Abstract
The importance of the vitamin B6-derived pyridoxal cofactor for human health has been established through more than 70 years of intensive biochemical research, revealing its fundamental roles in metabolism. B6 deficiency, resulting from nutritional limitation or impaired uptake from dietary sources, is associated with epilepsy, neuromuscular disease and neurodegeneration. Hereditary disorders of B6 processing are also known, and genetic defects in pathways involved in transport of B6 into the cell and its transformation to the pyridoxal-5′-phosphate enzyme cofactor can contribute to cardiovascular disease by interfering with homocysteine metabolism and the biosynthesis of vasomodulatory polyamines. Compared to the processes involved in cellular uptake and processing of the B6 vitamers, trafficking of the PLP cofactor across intracellular membranes is very poorly understood, even though the availability of PLP within subcellular compartments (particularly the mitochondrion) may have important health implications. The aim of this review is to concisely summarize the state of current knowledge of intracellular trafficking of PLP and to identify key directions for future research.
Keywords: Pyridoxal 5′-phosphate, vitamin B6, cofactor trafficking, mitochondria, iron homeostasis, heme biosynthesis
INTRODUCTION
The discovery of the B6 cofactor (pyridoxal 5′-phosphate, PLP) more than seventy years ago [1–3] initiated decades of fundamental biochemical research that has defined the unique role of that cofactor in enzyme catalysis [4–6], including activation of amine functional groups in metabolites [7,8] and, in the case of glycogen phosphorylase, activation of inorganic phosphate for phosphorolytic cleavage of glycosidic bonds [9]. PLP-dependent enzymes are now known to be an extraordinarily diverse family performing many essential metabolic functions in amino acid biosynthesis and catabolism, 1-carbon metabolism, membrane lipid biosynthesis, production of neurotansmitters and biogenic polyamines, as well as glycogen cycling and iron metabolism (iron-sulfur cluster and heme biosynthesis) (Table 1). While only PLP (and its congener, pyridoxamine 5′-phosphate (PMP)) can directly function in catalysis, the extended B6 family includes multiple vitamer forms, differing in phosphorylation state and modification of the 4′ carbon (Figure 1).
Table 1.
Subcellular localization of pyridoxal 5′-phosphate dependent enzymes in Saccharomyces cerevisiae.
| Enzyme Name | Abbreviation | EC No. | Gene IDa | Genetic Locusa |
|---|---|---|---|---|
| 1. Cytoplasm | ||||
| Aspartate aminotransferase | AAS | 2.6.1.1 | Aat2 | YLR027C |
| Aromatic aminotransferase I | ArAT | 2.6.1.57 | Aro8 | YGL202W |
| Aromatic aminotransferase II | ArAT | 2.6.1.57 | Aro9 | YHR137W |
| 7,8-Diamino-pelargonic acid aminotransferase | DAPA | 2.6.1.62 | Bio3 | YNR058W |
| Kynurenine aminotransferase | KAT | 2.6.1.7 | Bna3 | YJL060W |
| Kynureninase | KYNU | 3.7.1.3 | Bna5 | YLR231C |
| L-ornithine transaminase | OAT | 2.6.1.13 | Car2 | YLR438W |
| Cystathionine γ-lyase | CTH | 4.4.1.1 | Cys3 | YAL012W |
| Cystathionine β-synthase | CBS | 4.2.1.22 | Cys4 | YGR155W |
| Dihydrosphingosine phosphate lyase | 4.1.2.27 | Dpl1 | YDR294C | |
| Glutamate decarboxylase | GAD | 4.1.1.15 | Gad1 | YMR250W |
| Glycogen phosphorylase | GP | 2.4.1.1 | Gph1 | YPR160W |
| Histidinol-phosphate aminotransferase | 2.6.1.9 | His5 | YIL116W | |
| Cysteine-S-conjugate β-lyase | 4.4.1.13 | Irc7 | YFR055W | |
| Serine palmitoyltransferase | 2.3.1.50 | Lcb1 | YMR296C | |
| Serine palmitoyltransferase | 2.3.1.50 | Lcb2 | YDR062W | |
| Bifunctional cysteine synthase/O-acetylhomoserine aminocarboxypropyltransferase | 2.5.1.47 | Met17 | YLR303W | |
| 3-Phosphoserine aminotransferase | PSAT | 2.6.1.52 | Ser1 | YOR184W |
| Serine hydroxymethyltransferase | SHT | 2.1.2.1 | Shm2 | YLR058C |
| Branched-chain amino acid aminotransferase | BCAT | 2.6.1.42 | Bat2 | YJR148W |
| γ-aminobutyrate (GABA) transaminase | 2.6.1.19 | Uga1 | YGR019W | |
| 2-Aminoadipate transaminase | AadAT | 2.6.1.39 | YER152C | |
| Ornithine decarboxylase | ODC | 4.1.1.17 | Spe1 | YKL184W |
| S-adenosylmethionine decarboxylase | AdoMetDC | 4.1.1.50 | Spe2 | YOL052C |
| Phosphatidylserine decarboxylase | PSD | 4.1.1.65 | Psd1 | YNL169C |
| 2. Mitochondria | ||||
| Aspartate aminotransferase | AAT | 2.6.1.1 | Aat1 | YKL106W |
| Alanine:glyoxylate aminotransferase | AGAT | 2.6.1.44 | Agx1 | YFL030W |
| Alanine transaminase | ALT | 2.6.1.2 | Alt1 | YLR089C |
| Acetylornithine aminotransferase | 2.6.1.11 | Arg8 | YOL140W | |
| Kynurenine aminotransferase | KAT | 2.6.1.7 | Bna3 | YJL060W |
| L-serine (L-threonine) deaminase (catabolic) | 4.3.1.19 | Cha1 | YCL064C | |
| Glycine decarboxylase complex | GCC | 2.1.2.10 | Gcv2 | YMR189W |
| 5-Aminolevulinate synthase | ALAS | 2.3.1.37 | Hem1 | YDR232W |
| Threonine deaminase | TD | 4.3.1.19 | Ilv1 | YER086W |
| Cysteine desulfurase | 2.8.1.7 | Nfs1 | YCL017C | |
| Serine hydroxymethyltransferase | SHT | 2.1.2.1 | Shm1 | YBR263W |
| Branched-chain amino acid aminotransferase | BCAT | 2.6.1.42 | Bat1 | YHR208W |
| 3. Peroxisome | ||||
| Alanine:glyoxylate aminotransferase | AGAT | 2.6.1.44 | Agx1 | YFL030W |
Gene ID and genetic locus from Saccharomyces cerevisiae genome data.
