Abstract
Protection of substantia nigra (SN) dompaminergic (DA) neurons by neurotrophic factors (NTF) is one of the promising strategies in Parkinson’s disease (PD) therapy. A major clinical challenge for NTF-based therapy is that NTFs need to be delivered into the brain via invasive means, which often shows limited delivery efficiency. The nose to brain pathway is a non-invasive brain drug delivery approach developed in recent years. Of particular interest is the finding that intranasal insulin improves cognitive functions in Alzheimer’s patients. In vitro, insulin has been shown to protect neurons against various insults. Therefore, the current study was designed to test whether intranasal insulin could afford neuroprotection in the 6-hydroxylase dopamine (6-OHDA)-based rat PD model. 6-OHDA was injected into the right side of striatum to induce a progressive DA neuronal lesion in the ipsilateral SN pars compact (SNc). Recombinant human insulin was applied intranasally to rats starting from 24 h post lesion, once per day, for 2 weeks. A battery of motor behavioral tests was conducted on day 8 and 15. The number of DA neurons in the SNc was estimated by stereological counting. Our results showed that 6-OHDA injection led to significant motor deficits and a 53% of DA neuron loss in the ipsilateral side of injection. Treatment with insulin significantly ameliorated 6-OHDA-induced motor impairments, as shown in improved locomotor activity, tapered/ledged beam walking performance, Vibrissa-elicited forelimb-placing, initial steps, as well as methamphetamine-induced rotational behavior. Consistent with behavioral improvements, insulin treatment provided a potent protection of DA neurons in the SNc against 6-OHDA neurotoxicity, as shown by a 74.8 % of increase in tyrosine hydrolase (TH) positive neurons compared to the vehicle group. Intranasal insulin treatment did not affect body weight and blood glucose levels. In conclusion, our study showed that intranasal insulin provided strong neuroprotection in the 6-OHDA rat PD model, suggesting that insulin signaling may be a novel therapeutic target in a broad neurodegenerative disorders.
Keywords: insulin, dopaminergic neurons, striatum, substantia nigra, neurotrophin, Parkinson’s Disease
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder in aged population. Despite significant progresses towards better management of symptoms, there is a lack of effective preventive and therapeutic approaches. Neurotrophic factor (NTF) therapy has shown great promise in preclinical PD models (Hegarty et al., 2014; Rodrigues et al., 2014; d’Anglemont de Tassigny et al., 2015). However, recent clinical trials showed limited efficacy (Kalia et al., 2015). One of the factors contributing to the discrepancy between animal studies and clinical trials might be related to limited bioavailability of NTFs in the substantia nigra (SN) using invasive drug delivery approaches, rather than lack of true biological effectiveness. Hence, there is a great research need to overcome current technical hurdles of the brain delivery, in order to achieve therapeutic and sustainable levels of NTFs in the SN. Meanwhile, it is also important to explore new neuroprotective reagents.
Insulin is a hormone synthesized by the beta cells of the pancreas, and its major biological action in the periphery is to regulate blood glucose levels via stimulating glucose uptake in the liver, muscle, and adipose tissue. Since the energy metabolism of the brain is independent of insulin whereas insulin receptors are expressed by neural cells, it has been proposed that insulin might play physiological roles in the CNS (Banks et al., 2012). In fact, emerging evidence suggests that insulin plays important physiological roles in the brain by modulating high brain functions including cognition and appetite. For example, it has been demonstrated that intranasal insulin could enhance cognition in healthy human volunteers (Benedict et al., 2004; Reger et al., 2008) as well as Alzheimer’s patients (Reger et al., 2008). Insulin could be detected in the cerebrospinal fluid (CSF) within minutes after nasal application, suggesting that intranasal application is a practical means to deliver insulin into the brain parenchyma. Currently, several clinical trials are being conducted for intranasal insulin therapy in AD.
Insulin is closely related to insulin-like growth factor-1 (IGF-1), which is a well-known growth and trophic factor. In the brain, IGF-1 plays an important role in neurodevelopment as well as in recovery during CNS disorders (Fernandez and Torres-Aleman, 2012). Since both insulin receptor (IR) and IGF-1 receptors (IGF-1R) are widely expressed in the brain, and their intracellular signaling pathways largely overlap, insulin could function as a neuroprotective agent once it reaches brain parenchyma at sufficient levels. Currently, direct evidence supporting this notion is limited to in vitro studies. For instance, it has been shown that insulin could protect neurons against a variety of insults, including glucose-oxygen deprivation (Sun et al., 2010), excitotoxicity (Kim and Han, 2005), and oxidative stress (Ribeiro et al., 2014). The neuroprotective action of insulin in vivo, however, remains unexplored. Therefore, the current study was aimed to test whether intranasal insulin administration affords neuroprotection in a 6-hydroxydopamine (6-OHDA)-lesioned rat PD model.
Experimental procedures
1. Animals
Male Sprague-Dawley rats (250 g) were obtained from Harlan Laboratories (Indianapolis, IN). Animals arrived in the laboratory a week before experiments. All procedures for animal care were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee at the University of Mississippi Medical Center. A total of 64 rats were used in this study.
2. Intrastriatal injection of 6-OHDA
One hour prior to surgery, rats received an intraperitoneal (i.p.) injection of desipramine HCl (25 mg/kg) to block the uptake of 6-OHDA into noradrenergic terminals (Ling et al., 2004). Rats were anesthetized with isofluorane and were placed on a stereotaxic frame with a rat adaptor. A small hole was made on the skull by a dental drill, and a 10 µl Hamilton syringe was descended into the right striatum. The coordinates used were as following: 0.7 mm anterior to the bregma, 3 mm lateral to the sagittal suture, and 5 mm ventral to the dura (Paxinos et al., 1985). A total of 20 µg of 6-OHDA (in 4 µl saline with 0.2 mg/ml of ascorbic acid) was infused into the right striatum, over a period of 4 min. The needle was kept for additional 5 min before being slowly retrieved. The wound was closed with wound clips and animals were returned to their cages. The control rats received the same amount of vehicle (saline with ascorbic acid).
3. Intranasal insulin treatment
Starting 24 h post-surgery, rats were treated with recombinant human insulin (Cell Sciences, CRI505, Canton, MA) via intranasal application, as previously described (Marks et al., 2009; Cai et al., 2011). Under lightly anesthesia with isoflurane, the animal was held in an upright position and 200 µg of insulin (in 20 µl PBS) was applied to each naris using a 10 µl fine pipette tip. Hence, each rat received a total of 400 µg insulin per day. The daily treatment continued until day 14 post-surgery. Rats in the control group received the same amount of sterile PBS under light anesthesia. Thus, there were 4 experimental groups in this study: saline (intrastriatal injection) + vehicle (intranasal), saline + insulin, 6-OHDA + vehicle, and 6-OHDA + insulin. The Methamphetamine (Meth)-induced rotation test requires that half of the animals in each treatment group to be injected with saline (used as the vehicle for Meth) for calculating the baseline rotations. Therefore, 16 animals were included in each treatment group. All these animals were used in blood glucose and body weight measurements, and all behavioral tests except rotation test. Ten animals per treatment group were used for stereological cell counting study (see below).
