Abstract
Recent work has suggested separate developmental periods within the broader framework of adolescence, with data suggesting distinct alterations and vulnerabilities within these intervals. While previous research has suggested reduced sensitivity to the aversive effects of alcohol in adolescence relative to adults, a more detailed ontogeny of this effect has yet to be conducted. The adolescent brain undergoes significant transitions throughout adolescence, including in regions linked with drug reward and aversion. The current study aimed to determine the ontogeny of ethanol aversion by utilizing a conditioned taste aversion procedure at six different ages to test the hypothesis that the transitions into, through, and out of adolescence are associated with ontogenetic alterations in sensitivity to the aversive properties of ethanol. Non-deprived animals given Boost® as the conditioned stimulus (CS) were used in Experiment 1, whereas Experiment 2 used water-restricted animals provided with a saccharin/sucrose solution as the CS. In both experiments, an attenuated sensitivity to the aversive properties of ethanol was evident in adolescents compared to adults, although more age differences were apparent in water deprived animals than when a highly palatable CS was given to ad libitum animals. Overall, the data suggest an attenuated sensitivity to the aversive properties of ethanol that is most pronounced during pre- and early adolescence, declining thereafter to reach the enhanced aversive sensitivity of adults.
Keywords: Adolescence, Conditioned Taste Aversion, Ethanol, Water Deprivation
Introduction
Adolescence is an ontogenetic stage defined by age-specific alterations that are highly conserved across species, including neural, hormonal, and physical transformations. Changes in behavior are also noted, including adolescent-associated increases in social activity, novelty seeking, and risk taking (See Spear, 2000 and 2010 for a review). These and other age-typical behavioral characteristics of adolescence, including greater levels of alcohol consumption than are seen in adulthood, are evident across a variety of mammalian species, (see Spear, 2000, 2010 for a review). Given such across-species similarities, rodent models of adolescence have long been employed for the study of contributors to adolescent-typical behavioral and cognitive functions. For example, based on relative timing of biological, behavioral, and hormonal measures, the postnatal day (P) 25-P42 interval in rats has been suggested to be roughly analogous to the early-mid adolescent period in human (10–18 years), while P43-P55 mirrors approximately the age range from 18–25 years, or late adolescence/emerging adulthood in humans (C. S. Vetter-O’Hagen & Spear, 2012a). When puberty is indexed via vaginal opening (females) and balano preputial skinfold separation and sperm in the seminiferous tubules (males), female rats exhibit pubertal maturation by P36, with a majority of males exhibiting signs of maturation by P40 (C. S. Vetter-O’Hagen & Spear, 2012b). Thus, although the terms adolescence and puberty are often used synonymously, the two represent different transitions, with puberty classically referring to the attainment of sexual maturation (Graber & Brooks-Gunn, 1998) which generally occurs during early-mid adolescence, whereas adolescence encompasses the broader transition from childhood to adulthood, thus encompassing a greater range of developmental alterations (Blakemore, Burnett, & Dahl, 2010; Sisk & Foster, 2004).
Initiation of alcohol use commonly occurs among youth during adolescence (Faden, 2006). This may be problematic given that earlier ages of drinking initiation predict later problems with dependence (although this association is not necessarily causal – e.g. Dawson et al., 2008). Given that marked elevations in per occasion alcohol consumption among adolescents relative to adults are evident not only in humans (Hughes, 2010; Masten, Faden, Zucker, & Spear, 2009), but in other mammalian species such as rodents (Doremus, Brunell, Rajendran, & Spear, 2005; Vetter, Doremus-Fitzwater, & Spear, 2007), rodent models have been used to investigate potential factors contributing to the elevated ethanol intake of adolescence. Substantial alterations in activity of, and connectivity to, areas essential for processing rewarding (e.g. NAc) and aversive (e.g. amygdala) stimuli during adolescence (e.g. see Crews, He and Hodge, 2007 for a review) offer one possible framework contributing to age-specific responses to drug stimuli. Indeed, studies have determined adolescents to be relatively insensitive to many ethanol effects when compared to adults, particularly to ethanol effects such as its aversive properties which likely serve as cues to limit intake (for review, see Doremus-Fitzwater et al., 2010; Spear and Varlinskaya, 2005). However, whereas adolescents show an attenuated sensitivity to ethanol-induced sedative (Silveri & Spear, 1998), motor impairing (White et al., 2002), social disrupting (Varlinskaya & Spear, 2002) and aversive (Anderson, Varlinskaya, & Spear, 2010) effects, they conversely exhibit enhanced sensitivity to a few ethanol effects, including ethanol-induced social facilitation (Varlinskaya & Spear, 2006), memory impairments (Markwiese, Acheson, Levin, Wilson, & Swartzwelder, 1998), and its rewarding properties (Pautassi, Myers, Spear, Molina, & Spear, 2008; Ristuccia & Spear, 2008). This pattern of reduced sensitivity to aversive but increased sensitivity to rewarding properties is often associated with elevations in ethanol intake (Green & Grahame, 2008).
Another factor that could affect the sensitivity and intake of alcohol is sex. Female rodents demonstrate elevated ethanol intake relative to males beginning in late adolescence (Lancaster et al., 1996; Truxell, Molina, & Spear, 2007) and continuing into adulthood (Doremus et al., 2005; Vetter et al., 2007; C. Vetter-O’Hagen et al., 2009). Gonadal hormones may play a role in these differences, given that gonadectomy in males, either pre-pubertally or in adulthood, induces female-typical patterns of ethanol intake (C. S. Vetter-O’Hagen & Spear, 2011), with male-typical intake restored by testosterone replacement (C. S. Vetter-O’Hagen, Sanders, & Spear, 2011). It is possible that sex differences in ethanol sensitivity may occur around puberty that serve to promote emergence of sex differences in intake. If so, differences in the timing of puberty between males and females may lead to different ontogenetic patterns of ethanol sensitivity between males and females.
Reviews by Spear and Brake (1983) and Spear (2015) suggest the possibility of developmental dissociations in drug sensitivity and vulnerability during ages leading up to and continuing through adolescence into adulthood. Preadolescent/juvenile animals sometimes have been found to display patterns of behavior and drug sensitivity more similar to their adult counterparts than do adolescents, suggesting a developmental dissociation specific to adolescence (see Spear and Brake, 1983 for a review). For example, this ontogenetic dissociation, with adolescents being different than their younger or older counterparts, has been seen in response to amphetamine, with preadolescent rats expressing a pattern of taste aversion more similar to older animals than to adolescents (Infurna & Spear, 1979). Whether such an ontogenetic dissociation would be evident with ethanol has yet to be explored. Electrophysiological data from chronic exposure studies in animals have, however, suggested separable sensitivities within adolescence, with more marked alterations in the hippocampus following P30-40 ethanol exposure compared to P35-40 (Slawecki, Betancourt, Cole, & Ehlers, 2001). Likewise, intermittent intraperitoneal (i.p.) ethanol injections during P30-43, but not P45-57, resulted in later elevations in ethanol consumption relative to control animals (Alaux-Cantin et al., 2013), further highlighting separate periods of vulnerability within adolescence. These data are reminiscent of research showing higher rates of alcohol dependence among individuals who initiated drinking prior to age 14 compared to those who began drinking after age 20 (Grant & Dawson, 1997), with the later incidence of alcohol dependence greater for those first using alcohol at age 11–12 (15.9%) than at 13–14 (9%), and rapidly declining thereafter to a 1% incidence rate in those who began drinking age at 19 and older (DeWit, Adlaf, Offord, & Ogborne, 2000).