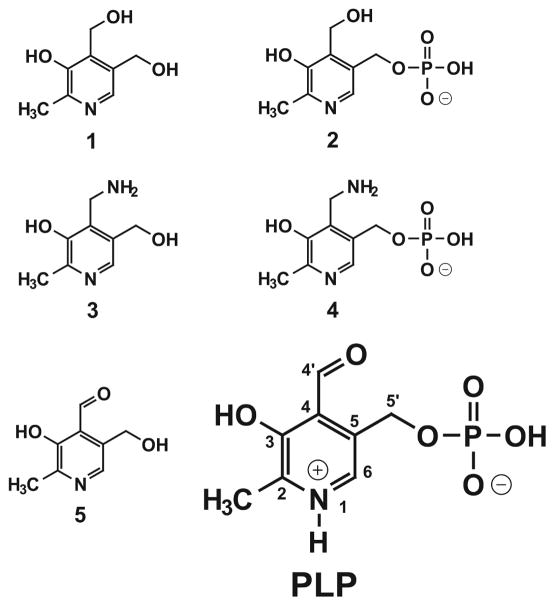
Figure 1.
Structures of B6 vitamers. PLP, pyridoxal 5′-phosphate, indicating the numbering scheme for the B6 ring system; (1) pyridoxine (PN); (2) pyridoxine 5′-phosphate (PNP); (3) pyridoxamine (PM); (4) pyridoxamine 5′-phosphate (PMP); (5) pyridoxal (PL).
The biosynthesis and metabolic interconversion of these vitamers has recently been worked out in detail, revealing two major biosynthetic pathways [10–13]. Vertebrates (including humans) lack both pathways, and thus rely on a salvage pathway to convert B6 provided by dietary sources or commensal intestinal flora into the enzyme cofactor, PLP. While not all organisms are able to synthesize the B6 cofactor, the ability to utilize environmental B6 through efficient uptake and salvage pathways appears to be universal [14]. Localization of these pathways has important implications for cellular metabolism. In particular, the exclusive localization of the salvage pathway to the cytoplasm of eukaryotic cells makes delivery of the membrane-impermeable PLP cofactor to enzymes in other subcellular compartments (particularly the mitochondrion) critically dependent on efficient mechanisms for intra-cellular cofactor trafficking. This requirement for intracellular PLP transport systems has been generally neglected and represents an important area for future research.
UPTAKE AT THE PLASMA MEMBRANE
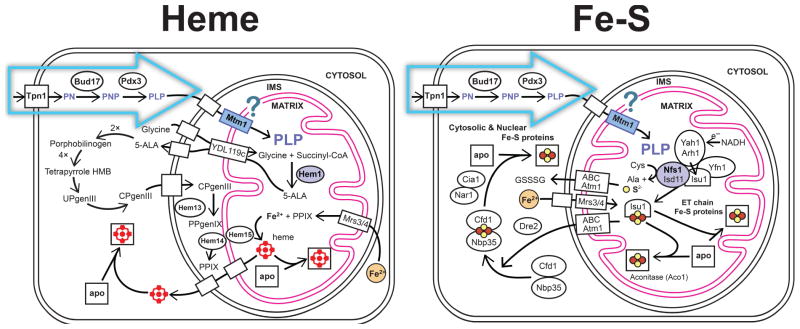
The B6 story starts at the cell surface. Utilization of environmental sources of B6 is a universal feature of life, and specific transport systems exist for cellular uptake of B6 vitamers (Figure 2). In the yeast Saccharomyces cerevisiae, Tpn1p, a member of the purine-cytosine permease subfamily in the major facilitator superfamily, has been identified as the plasma membrane pyridoxine (vitamin B6) transporter [15,16]. Transport studies have demonstrated that Tpn1p is a unique, high affinity (Km = 0.55 μM) pyridoxine carrier with broad substrate specificity for unphosphorylated B6 vitamers (including pyridoxine (PN), pyridoxamine (PM), and pyridoxal (PL)), utilizing a proton symport mechanism to concentrate B6 in the cell. Tpn1p is an integral membrane protein, predicted to be comprised of 12 transmembrane regions based on hydropathy analysis of the sequence.
