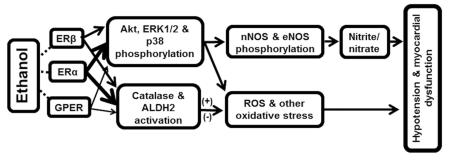
Abstract
Our previous studies showed that ethanol elicited estrogen (E2)-dependent myocardial oxidative stress and dysfunction. In the present study we tested the hypothesis that E2 signaling via the estrogen receptor (ER), ERα, mediates this myocardial detrimental effect of alcohol. To achieve this goal, conscious female rats in proestrus phase (highest endogenous E2 level) received a selective ER antagonist (200 μg/kg; i.v) for ERα (MPP), ERβ (PHTPP) or GPER (G15) or saline 30 min before ethanol (1g/kg; i.v) or saline infusion. ERα blockade virtually abrogated ethanol-evoked myocardial dysfunction and hypotension, while ERβ blockade had little effect on the hypotensive response, but caused delayed attenuation of the ethanol-evoked reductions in left ventricular developed pressure and the rate of left ventricle pressure rise. GPER blockade caused delayed attenuation of all cardiovascular effects of ethanol. All three antagonists attenuated the ethanol-evoked increases in myocardial catalase and ALDH2 activities, Akt, ERK1/2, p38, eNOS and nNOS phosphorylation, except for a lack of effect of PHTPP on p38. Finally, all three ER antagonists attenuated ethanol-evoked elevation in myocardial ROS, but this effect was most notable with ERα blockade. In conclusion, ERα plays a greater role in, and might serve as a molecular target for ameliorating, the E2 dependent myocardial oxidative stress and dysfunction caused by ethanol.
Keywords: Estrogen receptor subtypes, ethanol, blood pressure, myocardial function, oxidative stress
Graphical abstract
Introduction
Acutely administered ethanol lowers blood pressure and depresses myocardial function in proestrus rats (highest endogenous estrogen, E2, level) (Ibrahim et al., 2014). These responses are virtually absent in ovariectomized (OVX) rats, and are fully restored within minutes after acute E2 administration (El-Mas and Abdel-Rahman, 2014). The E2-dependence of the ethanol-evoked hypotension is clinically relevant because moderate ethanol consumption lowers blood pressure in young, but not in older women (Klatsky et al., 1990). However, the mechanisms of E2 mediation of the myocardial dysfunction and hypotensive effects of ethanol, and in particular the role of each the E2 receptor (ER) subtypes, remain unknown. The three ER subtypes, ERα, ERβ and the G protein-coupled estrogen receptor 1 (GPER) are distributed throughout the cardiovascular system, and act as important regulators of myocardial function by genomic and rapid non-genomic signaling mechanisms (Meyer et al., 2006, Meyer et al., 2011). Importantly, our recent findings that estrogen non-genomic effects are involved in ethanol-evoked myocardial depression and hypotension in female (El-Mas and Abdel-Rahman, 2014) and male (El-Mas and Abdel-Rahman, 2015) rats implicate one or more of the ER subtypes in the acute E2-dependent myocardial depressant effect of ethanol.
It is also important to identify the ER subtype implicated in E2 enhancement of the activity of two myocardial enzymes, catalase and aldehyde dehydrogenase 2 (ALDH2), which confer cardio-protection (Turdi et al., 2007, Kandadi et al., 2012), but also catalyze ethanol oxidation to acetaldehyde and acetate, respectively (Soffia and Penna, 1987, Kinoshita et al., 2001). Therefore, these 2 myocardial enzymes seem to play important roles in the E2-dependent molecular events that ultimately lead to ethanol-evoked myocardial oxidative stress and dysfunction. Despite this knowledge, there are no studies on the ER subtype(s) implicated in E2 exacerbation of ethanol deleterious effects on myocardial redox state and function.
It is likely that ERα plays a greater role in the E2-dependent cardiovascular effects of ethanol for two reasons. First, ERα is mostly recognized as the ER that mediates the rapid ER signaling following translocation to the cell membrane (Kiss et al., 2005, Mendez-Bolaina et al., 2007, Pedram et al., 2007), although ERβ has also been implicated (Chambliss et al., 2002, Jazbutyte et al., 2008). Second, ERα mediates E2 enhancement of PI3K-Akt-NOS signaling (Cheskis et al., 2008, Liu et al., 2009), and the latter is implicated in the E2-dependent acute hypotensive effect of ethanol (El-Mas et al., 2009). Nonetheless, given the crosstalk between ERα, ERβ and GPER (Filice et al., 2009, Haas et al., 2009) it will be important to elucidate the role of the 3 of ERs subtypes in E2-dependent myocardial dysfunction caused by ethanol.
The present study tested the hypothesis that ERα plays a pivotal role in the E2-dependent molecular events that mediate ethanol-evoked myocardial oxidative stress and dysfunction in female rats. To achieve this goal, we conducted hemodynamic studies in conscious proestrus rats that received ethanol in the absence or presence of selective ERα, ERβ or GPER blockade. These integrative studies were complemented with ex vivo molecular studies. Investigating the hemodynamic and biochemical effects of the selective pharmacological antagonists in the presence of the highest E2 level (proestrus rats) provided new information on the roles of the three ER subtypes in regulating the catalytic activity of myocardial catalase and ALDH2. Our hemodynamic and biochemical data support our hypothesis, and identify ERα blockade as a potential protective modality against the E2-dependent myocardial dysfunction caused by ethanol.
Materials and methods
Female Sprague-Dawley rats (200-250 g, Charles River, Raleigh, NC) were housed at a constant temperature of 23 ± 1°C, humidity of 50±10 %, and a 12-h light/dark cycle. Animals were allowed free access to food (Prolab Rodent Chow, Granville Milling, Creedmoor, NC) and water provided ad libitum. All the surgical procedure, experimental, and post-operative care procedures were performed in accordance with, and approved by the East Carolina University Institutional Animal Care and Use Committee and in accordance with the Guide for the Care and Use of Laboratory Animals (2011).
Surgery
The surgery was conducted under anesthesia induced by ketamine (90 mg/kg) and xylazine (10 mg/kg i.p.), and sterile conditions. Buprenorphine (0.03 mg/kg; s.c) was administered 30 min before surgery. Postoperative care included another dose of buprenorphine and durapen (10,000 unites/kg). Each rat was housed in a separate cage after surgery and was allowed at least one day for recovery before conducting the experiment.
