Abstract
Background
To investigate the prevalence of undiagnosed HIV infections in an emergency department (ED) with an established screening program.
Methods
Evaluation of the prevalence and risk factors for HIV from an 8-week (6/24/2007 – 8/18/2007) identity-unlinked HIV serosurvey, conducted at the same time as an ongoing opt-in rapid oral-fluid HIV screening program. Testing facilitators offering 24/7 bedside rapid testing to patients aged 18–64 years, with concordant collection of excess sera collected as part of routine clinical procedures. Known HIV positivity was determined by (1) chart review or self-report from the screening program, and/or (2) presence of antiretrovirals in serum specimens.
Results
Among 3,207 patients, 1,165 (36.3%) patients were offered an HIV test. Among those offered, 567 (48.7%) consented to testing. Concordance identity-unlinked study revealed that the prevalence of undiagnosed infections was: 2.3% in all patients; 1.0% in those offered testing versus 3.0% in those not offered testing (p<0.001); and 1.3% in those who declined testing compared to 0.4% in those who were tested (p=0.077). Higher median viral loads were observed in those not offered testing (14,255 copies/mL, IQR: 1,147 – 64,354) versus those offered testing (1,865 copies/mL, IQR: undetectable – 21,786) but the difference was not statistically significant.
Conclusions
High undiagnosed HIV prevalence was observed in ED patients who were not offered HIV testing and those who declined testing, compared to those who were tested. This indicates that even with an intensive facilitator-based rapid HIV screening model, significant missed opportunities remain with regard to identifying undiagnosed infections in the ED.
Keywords: HIV, Emergency Department, Undiagnosed Infection, HIV Screening
Introduction
The Centers for Disease Control and Prevention (CDC) estimates that 14% of 1.2 million HIV-infected individuals in U.S. were unaware of their positive serostatus [1]. Numerous previous studies have demonstrated that U.S. emergency departments (EDs) are the leading sites of encounter for “late-testers” and the most common site of ‘missed opportunities’ for HIV testing in medical settings [2, 3]. In order to better understand the magnitude of the hidden epidemic, ED investigators pioneered identity unlinked seroprevalence methodologies [4–7], and demonstrated high prevalence of unrecognized HIV infections, both historically and more recently. Since 2006 CDC revised recommendations for HIV testing in health-care settings [8], numerous U.S. EDs have established organized HIV testing programs to help find those with unrecognized HIV, with some success [9, 10].
Unfortunately, in spite of intensive national efforts, ED HIV screening programs still fail to identify many infected patients. A recent blinded seroprevalence study, conducted in the context of an ongoing non-targeted counselor-based HIV testing program in one ED in Washington, DC, found that the prevalence of undiagnosed HIV infection was 3 times higher amongst those who declined testing, as compared with those who accepted [11]. Notably, that study was conducted in a setting which used an opt-out approach for HIV screening (which in practice may not be always be the norm [9], due to state regulations and/or pragmatic issues), and assessed seroprevalence only on the select subset of patients who were considered ‘eligible’ to participate in opt-out screening, namely those with non-urgent medical conditions, and were deemed ‘able to communicate with ‘normal’ mental status. Thus, gaps remain with regard to defining the full spectrum of potential missed opportunities for HIV testing in EDs (including a more comprehensive assessment of demographic and clinical characteristics of those patients). Further study would be informative for optimizing strategies for HIV detection in ED settings.
Situated in a high HIV prevalence area in Baltimore City, our ED has implemented programmatic rapid HIV screening since 2005 [12]. During the summer of 2007, we conducted an identity-unlinked HIV seroprevalence study, simultaneous with 24/7 dedicated testing facilitator-based HIV testing. Our objectives were to: (1) determine the prevalence of undiagnosed HIV infections in the context of an active dedicated testing facilitator driven HIV opt-in non-targeted screening program; and (2) define and compare characteristics of patients with undiagnosed HIV infections, among those who were offered (versus not offered) HIV testing; and among those among who did (versus did not) consent to having an HIV test performed.
Methods
Our study was conducted in an academic adult ED in Baltimore City, which in 2007 saw approximately 60,000 visits annually. The ED serves an urban, inner-city population with a historically high HIV prevalence of 11 to 12%, and an HIV incidence of 0.56% to 0.94% per year [6].
