Abstract
Phosgene (CG), a toxic inhalation and industrial hazard, causes bronchoconstriction, vasoconstriction and associated pathological effects that could be life threatening. Ion channels of the transient receptor potential (TRP) family have been identified to act as specific chemosensory molecules in the respiratory tract in the detection, control of adaptive responses and initiation of detrimental signaling cascades upon exposure to various toxic inhalation hazards (TIH); their activation due to TIH exposure may result in broncho- and vasoconstriction. We studied changes in the regulation of intracellular free Ca2+ concentration ([Ca2+]i) in cultures of human bronchial smooth muscle cells (BSMC) and human pulmonary microvascular endothelial cells (HPMEC) exposed to CG (16 ppm, 8 min), using an air/liquid interface exposure system. CG increased [Ca2+]i (p<0.05) in both cell types, The CG-induced [Ca2+]i was blocked (p<0.05) by two types of TRP channel blockers, SKF-96365, a general TRP channel blocker, and RR, a general TRPV (vanilloid type) blocker, in both BSMC and HPMEC. These effects correlate with the in vivo efficacies of these compounds to protect against lung injury and 24 hr lethality from whole body CG inhalation exposure in mice (8-10 ppm × 20 min). Thus the TRP channel mechanism appears to be a potential target for intervention in CG toxicity.
Keywords: Phosgene, TRP channels, inhalation, calcium signaling, respiratory toxicology, SKF, therapeutic
1. INTRODUCTION
1.1 Phosgene as a Toxic Inhalation Hazard
Phosgene (CG, COCl2) is an industrial chemical used in the manufacturing of multiple compounds e.g., pharmaceuticals, dyes, and polyfoam rubber products. However, CG is highly toxic and its inhalation exposure can compromise the entire respiratory tract (Miller and Chang 2003, Tuorinsky and Sciuto 2008). As such CG was used as a chemical weapon during World War I Moreover, since CG is very easy to make, it also is a concern as a potential terrorist threat. Following a latent period of about 6 - 8 hr), CG initiates detrimental physiological effects such as bronchoconstriction, bronchitis, vasoconstriction, pulmonary edema, etc., followed by late effects such as inflammation, pulmonary and airway remodeling and asphyxia. All these eventually may develop into chronic obstructive pulmonary disease (COPD) leading to death by suffocation (Borak and Diller 2001, Pauluhn, Carson et al. 2007). However, current therapeutic options are limited to symptomatic treatment for pulmonary edema and pulmonary dysfunction, e.g., administration of leukotriene inhibitor, ibuprofen, aminophylline, or isoproterenol N-acetyl cysteine, but mainly by mechanical ventilation (Sciuto and Hurt 2004, Pauluhn, Carson et al. 2007).
1.2. TRP Inhibitors as Targeted Therapy against CG Inhalation Toxicity
The mechanism of CG-induced lung injury has eluded investigators for decades. TRP channels consisting of a group of non-selective cation channels with some subtypes preferentially conducting calcium ions are expressed throughout the airways from nasal mucosa to the alveolar-capillary system (Owsianik, Talavera et al. 2006, Grace, Baxter et al. 2014). These TRP channels are involved in many physiological and pathological systems in the airways and also throughout the body (Nilius, Owsianik et al. 2007, Billeter, Hellmann et al. 2014, Grace, Baxter et al. 2014). Certain TRP channels are known to be involved in both chemosensory functions and detrimental signaling cascades following exposures to toxic inhalation hazards(e.g., Cl2, CG, sulfur mustard, methyl isocyanate, SO2, etc.) (Bessac and Jordt 2010, Li, Kanju et al. 2011, Buch, Schafer et al. 2013). This study looked at subtypes of TRP channels, TRPC (canonical type), TRPV1 and TRPV4 (vanilloid type), and TRPA1 (ankyrin type) that have been reported to play a role in physiological and pathological mechanisms in the airway (see Table 1). These are cation conducting channels with some preference for Ca2+. TRPV5 and TRPV6 channels have relatively higher specificity for Ca2+ ions; however, they were not studied here. Intracellular Ca2+ homeostasis is important in the regulation of respiratory function. Alterations in intracellular Ca2+ homeostasis following inhalation exposure to respiratory toxicants or other stimuli cause chemosensory responses (i.e., cough, sneezing, pain) and may contribute to bronchoconstriction, vasoconstriction and altered alveolar and vascular membrane barrier permeability; this may cause enhanced bronchopressure and pulmonary hypertension (Nilius, Owsianik et al. 2007, Bessac and Jordt 2010, Li, Kanju et al. 2011, Buch, Schafer et al. 2013, Billeter, Hellmann et al. 2014, Grace, Baxter et al. 2014).
Table 1.