The method of arterial and left ventricular cannulation was detailed in our recent study (Ibrahim et al., 2014). Briefly, gas sterilized catheters, consisting of 5 cm PE10 tubing bonded to 15 cm PE50 tubing, filled with saline, were placed into the abdominal aorta/vena cava via the femoral artery/vein for blood pressure measurement and i.v. administration of pharmacological intervention (ER selective antagonist, ethanol infusion or vehicle). A 10 cm PE50 tubing was placed into left ventricle via the right carotid artery for the measurement of left ventricular function. All the catheters were tunneled subcutaneously and exited at the back of the neck between scapulars, and fixed with 3M VetbondTM tissue adhesive. Catheters were plugged with sterile stainless steel pins. Incisions were closed by surgical clips and swabbed with povidone-iodine solution.
Hemodynamic recording
Measurements of left ventricular function and blood pressure were conducted in conscious freely moving rats as in our previous study (Ibrahim et al., 2014). Briefly, on the day of experiment, the animal was allowed to adapt to the environment for at least 1 hour. The arterial and left ventricular catheters were flushed with heparinized saline (100 IU/ml) and connected to Gould-Statham pressure transducers (Oxnard-CA). Blood pressure and left ventricular indices were simultaneously recorded by ML870 (PowerLab 8/30), and analyzed using LabChart (v.7) pro software (AD Instruments, Colorado Spring, CO). The baseline values were obtained at least 30 min after stabilization period following catheter connection to the transducers.
Determination of plasma ethanol concentration
Heparinized blood was centrifuged at 5000 rpm for 10 minutes and ethanol content was determined by the enzymatic method (Kristoffersen et al., 2005, El-Mas et al., 2008) with modification. Briefly, 150 μl of buffer (sodium pyrophosphate 74.5mM, semicarbazide hydrochloride 74 mM glycine 22.4 mM), 25 μl of β-Nicotinamide adenine dinucleotide (25.1mM), 25 μl of perchloric acid (2%) and 5 μl of sample or ethanol standard, was added in 96-well plate, respectively. The reaction was started by adding 10 μl of alcohol dehydrogenase (0.34M). Absorbance was detected by spectrophotometer at 340 nm after 70 min reaction at room temperature with shaking. The concentration of ethanol was determined from a calibration curve.
Measurements of myocardial catalase and ALDH2 activity
Myocardial tissue was homogenized in phosphate-buffered saline (PBS) on ice and centrifuged at 4°C , at 10,000 rpm for 10 min. A 5μl of sample protein was used to evaluate catalytic activity by Colorimetric catalase assay kit (sigma), and 500μg sample protein for evaluation of ALDH2 activity by Mitochondrial ALDH2 activity assay kit (Abcam) according to the manufacturer’s protocol and our studies (El-Mas et al., 2012, Ibrahim et al., 2014).
Measurement of myocardial nitrite/nitrate (NOx) level
Myocardial tissue was homogenized in PBS on ice and centrifuged at 5000 rpm for 10 min. The upper supernatant was collected for fluorometric measurement of NOx (Cayman Chemical Company, Ann Arbor, MI) as described in our previous studies (El-Mas et al., 2012).
Measurement of myocardial ROS level
Myocardial ROS levels were measured by a fluorometric assay using 2′,7′-dichlorofluorescin diacetate (DCFH-DA, Molecular Probes). Briefly, myocardial tissue was homogenized in PBS on ice and centrifuged at 5000 rpm for 10 min. DCFH-DA (25 μM) was added to 250 μg of sample protein supernatant. The fluorescence intensities were measured in a 96-well plate every 5 min for 30 min with Fluoromax-3 spectrofluorometer set at 485 nm and 530 nm for excitation and emission wavelengths, respectively, at 37°C as repo rted (McGee and Abdel-Rahman, 2012).
Western blot
The protocols from our recent study (Ibrahim et al., 2014) were followed for measurements of Akt, ERK1/2, p38, nNOS and eNOS phosphorylation. Left ventricular tissue was homogenized on ice in a lysis buffer with protease inhibitor cocktail (Roche, IN), centrifuged (12,000 g for 15 min). Protein (80 μg) was separated by 4-12% gel electrophoresis (Novex Tis-Glycine gel, Life Technologies, CA) and semi dry transferred to nitrocellulose membranes, which were then blocked in Odyssey blocking buffer (LI-COR Biosciences, Lincoln, NE) for 2 hours. The membranes were incubated overnight at 4°C with a mixture of mouse anti-p-Akt (1:500) and rabbit anti-Akt (1:500, Cell Signaling, Danvers, MA), mouse anti-p-ERK1/2 (1:500) and ERK1/2 antibodies (1:500, Cell Signaling, Danvers, MA), mouse anti-p-p38 (1:500) and rabbit anti-p38 (1:500, Cell Signaling, Danvers, MA), rabbit anti-p-nNOS (Ser1417) (1:200) and mouse polyclonal anti-nNOS antibody (1:200, BD Biosciences, San Jose, CA), rabbit anti-p-eNOS (1:200, Thermo Fisher Scientific, Waltham, MA) and mouse polyclonal anti-eNOS antibody (1:200). Then the membranes were incubated for 60 min with mixture containing IRDye680-conjugated goat anti-mouse and IRDye800-conjugated goat anti-rabbit (1:15000, LICOR Biosciences). Bands were detected by Odyssey Infrared Imager and quantified by integrated intensities with Odyssey application software version 3 (LI-COR Biosciences).
Experimental groups and protocols
Experiment 1. Effect of selective ERα, ERβ or GPER blockade on ethanol-evoked myocardial depression and hypotension in proestrus female rats
The objective of this experiment was to elucidate the role of each ER subtype in the E2-dependent myocardial depressant effect of ethanol (1g/kg, i.v. infusion for 30 min). Based on our recent findings that implicated rapid ER signaling in ethanol evoked myocardial depression (El-Mas and Abdel-Rahman, 2014), we investigated the impact of acute blockade of ERα, ERβ or GPER on ethanol-evoked myocardial dysfunction and hypotension in 56 proestrus female rats, divided into 8 groups (n=6-8 rats each). The proestrus phase was determined by vaginal swab microscopy (Weihe, 1987). All rats were used in the conscious state 1-2 days after left ventricular and vascular catheterization as described under methods. At least 30 min was allowed for blood pressure and cardiac indices to stabilize at baseline following catheters connection to transducers, and before drug or vehicle administration. Six groups were divided in 3 pairs with each pair receiving a dose (200 μg/kg; i.v) of the ERα (MPP), ERβ (PHTPP) or GPER (G15) antagonist 30 min before ethanol (antagonist + ethanol) or an equal volume of saline (antagonist + saline). The remaining 2 groups received saline, vehicle for ER antagonists, followed by ethanol (saline + ethanol) or its vehicle, saline (control, saline + saline). The doses of the ER antagonists were based on reported studies (Eriksson et al., 1996, Santollo and Eckel, 2009, Weil et al., 2010, Lahm et al., 2012) and the dose of ethanol and route of administration were based on our recent study in the same model system (El-Mas and Abdel-Rahman, 2014). Mean arterial pressure (MAP), heart rate (HR), left ventricular developed pressure (LVDP) and the maximum rate of left ventricular pressure rise (dP/dtmax) were recorded during the ER antagonist pretreatment period, the 30 min of ethanol or saline infusion, and for 60 min thereafter. Data were analyzed by Lab chart. At the conclusion of the hemodynamic measurements, the rats were euthanized, tissue were collected, flash-frozen, and stored at −80°C for conducting the biochemical/molecular studies as described under methods. Notably, the adopted experimental approach also permitted investigation of the impact of the selective blockade of each ER subtype on hemodynamic function and on the myocardial enzymes that regulate the redox status.
