma in a Japanese population. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2014;44:1327–1334. doi: 10.1111/cea.12415. [DOI] [PubMed] [Google Scholar]
- 180.Sahu S, Lynn WS. Hyaluronic acid in the pulmonary secretions of patients with asthma. Biochem J. 1978;173:565–568. doi: 10.1042/bj1730565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bousquet J, Chanez P, Lacoste JY, Enander I, Venge P, Peterson C, Ahlstedt S, Michel FB, Godard P. Indirect evidence of bronchial inflammation assessed by titration of inflammatory mediators in BAL fluid of patients with asthma. J Allergy Clin Immunol. 1991;88:649–660. doi: 10.1016/0091-6749(91)90159-l. [DOI] [PubMed] [Google Scholar]
- 182.Ghosh S, Hoselton SA, Wanjara SB, Carlson J, McCarthy JB, Dorsam GP, Schuh JM. Hyaluronan stimulates ex vivo B lymphocyte chemotaxis and cytokine production in a murine model of fungal allergic asthma. Immunobiology. 2015;220:899–909. doi: 10.1016/j.imbio.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liang J, Jiang D, Jung Y, Xie T, Ingram J, Church T, Degan S, Leonard M, Kraft M, Noble PW. Role of hyaluronan and hyaluronan-binding proteins in human asthma. J Allergy Clin Immunol. 2011;128:403–411 e403. doi: 10.1016/j.jaci.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ohkawara Y, Tamura G, Iwasaki T, Tanaka A, Kikuchi T, Shirato K. Activation and transforming growth factor-beta production in eosinophils by hyaluronan. Am J Respir Cell Mol Biol. 2000;23:444–451. doi: 10.1165/ajrcmb.23.4.3875. [DOI] [PubMed] [Google Scholar]
- 185.Zhu Z, Lee CG, Zheng T, Chupp G, Wang J, Homer RJ, Noble PW, Hamid Q, Elias JA. Airway inflammation and remodeling in asthma. Lessons from interleukin 11 and interleukin 13 transgenic mice. Am J Respir Crit Care Med. 2001;164:S67–70. doi: 10.1164/ajrccm.164.supplement_2.2106070. [DOI] [PubMed] [Google Scholar]
- 186.Reeves SR, Kolstad T, Lien TY, Elliott M, Ziegler SF, Wight TN, Debley JS. Asthmatic airway epithelial cells differentially regulate fibroblast expression of extracellular matrix components. J Allergy Clin Immunol. 2014;134:663–670 e661. doi: 10.1016/j.jaci.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dentener MA, Vernooy JH, Hendriks S, Wouters EF. Enhanced levels of hyaluronan in lungs of patients with COPD: relationship with lung function and local inflammation. Thorax. 2005;60:114–119. doi: 10.1136/thx.2003.020842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kalay N, Elcik D, Canatan H, Kaya MG, Yarlioglues M, Oguzhan A, Dweik RA, Aytekin M. Elevated plasma hyaluronan levels in pulmonary hypertension. The Tohoku journal of experimental medicine. 2013;230:7–11. doi: 10.1620/tjem.230.7. [DOI] [PubMed] [Google Scholar]
- 189.Karmouty-Quintana H, Weng T, Garcia-Morales LJ, Chen NY, Pedroza M, Zhong H, Molina JG, Bunge R, Bruckner BA, Xia Y, Johnston RA, Loebe M, Zeng D, Seethamraju H, Belardinelli L, Blackburn MR. Adenosine A2B receptor and hyaluronan modulate pulmonary hypertension associated with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2013;49:1038–1047. doi: 10.1165/rcmb.2013-0089OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Klagas I, Goulet S, Karakiulakis G, Zhong J, Baraket M, Black JL, Papakonstantinou E, Roth M. Decreased hyaluronan in airway smooth muscle cells from patients with asthma and COPD. Eur Respir J. 2009;34:616–628. doi: 10.1183/09031936.00070808. [DOI] [PubMed] [Google Scholar]
- 191.Cantor J, Armand G, Turino G. Lung hyaluronan levels are decreased in alpha-1 antiprotease deficiency COPD. Respir Med. 2015;109:656–659. doi: 10.1016/j.rmed.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 192.Bracke KR, Dentener MA, Papakonstantinou E, Vernooy JH, Demoor T, Pauwels NS, Cleutjens J, van Suylen R, Joos GF, Brusselle GG, Wouters EF. Enhanced Deposition of Low Weight Hyaluronan in Lungs of Cigarette Smoke-Exposed Mice. Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2008-0424OC. [DOI] [PubMed] [Google Scholar]
- 193.Eurlings IM, Dentener MA, Mercken EM, de Cabo R, Bracke KR, Vernooy JH, Wouters EF, Reynaert NL. A comparative study of matrix remodeling in chronic models for COPD; mechanistic insights into the role of TNF-alpha. Am J Physiol Lung Cell Mol Physiol. 2014;307:L557–565. doi: 10.1152/ajplung.00116.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tazaki G, Kondo T, Tajiri S, Tsuji C, Shioya S, Tanigaki T. Functional residual capacity and airway resistance in rats of COPD model induced by systemic hyaluronidase. Tokai J Exp Clin Med. 2006;31:125–127. [PubMed] [Google Scholar]
- 195.Cantor J, Shteyngart B. Does lysozyme play a role in the pathogenesis of COPD? Medical hypotheses. 2015;84:551–554. doi: 10.1016/j.mehy.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 196.Cantor JO, Cerreta JM, Ochoa M, Ma S, Chow T, Grunig G, Turino GM. Aerosolized hyaluronan limits airspace enlargement in a mouse model of cigarette smoke-induced pulmonary emphysema. Exp Lung Res. 2005;31:417–430. doi: 10.1080/01902140590918669. [DOI] [PubMed] [Google Scholar]
- 197.Cantor JO, Cerreta JM, Ochoa M, Ma S, Liu M, Turino GM. Therapeutic effects of hyaluronan on smoke-induced elastic fiber injury: does delayed treatment affect efficacy? Lung. 2011;189:51–56. doi: 10.1007/s00408-010-9271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cantor JO. Potential therapeutic applications of hyaluronan in the lung. Int J Chron Obstruct Pulmon Dis. 2007;2:283–288. [PMC free article] [PubMed] [Google Scholar]
