Abstract
Diisocyanate chemicals essential to polyurethane production are a well-recognized cause of occupational asthma. The pathogenesis of diisocyanate-induced asthma, including the pathways by which the chemical is taken up and its distribution in exposed tissue, especially the lung, remain unclear. We developed an antiserum with specificity for methylene diphenyl diisocyanate (MDI) the most abundantly produced and utilized diisocyanate world-wide, and established its ability to detect MDI in situ. Polyclonal MDI-specific IgG were induced by immunizing rabbits with MDI-conjugated to keyhole limpet hemocyanin (KLH) emulsified in complete Freund’s adjuvant, followed by two booster injections with incomplete Freund’s adjuvant. The antiserum contains IgG that recognize a variety of different MDI conjugated proteins, but not unconjugated or mock exposed proteins by dot blot analysis. The antiserum further demonstrates specificity for proteins conjugated with MDI, but not other commonly used diisocyanates. Immunochemical studies with cytospun airway cells and formalin-fixed paraffin embedded lung tissue sections from mice intranasally exposed to MDI (as reversibly reactive glutathione conjugates, e.g. GSH-MDI) demonstrated the antiserum’s ability to detect MDI in tissue samples. The data demonstrate penetration of MDI into the lower airways, localized deposition in the epithelial region surrounding airways, and uptake by alveolar macrophages. The new immunochemical reagent should be useful for further studies delineating the uptake and tissue distribution of MDI, especially as it relates to adverse health effects from exposure.
Keywords: Methylene-diphenyl-diisocyanate (MDI), antiserum, airways, occupational asthma
1. INTRODUCTION
Diisocyanates, industrial chemicals with many important uses, are among the most commonly recognized causes of occupational asthma world-wide (Allport, Gilbert et al. 2003, Wisnewski, Liu et al. 2008). Methylene diphenyl diisocyanate (MDI) is the most abundantly produced and consumed diisocyanate, with applications in the generation of rigid foams and as a binding agent (Allport, Gilbert et al. 2003). Other diisocyanates, such as hexamethylene diisocyanate (HDI) and toluene diisocyanate (TDI) are less commonly used and have specialized applications as protective coatings and in the generation of “softer” flexible polyurethane foam (Allport, Gilbert et al. 2003, Wisnewski, Liu et al. 2008).
The pathogenesis of diisocyanate-induced asthma remains unclear, in part due to uncertainty regarding the reactivity of these chemicals with “self” molecules at the major sites of exposure. Animal studies with radio-isotope labeled diisocyanates suggest that a portion of inhaled chemical remains bound within lung tissue, however the distribution in different sub-anatomical locations remains unclear (Kennedy, Wilson et al. 1994). Redlich et al detected HDI along the airway epithelium of an exposed worker using polyclonal antiserum raised against HDI conjugated to ovalbumin (Redlich, Karol et al. 1997). Recent studies with TDI-specific monoclonal antibodies have demonstrated that TDI binds to proteins in the skin, which may be an important exposure route for inducing systemic immune sensitization (Nayak, Hettick et al. 2014). Studies detecting MDI in situ, however, are lacking, as necessary reagents have yet to be generated and characterized.
In this report, we develop a rabbit antiserum with specificity for MDI and demonstrate its utility for detecting the chemical in lung tissue from exposed animals. Importantly, the polyclonal serum includes anti-MDI IgG antibodies whose epitopes are not destroyed by formalin fixation, allowing immunochemical detection of MDI in samples processed according to routine histological procedures (e.g. formalin-fixed paraffin-embedded). The potential applications of the new anti-MDI polyclonal serum toward understanding disease pathogenesis and as evidence of chemical exposure are discussed.