Experiment 2. Effects of selective ER subtype blockade on modulators of myocardial redox status and ethanol metabolizing enzymes
These ex vivo studies were conducted to elucidate, for the first time, the role of each one of the 3 ER subtype in modulating myocardial catalase and ALDH2 activities and mediators of oxidative stress (mitogen-activated protein kinases, MAPKs) in the absence and presence of ethanol. Blood and myocardial tissues were obtained from all rats employed in experiment 1 for conducting these biochemical studies as well as blood ethanol concentration as detailed under methods and in our recent studies (Ibrahim et al., 2014).
Drugs
Ethanol (Midwest Grain Products Co., Weston, MO) was diluted in saline. 1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride (MPP dihydrochloride) and 4-[2-Phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (PHTPP) were purchased from Tocris Bioscience (Bristol, BS11 0QL, UK). (3aS,4R,9bR)-4-(6-bromo-1,3-benzodioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolone (G15) were purchased from Cayman Chemical (Ann Arbor, MI, USA). The ER antagonist was firstly dissolved in DMSO for stock solution. The stock solution was diluted (1:50) in saline for working solution.
Data analysis and statistics
Values are presented as mean ± SEM. Data were analyzed by one-way ANOVA or Student’s unpaired t test using Prism version 5 (GraphPad Software, Inc. La Jolla, CA). P < 0.05 was considered significant.
Results
Selective ERα blockade fully, while ERβ or GPER blockade partially, attenuated ethanol-evoked myocardial depression and hypotension
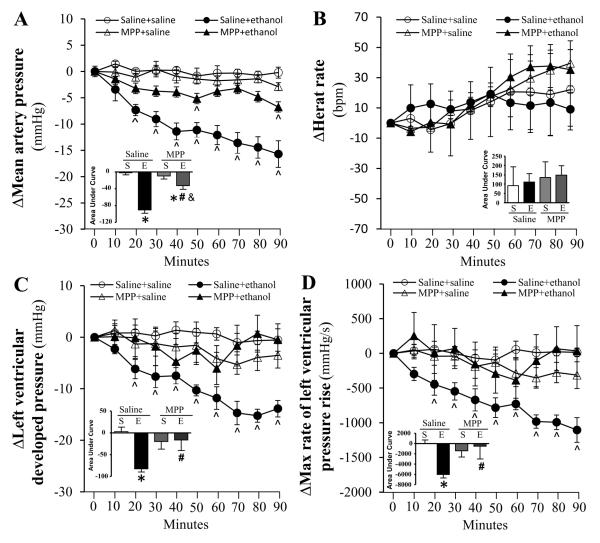
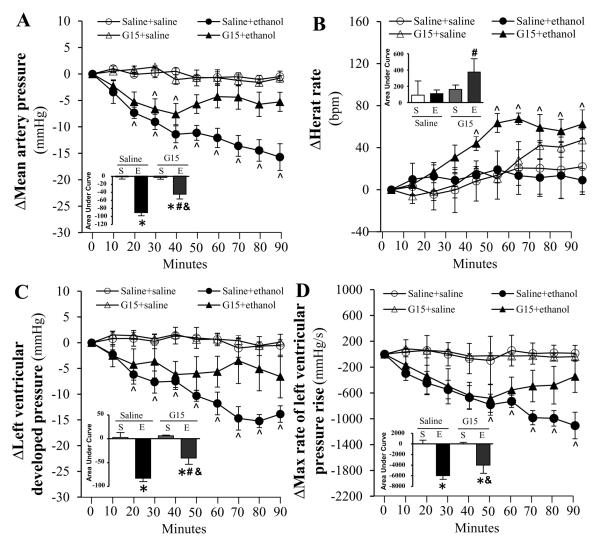
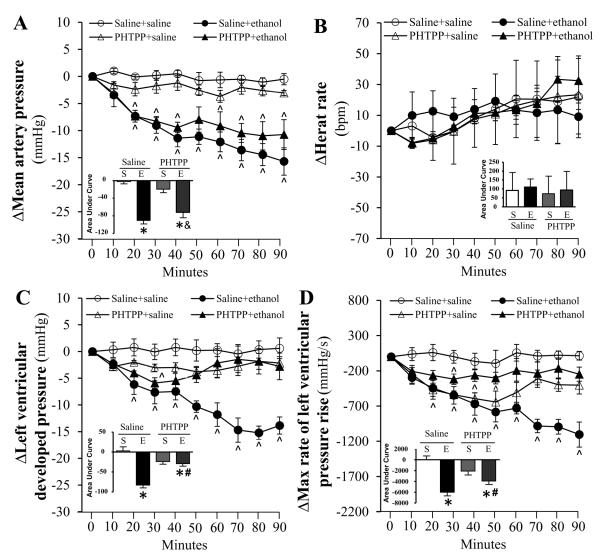
Compared to saline, none of the selective ER antagonists (200 μg/kg, each) influenced the measured hemodynamic variables (MAP, HR, LVDP and dP/dtmax) throughout the course of the study (Figs. 1-3). Pretreatment with the selective ERα antagonist (MPP) abrogated (p < 0.05) the reductions in blood pressure (Fig. 1A), LVDP (Fig. 1C) and dP/dtmax (Fig. 1D) caused by ethanol (1 g/kg). ERβ (PHTPP) or GPER (G15) blockade caused less evident and delayed attenuation of ethanol-evoked reductions in blood pressure (Figs. 2A, 3A), LVDP (Figs. 2C, 3C) and dP/dtmax (Figs. 2D, 3D). Only G15 (Fig. 3B), but not MPP (Fig. 1B) or PHTPP (Fig. 2B), significantly (p < 0.05) elevated the HR in the presence of ethanol.
Figure 1.