Specific TRP Channels related to respiratory diseases
| Channel | Possible Disease Connection | Reference |
|---|---|---|
| TRP C1/C6 | Asthma, bronchial hyper responsiveness, COPD, idiopathic pulmonary arterial hypertension, heart hypertrophy, mucus hypersecretion | (Nilius, Owsianik et al. 2007, Sel, Rost et al. 2008, Banner, Igney et al. 2011, Grace, Baxter et al. 2014, Kaneko and Szallasi 2014) |
| TRP V1 | Asthma, chronic pain, chronic cough, enhanced airway inflammation and bronchial hyper-reactivity, important sensor of noxious stimuli and tissue damage, bronchoconstriction, | (Caterina, Leffler et al. 2000, Helyes, Elekes et al. 2007, Nilius, Owsianik et al. 2007, Banner, Igney et al. 2011, Billeter, Hellmann et al. 2014, Grace, Baxter et al. 2014, Kaneko and Szallasi 2014) |
| TRP V4 | Chronic pain, asthma, bronchial hyperresponsiveness, hypertension, lung edema by control of epithelial and endothelial barrier function, acute respiratory distress syndrome, COPD, inflammatory airway disease, ventilator induced airway injury | (Michael, Wenjie et al., Freichel, Suh et al. 2001, Liedtke and Friedman 2003, Suzuki, Mizuno et al. 2003, Nilius, Owsianik et al. 2007, Banner, Igney et al. 2011, Grace, Baxter et al. 2014, Jurek, Hirano-Kobayashi et al. 2014, Kaneko and Szallasi 2014) |
| TRP A1 | Chronic pain, asthma, COPD, important sensor of noxious stimuli and tissue damage, allergic and non-allergic airway hyper-responsiveness, increased bronchoconstriction | (Bautista, Jordt et al. 2006, Nilius, Owsianik et al. 2007, Caceres, Brackmann et al. 2009, Banner, Igney et al. 2011, Balakrishna, Song et al. 2014, Billeter, Hellmann et al. 2014, Grace, Baxter et al. 2014, Kaneko and Szallasi 2014) |
TRP channels integrate multiple stimuli and activate downstream cellular signal pathways via Ca2+ entry and/or membrane depolarization and play an essential role in contraction and relaxation of the vascular system (Firth, Remillard et al. 2007, Guibert, Marthan et al. 2007, Guibert, Ducret et al. 2011, Yue, Xie et al. 2015)(Yue, 2015). CG has been reported to cause a substantial increase in intracellular free calcium (Werrlein, Kirby et al. 1999). A direct link between TRP channel mediated increase in intracellular Ca2+ and vasoconstriction and/or bronchoconstriction has not been established. We propose here a hypothetical mechanism suggesting a possible regulatory role of TRP channels in the overall mechanism of intracellular Ca2+ increase via different types of Ca2+ channels to include the voltage-gated Ca2+ channels as follows. The possible mechanism for the change in intracellular calcium may involve a combination of influx of extracellular Ca2+ ions and triggered release of intracellular Ca2+ ions stores. CG interaction with TRPA1 and/or T-type calcium channels is thought to cause the influx of Ca2+ ions through the plasma membrane. Consequently an increased intracellular Ca2+ concentration may somehow activate Ca2+ release from intracellular stores. The depletion of intracellular stores of Ca2+ ions may then signal extracellular Ca2+ entry through TRPV/C channels and/or L-type calcium channels in order to replenish the depleted Ca2+ stores. There has been evidence that TRPV and TRPC channels contribute to vasoconstriction via both store operated calcium channels and receptor mediated channels (Firth, Remillard et al. 2007, Guibert, Marthan et al. 2007, Guibert, Ducret et al. 2011, Yue, Xie et al. 2015). Moreover, it has been proposed that a substantial increase in intracellular free Ca2+ activates signaling pathways involved in cellular function that could be harmful, including depolarization of muscle cells causing vaso- and bronchoconstriction (Firth, Remillard et al. 2007, Guibert, Marthan et al. 2007, Guibert, Ducret et al. 2011, Yue, Xie et al. 2015).
Inhibitors of TRPA1, TRPV1 and TRPV4 have been developed to prevent activation of chemosensory TRP channels that are believed to be involved in pulmonary edema (Nilius, Owsianik et al. 2007, Billeter, Hellmann et al. 2014, Grace, Baxter et al. 2014, Jurek, Hirano-Kobayashi et al. 2014) and abnormal blood pressure as well as abnormal fluid and electrolyte balance resulting from COPD (Li, Kanju et al. 2011), heart failure (Thorneloe, Cheung et al. 2012), chronic asthma (Lee and Gu 2009), exposure to isocyanates and tear gases (Bessac, Sivula et al. 2009), and exposure to chlorine gas (Balakrishna, Song et al. 2014). Typically acute reactions to CG from its exposure at levels higher than the odor threshold and standard permissible levels include irritation, bronchoconstriction, and vasoconstriction, disruption of the alveolar membrane barrier function, pulmonary edema, asthma-like symptoms and respiratory distress. TRP channels have been proposed to represent specific targets of TIHs e.g., gases such as Cl2, CG, sulfur mustard, methyl isocyanate, SO2, chlorine etc. (Bessac and Jordt 2010, Li, Kanju et al. 2011, Buch, Schafer et al. 2013, Balakrishna, Song et al. 2014). Involvement of different subtypes of TRP channels in different respiratory and cardiac disorders have been proposed as shown in Table 1.
We investigated the possible role of TRP channels in the mechanism of CG toxicity first using in vitro cell culture models and selected TRP inhibitors. These studies were designed for a proof of concept that TRP channels might be involved in the toxicity of CG and that the TRP channels could be possible therapeutic targets for intervention against CG-induced pulmonary injury and lethality in vivo in mice. The particular TRP inhibitors studied were SKF-96365 (SKF), a general TRP channel blocker; and RR which is a general TRPV (vanilloid type) channel blocker. This manuscript discusses both the in vitro and in vivo results on the role of TRP channels in CG toxicity and the identification of prospective therapeutic targets for intervention of CG inhalation toxicity in animals. It should be noted that the TRP channel inhibitors used here lack the absolute specificity for the types of channels as described above. Therefore, a complete validation of the concept is dependent on additional confirmatory experiments using more reliable channel type-specific inhibitors; these are being pursued in our laboratory at present.
2. METHODS
2.1. Cell Culture Models
Frozen stock of human pulmonary microvascular endothelial cells (HPMEC) (ScienCell Research Laboratories, Carlsbad, CA) or human bronchial smooth muscle cells (BSMC, Lonza, Walkersville, MD) was cultured in tissue culture flasks or Corning Transwell™ plates (Fisher, Pittsburgh, PA) pretreated with bovine plasma fibronectin. Frozen stock cells were cultured in appropriate medium according to the manufacturers’ instructions. HPMEC were cultured in endothelial cell medium (Lonza, Walkersville, MD) supplemented with endothelial cell growth supplements, penicillin/streptomycin (ScienCell) and fetal bovine serum (FBS; Life Technologies, Grand Island NY). BSMC were cultured in SMBM medium (Lonza, Walkersville, MD) supplemented with growth factors (hEGF, insulin, hFGF-B), gentamicin/amphotericin (Lonza) and FBS. At 70-80% confluency, the monolayer cells were sub-cultured in appropriate vessels to about 80-90% confluence and were used in the experiments.