4. Blood glucose level and body weight measurement
Blood glucose levels were measured using a Contour Blood Glucose Monitoring System (Bayer, Germany). A small drop of blood obtained from rat tail veins was applied to a test strip and the glucose level was read out immediately. A baseline level was determined 24 h before surgery. Blood glucose was measured 30 min after insulin administration, once a day until day 15. The body weight of animals was recorded daily.
5. Behavioral tests
A battery of motor behavioral tests were conducted to assess nigrostriatal DA system integrity (Tillerson et al., 2002; Fleming et al., 2004; Schallert T., 2005; Woodlee et al., 2005). Except METH-induced rotational behavior, all other behavioral tests were conducted at 1 day before (day 0), 8 and 15 days after 6-OHDA injection.
5.1. Locomotion
Locomotor activity was measured using the ANY-maze Video Tracking System (Stoelting Co., Wood Dale, IL, USA). Rats were placed in activity chambers (42 × 25 × 40 cm3) in a quiet room with dimmed light. The total distance traveled by the testing animal was recorded during a 60-min testing period.
5.2. Exposure rearing test
The exposure rearing test was conducted by placing rats in an upright transparent Plexiglas cylinder (20 cm in diameter and 30 cm in height). The cylinder is high enough to prevent rats from reaching the top, and wide enough to allow 2 cm gap between the base of the tail and the cylinder wall when the rat is touching the wall using its forelimbs. Once inside the cylinder, rats typically rear and engage in exploratory behavior by placing their forelimbs along the cylinder wall. The rearing activities were recorded and the number of rearing events (against the wall) was counted in a 5 minutes period.
5.3. Tapered/ledged beam walking test
Sensorimotor function of hindlimbs was evaluated using a tapered/ledged beam test (Schallert T., 2005; Zhao et al., 2005). Foot-faults (slips) made with the hindlimbs can be measured as an index of hindlimb function. The tapered/ledged beam consists of the staging area (6 × 15 cm), trapezoid walking beam (6 and 1.5 cm wide and 135 cm long) and off-loading area (1.5 × 15 cm). The entire apparatus were elevated to a height of 50 cm above the floor. The beam walking apparatus consist of a tapered beam with underhanging ledges (2 cm wide, dropped 2 cm below the upper beam surface) on each side to permit foot faults without falling. Bedding from the animal’s home cage was placed at the end of beam to encourage movement. A bright light was placed above the start point to motivate the rats to traverse the beam. The rats’ performance was analyzed by calculating the slip ratio of the hindlimb (number of slips/number of total steps). The time each animal spent to traverse the beam and join their littermates was also recorded. Steps onto the ledge were scored as a full slip, while a half slip was given if the limbs touched the side of the beam. The slip ratio for each hindlimb was recorded. The mean value from three trials was used for statistical analysis.
5.4. Vibrissa-elicited forelimb-placing test
The vibrissa-elicited forelimb-placing is a useful tool to assess sensorimotor function of the forelimbs in rats. Rats use their vibrissae to gain bilateral information from the proximal environment and this information is integrated between the hemispheres. Disruption of the nigrostriatal system integrity leads to deficits in forelimb placing upon stimulation of the rat’s vibrissae (De Ryck et al., 1992; Woodlee et al., 2005). In this cross-midline test of forelimb placing, the animal was gently held by its torso, but was turned sideways so that the vibrissae were perpendicular to the surface of the table. The now downwardly oriented limb was gently restrained by the experimenter as the downwardly oriented vibrissae were brushed against a table edge once per trial for 10 trials. The percentage of trials in which the rat successfully placed its other forepaw onto the tabletop was recorded for each side. Intact animals place the forelimbs of both sides quickly onto the counter top with 100% success in this test, while damages to the nigrostriatal system lead to a decrease in the successful rate of placing response of the contralateral side. If an animal struggled during testing, the data were not included in the overall analysis. The percentage of successful placing response for each forelimb was calculated.
5.5. Movement initiation test
Movement initiation for each forelimb was assessed to test the forelimb akinesia (Tillerson et al., 2002; Fleming et al., 2004; Woodlee et al., 2005). The testing rat was held by its torso and its hindlimbs, and one forelimb was lifted above the surface of the table so that the weight of the animal’s body was supported with the other forelimb alone. The animal was allowed to initiate stepping movements in a 60 sec period for one forelimb and then the other in a balanced order. The time to initiate one step was recorded for each forelimb.
5.6. METH-induced locomotion
The test was performed on day 15 after 6-OHDA injection. Rats were first placed in the testing chambers to allow animals acclimatize their surroundings. One hour later, rats received saline or METH (2.5 mg/kg, s.c.) injection, and their activities were recorded for 90 min. Full 360° turns in the direction both ipsilateral (rotation towards the lesion) and contralateral to the lesion were counted. The results were expressed as net rotations towards the lesion side (ipsilateral turns – contralateral turns).
6. Stereological assessment of DA neurons in the SNc
After completion of the behavioral tests on day 15, rats were deeply anesthetized with isoflurane and perfused intracardially with 0.9% saline followed by 4% paraformaldehyde (PFA). Brains were post-fixed with 4% PFA for additional 24 h at 4 °C, and were immersed in sucrose solutions (sequential in 10%, 20% and 30% of sucrose solution in PBS, each for 24 h) for cytoprotection. Coronary free-floating sections at 40 µm of thickness were then prepared using a freezing slide microtome (SM2000R, Leica Biosystems, IL., USA). The number of DA neurons in the SNc was assessed using an unbiased stereological approach.