Conditioned taste aversion (CTA) is a method often used to measure the dysphoric effects of ethanol. In this procedure, ingestion of a novel flavor (CS) is paired with the effects of a specific drug (unconditioned stimulus, US). When the animals are later given an opportunity to consume the CS, the degree to which the animal avoids the solution is used to index the relative dysphoria experienced to the US during the CS/US pairing(s). Our laboratory has previously demonstrated a relative insensitivity to ethanol induced CTA in adolescents, with a higher dose (Anderson et al., 2010; C. Vetter-O’Hagen et al., 2009) or more CS-US pairings (Anderson et al., 2010) needed to produce attenuated intake of the CS in adolescents than adults. Given that the overall hedonic value of a drug is thought to be a function of the balance between its rewarding and aversive effects (Riley, 2011; M. Schuckit, Smith, Pierson, Danko, & Beltran, 2006; M. A. Schuckit et al., 2009; Verendeev & Riley, 2012), the relative insensitivity of adolescents to the aversive effects of ethanol may contribute to the enhancement of ethanol intake at this time.
While there is convincing evidence from both our laboratory and others that mid-adolescents are less sensitive to CTA to ethanol than are adults (Anderson et al., 2010; Holstein, Spanos, & Hodge, 2011; Schramm-Sapyta et al., 2010; C. Vetter-O’Hagen et al., 2009), the precise ontogeny of these aversive properties has yet to be identified. The purpose of the current study was to determine whether the insensitivity of adolescents to ethanol CTA relative to adults reflects a developmental dissociation or merely an ontogenetic increase in sensitivity to ethanol’s aversive consequences. Thus, in this study, CTA to a range of ethanol doses was assessed across a broad age range-- at ages chosen to represent pre-adolescents/juveniles (P23-25), early (P28-30), mid (P35-37), and late (P42-44) adolescents, “emerging adults” (P52-54), and adults (P72-74), based on prior literature showing discrete developmental windows within adolescence (e.g. Vetter-O’Hagen et al., 2012; Spear, 2015). Both males (Experiments 1 and 2a) and females (Experiment 2b) were examined.
Methods
General Methods
Sprague-Dawley male and female rats bred and reared in our colony at Binghamton University were used in this study. The day after birth, all litters were culled to 8–10 pups and housed with their dams until weaning on P21, at which time animals were pair-housed with same-sex littermates. Animals were maintained in a temperature controlled (20–22° C) vivarium on a 12-/12-h light/dark cycle (lights on at 0700) with ad libitum access to food (Purina Rat Chow, Lowell, MA) and tap water. All procedures were conducted in accordance with guidelines established by the National Institutes of Health using protocols approved by the Binghamton University Institutional Animal Care and use Committee.
Experiment 1
Design
The design of this experiment was a 6 Age (pre-adolescent/juvenile (pre: P23-25), early (P28-30), mid (P35-37), late (P42-44) adolescent, emerging adult (emerging: P52-54) and adult (P72-74)) X 6 ethanol Dose (0.0, 0.5, 1.0, 1.5, 2.0 and 2.5 g/kg) factorial. Initial sample sizes were 8–10 male rats per group. Animals were assigned to groups randomly, with the constraint that no more than one animal from any given litter was placed in a particular test group to avoid confounding litter with treatment effects (Zorrilla, 1997).
Procedure
CTA testing was conducted using procedures designed and adapted in our lab to minimize stress. Two days prior to conditioning, each animal was pair-housed with a same age and sexed animal from a different litter to permit housing pairs to be assigned the same dose condition. All CTA conditioning and test procedures were conducted in a novel testing cage, with the same cage used for conditioning and testing. Chocolate Boost® was used as the conditioned stimulus (CS), with adolescents on ad lib food and water readily consuming the solution (unpublished data), thereby removing the need for water deprivation. Prior to the onset of each 60-minute conditioning session, animals were weighed and each housing pair separated in a novel-cage with a wire-mesh divider for 15 minutes before exposure to tastant. Separating the animals in this way allowed for measurement of individual consumption without the stress of isolate housing, and has been used previously in our laboratory (e.g. Anderson, Varlinskaya and Spear, 2010; Saalfield and Spear, 2015). At the onset of training on conditioning day, each animal was provided with one bottle containing chocolate Boost®. Immediately following the 60-minute access to Boost®, the bottle was removed and the animal was injected with the designated dose of ethanol via intraperitoneal (i.p.) administration of a 20% (v/v) solution in physiological saline, with the 0 dose controls injected with 0.9% saline isovolumetric to the highest dose of ethanol administered. Each housing pair received the same drug challenge, and remained separated by the wire-mesh divider for an additional 15 minutes post-injection, after which they were returned to their homecage. Testing occurred two days after conditioning. For testing, cage mates were again separated in the same novel cage with a wire mesh divider used during conditioning, and 15 min later, provided with a bottle of Boost® for a one-hour test period.
Data Analysis
Intake of the CS in mls on both conditioning and test day were determined. Animals that consumed less than 1 ml of the CS on conditioning day were excluded from analysis, as were animals whose measured consumption was ≥ 2 standard deviations (S.D.) from the mean either during conditioning or testing (and hence likely to reflect bottle leakage). In total, data from 17 animals were excluded (13 animals for failing to consume 1 mL on conditioning day, and 4 for consumption greater than 2 S.D. above the mean, likely reflecting bottle leakage), with no more than 2 animals excluded per group, resulting in a final n of 7–10 per group.
Analyses of variance (ANOVAs) of baseline intake revealed age differences in intake on conditioning day in all studies. Because of these differences, along with apparent age differences in ‘neophobia’ and its habituation [indexed via baseline-to-test day intake of saline animals], the data were analyzed in two ways. Test day intake from animals at each age was converted into a percent saline control of animals at that age ([individual intake/mean intake of the age-appropriate saline group]*100), with simple effects analyses using one-way ANOVAs at each age and subsequent Dunnett’s post-hoc tests to determine effective doses at each age for producing CTA, and Fisher’s tests to assess differences in aversion across age at each dose. Raw intake data were also analyzed by a 6 Age by 4 Dose X 2 (conditioning versus test) Day repeated measures ANOVA to explore changes in consumption after conditioning on a within-subject basis, with Fisher’s post-hoc tests used to explore interactions and determine doses that induced decreased intake on test day relative to conditioning day at each age. Findings were considered significant at p ≤ 0.05.