Our non-targeted, rapid, oral fluid (OraQuick Advanced) opt-in HIV screening program aimed to offer free HIV testing to as many as possible ED patients who met the following criteria: ages 18–64 years; not critically ill; no previous diagnosis of HIV; no HIV test in the past 3 months; and able to provide informed consent. The operational model of screening program evolved over time based on the staffing model employed and varied logistical considerations, including level of funding to support our supplementary staff [12]. In the summer of 2007 (concordant with the time that this study was carried out), the screening program operated with trained facilitators who offered HIV testing to eligible ED patients, 24 hours a day, 7 days a week. These facilitators performed abbreviated pre- and post-test counseling, consent for HIV testing, and collected oral swab specimens which were tested in the ED satellite laboratory by dedicated laboratorians. One full-time dedicated HIV program coordinator, was responsible for linkage to care for any newly diagnosed HIV-infected patients. Details of the testing program have been described elsewhere [12].
An identity-unlinked HIV seroprevalence study was carried out during the summer of 2007 (8 week duration). All ED patients ≥ 18 years who had blood drawn for clinical purposes (and in whom excess sample was available for HIV testing) were included and evaluated using an identity-unlinked seroprevalence methodology [5]. Briefly, basic demographic and clinical data including HIV relevant information was extracted prior to sample de-identification. De-identified samples were then tested for HIV by third-generation enzyme immunoassays (ELISA); all positives were confirmed by Western blot followed by RNA viral load (VL) testing using Roche Amplicor v1.5 which has a limit of detection of 400 copies/ml (Roche, Indianapolis, IN). Antiretrovirals (ARVs) in serum specimens were detected using ultra-performance liquid chromatography-tandem mass spectrometry by the Clinical Pharmacology Laboratory at our institution. Known HIV positivity was determined by (1) both chart review and review of self-report from the HIV screening program (as applicable), and/or (2) presence of ARVs in serum specimens. The study was approved by the Johns Hopkins University School of Medicine Institutional Review Board. Use of an identity-unlinked methodology for determining HIV seroprevalence, is permitted via a consent waiver [5, 13] and involves accessing excess waste clinical blood specimens and paring that with de-identified demographic and administrative data, for purposes of understanding HIV epidemiology in the population.
Two-sided p<0.05 was considered statistically significant. Chi-squared tests or Fisher’s exact tests were performed to determine prevalence ratio or relative differences in proportion of undiagnosed HIV cases by their status in the screening program. Non-parametric Wilcoxon rank sum tests were performed to determine the differences in HIV RNA VLs by screening program group using SAS version 9.3 (SAS Institute Inc., Cary, North Carolina).
Results
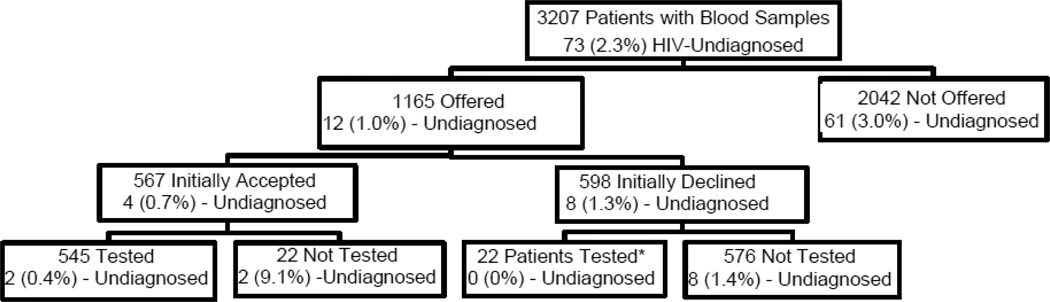
During the 8-week study period, there were 9,179 ED visits and 4,475 serum specimens collected from 7,254 unique patients. After excluding multiple specimens from the same visits and repeat visits, 3,399 unique patients were included. After excluding 192 known positive patients, there were 3,207 unique patients remaining, whom were included for the analysis. Among those patients, 1,165 (36.3%) patients were offered an HIV test during their ED visits, while 2,042 (63.7%) were not. Of those offered a test, 598 (51.3%) patients declined, while 567 (48.7%) accepted the test. There were 22 patients who accepted the test but were not tested, and another (i.e. distinct group of) 22 patients who initially declined the test, but were later tested as part of their care in the ED. The overall prevalence of undiagnosed HIV infection was 2.3% (73/3207) (Figure 1).
