Abstract
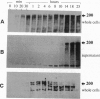
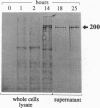
Bordetella pertussis, the human pathogen of whooping cough, when grown at 22 degrees C is nonvirulent and unable to bind eukaryotic cells. In response to a temperature shift to 37 degrees C, the bacterium acquires the ability to bind eukaryotic cells in a time-dependent fashion. By studying in vitro the temperature-induced transition, from the nonvirulent to the virulent state, we found that binding to CHO cells is mediated by the Arg-Gly-Asp-containing domain of filamentous hemagglutinin (FHA), a protein with multiple binding specificities. This protein is synthesized as a 367-kDa polypeptide within 10 min after temperature shift, but requires 2 hr before it is detected on the bacterial cell surface and starts to bind CHO cells. Mutations affecting the cell surface export of FHA abolish bacterial adhesion to CHO cells, while mutations in the outer membrane protein pertactin strongly reduce binding. This suggests that multiple chaperon proteins are required for a correct function of FHA. Finally, several hours after maximum binding efficiency is achieved, the N-terminal 220-kDa portion of FHA that contains the binding regions is cleaved off, possibly to release the bacteria from the bound cells and facilitate spreading. The different forms of FHA may play different roles during bacterial infection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aricò B., Scarlato V., Monack D. M., Falkow S., Rappuoli R. Structural and genetic analysis of the bvg locus in Bordetella species. Mol Microbiol. 1991 Oct;5(10):2481–2491. doi: 10.1111/j.1365-2958.1991.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Aricó B., Miller J. F., Roy C., Stibitz S., Monack D., Falkow S., Gross R., Rappuoli R. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles I. G., Dougan G., Pickard D., Chatfield S., Smith M., Novotny P., Morrissey P., Fairweather N. F. Molecular cloning and characterization of protective outer membrane protein P.69 from Bordetella pertussis. Proc Natl Acad Sci U S A. 1989 May;86(10):3554–3558. doi: 10.1073/pnas.86.10.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Magistris M. T., Romano M., Nuti S., Rappuoli R., Tagliabue A. Dissecting human T cell responses against Bordetella species. J Exp Med. 1988 Oct 1;168(4):1351–1362. doi: 10.1084/jem.168.4.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenighini M., Relman D., Capiau C., Falkow S., Prugnola A., Scarlato V., Rappuoli R. Genetic characterization of Bordetella pertussis filamentous haemagglutinin: a protein processed from an unusually large precursor. Mol Microbiol. 1990 May;4(5):787–800. doi: 10.1111/j.1365-2958.1990.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Glaser P., Ladant D., Sezer O., Pichot F., Ullmann A., Danchin A. The calmodulin-sensitive adenylate cyclase of Bordetella pertussis: cloning and expression in Escherichia coli. Mol Microbiol. 1988 Jan;2(1):19–30. [PubMed] [Google Scholar]
- Gross R., Aricò B., Rappuoli R. Genetics of pertussis toxin. Mol Microbiol. 1989 Jan;3(1):119–124. doi: 10.1111/j.1365-2958.1989.tb00111.x. [DOI] [PubMed] [Google Scholar]
- Gross R., Rappuoli R. Positive regulation of pertussis toxin expression. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3913–3917. doi: 10.1073/pnas.85.11.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LACEY B. W. Antigenic modulation of Bordetella pertussis. J Hyg (Lond) 1960 Mar;58:57–93. doi: 10.1017/s0022172400038134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. K., Roberts A. L., Finn T. M., Knapp S., Mekalanos J. J. A new assay for invasion of HeLa 229 cells by Bordetella pertussis: effects of inhibitors, phenotypic modulation, and genetic alterations. Infect Immun. 1990 Aug;58(8):2516–2522. doi: 10.1128/iai.58.8.2516-2522.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leininger E., Ewanowich C. A., Bhargava A., Peppler M. S., Kenimer J. G., Brennan M. J. Comparative roles of the Arg-Gly-Asp sequence present in the Bordetella pertussis adhesins pertactin and filamentous hemagglutinin. Infect Immun. 1992 Jun;60(6):2380–2385. doi: 10.1128/iai.60.6.2380-2385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leininger E., Roberts M., Kenimer J. G., Charles I. G., Fairweather N., Novotny P., Brennan M. J. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):345–349. doi: 10.1073/pnas.88.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. M., Brennan M. J., David J. L., Carter P. H., Cowell J. L., Manclark C. R. Comparison of type 2 and type 6 fimbriae of Bordetella pertussis by using agglutinating monoclonal antibodies. Infect Immun. 1988 Dec;56(12):3184–3188. doi: 10.1128/iai.56.12.3184-3188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C., Geoffroy M. C., Renauld G. Common accessory genes for the Bordetella pertussis filamentous hemagglutinin and fimbriae share sequence similarities with the papC and papD gene families. EMBO J. 1992 Sep;11(9):3175–3183. doi: 10.1002/j.1460-2075.1992.tb05394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locht C., Keith J. M. Pertussis toxin gene: nucleotide sequence and genetic organization. Science. 1986 Jun 6;232(4755):1258–1264. doi: 10.1126/science.3704651. [DOI] [PubMed] [Google Scholar]
- Menozzi F. D., Gantiez C., Locht C. Interaction of the Bordetella pertussis filamentous hemagglutinin with heparin. FEMS Microbiol Lett. 1991 Feb;62(1):59–64. doi: 10.1111/j.1574-6968.1991.tb04417.x. [DOI] [PubMed] [Google Scholar]