Results
Baseline Intake (Table 1)
Table 1.
Conditioning day intake across Age in Experiments 1 and 2 in both mls and mls/kg (S.E.M.). Data points marked with differing letters are significantly different from one another within each row (p ≤ 0.05)
| Pre P23-25 |
Early P28-30 |
Mid P35-37 |
Late P42-44 |
Emerging P52-54 |
Adult P72-74 |
||
|---|---|---|---|---|---|---|---|
| Experiment 1 ♂ Boost | ml | 4.83 ±0.5a | 5.08±0.55 a | 4.67±0.73 a | 6.97±1.28 b | 8.25±1.53 b | 8.16±1.4 b |
| ml/kg | 62.1±2.65 a | 45.23±2.17 b | 26.53±1.68 c | 29.73±2.32 c | 27.01±2.16 c | 19.85±1.47 d | |
| Experiment 2a ♂ SS + 50% water dep | ml | 4.87±.33a | 7.28±0.67b | 7.18±0.78b | 9.63±1.0c | 10.56±1.47c | 11.19±0.99c |
| ml/kg | 67.09±2.8 a | 70.51±3.32 a | 45.19±3.06 b | 45.47±2.5 b | 35.69±2.41 c | 29.05±1.47 c | |
| Experiment 2b ♀ SS + 50% water dep | ml | 3.91±0.19a | 5.04±0.31a | 7.74±0.29b | 7.53±0.6b | 7.42±0.46 c | 9.05±0.56c |
| ml/kg | 60.4±3.24 a | 53.53±3.21 a | 56.22±2.32 a | 44.57±3.48 b | 36.63±2.54 b | 37.2±2.31 b |
The ANOVA of baseline intake revealed a main effect of Age (F[5,278] = 12.99, p < 0.001), with late adolescent, emerging adulthood, and adult animals consuming significantly more mls of the CS compared to the three youngest ages; when intakes were converted to ml/kg body weight, these age differences, not surprisingly, were reversed.
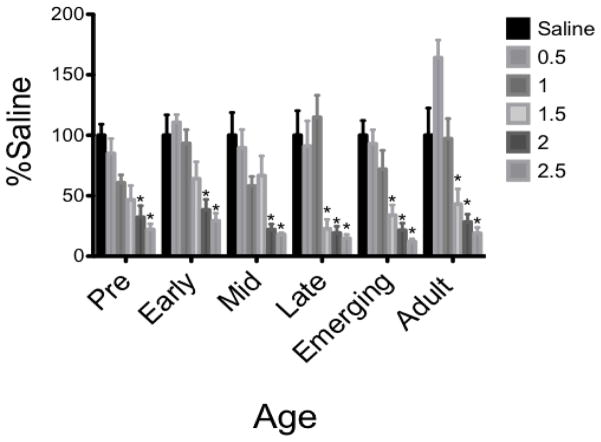
Test Intake (Figure 1)
Figure 1.
In males given Boost® as the CS, the 3 youngest age groups required a higher ethanol dose (2 g/kg) to express CTA when compared to the 3 older ages (1.5 g/kg).
*denotes significant difference from age-matched saline control
The ANOVA of % saline intake during the test session revealed a Dose X Age interaction (F[25,278] = 1.54, p = 0.05). Dunnett’s planned comparisons revealed two distinct patterns of ethanol consumption, with the 3 youngest ages (pre-adolescence, early and mid adolescence) displaying an attenuated sensitivity to the aversive properties of ethanol (i.e. greater Boost® consumption) compared to the 3 older age groups (late adolescence, emerging adulthood and adults), with CTAs not emerging until 2 g/kg and 1.5 g/kg between these groupings, respectively. Fisher post hoc analysis revealed adults consumed more Boost® at test than all other groups following of 0.5 g/kg ethanol, while the late animals consumed more Boost® than the pre- and early-groups at 1 g/kg ethanol. In contrast, the late animals consumed significantly less than the early and mid groups after 1.5 g/kg ethanol.
The repeated measures ANOVA conducted on conditioning versus test day intake revealed a significant interaction between Day, Age, and Dose (F25, 279) = 2.45, P < 0.001). Fisher pot hoc tests found that pre animals decreased their intake following conditioning with only the highest ethanol dose (2.5 g/kg), while early and mid animals decreased intake following conditioning with both 2 and 2.5 g/kg ethanol, late and emerging animals after doses of 1.5, 2 and 2.5 g/kg, and adults after 2 and 2.5 g/kg.
Experiment 2
In Experiment 1, in contrast to much of the CTA literature (Anderson et al., 2010; Morales & Spear, 2013; Saalfield & Spear, 2015; Schramm-Sapyta et al., 2010), few notable age differences emerged, with animals at all ages exhibited relatively high baseline intake of the CS even though animals were not fluid restricted, likely due to the highly palatable and caloric nature of the CS, Boost® (see Table 1). It is possible that this high CS palatability could have influenced the ontogenetic patterns observed. Specifically, the finding that adult animals did not display CTA following administration of 1 g/kg ethanol, an effect consistently seen in our lab (Anderson et al., 2010; C. Vetter-O’Hagen et al., 2009) was concerning, as was the possibility of diminished sensitivity using this CS, given relatively moderate age differences. Consequently, Experiment 2 was conducted to extend the results to more typical fluid deprivation conditions using procedures common to our laboratory for CTA testing. Methods were identical to Experiment 1 except “supersaccharin” (SS) was used as the CS, animals were 50% water deprived overnight prior to conditioning and test to promote adequate intake of the CS, and the lowest (0.5 g/kg) and highest (2.5 g/kg) doses were excluded based on findings from Experiment 1 showing the same response across all ages to each of these doses. Both males (Experiment 2a) and females (Experiment 2b) were examined to assess the ontogeny of ethanol CTA in each sex. These experiments were conducted sequentially at separate times, and hence were analyzed and are presented separately.
Experiment 2a
Design
The design of this experiment was a 6 Age X 4 ethanol dose (0.0, 1.0, 1.5, and 2.0 g/kg) factorial. Eight to 10 males were assigned to each group.