Time course of the effects of ERα blockade, by MPP, on ethanol (1g/kg; i.v)-evoked changes in mean arterial pressure (A), heart rate (B), left ventricular developed pressure (C), and the maximum rate of left ventricular pressure rise (D) in conscious female proestrus rats. Bar graphs represent the area under curve for saline (S) and ethanol (E) groups in the presence of the ER antagonist or its vehicle (saline). Values are mean ± SEM. * p < 0.05, versus saline + saline; # p < 0.05, versus saline + ethanol; & p < 0.05, versus antagonist + saline.
Figure 3.
Time course of the effect of GPER blockade, by G15, on ethanol (1g/kg; i.v)-evoked changes in mean arterial pressure (A), heart rate (B), left ventricular developed pressure (C), and the maximum rate of left ventricular pressure rise (D) in conscious female proestrus rats. Bar graphs represent the area under curve for saline (S) and ethanol (E) groups in the presence of the ER antagonist or its vehicle (saline). Values are mean ± SEM. * p < 0.05, versus saline + saline; # p < 0.05, versus saline + ethanol. & p < 0.05, versus antagonist + saline.
Figure 2.
Time course of the effects of ERβ blockade, by PHTPP, on ethanol (1g/kg; i.v)-evoked changes in mean arterial pressure (A), heart rate (B), left ventricular developed pressure (C), and the maximum rate of left ventricular pressure rise (D) in conscious female proestrus rats. Bar graphs represent the area under curve for saline (S) and ethanol (E) groups in the presence of the ER antagonist or its vehicle (saline). Values are mean ± SEM. * p < 0.05, versus saline + saline; # p < 0.05, versus saline + ethanol; & p < 0.05, versus antagonist + saline.
ERα, ERβ or GPER selective blockade abrogated ethanol enhancement of myocardial catalase and ALDH2 activity
Ethanol significantly (p < 0.05) enhanced myocardial catalase (Fig. 4A) and ALDH2 (Fig. 4B) in proestrus rats. Pretreatment with any of the 3 selective ER blockers attenuated (p < 0.05) the ethanol enhancement of the catalytic activity of both enzymes (Figs. 4A, B). When administered alone, each selective ER blocker significantly (p < 0.05) and similarly enhanced myocardial ALDH2 activity (Fig. 4B), while myocardial catalase activity was significantly (p < 0.05) reduced only by MPP (Fig. 4A). Blood alcohol concentration (81.3 ± 0.74, mg/dL), measured at the time of tissue collection, was significantly (p < 0.05) lower following PHTPP (55.3 ± 3.1 mg/dL), but was not influenced by G15 (92.5 ± 6.3 mg/dL) or MPP (85.8 ± 5.8 mg/dL) pretreatment.
Figure 4.
Effect of prior ERα (MPP) ERβ (PHTPP) or GPER (G15) selective blockade on catalase (A) and aldehyde dehydrogenase 2 (B) catalytic activity in myocardial tissues collected from ethanol or saline treated proestrus rats. Values are mean ± SEM. * p < 0.05, versus saline + saline; # p < 0.05, versus saline + ethanol; & p < 0.05, versus antagonist + saline.
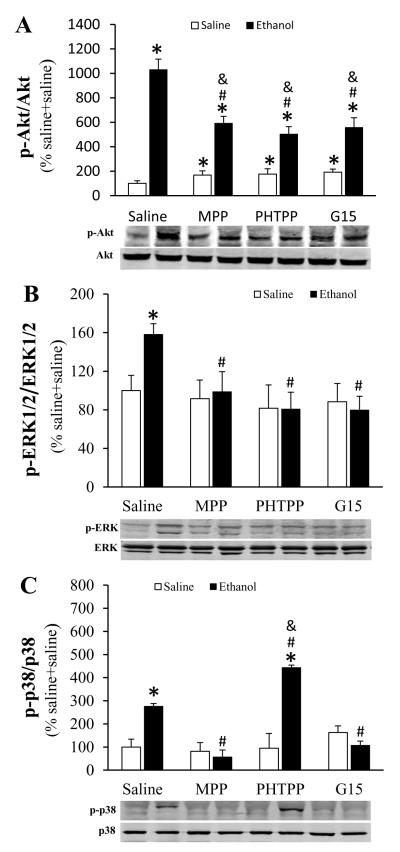
ERα, ERβ or GPER selective blockade differentially influenced ethanol-evoked MAPK phosphorylation in the myocardium
Ethanol significantly (p < 0.05) enhanced the phosphorylation of myocardial Akt, ERK1/2 and p38 (Fig. 5). Pretreatment with MPP, PHTPP or G15 significantly (p < 0.05) attenuated ethanol-evoked Akt (Fig. 5A) and ERK1/2 (Fig. 5B) phosphorylation. Further, MPP and G15 abrogated, while PHTPP significantly (p < 0.05) enhanced, ethanol-induced p38 phosphorylation (Fig. 5C). Compared with saline, MPP, PHTPP or G15 significantly (p < 0.05) enhanced basal Akt phosphorylation (Fig. 5A), but had no effect on basal ERK1/2 (Fig. 5B) or p38 (Fig. 5C) phosphorylation.
Figure 5.
Effect of prior ERα (MPP) ERβ (PHTPP) or GPER (G15) selective blockade on protein kinase B (Akt, A), extracellular signal-regulated kinase 1 and 2 (ERK1/2, B) and p38 mitogen-activated protein kinases (p38, C) phosphorylation in myocardial tissues collected from ethanol or saline treated proestrus rats. Representative bands are shown for phosphorylated Akt (p-Akt), p-ERK1/2 and p-p38 and the corresponding total protein measured by western blot. Values are mean ± SEM. * p < 0.05, versus saline + saline; # p < 0.05, versus saline + ethanol; & p < 0.05, versus antagonist + saline.
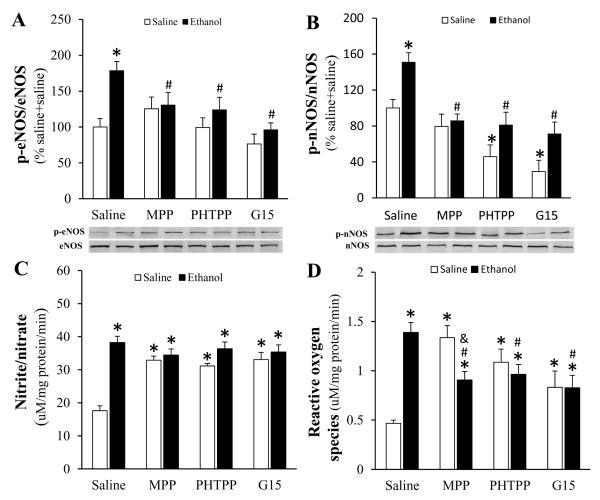
ERs blockade abrogated ethanol-evoked enhancement of eNOS and nNOS phosphorylation and ROS generation
Ethanol significantly (p < 0.05) increased eNOS (Fig. 6A) and nNOS (Fig. 6B) phosphorylation as well as NOx (Fig. 6C) and ROS (Fig. 6D) levels in the myocardium. All 3 ER subtype antagonists abolished (p < 0.05) ethanol-evoked eNOS (Fig. 6A) and nNOS (Fig. 6B) phosphorylation. A significant (p < 0.05) increase in basal NOx level, caused by each of the ER subtype antagonists, masked ethanol evoked increases in NOx (Fig. 6C). While all ER subtype antagonists attenuated (p < 0.05) ethanol-evoked increase in myocardial ROS, this attenuation was most evident with ERα blockade. The latter also produced the greatest increase in myocardial ROS caused by the selective ER antagonists in the absence of ethanol (Fig. 6D).