Figure 2.
Pathways for B6 transport and processing in eukaryotic cells. Uptake of pyridoxine at the plasma membrane allows conversion to PLP within the cytoplasm. Subsequent trafficking of the pre-formed PLP cofactor to the mitochondrion is required for essential processes of heme biosynthesis (Left) and Fe-S cluster biogenesis (Right). Yeast (Saccharomyces cerevisiae) gene IDs are used to identify specific elements of these pathways.
Two homologous transport systems have been found in human cells [17–19], with distinct substrate specificity patterns. One system has been reported to be specific for unphosphorylated B6 vitamers (PN and PM) while the other may support uptake of the phosphorylated form (PLP) as well. Genetic identification and detailed characterization of these carriers is still lacking.
SALVAGE PATHWAYS
The capacity of a cell to utilize exogenous PN and PM entirely depends upon its ability to convert those vitamers to PLP through the salvage pathway (Figure 2)[14]. Phosphorylation of the 5′ hydroxymethyl group by the broad-specificity pyridoxine (pyridoxal, pyridoxamine) kinase (PNK, yeast gene ID Bud17p)[14,20,21] efficiently traps the vitamer within the cell, since, in contrast to the precursor (PN), the charged product PNP is membrane-impermeable. Trapping by phosphorylation is a general principle in cellular metabolism (“the importance of being charged” [22]) which also restricts the phosphorylated product to the subcellular compartment in which it is formed. Subsequent oxidation of the 4′ hydroxymethyl group of PNP by pyridoxine oxidase (PNO, Pdx3p) [23] is required to form the functional cofactor.
Release of the PLP cofactor product from PNK appears to be very slow, leading to a suggestion that the enzyme may function as a chaperone [24] and the presence of a secondary PLP binding site on the surface of PNO [23] may reflect a chaperone role for that enzyme, as well. In solution, free PLP is subject to hydrolysis of the phosphoester bond (particularly at lower pH, or in the presence of phosphatases) and other reactions, including nonspecific imine formation by reaction with amine groups in amino acids and proteins. A PNK-PLP (or PNO-PLP) chaperone complex could suppress these side reactions and facilitate delivery of the cofactor to cognate apoenzymes for activation.
Formation of PLP (by de novo biosynthesis or salvage pathways) is a uniquely cytoplasmic function in the eukaryotic cell, since the enzymes involved in those pathways have an exclusively cytoplasmic localization. This localization pattern is consistent with the observation that most, but not all, of the PLP-dependent enzymes in the cell are also localized in the cytoplasm (Table 1). However, some PLP-dependent enzymes reside in other compartments, and, since PLP is membrane-impermeable, internal PLP trafficking pathways must be present to supply these enzymes with cofactor.
INTRACELLULAR TRAFFICKING
The presence of PLP-dependent enzymes in both the mitochondrion and peroxisome (Table 1) implies that there must be a mechanism for delivering the cofactor to enzymes in those compartments. Since the PLP cofactor is membrane-impermeable, membrane transport systems must exist to supply those two organelles with the PLP cofactor. Both compartments are bounded by bilayer membranes, but there are significant differences that make the two cases distinct.
The peroxisome is bounded by a single bilayer membrane, containing membrane-spanning pores (porin-like channels) that permit diffusion of small metabolites across the membrane [25–28]. These pores appear to mediate diffusional transport of a variety of small solutes [29–31] but it is unclear whether pores or specific carriers mediate the transport of cofactors (including coenzyme A, nucleotides and NAD+)[32–34]. The organization of the mitochondrion is more complex, with two concentric bilayer membranes [35,36]. The outer mitochondrial membrane (OMM), like the peroxisomal membrane, contains pores (VDACs) that allow free diffusion of molecules up to about 5 kDa [25–28], ensuring availability of cytoplasmic PLP to proteins in the mitochondrial intermembrane space (IMS). In contrast, the inner mitochondrial membrane (IMM) forms a strict permeability barrier preventing free diffusion of any molecules other than O2 and CO2.
MITOCHONDRIAL PLP TRANSPORT
Trafficking of B6 to the mitochondrion was first investigated more than 30 years ago, using radiotracer methods to monitor uptake of PN and PLP in isolated rat liver mitochondria [37,38]. Pyridoxine was found to permeate the mitochondrial membranes by simple diffusion, indicated by non-saturating uptake kinetics and equilibration across the membrane rather than concentration against a gradient. However, this diffusion process is unlikely to be biologically important, both because of the limited availability of free pyridoxine in the cell, and because of the lack of pyridoxine-processing salvage enzymes in the mitochondrial matrix, as described above.
