Abstract
High rates of comorbidity for anxiety and alcohol-use disorders suggest a causal relationship between these conditions. Previous work demonstrates basal anxiety levels in outbred Long-Evans rats correlate with differences in voluntary ethanol consumption and that amygdalar Neuropeptide Y (NPY) systems may play a role in this relationship. The present work explores the possibility that differences in sensitivity to ethanol’s anxiolytic effects contribute to differential ethanol self-administration in these animals and examines the potential role of central and peripheral NPY in mediating this relationship. Animals were first exposed to the elevated plus maze (EPM) to assess individual differences in anxiety-like behaviors and levels of circulating NPY and corticosterone (CORT). Rats were then tested for anxiety-like behavior in the light-dark box (LD box) following acute ethanol treatment (1 g/kg; intraperitoneally [i.p.]), and neuronal activation in the amygdala and bed nucleus of the stria terminalis (BNST) was assessed using Fos immunohistochemistry. EPM exposure increased plasma CORT levels without altering plasma NPY levels. Acute ethanol treatment significantly increased light-dark transitions and latency to re-enter the light arena, but no differences were seen between high- and low-anxiety groups and no correlations were found between anxiety-like behaviors in the EPM and LD box. Acute ethanol treatment significantly increased Fos immunoreactivity in the BNST and the central amygdala. Although NPY neurons were not significantly activated following ethanol exposure, in saline-treated animals lower levels of anxiety-like behavior in the LD box (more time in the light arena and more transitions) were correlated with higher NPY-positive cell density in the central amygdala. Our results suggest that activation of the CeA and BNST are involved in the behavioral expression of ethanol-induced anxiolysis, and that differences in basal anxiety state may be correlated with NPY systems in the extended amygdala.
Keywords: anxiety, BNST, amygdala, NPY, individual differences
Introduction
Anxiety disorders frequently co-occur with alcohol-use disorders (AUDs), with 75% of individuals that abuse alcohol having a current or previous diagnosis of an anxiety disorder (Kushner, Abrams, & Borchardt, 2000; Kushner et al., 2011; Swendsen et al., 2010). Anxiety relief is often cited as a motivation to consume alcohol, and both human and animal studies have demonstrated that acute ethanol reduces anxiety (Eckardt et al., 1998; Kushner et al., 2000; Wilson, Burghardt, Ford, Wilkinson, & Primeaux, 2004). Additionally, it has been suggested that chronic anxiety symptoms may promote alcohol abuse and the development of alcohol dependence (Koob, 2003), as preclinical studies show that elevated innate anxiety states are associated with increased drinking in two-bottle choice paradigms (Spanagel et al., 1995; Primeaux, Wilson, Bray, York, & Wilson, 2006). In contrast, repeated ethanol exposure and withdrawal can result in elevated measures of anxiety (Kliethermes, 2005; Valdez et al., 2002), suggesting that chronic, heavy ethanol use may contribute to the development of anxiety disorders. While many studies have examined the relationship between withdrawal-induced anxiety and the progression from moderate ethanol consumption to ethanol abuse and dependence, it remains unclear to what extent pre-existing anxiety disorders contribute to the development of alcohol-use disorders.
A number of preclinical studies suggest that ethanol preference may be related to anxiety-like behavioral phenotype. Some of the rodent lines selectively bred for high or low ethanol consumption show differences in anxiety-like behaviors. Both alcohol-preferring (P) rats and Sardinian alcohol-preferring (sP) rats demonstrate more anxiety-like behavior on the elevated plus maze (EPM) than the associated non-preferring (NP and sNP) lines (Colombo et al., 1995; Stewart, Gatto, Lumeng, Li, & Murphy, 1993). Interestingly, outbred rats appear to show a similar association. Previous work from our laboratory has shown that Long-Evans rats show highly variable anxiety-like behavior on the EPM, and rats characterized as having a high-anxiety phenotype had higher preference scores for ethanol in a 24-h two-bottle choice paradigm, as compared to low-anxiety animals (Primeaux et al., 2006). A comparable relationship was found in Wistar rats, with high-anxiety animals having higher ethanol intake and ethanol preference than low-anxiety animals (Spanagel et al., 1995). However, the relationship between anxiety-like behaviors and ethanol consumption may be dependent on the ethanol drinking paradigm used. In an acute voluntary ethanol consumption paradigm based on the murine drinking-in-the-dark model (Rhodes, Best, Belknap, Finn, & Crabbe, 2005), Long-Evans rats characterized as having a high-anxiety phenotype consumed significantly less ethanol than low-anxiety animals (White, Ford, Fadel, & Wilson, 2009). Additionally, rats consuming different amounts of ethanol in this limited-access paradigm showed differential anxiolytic effects on the elevated plus maze (Sharko, Kaigler, Fadel, & Wilson, 2013). Although these data indicate that individual differences in anxiety measures are associated with differences in ethanol preference and consumption patterns, further work is needed to clarify if these behavioral phenotypes are related.
One of the primary factors thought to influence ethanol consumption levels is sensitivity to ethanol’s subjective effects, and we hypothesized that differences in sensitivity to the behavioral and neurobiological effects of ethanol may contribute to the observed differences in drinking behavior between high- and low-anxiety animals. Therefore, the first goal of this study was to determine if individual differences in baseline anxiety (as measured on the EPM) influence the acute anxiolytic effects of ethanol in the LD box. This design allowed for comparisons of measures of anxiety-like behavior in the EPM and the LD box in both saline- and ethanol-treated animals. Two behavioral tests were used as prior studies have demonstrated that re-exposure to tests like the EPM cannot be used to test drug responses, since EPM exposure produces one-trial tolerance and reduces the observed anxiolytic-like effects of benzodiazepines and ethanol in subsequent trials (File, Mabbutt, & Hitchcott, 1990; Bertoglio & Carobrez, 2002). We chose to use the LD box to assess ethanol-induced changes in anxiety because percent open arm time (%OAT) on the EPM has been shown to correlate with anxiety-like behaviors in the LD box (McCool & Chappell, 2007). We also analyzed the peripheral stress responses following the initial exposure to the EPM to examine if stress-induced changes in CORT or NPY levels correlated with the individual differences in anxiety-like responses in the EPM and/or ethanol-induced responses. Current evidence demonstrates a critical role for the NPY system in mediating both anxiety-related responses and the acute effects of alcohol (Sharko, Fadel, & Wilson, 2013).