Figure 1.
Proportion of Undiagnosed HIV Cases in Urban Emergency Department Patients in an Identity-Unlinked Seroprevalence Study by Emergency Department-Based Rapid HIV Screening Program Status, 2007
* Patients who were tested as part of their clinical care in the ED based on later requests by patients or providers outside of the initial ED screening program
Significant differences were observed when comparing those who were offered HIV testing versus those who were not, with regard to age, gender, race, and payor type (Table 1). After excluding patients > 64 years of old (i.e. outside of 2006 CDC recommended age group for HIV testing), age was not found to be significantly associated with whether a test was offered or not. Patients who were offered an HIV test were more likely to be African American but less likely to be publicly insured than those who were not offered an HIV test.
Table 1.
Characteristics of Emergency Department (ED) Patients by ED-Based Rapid HIV Screening Program Status, 2007
| Characteristics | Categories | HIV Screening | |
|---|---|---|---|
| Offered N=1165 |
Not Offered N=2042 |
||
| Age (years)* | 18–24 | 153 (13) | 225 (11) |
| 25–34 | 214 (18) | 328 (16) | |
| 35–44 | 278 (24) | 391 (19) | |
| 45–54 | 279 (24) | 398 (19) | |
| 55–64 | 209 (18) | 236 (12) | |
| ≥ 65 | 32 (3) | 462 (23) | |
| Unknown | 0 (0) | 2 (0) | |
| Gender | Female | 658 (56) | 1105 (54) |
| Male | 507 (44) | 937 (46) | |
| Race* | African American | 817 (70) | 1309 (64) |
| White | 296 (25) | 600 (29) | |
| Hispanic | 18 (2) | 37 (2) | |
| Other | 25 (2) | 72 (4) | |
| Unknown | 9 (1) | 24 (1) | |
| Triage Acuity Level* | 1 | 27 (2) | 140 (7) |
| 2 | 335 (29) | 605 (30) | |
| 3 | 701 (60) | 1168 (57) | |
| 4 | 83 (7) | 84 (4) | |
| 5 | 0 (0) | 7 (0) | |
| Unknown | 19 (2) | 38 (2) | |
| Health Insurance* | Public | 179 (15) | 527 (26) |
| Private | 573 (49) | 811 (40) | |
| Self-Pay | 390 (33) | 655 (32) | |
| Other | 23 (2) | 49 (2) | |
| Disposition* | Admit | 420 (36) | 832 (41) |
| Discharged | 687 (59) | 1098 (54) | |
| Against Medical Advice | 30 (3) | 36 (2) | |
| Left Without Being Seen | 7 (1) | 22 (1) | |
| Referral | 2 (0) | 12 (1) | |
| Expired | 0 (0) | 4 (0) | |
| Other | 19 (2) | 38 (2) | |
p<0.05
The prevalence of undiagnosed HIV was 3 times higher in those who were not offered, versus those who were offered (3.0% versus 1.0%, respectively, prevalence ratio: 2.90, 95% CI: 1.57, 5.36, p<0.001). Further, after excluding those > 64 years of age, the undiagnosed prevalence of HIV was even higher at 3.8%, amongst those not offered testing. No statistically significant differences were observed with regard to age, gender, race, triage acuity level, or medical insurance payor type amongst patients with undiagnosed HIV infection, according to whether or not they were offered a test (Table 2). However, significant difference was observed in the distribution of types of chief complaint when comparing those who were offered verses not offered, an HIV test (p=0.004, Fisher’s exact test) (Table 3).
Table 2.