A different type of transport behavior was observed for PLP, which was rapidly taken up by the isolated mitochondria. PLP first accumulated in the IMS, and subsequently entered the mitochondrial matrix in a concentrative process. Mitochondrial uptake of PLP was found to be “passive”. i.e., insensitive to inhibitors and uncouplers of oxidative phosphorylation, providing evidence for carrier-mediated PLP transport uncoupled from ATP synthesis. Surprisingly, this earlier work has not been extended by further studies.
The lack of additional studies may at least partly relate to the difficulty of mitochondrial PLP uptake measurements. The earlier experiments required challenging measurements of the [14C]-PLP/3H2O ratio to demonstrate concentration of the cofactor, and fractionation experiments to define the distribution of the radionuclide in different mitochondrial compartments. In addition, PLP is a reactive aldehyde that can nonspecifically react with a variety of amines, complicating the interpretation of results.
IN ORGANELLO PLP TRANSPORT ASSAY
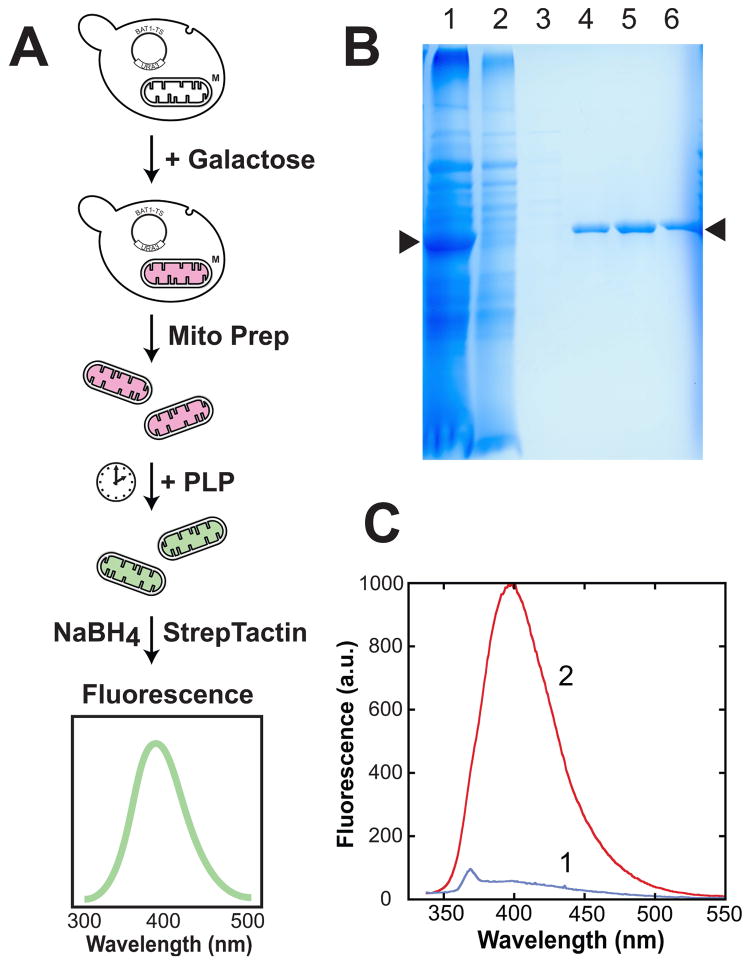
Innovative new approaches may be required to advance the field, and ultimately elucidate the mechanisms of mitochondrial PLP trafficking. In particular, molecular characterization of this essential cellular process may depend on availability of a simple and effective in organello assay for mitochondrial PLP transport, providing an alternative to the radiotracer experiments described above. The radiotracer methods used in the original studies of mitochondrial PLP transport are relatively challenging, involving complex assay protocols, and the reactivity of the PLP aldehyde towards protein lysine side chains and aminophospholipids can result in nonspecific binding, complicating analysis. It may be possible to address both issues by using a matrix-localized reporter protein to monitor membrane transport, as illustrated in Figure 3. Mitochondrial branched chain amino transferase (BCAT, Bat1p)[39,40] can be overexpressed in yeast mitochondria, which become loaded with high levels of the Bat1p apoprotein as protein import overwhelms PLP availability in that compartment (Figure 3, B&C). Mitochondria can be isolated by standard methods [41,42] and used for in organello uptake assays, fixing the localization of PLP at the endpoint by borohydride reduction of protein conjugates [43]. Affinity purification of tagged mBAT-TwinStrep (Figure 3B) then allows a direct read-out of the conjugated pyridoxine content by combined fluorescence and absorption measurements (Figure 3C). The results shown in Figure 3C indicate that overexpression of mBAT-TwinStrep protein in yeast mitochondria leads to accumulation of nearly pure apoprotein.
Figure 3.