It has been hypothesized that overlapping neurobiological mechanisms may account for the high rates of comorbidity seen for anxiety disorders and AUDs. Many of the brain regions that mediate the behavioral effects of ethanol also mediate responses to anxiogenic stimuli, particularly the central (CeA) and basolateral (BLA) nuclei of the amygdala and the bed nucleus of the stria terminalis (BNST). Acute ethanol treatment has been shown to alter neurotransmission in each of these regions (Roberto, Madamba, Moore, Tallent, & Siggins, 2003; Silberman, Shi, Brunso-Bechtold, & Weiner, 2008; Kash, Matthews, & Winder, 2008; Weitlauf, Egli, Grueter, & Winder, 2004; Lack, Ariwodola, Chappell, Weiner, & McCool, 2008), suggesting that these regions may be of particular importance in mediating the relationship between ethanol and anxiety. The second goal of this work was to identify differences in amygdalar and BNST activation that may underlie any behavioral differences in response to an anxiolytic dose of ethanol. We previously found that activation of the central amygdala and BNST was positively correlated with behavioral measures of anxiolysis following voluntary ethanol consumption (Sharko et al., 2013b). Using immunohistochemical techniques, we examined expression of Fos, an immediate-early gene commonly used as a marker for neuronal activation (Curran & Morgan, 1995), in the amygdala and the BNST in control and ethanol-treated rats, as well as activation of NPY-containing neurons in these regions.
Methods
Animals
Adult male Long-Evans rats (175–200 g; Harlan, Indianapolis, IN) were singly housed and maintained on a 12-h light-dark cycle (lights on at 1:00 AM) with ad libitum access to food and water. Animals were habituated to daily handling before the experiment, and body weight, water consumption, and food consumption were monitored for the duration of the experiment. All procedures were approved by the University of South Carolina Institutional Animal Care and Use Committee.
Responses in the elevated plus maze
One week before examining the acute effects of ethanol in the LD transition box, animals were tested in the EPM to assess their pre-existing anxiety levels in a similar test of novelty. Blood samples were taken before and after EPM testing to analyze EPM-induced increases in both CORT and NPY. Animals (N = 30) were tested on the EPM during the late light portion of the light-dark cycle. The apparatus consisted of four arms (56 × 10 cm) made of black Plexiglas® with gray matting, elevated 50 cm above the floor. Open arms had a 1 cm lip and closed arms had 40-cm tall black walls. Rats were placed on the maze in the center square facing an open arm and test sessions lasted 5 min. Behavior was tracked using the EthoVision XT automated tracking system (Noldus, Leesburg, VA). Percent open-arm time (%OAT), the ratio of time spent in the open arms over the time spent in all arms, and percent open-arm entries (%OAE) were calculated to assess anxiety-like behavior. Distance traveled and closed-arm entries were used to assess spontaneous locomotor behavior. For analysis purposes, animals were divided into high- and low-anxiety groups based on median split of percent open-arm time.
One week prior to EPM testing, animals were lightly restrained and 100 μL of blood was collected from a small nick in the tail vein. A second 100 μL tail blood sample was collected in the same way immediately following EPM testing. All blood samples were collected in tubes prepared with aprotinin and EDTA, then centrifuged, and plasma was stored at −20 °C until extraction and analysis. Plasma corticosterone levels were assessed in whole plasma using a CORT-specific ELISA kit (Enzo, Farmingdale, NY). For plasma NPY analysis, plasma samples were extracted over C-18 spin columns (Thermo, Rockford, IL), and NPY protein levels were analyzed from extracts using an NPY-specific ELISA kit (Bachem, Torrance, CA).
Acute ethanol effects in the light-dark transition box
One week after EPM testing, animals were tested in a light-dark transition box, which consisted of two connected chambers: a well-lit open-topped 30 × 30-cm chamber with clear Plexiglas walls® (30 cm) and a smaller 30 × 15-cm dark chamber with black Plexiglas® walls (30 cm). Ten minutes prior to LD testing, animals (N = 14) were administered 1 g/kg ethanol (N = 14; 10% v/v; i.p.) or an equivalent dose of saline vehicle (N = 16). Each treatment group had equal numbers of high- and low-anxiety rats. LD Box test sessions were performed late during the light cycle and lasted 5 min, to correspond to the duration of EPM testing. Animals were placed in the center of the light arena, facing away from the dark box. Behavior was tracked using EthoVision XT automated tracking system (Noldus, Leesburg, VA). Time spent in the light box, transition frequency, latency to enter the dark box, and distance traveled in the light chamber were assessed.
Immunohistochemistry
Two hours after light-dark box testing, animals were deeply anesthetized using isoflurane inhalation. This time point was selected as optimal for seeing changes in Fos protein and assessing neuronal activation following a challenge (Nestler, Barrot, & Self, 2001). Rats were transcardially perfused with ice-cold 0.1 M phosphate-buffered saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were removed from the skulls and post-fixed for 24 h in 4% paraformaldehyde. Brains were transferred to 15% sucrose in 0.1 M phosphate buffer and stored at 4 °C until sectioning. Coronal brains sections (45 μm) were cut on a microtome (Microm, Waldorf, Germany) and stored in anti-freezing solution (30% sucrose/30% ethylene glycol in 0.1 M phosphate buffer) at −20 °C until processing for immunohistochemistry.