- 199.Riise GC, Schersten H, Nilsson F, Ryd W, Andersson BA. Activation of eosinophils and fibroblasts assessed by eosinophil cationic protein and hyaluronan in BAL. Association with acute rejection in lung transplant recipients. Chest. 1996;110:89–96. doi: 10.1378/chest.110.1.89. [DOI] [PubMed] [Google Scholar]
- 200.Todd JL, Wang X, Sugimoto S, Kennedy VE, Zhang HL, Pavlisko EN, Kelly FL, Huang H, Kreisel D, Palmer SM, Gelman AE. Hyaluronan contributes to bronchiolitis obliterans syndrome and stimulates lung allograft rejection through activation of innate immunity. Am J Respir Crit Care Med. 2014;189:556–566. doi: 10.1164/rccm.201308-1481OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Johnsson C, Tufveson G. Serum hyaluronan–a potential marker of cardiac allograft rejection? The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2006;25:544–549. doi: 10.1016/j.healun.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 202.Zhang W, Gao L, Qi S, Liu D, Xu D, Peng J, Daloze P, Chen H, Buelow R. Blocking of CD44-hyaluronic acid interaction prolongs rat allograft survival. Transplantation. 2000;69:665–667. doi: 10.1097/00007890-200002270-00032. [DOI] [PubMed] [Google Scholar]
- 203.Knoflach A, Magee C, Denton MD, Kim KS, Buelow R, Hancock WW, Sayegh MH. Immunomodulatory functions of hyaluronate in the LEW-to-F344 model of chronic cardiac allograft rejection. Transplantation. 1999;67:909–914. doi: 10.1097/00007890-199903270-00020. [DOI] [PubMed] [Google Scholar]
- 204.Johnsson C, Hallgren R, Elvin A, Gerdin B, Tufveson G. Hyaluronidase ameliorates rejection-induced edema. Transplant international: official journal of the European Society for Organ Transplantation. 1999;12:235–243. doi: 10.1007/s001470050216. [DOI] [PubMed] [Google Scholar]
- 205.Inokoshi Y, Tanino Y, Wang X, Sato S, Fukuhara N, Nikaido T, Fukuhara A, Saito J, Frevert CW, Munakata M. Clinical significance of serum hyaluronan in chronic fibrotic interstitial pneumonia. Respirology. 2013;18:1236–1243. doi: 10.1111/resp.12144. [DOI] [PubMed] [Google Scholar]
- 206.van der Windt GJ, Florquin S, de Vos AF, van’t Veer C, Queiroz KC, Liang J, Jiang D, Noble PW, van der Poll T. CD44 deficiency is associated with increased bacterial clearance but enhanced lung inflammation during Gram-negative pneumonia. Am J Pathol. 2010;177:2483–2494. doi: 10.2353/ajpath.2010.100562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Collins SL, Black KE, Chan-Li Y, Ahn YH, Cole PA, Powell JD, Horton MR. Hyaluronan fragments promote inflammation by down-regulating the anti-inflammatory A2a receptor. Am J Respir Cell Mol Biol. 2011;45:675–683. doi: 10.1165/rcmb.2010-0387OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Guechot J, Poupon RE, Giral P, Balkau B, Giboudeau J, Poupon R. Relationship between procollagen III aminoterminal propeptide and hyaluronan serum levels and histological fibrosis in primary biliary cirrhosis and chronic viral hepatitis C. J Hepatol. 1994;20:388–393. doi: 10.1016/s0168-8278(94)80013-8. [DOI] [PubMed] [Google Scholar]
- 209.Neuman MG, Cohen LB, Nanau R. Hyaluronic acid as a non-invasive biomarker of liver fibrosis. Clin Biochem. 2015 doi: 10.1016/j.clinbiochem.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 210.Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol. 2013;59:236–242. doi: 10.1016/j.jhep.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 211.Rostami S, Parsian H. Hyaluronic Acid: from biochemical characteristics to its clinical translation in assessment of liver fibrosis. Hepat Mon. 2013;13:e13787. doi: 10.5812/hepatmon.13787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Arain SA, Meo SA, Jamal Q. Serum hyaluronic acid level does not reliably differentiate minimal and significant liver disease in chronic hepatitis C. Saudi Med J. 2011;32:1241–1245. [PubMed] [Google Scholar]
- 213.Wolf D, Schumann J, Koerber K, Kiemer AK, Vollmar AM, Sass G, Papadopoulos T, Bang R, Klein SD, Brune B, Tiegs G. Low-molecular-weight hyaluronic acid induces nuclear factor-kappaB-dependent resistance against tumor necrosis factor alpha-mediated liver injury in mice. Hepatology. 2001;34:535–547. doi: 10.1053/jhep.2001.27218. [DOI] [PubMed] [Google Scholar]
- 214.Kimura K, Nagaki M, Kakimi K, Saio M, Saeki T, Okuda Y, Kuwata K, Moriwaki H. Critical role of CD44 in hepatotoxin-mediated liver injury. J Hepatol. 2008;48:952–961. doi: 10.1016/j.jhep.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 215.Papakonstantinou E, Roth M, Block LH, Mirtsou-Fidani V, Argiriadis P, Karakiulakis G. The differential distribution of hyaluronic acid in the layers of human atheromatic aortas is associated with vascular smooth muscle cell proliferation and migration. Atherosclerosis. 1998;138:79–89. doi: 10.1016/s0021-9150(98)00006-9. [DOI] [PubMed] [Google Scholar]
- 216.Fischer JW, Schror K. Regulation of hyaluronan synthesis by vasodilatory prostaglandins. Implications for atherosclerosis. Thromb Haemost. 2007;98:287–295. [PubMed] [Google Scholar]
- 217.Chai S, Chai Q, Danielsen CC, Hjorth P, Nyengaard JR, Ledet T, Yamaguchi Y, Rasmussen LM, Wogensen L. Overexpression of hyaluronan in the tunica media promotes the development of atherosclerosis. Circ Res. 2005;96:583–591. doi: 10.1161/01.RES.0000158963.37132.8b. [DOI] [PubMed] [Google Scholar]
- 218.Nagy N, Freudenberger T, Melchior-Becker A, Rock K, Ter Braak M, Jastrow H, Kinzig M, Lucke S, Suvorava T, Kojda G, Weber AA, Sorgel F, Levkau B, Ergun S, Fischer JW. Inhibition of hyaluronan synthesis accelerates murine atherosclerosis: novel insights into the role of hyaluronan synthesis. Circulation. 2010;122:2313–2322. doi: 10.1161/CIRCULATIONAHA.110.972653. [DOI] [PubMed] [Google Scholar]