2.2. VitroCell Air/Liquid Exposure System
An air/liquid interface exposure apparatus was purchased from VitroCell Systems (Waldkirch, Germany) and teamed with a Brooks Instruments (Hatfield, PA) mass flow controller (MFC) and KNF Neuberger (Trenton, NJ) vacuum pump to create a compact exposure setup. CG mixtures at 16 ppm were produced by mixing 2% CG in nitrogen with dilution air from a compressor (Jun Air, Norresund by, Denmark). Gas ratios were controlled by additional Brooks MFC units and gas concentrations verified with an FTIR (Gasmet, La Prairie, Quebec, Canada). Gas mixture, exposure flow rates, and system vacuum were controlled and monitored using a custom virtual instrument created in LabVIEW (National Instruments, Austin, TX).
2.3. CG Exposure of Cultured Cells
Cell cultures were grown on Transwell™ inserts, and the apical medium was aspirated to produce an air/liquid interface at the cell surface. Cultures were exposed to CG at concentrations of 16 ppm for 8 minutes at a flow rate of 8.3 ml/minute. Immediately following exposure, cells were returned to culture conditions, but with medium containing either no treatment or treatment with either SKF (5 or 50 μM) (Glass, Pocock et al. 2005) or RR (20 or 100 μM) (Cordova, Werner et al. 2011). Intracellular calcium assays were used to study the effects of CG on each cell model.
2.4. Intracellular Calcium Level and Calcium Uptake Assays
Ca2+ movement was monitored by direct measurement of [Ca2+]i using a fluorometric method. The Fluo-4 Direct™ calcium uptake assay (Invitrogen™, Grand Island NY) was chosen due to the key components of the kit that reduce the background fluorescence and was performed according to the manufacturer's instructions. Briefly, 2X Fluo-4 Direct™ calcium assay buffer (1M HEPES in Hanks buffered saline solution, pH 7.3) with 5 mM probenecid was thawed and mixed. The media was removed from monolayer cultured cells and replaced with resting buffer (5.9 KCl mM resting buffer). The 2X Fluo-4 Direct™ calcium assay buffer with 5mM probenecid was added in equal volumes to the sample volume and incubated at 37°C for approximately 30 minutes. The fluorescence was measured using a M4 SpectraMax microplate reader and SoftMax Pro software (Molecular Devices, Sunnyvale, CA). Cells were exposed to CG under a USAMRICD-approved SOP. The plate was incubated at 37°C, and the fluorescence measured using the M4 microplate reader. Measurements were taken every 3-5 minutes for up to 2 hours. The average baseline fluorescence for each well was subtracted from the control and experimental relative fluorescence units (RFU). The data was represented as the % of experimental RFU compared to RFU of cells exposed to air alone.
2.5. Whole-Body CG Exposure in Mice
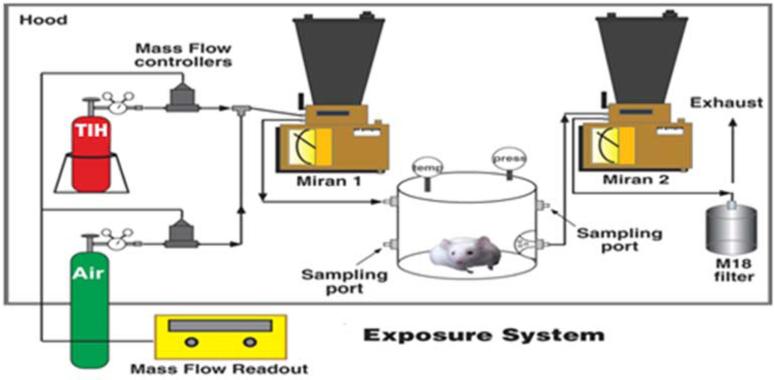
Groups of 40 CD-1 male mice weighing between 20–32 g (Charles River, Wilmington, MA) were placed in a Plexiglas™ chamber with a volume of 15.8 L and exposed whole-body to mixtures of CG (2.0% CG: 98% N2, Matheson Gas Products, Baltimore, MD) and filtered clean air at a rate of 20 L/min for 20 min for a concentration × time (Ct) amount of 0 (air only) or 10 ppm CG (200 ppm*min) (see Figure 1). All elements of the exposures were performed in an approved laboratory fume hood. CG was metered through a Brooks mass flow controller at a rate dependent on the desired concentration and mixed with air and then passed through an infrared spectrometer (Miran 1A, Foxboro Co, Sharon, MA) equipped with a real-time analog output and into the exposure chamber. Out-flowing gas from the chamber was passed through a Fourier transformed infrared spectrometer (FTIR, Gasmet, La Prairie, Quebec, Canada) to determine the concentration of CG exiting the chamber. Effluents from the exposure system passed first through an M18 filter and then through standard activated-charcoal fume hood filters. Following exposure mice received either no treatment or treatment with either SKF (10 mg/kg) (Glass, Pocock et al. 2005) or RR (3, 6, and 9 mg/kg) (Cordova, Werner et al. 2011) which is ≤ 350 μM and ≤ 100 μM in a 30 g mouse respectively, administered ip 20 min after CG exposure. Note: We don't know the distribution of RR in the mouse; final doses will probably be less than that calculated.
Figure 1. Toxic inhalation hazard exposure system.
Twenty-five to thirty-five g male mice were exposed to mixture of CG, 10% balance N2 and filtered room air to the desired concentration using a Brooks mass flow controller at a rate dependent on the desired concentration and mixed with air and then passed through a Miran infrared spectrometer equipped with a real-time analog output and into the exposure chamber. Dosing was controlled by Labview software and phosgene gas concentration exiting the chamber was determined using a GASMET FTIR gas analyzer.