2. METHODS
2.1 Chemicals and reagents
H2O2, the diisocyanates, 4,4′-diphenylmethane diisocyanate (MDI), 1,6 hexamethylene diisocyanate (HDI), and an 80:20 ratio mixture of 2,4:2,6 toluene diisocyanate (TDI), albumins (from human, bovine and mouse), tropomyosin, keyhole-limpet hemocyanin (KLH), Tween 20, tetramethylbenzidine (TMB) liquid substrate solution, complete and incomplete Freund’s adjuvant were from Sigma-Aldrich (St. Louis, MO). Biotin-labeled mouse monoclonal anti-rabbit IgG (6C1A8) with no cross-reactivity to IgG from other species was from ProSci Inc. (Poway, CA). Streptavidin-HRP and peroxidase conjugated anti-rabbit IgG were from BD Biosciences (San Jose, CA). Biotinylated goat anti-human albumin was from Bethyl (Montgomery, TX) and protein-A purified anti-IL-33 polyclonal rabbit IgG was from Enzo Life Sciences (Farmingdale, NY). Cyanogen bromide activated sepharose 4B was from Amersham Biosciences (Uppsala, Sweden) and protein A affinity columns were from BioRad (Hercules, CA). Biotin blocking solution was from Life Technologies (Eugene, OR). Diaminobenzidine (DAB) substrate, hematoxylin, and histomount were from a HistoMouse-MAX staining Kit (Invitrogen, Frederick, MD). Protein A sepharose 4B (InVitrogen). Normal rabbit and goat serum were from ThermoFisher Scientific (Waltham, MA). Amersham Hyperfilm MP from GE Healthcare Bio-Sciences (Pittsburgh, PA) was used in developing dot blots.
2.2 Generation of diisocyanate-conjugated proteins
Diisocyanate conjugated proteins were prepared as previously described (Wisnewski, Liu et al. 2008, Wisnewski, Liu et al. 2010). Briefly, proteins were dissolved in 20 mM phosphate buffered saline pH 7.4 at a concentration of 5 mg/ml. Diisocyanates were prediluted to 10% w/v in acetone, and 10 μl/ml of 10% diisocyanate was added to protein solutions to achieve a final concentration of 0.1% (w/v) diisocyanate. Diisocyanate-protein reactions were mixed vigorously and rotated end-over-end for 2 hours at room temperature. Following centrifugation at 10,000 g, the supernatant was recovered and dialyzed against PBS to remove unreacted and/or hydrolyzed chemical as well as potential low molecular weight diisocyanate polymers. Following dialysis, samples were again centrifuged at 10,000 g, sterile filtered (0.2 μm), and stored at −80°C. Diisocyanate conjugation to proteins was verified by changes in the different proteins’ electrophoretic mobility and by mass spectrometry as previously described (Wisnewski, Liu et al. 2008, Wisnewski, Liu et al. 2010). Control unconjugated proteins were “mock exposed” to 10 μl/ml of acetone without diisocyanate and identically processed.
2.3 Generation of polyclonal MDI-specific rabbit serum
To generate MDI-specific polyclonal rabbit serum, New Zealand White rabbits (Harlan Laboratories, Indianapolis, IN) were immunized subcutaneously with 500 μg of 4,4′-MDI-conjugated KLH emulsified in complete Freund’s adjuvant, followed by two booster shots of 250 μg of MDI-KLH in incomplete Freund’s adjuvant on days 14 and 28. Terminal bleed serum was collected on day 72 from 2 different rabbits, pooled, and depleted of anti-KLH immunoglobulins by affinity chromatography using KLH-coupled sepharose 4B. For immunochemistry studies, the IgG fraction was further purified by protein A affinity chromatography. All animal studies were conducted in accordance with institutional guidelines.
2.4 Dot blot analyses
One microliter of protein solutions (5mg/ml, except for tropomyosin, 1 mg/ml) in PBS were spotted onto nitrocellulose membrane. Membranes were blotted with a 1:1000 dilution of anti-MDI polyclonal rabbit serum followed by a 1:2000 dilution of peroxidase conjugated anti-rabbit IgG. In studies with human albumin conjugated with different diisocyanates, dot blots were similarly probed with anti-MDI polyclonal serum, then subsequently stripped, and reprobed with biotinylated goat anti-human albumin, followed by peroxidase conjugated streptavidin. Replicate samples were also blotted with a 1:1000 dilution of pre-immune rabbit serum, normal rabbit serum and 2 μg/ml of polyclonal anti-IL-33 rabbit IgG. All bots were blocked with 3% dry milk in tris buffered saline (TBS) pH 7.4, washed with TBS containing 0.05% Tween 20, and developed with TMB liquid substrate, before exposure to film and processing. In some experiments, proteins were stained with 0.1% Ponceau S, and then washed before blotting with specific antibodies.