Procedure
Two days prior to conditioning, each pair of animals was 50% water restricted. To calculate water restriction, water intake for each pair of animals over the previous 24-hour period was measured and half of this amount provided for the following 24-hours. Twenty-four hours after the onset of the water restriction period, the conditioning session occurred. All conditioning, testing and analysis procedures were the same as Experiment 1, with the exception that a solution of supersaccharin (SS) (3% sucrose, 0.125% saccharin in water; modified from Ji et al., 2008; see Morales et al., 2014) replaced Boost® as the CS. Following the conditioning session, each pair of animals was provided with ad libitum access to a fresh bottle of water. The following day, animals again began 24 hours of 50% water restriction, with the test session occurring the following day.
Data Analysis
All data analysis procedures were identical to those in Experiment 1. Seven animals were removed from analysis due to failure to consume 1 ml of the CS on conditioning day, resulting in a final n of 7–10 per group.
Results
Experiment 2a (males)
Baseline Intake (Table 1)
The ANOVA of baseline intake in mls revealed a main effect of Age (F[5,167] = 20.77, p < 0.001), with pre adolescent males consuming the least amount of the CS, and late adolescent, emerging adulthood, and adult males consuming significantly more of the CS compared to early and mid adolescents.
Test Intake (Figure 2)
Figure 2.
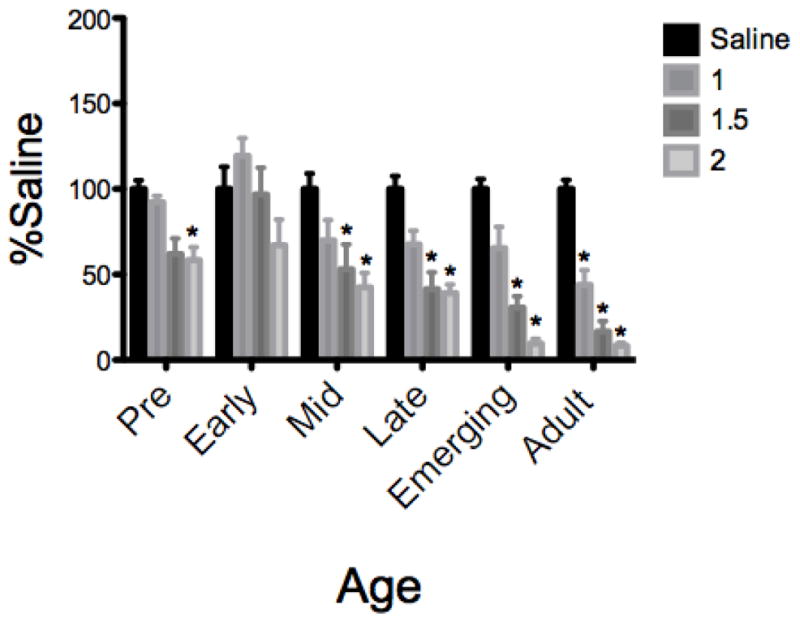
In males given SS as the CS, notable age differences across age were evident in ethanol CTA, with early adolescents failing to develop a CTA, preadolescents displaying significant CTA at only the 2 g/kg dose, and CTA expressed among older adolescents beginning at 1.5 g/kg, and among adults at all doses.
* denotes significant difference from age-matched saline control
The ANOVA of % saline intake during the test session revealed a Dose X Age interaction (F[15,163] =2.39, p = 0.004). Dunnett’s planned comparisons revealed that early adolescents did not show significant CTA at any dose, whereas pre-adolescents exhibited CTA at only the 2.0 g/kg dose, with a trend (p < 0.07) for CTA at the 1.5 g/kg dose. Older animals were more sensitive to ethanol CTA, with mid, late and emerging adults displaying a significant CTA at doses of 1.5 g/kg and above and adults showing CTA at all doses. Fisher post hoc analysis revealed that early adolescent males had significantly higher intakes than pre and mid males at the 1 and 1.5 doses, and greater intake than late, emerging and adult males at all 3 doses. Similar patterns of sensitivity were seen between emerging and adult males, with both of these groups showing significantly greater attenuations in intake than all younger groups.
Similar findings were obtained from the repeated measure ANOVA of the intake data on conditioning and test days. Fisher’s post-hoc analyses to explore the locus of the the significant interaction between Day, Age, and Dose (F(15, 160) = 2.83, P < 0.001) revealed that pre and early adolescent males failed to develop CTA following any of the administered doses, whereas mid, late and emerging males developed CTA following conditioning with 1.5 and 2 g/kg. Adult males displayed the greatest sensitivity, with significant decreases in intake on test day after conditioning with all doses of ethanol (1, 1.5 and 2 g/kg).
Experiment 2b (Females)
Methods
Design
The design of this experiment was the same as Experiment 2a, a 6 Age X 4 ethanol dose (0.0, 1.0, 1.5, and 2.0 g/kg) factorial, with eight to 10 females assigned to each group.
Procedure
The procedure of Experiment 2b was identical to that of Experiment 2a. Following testing, a vaginal smear was collected from each female to estimate stage of estrous cycle.
Data Analysis
All data analysis procedures were identical to those in Experiment 1. Five animals were removed from analysis due to failure to consume 1 ml of the CS on conditioning day, resulting in a final n of 7–10 per group
Baseline Intake (Table 1)
The ANOVA of baseline intake in mls revealed a main effect of Age (F[5,168] = 22.3, p < 0.001), with pre- and early adolescent females consuming significantly less of the CS when compared to the four oldest ages.
Test Intake (Figure 3)
Figure 3.
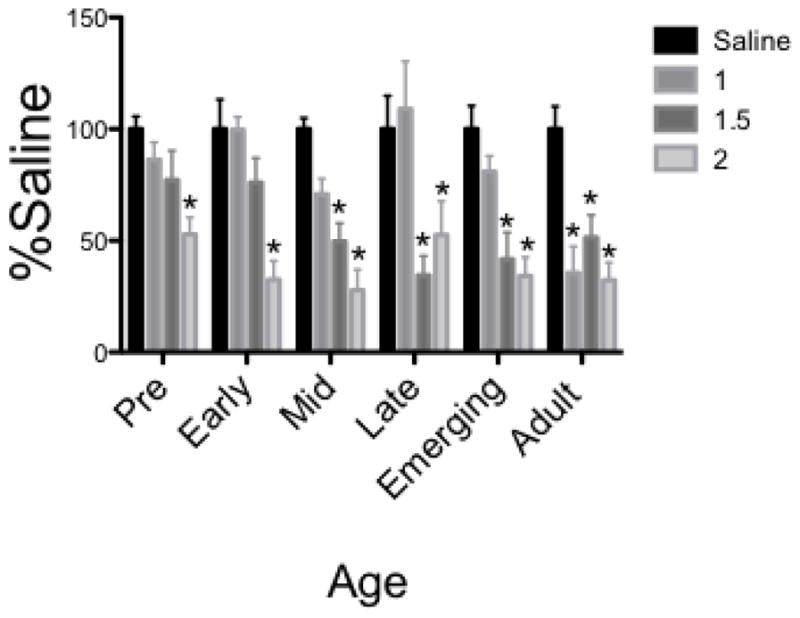
In females given SS as the CS, early and preadolescents displayed significant CTA at only the 2 g/kg dose, older adolescents beginning at a dose of 1.5 g/kg, and adults at all doses. * denotes significant difference from age-matched saline control
The ANOVA of % saline intake during the test session revealed a Dose X Age interaction (F[15,167] =2.32, p = 0.005). Dunnett’s planned comparisons revealed that early and pre-adolescent females exhibited CTA at only the 2.0 g/kg dose. Older females were more sensitive to ethanol CTA, with mid, late and emerging females displaying significant CTA at doses of 1.5 g/kg and above and adult females showing CTA at all doses. Fisher post hoc analyses revealed that adult females showed greater intake suppression than females at all other ages at the 1 g/kg dose, whereas both late and emerging females showed significantly greater intake suppression than pre and early females at the 1.5 g/kg dose.