2.6. Histopathology
Mice were anesthetized by an intraperitoneal injection of sodium pentobarbital (50 mg/kg). When the animals were nonresponsive to toe pinch, the thoracic cavity was opened. After removing the ventral portion of the chest wall and freeing the trachea from the surrounding muscles, a needle (0.6 mm X 20 mm) attached to a 3 cc syringe was inserted into the right ventricle. With the left atrium severed, normal saline (0.9% sodium chloride) was injected slowly into the heart until the blood was flushed out of the lungs. A small incision was made on top of the exposed trachea. Using a blunted 18 gauge needle, fixative was slowly instilled through the trachea, which had been ligated with a suture to prevent leakage, until the lungs inflated and there was back flow through the opening. For light microscopic examination, 4% paraformaldehyde and 1% glutaraldehyde (Karnovsky's) mixture was used. Following removal from the thoracic cavity, lungs were post-fixed in Karnovsky's for 2-3 hr and then processed in paraffin. Sections from each lung lobe cut at 4 microns were stained initially with H&E for evaluating the extent of pathology. Lung pathology was graded from 0 to 4 (0 indicates no changes; 1, minimal lesions affecting 1–10% of the area; 2, scattered or random changes affecting 11–25% of the area; 3, moderate changes affecting 25–45%; and 4, severe tissue changes affecting >50% of the area). The pulmonary parameters evaluated included alveolar edema, alveolar fibrin, alveolar hemorrhage, pulmonary congestion, alveolar cellular infiltrates, alveolar epithelial necrosis, bronchiolar infiltrates, bronchiolar epithelial necrosis, smooth muscle thickness, interstitial cellular infiltrates and edema, perivascular cellular infiltrates and edema, and perivascular fibrin. Ten microscopic fields with lung parenchyma were digitally captured by light microscopy at 200X magnification and graded. The median of the grades was used to generate a composite grade for each mouse. The qualitative histological analysis of coded slides was performed by a pathologist blinded to the coding of the slides.
2.7. Statistical Analysis
Relative fluorescence units (Fluo-4 assay) were averaged and expressed as the mean ± standard deviation. The averages of the CG exposed with and without treatment by SKF or RR were normalized to respective controls (no CG) and presented as % of control for each experiment. The animals were randomized to each exposure group. The differences between exposure groups and post-exposure time point were determined using a two-factor analysis of variance (ANOVA) followed by comparison of exposure group means and time point means using the Tukey's multiple comparison test. Survival rates were compared using Fisher's exact test (one-tailed) at the 24 hr endpoint of the study. Mean survival times were compared using a Kaplan-Meier survival analysis. Morphological differences between control and experimental samples were determined as either present or absent; the statistical significance of this binomial response was considered using either a Fisher's exact or Chi Square test. Data was statistically significant when p-values were less than or equal to 0.05 and were designated as * p<0.05, ** p<0.01, or *** p<0.001.
3. RESULTS AND DISCUSSION
3.1. In vitro effects of CG on intracellular Ca2+ and identifying the types of Ca2+ channels involved in CG toxicity
The working hypothesis was as follows: (a) one of the cellular mechanisms of action of CG is via increasing intracellular free Ca2+ concentration ([Ca2+]i), which could be responsible for the vasoconstriction and/or bronchoconstriction effects of CG and (b) preventing CG-induced [Ca2+]i increase by Ca2+ channel blockers might counteract CG toxicity both in vitro and in vivo. Previous in vitro studies on the mechanism of CG toxicity have shown that CG causes an increase in IL-8 as well as a reduction in cell viability and metabolic activity in the immortalized human bronchial epithelial cells 16HBEs (Olivera and Sciuto 2009), small airway cells (Cowan, Smith et al. 2004), and in the lung epithelial carcinoma cell line A549 (Wijte, Alblas et al. 2011). Moreover, sublethal doses of CG was reported to cause a substantial increase in intracellular free calcium in sheep pulmonary endothelial cells (Werrlein, Kirby et al. 1999).
Since Ca2+ regulates muscle contraction and relaxation, we have studied the role of Ca2+ channels to include the TRP channels in CG pathophysiology. BSMC and HPMEC were exposed to 16 ppm, 8 min using the VitroCell air/liquid exposure system. This exposure model has been established at the US Army Medical Research Institute of Chemical Defense for a number of years and has been shown to adversely affect both cellular viability and epithelial barrier function when various cell models are exposed to 4 to 64 ppm phosgene (Olivera and Sciuto 2009). TRP channels are involved in signal transduction of various pathways following activation and involve cells that are not directly exposed to air, e.g. macrophages, endothelial cells, vascular endothelial cells (Freichel, Suh et al. 2001, Alvarez, King et al. 2006, Willette, Bao et al. 2008, Grace, Baxter et al. 2014, Jurek, Hirano-Kobayashi et al. 2014). CG is hydrophobic in nature and, therefore, it passes through the upper respiratory tract with less absorption and concentrates mostly in the deep lung to cause toxicity in the alveoli and microvascular system. The microvasculature is responsible for feeding the alveoli and nearby epithelial and pro-inflammatory cells. The septum is very thin between the alveoli and microvascular system; moreover, CG has been shown to cross this barrier (Borak and Diller 2001). CG is edemagenic, i.e. there is a link between pulmonary edema and CG exposure, the causative effect being a loss of barrier integrity. This is thought to result from the initiation of various toxic signaling mechanisms, including changes in intracellular calcium (Werrlein, Kirby et al. 1999, Sciuto and Hurt 2004, Pauluhn, Carson et al. 2007). CG is also known to cause vaso- and bronchoconstriction (Borak and Diller 2001, Pauluhn, Carson et al. 2007) through possible calcium signaling (Werrlein, Kirby et al. 1999). Our choice of BSMC and HPMEC are representative cellular models of these physiological parameters.