Loading yeast mitochondria with recombinant Bat1p for in organello assay of PLP transport. (A) Assay scheme: yeast containing an expression vector encoding the mitochondrial branched chain amino transferase (Bat1) fused to a C-terminal TwinStrep affinity tag (pYES2mBAT-TwinStrep) are induced overnight (2% galactose, 2% lactate, 0.2% raffinose) and mitochondria isolated by subcellular fractionation (homogenization, differential centrifugation). Isolated mitochondria are suspended in isosmotic buffer and treated with PLP with rotisserie agitation. A timecourse may be generated by fixing the PLP bound to protein using borohydride reduction at the endpoint. StrepTactin affinity purification of the mBAT-StrepTag protein followed by fluorescence analysis permits quantitation of cofactor content of the protein. (B) Purification of recombinant mBAT-TwinStrep protein from isolated yeast mitochondria. (1) intact isolated mitochondria, showing high level expression of recombinant mBAT-TwinStrep protein; (2) StrepTactin flow-through; (3) StrepTactin wash; (4–6) StrepTactin elution with desthiobiotin. Arrows (▶) identify the location of the recombinant mBAT-TwinStrep protein. (C) Fluorescence spectra for affinity-purified mBAT-TwinStrep isolated from yeast mitochondria before (1) or after (2) incubation with PLP followed by reductive trapping with borohydride in the presence of 1 mM PMSF. Both samples 35 μg/mL protein in 100 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA.
PLP-OME PROTEOMICS ANALYSIS
The identity of the molecular targets for PLP inside the mitochondrion is another interesting question with important biomedical implications. PLP transported into the mitochondrial matrix appears to be completely bound to protein, with no detectable free cofactor [38]. Since the distribution of the cofactor among protein complexes determines its metabolic effects, defining the PLP-linked proteome, the ‘PLP-ome’, may lead to deeper insight into the essential roles of the PLP cofactor in health and disease. The aim of a complete PLP-ome proteomics analysis would to determine the distribution of PLP over the protein population, identifying binding sites and evaluating the degree of cofactor loading. Although the PLP cofactor is labile, PLP-protein adducts can be conveniently stabilized by borohydride reduction, converting the PLP imine (Schiff base) linkage to a hydrolytically stable amine [43]. The covalently-conjugated cofactor in the product remains attached through typical procedures for protein separation and analysis, allowing detailed characterization of the complex. A relatively simple solution to PLP-ome analysis that has already been described involves Western blot detection of PLP-protein conjugates following borohydride reduction using anti-pyridoxine (conjugated) antibodies, revealing the pattern of labeling over the entire protein population [43]. However, this method is fairly insensitive, does not provide a direct identification of the protein partner, and does not lend itself to a quantitative treatment of the data. Advanced methods of multiplexed mass spectrometry building on recent advances in phosphoproteomics analysis [44–48] are likely to be much more effective, providing information on cofactor loading and potentially defining the PLP binding site(s) within the protein.
Direct enzymatic analysis can also provide information on PLP binding reflected in the increase in catalytic activity following addition of the cofactor. This approach has been used to evaluate the degree of PLP loading for the mitochondrial matrix enzyme 5-aminolevulinate synthase (ALAS), which catalyzes a key step in heme biosynthesis (Figure 2), indicating that at least in some cases PLP availability limits the activity of mitochondrial enzymes [43]. Since the PLP cofactor can dissociate from protein complexes and redistribute on the timescale of enzyme assays, potential sources of the cofactor in the assay mixture need to be carefully controlled.
CARRIER ANALYSIS: IN VITRO ASSAY OF PLP TRANSPORT
The evidence for a specific transport pathway for mitochondrial uptake of PLP implies existence of a carrier that may be isolated for detailed molecular characterization. Over the past decades, intensive research has elucidated the mechanisms of mitochondrial transport, and the important role of mitochondrial carrier proteins (MCPs) in these processes [49–53]. The yeast MCP superfamily includes 35 members, including many whose functions have been assigned by genetic analysis or direct assays. A PLP carrier function has recently been proposed for a yeast MCP (Mtm1p) based on the knock-out phenotype and direct analysis of cofactor binding [43,54]. The purified recombinant protein tightly binds PLP (KD = 0.6 μM (fluorescence titration); KD,1 = 2 μM, KD,2 = 47 μM (isothermal titration calorimetry, ITC)) while no binding of Mn+2, methionine, cysteine or glutathione could be detected by ITC (unpublished results).
Direct evidence for a PLP transport function for Mtm1p (or another carrier) will require an effective in vitro transport assay. As described above, the design of an effective PLP transport assay is complicated by the reactivity of the 4′ aldehyde, since transport across the membrane needs to be distinguished from nonspecific attachment of the cofactor to proteins or aminophospholipids on the outer surface of a membrane system, a problem that is not usually encountered in membrane carrier in vitro assays.