- 219.Cuff CA, Kothapalli D, Azonobi I, Chun S, Zhang Y, Belkin R, Yeh C, Secreto A, Assoian RK, Rader DJ, Pure E. The adhesion receptor CD44 promotes atherosclerosis by mediating inflammatory cell recruitment and vascular cell activation. J Clin Invest. 2001;108:1031–1040. doi: 10.1172/JCI12455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Zhao L, Lee E, Zukas AM, Middleton MK, Kinder M, Acharya PS, Hall JA, Rader DJ, Pure E. CD44 expressed on both bone marrow-derived and non-bone marrow-derived cells promotes atherogenesis in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28:1283–1289. doi: 10.1161/ATVBAHA.108.165753. [DOI] [PubMed] [Google Scholar]
- 221.Mine S, Okada Y, Kawahara C, Tabata T, Tanaka Y. Serum hyaluronan concentration as a marker of angiopathy in patients with diabetes mellitus. Endocr J. 2006;53:761–766. doi: 10.1507/endocrj.k05-119. [DOI] [PubMed] [Google Scholar]
- 222.Mahadevan P, Larkins RG, Fraser JR, Fosang AJ, Dunlop ME. Increased hyaluronan production in the glomeruli from diabetic rats: a link between glucose-induced prostaglandin production and reduced sulphated proteoglycan. Diabetologia. 1995;38:298–305. doi: 10.1007/BF00400634. [DOI] [PubMed] [Google Scholar]
- 223.Bertheim U, Engstrom-Laurent A, Hofer PA, Hallgren P, Asplund J, Hellstrom S. Loss of hyaluronan in the basement membrane zone of the skin correlates to the degree of stiff hands in diabetic patients. Acta Derm Venereol. 2002;82:329–334. doi: 10.1080/000155502320624041. [DOI] [PubMed] [Google Scholar]
- 224.Cechowska-Pasko M, Palka J, Bankowski E. Decreased biosynthesis of glycosaminoglycans in the skin of rats with chronic diabetes mellitus. Exp Toxicol Pathol. 1999;51:239–243. doi: 10.1016/S0940-2993(99)80105-5. [DOI] [PubMed] [Google Scholar]
- 225.Mahadevan P, Larkins RG, Fraser JR, Dunlop ME. Effect of prostaglandin E2 and hyaluronan on mesangial cell proliferation. A potential contribution to glomerular hypercellularity in diabetes. Diabetes. 1996;45:44–50. doi: 10.2337/diab.45.1.44. [DOI] [PubMed] [Google Scholar]
- 226.Marutsuka K, Woodcock-Mitchell J, Sakamoto T, Sobel BE, Fujii S. Pathogenetic implications of hyaluronan-induced modification of vascular smooth muscle cell fibrinolysis in diabetes. Coron Artery Dis. 1998;9:177–184. doi: 10.1097/00019501-199809040-00002. [DOI] [PubMed] [Google Scholar]
- 227.Kodama K, Horikoshi M, Toda K, Yamada S, Hara K, Irie J, Sirota M, Morgan AA, Chen R, Ohtsu H, Maeda S, Kadowaki T, Butte AJ. Expression-based genome-wide association study links the receptor CD44 in adipose tissue with type 2 diabetes. Proc Natl Acad Sci U S A. 2012;109:7049–7054. doi: 10.1073/pnas.1114513109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Weiss L, Slavin S, Reich S, Cohen P, Shuster S, Stern R, Kaganovsky E, Okon E, Rubinstein AM, Naor D. Induction of resistance to diabetes in non-obese diabetic mice by targeting CD44 with a specific monoclonal antibody. Proc Natl Acad Sci U S A. 2000;97:285–290. doi: 10.1073/pnas.97.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Soulie P, Chassot A, Ernandez T, Montesano R, Feraille E. Spatially restricted hyaluronan production by Has2 drives epithelial tubulogenesis in vitro. Am J Physiol Cell Physiol. 2014;307:C745–759. doi: 10.1152/ajpcell.00047.2014. [DOI] [PubMed] [Google Scholar]
- 230.Nishikawa K, Andres G, Bhan AK, McCluskey RT, Collins AB, Stow JL, Stamenkovic I. Hyaluronate is a component of crescents in rat autoimmune glomerulonephritis. Lab Invest. 1993;68:146–153. [PubMed] [Google Scholar]
- 231.Yung S, Tsang RC, Leung JK, Chan TM. Increased mesangial cell hyaluronan expression in lupus nephritis is mediated by anti-DNA antibody-induced IL-1beta. Kidney Int. 2006;69:272–280. doi: 10.1038/sj.ki.5000042. [DOI] [PubMed] [Google Scholar]
- 232.Kubo M, Kikuchi K, Yazawa N, Fujimoto M, Tamaki T, Tamaki K. Increased serum concentration of hyaluronate in dermatomyositis patients. Arch Dermatol Res. 1998;290:579–581. doi: 10.1007/s004030050355. [DOI] [PubMed] [Google Scholar]
- 233.Hallgren R, Engstrom-Laurent A, Nisbeth U. Circulating hyaluronate. A potential marker of altered metabolism of the connective tissue in uremia. Nephron. 1987;46:150–154. doi: 10.1159/000184331. [DOI] [PubMed] [Google Scholar]
- 234.Turney JH, Davison AM, Forbes MA, Cooper EH. Hyaluronic acid in end-stage renal failure treated by haemodialysis: clinical correlates and implications. Nephrol Dial Transplant. 1991;6:566–570. doi: 10.1093/ndt/6.8.566. [DOI] [PubMed] [Google Scholar]
- 235.Stenvinkel P, Heimburger O, Wang T, Lindholm B, Bergstrom J, Elinder CG. High serum hyaluronan indicates poor survival in renal replacement therapy. Am J Kidney Dis. 1999;34:1083–1088. doi: 10.1016/S0272-6386(99)70014-4. [DOI] [PubMed] [Google Scholar]
- 236.Edward M, Fitzgerald L, Thind C, Leman J, Burden AD. Cutaneous mucinosis associated with dermatomyositis and nephrogenic fibrosing dermopathy: fibroblast hyaluronan synthesis and the effect of patient serum. Br J Dermatol. 2007;156:473–479. doi: 10.1111/j.1365-2133.2006.07652.x. [DOI] [PubMed] [Google Scholar]
- 237.Jun Z, Hill PA, Lan HY, Foti R, Mu W, Atkins RC, Nikolic-Paterson DJ. CD44 and hyaluronan expression in the development of experimental crescentic glomerulonephritis. Clin Exp Immunol. 1997;108:69–77. doi: 10.1046/j.1365-2249.1997.d01-977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Matsuda M, Shikata K, Shimizu F, Suzuki Y, Miyasaka M, Kawachi H, Kawashima H, Wada J, Sugimoto H, Shikata Y, Ogawa D, Tojo SJ, Akima K, Makino H. Therapeutic effect of sulphated hyaluronic acid, a potential selectin-blocking agent, on experimental progressive mesangial proliferative glomerulonephritis. J Pathol. 2002;198:407–414. doi: 10.1002/path.1209. [DOI] [PubMed] [Google Scholar]