Sections were washed in Tris-buffered saline prior to free-floating immunohistochemical processing. Following blocking, sections were agitated with goat Fos antibody (1:1000; Santa Cruz, Santa Cruz, CA), followed by biotinylated donkey anti-goat secondary antibody (1:1000 Jackson Immuno, West Grove, PA) and horseradish peroxidase-streptavidin (1:1600; Jackson Immuno, West Grove, PA). Labeling was visualized using nickel/cobalt-enhanced diaminobenzidine (DAB) to produce a blue-black precipitate confined to the nucleus of Fos immunoreactive neurons. Sections were then incubated with rabbit-NPY antibody (1:4000; Peninsula, Los Gatos, CA), followed by unlabeled donkey anti-rabbit secondary antibody (1:200; Jackson Immuno) and rabbit peroxidase anti-peroxidase (1:400; Covance, Princeton, NJ). Labeling was visualized with DAB to produce a brown precipitate in the cytoplasm of NPY-positive neurons. Sections were viewed at 10X–40X magnification under brightfield illumination (Nikon, Melville, NY). Images were acquired using a digital camera, and immunoreactivity was quantified using Neurolucida Microbright Field System (MBF Biosciences, Williston, VT).
Two sections were analyzed for BNST (coordinates: 0.20 to −0.40 from bregma) and four sections were analyzed for amygdala (coordinates: −1.80 to −3.60 from bregma) (Paxinos & Watson, 1986). Measurements were taken from the left and right side of each brain section and expressed as positive cell counts over area of region counted (counts/mm2). Measurements from individual brain regions for each animal were averaged to produce a single value for a given region in each animal.
Data analysis and statistics
For analysis purposes, animals were divided into high- and low-anxiety individuals based on a median split of %OAT on the EPM. ANOVA with repeated measures was used to compare pre- and post-EPM plasma levels of CORT and NPY. Two-way analysis of variance (ANOVA) was used to compare behavioral measures in the LD box across treatment (saline versus ethanol) and trait (high- and low-anxiety) groups. Since no significant differences were observed between high- and low-anxiety groups in the LD box, Fos and NPY-positive cell counts were analyzed using t tests to compare saline- and ethanol-treated groups. Correlation statistics were also used to compare behavioral measures, NPY/Fos-positive cell counts, and plasma corticosterone and NPY levels. All significance levels were set to p < 0.05.
Results
Responses to novelty in the EPM
As we have observed previously, a median split of %OAT in the EPM demonstrates a significant difference in anxiety-like behaviors (t[28] = 5.576, p < 0.0001) between high-anxiety (%OAT = 1.5 ± 0.7%) and low-anxiety (%OAT = 23.8 ± 3.9%) phenotypes. Plasma samples collected before and after EPM testing were analyzed for NPY and corticosterone concentrations to determine if differences in anxiety-like behavior were associated with either baseline differences in these markers or differential changes in these markers following a mild anxiogenic stimulus (EPM exposure). Two-way repeated-measure ANOVAs revealed that EPM exposure significantly increased plasma CORT levels (F[1,28] = 97.16; p < 0.0001), with post-EPM CORT levels (mean = 174.9 ± 5.06 ng/ml) almost double baseline levels (mean = 89.21 ± 7.28 ng/ml), but had no significant effect on plasma NPY levels (F[1,28] = 0.3280; p = 0.57). No significant differences in plasma CORT levels (F[1,28] = 0.2653; p = 0.61) or plasma NPY levels (F[1,28] = 0.1290; p = 0.72) were detected between high- and low-anxiety animals, nor were there any significant interactions between anxiety phenotype and sampling time point for either CORT or NPY. Additionally, no correlations were found between anxiety-like behaviors on the EPM (%OAT and %OAE) and either baseline or post-EPM plasma CORT and plasma NPY levels.
Light-dark transition box
Two-way ANOVAs detected an overall anxiolytic effect of ethanol on several measures in the LD box (Fig. 1), but no significant effect of anxiety phenotype (as assessed in the EPM) and no interaction between anxiety phenotype and ethanol effects for any behavior in the LD box. Additionally, planned comparisons showed no differences in anxiety-like behaviors in the LD box between high- and low-anxiety groups following either saline or ethanol treatment. As high- and low-anxiety groups did not differ in measures from the LD box, animals were collapsed into saline and ethanol groups for graphical representations (Fig. 1). Comparisons of %OAT and %OAE (EPM) with behavioral measures from the LD box (time spent in the light arena, transition frequency, and latency to first transition) yielded no significant correlations between anxiety-like behaviors in these two tests. However, a significant positive correlation was found between measures of general locomotor behavior (distance traveled) in the EPM and LD box (r = 0.54; p < 0.05; data not shown) for control animals, but not among ethanol-treated animals.
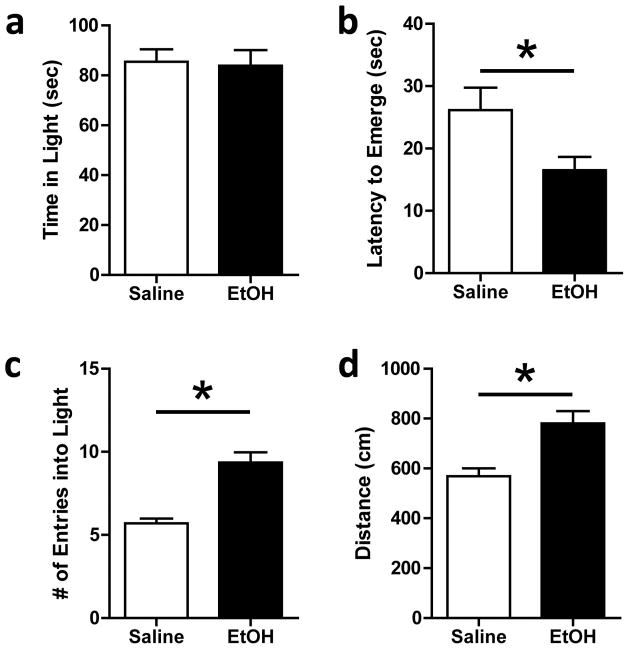
Fig. 1.