DA neurons in the SN were identified by tyrosine hydrolase (TH) immunostaining. A pilot immunostaining using a conventional protocol was performed to assess antibody penetration, which is a critical step for accurate stereological cell counting. For this purpose, image stacks with a step size of 1 µm were acquired to cover the full thickness of the section under a motorized microscope (Nikon Nie, NY., USA), and TH+ cells were examined by Stereoinvestigator software (MBF Bioscience, Williston, VT, USA). Following this protocol, it appeared that the TH antibody was only able to penetrate ∼10 µm on each side of the section, thus leaving about 10 µm in the center unstained. This problem persisted after increasing triton concentration (up to 0.8%) in the buffer and/or extending incubation time (up to 1 wk). We later overcame this technical issue by pre-treating sections with protease K. Our modified immunostaining protocol yielded excellent antibody penetration into the full thickness of the sections. Briefly, brain sections were washed in PBS and then blocked in 10% normal goat serum in PBS for 30 min. Sections were treated with protease K (EMD Millipore Billerica, MA, USA) diluted 1:50 in PBS for 1 hr at RT. Following washing in large volume of PBS, sections were incubated with anti-TH antibody (EMD Millipore) at 1:1000 dilution in PBS containing 7.5% goat serum and 0.2% triton, overnight at RT. The next day, sections were washed 3 times in PBS and then incubated with Alex fluo-488-conjugated anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA., USA) in PBS containing 7.5% goat serum. After washing, sections were mounted on slides and air dried. Fluorescence mounting medium containing DAPI was then applied on sections, and sections were covered with coverglass.
A pilot study was conducted to determine optimal parameters for stereological estimation of TH+ cells in the SNc. Various configurations in the size of systemic random grid, the counting frame, the guard zone, and the optical dissection probe depth, were tested to satisfy the following requirement: 1) 0–5 cells identified within the counting frame; 2) a minimum of 500 TH+ neurons counted in the entire SNc; and 3) the coefficient of error (CE), which is used to estimate the precision of stereological cell counting, is less than 0.1. Based on these requirements, the following parameters were found to be appropriate: systematic random grid size at 160 µm × 160 µm, a counting frame at 140 µm × 110 µm, the guard zone at 2 µm, and the probe depth at 16 µm. These parameters were used across all samples. Under SRS image acquisition workflow, the anatomical boundaries of SNc were outlined for each coronary plane under 4× objective lens. Typically 10 sections (in a series of every 6th section encompassing the entire SN region) were used for stereological cell counting. Using the pre-determined parameters as described above, image stacks across the X, Y, and Z axis were acquired for both the FITC channel (TH+ cells) and DAPI channel (nuclei). An optical fractionator probe was then employed to count TH+ neurons in the SNc. As a general rule, TH+ cells that were either inside the counting frame or touching the top and right green borders were counted, while those touching the left and bottom red borders were not counted. Upon completion of cell counting in all sections, the total number of TH+ cells in the entire SNc was calculated by the software, based on the section thickness, the serial section intervals, as well as the total cell counting. A total of 10 rats per treatment group were initially used to prepare brain sections for stereological study. However, due to technical issues, sections from one rat brain in the 6-OHDA+insulin group were found to be unusable for stereology. Therefore, nine animals were used in this group while 10 in other three treatment groups.
7. Statistics
Data were analyzed using SigmaPlot software (version 12.0), and were presented as the mean ± standard error of mean (SEM). One way analysis of variance (ANOVA) followed by post-hoc Student-Newman-Keuls test was performed to compare differences among multiple treatments for stereological cell counting and METH-induced rotational behavior data. Other behavioral data were analyzed by two-way repeated measures ANOVA followed by the Student-Newman-Keuls test. Results with a p < 0.05 were considered statistically significant.
Results
1. Intranasal insulin significantly ameliorated 6-OHDA-induced motor behavioral impairments
Using a comprehensive set of motor behavioral tests, our results showed that insulin treatment resulted in significant functional recovery in the 6-OHDA-lesioned rats.
1.1. Locomotion
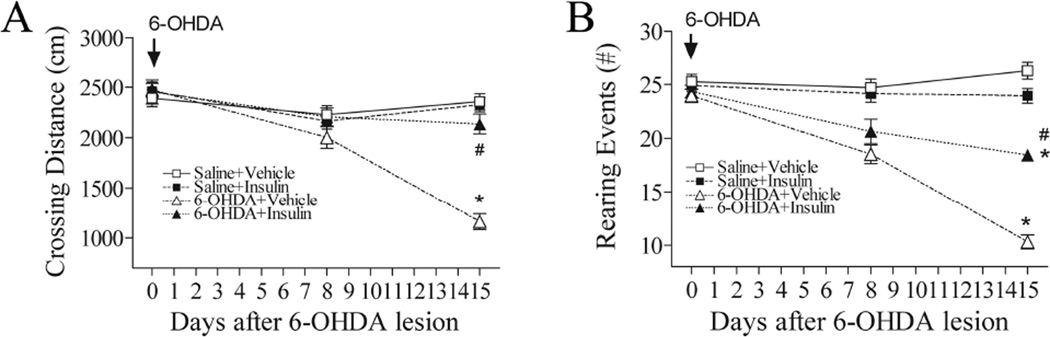
A significant decline of locomotor activity (the crossing distances in Fig. 1A) was observed in rats on day 15 post 6-OHDA lesion (p<0.05). There was no significant impairment on day 8, suggesting that locomotor deficits occurred in the second week after 6-OHDA lesion. Intranasal insulin treatment significantly attenuated 6-OHDA-induced locomotor activity deficit (p<0.05).
Fig. 1.
Intranasal insulin significantly ameliorates locomotor and exposure rearing impairment in rats subjected to unilaterally 6-OHDA lesion. 6-OHDA treatment significantly reduced both crossing distance (A: Open field test) and rearing events (B: Exposure rearing response) on day 15. Treatment with intranasal insulin significantly ameliorated deficits in both locomotion and rearing events in 6-OHDA-lesion rats. Data were analyzed by two-way repeated measures ANOVA followed by Student-Newman-Keuls test. N=16 in each group. * P<0.05 represents significant difference between the 6-OHDA+vehicle and the saline+vehicle group, or between the 6-OHDA+vehicle and the saline+insulin group, on the same post-treatment day. # P<0.05 represents significant difference between the 6-OHDA+insulin group and the 6-OHDA+vehicle group on the same post-treatment day.
1.2. Rearing events
The rearing events were significantly reduced in the 6-OHDA exposed rats on day 15 (p<0.05) (Fig. 1B). Similar to locomotion, it was not altered on day 8. Insulin treatment significantly attenuated 6-OHDA-induced exposure rearing deficit (p<0.05).