Figure 6.
Effect of prior ERα (MPP) ERβ (PHTPP) or GPER (G15) on endothelial nitric oxide synthase (eNOS, A) and neuronal NOS (nNOS, B) phosphorylation and on nitrite/nitrate (C) and reactive oxygen species (ROS, D) levels in myocardial tissues collected from ethanol or saline treated proestrus rats. Representative bands are shown for phosphorylated eNOS (p-eNOS) and p-nNOS measured by western blot. Values are mean ± SEM. * p < 0.05, versus saline + saline; # p < 0.05, versus saline + ethanol; & p < 0.05, versus antagonist + saline.
Discussion
In the present study we elucidated the roles of ERα, ERβ and GPER in the E2-dependent myocardial oxidative stress/dysfunction caused by ethanol in proestrus rats. The most important findings of this study are: (1) ERα blockade (MPP) virtually abolished ethanol-evoked myocardial dysfunction and hypotension, (2) ERβ (PHTPP) or GPER (G15) blockade had little effect on the hypotensive response and produced delayed attenuation of ethanol-evoked reductions in LVDP and dP/dtmax, (3) All ER subtype antagonists abrogated ethanol-evoked activation of myocardial ALDH2 and catalase, (4) ER subtype antagonists varied in their ability to attenuate ethanol-evoked phosphorylation of myocardial MAPKs, NOx and ROS generation. While ERα is the major mediator of myocardial oxidative stress/dysfunction and hypotension, ERβ and GPER also contribute to the maintenance of these E2 dependent deleterious effects of ethanol in proestrus rats.
Endogenous E2 directly modulates HR and cardiac contractility (Li et al., 2000, Mercuro et al., 2000), and the 3 ER subtypes are expressed in the myocardium (Meyer et al., 2006, Meyer et al., 2011). To our knowledge, this is the first elucidation of the role of ERα, ERβ and GPER in E2 dependent cardiovascular and biochemical effects of ethanol in proestrus rats. We adopted a pharmacological approach that permitted selective individual blockade of each of the 3 ER subtypes (Eriksson et al., 1996, Santollo and Eckel, 2009, Weil et al., 2010, Lahm et al., 2012) in the presence of the highest endogenous E2 levels (proestrus phase). We show that ERα blockade (MPP) produced the most prominent attenuation of the ethanol-evoked reductions in myocardial function and blood pressure (Fig.1), which suggests a pivotal role for ERα in these deleterious effects. This notion is further supported by the full expression of ethanol-evoked reduction in myocardial contractility index (dP/dtmax) during the first 40-50 min in the presence of ERβ or GPER blockade (Figs. 2 and 3). However, these findings might also implicate ERβ and GPER in the delayed myocardial depressant effect of ethanol and raise the interesting possibility of a crosstalk between the 3 ER subtypes in this phenomenon. Notably, crosstalk and overlapping functions exist between the ER subtypes with regards to physiological modulation of cardiac function (Matthews and Gustafsson, 2003, Filice et al., 2009, Meyer et al., 2009). These findings are exciting and could be clinically relevant given the proposed use of MPP as ovarian cancer therapeutic (Chan et al., 2014).
Results of the present study showed that the reductions in myocardial contractility indices (dP/dtmax and LVDP) and blood pressure were tightly related (Fig.1), which agrees with our reported findings (El-Mas and Abdel-Rahman, 2014, Ibrahim et al., 2014), and supports a major role for myocardial depression in ethanol-evoked hypotension. However, the preserved ethanol-evoked hypotension despite the late recovery of cardiac indices in ERβ-blocked rats might be explained, at least partly, by ERα or GPER mediated vasodilation. This possibility is supported by the greater involvement of vascular ERα and GPER, than ERβ, in the NO-dependent vasodilation (Reslan et al., 2013, Kim et al., 2014). Notably, while eNOS and nNOS are expressed in the myocardium and vasculature (Fukumura et al., 2001, Martin et al., 2006, Lekontseva et al., 2011), their roles in cell signaling and function vary in different tissues. Therefore, it is possible that the differences in ER subtype modulation of eNOS and nNOS in the myocardium and vasculature account for the preservation of ethanol-evoked hypotension in ERβ-blocked rats in the present study.
Ethanol enhancement of myocardial MAPKs underlies the E2-dependent myocardial dysfunction as a result of increases in NOS phosphorylation (NOx level) and ROS generation (El-Mas et al., 2009, El-Mas and Abdel-Rahman, 2014, Ibrahim et al., 2014). In addition to confirming these findings (Figs. 5, 6), the current study presents important data on the roles of the ER subtypes in these molecular events in the absence and presence of ethanol. First, all three ER subtypes contributed to enhancement of Akt (Fig. 5A), ERK1/2 (Fig. 5B), eNOS (Fig. 6A) and nNOS (Fig. 6B) phosphorylation, but only ERα and GPER contributed to ethanol enhancement of p38 phosphorylation (Fig. 5C). Surprisingly, despite their ability to significantly attenuate eNOS (Fig. 6A) and nNOS (Fig. 6B) phosphorylation, none of the ER antagonists attenuated the increase in NOx (Fig. 6C), caused by ethanol. Notably, an unexpected finding, in the absence of ethanol, was the substantial increase in myocardial NOx caused by each of the ER antagonists despite the associated reductions in nNOS phosphorylation (Fig. 6). This unexpected result might reflect a compensatory “summation” of NOx produced by eNOS phosphorylation via the two remaining ERs when the third ER subtype is blocked (Fig. 6D). This notion is supported by the significant increase in Akt phosphorylation observed following each selective ER subtype blockade (Fig. 5A) and the ability of phosphorylated Akt to activate eNOS and increase NOx production (Raphael et al., 2010). These biochemical responses triggered by ER blockade may have masked ethanol-evoked increase in myocardial NOx (Fig. 6C).