Characteristics of 73 Undiagnosed HIV-Infected Patients in an Urban Emergency Department (ED) by ED-Based Rapid HIV Screening Program Status, 2007
| Characteristics | Categories | HIV Screening | |
|---|---|---|---|
| Offered N=12 |
Not Offered N=61 |
||
| Age (years) | 18–24 | 0 (0) | 1 (2) |
| 25–34 | 1 (8) | 12 (20) | |
| 35–44 | 3 (25) | 25 (41) | |
| 45–54 | 5 (42) | 18 (30) | |
| 55–64 | 2 (17) | 4 (7) | |
| ≥ 65 | 1 (8) | 1 (2) | |
| Gender | Female | 5 (42) | 26 (43) |
| Male | 7 (58) | 35 (57) | |
| Race | African American | 11 (92) | 52 (85) |
| White | 1 (8) | 7 (11) | |
| Other | 0 (0) | 1 (2) | |
| Unknown | 0 (0) | 1 (2) | |
| Triage Acuity Level | 1 | 0 (0) | 4 (7) |
| 2 | 3 (25) | 16 (26) | |
| 3 | 9 (75) | 38 (62) | |
| 4 | 0 (0) | 3 (5) | |
| 5 | 0 (0) | 0 (0) | |
| Health Insurance | Public | 2 (17) | 16 (26) |
| Private | 7 (58) | 28 (46) | |
| Self-Pay | 3 (25) | 16 (26) | |
| Other | 0 (0) | 1 (2) | |
| Disposition | Admit | 4 (33) | 31 (51) |
| Discharged | 6 (50) | 29 (48) | |
| Against Medical Advice | 2 (17) | 1 (2) | |
Table 3.
Chief Complaints of Emergency Department Visits by 73 Undiagnosed HIV-Infected Patients in an Urban Adult Emergency Department, June – August 2007
| Chief Complaints | Number | HIV screening | |
|---|---|---|---|
| N=73 | Offered N=12 |
Not Offered N=61 |
|
| Pain | 15 (21) | 1 (8) | 14 (23) |
| Altered Mental / Psychiatric | 13 (18) | 0 (0) | 13 (21) |
| Fever / Sore Throat / Vomiting / Abscess / Other infectious disease-related | 13 (18) | 1 (8) | 12 (20) |
| Chest Pain / Short of Breath | 11 (15) | 4 (33) | 7 (12) |
| Injury / Accident | 6 (8) | 0 (0) | 6 (10) |
| Headache / Migraine | 4 (5) | 3 (25) | 1 (2) |
| Dizziness / Syncope | 2 (3) | 1 (8) | 1 (2) |
| Weakness / Numbness | 3 (4) | 1 (8) | 2 (3) |
| Other | 6 (8) | 1 (8) | 5 (8) |
p<0.05, Fisher’s exact test, for the distribution of chief complaint category by HIV screening offered status
Among those who were offered an HIV test, marginally significant differences in the prevalence of undiagnosed HIV infection were observed according to patient’s acceptance status. Eight (1.3%) of 576 patients who declined the testing (and were not tested) were HIV-infected versus two (0.4%) of 545 patients who accepted and were tested for HIV (prevalence ratio: 3.79, 95% CI: 0.81, 17.74, p=0.077). Two (9.1%) of 22 patients who initially accepted the test (but were ultimately not tested during their ED visit), and none of the 22 patients who initially declined testing (but were later testing as part of their clinical care) were HIV-infected.
The average mean HIV RNA VLs amongst the 73 undiagnosed HIV-infected patients was 110,340 copies/mL (range: undetectable – 3,396,395); the median VLs was 10,814 copies/mL [interquartile range (IQR): 749 – 44,660]. Higher mean and median VLs were observed in those who were not offered (mean: 129,005±455,391 copies/mL; median: 14,255 copies/mL, IQR: 1,147 – 64,354), versus those who were offered (15,463±29,226 copies/mL; median: 1,865 copies/mL, IQR: undetectable – 21,786) but the difference was not statistically significant (p=0.103, Wilcoxon rank sums test). Eighteen (25%) patients with undiagnosed HIV infection had VLs > 50,000 copies/mL. Of these, seventeen were not offered testing and only one was offered and tested by the program. There was no significant difference in proportion of patients with high HIV VLs (>50,000 copies/mL) by HIV testing offer status (not offered: 28% versus offered: 8%, p=0.282).