2.5 Immunochemistry
Studies were performed on replicate samples of cytospun airway cells obtained by bronchoalveolar lavage (BAL) and lung tissue sections of MDI exposed or control mice acquired during prior studies as previously reported (Wisnewski, Liu et al. 2015). Cytospun BAL cells fixed with −20°C methanol and formalin-fixed paraffin-embedded tissue sections were blocked with 20% goat serum and stained with 2 μg/ml of KLH-depleted, protein-A affinity purified anti-MDI rabbit IgG, or 2 μg/ml of isotype control protein-A purified polyclonal rabbit IgG raised against IL-33 or 2 μg/ml protein-A purified normal rabbit IgG (not shown). Endogenous peroxidase was quenched before analysis using H2O2, and endogenous biotin was blocked before addition of biotin-labeled mouse monoclonal anti-rabbit IgG (with no cross-reactivity to other species IgG) using commercially available “biotin-blocking solution” (see Section 2.1 above). Slides were further developed with peroxidase-labeled streptavidin, DAB substrate, and hematoxylin counterstain. Micrographs were taken under a Nikon Eclipse NI-U model microscope (Tokyo, Japan) equipped with a Nikon DS-Ri2 image capture system and loaded into Nikon’s NIS-Elements Basic Research software program for image analysis.
3. RESULTS
3.1 MDI antisera’s specificity for MDI conjugated vs. unconjugated proteins
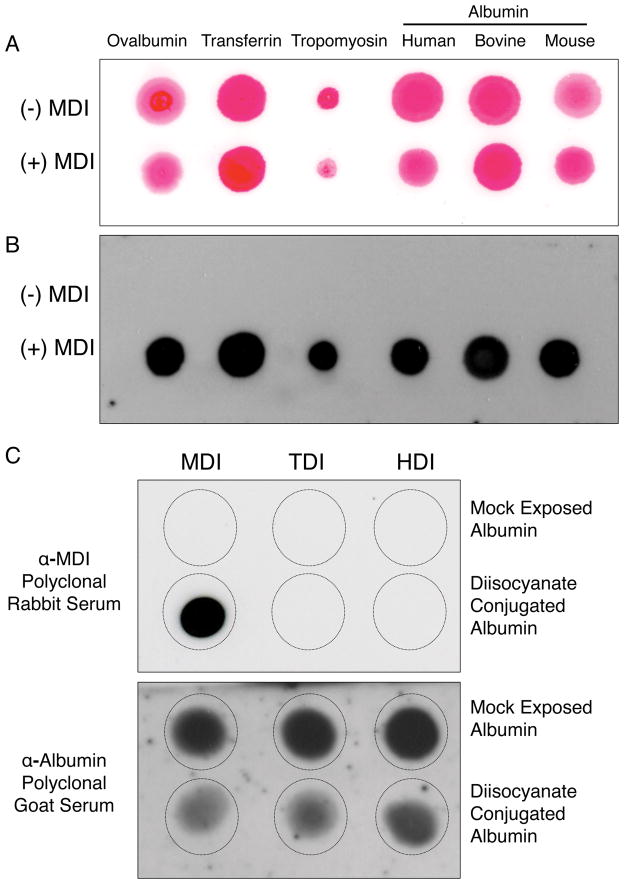
The presence of MDI-specific IgG in the polyclonal rabbit serum was first assessed by dot blot analysis. As shown (Figure 1A and B) the MDI antisera contained rabbit IgG that recognized MDI in a hapten-like manner; the antisera bound to a variety of different MDI conjugated proteins, regardless of the protein “carrier”, but did not bind the corresponding unconjugated (mock reacted) proteins.
Figure 1.
Specificity of MDI antisera. Different MDI-conjugated (+ MDI) or unconjugated/mock exposed (− MDI) proteins were spotted onto nitrocellulose membranes, stained for total protein using Ponceau-S (Panel A), and then blotted (Panel B) with a 1:1000 dilution of polyclonal anti-MDI serum, and reagents to detect rabbit IgG. In Panel C, mock exposed control albumin samples, or albumin conjugated with MDI, TDI or HDI (as labeled) were spotted onto nitrocellulose membranes and probed with MDI antisera or biotinylated anti-human albumin (as labeled) and developed with reagents that detect rabbit IgG or biotin respectively. No signal was observed in dot blots with control, pre-immune, normal rabbit serum, or polyclonal rabbit anti-IL-33 (data not shown).
3.2 MDI antisera’s specificity for MDI vs. other diisocyanates
We next tested the specificity of the rabbit polyclonal antisera for MDI vs. other diisocyanates commonly used industrially, namely TDI and HDI. As shown (Figure 1C), the MDI antisera contained IgG that bound to albumin conjugated with MDI, but not HDI or TDI, or unconjugated (mock exposed) albumin preparations. In contrast, control biotinylated anti-human-albumin goat IgG recognizes albumin bound by all 3 diisocyanates; however binding is reduced following diisocyanate conjugation, likely due to chemical modification of the native albumin structure as previously described (Wisnewski, Liu et al. 2008, Wisnewski, Liu et al. 2010).