Reported studies implicated MAPK phosphorylation in oxidative stress (Gaitanaki et al., 2003, Jayakumar et al., 2006), and our previous study causally linked the Akt-ERK1/2 pathway to the E2-dependent myocardial depressant effect of ethanol (El-Mas et al., 2009). In agreement with the latter, ethanol significantly increased Akt and ERK1/2 phosphorylation (Fig. 5A, B) as well as p38 (Fig. 5C), which is also implicated in oxidative stress (Gaitanaki et al., 2003, Umoh et al., 2014). Here, we show that all 3 ER subtypes contributed to these molecular events except for ERβ because its blockade further increased p38 phosphorylation (Fig. 5). The reason for the latter finding is not known because it occurred in the absence of any change in p38 phosphorylation by PHTPP (Fig. 5C). Notably, only Akt phosphorylation was significantly increased, albeit to different degrees, by ER subtype blockade (Fig. 5A). These findings suggest that the three ER subtypes mediate, at least partly, ethanol evoked oxidative stress via MAPK phosphorylation. While further studies are needed to discern the role of ERβ in ethanol effect on p38 phosphorylation, these pharmacological and biochemical findings support a pro-oxidant role for E2, in the presence of ethanol, mediated via MAPK phosphorylation (Ibrahim et al., 2014).
It was also important to elucidate the roles of the ER subtypes in modulating the catalytic activity of two major antioxidant enzymes, catalase and ALDH2 because of their: (i) contribution to the myocardial redox state (Klyosov et al., 1996, Chen et al., 2008, Day, 2009); (ii) involvement in ethanol metabolism (Soffia and Penna, 1987, Kinoshita et al., 2001), and (iii) induction by ethanol in the myocardium of female rats (El-Mas and Abdel-Rahman, 2014, Ibrahim et al., 2014). We show that ERα, ERβ or GPER blockade attenuated ethanol-evoked increases in catalase (Fig. 4A) and ALDH2 (Fig. 4B) catalytic activity. However, MPP was the only ER subtype antagonist that attenuated basal myocardial catalase activity (Fig. 4A). This new finding supports ERα mediation of the nongenomic E2 enhancement of myocardial catalase activity in our recent study (El-Mas and Abdel-Rahman, 2015), and might explain the greatest increase in myocardial ROS following ERα, compared to ERβ or GPER, blockade (Fig. 6D). On the other hand, each of the 3 ER antagonists paradoxically increased ALDH2 activity (Fig. 4B). Whether this is a compensatory response remains to be investigated. Notably, while each ER subtype might serve specific function, there is clear overlap in their functions (Matthews and Gustafsson, 2003, Filice et al., 2009, Meyer et al., 2009). Nonetheless, such ALDH2 induction might explain the ability of MPP, PHTPP or G15 to abrogate ethanol evoked myocardial oxidative stress, which was most notable with MPP (Fig. 6D). This biochemical response might also explain the ability of MPP to attenuate ethanol-evoked myocardial dysfunction as well as a similar, but delayed effect by PHTPP and G15, discussed above. This notion is supported by the ability of pharmacologic induction of ALDH2 by activation of epsilon protein kinase C to attenuate ethanol-evoked cardiotoxicity (Churchill et al., 2009).
It is imperative to comment on a limitation of our study that necessitates interpreting our findings with caution because the loss of ER subtype function might influence signaling via the 2 other ERs, a shared limitation with gene knockout strategy. We acknowledge that while each of the 3 ER subtypes has a certain function, cell signaling interaction and overlapping functions exist between the 3 ER subtypes (Matthews and Gustafsson, 2003, Filice et al., 2009, Meyer et al., 2009). Nonetheless, our pharmacological approach made it feasible to investigate the impact of loss the nongenomic component of a particular ER subtype on cardiovascular function in the absence or presence of ethanol. This is important because the nongenomic mechanisms mediate, at least partly, the E2-dependent adverse hemodynamic effects of ethanol (El-Mas and Abdel-Rahman, 2014).
Overall, the current study underscores the importance of ERα signaling in ethanol-evoked myocardial dysfunction and hypotension in proestrus rats. The findings support our hypothesis that oxidative stress creates a cellular environment that transforms estrogen from being an antiinflammatory into a proinflammatory hormone. This notion is supported by the inability of ethanol to cause myocardial dysfunction, despite its induction of oxidative stress, unless endogenous or exogenous E2 is present in female (El-Mas and Abdel-Rahman, 2014, Ibrahim et al., 2014) or male (El-Mas and Abdel-Rahman, 2015). Similarly, the E2-evoked coronary vasodilation is transformed into vasoconstriction in presence of oxidative stress (White et al., 2005). Finally, the findings identify ER, particularly ERα, blockade as a potential protective modality against E2-dependent myocardial dysfunction caused by ethanol consumption.
Highlights.
ERα plays the major role in the E2-dependent ethanol-evoked myocardial dysfunction.
ERα, but not ERβ or GPER, blockade inhibits myocardial catalase activity.
All 3 ER subtypes contribute to the E2-dependent ethanol-evoked ALDH2 activation.
ERα blockade caused most evident attenuation of ethanol-evoked oxidative stress.
Acknowledgments
This work was supported by Grant 2R01 AA014441-9 from the National Institute on Alcohol Abuse and Alcoholism.
The authors thank Ms. Kui Sun for her technical assistance.