Discussion
In our ED, which has a known historically high prevalence of HIV infections among its patients [5, 6, 12, 14], we found a significantly higher prevalence of undiagnosed HIV among patients who were not offered HIV testing, versus those who were offered; this, in spite of the existence of a concordant 24/7 dedicated testing facilitator-based opt-in rapid ED-based HIV screening program. While the broad goals of our screening program was to offer and test as many eligible patients as possible, our facilitator model resulted in only 36% of all patients (or 42% of the age eligible patients i.e. those < 65 years of age) being offered a test. Although those numbers seem relatively low, the percentage of HIV test offer are comparable to the average (39%) reported by other peer-reviewed studies [15]. The proportion (47%, 545/1165) of patients who were tested among those offered in our study was also comparable to the average (42%) reported [15]. Although we were unable to systematically gather reasons for not offering an HIV test to patients, empiric observations indicate that reasons for non-offer included presence of individual patient exclusion criteria (i.e. critically ill, unable to consent, patient self-reported HIV testing history within previous 3 months, and known HIV positivity status), as well as the relative lack of personnel resources required to approach each potentially eligible patient. Recent published studies suggest that an integrated, streamlined, and automatic electronic opt-out approach, which can eliminate barriers associated with HIV testing offer, may minimize the observed discordance between undiagnosed HIV infections and the underlying HIV seroprevalence [16–18].
Of the total number of undiagnosed HIV infected patients who came to our ED during the study period, 61/73 (84%) were not offered a test for HIV. Notably, a significantly higher proportion of patients who were not offered an HIV test were admitted to the hospital than those who were offered. In addition, the majority of undiagnosed HIV-infected patients who were not offered an HIV test were admitted. Approximately 75% (45/61) of undiagnosed HIV-infected patients who were not offered an HIV test had chief complaint which included either infectious disease related conditions, pain, injury/accidents, altered mental status/psychiatric-related conditions while only 17% (2/12) of those who were offered had one of these chief complaints. This suggests that those with undiagnosed infections in our ED were more likely to be those with critical illness and/or were conditions which precluded our facilitators from providing informed consent (based on our program criteria from being eligible to approach). It is possible that our facilitators were over-conservatively with regard to interpreting eligibility criteria. Opt-out approaches for HIV test (at the point of triage and by the clinical staff) could help overcome at least some of those gaps. Alternatively, addition of scaled-up routine inpatient screening programs for all ED admitted patients could minimize potential missed ED opportunity for identifying those with undiagnosed infections [19]. In our own ED, that could have resulted in an additional 31 undiagnosed infections (or 51% of the total undiagnosed who were not offered) being detected. Based on use of identity-unlinked methodology employed here, we are unable to know whether those individuals were diagnosed or not in our inpatient setting. However, previous studies from our own site and others, suggest shortfalls and opportunities remain [20, 21]. There were some additional observations based on chief complaints, with regard to whether patients with undiagnosed HIV were offered an HIV test or not (i.e. those with chief complaints including chest pain/short of breath, headache/migraine, and dizziness/syncope had a relatively higher chance of being offered an HIV test, versus those who presented with infectious diseases, pain, injury, and/or altered mental status-related chief complaints). Although it is impossible to say with certainty why this trend exists, one possible explanation is that certain chief complaints make it more likely that test facilitators can interact with the patients over the course of their ED stay (e.g. those patients with headache or dizziness may clinically improve during their stay, and those with chest pain/short of breath may have longer lengths of stay (e.g. in observation areas), providing opportunities for screening encounter with HIV testing facilitators.
Our findings are consistent with what has been previously described in the literature, i.e. there are significant proportions of ED patients who decline HIV testing in spite of being offered testing, and particularly when opt-in approaches are employed [22]. Further, among those who decline testing, our findings are consistent with what we and others have previously reported, namely, that among those who decline testing there are a significantly higher prevalence of patients with traditional ‘high risk’ behaviors [23] and a higher prevalence of undiagnosed (or hidden) infections [11]. Of note, due to the relatively small number of undiagnosed infections in those patients who were offered testing in our study, we observed only a marginally statistically significant difference in the prevalence of undiagnosed HIV infection between those who declined and those who accepted testing. However, the prevalence ratio between two groups was approximately 4, which is comparable to that in an ED testing program in Washington DC [11]. Systematic destigmatization of HIV testing and reduction of testing barriers approach, e.g. routine opt-out testing [16], would likely lower the prevalence ratio of undiagnosed infection, between those tested and those not tested. Alternatively, novel approaches to increase test acceptance, such as computerized registration/triage kiosks could be considered and scaled-up, as they have already been shown to increase proportions of HIV test acceptance, both overall and amongst high-risk patients [23]. Such approaches could be particularly important in states where opt-out approaches for HIV testing are not yet permitted by state law.