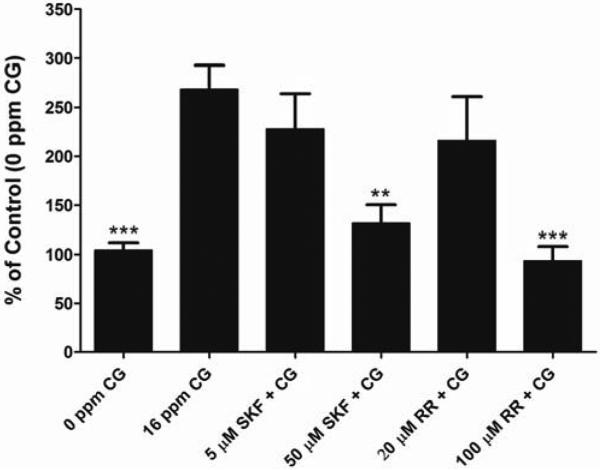
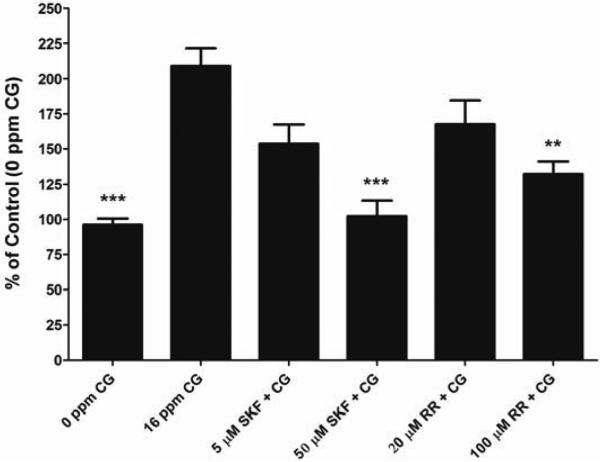
For our study, the toxicity of phosgene was assessed by live/dead cell viability analysis along with gross observations using light microscopy (data not shown). We observed that there was a dose-dependent decrease in cell viability when exposed to CG. For further experiments we chose the 16 ppm for 8 min as exposure dose since it gave us significant changes in [Ca2+]i with the lowest change in cell viability to minimize the loss of cells from the Transwell matrix following exposure to CG. Even though we are unable to correlate to a specific concentration of intracellular calcium, a significant increase in intracellular calcium is enough to trigger signaling pathways involved in cellular function that could be harmful. Our in vitro studies show that CG (16 ppm, 8 min) increases [Ca2+]i in cultured BSMC (Figure 2) and HPMEC (Figure 3). CG-induced Ca2+ increase is reduced by SKF (50 μM) and RR (100 μM) in BSMC (Figure 2) as well as in HPMEC (Figure 3) (p<0.01 when compared to CG alone), when given directly following CG exposure. Moreover the [Ca2+]i in CG-exposed cells treated with SKF (50 μM) and RR (100 μM) was not significantly different from that in control cells (no CG). There was a decrease in [Ca2+]i observed with 5 μM SKF and 20 μM RR in both BSMC and HPMECs; however it was not significant. Since SKF and the compound RR have been proposed as general TRP and TRP V blockers, respectively, our results indicate the TRP channel mechanisms as possible therapeutic targets for CG inhalation injury.
Figure 2. Effect of CG on [Ca2+]i in BSMC and the effect of SKF 96365 and RR on change in [Ca2+]i.
BSMC were grown to a 90% confluency on Transwell™ inserts and were exposed to air (0 ppm CG, 8 min) or CG (16 ppm, 8 min). SKF and RR were given directly following CG exposure. [Ca2+]i was monitored with Fluo-4 Ca2+ indicator assay. Data is represented as % of air Control and presented as mean ± SD. **p < 0.01 and *** p < 0.001 when compared to 16 ppm CG alone. Five μM SKF and 20 μM RR were significantly different from control (air) p<0.05. n ≥ 12
Figure 3. Effect of CG on [Ca2+]i in HPMEC and the effect of SKF 96365 and RR on change in [Ca2+]i.
HPMEC were grown to a 90% confluency on fibronectin-coated Transwell™ inserts and were exposed to air (0 ppm CG, 8 min) or CG (16 ppm, 8 min). SKF and RR were given directly following CG exposure. [Ca2+]i was monitored with Fluo-4 Ca2+ indicator assay. Data is represented as % of air Control and presented as mean ± SD. **p < 0.01 and *** p < 0.001 when compared to 16 ppm CG alone. Five μM SKF and 20 μM RR were significantly different from control (air) p<0.05. n ≥ 12.
3.2. The effects of TRP channel inhibitors on CG-inhalation toxicity
This part of the study involved evaluating in live animals, here in mice, prospective therapeutic candidate compounds against toxicity due to CG. The mouse CG exposure animal model established at USAMRICD served as the CG inhalation model (Duniho, Martin et al. 2002). This exposure model results in lung pathology similar to that seen in humans following phosgene exposure, to include inflammation of bronchial and alveolar structures, disruption of normal lung architecture and delayed appearances of pulmonary edema, which has been well documented (Duniho, Martin et al. 2002, Sciuto and Hurt 2004, Pauluhn, Carson et al. 2007). For this specific study, CG (LD50 Ct @10 ppm, 10 min) resulted in latent pulmonary edema formation, tissue hypoxia, hypoxemia, and uncompensated respiratory acidosis, yielding a mortality rate of approximately 55% at 24 hr after CG inhalation. Both SKF and RR were tested in the initial screening of test compounds for this exposure model. CG control had a significantly lower survival rate at 24 hr, 43% (13/30) compared to a CG+SKF, 70% (21/30), p=.034. Moreover, CG control had a significantly lower survival rate at 24 hr, 36.7% (11/30), compared to CG+ 3, 6, and 9 mg/kg RR, 66.7% (20/30), 73.3% (22/30) and 70% (21/30) respectively. RR was the more efficacious of the two compounds; therefore, RR continued into the next phase for further studies, whereas no further studies were done with SKF.