3.3. Detection of MDI within airway cells from MDI-GSH exposed mice
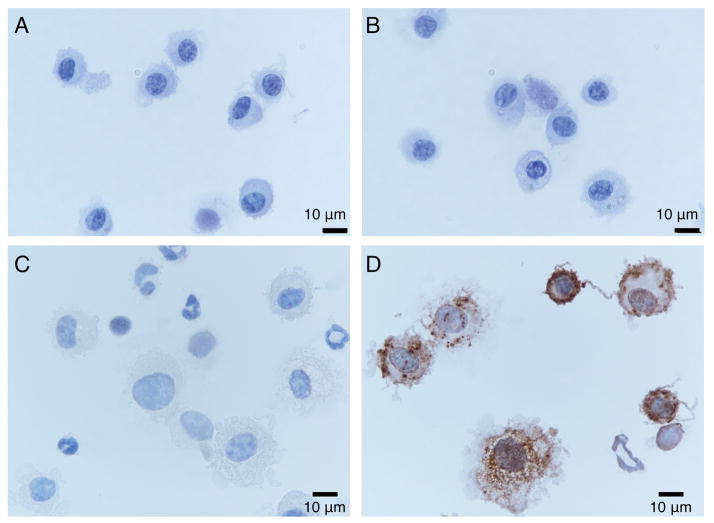
We tested the ability of the MDI antisera to detect MDI in airway cells recovered by bronchoalveolar lavage (BAL). Immunochemical staining of cytospun BAL cells from mice sensitized and exposed to MDI, as reversibly reactive MDI-GSH conjugates (Wisnewski, Liu et al. 2015), identified MDI primarily within the cytoplasm of cells with alveolar macrophage or dendritic cell morphology, but not polymorphonuclear or lymphocyte morphology (Figure 2). In contrast, no staining was observed in BAL cells recovered from MDI sensitized mice that had been exposed to inactivated (e.g. hydrolyzed) MDI.
Figure 2.
Immunochemical detection of MDI in BAL cells. Lower airway cells from MDI skin sensitized mice exposed to inactivated (hydrolyzed) MDI (Panels A & B) or MDI-GSH (Panels C & D) were stained with protein-A affinity purified control (anti-IL-33) rabbit polyclonal antisera (Panels A & C) or KLH-depleted, protein-A affinity purified MDI antisera (Panels B & D). Note dark staining within cytoplasm of cells with alveolar macrophage and dendritic cell-like morphology.
3.4 Detection of MDI in lung tissue from MDI-GSH exposed mice
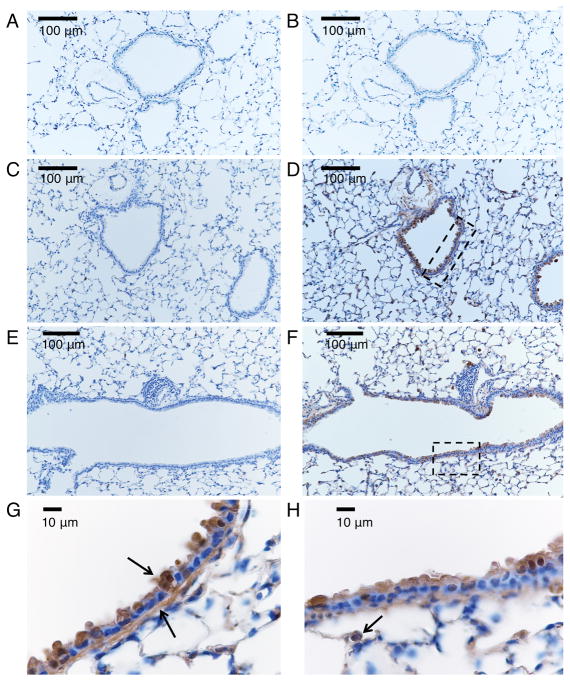
We further tested the ability of MDI antisera to detect MDI in situ in lung tissue from mice that had been exposed to MDI-GSH (Wisnewski, Liu et al. 2015). As shown in Figure 3, immunochemical staining of formalin-fixed paraffin embedded tissue sections identified MDI localized primarily within epithelial cells, along the basement membrane lining the bronchial airways, and to a lesser extent, alveolar epithelial cells. Additional staining was also noted in cells that resemble alveolar macrophages, consistent with data from studies of BAL cells shown in Figure 2. The data validate the recently described effectiveness of delivering reactive MDI to the lower airways in rodents, via intranasal application of reversibly reactive MDI-GSH conjugates (Wisnewski, Liu et al. 2015).