Light-dark box behaviors. a) Ethanol treatment did not alter total time spent in the light arena. b) Ethanol-treated animals showed a decreased latency to emerge from the dark arena, as compared to saline-treated animals (*p < 0.0001). c) Ethanol treatment significantly increased the number of transitions made between the light and dark arenas (*p < 0.05). d) Distance traveled in light arena was significantly greater in ethanol-treated animals compared to saline-treated animals (*p < 0.001) (N = 7–8)
Although mean time spent in the light arena was not significantly different between the saline and ethanol-treated groups (Fig. 1a; F[1,26] = 0.038; p = 0.85), ethanol treatment significantly decreased latency to emerge from the dark arena (Fig. 1b; F[1,26] = 4.579; p < 0.05) and significantly increased the number of transitions between the light and dark arenas (Fig. 1c; F[1,26] = 31.85; p < 0.0001). Ethanol-treated animals also exhibited greater locomotor behavior in the light arena than saline-treated animals (Fig. 1d; F[1,26] = 13.70; p < 0.005).
Fos and NPY expression
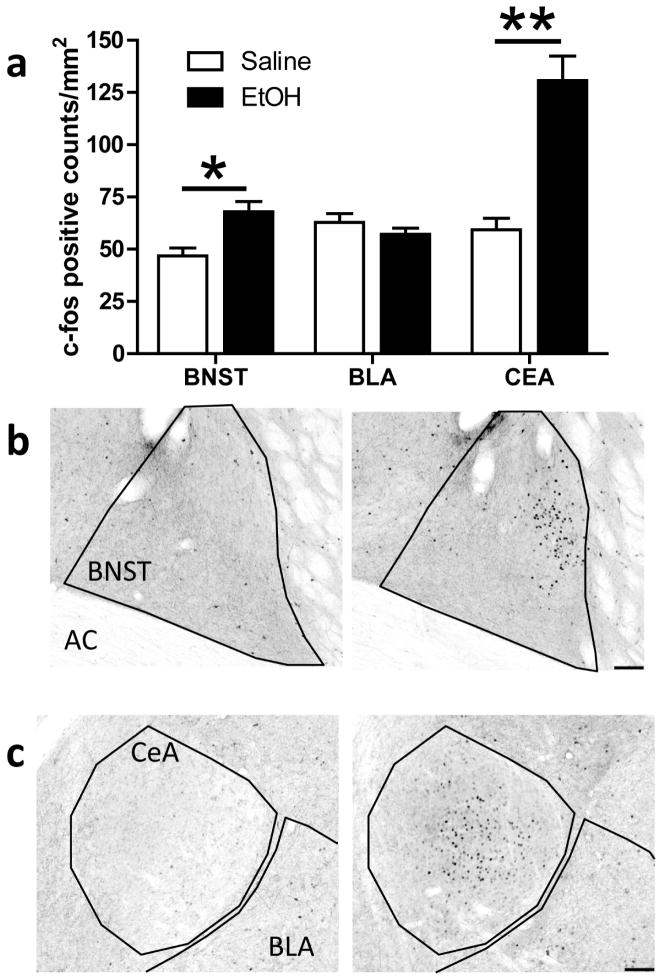
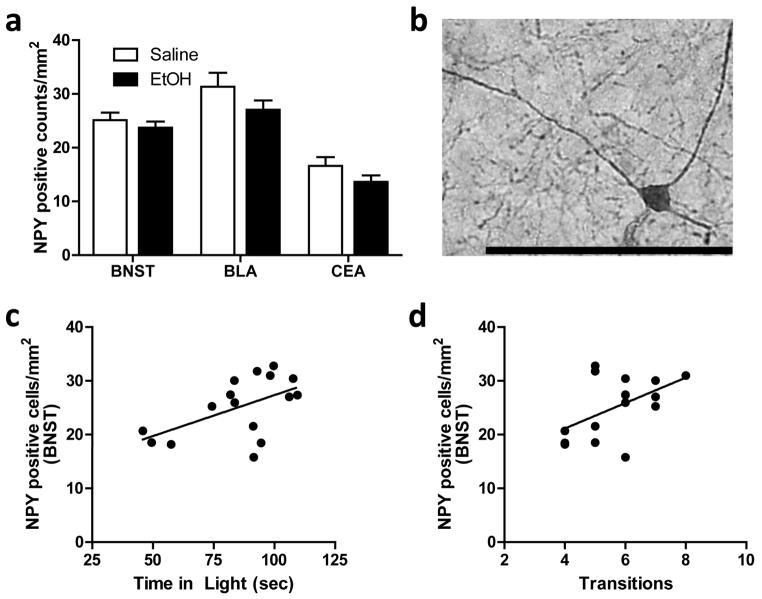
Ethanol-treated animals showed significantly more Fos-positive cell counts in both the BNST (Fig. 2a; p < 0.005) and the CeA (Fig. 2a; p < 0.0001), compared to saline-treated animals. In the BNST, Fos immunoreactivity was restricted to the dorsolateral region (Fig. 2b), while in the CeA, Fos immunoreactivity was found in both the medial and lateral divisions (Fig. 2c). No significant effect of ethanol treatment was found in the BLA (Fig. 2a). Additionally, no significant correlations were found between Fos-positive cell counts in these brain regions and any LD Box behavior. No significant main effect of ethanol treatment on the density of NPY-positive neurons was detected for the BNST, CeA, or BLA (Fig. 3a), and NPY-positive neurons did not show significant Fos co-immunoreactivity in any of the brain regions examined. Fig. 3b shows a representative photomicrograph of NPY-positive staining in the amygdala (40X magnification). However, correlational analyses showed positive relationships between the number of NPY-positive cells in the BNST and both time spent in the light arena (Fig. 3c; r = 0.54; p < 0.05) and transition frequency (Fig. 3d; r = 0.51; p < 0.05) among saline-treated animals; no correlations were found between these measures in ethanol-treated animals.
Fig. 2.