The pattern of results obtained from the repeated measures ANOVA of conditioning and test day intake in these females revealed similar findings, although the pre and early adolescent females were found to be even less sensitive to ethanol CTA in these analyses. A significant interaction between Day, Age, and Dose (F(15, 167) = 4.73, p < 0.001) emerged in this repeated measure ANOVA, with Fisher post hoc analyses revealing that pre and early adolescent females failed to develop CTA following any of the doses administered, whereas mid and emerging females developed an aversion following the 1.5 and 2 g/kg doses. Late adolescent females displayed decreased intake following 1.5 g/kg although the 2.0 g/kg dose failed to reach signifcance (p=0.1), likely due to the large variability within that group. Adult females developed an aversion to all doses of ethanol (1, 1.5 and 2 g/kg).
Estrous Cycle
The effect of estrus cycle on test day intake was analyzed using a 5 Age X 4 estrous cycle (proestrus, estrus, metestrus, and diestrus) ANOVA. Pre-adolescents were excluded from analysis due to no animals having reached age of vaginal opening. No significant effects emerged in this analysis (F(5, 177) = 1.28, p = 0.27).
Discussion
Across both experiments, adolescents were less sensitive to the aversive effects of ethanol compared to their adult counterparts. In experiment 1, only modest age differences were observed, with younger animals being generally less sensitive to the aversive effects of ethanol than their more mature counterparts. Due to the highly palatable and caloric nature of the CS (Boost®) used in Experiment 1, a second study was conducted to expand upon these results using a ‘supersac’ solution as the CS in 50% water restricted males and females. Preliminary data from our lab has shown both adolescents (P25, P32, P42) and adults (P70) to readily consume Boost® without any water deprivation, whereas less than 1 ml is consumed, on average, in animals of these ages when given SS in their natural, non-deprived state. In Experiments 2a and b, pre and early adolescent males and females either failed to develop CTA or showed it only at the highest dose, distinguishing these ages as the most resistant to the aversive effects of ethanol. Post hoc analysis revealed that in males, early adolescents consumed significantly more of the CS at test compared to pre and mid males at the 1 and 1.5 doses, and than late, emerging and adult males at all 3 doses, with emerging and adult animals displaying the greatest sensitivity to ethanol CTA. Post hoc analyses across age revealed that in females, the 3 youngest ages (pre, early, and mid) did not differ significantly in their sensitivity to ethanol CTA, but all consumed more of the CS after ethanol conditioning than the 3 older ages (late, emerging, and adult). Both experiments confirm prior findings that adolescents are less sensitive overall than mature animals to the aversive properties of ethanol as indexed via CTA (Anderson et al., 2010; C. Vetter-O’Hagen et al., 2009), while expanding these data to define pre and early adolescence as the period of least sensitivity to these aversive effects.
It should be noted that the insensitivity of adolescents to ethanol’s aversive properties is not only evident with ethanol, but extends to CTAs assessed with other drugs of abuse as well, including cocaine (Schramm-Sapyta, Morris, & Kuhn, 2006), tetrahydrocannabinol (THC) (Schramm-Sapyta et al., 2007), and nicotine (Shram, Funk, Li, & Le, 2006). Together, these data suggest that adolescents show a general reduction in sensitivity to drug-induced CTA across a variety of drug classes, suggesting that adolescents may generally process aversive stimuli differently when compared to their adult counterparts. An alternative explanation is that animals of this age may less effectively associate the aversive properties of a drug with the CS with which it was paired. This possibility seems unlikely, however, given data showing that within the same experimental series, adolescents exhibited weaker CTA to nicotine than adults, but greater sensitivity to its rewarding properties as indexed via nicotine-induced conditioned place preference (Shram et al., 2006). There is additional literature showing greater Pavlovian conditioning to the rewarding properties of drugs such as nicotine and cocaine in adolescents (Badanich, Adler, & Kirstein, 2006; Brenhouse & Andersen, 2008; Shram et al., 2006; Torres, Tejeda, Natividad, & O’Dell, 2008; Zakharova, Leoni, Kichko, & Izenwasser, 2009; Zakharova, Wade, & Izenwasser, 2009). Another alternative explanation of the developmental differences observed in the present study is that they may reflect age differences in non-specific effects of ethanol exposure (e.g., ethanol-induced shift in taste sensitivity, habituation of taste neophobia, circadian rhythms, etc.). This possibility could have been addressed with inclusion of a non-paired control group that was not included in these studies.
Adolescence has widely been defined as a broad developmental period bridging the gap between childhood and adulthood. More recently, adolescence has been partitioned into separate periods of vulnerability (See Spear, 2015 for a review). The current data support this developmental dissociation, and extend prior research by highlighting early adolescence (and the interval leading into adolescence) as a time of particularly reduced sensitivity to ethanol’s aversive effects. Among both males and females, adolescent resistance to the aversive effects of ethanol was most marked during the pre adolescence/juvenile and early adolescent periods. These findings complement prior data on human youth showing early adolescence to be a particularly vulnerable period (e.g. Grant and Dawson, 1997), with for instance individuals who begin drinking prior to age 13 being especially likely to express later binge (>5 drinks) and extreme (>10 drinks/occasion) drinking (Hingson & Zha, 2009). In rodent studies as well, early adolescence appears to be a time of particular vulnerability to lasting effects of ethanol (see Spear, 2015), with, for example, repeated ethanol exposure early in adolescence resulting in persistence of adolescent-like sensitivities to ethanol in adulthood, alterations not evident in those exposed late in adolescence (e.g. Varlinskaya, Truxell, & Spear, 2014; see Spear, 2015 for review). Although alternative measures of ethanol’s aversive properties (conditioned place aversion (CPA)) have suggested late adolescence as a period of diminished sensitivity (Philpot, Badanich, & Kirstein, 2003), multiple studies have suggested that CTA paradigms are more readily able to detect a substance’s aversive qualities than CPA (Risinger & Cunningham, 2000) or that the two tests do not comparably measure aversion (Gore-Langton, Flax, Pomfrey, Wetzell, & Riley, 2015).