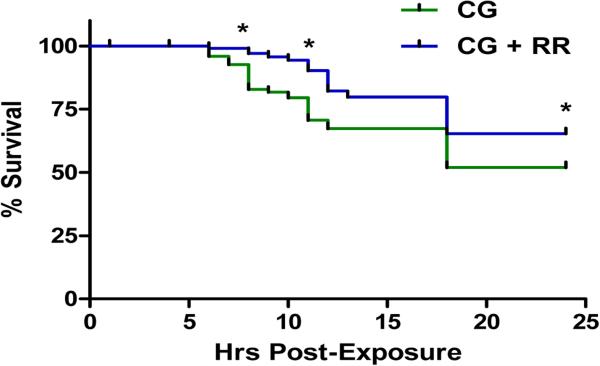
In the extended phase studies, RR was administered at 20 min following CG exposure. Animals that received RR after CG exposure had significantly greater survival throughout the 24 hr period observed when compared to other test compounds in the study (Figure 4). RR treated animals seemed to have the ability to compensate for the CG-induced respiratory acidosis at early time points (4 to 8 hr post-exposure) and their pH levels were able to adjust to near control levels at 8 hr post-exposure (data not shown). Histopathology results showed a reduction in the CG-induced pathology at both 12 and 24 hr post-exposure (Table 2). However, at 24 hr, there was an increase in inflammatory cells (52%) in the alveolar exudates. As already stated, RR is known as a general TRPV channel blocker; thus, these observations correlate well with the studies involving TRPV1 and TRPV4 channels and increased permeability of pulmonary vascular and epithelial barriers in respiratory diseases see, Table 1. Based on the role of the TRP channels involved primarily in airway pathologies as described in literature, we speculate that the inhibition of TRPV4 is probably responsible for the decrease in edema and also the increase in survivability. However, this is not confirmed is this study since RR lacks absolute specificity for individual channel types.
Figure 4. Percent survival following CG inhalation exposure.
Percent survival represented as % median across time. CG had a steady decrease in survival from 8-24 hr. At 24 hr, 47% of the non-treated animals were alive. RR was able to significantly delay and reduce mortality following CG exposure from 8-24 hr post-exposure with 69% of animals surviving at 24 hr post-exposure. (*) significantly different from air non-treated group (air control) for average % survival.
Table 2. Effect of RR on histopathological scores in the lung following CG inhalation exposure in mice at specified time points.
Inhaled CG resulted in the typical pathology of TIH-induced lung injury, and the main pathological characteristics scored are listed in the table below. The effects on the lung histopathological scores of administration of RR after CG inhalation exposure at 12 and 24 hr post-exposure were analyzed, and the median grade of these symptoms following administration when compared to CG alone are shown below.
| Time | 12 hr | 24 hr | |||
|---|---|---|---|---|---|
| Treatment | PR | PN | PR | PN | |
| Perivascular edema, +/− inflammation | 0 | 0 | 0 | 1 | |
| Alveolar exudate | Edema | 0 | 0 | 0 | 1 |
| Fibrin | 1 | 1 | 2 | 3 | |
| Hemorrhage | 0 | 0 | 0 | 0 | |
| Inflammatory cells | 0 | 0 | 1 | 1 | |
| Alveolar epithelial necrosis | 0 | 0 | 0 | 0 | |
| Interstitial cellular infiltrate & edema | 1 | 1 | 2 | 2 | |
| Bronchiolar exudate | 0 | 1 | 1 | 3 | |
| Bronchiolar epithelial necrosis | 0 | 0 | 0 | 1 | |
| Perbronchiolar PMN infiltrates & edema | 0 | 0 | 0 | 0 | |
PR = phosgene + RR; PN = phosgene no treatment n=10
There is a decrease in the positive effects of RR treatment in both survivability and histopathology around 12 hr following CG exposure. This is most likely due to a decrease in drug levels over time. This would suggest that administration a second dose of RR around the 8-12 hr time point.. This agrees with what was reported when inhaled RR was used to prevent ventilator-induced lung edema, where the protection provided by RR alone was efficient for less than two hours unless the RR therapy was administered in a sustained released delivery system using inhaled poly-lactic-co-glycolic acid (PLGA) nanoparticles (Jurek, Hirano-Kobayashi et al. 2014).
4. CONCLUSIONS
TRP channels have been proposed by other investigators as specific as well as sensitive target molecules in the lung and pulmonary vasculature for inhalation exposure to toxic challenges (Banner, Igney et al. 2011, Buch, Schafer et al. 2013, Balakrishna, Song et al. 2014, Billeter, Hellmann et al. 2014, Grace, Baxter et al. 2014). As indicated above, these molecules serve important functions as TIH chemosensors and in signaling pathways for targeted interventions (see background). Based on studies in literature, we suggest that the inhibition of TRPV channels to include TRPV1 and TPV4 is responsible for the decrease in edema and also the increase in survivability, with the inhibition of TRPV4 being more important due to involvement of edema formation in the lower lungs in CG toxicity. The RR inhibitor lacks the absolute specificity for either TRPV1 or TRPV4 channels. Therefore, a complete validation of the concept is dependent on additional confirmatory experiments using more reliable channel type-specific inhibitors; these are being pursued in our laboratory at present. Our results also suggested that administering at least a second dose of RR around the 8-12 hr time point after CG exposure might be efficacious since the positive effects seen with histopathology began to diminish at 12 hr post-exposure. Further studies using other routes of administration and sustained released delivery systems should improve the efficacy of TRP channel inhibitors in CG-induced lung injuries (Grace, Baxter et al. 2014). The results discussed here are geared toward establishing a mechanism-based therapeutic strategy for CG inhalation exposures. Our results suggest that the TRP channel mediated mechanisms are possible therapeutic targets for a CG toxicity intervention.
Highlights.
Phosgene (CG) increases intracellular Ca2+ ([Ca2+]i) in cultured BSMC and HPMEC.
TRP channel inhibitors (general TRP, TRPV) prevent CG-induced [Ca2+]i increase.
TRP channel inhibitors protect mice against a 24-hour lethal CG inhalation dose.
TRP channels appear to play a role in CG toxicity both in vitro and in vivo.
TRP channels are possible targets for intervention against CG toxicity.