Abbreviations
- E2
estrogen
- ERα
estrogen receptor alpha
- ERβ
estrogen receptor beta
- GPER
G protein-coupled estrogen receptor
- MPP
1,3-Bis(4-hydroxyphenyl)-4-methyl-5-[4-(2-piperidinylethoxy)phenol]-1H-pyrazole dihydrochloride
- PHTPP
4-[2-Phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol
- G15
(3aS,4R,9bR)-4-(6-bromo-1,3-benzodioxol-5-yl)-3a,4,5,9b-tetrahydro-3H-cyclopenta[c]quinolone
- ALDH2
aldehyde dehydrogenase 2 family
- Akt
protein kinase B
- ERK1/2
extracellular signal-regulated protein kinases 1 and 2
- p38
mitogen-activated protein kinases
- eNOS
endothelial nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- ROS
reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERbeta has nongenomic action in caveolae. Mol Endocrinol. 2002;16:938–946. doi: 10.1210/mend.16.5.0827. [DOI] [PubMed] [Google Scholar]
- Chan KK, Leung TH, Chan DW, Wei N, Lau GT, Liu SS, Siu MK, Ngan HY. Targeting estrogen receptor subtypes (ERalpha and ERbeta) with selective ER modulators in ovarian cancer. J Endocrinol. 2014;221:325–336. doi: 10.1530/JOE-13-0500. [DOI] [PubMed] [Google Scholar]
- Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheskis BJ, Greger J, Cooch N, McNally C, McLarney S, Lam HS, Rutledge S, Mekonnen B, Hauze D, Nagpal S, Freedman LP. MNAR plays an important role in ERa activation of Src/MAPK and PI3K/Akt signaling pathways. Steroids. 2008;73:901–905. doi: 10.1016/j.steroids.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Churchill EN, Disatnik MH, Mochly-Rosen D. Time-dependent and ethanol-induced cardiac protection from ischemia mediated by mitochondrial translocation of varepsilonPKC and activation of aldehyde dehydrogenase 2. J Mol Cell Cardiol. 2009;46:278–284. doi: 10.1016/j.yjmcc.2008.09.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day BJ. Catalase and glutathione peroxidase mimics. Biochem Pharmacol. 2009;77:285–296. doi: 10.1016/j.bcp.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Nongenomic effects of estrogen mediate the dose-related myocardial oxidative stress and dysfunction caused by acute ethanol in female rats. Am J Physiol Endocrinol Metab. 2014;306:E740–747. doi: 10.1152/ajpendo.00465.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Abdel-Rahman AA. Estrogen modulation of the ethanol-evoked myocardial oxidative stress and dysfunction via DAPK3/Akt/ERK activation in male rats. Toxicol Appl Pharmacol. 2015 doi: 10.1016/j.taap.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Fan M, Abdel-Rahman AA. Endotoxemia-mediated induction of cardiac inducible nitric-oxide synthase expression accounts for the hypotensive effect of ethanol in female rats. J Pharmacol Exp Ther. 2008;324:368–375. doi: 10.1124/jpet.107.127498. [DOI] [PubMed] [Google Scholar]
- El-Mas MM, Fan M, Abdel-Rahman AA. Facilitation of myocardial PI3K/Akt/nNOS signaling contributes to ethanol-evoked hypotension in female rats. Alcohol Clin Exp Res. 2009;33:1158–1168. doi: 10.1111/j.1530-0277.2009.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Mas MM, Fan M, Abdel-Rahman AA. Differential modulation by vascular nitric oxide synthases of the ethanol-evoked hypotension and autonomic dysfunction in female rats. Alcohol. 2012;46:727–735. doi: 10.1016/j.alcohol.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson CJ, Fukunaga T, Sarkola T, Lindholm H, Ahola L. Estrogen-related acetaldehyde elevation in women during alcohol intoxication. Alcohol Clin Exp Res. 1996;20:1192–1195. doi: 10.1111/j.1530-0277.1996.tb01110.x. [DOI] [PubMed] [Google Scholar]
- Filice E, Recchia AG, Pellegrino D, Angelone T, Maggiolini M, Cerra MC. A new membrane G protein-coupled receptor (GPR30) is involved in the cardiac effects of 17beta-estradiol in the male rat. J Physiol Pharmacol. 2009;60:3–10. [PubMed] [Google Scholar]
- Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaitanaki C, Konstantina S, Chrysa S, Beis I. Oxidative stress stimulates multiple MAPK signalling pathways and phosphorylation of the small HSP27 in the perfused amphibian heart. J Exp Biol. 2003;206:2759–2769. doi: 10.1242/jeb.00483. [DOI] [PubMed] [Google Scholar]
- Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, Barton M. Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circ Res. 2009;104:288–291. doi: 10.1161/CIRCRESAHA.108.190892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim BM, Fan M, Abdel-Rahman AA. Oxidative stress and autonomic dysregulation contribute to the acute time-dependent myocardial depressant effect of ethanol in conscious female rats. Alcohol Clin Exp Res. 2014;38:1205–1215. doi: 10.1111/acer.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar AR, Panickar KS, Murthy Ch R, Norenberg MD. Oxidative stress and mitogen-activated protein kinase phosphorylation mediate ammonia-induced cell swelling and glutamate uptake inhibition in cultured astrocytes. J Neurosci. 2006;26:4774–4784. doi: 10.1523/JNEUROSCI.0120-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbutyte V, Arias-Loza PA, Hu K, Widder J, Govindaraj V, von Poser-Klein C, Bauersachs J, Fritzemeier KH, Hegele-Hartung C, Neyses L, Ertl G, Pelzer T. Ligand-dependent activation of ER{beta} lowers blood pressure and attenuates cardiac hypertrophy in ovariectomized spontaneously hypertensive rats. Cardiovasc Res. 2008;77:774–781. doi: 10.1093/cvr/cvm081. [DOI] [PubMed] [Google Scholar]
- Kandadi MR, Yu X, Frankel AE, Ren J. Cardiac-specific catalase overexpression rescues anthrax lethal toxin-induced cardiac contractile dysfunction: role of oxidative stress and autophagy. BMC Med. 2012;10:134. doi: 10.1186/1741-7015-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Young BD, Bender JR. Endothelial estrogen receptor isoforms and cardiovascular disease. Mol Cell Endocrinol. 2014;389:65–70. doi: 10.1016/j.mce.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita H, Jessop DS, Finn DP, Coventry TL, Roberts DJ, Ameno K, Jiri I, Harbuz MS. Acetaldehyde, a metabolite of ethanol, activates the hypothalamic-pituitary-adrenal axis in the rat. Alcohol Alcohol. 2001;36:59–64. doi: 10.1093/alcalc/36.1.59. [DOI] [PubMed] [Google Scholar]
- Kiss AL, Turi A, Mullner N, Kovacs E, Botos E, Greger A. Oestrogen-mediated tyrosine phosphorylation of caveolin-1 and its effect on the oestrogen receptor localisation: an in vivo study. Mol Cell Endocrinol. 2005;245:128–137. doi: 10.1016/j.mce.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Klatsky AL, Armstrong MA, Friedman GD. Risk of cardiovascular mortality in alcohol drinkers, ex-drinkers and nondrinkers. Am J Cardiol. 1990;66:1237–1242. doi: 10.1016/0002-9149(90)91107-h. [DOI] [PubMed] [Google Scholar]