- 239.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 240.de la Motte CA, Kessler SP. The role of hyaluronan in innate defense responses of the intestine. Int J Cell Biol. 2015;2015:481301. doi: 10.1155/2015/481301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kessler S, Rho H, West G, Fiocchi C, Drazba J, de la Motte C. Hyaluronan (HA) deposition precedes and promotes leukocyte recruitment in intestinal inflammation. Clin Transl Sci. 2008;1:57–61. doi: 10.1111/j.1752-8062.2008.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.de La Motte CA, Hascall VC, Calabro A, Yen-Lieberman B, Strong SA. Mononuclear leukocytes preferentially bind via CD44 to hyaluronan on human intestinal mucosal smooth muscle cells after virus infection or treatment with poly(I.C) J Biol Chem. 1999;274:30747–30755. doi: 10.1074/jbc.274.43.30747. [DOI] [PubMed] [Google Scholar]
- 243.Bandyopadhyay SK, de la Motte CA, Kessler SP, Hascall VC, Hill DR, Strong SA. Hyaluronan-mediated leukocyte adhesion and dextran sulfate sodium-induced colitis are attenuated in the absence of signal transducer and activator of transcription 1. Am J Pathol. 2008;173:1361–1368. doi: 10.2353/ajpath.2008.080444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kessler SP, Obery DR, de la Motte C. Hyaluronan Synthase 3 Null Mice Exhibit Decreased Intestinal Inflammation and Tissue Damage in the DSS-Induced Colitis Model. Int J Cell Biol. 2015;2015:745237. doi: 10.1155/2015/745237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Collins CB, Ho J, Wilson TE, Wermers JD, Tlaxca JL, Lawrence MB, Solga M, Lannigan J, Rivera-Nieves J. CD44 deficiency attenuates chronic murine ileitis. Gastroenterology. 2008;135:1993–2002. doi: 10.1053/j.gastro.2008.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Albeiroti S, Ayasoufi K, Hill DR, Shen B, de la Motte CA. Platelet hyaluronidase-2: an enzyme that translocates to the surface upon activation to function in extracellular matrix degradation. Blood. 2015;125:1460–1469. doi: 10.1182/blood-2014-07-590513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zheng L, Riehl TE, Stenson WF. Regulation of colonic epithelial repair in mice by Toll-like receptors and hyaluronic acid. Gastroenterology. 2009;137:2041–2051. doi: 10.1053/j.gastro.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Riehl TE, Foster L, Stenson WF. Hyaluronic acid is radioprotective in the intestine through a TLR4 and COX-2-mediated mechanism. Am J Physiol Gastrointest Liver Physiol. 2012;302:G309–316. doi: 10.1152/ajpgi.00248.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.McAtee CO, Barycki JJ, Simpson MA. Emerging roles for hyaluronidase in cancer metastasis and therapy. Adv Cancer Res. 2014;123:1–34. doi: 10.1016/B978-0-12-800092-2.00001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Tian X, Azpurua J, Hine C, Vaidya A, Myakishev-Rempel M, Ablaeva J, Mao Z, Nevo E, Gorbunova V, Seluanov A. High-molecular-mass hyaluronan mediates the cancer resistance of the naked mole rat. Nature. 2013;499:346–349. doi: 10.1038/nature12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Weber GF, Bronson RT, Ilagan J, Cantor H, Schmits R, Mak TW. Absence of the CD44 gene prevents sarcoma metastasis. Cancer Res. 2002;62:2281–2286. [PubMed] [Google Scholar]
- 252.Godar S, Ince TA, Bell GW, Feldser D, Donaher JL, Bergh J, Liu A, Miu K, Watnick RS, Reinhardt F, McAllister SS, Jacks T, Weinberg RA. Growth-inhibitory and tumor- suppressive functions of p53 depend on its repression of CD44 expression. Cell. 2008;134:62–73. doi: 10.1016/j.cell.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Zhang S, Wu CC, Fecteau JF, Cui B, Chen L, Zhang L, Wu R, Rassenti L, Lao F, Weigand S, Kipps TJ. Targeting chronic lymphocytic leukemia cells with a humanized monoclonal antibody specific for CD44. Proc Natl Acad Sci U S A. 2013;110:6127–6132. doi: 10.1073/pnas.1221841110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Cresta F, Naselli A, Favilli F, Casciaro R. Inhaled hypertonic saline+hyaluronic acid in cystic fibrosis with asthma-like symptoms: a new therapeutic chance. BMJ Case Rep. 2013;2013 doi: 10.1136/bcr-2013-009042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ros M, Casciaro R, Lucca F, Troiani P, Salonini E, Favilli F, Quattrucci S, Sher D, Assael BM. Hyaluronic acid improves the tolerability of hypertonic saline in the chronic treatment of cystic fibrosis patients: a multicenter, randomized, controlled clinical trial. J Aerosol Med Pulm Drug Deliv. 2014;27:133–137. doi: 10.1089/jamp.2012.1034. [DOI] [PubMed] [Google Scholar]
- 256.Engelhardt PF, Morakis N, Daha LK, Esterbauer B, Riedl CR. Long-term results of intravesical hyaluronan therapy in bladder pain syndrome/interstitial cystitis. Int Urogynecol J. 2011;22:401–405. doi: 10.1007/s00192-010-1294-y. [DOI] [PubMed] [Google Scholar]
- 257.Liu YY, Lee CH, Dedaj R, Zhao H, Mrabat H, Sheidlin A, Syrkina O, Huang PM, Garg HG, Hales CA, Quinn DA. High-molecular-weight hyaluronan–a possible new treatment for sepsis-induced lung injury: a preclinical study in mechanically ventilated rats. Crit Care. 2008;12:R102. doi: 10.1186/cc6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Toole BP, Ghatak S, Misra S. Hyaluronan oligosaccharides as a potential anticancer therapeutic. Curr Pharm Biotechnol. 2008;9:249–252. doi: 10.2174/138920108785161569. [DOI] [PubMed] [Google Scholar]