Figure 3.
Immunochemical detection of MDI in situ in the airway. Lung tissue sections from mice exposed via the airways to control inactivated (e.g. hydrolyzed) MDI (A, B) or MDI-GSH conjugates (C–H) were stained with control (anti-IL-33) rabbit serum (A, C, E) or anti-MDI rabbit serum (B, D, F, G, H) as described in the methods section and detected via DAB (brown) peroxidase stain, with blue hematoxylin counter stain. Panels A–F were photographed under 200X magnification. Panels G and H, taken under 1000X magnification, highlight areas shown in the rectangles in Panels D and F respectively. Arrows highlight staining of cells lining the airways, the basement membrane and an alveolar macrophage.
4. DISCUSSION
The generation and characterization of a new immunologic reagent for the detection of MDI, an important occupational allergen, is described in this report. Anti-MDI polyclonal serum was developed by immunizing rabbits with KLH covalently conjugated with MDI, and subsequently evaluated via dot blots and immunocytochemical studies. The antiserum contains IgG that recognizes MDI in a hapten-like manner, and binds to MDI when conjugated to a diverse array of carrier proteins including albumin, tropomyosin, transferrin, and ovalbumin. The antiserum is specific for MDI, and does not cross-react with (proteins conjugated with) other commonly used isocyanates, including TDI and HDI. Immunocytochemical studies demonstrate the antiserum’s capacity to detect MDI in situ in formalin-fixed paraffin-embedded tissue sections from the lungs of exposed animals. The new reagent will serve as a useful probe for research studies evaluating the pathogenesis of MDI exposure induced asthma, especially the uptake and distribution of chemical, which continues to remain unclear despite decades of research.
The present immunocytochemical studies with anti-MDI polyclonal antiserum expand upon recent studies demonstrating the capacity of reversibly reactive MDI-GSH conjugates to induce eosinophilic airway inflammation in sensitized animals (Wisnewski, Liu et al. 2015). The data confirm penetration of MDI to the lower airways following intranasal application of MDI-GSH conjugates under light anesthesia; unlike prior studies, which have found limited lower airway penetration of pure isocyanate vapors (or solutions in organic solvent), presumably due to the scrubbing effects of rodent upper airways (Kennedy, Singh et al. 1993, Kennedy, Wilson et al. 1994, Pauluhn 2014). The findings validate the hypothesis that MDI-GSH conjugates provide an effective approach for delivering a reactive form of MDI to the lower airways, overcoming barriers that have limited animal studies of MDI asthma pathogenesis in the past.
The present data identify airway cells, airway basement membrane, alveolar macrophages, and to a lesser extent alveolar epithelial cells, as targets for MDI in the lower airway. These findings are consistent with limited prior studies in humans, biophysical aspects of the airway microenvironment, and accumulating data on monocyte/macrophage responses to isocyanates in vitro and in vivo (Redlich, Karol et al. 1997, Wisnewski, Liu et al. 2008, Wisnewski, Liu et al. 2015). Airway epithelial cells have been hypothesized as a primary reaction target for diisocyanates given their location at the air/liquid interface of the lung. Isocyanate reactivity with human airway cells in vivo and in vitro has been reported following HDI exposure, but similar studies have not been undertaken with MDI (Redlich, Karol et al. 1997). Reactivity of MDI with the bronchial basement membrane has not been previously recognized, but specific components, including laminin and elastin have been proposed as targets for diisocyanate conjugation (Nayak, Hettick et al. 2014, Hulst, Verstappen et al. 2015). Deposition of isocyanate along the bronchial basement membrane might result in a stable/persistent reaction product capable of eliciting immune responses long after exposure ceases. Uptake of MDI by alveolar macrophages is consistent with the well-recognized phagocytic capacity of these cells and their role in airway homeostasis. The effects of MDI (and other diisocyanates) on airway monocytes/macrophages and related antigen presenting cells remains unclear, but could play an important role in asthma pathogenesis (Wisnewski, Liu et al. 2015). In vitro studies with human monocytes that have phagocytized isocyanate conjugated proteins have documented marked changes in gene expression patterns, some of which have been linked to adverse clinical outcomes from occupational diisocyanate exposure (Wisnewski, Liu et al. 2008). Thus, the present immunocytochemical identification of MDI in situ in the lungs of animals exposed to MDI (as reversibly reactive GSH conjugates) validates limited prior studies, and leading hypotheses on chemical reactivity/deposition in vivo.