- Miller J. F., Mekalanos J. J., Falkow S. Coordinate regulation and sensory transduction in the control of bacterial virulence. Science. 1989 Feb 17;243(4893):916–922. doi: 10.1126/science.2537530. [DOI] [PubMed] [Google Scholar]
- Nicosia A., Perugini M., Franzini C., Casagli M. C., Borri M. G., Antoni G., Almoni M., Neri P., Ratti G., Rappuoli R. Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4631–4635. doi: 10.1073/pnas.83.13.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappuoli R., Aricó B., Scarlato V. Thermoregulation and reversible differentiation in Bordetella: a model for pathogenic bacteria. Mol Microbiol. 1992 Aug;6(15):2209–2211. doi: 10.1111/j.1365-2958.1992.tb01395.x. [DOI] [PubMed] [Google Scholar]
- Relman D. A., Domenighini M., Tuomanen E., Rappuoli R., Falkow S. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relman D., Tuomanen E., Falkow S., Golenbock D. T., Saukkonen K., Wright S. D. Recognition of a bacterial adhesion by an integrin: macrophage CR3 (alpha M beta 2, CD11b/CD18) binds filamentous hemagglutinin of Bordetella pertussis. Cell. 1990 Jun 29;61(7):1375–1382. doi: 10.1016/0092-8674(90)90701-f. [DOI] [PubMed] [Google Scholar]
- Roberts M., Fairweather N. F., Leininger E., Pickard D., Hewlett E. L., Robinson A., Hayward C., Dougan G., Charles I. G. Construction and characterization of Bordetella pertussis mutants lacking the vir-regulated P.69 outer membrane protein. Mol Microbiol. 1991 Jun;5(6):1393–1404. doi: 10.1111/j.1365-2958.1991.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Sato Y., Cowell J. L., Sato H., Burstyn D. G., Manclark C. R. Separation and purification of the hemagglutinins from Bordetella pertussis. Infect Immun. 1983 Jul;41(1):313–320. doi: 10.1128/iai.41.1.313-320.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saukkonen K., Burnette W. N., Mar V. L., Masure H. R., Tuomanen E. I. Pertussis toxin has eukaryotic-like carbohydrate recognition domains. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):118–122. doi: 10.1073/pnas.89.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saukkonen K., Cabellos C., Burroughs M., Prasad S., Tuomanen E. Integrin-mediated localization of Bordetella pertussis within macrophages: role in pulmonary colonization. J Exp Med. 1991 May 1;173(5):1143–1149. doi: 10.1084/jem.173.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlato V., Aricò B., Prugnola A., Rappuoli R. Sequential activation and environmental regulation of virulence genes in Bordetella pertussis. EMBO J. 1991 Dec;10(12):3971–3975. doi: 10.1002/j.1460-2075.1991.tb04967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarlato V., Prugnola A., Aricó B., Rappuoli R. Positive transcriptional feedback at the bvg locus controls expression of virulence factors in Bordetella pertussis. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6753–6757. doi: 10.1073/pnas.87.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahin R. D., Brennan M. J., Li Z. M., Meade B. D., Manclark C. R. Characterization of the protective capacity and immunogenicity of the 69-kD outer membrane protein of Bordetella pertussis. J Exp Med. 1990 Jan 1;171(1):63–73. doi: 10.1084/jem.171.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainer D. W., Scholte M. J. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol. 1970 Oct;63(2):211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- Stibitz S., Weiss A. A., Falkow S. Genetic analysis of a region of the Bordetella pertussis chromosome encoding filamentous hemagglutinin and the pleiotropic regulatory locus vir. J Bacteriol. 1988 Jul;170(7):2904–2913. doi: 10.1128/jb.170.7.2904-2913.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomanen E., Towbin H., Rosenfelder G., Braun D., Larson G., Hansson G. C., Hill R. Receptor analogs and monoclonal antibodies that inhibit adherence of Bordetella pertussis to human ciliated respiratory epithelial cells. J Exp Med. 1988 Jul 1;168(1):267–277. doi: 10.1084/jem.168.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urisu A., Cowell J. L., Manclark C. R. Filamentous hemagglutinin has a major role in mediating adherence of Bordetella pertussis to human WiDr cells. Infect Immun. 1986 Jun;52(3):695–701. doi: 10.1128/iai.52.3.695-701.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J Infect Dis. 1984 Aug;150(2):219–222. doi: 10.1093/infdis/150.2.219. [DOI] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L., Myers G. A., Falkow S. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect Immun. 1983 Oct;42(1):33–41. doi: 10.1128/iai.42.1.33-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A. A., Hewlett E. L. Virulence factors of Bordetella pertussis. Annu Rev Microbiol. 1986;40:661–686. doi: 10.1146/annurev.mi.40.100186.003305. [DOI] [PubMed] [Google Scholar]
- Willems R. J., van der Heide H. G., Mooi F. R. Characterization of a Bordetella pertussis fimbrial gene cluster which is located directly downstream of the filamentous haemagglutinin gene. Mol Microbiol. 1992 Sep;6(18):2661–2671. doi: 10.1111/j.1365-2958.1992.tb01443.x. [DOI] [PubMed] [Google Scholar]
- Willems R., Paul A., van der Heide H. G., ter Avest A. R., Mooi F. R. Fimbrial phase variation in Bordetella pertussis: a novel mechanism for transcriptional regulation. EMBO J. 1990 Sep;9(9):2803–2809. doi: 10.1002/j.1460-2075.1990.tb07468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M. Adhesive recognition sequences. J Biol Chem. 1991 Jul 15;266(20):12809–12812. [PubMed] [Google Scholar]