Fos-positive cell counts in the BNST, BLA, and CeA. a) Acute ethanol exposure significantly increased Fos-positive cell counts in the BNST (*p < 0.005) and the CeA (**p < 0.0001), but not in the BLA. b–c) Representative photomicrographs (10X) of Fos immunoreactivity in the BNST (b) and CeA (c) of a saline- (left panel) and ethanol-treated (right panel) animal. Scale bar = 100 microns.
Fig. 3.
NPY-positive cell counts in the BNST, BLA, and CeA. a) No differences in NPY-positive cell counts between groups were seen in any of the regions examined: BNST, BLA, or CeA. b) Representative photomicrograph (40X) of NPY immunoreactivity in the CeA. Scale bar = 100 microns. c–d) Plots showing positive correlations between NPY-positive cell counts in the BNST and (c) time spent in the light arena (R2 = 0.2960) and (d) number of light-dark transitions (R2 = 0.2607).
Discussion
Although the hereditary and demographic factors that increase risk for alcohol abuse and dependence are well known (Swendsen et al., 2009; Kalaydjian et al., 2009; Merikangas, Leckman, Prusoff, Pauls, & Weissman, 1985), more subtle behavioral and neurobiological differences also contribute to susceptibility to alcohol-use disorders. Our laboratory has described an outbred Long-Evans rat model for examining the relationship between individual differences in basal anxiety-like behavior and vulnerability to excessive alcohol use and alcohol abuse. Using this model, we have previously shown that individual differences in ethanol consumption in a limited-access paradigm induce differential anxiolytic effects in the EPM (Sharko et al., 2013b), and that differences in anxiety phenotype correlate with differences in voluntary ethanol consumption (Primeaux et al., 2006). This report expands these findings but suggests that individual differences in anxiety state, as assessed in the EPM, do not predict differential effects of acutely administered ethanol in the LD box. These low doses of ethanol produced anxiolytic effects in several measures in the LD box and activated neurons in the central amygdala and BNST in a manner similar to that seen previously in rats self-administering ethanol (Sharko et al., 2013a). Although ethanol treatment did not significantly activate NPY-containing neurons, individual differences in time spent in the light and in number of light-dark transitions among saline-treated animals were positively correlated with the density of NPY-containing neurons in the BNST, suggesting these NPY-containing neurons may help regulate basal anxiety state, but perhaps not the acute anxiolytic effects of ethanol.
Overall, acute ethanol administration had an anxiolytic effect in the LD box. Ethanol-treated animals had significantly more transitions between the light and dark arenas and shorter latency to leave the dark chamber as compared to saline-treated animals, but ethanol treatment did not significantly alter other measures of anxiety-like behavior in this test. Prior reports of ethanol’s effects on behavior in the LD box have been inconsistent, making it difficult to clearly determine the best measure of the anxiolytic effects of ethanol in the LD box. Ethanol self-administration increased transitions and light-zone time and decreased emergence latency in rats (Gallate, Morley, Ambermoon, & McGregor, 2003), while injected ethanol either increased transitions without changing light-zone time or emergences (Correa, Manrique, Font, Escrig, & Aragon, 2008), or had no effect on any of these measures (Grisel, Bartels, Allen, & Turgeon, 2008) in mice. Although positive correlations between anxiety-like behaviors in the EPM and LD box have been reported (McCool & Chappell, 2007), we found no significant correlations between %OAT on the EPM and any behavioral measures in the LD box in either saline- or ethanol-treated groups. Further, individual differences in anxiety-like behavior in the EPM do not appear to translate into differences in anxiety-related behaviors in the LD box.
A number of studies indicate that sensitivity to the anxiolytic effects of ethanol positively correlate with ethanol consumption. High-ethanol consuming P rats are more sensitive to the anxiolytic properties of ethanol than low-ethanol consuming NP rats (Stewart et al., 1993), and adolescent rats, who typically consume more ethanol than adult rats, also show increased sensitivity to the anxiety-reducing effects of ethanol as compared to adult rats (Varlinskaya & Spear, 2012). However, rats selectively bred for high (HAB) and low (LAB) anxiety behavior show the opposite relationship. HAB rats are more sensitive to ethanol-induced anxiolysis than the LAB rats, but consume less ethanol (Henniger, Spanagel, Wigger, Landgraf, & Holter, 2002). These data suggest that sensitivity to ethanol’s anxiolytic properties may not be related specifically to ethanol consumption patterns, but it must be noted that different ethanol access paradigms were used in these experiments, and as we have seen in our own work, this may influence ethanol consumption patterns (Primeaux et al., 2006; White et al., 2009).
Interestingly, the correlation between innate anxiety-like behavior and sensitivity to the anxiolytic effects of ethanol are consistent across these three models, with HAB rats, P rats, and adolescents showing greater basal anxiety-like behavior, in addition to greater sensitivity to ethanol-induced anxiolysis, as compared to LAB rats, NP rats, and adult rats, respectively (Henniger et al., 2002; Stewart et al., 1993; Lynn & Brown, 2010). These results suggest that this relationship may be pertinent to anxiety-induced ethanol consumption. While we did see an overall anxiolytic effect of ethanol during the LD box testing, we found no differences in sensitivity to the anxiolytic effects of ethanol between high- and low-anxiety groups as assessed in the EPM. These data indicate that initial anxiety phenotype, at least as assessed using the EPM, is not predictive of sensitivity to the anxiolytic effects of ethanol in the LD box. Although the EPM and LD box both assess unconditioned anxiety-like responses by measuring avoidance behaviors, factor analyses suggest these two apparatuses also assess unique aspects of anxiety-like phenotypes. Specifically, EPM responses were reported to be more related to exploration, while LD box behaviors were more related to neophobia (Belzung & Le Pape, 1994). Subtle differences between these behavioral assays may be a confound in our experiments. Several other factors may contribute to the lack of an observable difference in behavioral responses to ethanol in high- and low-anxiety rats as well.