Acknowledgments
Funding: This research was supported by an interagency agreement between NIH/NIAID (Y1-A1-6179-04) and the USAMRICD (A120-B.P2009-03). Additionally this research was supported in part by an appointment to the Postgraduate Research Participation Program at the U.S. Army Medical Research Institute of Chemical Defense administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and U.S. Army Medical Research and Materiel Command (USAMRMC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The views expressed in this article are those of the author(s) and do not reflect the official policy of the Department of Army, Department of Defense, or the U.S. Government. The experimental protocol was approved by the Animal Care and Use Committee at the United States Army Medical Research Institute of Chemical Defense, and all procedures were conducted in accordance with the principles stated in the Guide for the Care and Use of Laboratory Animals and the Animal Welfare Act of 1966 (P.L. 89-544), as amended,
REFERENCES
- Alvarez DF, King JA, Weber D, Addison E, Liedtke W, Townsley MI. Transient receptor potential vanilloid 4-mediated disruption of the alveolar septal barrier: a novel mechanism of acute lung injury. Circ Res. 2006;99(9):988–995. doi: 10.1161/01.RES.0000247065.11756.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishna S, Song W, Achanta S, Doran SF, Liu B, Kaelberer MM, Yu Z, Sui A, Cheung M, Leishman E, Eidam HS, Ye G, Willette RN, Thorneloe KS, Bradshaw HB, Matalon S, Jordt SE. TRPV4 inhibition counteracts edema and inflammation and improves pulmonary function and oxygen saturation in chemically induced acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2014;307(2):L158–172. doi: 10.1152/ajplung.00065.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banner KH, Igney F, Poll C. TRP channels: emerging targets for respiratory disease. Pharmacol Ther. 2011;130(3):371–384. doi: 10.1016/j.pharmthera.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124(6):1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bessac BF, Jordt SE. Sensory detection and responses to toxic gases: mechanisms, health effects, and countermeasures. Proc Am Thorac Soc. 2010;7(4):269–277. doi: 10.1513/pats.201001-004SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessac BF, Sivula M, von Hehn CA, Caceres AI, Escalera J, Jordt SE. Transient receptor potential ankyrin 1 antagonists block the noxious effects of toxic industrial isocyanates and tear gases. FASEB J. 2009;23(4):1102–1114. doi: 10.1096/fj.08-117812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeter AT, Hellmann JL, Bhatnagar A, Polk HC., Jr. Transient receptor potential ion channels: powerful regulators of cell function. Ann Surg. 2014;259(2):229–235. doi: 10.1097/SLA.0b013e3182a6359c. [DOI] [PubMed] [Google Scholar]
- Borak J, Diller WF. Phosgene exposure: mechanisms of injury and treatment strategies. J Occup Environ Med. 2001;43(2):110–119. doi: 10.1097/00043764-200102000-00008. [DOI] [PubMed] [Google Scholar]
- Buch T, Schafer E, Steinritz D, Dietrich A, Gudermann T. Chemosensory TRP Channels in the Respiratory Tract: Role in Toxic Lung Injury and Potential as “Sweet Spots” for Targeted Therapies. Springer; Berlin Heidelberg: 2013. [DOI] [PubMed] [Google Scholar]
- Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D'Amours M, Witek JS, Fanger CM, Chong JA, Hayward NJ, Homer RJ, Cohn L, Huang X, Moran MM, Jordt SE. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci U S A. 2009;106(22):9099–9104. doi: 10.1073/pnas.0900591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Cordova MM, Werner MF, Silva MD, Ruani AP, Pizzolatti MG, Santos AR. Further antinociceptive effects of myricitrin in chemical models of overt nociception in mice. Neurosci Lett. 2011;495(3):173–177. doi: 10.1016/j.neulet.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Cowan FM, Smith WJ, Moran TS, Paris MM, Williams AD, Sciuto AM. Sulfur mustard- and phosgene-increased IL-8 in human small airway cell cultures. US Army Medical Research Institute of Chemical Defense; Aberden Proving Ground, MD: 2004. [Google Scholar]
- Duniho SM, Martin J, Forster JS, Cascio MB, Moran TS, Carpin LB, Sciuto AM. Acute changes in lung histopathology and bronchoalveolar lavage parameters in mice exposed to the choking agent gas phosgene. Toxicol Pathol. 2002;30(3):339–349. doi: 10.1080/01926230252929918. [DOI] [PubMed] [Google Scholar]
- Firth AL, Remillard CV, Yuan JX-J. TRP channels in hypertension. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2007;1772(8):895–906. doi: 10.1016/j.bbadis.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freichel M, Suh SH, Pfeifer A, Schweig U, Trost C, Weissgerber P, Biel M, Philipp S, Freise D, Droogmans G, Hofmann F, Flockerzi V, Nilius B. Lack of an endothelial store-operated Ca2+ current impairs agonist-dependent vasorelaxation in TRP4−/− mice. Nat Cell Biol. 2001;3(2):121–127. doi: 10.1038/35055019. [DOI] [PubMed] [Google Scholar]
- Glass CA, Pocock TM, Curry FE, Bates DO. Cytosolic Ca2+ concentration and rate of increase of the cytosolic Ca2+ concentration in the regulation of vascular permeability in Rana in vivo. J Physiol. 2005;564(Pt 3):817–827. doi: 10.1113/jphysiol.2005.083220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace MS, Baxter M, Dubuis E, Birrell MA, Belvisi MG. Transient receptor potential (TRP) channels in the airway: role in airway disease. Br J Pharmacol. 2014;171(10):2593–2607. doi: 10.1111/bph.12538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guibert C, Ducret T, Savineau J-P. Expression and physiological roles of TRP channels in smooth muscle cells. Transient Receptor Potential Channels. Springer. 2011:687–706. doi: 10.1007/978-94-007-0265-3_36. [DOI] [PubMed] [Google Scholar]