1.3. Tapered/ledged beam walking test
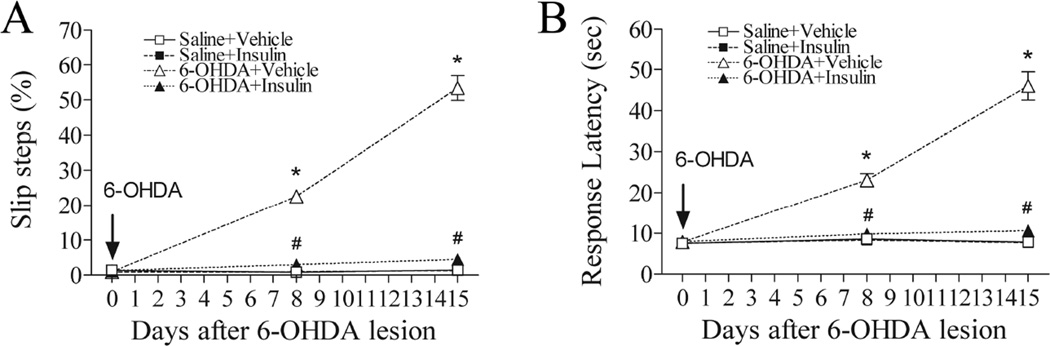
All control animals successfully performed this test without making any errors during walking. However, a significantly increased incidence of slip was observed in the 6-OHDA-exposed rats on day 8 (p<0.05), and it was further increased on day 15 (p<0.05) (Fig. 2A). In addition to increased incidence of faulty steps, the response latency was also increased significantly in the 6-OHDA-treated rats (p<0.05) (Fig. 2B). Intranasal insulin treatment led to a near complete recovery on both steps and response latency in beam walking performance test (p<0.05) (Fig. 2A&2B).
Fig. 2.
Intranasal insulin significantly improved motor performance in tapered/ledged beam walking tests in 6-OHDA lesion rats. At both day 8 and 15, 6-OHDA lesioned rats showed significantly increased incidence of slip steps and response latency as compared to the control. Treatment with intranasal insulin completely reversed 6-OHDA-induced motor impairment. Data were analyzed by two-way repeated measures ANOVA followed by Student-Newman-Keuls test. N=16 in each group. * P<0.05 represents significant difference between the 6-OHDA+vehicle and the saline+vehicle group, or between the 6-OHDA+vehicle and the saline+insulin group, on the same post-treatment day. # P<0.05 represents significant difference between the 6-OHDA+insulin group and the 6-OHDA+vehicle group on the same post-treatment day.
1.4. Vibrissae-initiated forelimb placement test
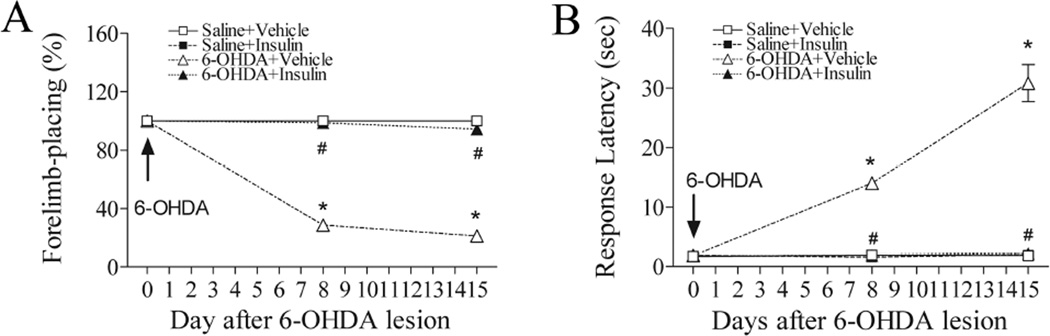
As shown in Fig. 3A, the contralateral forelimb placing was markedly impaired on both day 8 and 15 (p<0.05), in the 6-OHDA-injected rats upon stimulating the animal’s ipsilateral vibrissae. Insulin treatment abolished the 6-OHDA-induced deficits in this test (p<0.05).
Fig 3.
Intranasal insulin significantly improved forelimb sensorimotor deficits. A marked decrease in the successful rate of forelimb placing in response to vibrissa stimulation was observed in the 6-OHDA-lesioned rats on day 8 and 15 (A). A significant, progressive increase of the response latency to initiate steps was also observed in 6-OHDA-lesioned rats (B). Daily treatment with intranasal insulin significantly increased the success rate of forelimb-placing (A), and reduced the response latency to initiate steps (B). N=16 in each group. * P<0.05 represents significant difference between the 6-OHDA+vehicle and the saline+vehicle group, or between the 6-OHDA+vehicle and the saline+insulin group, on the same post-treatment day. # P<0.05 represents significant difference between the 6-OHDA+insulin group and the 6-OHDA+vehicle group on the same post-treatment day.
1.5. Movement initiation test
The movement initiation ability was significantly impaired on day 8 post 6-OHDA lesion (p<0.05), and further deteriorated on day 15 (p<0.05) (Fig. 3B). Intranasal insulin treatment completely restored the ability to initiate forelimb movement (p<0.05).
1.6. METH-induced rotational behavior
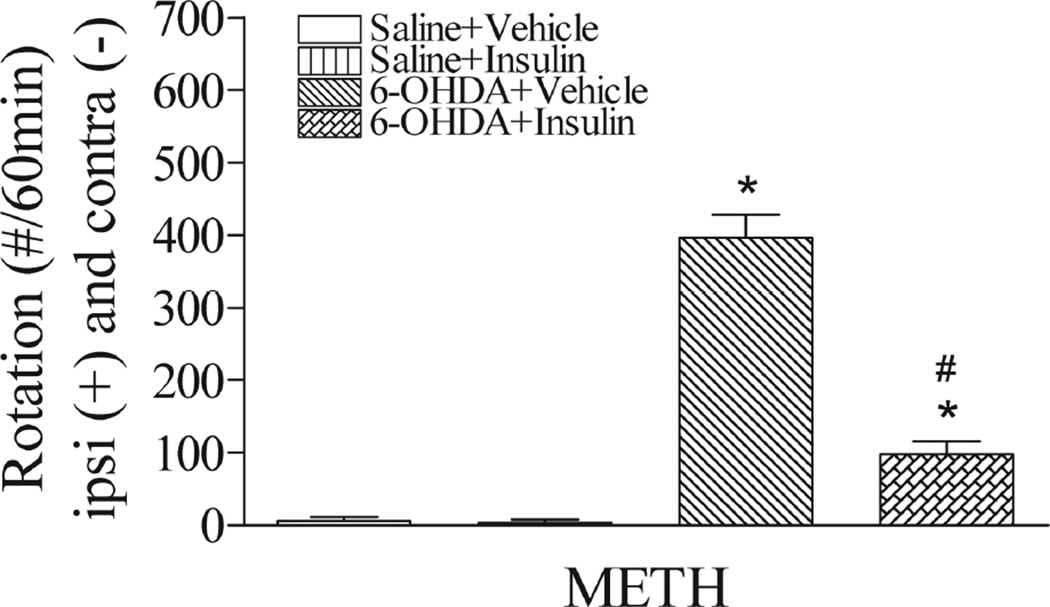
The rotation test is one of the most reliable tests used in rodent neurotoxin PD models with ipsilateral lesion (Bove et al., 2005). Upon METH challenge, the 6-OHDA-lesioned rats showed significantly increased rotations towards lesion side (p<0.05) (Fig. 4). Intranasal insulin treatment significantly reduced rotations as compared to the 6-OHDA+vehicle rats (p<0.05).