- 259.Ogawa D, Shikata K, Matsuda M, Akima K, Iwahashi M, Okada S, Tsuchiyama Y, Shikata Y, Wada J, Makino H. Sulfated hyaluronic acid, a potential selectin inhibitor, ameliorates experimentally induced crescentic glomerulonephritis. Nephron Exp Nephrol. 2005;99:e26–32. doi: 10.1159/000081795. [DOI] [PubMed] [Google Scholar]
- 260.Hintze V, Moeller S, Schnabelrauch M, Bierbaum S, Viola M, Worch H, Scharnweber D. Modifications of hyaluronan influence the interaction with human bone morphogenetic protein-4 (hBMP-4) Biomacromolecules. 2009;10:3290–3297. doi: 10.1021/bm9008827. [DOI] [PubMed] [Google Scholar]
- 261.Hintze V, Miron A, Moeller S, Schnabelrauch M, Wiesmann HP, Worch H, Scharnweber D. Sulfated hyaluronan and chondroitin sulfate derivatives interact differently with human transforming growth factor-beta1 (TGF-beta1) Acta Biomater. 2012;8:2144–2152. doi: 10.1016/j.actbio.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 262.Liao YH, Jones SA, Forbes B, Martin GP, Brown MB. Hyaluronan: pharmaceutical characterization and drug delivery. Drug Deliv. 2005;12:327–342. doi: 10.1080/10717540590952555. [DOI] [PubMed] [Google Scholar]
- 263.Cai S, Alhowyan AA, Yang Q, Forrest WC, Shnayder Y, Forrest ML. Cellular uptake and internalization of hyaluronan-based doxorubicin and cisplatin conjugates. J Drug Target. 2014;22:648–657. doi: 10.3109/1061186X.2014.921924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Montagner IM, Merlo A, Carpanese D, Zuccolotto G, Renier D, Campisi M, Pasut G, Zanovello P, Rosato A. Drug conjugation to hyaluronan widens therapeutic indications for ovarian cancer. Oncoscience. 2015;2:373–381. doi: 10.18632/oncoscience.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Montagner IM, Merlo A, Zuccolotto G, Renier D, Campisi M, Pasut G, Zanovello P, Rosato A. Peritoneal tumor carcinomatosis: pharmacological targeting with hyaluronan-based bioconjugates overcomes therapeutic indications of current drugs. PloS one. 2014;9:e112240. doi: 10.1371/journal.pone.0112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Banzato A, Bobisse S, Rondina M, Renier D, Bettella F, Esposito G, Quintieri L, Melendez-Alafort L, Mazzi U, Zanovello P, Rosato A. A paclitaxel-hyaluronan bioconjugate targeting ovarian cancer affords a potent in vivo therapeutic activity. Clin Cancer Res. 2008;14:3598–3606. doi: 10.1158/1078-0432.CCR-07-2019. [DOI] [PubMed] [Google Scholar]
- 267.Cai S, Xie Y, Bagby TR, Cohen MS, Forrest ML. Intralymphatic chemotherapy using a hyaluronan-cisplatin conjugate. J Surg Res. 2008;147:247–252. doi: 10.1016/j.jss.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Song S, Qi H, Xu J, Guo P, Chen F, Li F, Yang X, Sheng N, Wu Y, Pan W. Hyaluronan-based nanocarriers with CD44-overexpressed cancer cell targeting. Pharm Res. 2014;31:2988–3005. doi: 10.1007/s11095-014-1393-4. [DOI] [PubMed] [Google Scholar]
- 269.Lu X, Huang X. Design and syntheses of hyaluronan oligosaccharide conjugates as inhibitors of CD44-Hyaluronan binding. Glycoconj J. 2015 doi: 10.1007/s10719-015-9597-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.El-Dakdouki MH, Xia J, Zhu DC, Kavunja H, Grieshaber J, O’Reilly S, McCormick JJ, Huang X. Assessing the in vivo efficacy of doxorubicin loaded hyaluronan nanoparticles. ACS Appl Mater Interfaces. 2014;6:697–705. doi: 10.1021/am404946v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Gibbs P, Clingan PR, Ganju V, Strickland AH, Wong SS, Tebbutt NC, Underhill CR, Fox RM, Clavant SP, Leung J, Pho M, Brown TJ. Hyaluronan-Irinotecan improves progression-free survival in 5-fluorouracil refractory patients with metastatic colorectal cancer: a randomized phase II trial. Cancer Chemother Pharmacol. 2011;67:153–163. doi: 10.1007/s00280-010-1303-3. [DOI] [PubMed] [Google Scholar]
- 272.Yang JA, Kong WH, Sung DK, Kim H, Kim TH, Lee KC, Hahn SK. Hyaluronic acid-tumor necrosis factor-related apoptosis-inducing ligand conjugate for targeted treatment of liver fibrosis. Acta Biomater. 2015;12:174–182. doi: 10.1016/j.actbio.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 273.Jederstrom G, Andersson A, Grasjo J, Sjoholm I. Formulating insulin for oral administration: preparation of hyaluronan-insulin complex. Pharm Res. 2004;21:2040–2047. doi: 10.1023/b:pham.0000048195.69304.ff. [DOI] [PubMed] [Google Scholar]
- 274.Jederstrom G, Grasjo, Nordin A, Sjoholm I, Andersson A. Blood glucose-lowering activity of a hyaluronan-insulin complex after oral administration to rats with diabetes. Diabetes Technol Ther. 2005;7:948–957. doi: 10.1089/dia.2005.7.948. [DOI] [PubMed] [Google Scholar]
- 275.Nakamura T, Takagaki K, Shibata S, Tanaka K, Higuchi T, Endo M. Hyaluronic-acid-deficient extracellular matrix induced by addition of 4- methylumbelliferone to the medium of cultured human skin fibroblasts. Biochem Biophys Res Commun. 1995;208:470–475. doi: 10.1006/bbrc.1995.1362. [DOI] [PubMed] [Google Scholar]
- 276.Kultti A, Pasonen-Seppanen S, Jauhiainen M, Rilla KJ, Karna R, Pyoria E, Tammi RH, Tammi MI. 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp Cell Res. 2009;315:1914–1923. doi: 10.1016/j.yexcr.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 277.Nagy N, Kuipers HF, Frymoyer AR, Ishak HD, Bollyky JB, Wight TN, Bollyky PL. 4-methylumbelliferone treatment and hyaluronan inhibition as a therapeutic strategy in inflammation, autoimmunity, and cancer. Frontiers in immunology. 2015;6:123. doi: 10.3389/fimmu.2015.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Abate A, Dimartino V, Spina P, Costa PL, Lombardo C, Santini A, Del Piano M, Alimonti P. Hymecromone in the treatment of motor disorders of the bile ducts: a multicenter, double-blind, placebo-controlled clinical study. Drugs Exp Clin Res. 2001;27:223–231. [PubMed] [Google Scholar]