Similar, but somewhat different results were obtained between Experiments 1 and 2a, with water restriction being a major difference between studies. Deprivation has long been known to affect CTA development (see Grigson et al., 2009 for references). Food deprivation has been shown to result in attenuated CTA to amphetamine and chlordiazepoxide (Bell, Thiele, Seeley, Bernstein, & Woods, 1998) when compared to free-feeding animals. Furthermore, the suppressive effects of drugs of abuse in water replete animals have been reported to result in either reduced (morphine: Grigson, Lyuboslavsky, Tanase, and Wheeler, 1999) or comparable (heroin: Grigson, Twining and Carelli, 2000) suppression of CS intake when compared with water restricted animals. In agreement with these findings, ethanol-induced CTA was attenuated at some ages in the non-deprived males of Experiment 1 relative to the water-restricted males in Experiment 2a. CTA, however, in deprived subjects can also be affected by the caloric nature of the CS, with suppression of intake being greater when using a non- or low caloric CS, as the CS would not be offsetting the deprivation state. The caloric value of our two CSs differed greatly between our experiments, with Boost® (1.01 calories/ml) being much more calorically dense than SS (0.12 calories/ml); therefore, the increased intake at test seen in our adolescent animals was likely due to a reduced sensitivity to the aversive properties, rather than a biological drive to continue consuming the solution due to the low caloric value of SS. As water restriction was only used with SS in Experiment 2, and since the CS was not held constant across the two experiments, it is impossible to decipher whether the reduced caloric nature of the CS or the water restriction per se led to the expression of more age effects among males in Experiment 2a than 1. With the growing ethanol-induced CTA literature utilizing water deprivation as a way to ensure adequate intake of the CS on conditioning day, it may be beneficial for future work to directly compare water-replete and water-restricted animals for ethanol CTA under circumstances of calorically different CSs to determine the effect of these variables on ethanol CTA.
Experiment 2b examined females to assess whether similar ontogenetic patterns of aversion were observed in females as in males. Prior research has reported both attenuated (Chambers, Sengstake, Yoder, & Thornton, 1981; Sherrill, Berthold, Koss, Juraska, & Gulley, 2011) and enhanced (Morales et al., 2014; Morales & Spear, 2013) sensitivity to aversive effects of drugs of abuse in adolescent and adult females when compared to their male counterparts. Current data support generally similar ontogenetic patterns of ethanol aversion, with early adolescents displaying reduced CTA compared to older animals in both sexes. It is possible that the rise of gonadal hormones could have increased sensitivity to ethanol’s aversive effects. If the aversive properties of ethanol were puberty-dependent, however, it might be anticipated that a developmental shift in ethanol aversion would have continued in late adolescent animals concurrent with the continued rise in gonadal hormones (see Vetter O’Hagen & Spear, 2012b), an effect not seen in the current data. Further support for the notion that gonadal hormones may not exert notable effects on ethanol’s aversive effects are data showing that removal of the gonads during adolescence had no impact on CTA acquisition in adulthood in either males or females (Kim and Spear, 2015).
Another possible contributor to the ontogenetic difference in ethanol CTA is the considerable maturational changes in the brain that occurs throughout adolescence (e.g. see Spear, 2000 for a review). Prominent developmental alterations include ontogenetic increases in control of subcortical regions by the prefrontal cortex (Crews et al., 2007), as well as maturational changes in connectivity between subcortical regions critical for processing rewarding (e.g., nucleus accumbens) and aversive (e.g., the amygdala) stimuli during adolescence, with such regions shown, under certain circumstances, to exhibit greater reactivity to rewarding and emotional/affective stimuli than evident in adulthood (see Spear, 2011). It is possible that the maturational changes in reward and aversion neurocircuitry may contribute to increased alcohol intake in adolescence, as the overall hedonic value of a drug is thought to be a function of the balance between its rewarding and aversive effects (Riley, 2011; M. Schuckit et al., 2006; M. A. Schuckit et al., 2009; Verendeev & Riley, 2012). The specific ontogenetic patterns of neuronal activation in brain areas linked to ethanol reward and aversion, however, are still largely unknown, and would lead to a better understanding of the impact of developmental alterations in the brain on adolescent drug and alcohol use.
The current study demonstrates the robustness of the adolescent insensitivity to the aversive effects of ethanol, with this effect evident regardless of the caloric nature of the CS or whether or not animals are fluid deprived. Among both males and females, general ontogenetic increases in ethanol’s aversive effects were observed, with adolescent resistance to the aversive effects of ethanol most marked leading into and during early adolescence. Nevertheless, the relative insensitivity of young adolescents to the aversive effects of ethanol, possibly coupled with an increased sensitivity to its rewarding effects, may act as permissive factors contributing to the initiation and maintenance of drinking during development. This general resistance to the aversive effects of drugs, when coupled with other adolescent-typical behaviors (e.g. increased novelty seeking and risk-taking, see Spear, 2000, 2010 for review), may contribute to the initiation and persistence of drug and alcohol use during adolescence. Drug abuse is thought to be associated with the relative balance between the rewarding and aversive properties of the drug, with attenuated sensitivity to aversive effects often associated with increased drug use (see Riley, 2011 for a review), perhaps more strongly so than an enhanced sensitivity to its rewarding effects per se (at least in the case of ethanol – see Green & Grahame, 2008 among others). Overall, pre- and early adolescents display a marked insensitivity to ethanol’s aversive properties, highlighting this time as a crucial period for early prevention efforts.
Highlights.
Early adolescents are relatively insensitive to ethanol’s aversive properties.
Similar ontogenetic increases in ethanol CTA were seen in males and females.
Fewer age differences were seen using a highly palatable CS in non-deprived animals.
Acknowledgments
“Research reported in this publication was supported by the National Institute On Alcohol Abuse And Alcoholism of the National Institutes of Health under Award Number P50AA017823. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.”