During the 8-week study period, our screening program only detected 2.7% (2/73) of the undiagnosed HIV infected population who passed through our ED. Two individuals were offered testing, but ultimately not tested during their ED stay. There are multiple possible explanations for what might have occurred with those patients, including that the patient decided to leave the ED prior to their test being performed, or they disclosed that they were HIV-positive to the facilitator, after agreeing to a test, and were not repeat tested (a known phenomenon we have recurrently observed in our ED). As noted above, due to the identity-unlinked nature of this study, we are unable to determine the definitive reason for those cases where testing did not occur. The remaining 69 undiagnosed patients were either not offered a test, or were offered but declined. Despite the observed lack of statistical significance, there was a trend towards higher mean and median VLs and a higher proportion of high VLs (>50,000 copies/mL) in those who were not offered an HIV test, versus those who were offered. This is a particularly important finding with regard to defining strategies to bridge the observed gaps, since HIV VL is known to be one of the most important predictors for HIV transmission [24]. Specifically, rates of HIV transmission via sexual transmission is 9.03 per 100 person-years for those with a VL >50,000 copies/mL [25]. In addition, estimates suggest that approximately eight HIV infections could be averted per 100 persons who were aware of their infection (versus those who were unaware) in a year [26]. Thus, from our study, 17 ED patients who had undiagnosed HIV infection and high VLs would likely generate approximately two new infections per year if they remained ‘hidden’, further driving the local HIV epidemic.
Our study is susceptible to several limitations. First, we may have underestimated known HIV positivity status, since this was coded based solely our institutional electronic medical record (EMR) review and self-report. Patients might have been diagnosed with HIV in other settings or testing venue but their infection status may not have been documented in our hospital EMR. Patients could also choose not to disclose their HIV positivity status to the testing staff. To offset this potential misclassification bias and minimize our underestimate of ‘known’ HIV, we did include laboratory test to detect the presence of ARVs; however that would only pertain to those who were on therapy so potential biases remain. Second, our estimates cannot be generalized to our entire ED population due to selection bias. The study period was restricted to an 8 week period over one summer and the study was also restricted to those with blood specimens, thus included only about 7% of annual ED visits. Annual data as well as seasonal trend regarding undiagnosed HIV infection in our population are not available. Those with blood specimens were likely to be older or female, but less likely to be African American, as compared to those without (data not shown). Therefore, the prevalence in those without blood specimens (young African American men) could likely be higher according to the data from our previous seroprevalence studies and local HIV epidemic in Baltimore City. However, the true undiagnosed HIV prevalence remains unknown for those patients who did not have blood drawn. Third, our study was also reliant on the availability of waste blood specimens. We believe that any potential biases associated with that were relatively small, however, since nearly all patients in our ED (based on our observed experience) who have blood drawn have sufficient excess serum for HIV testing in the identity-unlinked study. Fourth, new EMR technologies (e.g. electronic prompt from triage) and HIV diagnostic methods (i.e. fourth-generation ELISA) have been introduced into our ED in 2013. Further the current staffing and operational model for HIV testing in our ED (i.e. triage-integrated nurse-driven HIV testing) [27] has significantly changed from what we describe herein from 2007 (i.e. 24/7 dedicated testing facilitator-based HIV testing). It is thus possible that the high rates of undiagnosed HIV observed here (amongst those not offered an HIV test) may not be present with our current screening program; future research will be required to address that. Nevertheless, our findings regarding ‘missed opportunities’ are important for those EDs which continue to provide HIV screening via a parallel facilitator-based model. Finally, our findings cannot be generalized to other EDs in the U.S. Accordingly, results should be interpreted with caution since local HIV epidemic and ED-based HIV screening program might be different from ours.
In conclusion, we observed a disproportionately high prevalence of undiagnosed HIV infection in ED patients amongst patients who were not offered HIV screening and in those who declined screening, versus those who accepted testing. This indicates that even with an intensive opt-in testing facilitator-based rapid HIV screening model, significant missed opportunities remain with regard to identifying undiagnosed HIV-infected individuals in the ED. These findings provide conceptual framework and model for exploring the effectiveness of ED-based HIV screening programs. Opt-out, clinical staff-driven, registration/triage-based integrated routine HIV testing models which helps to de-stigmatize HIV by offering testing to every eligible patient are being evaluated currently [16, 17] and have already been shown to increase HIV test offer and test acceptance, to be expected helping curtail the ‘missed opportunities’ we observed in this study.