- 279.McKallip RJ, Hagele HF, Uchakina ON. Treatment with the hyaluronic acid synthesis inhibitor 4-methylumbelliferone suppresses SEB-induced lung inflammation. Toxins (Basel) 2013;5:1814–1826. doi: 10.3390/toxins5101814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.McKallip RJ, Ban H, Uchakina ON. Treatment with the hyaluronic Acid synthesis inhibitor 4-methylumbelliferone suppresses LPS-induced lung inflammation. Inflammation. 2015;38:1250–1259. doi: 10.1007/s10753-014-0092-y. [DOI] [PubMed] [Google Scholar]
- 281.Mueller AM, Yoon BH, Sadiq SA. Inhibition of hyaluronan synthesis protects against central nervous system (CNS) autoimmunity and increases CXCL12 expression in the inflamed CNS. J Biol Chem. 2014;289:22888–22899. doi: 10.1074/jbc.M114.559583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Colombaro V, Decleves AE, Jadot I, Voisin V, Giordano L, Habsch I, Nonclercq D, Flamion B, Caron N. Inhibition of hyaluronan is protective against renal ischaemia-reperfusion injury. Nephrol Dial Transplant. 2013;28:2484–2493. doi: 10.1093/ndt/gft314. [DOI] [PubMed] [Google Scholar]
- 283.Yoshioka Y, Kozawa E, Urakawa H, Arai E, Futamura N, Zhuo L, Kimata K, Ishiguro N, Nishida Y. Suppression of hyaluronan synthesis alleviates inflammatory responses in murine arthritis and in human rheumatoid synovial fibroblasts. Arthritis Rheum. 2013;65:1160–1170. doi: 10.1002/art.37861. [DOI] [PubMed] [Google Scholar]
- 284.Piccioni F, Fiore E, Bayo J, Atorrasagasti C, Peixoto E, Rizzo M, Malvicini M, Tirado-Gonzalez I, Garcia MG, Alaniz L, Mazzolini G. 4-Methylumbelliferone inhibits hepatocellular carcinoma growth by decreasing IL-6 production and angiogenesis. Glycobiology. 2015;25:825–835. doi: 10.1093/glycob/cwv023. [DOI] [PubMed] [Google Scholar]
- 285.Piccioni F, Malvicini M, Garcia MG, Rodriguez A, Atorrasagasti C, Kippes N, Piedra Buena IT, Rizzo MM, Bayo J, Aquino J, Viola M, Passi A, Alaniz L, Mazzolini G. Antitumor effects of hyaluronic acid inhibitor 4-methylumbelliferone in an orthotopic hepatocellular carcinoma model in mice. Glycobiology. 2012;22:40–410. doi: 10.1093/glycob/cwr158. [DOI] [PubMed] [Google Scholar]
- 286.Adams L. Adjuvants to local anaesthesia in ophthalmic surgery. Br J Ophthalmol. 2011;95:1345–1349. doi: 10.1136/bjo.2010.188789. [DOI] [PubMed] [Google Scholar]
- 287.Yasuda T, Ribeiro LG, Maroko PR. Effect of hyaluronidase on experimental cerebral infarct size and mortality. Lab Invest. 1982;46:400–404. [PubMed] [Google Scholar]
- 288.Maroko PR, Davidson DM, Libby P, Hagan AD, Braunwald E. Effects of hyaluronidase administration on myocardial ischemic injury in acute infarction. A preliminary study in 24 patients. Ann Intern Med. 1975;82:516–520. doi: 10.7326/0003-4819-82-4-516. [DOI] [PubMed] [Google Scholar]
- 289.Roberts R, Braunwald E, Muller JE, Croft C, Gold HK, Hartwell TD, Jaffe AS, Mullin SM, Parker C, Passamani ER, et al. Effect of hyaluronidase on mortality and morbidity in patients with early peaking of plasma creatine kinase MB and non-transmural ischaemia. Multicentre investigation for the limitation of infarct size (MILIS) Br Heart J. 1988;60:290–298. doi: 10.1136/hrt.60.4.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80:1921–1943. doi: 10.1016/j.lfs.2007.02.037. [DOI] [PubMed] [Google Scholar]
- 291.Furuya T, Yamagata S, Shimoyama Y, Fujihara M, Morishima N, Ohtsuki K. Biochemical characterization of glycyrrhizin as an effective inhibitor for hyaluronidases from bovine testis. Biol Pharm Bull. 1997;20:973–977. doi: 10.1248/bpb.20.973. [DOI] [PubMed] [Google Scholar]
- 292.Harunari E, Imada C, Igarashi Y, Fukuda T, Terahara T, Kobayashi T. Hyaluromycin, a new hyaluronidase inhibitor of polyketide origin from marine Streptomyces sp. Mar Drugs. 2014;12:491–507. doi: 10.3390/md12010491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Orlent H, Hansen BE, Willems M, Brouwer JT, Huber R, Kullak-Ublick GA, Gerken G, Zeuzem S, Nevens F, Tielemans WC, Zondervan PE, Lagging M, Westin J, Schalm SW. Biochemical and histological effects of 26 weeks of glycyrrhizin treatment in chronic hepatitis C: a randomized phase II trial. J Hepatol. 2006;45:539–546. doi: 10.1016/j.jhep.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 294.Yanagawa Y, Ogura M, Fujimoto E, Shono S, Okuda E. Effects and cost of glycyrrhizin in the treatment of upper respiratory tract infections in members of the Japanese maritime self-defense force: Preliminary report of a prospective, randomized, double-blind, controlled, parallel-group, alternate-day treatment assignment clinical trial. Curr Ther Res Clin Exp. 2004;65:26–33. doi: 10.1016/S0011-393X(04)90002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Jacobetz MA, Chan DS, Neesse A, Bapiro TE, Cook N, Frese KK, Feig C, Nakagawa T, Caldwell ME, Zecchini HI, Lolkema MP, Jiang P, Kultti A, Thompson CB, Maneval DC, Jodrell DI, Frost GI, Shepard HM, Skepper JN, Tuveson DA. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut. 2013;62:112–120. doi: 10.1136/gutjnl-2012-302529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Mummert ME, Mohamadzadeh M, Mummert DI, Mizumoto N, Takashima A. Development of a peptide inhibitor of hyaluronan-mediated leukocyte trafficking. J Exp Med. 2000;192:769–779. doi: 10.1084/jem.192.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Martin SF, Dudda JC, Bachtanian E, Lembo A, Liller S, Durr C, Heimesaat MM, Bereswill S, Fejer G, Vassileva R, Jakob T, Freudenberg N, Termeer CC, Johner C, Galanos C, Freudenberg MA. Toll-like receptor and IL-12 signaling control susceptibility to contact hypersensitivity. J Exp Med. 2008;205:2151–2162. doi: 10.1084/jem.20070509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Mummert ME, Mummert D, Edelbaum D, Hui F, Matsue H, Takashima A. Synthesis and surface expression of hyaluronan by dendritic cells and its potential role in antigen presentation. J Immunol. 2002;169:4322–4331. doi: 10.4049/jimmunol.169.8.4322. [DOI] [PubMed] [Google Scholar]