- Klyosov AA, Rashkovetsky LG, Tahir MK, Keung WM. Possible role of liver cytosolic and mitochondrial aldehyde dehydrogenases in acetaldehyde metabolism. Biochemistry. 1996;35:4445–4456. doi: 10.1021/bi9521093. [DOI] [PubMed] [Google Scholar]
- Kristoffersen L, Skuterud B, Larssen BR, Skurtveit S, Smith-Kielland A. Fast quantification of ethanol in whole blood specimens by the enzymatic alcohol dehydrogenase method. Optimization by experimental design. J Anal Toxicol. 2005;29:66–70. doi: 10.1093/jat/29.1.66. [DOI] [PubMed] [Google Scholar]
- Lahm T, Albrecht M, Fisher AJ, Selej M, Patel NG, Brown JA, Justice MJ, Brown MB, Van Demark M, Trulock KM, Dieudonne D, Reddy JG, Presson RG, Petrache I. 17beta-Estradiol attenuates hypoxic pulmonary hypertension via estrogen receptor-mediated effects. Am J Respir Crit Care Med. 2012;185:965–980. doi: 10.1164/rccm.201107-1293OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekontseva O, Chakrabarti S, Jiang Y, Cheung CC, Davidge ST. Role of neuronal nitric-oxide synthase in estrogen-induced relaxation in rat resistance arteries. J Pharmacol Exp Ther. 2011;339:367–375. doi: 10.1124/jpet.111.183798. [DOI] [PubMed] [Google Scholar]
- Li HY, Bian JS, Kwan YW, Wong TM. Enhanced responses to 17beta-estradiol in rat hearts treated with isoproterenol: involvement of a cyclic AMP-dependent pathway. J Pharmacol Exp Ther. 2000;293:592–598. [PubMed] [Google Scholar]
- Liu CJ, Lo JF, Kuo CH, Chu CH, Chen LM, Tsai FJ, Tsai CH, Tzang BS, Kuo WW, Huang CY. Akt mediates 17beta-estradiol and/or estrogen receptor-alpha inhibition of LPS-induced tumor necresis factor-alpha expression and myocardial cell apoptosis by suppressing the JNK1/2-NFkappaB pathway. J Cell Mol Med. 2009;13:3655–3667. doi: 10.1111/j.1582-4934.2009.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SR, Emanuel K, Sears CE, Zhang YH, Casadei B. Are myocardial eNOS and nNOS involved in the beta-adrenergic and muscarinic regulation of inotropy? A systematic investigation. Cardiovasc Res. 2006;70:97–106. doi: 10.1016/j.cardiores.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Matthews J, Gustafsson JA. Estrogen signaling: a subtle balance between ER alpha and ER beta. Mol Interv. 2003;3:281–292. doi: 10.1124/mi.3.5.281. [DOI] [PubMed] [Google Scholar]
- McGee MA, Abdel-Rahman AA. Enhanced vascular neuronal nitric-oxide synthase-derived nitric-oxide production underlies the pressor response caused by peripheral N-methyl-D-aspartate receptor activation in conscious rats. J Pharmacol Exp Ther. 2012;342:461–471. doi: 10.1124/jpet.112.194464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Bolaina E, Sanchez-Gonzalez J, Ramirez-Sanchez I, Ocharan-Hernandez E, Nunez-Sanchez M, Meaney-Mendiolea E, Meaney A, Asbun-Bojalil J, Miliar-Garcia A, Olivares-Corichi I, Ceballos-Reyes G. Effect of caveolin-1 scaffolding peptide and 17beta-estradiol on intracellular Ca2+ kinetics evoked by angiotensin II in human vascular smooth muscle cells. Am J Physiol Cell Physiol. 2007;293:C1953–1961. doi: 10.1152/ajpcell.00519.2006. [DOI] [PubMed] [Google Scholar]
- Mercuro G, Podda A, Pitzalis L, Zoncu S, Mascia M, Melis GB, Rosano GM. Evidence of a role of endogenous estrogen in the modulation of autonomic nervous system. Am J Cardiol. 2000;85:787–789. A789. doi: 10.1016/s0002-9149(99)00865-6. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Haas E, Barton M. Gender differences of cardiovascular disease: new perspectives for estrogen receptor signaling. Hypertension. 2006;47:1019–1026. doi: 10.1161/01.HYP.0000223064.62762.0b. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Haas E, Prossnitz ER, Barton M. Non-genomic regulation of vascular cell function and growth by estrogen. Mol Cell Endocrinol. 2009;308:9–16. doi: 10.1016/j.mce.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Prossnitz ER, Barton M. The G protein-coupled estrogen receptor GPER/GPR30 as a regulator of cardiovascular function. Vascul Pharmacol. 2011;55:17–25. doi: 10.1016/j.vph.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- Raphael J, Gozal Y, Navot N, Zuo Z. Hyperglycemia inhibits anesthetic-induced postconditioning in the rabbit heart via modulation of phosphatidylinositol-3-kinase/Akt and endothelial nitric oxide synthase signaling. J Cardiovasc Pharmacol. 2010;55:348–357. doi: 10.1097/FJC.0b013e3181d26583. [DOI] [PubMed] [Google Scholar]
- Reslan OM, Yin Z, do Nascimento GR, Khalil RA. Subtype-specific estrogen receptor-mediated vasodilator activity in the cephalic, thoracic, and abdominal vasculature of female rat. J Cardiovasc Pharmacol. 2013;62:26–40. doi: 10.1097/FJC.0b013e31828bc88a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santollo J, Eckel LA. Effect of a putative ERalpha antagonist, MPP, on food intake in cycling and ovariectomized rats. Physiol Behav. 2009;97:193–198. doi: 10.1016/j.physbeh.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soffia F, Penna M. Ethanol metabolism by rat heart homogenates. Alcohol. 1987;4:45–48. doi: 10.1016/0741-8329(87)90059-0. [DOI] [PubMed] [Google Scholar]
- Turdi S, Li Q, Lopez FL, Ren J. Catalase alleviates cardiomyocyte dysfunction in diabetes: role of Akt, Forkhead transcriptional factor and silent information regulator 2. Life Sci. 2007;81:895–905. doi: 10.1016/j.lfs.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Umoh NA, Walker RK, Al-Rubaiee M, Jeffress MA, Haddad GE. Acute alcohol modulates cardiac function as PI3K/Akt regulates oxidative stress. Alcohol Clin Exp Res. 2014;38:1847–1864. doi: 10.1111/acer.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihe WH. The laboratory rat. In: Pool T, editor. UFAW Handbook on the Care and Management of Laboratory Animals. 6th Ed. Longman Scientific and Technical; Harlow, UK: 1987. 6th Ed. [Google Scholar]
- Weil BR, Manukyan MC, Herrmann JL, Wang Y, Abarbanell AM, Poynter JA, Meldrum DR. Signaling via GPR30 protects the myocardium from ischemia/reperfusion injury. Surgery. 2010;148:436–443. doi: 10.1016/j.surg.2010.03.011. [DOI] [PubMed] [Google Scholar]
- White RE, Han G, Dimitropoulou C, Zhu S, Miyake K, Fulton D, Dave S, Barman SA. Estrogen-induced contraction of coronary arteries is mediated by superoxide generated in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2005;289:H1468–1475. doi: 10.1152/ajpheart.01173.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]