Fig. 4.
Intranasal insulin significantly reduced METH-induced rotational behaviors in 6-OHDA-lesioned rats. The METH-induced rotation was calculated as the net value by subtracting the contralateral rotation from the ipsilateral rotation assessed on day 15 post-lesion. 6-OHDA-lesioned rats showed a markedly increased rotation towards the lesion side, which was significantly reduced by treatment with intranasal insulin. N=8 in each group. * P<0.05 represents significant difference between the 6-OHDA+vehicle and the saline+vehicle group, or between the 6-OHDA+vehicle and the saline+insulin group. # P<0.05 represents significant difference between the 6-OHDA+insulin group and the 6-OHDA+vehicle group.
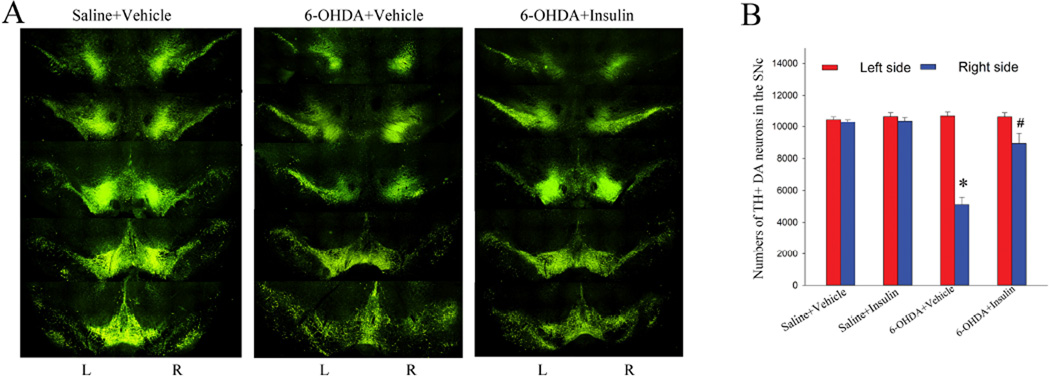
2. Intranasal insulin significantly protected DA neurons against 6-OHDA neurotoxicity in the SN
Following motor behavioral tests, we then assessed DA neuron survival in the SNc, using an unbiased stereological cell counting approach. Intrastriatal 6-OHDA injection led to a retrograde lesion to DA neurons in the SNc. In agreement with early studies suggesting a rather modest damage to the DA neurons in this model, our data showed that there was a 53% loss of DA neurons in the ipsilateral (right) SNc of 6-OHDA-injected rats (5110 ± 464 cells vs 10302 ±157 cells in the right side of control rats). We did not find DA neuron loss in the contralateral SNc (10714 ± 246 cells compared to 10473±178 cells of the left side of saline+vehicle rats). Treatment with insulin significantly ameliorated 6-OHDA-induced DA neuron loss in the ipsilateral SNc (8931±626 cells) (Fig. 5B). There was no difference between saline+vehicle and saline+insulin groups (10669±239 cells left side; 10388±217 cells right side) (P>0.05), on either side of the SNc. The CE values for all groups were less than 0.1 (range from 0.075 to 0.096).
Fig. 5.
Intranasal insulin treatment protected nigral DA neurons against 6-OHDA neurotoxicity. A: representative micrographs illustrate TH immunostaining in the SN regions. 6-OHDA lesion rats showed a marked loss of TH+ DA neurons in the ipsilateral side, primarily located in the SNc but also noticeable in the SN reticulate and VTA regions. In contrast, TH immunostaining in the contralateral side was not affected. Treatment with insulin protected TH+ neurons in the ipsilateral SNc against 6-OHDA lesion. B: Stereological cell counting to estimate numbers of DA neurons in both side of the SNc. 6-OHDA exposure led to a 53% loss of DA neurons in the ipsilateral SNc, while insulin treatment significantly increased DA neuron survival in the 6-OHDA-exposed animals (P<0.05). N=10 for the saline+vehicle, saline+insulin, and 6-OHDA+vehicle groups; n=9 for the 6-OHDA+insulin group. * P<0.05 vs the right side of the saline+vehicle or saline+insulin group; #p<0.05 vs the right side of the 6-OHDA+vehicle group.
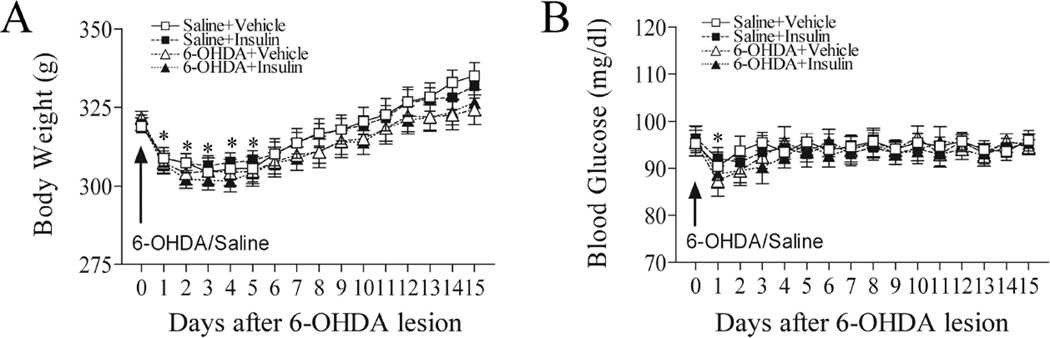
3. Intranasal insulin treatment did not alter body weight and blood glucose levels
As shown in Fig. 6A, animals in all experimental groups lost weight during the first few days after surgery. However, animals were able to regain their body weight 4 days after the surgery (p>0.05 compared to day 0). Similarly, blood glucose levels in all experimental animals, including the controls, dropped at 24 h post-surgery, but were recovered to pre-surgery levels afterwards (Fig. 6B). There were no significant differences in blood glucose levels among any experimental groups at any time-points assessed (p>0.05) (Fig. 6B).
Fig. 6.
Intranasal insulin did not affect body weight and blood glucose levels. A: The body weight of all experimental animals decreased in the first few days after the surgery; however, all animals were able to regain body weight to the pre-surgery levels afterward. There were no significant differences among groups. Similarly, blood glucose levels dropped sharply in all animals at 24 h post-surgery, but returned to pre-surgery levels afterwards. There were no significant differences of blood glucose levels among treatment groups. *P<0.05 vs before surgery. N=16 in each group.