- 300.Stober VP, Szczesniak C, Childress Q, Heise RL, Bortner C, Hollingsworth JW, Neuringer IP, Palmer SM, Garantziotis S. Bronchial epithelial injury in the context of alloimmunity promotes lymphocytic bronchiolitis through hyaluronan expression. Am J Physiol Lung Cell Mol Physiol. 2014;306:L1045–1055. doi: 10.1152/ajplung.00353.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Mummert ME, Mummert DI, Ellinger L, Takashima A. Functional roles of hyaluronan in B16-F10 melanoma growth and experimental metastasis in mice. Mol Cancer Ther. 2003;2:295–300. [PubMed] [Google Scholar]
- 302.Guan H, Nagarkatti PS, Nagarkatti M. Blockade of hyaluronan inhibits IL-2-induced vascular leak syndrome and maintains effectiveness of IL-2 treatment for metastatic melanoma. J Immunol. 2007;179:3715–3723. doi: 10.4049/jimmunol.179.6.3715. [DOI] [PubMed] [Google Scholar]
- 303.Tolg C, Hamilton SR, Zalinska E, McCulloch L, Amin R, Akentieva N, Winnik F, Savani R, Bagli DJ, Luyt LG, Cowman MK, McCarthy JB, Turley EA. A RHAMM mimetic peptide blocks hyaluronan signaling and reduces inflammation and fibrogenesis in excisional skin wounds. Am J Pathol. 2012;181:1250–1270. doi: 10.1016/j.ajpath.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Rizzardi AE, Vogel RI, Koopmeiners JS, Forster CL, Marston LO, Rosener NK, Akentieva N, Price MA, Metzger GJ, Warlick CA, Henriksen JC, Turley EA, McCarthy JB, Schmechel SC. Elevated hyaluronan and hyaluronan-mediated motility receptor are associated with biochemical failure in patients with intermediate-grade prostate tumors. Cancer. 2014;120:1800–1809. doi: 10.1002/cncr.28646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Piotrowicz RS, Damaj BB, Hachicha M, Incardona F, Howell SB, Finlayson M. A6 peptide activates CD44 adhesive activity, induces FAK and MEK phosphorylation, and inhibits the migration and metastasis of CD44-expressing cells. Mol Cancer Ther. 2011;10:2072–2082. doi: 10.1158/1535-7163.MCT-11-0351. [DOI] [PubMed] [Google Scholar]
- 306.Ugarte-Berzal E, Bailon E, Amigo-Jimenez I, Albar JP, Garcia-Marco JA, Garcia-Pardo A. A novel CD44-binding peptide from the pro-matrix metalloproteinase-9 hemopexin domain impairs adhesion and migration of chronic lymphocytic leukemia (CLL) cells. J Biol Chem. 2014;289:15340–15349. doi: 10.1074/jbc.M114.559187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Finlayson M. Modulation of CD44 Activity by A6-Peptide. Frontiers in immunology. 2015;6:135. doi: 10.3389/fimmu.2015.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Charrad RS, Gadhoum Z, Qi J, Glachant A, Allouche M, Jasmin C, Chomienne C, Smadja-Joffe F. Effects of anti-CD44 monoclonal antibodies on differentiation and apoptosis of human myeloid leukemia cell lines. Blood. 2002;99:290–299. doi: 10.1182/blood.v99.1.290. [DOI] [PubMed] [Google Scholar]
- 309.Song G, Liao X, Zhou L, Wu L, Feng Y, Han ZC. HI44a, an anti-CD44 monoclonal antibody, induces differentiation and apoptosis of human acute myeloid leukemia cells. Leuk Res. 2004;28:1089–1096. doi: 10.1016/j.leukres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 310.Guo Y, Ma J, Wang J, Che X, Narula J, Bigby M, Wu M, Sy MS. Inhibition of human melanoma growth and metastasis in vivo by anti-CD44 monoclonal antibody. Cancer Res. 1994;54:1561–1565. [PubMed] [Google Scholar]
- 311.Breyer R, Hussein S, Radu DL, Putz KM, Gunia S, Hecker H, Samii M, Walter GF, Stan AC. Disruption of intracerebral progression of C6 rat glioblastoma by in vivo treatment with anti-CD44 monoclonal antibody. J Neurosurg. 2000;92:140–149. doi: 10.3171/jns.2000.92.1.0140. [DOI] [PubMed] [Google Scholar]
- 312.Haynes BF, Hale LP, Patton KL, Martin ME, McCallum RM. Measurement of an adhesion molecule as an indicator of inflammatory disease activity. Up-regulation of the receptor for hyaluronate (CD44) in rheumatoid arthritis. Arthritis Rheum. 1991;34:1434–1443. doi: 10.1002/art.1780341115. [DOI] [PubMed] [Google Scholar]
- 313.Mikecz K, Brennan FR, Kim JH, Glant TT. Anti-CD44 treatment abrogates tissue oedema and leukocyte infiltration in murine arthritis. Nat Med. 1995;1:558–563. doi: 10.1038/nm0695-558. [DOI] [PubMed] [Google Scholar]
- 314.Henke C, Bitterman P, Roongta U, Ingbar D, Polunovsky V. Induction of fibroblast apoptosis by anti-CD44 antibody: implications for the treatment of fibroproliferative lung disease. Am J Pathol. 1996;149:1639–1650. [PMC free article] [PubMed] [Google Scholar]
- 315.Seiter S, Weber B, Tilgen W, Zoller M. Down-modulation of host reactivity by anti-CD44 in skin transplantation. Transplantation. 1998;66:778–791. doi: 10.1097/00007890-199809270-00013. [DOI] [PubMed] [Google Scholar]
- 316.Wang F, Chen J, Shao W, Xie B, Wang Y, Lan T, Thorlacius H, Qi Z. Anti-CD44 monoclonal antibody inhibits heart transplant rejection mediated by alloantigen-primed CD4(+) memory T cells in nude mice. Immunol Invest. 2010;39:807–819. doi: 10.3109/08820139.2010.497833. [DOI] [PubMed] [Google Scholar]
- 317.Runnels HA, Weber GL, Min J, Kudlacz EM, Zobel JF, Donovan CB, Thiede MA, Zhang J, Alpert RB, Salafia MA, Milici AJ, Burdette D, Bell RR, Beebe JS, Xu X. PF-03475952: a potent and neutralizing fully human anti-CD44 antibody for therapeutic applications in inflammatory diseases. Adv Ther. 2010;27:168–180. doi: 10.1007/s12325-010-0010-0. [DOI] [PubMed] [Google Scholar]