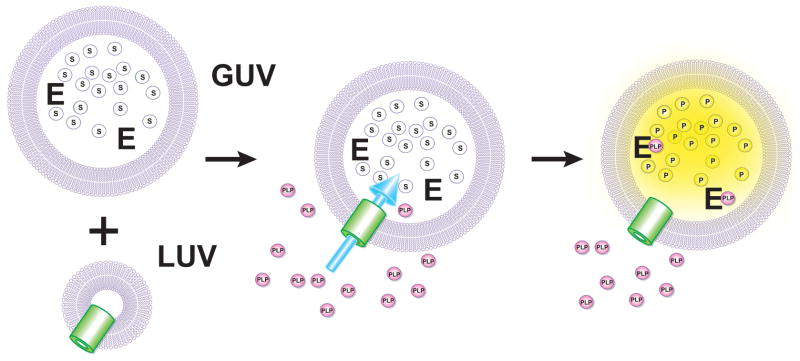
An approach to PLP membrane transport measurements based on catalytic amplification of the permeation signal through activation of an encapsulated PLP-dependent apoenzyme is shown in Figure 4. This hypothetical scheme utilizes a coupled assay to detect PLP in the lumen of a membrane-bounded vesicle and to monitor the progress of cofactor transport, using established membrane reconstitution and encapsulation methods [55–58]. While in principle, any PLP-dependent enzyme would be suitable, practical implementation of this type of assay will depend on both availability and stability of the apoenzyme, and availability of a suitable detection substrate. Escherichia coli tryptophanase (TnaAp, [EC 4.1.99.1])[59] appears to be a likely prospect for this application, since it can be prepared in large quantities as a 6×His tagged recombinant protein, can be rigorously converted to pure apoenzyme by dialysis against 1 M amino acid solution (e.g., D,L-alanine), and is highly stable as the apoprotein. A chromogenic substrate for TnaAp has been reported [60], as well as a fluorogenic substrate [61] which would greatly enhance the sensitivity of PLP detection.
Figure 4.
Hypothetical in vitro assay for PLP transport based on activation of an encapsulated apoenzyme. (Top) Purified carrier in proteoliposome is combined with giant unilamellar vesicles (GUV) containing encapsulated recombinant E. coli TnaAp (E) and a fluorogenic substrate (S). (B) Fusion of the proteoliposomes with the GUVs. (C) Transport of exogenous PLP leads to activation of TnaAp allowing fluorometric monitoring of PLP uptake.
PLP TRAFFICKING DEFECTS AND METABOLIC DISEASE
A number of important mitochondrial pathways, including those involved in amino acid biosynthesis, energy metabolism and metal homeostasis, require PLP for at least one step (Table 1, Figure 2). While there is some redundancy in pathways for amino acid processing between the cytoplasm and mitochondrial matrix (e.g., distinct cytoplasmic and mitochondrial isozymes for amino acid biosynthesis (Aat1p,Aat2p), catabolism (Bat1p,Bat2p), and C-1 metabolism (Shm1p, Shm2p)), the mitochondrial requirement for matrix PLP to support heme biosynthesis and Fe-S cluster biogenesis appears to be absolute, and may represent a critical feature linking PLP trafficking to health and disease. The connection between PLP availability and health is well-known [62–64], but the focus has generally been on dietary availability of the cofactor and cytoplasmic metabolism. Intracellular trafficking of PLP is clearly an essential function of the eukaryotic cell, one which has largely neglected, in spite of its fundamental biological importance. There is strong evidence linking defects in mitochondrial metabolite trafficking to metabolic dysfunction and human disease [65–67] indicating the importance of this area for future advances in cardiovascular health and understanding of neurodegenerative disease.
CONCLUSION
Enzymes that require the PLP cofactor are generally localized to the cytoplasm in eukaryotic cells, where conversion of the pyridoxine precursor occurs through the action of pyridoxine kinase and pyridoxine 5′ phosphate oxidase enzymes. The unique localization of the latter two enzymes to the cytoplasm means that any PLP-dependent enzymes residing in other compartments (including the mitochondrion) must acquire the cofactor through a mechanism involving transport of fully formed PLP, rendered impermeable to biological membranes by its charge and hydrophilicity. The clear link between mitochondrial PLP transport and essential pathways of metabolism, including heme biosynthesis and Fe-S cluster biogenesis (Figure 2), demonstrates the importance of this process for cell function. These crucial metabolic links may be revealed in hereditary disorders of PLP trafficking, or provide novel targets for drug development.
RESEARCH HIGHLIGHTS.