Footnotes
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alaux-Cantin S, Warnault V, Legastelois R, Botia B, Pierrefiche O, Vilpoux C, Naassila M. Alcohol intoxications during adolescence increase motivation for alcohol in adult rats and induce neuroadaptations in the nucleus accumbens. Neuropharmacology. 2013;67:521–531. doi: 10.1016/j.neuropharm.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Varlinskaya EI, Spear LP. Ethanol-induced conditioned taste aversion in male sprague-dawley rats: impact of age and stress. Alcohol Clin Exp Res. 2010;34(12):2106–2115. doi: 10.1111/j.1530-0277.2010.01307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;550(1–3):95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Bell SM, Thiele TE, Seeley RJ, Bernstein IL, Woods SC. Effects of food deprivation on conditioned taste aversions in rats. Pharmacol Biochem Behav. 1998;60(2):459–466. doi: 10.1016/s0091-3057(98)00024-0. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE. The role of puberty in the developing adolescent brain. Hum Brain Mapp. 2010;31(6):926–933. doi: 10.1002/hbm.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenhouse HC, Andersen SL. Delayed extinction and stronger reinstatement of cocaine conditioned place preference in adolescent rats, compared to adults. Behav Neurosci. 2008;122(2):460–465. doi: 10.1037/0735-7044.122.2.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers KC, Sengstake CB, Yoder RL, Thornton JE. Sexually dimorphic acquisition of a conditioned taste aversion in rats: effects of gonadectomy, testosterone replacement and water deprivation. Physiol Behav. 1981;27(1):83–88. doi: 10.1016/0031-9384(81)90303-6. [DOI] [PubMed] [Google Scholar]
- Crews F, He J, Hodge C. Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav. 2007;86(2):189–199. doi: 10.1016/j.pbb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcohol Clin Exp Res. 2008;32(12):2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157(5):745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29(10):1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72(1):114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faden VB. Trends in initiation of alcohol use in the United States 1975 to 2003. Alcohol Clin Exp Res. 2006;30(6):1011–1022. doi: 10.1111/j.1530-0277.2006.00115.x. [DOI] [PubMed] [Google Scholar]
- Gore-Langton JK, Flax SM, Pomfrey RL, Wetzell BB, Riley AL. Measures of the aversive effects of drugs: A comparison of conditioned taste and place aversions. Pharmacol Biochem Behav. 2015;134:99–105. doi: 10.1016/j.pbb.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Green AS, Grahame NJ. Ethanol drinking in rodents: is free-choice drinking related to the reinforcing effects of ethanol? Alcohol. 2008;42(1):1–11. doi: 10.1016/j.alcohol.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigson PS, Lyuboslavsky PN, Tanase D, Wheeler RA. Water-deprivation prevents morphine-, but not LiCl-induced, suppression of sucrose intake. Physiol Behav. 1999;67(2):277–286. doi: 10.1016/s0031-9384(99)00080-3. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC, Carelli RM. Heroin-induced suppression of saccharin intake in water-deprived and water-replete rats. Pharmacol Biochem Behav. 2000;66(3):603–608. doi: 10.1016/s0091-3057(00)00253-7. [DOI] [PubMed] [Google Scholar]
- Grigson PS, Twining RC, Freet CS, Wheeler RA, Geddes RI. Drug-Induced Suppression of Conditioned Stimulus Intake: Reward, Aversion, and Addiction. In: Reilly S, Schachtman TR, editors. Conditioned Taste Aversion: Neural and Behavioral Processes. New York: Oxford University Press; 2009. pp. 74–91. [Google Scholar]
- Hingson RW, Zha W. Age of drinking onset, alcohol use disorders, frequent heavy drinking, and unintentionally injuring oneself and others after drinking. Pediatrics. 2009;123(6):1477–1484. doi: 10.1542/peds.2008-2176. [DOI] [PubMed] [Google Scholar]
- Holstein SE, Spanos M, Hodge CW. Adolescent C57BL/6J mice show elevated alcohol intake, but reduced taste aversion, as compared to adult mice: a potential behavioral mechanism for binge drinking. Alcohol Clin Exp Res. 2011;35(10):1842–1851. doi: 10.1111/j.1530-0277.2011.01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A. State Estimates of Substance Abuse from the 2006–2007 National Surveys on Drug Use and Health. DIANE Publishing; 2010. [Google Scholar]
- Infurna RN, Spear LP. Developmental changes in amphetamine-induced taste aversions. Pharmacol Biochem Behav. 1979;11(1):31–35. doi: 10.1016/0091-3057(79)90293-4. [DOI] [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behav Pharmacol. 2008;19(1):1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EU, Spear LP. Sex-Dependent Consequences of Pre-Pubertal Gonadectomy: Social Behavior, Stress and Ethanol Responsivity. Behav Brain Res. 2015 doi: 10.1016/j.bbr.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster FE, Brown TD, Coker KL, Elliott JA, Wren SB. Sex differences in alcohol preference and drinking patterns emerge during the early postpubertal period. Alcohol Clin Exp Res. 1996;20(6):1043–1049. doi: 10.1111/j.1530-0277.1996.tb01945.x. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22(2):416–421. [PubMed] [Google Scholar]
- Masten AS, Faden VB, Zucker RA, Spear LP. A developmental perspective on underage alcohol use. Alcohol Res Health. 2009;32(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- Morales M, Schatz KC, Anderson RI, Spear LP, Varlinskaya EI. Conditioned taste aversion to ethanol in a social context: impact of age and sex. Behav Brain Res. 2014;261:323–327. doi: 10.1016/j.bbr.2013.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M, Spear LP. Differences in sensitivity to ethanol-induced conditioned taste aversions emerge after pre- or post-pubertal gonadectomy in male and female rats. Behav Brain Res. 2013;240:69–75. doi: 10.1016/j.bbr.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, McMorris BJ, Toumbourou JW, Hemphill SA, Donath S, Catalano RF. Puberty and the onset of substance use and abuse. Pediatrics. 2004;114(3):e300–306. doi: 10.1542/peds.2003-0626-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent but not adult rats exhibit ethanol-mediated appetitive second-order conditioning. Alcohol Clin Exp Res. 2008;32(11):2016–2027. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philpot RM, Badanich KA, Kirstein CL. Place conditioning: age-related changes in the rewarding and aversive effects of alcohol. Alcohol Clin Exp Res. 2003;27(4):593–599. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- Riley AL. The paradox of drug taking: the role of the aversive effects of drugs. Physiol Behav. 2011;103(1):69–78. doi: 10.1016/j.physbeh.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Risinger FO, Cunningham CL. DBA/2J mice develop stronger lithium chloride-induced conditioned taste and place aversions than C57BL/6J mice. Pharmacol Biochem Behav. 2000;67(1):17–24. doi: 10.1016/s0091-3057(00)00310-5. [DOI] [PubMed] [Google Scholar]