Discussion
This is the first study to demonstrate that intranasal insulin could afford a strong neuroprotection in a preclinical PD model. The potent pro-survival effect on DA neurons suggest that intranasal insulin may be a novel therapy for not only PD, but also other neurodegenerative disorders.
The idea that insulin could act as a trophic factor is not new, because insulin shares many biological similarities with IGF-1, which is a potent and universal trophic factor for cell survival. In the CNS, IGF-1 is critically involved in both normal neurodevelopment and regeneration during brain injury (Torres-Aleman, 2010). Because insulin shares 50% homology with IGF-1, whereas IR shares 66% homology with IGF-1R (Banks et al., 2012), it is not surprising that the downstream signaling pathways of IR and IGF-1R largely overlap. The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) and the Ras-MAPK extracellular signal-regulated kinases (Erk1/2) are two major transduction systems (Fernandez and Torres-Aleman, 2012) mediating survival and growth effects by insulin/IGF-1. Early studies have demonstrated that IR and insulin receptor substrates (IRS) are widely expressed in the brain in a regional and cell type-specific manner (Duarte et al., 2012; Fernandez and Torres-Aleman, 2012). Insulin can bind to both IR and IGF-1R; however, the binding affinity for IR is approximately ∼100–1000 higher than IGF-1R. Interestingly, the human brain also expresses a hybrid receptor comprising each chain of IR and IGF-1R, this enables the hybrid receptor to bind insulin and IGF-1 with similar affinity (Fernandez and Torres-Aleman, 2012). Theoretically, once reaching brain parenchyma, insulin could potentially activate IR, IGF-1R, and the hybrid receptor, leading to cell survival and growth. Currently, the expression of IR and the hybrid receptor at cellular level is not clear, although it has been reported that neurons express higher levels of IR than glial cells (Unger et al., 1991). It is worth mentioning that an early study by Figlewicz and colleagues demonstrated a large overlap between IR and TH immunoreacitvity in the VTA and SN regions (Figlewicz et al., 2003). All these evidence suggests that intranasal insulin could be a potential therapeutic agent to treat neurodegenerative disorders. Despite a lack of in vivo evidence, a number of in vitro studies have demonstrated that insulin could protect neuronal injury against various insults (Kim and Han, 2005; Sun et al., 2010; Ribeiro et al., 2014). To the best of our knowledge, this is the first study to demonstrate that intranasal insulin not only protects DA neurons from injury but also significantly ameliorates motor behavioral deficits in an animal model of PD.
Besides its trophic effect on neurons, insulin appears to have much diverse biological actions in the brain. For example, insulin could increase the expression of brain-derived neurotrophic factor (BDNF) in the hypothalamus (Negron et al., 2015). BDNF is a potent NTFs that has been found to be neuroprotective in preclinical PD models (Hegarty et al., 2014). Insulin has also been demonstrated to regulate cytokine release from cultured astrocytes and microglia, and reduce toxicity of activated microglia towards SH-SY5Y neuronal cells (Spielman et al., 2015). Interestingly, insulin is also anti-inflammatory in both the periphery (Jeschke et al., 2004) and the brain (Adzovic et al., 2015). Thus, a marked improvement of functional outcomes in rats observed in this study might also be attributed to indirect effects, at least partially.
The blood brain barrier (BBB) is notoriously known for its impermeability to larger molecules, which creates enormous challenge for brain drug delivery. While there are some success in enhancing central bioavailability of certain small molecules by modifying their chemical properties, nearly all large molecules such as peptides and proteins failed in this attempt (Begley, 2004). Therefore, seeking alternative brain delivery approaches is urgently needed to develop novel drug-based therapies for neurodegenerative disorders. One of the strategies is to enhance drug transport crossing the BBB by modifying their chemical configuration or formulation, such as using nanoparticles or polyethylene glycol (PEG). Alternatively, it is also possible to transiently enhance BBB permeability by novel techniques such as the ultrasound (Beccaria et al., 2015). All those approaches that are aimed at enhancing brain delivery by targeting BBB, however, are currently limited in animal studies. In contrast, the nose-to-brain pathway is a novel noninvasive brain drug delivery approach which bypass the BBB, making it especially useful for delivery of larger molecules such as peptides and/or proteins (Djupesland et al., 2013; Pardeshi and Belgamwar, 2013). Importantly, this approach has been tested in numerous clinical trials. The exact pathways and mechanisms by which intranasally delivered drugs can gain access to the brain parenchyma are not fully elucidated. It is generally acknowledged that the olfactory and trigeminal pathways are two major routes (Thorne et al., 2004; Thorne et al., 2008). One of the possible transport mechanisms is that peptides diffuse into the olfactory and trigeminal nerve terminals, which are in direct contact with the nasal mucus, are transported retrogradely into their respective cell bodies and then into olfactory bulb or brainstem. Alternatively, peptides are transported along the olfactory and trigeminal nerves through extracellular channels (created by ensheathing cells), rather than a simply slow axonal transport (Lochhead and Thorne, 2012), since intranasally administrated peptides could be detected in the CSF rather rapidly (as early as 15 minutes) while it seems that simple diffusion across brain parenchyma is unlikely to reach such a rapid distribution. Some researchers also suggested additional mechanisms, such as a “perivascular pump” mechanism powered by arterial pulsation and blood pressure to drive peptides rapidly distributed across the brain parenchyma via interstitial fluid (Hadaczek et al., 2006; Liu et al., 2012).
Although the brain delivery efficiency of peptides is likely related to their molecular mass, large proteins such as NTFs have been successfully delivered into brain via intranasal route (Bender et al., 2015). The molecular weight of the current FDA approved drugs for nasal application is relatively small, between 1,000–3,400 Da (Ozsoy et al., 2009). IGF-1, which is 7.5 kDa, was detected at significant levels in the neonatal rat brain after intranasal application (Lin et al., 2009), whereas intranasal IGF-1 has been demonstrated to be neuroprotective in a number of animal models of brain disorders, including stroke (Fletcher et al., 2009), neonatal brain injury (Cai et al., 2011), and Huntington’s disease (Lopes et al., 2014). Recently, it was reported that glial cell line-derived growth factor (GDNF), which is a much large protein with a molecular weight of 15 kDa, could be detected in the rat brain after nasal application (Bender et al., 2015). The same group had previously demonstrated that intranasal GDNF provided a significant neuroprotection in the 6-OHDA rat PD model (Migliore et al., 2014). Compared to IGF-1 and GDNF, insulin is smaller with a molecular weight of 5.8 kDa. A recent study reported that recombinant human insulin could be detected in many brain regions in rats within 30 minutes following intranasal application (Kamei and Takeda-Morishita, 2015).