One of these factors may be the lack of correlation between anxiety-like behaviors on the EPM and anxiety-like behaviors in the LD box. McCool and Chappel (2007) found a significant correlation between time in the open arm in the EPM and time in the light box in the LD box among Long-Evans rats, noting that the behaviors were less robustly expressed in the LD box. We, however, found no correlation between %OAT and anxiety-like behaviors in the LD box among saline-treated control animals. This difference could be the result of differences in experimental protocol or testing conditions. Unlike McCool and Chappel (2007), our animals did not undergo a surgical procedure prior to testing, but did receive an intraperitoneal injection (i.e., a mild stressor) 10 min before testing in the LD box. Mean open-arm time on the EPM in our experiments for high- and low-anxiety animals was 48 sec and 3 sec, respectively, as compared to 120 sec (high) and 30 sec (low) in McCool and Chappel, suggesting that either the prior surgery or the injection may be influencing subsequent measures of anxiety-like behaviors; alternatively, this may be a matter of differences in analysis parameters for the EPM. Unfortunately, a single exposure to the EPM apparatus eliminates the anxiolytic effects of a number of drugs, including ethanol, in subsequent trials (File et al., 1990; Bertoglio & Carobrez, 2002), prohibiting the use of the EPM for both determination of basal anxiety phenotype and ethanol-induced changes in anxiety-like behavior. It is also possible that like re-exposure to the EPM, which produces tolerance to the effects of anxiolytics, pre-exposure to the EPM may induce blunted ethanol effects in the LD Box one week later. A second concern is the dose of ethanol used. Although 1 g/kg is within the reported dose range (0.5 g/kg to 2 g/kg) and below the maximal dose for ethanol’s anxiolytic effects (Wilson et al., 2004; Bertoglio & Carobrez, 2002; Boerngen-Lacerda & Souza-Formigoni, 2000), this dose may be producing a ceiling effect in the LD box, with both high- and low-anxiety animals responding similarly. We feel this is unlikely given that blood ethanol concentrations for this dose at 10 min post-injection are between 0.1 and 0.2 ng/mL (Wilson et al., 2004), although blood ethanol analysis was not performed in this study to avoid the stress of a tail bleed that may have confounded our results assessing neuronal activation. It is possible that differential responses would be seen in low- and high-anxiety groups at other time points after ethanol administration or at higher ethanol doses. Blood ethanol levels peak 10 min after intraperitoneal injection, making it an ideal time point for analysis of ethanol effects, and in a pilot study, a 1.5 g/kg dose of ethanol produced noticeable ataxia and behavior in the LD box could not be scored. Finally, individual differences in sensitivity to the anxiolytic effects of ethanol based on anxiety phenotype may only be seen with self-administration rather than acute injection paradigms. This distinction could be related to the stress of injection procedures prior to testing during acute administration paradigms, although animals are extensively habituated to handling prior to testing and saline-treated animals similarly receive an intraperitoneal injection.
Ethanol treatment significantly increased Fos immunoreactivity in the CeA, as well as in the BNST, but did not alter Fos expression in the BLA. The patterns of Fos expression we found in the amygdala are in keeping with reports detailing the neuroanatomical targets of low to moderate doses of ethanol (0.5–2.0 g/kg) administered under acute conditions (Morales, Criado, Sanna, Henriksen, & Bloom, 1998; Bachtell, Wang, Freeman, Risinger, & Ryabinin, 1999; Thiele, van, & Bernstein, 1997; Chang, Patel, & Romero, 1995), and are similar to the activation pattern we saw after ethanol self-administration just prior to exposure to the EPM (Sharko et al., 2013b). Higher doses of ethanol (3 g/kg) have been shown to increase Fos immunoreactivity in the BNST, but most studies report no change in this region following lower doses of ethanol (Bachtell et al., 1999; Chang et al., 1995; Herring, Mayfield, Camp, & Alcantara, 2004; White et al., 2009; Sharko et al., 2013b). As in our previous study, the neuronal activation in the CeA may represent a combined effect of ethanol treatment and exposure to a mild anxiogenic stimulus, in this case the LD Box. The BNST has been implicated as preferentially mediating unconditioned anxiety-like responses as compared to conditioned-fear responses, which are more associated with amygdalar activity (Walker, Toufexis, & Davis, 2003). As ethanol treatment also increased Fos immunoreactivity in the BNST, increased neuronal activation in this region may be part of an anxiety-reducing pathway activated by anxiogenic stimuli, which is responsive to anxiolytic drugs. Ethanol treatment did not significantly alter NPY-positive cell counts or induce significant Fos accumulation in NPY-positive cells, suggesting these cells are not activated in response to an acute ethanol challenge. However, it may be that NPY neurons do not express c-Fos and that alternative measures, such as NPY release or expression of other immediate early genes, may better reflect acute NPY activation. Interestingly, a significant correlation was detected between the density of NPY neurons in the BNST and anxiety-like behavior in the LD box. Consistent with the notion that higher NPY levels are anxiolytic, greater numbers of NPY-positive neurons in the BNST were correlated with more time in the light arena and more transitions to the light in the LD Box. This is similar to decreased measures of anxiety seen in the EPM following increases in NPY expression by virus-mediated gene transfer (Primeaux et al., 2006). That these correlations were only detected in saline-treated control animals is consistent with NPY’s role in mediating basal anxiety levels. The lack of a correlation in the ethanol-treated animals may indicate that ethanol has an anxiolytic effect independent of NPY activity, and perhaps another neurotransmitter in the BNST is mediating the acute anxiolytic effects of ethanol. Alternatively, ethanol-induced changes in NPY activity and NPY-mediated anxiety-like responses may only become apparent after long-term ethanol exposure or the development of dependence (Gilpin, 2012). Overall, the findings support the notion that neuronal activation in the CeA, and perhaps in BNST, is central to the acute anxiety-reducing effects of ethanol in these behavioral paradigms. Ethanol is well known to influence physiological responses in the CeA. Through these effects ethanol may be altering physiological responses in downstream targets, including the BNST, to modulate anxiety-like responses (Gilpin & Roberto, 2012).
Highlights.