Acknowledgments
Funding Sources:
The study was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health. Dr. Kraus was supported by the 2007 Emergency Medicine Foundation (EMF) / Society for Academic Emergency Medicine (SAEM) Medical Student Research Grant to conduct the identity-unlinked seroprevalence study. The Johns Hopkins University Department of Emergency Medicine HIV Testing Program was funded in part by grants from Maryland Department of Health and Mental Hygiene. Dr. Hsieh is also supported in part by an NIH Award, K01AI100681 from NIAID to study HIV testing in emergency departments using a modeling approach. Dr. Rothman is currently supported in part by funding from the Gilead Focus Program which does not fund this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
We declare no competing interests.
Ethics committee approval
The Johns Hopkins University School of Medicine Institutional Review Board approved the identity-unlinked seroprevalence study as well as the ED-based HIV testing and linkage to care program.
References
- 1.Hall H, An Q, Tang T, Song R, Chen M, Green T, et al. Prevalence of Diagnosed and Undiagnosed HIV Infection - United States, 2008–2012. MMWR Morb Mortal Wkly Rep. 2015;64:657–662. [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Missed opportunities for earlier diagnosis of HIV infection--South Carolina, 1997–2005. MMWR Morb Mortal Wkly Rep. 2006;55:1269–1272. [PubMed] [Google Scholar]
- 3.Liddicoat R, Horton N, Urban R, Maier E, Christiansen D, Samet J. Assessing missed opportunities for HIV testing in medical settings. J Gen Intern Med. 2004;19:349–356. doi: 10.1111/j.1525-1497.2004.21251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goggin M, Davidson A, Cantril S, O'Keefe L, Douglas J. The extent of undiagnosed HIV infection among emergency department patients: results of a blinded seroprevalence survey and a pilot HIV testing program. J Emerg Med. 2000;19:13–19. doi: 10.1016/s0736-4679(00)00175-x. [DOI] [PubMed] [Google Scholar]
- 5.Kelen G, Fritz S, Qaqish B, Brookmeyer R, Baker J, Kline R, et al. Unrecognized human immunodeficiency virus infection in emergency department patients. N Engl J Med. 1988;318:1645–1650. doi: 10.1056/NEJM198806233182503. [DOI] [PubMed] [Google Scholar]
- 6.Laeyendecker O, Rothman R, Henson C, Horne B, Ketlogetswe K, Kraus C, et al. The effect of viral suppression on cross-sectional incidence testing in the johns hopkins hospital emergency department. J Acquir Immune Defic Syndr. 2008;48:211–215. doi: 10.1097/QAI.0b013e3181743980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moschella P, Hart K, Ruffner A, Lindsell C, Wayne D, Sperling M, et al. Prevalence of undiagnosed acute and chronic HIV in a lower-prevalence urban emergency department. Am J Public Health. 2014;104:1695–1699. doi: 10.2105/AJPH.2014.301953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Branson B, Handsfield H, Lampe M, Janssen R, Taylor A, Lyss S, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in healthcare settings. MMWR Recomm Rep. 2006;55:1–17. [PubMed] [Google Scholar]
- 9.Rothman R, Hsieh Y-H, Harvey L, Connell S, Lindsell C, Haukoos J, et al. 2009 US Emergency Department HIV Testing Practices. Ann Emerg Med. 2011;58:S3–S9. e4. doi: 10.1016/j.annemergmed.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. Results of the Expanded HIV Testing Initiative--25 jurisdictions, United States, 2007–2010. MMWR Morb Mortal Wkly Rep. 2011;60:805–810. [PubMed] [Google Scholar]