In summary, a new antiserum was developed with specificity for the occupational allergen, MDI, a chemical essential to numerous industrial processes. The polyclonal antiserum contains IgG that recognize MDI in a hapten-like manner and demonstrates utility for localizing the chemical in situ in tissue samples processed for routine histology (e.g. formalin-fixed paraffin embedded). The new MDI antiserum will be useful for future studies delineating the uptake and distribution of MDI, especially as it relates to the development of asthma and other adverse exposure outcomes.
Acknowledgments
This work was supported by funding from CDC/NIOSH from grants OH010494 and OH010438
ABBREVIATIONS
- BAL
bronchoalveolar lavage
- DAB
diaminobenzidine
- GSH
reduced glutathione
- HDI
hexamethylene diisocyanate
- KLH
keyhole limpet hemocyanin
- MDI
methylene diphenyl diisocyanate
- TMB
tetramethylbenzidine
- TBS
tris buffered saline
- TDI
toluene diisocyanate
Contributor Information
Adam V Wisnewski, Email: Adam.wisnewski@yale.edu.
Jian Liu, Email: Jian.liu@yale.edu.
References
- Allport DC, Gilbert DS, Outterside SM, editors. MDI and TDI: Safety, Health and the Environment: A Source Book and Practical Guide. Chichester Wiley, Wiley; 2003. [DOI] [Google Scholar]
- Hulst AG, Verstappen DR, van der Riet-Van Oeveren D, Vermeulen NP, Noort D. Mass spectrometric identification of isocyanate-induced modifications of keratins in human skin. Chem Biol Interact. 2015;237:141–150. doi: 10.1016/j.cbi.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Kennedy AL, Singh G, Alarie Y, Brown WE. Autoradiographic analyses of guinea pig airway tissues following inhalation exposure to 14C-labeled methyl isocyanate. Fundam Appl Toxicol. 1993;20(1):57–67. doi: 10.1093/toxsci/20.1.57. [DOI] [PubMed] [Google Scholar]
- Kennedy AL, Wilson TR, Stock MF, Alarie Y, Brown WE. Distribution and reactivity of inhaled 14C-labeled toluene diisocyanate (TDI) in rats. Arch Toxicol. 1994;68(7):434–443. doi: 10.1007/s002040050094. [DOI] [PubMed] [Google Scholar]
- Nayak AP, Hettick JM, Siegel PD, Anderson SE, Long CM, Green BJ, Beezhold DH. Toluene diisocyanate (TDI) disposition and co-localization of immune cells in hair follicles. Toxicol Sci. 2014;140(2):327–337. doi: 10.1093/toxsci/kfu079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauluhn J. Development of a respiratory sensitization/elicitation protocol of toluene diisocyanate (TDI) in Brown Norway rats to derive an elicitation-based occupational exposure level. Toxicology. 2014;319:10–22. doi: 10.1016/j.tox.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Redlich CA, Karol MH, Graham C, Homer RJ, Holm CT, Wirth JA, Cullen MR. Airway isocyanate-adducts in asthma induced by exposure to hexamethylene diisocyanate. Scand J Work Environ Health. 1997;23(3):227–231. doi: 10.5271/sjweh.203. [DOI] [PubMed] [Google Scholar]
- Wisnewski AV, Liu J, Colangelo CM. Glutathione reaction products with a chemical allergen, methylene-diphenyl diisocyanate, stimulate alternative macrophage activation and eosinophilic airway inflammation. Chem Res Toxicol. 2015;28(4):729–737. doi: 10.1021/tx5005002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski AV, Liu J, Redlich CA. Antigenic changes in human albumin caused by reactivity with the occupational allergen diphenylmethane diisocyanate. Anal Biochem. 2010;400(2):251–258. doi: 10.1016/j.ab.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisnewski AV, Liu Q, Liu J, Redlich CA. Human innate immune responses to hexamethylene diisocyanate (HDI) and HDI-albumin conjugates. Clin Exp Allergy. 2008;38(6):957–967. doi: 10.1111/j.1365-2222.2008.02982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]