- 318.Chen D, McKallip RJ, Zeytun A, Do Y, Lombard C, Robertson JL, Mak TW, Nagarkatti PS, Nagarkatti M. CD44-deficient mice exhibit enhanced hepatitis after concanavalin A injection: evidence for involvement of CD44 in activation-induced cell death. J Immunol. 2001;166:5889–5897. doi: 10.4049/jimmunol.166.10.5889. [DOI] [PubMed] [Google Scholar]
- 319.Stoop R, Kotani H, McNeish JD, Otterness IG, Mikecz K. Increased resistance to collagen-induced arthritis in CD44-deficient DBA/1 mice. Arthritis Rheum. 2001;44:2922–2931. doi: 10.1002/1529-0131(200112)44:12<2922::aid-art480>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 320.Wang X, Xu L, Wang H, Zhan Y, Pure E, Feuerstein GZ. CD44 deficiency in mice protects brain from cerebral ischemia injury. J Neurochem. 2002;83:1172–1179. doi: 10.1046/j.1471-4159.2002.01225.x. [DOI] [PubMed] [Google Scholar]
- 321.Tolg C, Hamilton SR, Morningstar L, Zhang J, Zhang S, Esguerra KV, Telmer PG, Luyt LG, Harrison R, McCarthy JB, Turley EA. RHAMM promotes interphase microtubule instability and mitotic spindle integrity through MEK1/ERK1/2 activity. J Biol Chem. 2010;285:26461–26474. doi: 10.1074/jbc.M110.121491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Foley JP, Lam D, Jiang H, Liao J, Cheong N, McDevitt TM, Zaman A, Wright JR, Savani RC. Toll-like receptor 2 (TLR2), transforming growth factor-beta, hyaluronan (HA), and receptor for HA-mediated motility (RHAMM) are required for surfactant protein A-stimulated macrophage chemotaxis. J Biol Chem. 2012;287:37406–37419. doi: 10.1074/jbc.M112.360982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Ma X, Pearce JD, Wilson DB, English WP, Edwards MS, Geary RL. Loss of the hyaluronan receptor RHAMM prevents constrictive artery wall remodeling. J Vasc Surg. 2014;59:804–813. doi: 10.1016/j.jvs.2013.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Kouvidi K, Berdiaki A, Nikitovic D, Katonis P, Afratis N, Hascall VC, Karamanos NK, Tzanakakis GN. Role of receptor for hyaluronic acid-mediated motility (RHAMM) in low molecular weight hyaluronan (LMWHA)-mediated fibrosarcoma cell adhesion. J Biol Chem. 2011;286:38509–38520. doi: 10.1074/jbc.M111.275875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Yamamura H, Zhang M, Markwald RR, Mjaatvedt CH. A heart segmental defect in the anterior-posterior axis of a transgenic mutant mouse. Dev Biol. 1997;186:58–72. doi: 10.1006/dbio.1997.8559. [DOI] [PubMed] [Google Scholar]
- 326.Williams DR, Jr, Presar AR, Richmond AT, Mjaatvedt CH, Hoffman S, Capehart AA. Limb chondrogenesis is compromised in the versican deficient hdf mouse. Biochem Biophys Res Commun. 2005;334:960–966. doi: 10.1016/j.bbrc.2005.06.189. [DOI] [PubMed] [Google Scholar]
- 327.Dours-Zimmermann MT, Maurer K, Rauch U, Stoffel W, Fassler R, Zimmermann DR. Versican V2 assembles the extracellular matrix surrounding the nodes of ranvier in the CNS. J Neurosci. 2009;29:7731–7742. doi: 10.1523/JNEUROSCI.4158-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Choocheep K, Hatano S, Takagi H, Watanabe H, Kimata K, Kongtawelert P, Watanabe H. Versican facilitates chondrocyte differentiation and regulates joint morphogenesis. J Biol Chem. 2010;285:21114–21125. doi: 10.1074/jbc.M109.096479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Little CB, Meeker CT, Hembry RM, Sims NA, Lawlor KE, Golub SB, Last K, Fosang AJ. Matrix metalloproteinases are not essential for aggrecan turnover during normal skeletal growth and development. Mol Cell Biol. 2005;25:3388–3399. doi: 10.1128/MCB.25.8.3388-3399.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Little CB, Meeker CT, Golub SB, Lawlor KE, Farmer PJ, Smith SM, Fosang AJ. Blocking aggrecanase cleavage in the aggrecan interglobular domain abrogates cartilage erosion and promotes cartilage repair. J Clin Invest. 2007;117:1627–1636. doi: 10.1172/JCI30765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Schledzewski K, Geraud C, Arnold B, Wang S, Grone HJ, Kempf T, Wollert KC, Straub BK, Schirmacher P, Demory A, Schonhaber H, Gratchev A, Dietz L, Thierse HJ, Kzhyshkowska J, Goerdt S. Deficiency of liver sinusoidal scavenger receptors stabilin-1 and -2 in mice causes glomerulofibrotic nephropathy via impaired hepatic clearance of noxious blood factors. J Clin Invest. 2011;121:703–714. doi: 10.1172/JCI44740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Huang SS, Liu IH, Smith T, Shah MR, Johnson FE, Huang JS. CRSBP-1/LYVE-l-null mice exhibit identifiable morphological and functional alterations of lymphatic capillary vessels. FEBS Lett. 2006;580:6259–6268. doi: 10.1016/j.febslet.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Fulop C, Szanto S, Mukhopadhyay D, Bardos T, Kamath RV, Rugg MS, Day AJ, Salustri A, Hascall VC, Glant TT, Mikecz K. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 2003;130:2253–2261. doi: 10.1242/dev.00422. [DOI] [PubMed] [Google Scholar]
- 334.Mahoney DJ, Mikecz K, Ali T, Mabilleau G, Benayahu D, Plaas A, Milner CM, Day AJ, Sabokbar A. TSG-6 regulates bone remodeling through inhibition of osteoblastogenesis and osteoclast activation. J Biol Chem. 2008;283:25952–25962. doi: 10.1074/jbc.M802138200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Swaidani S, Cheng G, Lauer ME, Sharma M, Mikecz K, Hascall VC, Aronica MA. TSG-6 protein is crucial for the development of pulmonary hyaluronan deposition, eosinophilia, and airway hyperresponsiveness in a murine model of asthma. J Biol Chem. 2013;288:412–422. doi: 10.1074/jbc.M112.389874. [DOI] [PMC free article] [PubMed] [Google Scholar]