Intracellular trafficking of the PLP cofactor is an essential biological function
Mitochondrial trafficking of PLP is required for heme and Fe-S cluster biosynthesis
Transport systems for intracellular trafficking of PLP are largely undefined
New approaches are required to investigate PLP trafficking mechanisms
Abbreviations
- MCP
mitochondrial carrier protein
- PLP
pyridoxal 5′-phosphate
- PN
pyridoxine
- PL
pyridoxal
- PM
pyridoxamine
- PMP
pyridoxamine 5′-phosphate
- ALAS
5-aminolevulinate synthase
- ITC
isothermal titration calorimetry
Footnotes
FUNDING
This work was partly supported by NIH grant R01 GM42680 to J.W.W.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ohdake S. Isolation of ‘Oryzanin’ (Antineuritic Vitamin) from Rice-polishings. Bull Agri Chem Soc Japan. 1932;8:11–46. [Google Scholar]
- 2.György P. Crystalline vitamin B6. J Am Chem Soc. 1938;60:983–984. [Google Scholar]
- 3.Rosenberg IH. A history of the isolation and identification of vitamin B(6) Ann Nutr Metab. 2012;61:236–238. doi: 10.1159/000343113. [DOI] [PubMed] [Google Scholar]
- 4.Mooney S, Leuendorf JE, Hendrickson C, Hellmann H. Vitamin B6: A Long Known Compound of Surprising Complexity. Molecules. 2009;14:329–351. doi: 10.3390/molecules14010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toney MD. Pyridoxal phosphate enzymology. Biochim Biophys Acta. 2011;1814:1405–1406. doi: 10.1016/j.bbapap.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Hellmann H, Mooney S. Vitamin B6: A Molecule for Human Health? Molecules. 2010;15:442–459. doi: 10.3390/molecules15010442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toney MD. Reaction specificity in pyridoxal enzymes. Arch Bioch Biophys. 2005;433:279–287. doi: 10.1016/j.abb.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 8.Percudani R, Peracchi A. A genomic overview of pyridoxal-phosphate-dependent enzymes. EMBO Rep. 2003;4:850–854. doi: 10.1038/sj.embor.embor914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helmreich EJ. How pyridoxal 5′-phosphate could function in glycogen phosphorylase catalysis. Biofactors. 1992;3:159–172. [PubMed] [Google Scholar]
- 10.Drewke C, Leistner E. Biosynthesis of vitamin B6 and structurally related derivatives. Vitam Horm. 2001;61:121–155. doi: 10.1016/s0083-6729(01)61004-5. [DOI] [PubMed] [Google Scholar]
- 11.Mittenhuber G. Phylogenetic analyses and comparative genomics of vitamin B6 (pyridoxine) and pyridoxal phosphate biosynthesis pathways. J Mol Microbiol Biotechnol. 2001;3:1–20. [PubMed] [Google Scholar]
- 12.Burns KE, Xiang Y, Kinsland CL, McLafferty FW, Begley TP. Reconstitution and biochemical characterization of a new pyridoxal-5′-phosphate biosynthetic pathway. J Am Chem Soc. 2005;127:3682–3683. doi: 10.1021/ja042792t. [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee T, Hanes J, Tews I, Ealick SE, Begley TP. Pyridoxal phosphate: biosynthesis and catabolism. Biochim Biophys Acta. 2011;1814:1585–1596. doi: 10.1016/j.bbapap.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 14.di Salvo ML, Contestabile R, Safo MK. Vitamin B(6) salvage enzymes: mechanism, structure and regulation. Biochim Biophys Acta. 2011;1814:1597–1608. doi: 10.1016/j.bbapap.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 15.Shane B, Snell EE. Transport and metabolism of vitamin B6 in the yeast Saccharomyces carlsbergensis 4228. J Biol Chem. 1976;251:1042–1051. [PubMed] [Google Scholar]
- 16.Stolz J, Vielreicher M. Tpn1p, the plasma membrane vitamin B6 transporter of Saccharomyces cerevisiae. J Biol Chem. 2003;278:18990–18996. doi: 10.1074/jbc.M300949200. [DOI] [PubMed] [Google Scholar]
- 17.Said HM, Ortiz A, Ma TY. A carrier-mediated mechanism for pyridoxine uptake by human intestinal epithelial Caco-2 cells: regulation by a PKA-mediated pathway. Am J Physiol Cell Physiol. 2003;285:C1219–C1225. doi: 10.1152/ajpcell.00204.2003. [DOI] [PubMed] [Google Scholar]
- 18.Said ZM, Subramanian VS, Vaziri ND, ad Said HM. Pyridoxine uptake by colonocytes: a specific and regulated carrier-mediated process. Am J Physiol Cell Physiol. 2008;294:C1192– C1197. doi: 10.1152/ajpcell.00015.2008. [DOI] [PubMed] [Google Scholar]
- 19.Schenker S, Johnson RF, Mahuren JD, Henderson GI, Coburn SP. Human placental vitamin B6 (pyridoxal) transport: normal characteristics and effects of ethanol. Am J Physiol. 1992;262:R966–R974. doi: 10.1152/ajpregu.1992.262.6.R966. [DOI] [PubMed] [Google Scholar]