- Guibert C, Marthan R, Savineau JP. Modulation of ion channels in pulmonary arterial hypertension. Curr Pharm Des. 2007;13(24):2443–2455. doi: 10.2174/138161207781368585. [DOI] [PubMed] [Google Scholar]
- Helyes Z, Elekes K, Nemeth J, Pozsgai G, Sandor K, Kereskai L, Borzsei R, Pinter E, Szabo A, Szolcsanyi J. Role of transient receptor potential vanilloid 1 receptors in endotoxin-induced airway inflammation in the mouse. Am J Physiol Lung Cell Mol Physiol. 2007;292(5):L1173–1181. doi: 10.1152/ajplung.00406.2006. [DOI] [PubMed] [Google Scholar]
- Jurek SC, Hirano-Kobayashi M, Chiang H, Kohane DS, Matthews BD. Prevention of ventilator-induced lung edema by inhalation of nanoparticles releasing ruthenium red. Am J Respir Cell Mol Biol. 2014;50(6):1107–1117. doi: 10.1165/rcmb.2013-0163OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, Szallasi A. Transient receptor potential (TRP) channels: a clinical perspective. Br J Pharmacol. 2014;171(10):2474–2507. doi: 10.1111/bph.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY, Gu Q. Role of TRPV1 in inflammation-induced airway hypersensitivity. Curr Opin Pharmacol. 2009;9(3):243–249. doi: 10.1016/j.coph.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Kanju P, Patterson M, Chew WL, Cho SH, Gilmour I, Oliver T, Yasuda R, Ghio A, Simon SA, Liedtke W. TRPV4-mediated calcium influx into human bronchial epithelia upon exposure to diesel exhaust particles. Environ Health Perspect. 2011;119(6):784–793. doi: 10.1289/ehp.1002807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/− mice. Proc Natl Acad Sci U S A. 2003;100(23):13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael M, Wenjie Y, Wolfgang L, Stefan U, Wolfgang MK. A46. VENTILATOR-INDUCED LUNG INJURY: RESEARCH AND CLINICAL ASPECTS. American Thoracic Society; TRPV4-deficiency Protects Overventilated Mice From Lung Edema But Not Inflammation. pp. A1676–A1676. [Google Scholar]
- Miller K, Chang A. Acute inhalation injury. Emerg Med Clin North Am. 2003;21(2):533–557. doi: 10.1016/s0733-8627(03)00011-7. [DOI] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol Rev. 2007;87(1):165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Olivera DS, Sciuto AM. D40. UNDERSTANDING INHALATIONAL DISASTERS. American Thoracic Society; 2009. In Vitro Model for Screening Therapeutics for Toxic Industrial Chemical Inhalation Injury. p. A5867. [Google Scholar]
- Owsianik G, Talavera K, Voets T, Nilius B. Permeation and selectivity of TRP channels. Annu Rev Physiol. 2006;68:685–717. doi: 10.1146/annurev.physiol.68.040204.101406. [DOI] [PubMed] [Google Scholar]
- Pauluhn J, Carson A, Costa DL, Gordon T, Kodavanti U, Last JA, Matthay MA, Pinkerton KE, Sciuto AM. Workshop summary: phosgene-induced pulmonary toxicity revisited: appraisal of early and late markers of pulmonary injury from animal models with emphasis on human significance. Inhal Toxicol. 2007;19(10):789–810. doi: 10.1080/08958370701479133. [DOI] [PubMed] [Google Scholar]
- Sciuto AM, Hurt HH. Therapeutic treatments of phosgene-induced lung injury. Inhal Toxicol. 2004;16(8):565–580. doi: 10.1080/08958370490442584. [DOI] [PubMed] [Google Scholar]
- Sel S, Rost BR, Yildirim AO, Sel B, Kalwa H, Fehrenbach H, Renz H, Gudermann T, Dietrich A. Loss of classical transient receptor potential 6 channel reduces allergic airway response. Clin Exp Allergy. 2008;38(9):1548–1558. doi: 10.1111/j.1365-2222.2008.03043.x. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278(25):22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- Thorneloe KS, Cheung M, Bao W, Alsaid H, Lenhard S, Jian MY, Costell M, Maniscalco-Hauk K, Krawiec JA, Olzinski A, Gordon E, Lozinskaya I, Elefante L, Qin P, Matasic DS, James C, Tunstead J, Donovan B, Kallal L, Waszkiewicz A, Vaidya K, Davenport EA, Larkin J, Burgert M, Casillas LN, Marquis RW, Ye G, Eidam HS, Goodman KB, Toomey JR, Roethke TJ, Jucker BM, Schnackenberg CG, Townsley MI, Lepore JJ, Willette RN. An orally active TRPV4 channel blocker prevents and resolves pulmonary edema induced by heart failure. Sci Transl Med. 2012;4(159):159ra148. doi: 10.1126/scitranslmed.3004276. [DOI] [PubMed] [Google Scholar]
- Tuorinsky S, Sciuto A. Toxic inhalation injury and toxic industrial chemicals. Medical aspects of chemical warfare. Office of the General Surgeon; M. Lenhart. Washington DC: 2008. pp. 339–370. [Google Scholar]
- Werrlein RJ, Kirby SD, Madren-Whalley JS. Phosgene-Induced Calcium Changes in Pulmanary Artery Endothelial Cells. In: Salem H, Kata SA, editors. Toxicicyt Assessment Alternatives: Methods, Issues, Opportunities. Humana Press; 1999. pp. 175–183. [Google Scholar]
- Wijte D, Alblas MJ, Noort D, Langenberg JP, van Helden HP. Toxic effects following phosgene exposure of human epithelial lung cells in vitro using a CULTEX(R) system. Toxicol In Vitro. 2011;25(8):2080–2087. doi: 10.1016/j.tiv.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, Turner GH, Ju H, Thomas H, Fishman CE, Sulpizio A, Behm DJ, Hoffman S, Lin Z, Lozinskaya I, Casillas LN, Lin M, Trout RE, Votta BJ, Thorneloe K, Lashinger ES, Figueroa DJ, Marquis R, Xu X. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: Part 2. J Pharmacol Exp Ther. 2008;326(2):443–452. doi: 10.1124/jpet.107.134551. [DOI] [PubMed] [Google Scholar]
- Yue Z, Xie J, Albert SY, Stock J, Du J, Yue L. Role of TRP channels in the cardiovascular system. American Journal of Physiology-Heart and Circulatory Physiology. 2015;308(3):H157–H182. doi: 10.1152/ajpheart.00457.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]