- 11.Czarnogorski M, Brown J, Lee V, Oben J, Kuo I, Stern R, et al. The Prevalence of Undiagnosed HIV Infection in Those Who Decline HIV Screening in an Urban Emergency Department. AIDS Res Treat. 2011:879065. doi: 10.1155/2011/879065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hsieh Y-H, Jung J, Shahan J, Pollack H, Hairston H, Moring-Paris D, et al. Outcomes and Cost Analysis of Three Operational Models for Rapid HIV Testing Services in an Academic Inner-City Emergency Department. Ann Emerg Med. 2011;58:S133–S139. doi: 10.1016/j.annemergmed.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 13.Kelen G, Hsieh Y-H, Rothman R, Patel E, Laeyendecker O, Marzinke M, et al. Improvements in the continuum of HIV care in an inner-city emergency department. AIDS. 2015 doi: 10.1097/QAD.0000000000000896. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelen G, Hexter D, Hansen K, Tang N, Pretorius S, Quinn T. Trends in human immunodeficiency virus (HIV) infection among a patient population of an inner-city emergency department: implications for emergency department-based screening programs for HIV infection. Clin Infect Dis. 1995;21:867–875. doi: 10.1093/clinids/21.4.867. [DOI] [PubMed] [Google Scholar]
- 15.Haukoos J. The impact of nontargeted HIV screening in emergency departments and the ongoing need for targeted strategies. Arch Intern Med. 2012;172:20–22. doi: 10.1001/archinternmed.2011.538. [DOI] [PubMed] [Google Scholar]
- 16.Hoxhaj S, Davila J, Modi P, Kachalia N, Malone K, Ruggerio M, et al. Using Nonrapid HIV Technology for Routine, Opt-out HIV Screening in a High-Volume Urban Emergency Department. Ann Emerg Med. 2011;58:S79–S84. doi: 10.1016/j.annemergmed.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 17.Geren K, Lovecchio F, Knight J, Fromm R, Moore E, Tomlinson C, et al. Identification of Acute HIV Infection Using Fourth-Generation Testing in an Opt-Out Emergency Department Screening Program. Ann Emerg Med. 2014;64:537–546. doi: 10.1016/j.annemergmed.2014.05.021. [DOI] [PubMed] [Google Scholar]
- 18.Wilbur L, Huffman G, Lofton S, Finnell J. The Use of a Computer Reminder System in an Emergency Department Universal HIV Screening Program. Ann Emerg Med. 2011;58:S71–S73. e71. doi: 10.1016/j.annemergmed.2011.03.028. [DOI] [PubMed] [Google Scholar]
- 19.Lubelchek R, Kroc K, Levine D, Beavis K, Roberts R. Routine, Rapid HIV Testing of Medicine Service Admissions in the Emergency Department. Ann Emerg Med. 2011;58:S65–S70. doi: 10.1016/j.annemergmed.2011.03.027. [DOI] [PubMed] [Google Scholar]
- 20.Voetsch A, Heffelfinger J, Yonek J, Patel P, Ethridge S, Torres G, et al. HIV screening practices in U.S. hospitals, 2009–2010. Public Health Rep. 2012;127:524–531. doi: 10.1177/003335491212700508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegel M, Kennedy L, Rexroth K, Lankford M, Turner M, McKnight A, et al. Better but not ideal acceptance of routine inpatient HIV point-of-care testing among veterans in a high prevalence area. J Acquir Immune Defic Syndr. 2010;55:205–210. doi: 10.1097/QAI.0b013e3181e15bf4. [DOI] [PubMed] [Google Scholar]
- 22.Haukoos J, Hopkins E, Bender B, Al-Tayyib A, Long J, Harvey J, et al. Use of kiosks and patient understanding of opt-out and opt-in consent for routine rapid human immunodeficiency virus screening in the emergency department. Acad Emerg Med. 2012;19:287–293. doi: 10.1111/j.1553-2712.2012.01290.x. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh Y-H, Gauvey-Kern M, Peterson S, Woodfield A, Deruggiero K, Gaydos C, et al. An emergency department registration kiosk can increase HIV screening in high risk patients. J Telemed Telecare. 2014;20:454–459. doi: 10.1177/1357633X14555637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinn T, Wawer M, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342:921–929. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 25.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 26.Hall H, Holtgrave D, Maulsby C. HIV transmission rates from persons living with HIV who are aware and unaware of their infection. AIDS. 2012;26:893–896. doi: 10.1097/QAD.0b013e328351f73f. [DOI] [PubMed] [Google Scholar]
- 27.Signer D, Peterson S, Hsieh Y-H, Haider S, Saheed M, Neira P, et al. Scaling Up HIV Testing in an Academic Emergency Department: An Integrated Testing Model with Fourth-Generation and Point-of-Care Testing. Public Health Rep. 2015 doi: 10.1177/00333549161310S110. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]