- Ristuccia RC, Spear LP. Autonomic responses to ethanol in adolescent and adult rats: a dose-response analysis. Alcohol. 2008;42(8):623–629. doi: 10.1016/j.alcohol.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalfield J, Spear L. Consequences of repeated ethanol exposure during early or late adolescence on conditioned taste aversions in rats. Dev Cogn Neurosci. 2015 doi: 10.1016/j.dcn.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Cha YM, Chaudhry S, Wilson WA, Swartzwelder HS, Kuhn CM. Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology (Berl) 2007;191(4):867–877. doi: 10.1007/s00213-006-0676-9. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, DiFeliceantonio AG, Foscue E, Glowacz S, Haseeb N, Wang N, … Kuhn CM. Aversive effects of ethanol in adolescent versus adult rats: potential causes and implication for future drinking. Alcohol Clin Exp Res. 2010;34(12):2061–2069. doi: 10.1111/j.1530-0277.2010.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Morris RW, Kuhn CM. Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacol Biochem Behav. 2006;84(2):344–352. doi: 10.1016/j.pbb.2006.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit M, Smith T, Pierson J, Danko G, Beltran IA. Relationships among the level of response to alcohol and the number of alcoholic relatives in predicting alcohol-related outcomes. Alcohol Clin Exp Res. 2006;30(8):1308–1314. doi: 10.1111/j.1530-0277.2006.00158.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Danko GP, Trim R, Bucholz KK, Edenberg HJ, … Dick DM. An evaluation of the full level of response to alcohol model of heavy drinking and problems in COGA offspring. J Stud Alcohol Drugs. 2009;70(3):436–445. doi: 10.15288/jsad.2009.70.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill LK, Berthold C, Koss WA, Juraska JM, Gulley JM. Sex differences in the effects of ethanol pre-exposure during adolescence on ethanol-induced conditioned taste aversion in adult rats. Behav Brain Res. 2011;225(1):104–109. doi: 10.1016/j.bbr.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Funk D, Li Z, Le AD. Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 2006;186(2):201–208. doi: 10.1007/s00213-006-0373-8. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. Decreased sensitivity to the hypnotic effects of ethanol early in ontogeny. Alcohol Clin Exp Res. 1998;22(3):670–676. doi: 10.1111/j.1530-0277.1998.tb04310.x. [DOI] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. The neural basis of puberty and adolescence. Nat Neurosci. 2004;7(10):1040–1047. doi: 10.1038/nn1326. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M, Cole M, Ehlers CL. Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Brain Res Dev Brain Res. 2001;128(1):63–72. doi: 10.1016/s0165-3806(01)00150-x. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Curr Opin Neurobiol. 2010;20(2):236–241. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP. The behavioral neuroscience of adolescence. New York: Norton; 2010. [Google Scholar]
- Spear LP. Adolescent alcohol exposure: Are there separable vulnerable periods within adolescence? Physiol Behav. 2015;148:122–130. doi: 10.1016/j.physbeh.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, Brake SC. Periadolescence: age-dependent behavior and psychopharmacological responsivity in rats. Dev Psychobiol. 1983;16(2):83–109. doi: 10.1002/dev.420160203. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- Sun SS, Schubert CM, Chumlea WC, Roche AF, Kulin HE, Lee PA, … Ryan AS. National estimates of the timing of sexual maturation and racial differences among US children. Pediatrics. 2002;110(5):911–919. doi: 10.1542/peds.110.5.911. [DOI] [PubMed] [Google Scholar]
- Torres OV, Tejeda HA, Natividad LA, O’Dell LE. Enhanced vulnerability to the rewarding effects of nicotine during the adolescent period of development. Pharmacol Biochem Behav. 2008;90(4):658–663. doi: 10.1016/j.pbb.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truxell EM, Molina JC, Spear NE. Ethanol intake in the juvenile, adolescent, and adult rat: effects of age and prior exposure to ethanol. Alcohol Clin Exp Res. 2007;31(5):755–765. doi: 10.1111/j.1530-0277.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- Tschann JM, Adler NE, Irwin CE, Jr, Millstein SG, Turner RA, Kegeles SM. Initiation of substance use in early adolescence: the roles of pubertal timing and emotional distress. Health Psychol. 1994;13(4):326–333. doi: 10.1037//0278-6133.13.4.326. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Acute effects of ethanol on social behavior of adolescent and adult rats: role of familiarity of the test situation. Alcohol Clin Exp Res. 2002;26(10):1502–1511. doi: 10.1097/01.ALC.0000034033.95701.E3. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Spear LP. Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Dev Psychobiol. 2006;48(2):146–161. doi: 10.1002/dev.20124. [DOI] [PubMed] [Google Scholar]
- Varlinskaya EI, Truxell E, Spear LP. Chronic intermittent ethanol exposure during adolescence: effects on social behavior and ethanol sensitivity in adulthood. Alcohol. 2014;48(5):433–444. doi: 10.1016/j.alcohol.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verendeev A, Riley AL. Conditioned taste aversion and drugs of abuse: history and interpretation. Neurosci Biobehav Rev. 2012;36(10):2193–2205. doi: 10.1016/j.neubiorev.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31(7):1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen C, Varlinskaya E, Spear L. Sex differences in ethanol intake and sensitivity to aversive effects during adolescence and adulthood. Alcohol Alcohol. 2009;44(6):547–554. doi: 10.1093/alcalc/agp048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Sanders KW, Spear LP. Evidence for suppressant effects of testosterone on sex-typical ethanol intake in male Sprague-Dawley rats. Behav Brain Res. 2011;224(2):403–407. doi: 10.1016/j.bbr.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. The effects of gonadectomy on age- and sex-typical patterns of ethanol consumption in Sprague-Dawley rats. Alcohol Clin Exp Res. 2011;35(11):2039–2049. doi: 10.1111/j.1530-0277.2011.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. The effects of gonadectomy on sex- and age-typical responses to novelty and ethanol-induced social inhibition in adult male and female Sprague-Dawley rats. Behav Brain Res. 2012a;227(1):224–232. doi: 10.1016/j.bbr.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter-O’Hagen CS, Spear LP. Hormonal and physical markers of puberty and their relationship to adolescent-typical novelty-directed behavior. Dev Psychobiol. 2012b;54(5):523–535. doi: 10.1002/dev.20610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS. Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav. 2002;73(3):673–677. doi: 10.1016/s0091-3057(02)00860-2. [DOI] [PubMed] [Google Scholar]
- Witt ED. Puberty, hormones, and sex differences in alcohol abuse and dependence. Neurotoxicol Teratol. 2007;29(1):81–95. doi: 10.1016/j.ntt.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Zakharova E, Leoni G, Kichko I, Izenwasser S. Differential effects of methamphetamine and cocaine on conditioned place preference and locomotor activity in adult and adolescent male rats. Behav Brain Res. 2009;198(1):45–50. doi: 10.1016/j.bbr.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharova E, Wade D, Izenwasser S. Sensitivity to cocaine conditioned reward depends on sex and age. Pharmacol Biochem Behav. 2009;92(1):131–134. doi: 10.1016/j.pbb.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP. Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol. 1997;30(2):141–150. doi: 10.1002/(sici)1098-2302(199703)30:2<141::aid-dev5>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]