Acute low dose ethanol treatment increases Fos activation in the BNST and CeA
Low NPY density in the BNST was correlated with high anxiety-like behavior in LD Box
Mild stress exposure did not alter circulating NPY levels
No significant correlation between anxiety-like behaviors in the EPM and LD Box
Acknowledgments
This work was supported by grants from the National Institute for Mental Health to JRF and MAW (MH063344) and the National Institute on Alcohol Abuse and Alcoholism to JRF and MAW (AA017361) and a VA Merit Award to MAW (1101 BX001374).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Bachtell RK, Wang YM, Freeman P, Risinger FO, Ryabinin AE. Alcohol drinking produces brain region-selective changes in expression of inducible transcription factors. Brain Res. 1999;847:157–165. doi: 10.1016/s0006-8993(99)02019-3. [DOI] [PubMed] [Google Scholar]
- Belzung C, Le Pape G. Comparison of different behavioral test situations used in psychopharmacology for measurement of anxiety. Physiol Behav. 1994;56:623–628. doi: 10.1016/0031-9384(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Bertoglio LJ, Carobrez AP. Anxiolytic effects of ethanol and phenobarbital are abolished in test-experienced rats submitted to the elevated plus maze. Pharmacol Biochem Behav. 2002;73:963–969. doi: 10.1016/s0091-3057(02)00958-9. [DOI] [PubMed] [Google Scholar]
- Boerngen-Lacerda R, Souza-Formigoni ML. Does the increase in locomotion induced by ethanol indicate its stimulant or anxiolytic properties? Pharmacol Biochem Behav. 2000;67:225–232. doi: 10.1016/s0091-3057(00)00360-9. [DOI] [PubMed] [Google Scholar]
- Chang SL, Patel NA, Romero AA. Activation and desensitization of Fos immunoreactivity in the rat brain following ethanol administration. Brain Res. 1995;679:89–98. doi: 10.1016/0006-8993(95)00210-h. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Lobina C, Reali R, Zocchi A, Fadda F, et al. Sardinian alcohol-preferring rats: a genetic animal model of anxiety. Physiol Behav. 1995;57:1181–1185. doi: 10.1016/0031-9384(94)00382-f. [DOI] [PubMed] [Google Scholar]
- Correa M, Manrique HM, Font L, Escrig MA, Aragon CM. Reduction in the anxiolytic effects of ethanol by centrally formed acetaldehyde: the role of catalase inhibitors and acetaldehyde-sequestering agents. Psychopharmacology (Berl) 2008;200:455–464. doi: 10.1007/s00213-008-1219-3. [DOI] [PubMed] [Google Scholar]
- Curran T, Morgan JI. Fos: an immediate-early transcription factor in neurons. J Neurobiol. 1995;26:403–412. doi: 10.1002/neu.480260312. [DOI] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, et al. Effects of moderate alcohol consumption on the central nervous system. Alcohol Clin Exp Res. 1998;22:998–1040. doi: 10.1111/j.1530-0277.1998.tb03695.x. [DOI] [PubMed] [Google Scholar]
- File SE, Mabbutt PS, Hitchcott PK. Characterisation of the phenomenon of “one-trial tolerance” to the anxiolytic effect of chlordiazepoxide in the elevated plus-maze. Psychopharmacology (Berl) 1990;102:98–101. doi: 10.1007/BF02245751. [DOI] [PubMed] [Google Scholar]
- Gallate JE, Morley KC, Ambermoon P, McGregor IS. The consequences of beer consumption in rats: acute anxiolytic and ataxic effects and withdrawal-induced anxiety. Psychopharmacology (Berl) 2003;166:51–60. doi: 10.1007/s00213-002-1291-z. [DOI] [PubMed] [Google Scholar]
- Gilpin NW. Corticotropin-releasing factor (CRF) and neuropeptide Y (NPY): effects on inhibitory transmission in central amygdala, and anxiety- & alcohol-related behaviors. Alcohol. 2012;46:329–337. doi: 10.1016/j.alcohol.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Roberto M. Neuropeptide modulation of central amygdala neuroplasticity is a key mediator of alcohol dependence. Neurosci Biobehav Rev. 2012;36:873–888. doi: 10.1016/j.neubiorev.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grisel JE, Bartels JL, Allen SA, Turgeon VL. Influence of beta-Endorphin on anxious behavior in mice: interaction with EtOH. Psychopharmacology (Berl) 2008;200:105–115. doi: 10.1007/s00213-008-1161-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henniger MS, Spanagel R, Wigger A, Landgraf R, Holter SM. Alcohol self-administration in two rat lines selectively bred for extremes in anxiety-related behavior. Neuropsychopharmacology. 2002;26:729–736. doi: 10.1016/S0893-133X(01)00408-0. [DOI] [PubMed] [Google Scholar]
- Herring BE, Mayfield RD, Camp MC, Alcantara AA. Ethanol-induced Fos immunoreactivity in the extended amygdala and hypothalamus of the rat brain: focus on cholinergic interneurons of the nucleus accumbens. Alcohol Clin Exp Res. 2004;28:588–597. doi: 10.1097/01.alc.0000122765.58324.6d. [DOI] [PubMed] [Google Scholar]
- Kalaydjian A, Swendsen J, Chiu WT, Dierker L, Degenhardt L, Glantz M, et al. Sociodemographic predictors of transitions across stages of alcohol use, disorders, and remission in the National Comorbidity Survey Replication. Compr Psychiatry. 2009;50:299–306. doi: 10.1016/j.comppsych.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash TL, Matthews RT, Winder DG. Alcohol inhibits NR2B-containing NMDA receptors in the ventral bed nucleus of the stria terminalis. Neuropsychopharmacology. 2008;33:1379–1390. doi: 10.1038/sj.npp.1301504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliethermes CL. Anxiety-like behaviors following chronic ethanol exposure. Neurosci Biobehav Rev. 2005;28:837–850. doi: 10.1016/j.neubiorev.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev. 2000;20:149–171. doi: 10.1016/s0272-7358(99)00027-6. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Wall MM, Krueger RF, Sher KJ, Maurer E, Thuras P, et al. Alcohol Dependence is Related to Overall Internalizing Psychopathology Load Rather than to Particular Internalizing Disorders: Evidence from a National Sample. Alcohol Clin Exp Res. 2011 doi: 10.1111/j.1530-0277.2011.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lack AK, Ariwodola OJ, Chappell AM, Weiner JL, McCool BA. Ethanol inhibition of kainate receptor-mediated excitatory neurotransmission in the rat basolateral nucleus of the amygdala. Neuropharmacology. 2008;55:661–668. doi: 10.1016/j.neuropharm.2008.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn DA, Brown GR. The ontogeny of anxiety-like behavior in rats from adolescence to adulthood. Dev Psychobiol. 2010;52:731–739. doi: 10.1002/dev.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Chappell A. Strychnine and taurine modulation of amygdala-associated anxiety-like behavior is ‘state’ dependent. Behav Brain Res. 2007;178:70–81. doi: 10.1016/j.bbr.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Leckman JF, Prusoff BA, Pauls DL, Weissman MM. Familial transmission of depression and alcoholism. Arch Gen Psychiatry. 1985;42:367–372. doi: 10.1001/archpsyc.1985.01790270057006. [DOI] [PubMed] [Google Scholar]
- Morales M, Criado JR, Sanna PP, Henriksen SJ, Bloom FE. Acute ethanol induces c-fos immunoreactivity in GABAergic neurons of the central nucleus of the amygdala. Brain Res. 1998;798:333–336. doi: 10.1016/s0006-8993(98)00457-0. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, Self DW. DeltaFosB: a sustained molecular switch for addiction. Proc Natl Acad Sci USA. 2001;98:11042–11046. doi: 10.1073/pnas.191352698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2. Academic Press; 1986. [DOI] [PubMed] [Google Scholar]
- Primeaux SD, Wilson SP, Bray GA, York DA, Wilson MA. Overexpression of neuropeptide Y in the central nucleus of the amygdala decreases ethanol self-administration in “anxious” rats. Alcohol Clin Exp Res. 2006;30:791–801. doi: 10.1111/j.1530-0277.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Moore SD, Tallent MK, Siggins GR. Ethanol increases GABAergic transmission at both pre- and postsynaptic sites in rat central amygdala neurons. Proc Natl Acad Sci USA. 2003;100:2053–2058. doi: 10.1073/pnas.0437926100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharko AC, Fadel JR, Wilson MA. Mechanisms and mediators of the relationship between anxiety disorders and alcohol use disorders: focus on amygdalar NPY. Journal of Addiction Research and Therapy. 2013;014 [Google Scholar]
- Sharko AC, Kaigler KF, Fadel JR, Wilson MA. Individual differences in voluntary ethanol consumption lead to differential activation of the central amygdala in rats: relationship to the anxiolytic and stimulant effects of low dose ethanol. Alcohol Clin Exp Res. 2013;37(Suppl 1):E172–E180. doi: 10.1111/j.1530-0277.2012.01907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Shi L, Brunso-Bechtold JK, Weiner JL. Distinct mechanisms of ethanol potentiation of local and paracapsular GABAergic synapses in the rat basolateral amygdala. J Pharmacol Exp Ther. 2008;324:251–260. doi: 10.1124/jpet.107.128728. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Montkowski A, Allingham K, Stohr T, Shoaib M, Holsboer F, et al. Anxiety: a potential predictor of vulnerability to the initiation of ethanol self-administration in rats. Psychopharmacology (Berl) 1995;122:369–373. doi: 10.1007/BF02246268. [DOI] [PubMed] [Google Scholar]
- Stewart RB, Gatto GJ, Lumeng L, Li TK, Murphy JM. Comparison of alcohol-preferring (P) and nonpreferring (NP) rats on tests of anxiety and for the anxiolytic effects of ethanol. Alcohol. 1993;10:1–10. doi: 10.1016/0741-8329(93)90046-q. [DOI] [PubMed] [Google Scholar]
- Swendsen J, Conway KP, Degenhardt L, Dierker L, Glantz M, Jin R, et al. Sociodemographic risk factors for alcohol and drug dependence: the 10-year follow-up of the national comorbidity survey. Addiction. 2009;104:1346–1355. doi: 10.1111/j.1360-0443.2009.02622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen J, Conway KP, Degenhardt L, Glantz M, Jin R, Merikangas KR, et al. Mental disorders as risk factors for substance use, abuse and dependence: results from the 10-year follow-up of the National Comorbidity Survey. Addiction. 2010;105:1117–1128. doi: 10.1111/j.1360-0443.2010.02902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, van DG, Bernstein IL. Ethanol-induced c-Fos expression in rat lines selected for low and high alcohol consumption. Brain Res. 1997;756:278–282. doi: 10.1016/s0006-8993(97)00228-x. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, et al. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Increases in anxiety-like behavior induced by acute stress are reversed by ethanol in adolescent but not adult rats. Pharmacol Biochem Behav. 2012;100:440–450. doi: 10.1016/j.pbb.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Weitlauf C, Egli RE, Grueter BA, Winder DG. High-frequency stimulation induces ethanol-sensitive long-term potentiation at glutamatergic synapses in the dorsolateral bed nucleus of the stria terminalis. J Neurosci. 2004;24:5741–5747. doi: 10.1523/JNEUROSCI.1181-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LC, Ford KA, Fadel JR, Wilson MA. Individual variation in anxiety and ethanol self-administration: a pheotypic analysis of limbic system neuronal activation. International Behavioral Neuroscience Society Meeting 2009 [Google Scholar]
- Wilson MA, Burghardt PR, Ford KA, Wilkinson MB, Primeaux SD. Anxiolytic effects of diazepam and ethanol in two behavioral models: comparison of males and females. Pharmacol Biochem Behav. 2004;78:445–458. doi: 10.1016/j.pbb.2004.04.017. [DOI] [PubMed] [Google Scholar]