A major concern regarding potential side effects of intranasal insulin therapy is the risk of hypoglycemia. In the current model, we did not observed significant drop of blood glucose levels at 30 min and 24 h following intranasal insulin application, suggesting a minimal effect on systemic glucose levels. This is consistent with report from human studies (Freiherr et al., 2013).
In conclusion, the current study demonstrated that intranasal insulin provides a strong neuroprotection in the 6-OHDA rat PD model. Given that intranasal insulin therapy has been consistently demonstrated to improve cognitive decline in AD patients, whereas a number of studies showed that insulin could protect neuronal injury in vitro, intranasal insulin may hold therapeutic potential for not only AD and PD, but also other neurodegenerative disorders.
Highlights.
Intranasal insulin treatment significantly ameliorates 6-OHDA-induced sensorimotor dysfunction in rats
Intranasal insulin treatment significantly reduced methamphetamine-induced rotation in 6-OHDA-lesion rats
Intranasal insulin treatment significantly increased DA neuron survival in the SN in 6-OHDA-lesion rats
Intranasal insulin treatment did not cause hypoglycemia in rats
Acknowledgments
This work is supported by a grant from Michael J. Fox foundation (to Y.P.) and a NIH grant NINDS R01NS080844 (to LW. F.)
Abbreviations
- CNS
central nervous system
- IGF-1
insulin-like growth factor-1
- IR
insulin receptor
- NTF
neurotrophic factors
- 6-OHDA
6-hydroxylase dopamine
- PD
Parkinson’s disease
- SN
substantia nigra
- SNc
substantia nigra pars compacta
- TH
tyrosine hydrolase
- VTA
ventral tegmental area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adzovic L, Lynn AE, D’Angelo HM, Crockett AM, Kaercher RM, Royer SE, Hopp SC, Wenk GL. Insulin improves memory and reduces chronic neuroinflammation in the hippocampus of young but not aged brains. J Neuroinflammation. 2015;12:63. doi: 10.1186/s12974-015-0282-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012;136:82–93. doi: 10.1016/j.pharmthera.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beccaria K, Canney M, Goldwirt L, Fernandez C, Piquet J, Perier MC, Lafon C, Chapelon JY, Carpentier A. Ultrasound-induced opening of the blood-brain barrier to enhance temozolomide and irinotecan delivery: an experimental study in rabbits. J Neurosurg. 2015;13:1–9. doi: 10.3171/2015.4.JNS142893. [DOI] [PubMed] [Google Scholar]
- Begley DJ. Delivery of therapeutic agents to the central nervous system: the problems and the possibilities. Pharmacol Ther. 2004;104:29–45. doi: 10.1016/j.pharmthera.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Bender TS, Migliore MM, Campbell RB, John Gatley S, Waszczak BL. Intranasal administration of glial-derived neurotrophic factor (GDNF) rapidly and significantly increases whole-brain GDNF level in rats. Neuroscience. 2015;303:569–576. doi: 10.1016/j.neuroscience.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology. 2004;29:1326–1334. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Bove J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson’s disease. NeuroRx. 2005;2:484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Fan LW, Lin S, Pang Y, Rhodes PG. Intranasal administration of insulin-like growth factor-1 protects against lipopolysaccharide-induced injury in the developing rat brain. Neuroscience. 2011;194:195–207. doi: 10.1016/j.neuroscience.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Anglemont de Tassigny X, Pascual A, Lopez-Barneo J. GDNF-based therapies, GDNF-producing interneurons, and trophic support of the dopaminergic nigrostriatal pathway. Implications for Parkinson’s disease. Front Neuroanat. 2015;9:10. doi: 10.3389/fnana.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ryck M, Van Reempts J, Duytschaever H, Van Deuren B, Clincke G. Neocortical localization of tactile/proprioceptive limb placing reactions in the rat. Brain Res. 1992;573:44–60. doi: 10.1016/0006-8993(92)90112-m. [DOI] [PubMed] [Google Scholar]
- Djupesland PG, Mahmoud RA, Messina JC. Accessing the brain: the nose may know the way. J Cereb Blood Flow Metab. 2013;33:793–794. doi: 10.1038/jcbfm.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte AI, Moreira PI, Oliveira CR. Insulin in central nervous system: more than just a peripheral hormone. J Aging Res. 2012;2012:384017. doi: 10.1155/2012/384017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez AM, Torres-Aleman I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci. 2012;13:225–239. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003;964:107–115. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Zhu C, Fernagut PO, Mehta A, DiCarlo CD, Seaman RL, Chesselet MF. Behavioral and immunohistochemical effects of chronic intravenous and subcutaneous infusions of varying doses of rotenone. Exp Neurol. 2004;187:418–429. doi: 10.1016/j.expneurol.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Fletcher L, Kohli S, Sprague SM, Scranton RA, Lipton SA, Parra A, Jimenez DF, Digicaylioglu M. Intranasal delivery of erythropoietin plus insulin-like growth factor-I for acute neuroprotection in stroke. Laboratory investigation. J Neurosurg. 2009;111:164–170. doi: 10.3171/2009.2.JNS081199. [DOI] [PubMed] [Google Scholar]
- Freiherr J, Hallschmid M, Frey WH, 2nd, Brunner YF, Chapman CD, Holscher C, Craft S, De Felice FG, Benedict C. Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS Drugs. 2013;27:505–514. doi: 10.1007/s40263-013-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadaczek P, Yamashita Y, Mirek H, Tamas L, Bohn MC, Noble C, Park JW, Bankiewicz K. The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther. 2006;14:69–78. doi: 10.1016/j.ymthe.2006.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegarty SV, O’Keeffe GW, Sullivan AM. Neurotrophic factors: from neurodevelopmental regulators to novel therapies for Parkinson’s disease. Neural Regen Res. 2014;9:1708–1711. doi: 10.4103/1673-5374.143410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeschke MG, Klein D, Bolder U, Einspanier R. Insulin attenuates the systemic inflammatory response in endotoxemic rats. Endocrinology. 2004;145:4084–4093. doi: 10.1210/en.2004-0592. [DOI] [PubMed] [Google Scholar]
- Kalia LV, Kalia SK, Lang AE. Disease-modifying strategies for Parkinson’s disease. Mov Disord. 2015;30:1442–1450. doi: 10.1002/mds.26354. [DOI] [PubMed] [Google Scholar]
- Kamei N, Takeda-Morishita M. Brain delivery of insulin boosted by intranasal coadministration with cell-penetrating peptides. J Control Release. 2015;197:105–110. doi: 10.1016/j.jconrel.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Han Y. Insulin inhibits AMPA-induced neuronal damage via stimulation of protein kinase B (Akt) J Neural Transm. 2005;112:179–191. doi: 10.1007/s00702-004-0163-6. [DOI] [PubMed] [Google Scholar]
- Lin S, Fan LW, Rhodes PG, Cai Z. Intranasal administration of IGF-1 attenuates hypoxic-ischemic brain injury in neonatal rats. Exp Neurol. 2009;217:361–370. doi: 10.1016/j.expneurol.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Z, Chang QA, Tong CW, Leurgans SE, Lipton JW, Carvey PM. Rotenone potentiates dopamine neuron loss in animals exposed to lipopolysaccharide prenatally. Exp Neurol. 2004;190:373–383. doi: 10.1016/j.expneurol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Liu Q, Shen Y, Chen J, Gao X, Feng C, Wang L, Zhang Q, Jiang X. Nose-to-brain transport pathways of wheat germ agglutinin conjugated PEG-PLA nanoparticles. Pharm Res. 2012;29:546–558. doi: 10.1007/s11095-011-0641-0. [DOI] [PubMed] [Google Scholar]
- Lochhead JJ, Thorne RG. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 2012;64:614–628. doi: 10.1016/j.addr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Lopes C, Ribeiro M, Duarte AI, Humbert S, Saudou F, Pereira de Almeida L, Hayden M, Rego AC. IGF-1 intranasal administration rescues Huntington’s disease phenotypes in YAC128 mice. Mol Neurobiol. 2014;49:1126–1142. doi: 10.1007/s12035-013-8585-5. [DOI] [PubMed] [Google Scholar]
- Marks DR, Tucker K, Cavallin MA, Mast TG, Fadool DA. Awake intranasal insulin delivery modifies protein complexes and alters memory, anxiety, and olfactory behaviors. J Neurosci. 2009;29:6734–6751. doi: 10.1523/JNEUROSCI.1350-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore MM, Ortiz R, Dye S, Campbell RB, Amiji MM, Waszczak BL. Neurotrophic and neuroprotective efficacy of intranasal GDNF in a rat model of Parkinson’s disease. Neuroscience. 2014;274:11–23. doi: 10.1016/j.neuroscience.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Negron AL, Beymer M, Yu G, Warren KA, Acosta-Martinez M. Prolonged hyperglycemia & hyperinsulinemia increases BDNF mRNA expression in the posterior ventromedial hypothalamus and the dorsomedial hypothalamus of fed female rats. Neuroscience. 2015;303:422–432. doi: 10.1016/j.neuroscience.2015.07.018. [DOI] [PubMed] [Google Scholar]
- Ozsoy Y, Gungor S, Cevher E. Nasal delivery of high molecular weight drugs. Molecules. 2009;14:3754–3779. doi: 10.3390/molecules14093754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardeshi CV, Belgamwar VS. Direct nose to brain drug delivery via integrated nerve pathways bypassing the blood-brain barrier: an excellent platform for brain targeting. Expert Opin Drug Deliv. 2013;10:957–972. doi: 10.1517/17425247.2013.790887. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985;13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Green PS, Baker LD, Cholerton B, Fishel MA, Plymate SR, Cherrier MM, Schellenberg GD, Frey WH, 2nd, Craft S. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis. 2008;13:323–331. doi: 10.3233/jad-2008-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro M, Rosenstock TR, Oliveira AM, Oliveira CR, Rego AC. Insulin and IGF-1 improve mitochondrial function in a PI-3K/Akt-dependent manner and reduce mitochondrial generation of reactive oxygen species in Huntington’s disease knock-in striatal cells. Free Radic Biol Med. 2014;74:129–144. doi: 10.1016/j.freeradbiomed.2014.06.023. [DOI] [PubMed] [Google Scholar]
- Rodrigues TM, Jeronimo-Santos A, Outeiro TF, Sebastiao AM, Diogenes MJ. Challenges and promises in the development of neurotrophic factor-based therapies for Parkinson’s disease. Drugs Aging. 2014;31:239–261. doi: 10.1007/s40266-014-0160-x. [DOI] [PubMed] [Google Scholar]
- Schallert TWMT. Motor systems: orienting and placing. In: Whishaw IQKB, editor. The Behaviour of the Laboratory Rat: A Handbook with Tests. New York: Oxford University Press; 2005. pp. 129–140. [Google Scholar]
- Spielman LJ, Bahniwal M, Little JP, Walker DG, Klegeris A. Insulin Modulates In Vitro Secretion of Cytokines and Cytotoxins by Human Glial Cells. Curr Alzheimer Res. 2015;12:684–693. doi: 10.2174/1567205012666150710104428. [DOI] [PubMed] [Google Scholar]
- Sun X, Yao H, Douglas RM, Gu XQ, Wang J, Haddad GG. Insulin/PI3K signaling protects dentate neurons from oxygen-glucose deprivation in organotypic slice cultures. J Neurochem. 2010;112:377–388. doi: 10.1111/j.1471-4159.2009.06450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne RG, Hanson LR, Ross TM, Tung D, Frey WH., 2nd Delivery of interferon-beta to the monkey nervous system following intranasal administration. Neuroscience. 2008;152:785–797. doi: 10.1016/j.neuroscience.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Pronk GJ, Padmanabhan V, Frey WH., 2nd Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–496. doi: 10.1016/j.neuroscience.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Tillerson JL, Cohen AD, Caudle WM, Zigmond MJ, Schallert T, Miller GW. Forced nonuse in unilateral parkinsonian rats exacerbates injury. J Neurosci. 2002;22:6790–6799. doi: 10.1523/JNEUROSCI.22-15-06790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Aleman I. Toward a comprehensive neurobiology of IGF-I. Dev Neurobiol. 2010;70:384–396. doi: 10.1002/dneu.20778. [DOI] [PubMed] [Google Scholar]
- Unger JW, Moss AM, Livingston JN. Immunohistochemical localization of insulin receptors and phosphotyrosine in the brainstem of the adult rat. Neuroscience. 1991;42:853–861. doi: 10.1016/0306-4522(91)90049-t. [DOI] [PubMed] [Google Scholar]
- Woodlee MT, Asseo-Garcia AM, Zhao X, Liu SJ, Jones TA, Schallert T. Testing forelimb placing “across the midline” reveals distinct, lesion-dependent patterns of recovery in rats. Exp Neurol. 2005;191:310–317. doi: 10.1016/j.expneurol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- Zhao CS, Puurunen K, Schallert T, Sivenius J, Jolkkonen J. Effect of cholinergic medication, before and after focal photothrombotic ischemic cortical injury, on histological and functional outcome in aged and young adult rats. Behav Brain Res. 2005;156:85–94. doi: 10.1016/j.bbr.2004.05.011. [DOI] [PubMed] [Google Scholar]