- 20.Safo MK, Musayev FN, di Salvo ML, Hunt S, Claude JB, Schirch V. Crystal structure of pyridoxal kinase from the Escherichia coli pdxK gene: implications for the classification of pyridoxal kinases. J Bacteriol. 2006;188:4542–4552. doi: 10.1128/JB.00122-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghatge MS, Contestabile R, di Salvo ML, Desai JV, Gandhi AK, Camara CM, Florio R, González IN, Parroni A, Schirch V, Safo MK. Pyridoxal 5′-phosphate is a slow tight binding inhibitor of E coli pyridoxal kinase. PLoS One. 2012;7:e41680. doi: 10.1371/journal.pone.0041680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westheimer FH. Why nature chose phosphates. Science. 1987;235:1173–1178. doi: 10.1126/science.2434996. [DOI] [PubMed] [Google Scholar]
- 23.Loubbardi A, Marcireau C, Karst F, Guilloton M. Sterol uptake induced by an impairment of pyridoxal phosphate synthesis in Saccharomyces cerevisiae: cloning and sequencing of the PDX3 gene encoding pyridoxine (pyridoxamine) phosphate oxidase. J Bacteriol. 1995;177:1817–1823. doi: 10.1128/jb.177.7.1817-1823.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.di Salvo ML, Safo MK, Musayev FN, Bossa F, Schirch V. Structure and mechanism of Escherichia coli pyridoxine 5′-phosphate oxidase. Biochim Biophys Acta. 2003;1647:76–82. doi: 10.1016/s1570-9639(03)00060-8. [DOI] [PubMed] [Google Scholar]
- 25.Benz R. Permeation of hydrophilic solutes through mitochondrial outer membranes: review on mitochondrial porins. Biochim Biophys Acta. 1994;1197:167–196. doi: 10.1016/0304-4157(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 26.Blachly-Dyson E, Forte M. VDAC Channels. IUBMB Life. 2001;52:113–118. doi: 10.1080/15216540152845902. [DOI] [PubMed] [Google Scholar]
- 27.Rostovtseva TK. VDAC structure, function, and regulation of Mitochondrial and Cellular Metabolism. Biochim Biophys Acta. 2012;1818:1437. doi: 10.1016/j.bbamem.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 28.Colombini M. VDAC structure, selectivity, and dynamics. Biochim Biophys Acta. 2012;1818:1457–1465. doi: 10.1016/j.bbamem.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakuraba H, Noguchi T. The permeation of pyridoxal 5′-phosphate in chicken liver peroxisomes. Arch Biochem Biophys. 1994;309:396–398. doi: 10.1006/abbi.1994.1130. [DOI] [PubMed] [Google Scholar]
- 30.Van Veldhoven P, Debeer LJ, Mannaerts GP. Water- and solute-accessible spaces of purified peroxisomes. Evidence that peroxisomes are permeable to NAD+ Biochem J. 1983;210:685–693. doi: 10.1042/bj2100685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Veldhoven PP, Just WW, Mannaerts GP. Permeability of the peroxisomal membrane to cofactors of beta-oxidation. Evidence for the presence of a pore-forming protein. J Biol Chem. 1987;262:4310–4318. [PubMed] [Google Scholar]
- 32.Antonenkov VD, Sormunen RT, Hiltunen JK. The rat liver peroxisomal membrane forms a permeability barrier for cofactors but not for small metabolites in vitro. J Cell Sci. 2004;117:5633–5642. doi: 10.1242/jcs.01485. [DOI] [PubMed] [Google Scholar]
- 33.Visser WF, van Roermund CW, Ijlst L, Waterham HR, Wanders RJ. Metabolite transport across the peroxisomal membrane. Biochem J. 2007;401:365–375. doi: 10.1042/BJ20061352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antonenkov VD, Hiltunen JK. Transfer of metabolites across the peroxisomal membrane. Biochim Biophys Acta. 2012;1822:1374–1386. doi: 10.1016/j.bbadis.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Schatz G. The Magic Garden. Ann Rev Biochem. 2007;76:673–678. doi: 10.1146/annurev.biochem.76.060806.091141. [DOI] [PubMed] [Google Scholar]
- 36.Okamoto K, Shaw JM. Mitochondrial Morphology and Dynamics in Yeast and Multicellular Eukaryotes. Ann Rev Genetics. 2005;39:503–536. doi: 10.1146/annurev.genet.38.072902.093019. [DOI] [PubMed] [Google Scholar]
- 37.Lui A, Lumeng L, Li TK. Metabolism of vitamin B6 in rat liver mitochondria. J Biol Chem. 1981;256:6041–6046. [PubMed] [Google Scholar]
- 38.Lui A, Lumeng L, Li TK. Transport of pyridoxine and pyridoxal 5′-phosphate in isolated rat liver mitochondria. J Biol Chem. 1982;257:14903–14906. [PubMed] [Google Scholar]
- 39.Kispal G, Steiner H, Court DA, Rolinski B, Lill R. Mitochondrial and cytosolic branched-chain amino acid transaminases from yeast, homologs of the myc oncogene-regulated Eca39 protein. J Biol Chem. 1996;271:24458–24464. doi: 10.1074/jbc.271.40.24458. [DOI] [PubMed] [Google Scholar]
- 40.Prohl C, Kispal G, Lill R. Branched-chain-amino-acid transaminases of yeast Saccharomyces cerevisiae. Methods Enzymol. 2000;324:365–375. doi: 10.1016/s0076-6879(00)24246-8. [DOI] [PubMed] [Google Scholar]
- 41.Meisinger C, Pfanner N, Truscott KN. Isolation of yeast mitochondria. Methods Mol Biol. 2006;313:33–39. doi: 10.1385/1-59259-958-3:033. [DOI] [PubMed] [Google Scholar]
- 42.Boldogh IR, Pon LA. Purification and subfractionation of mitochondria from the yeast Saccharomyces cerevisiae. Methods Cell Biol. 2007;80:45–64. doi: 10.1016/S0091-679X(06)80002-6. [DOI] [PubMed] [Google Scholar]
- 43.Whittaker MM, Penmatsa A, Whittaker JW. The Mtm1p carrier and pyridoxal 5′-phosphate cofactor trafficking in yeast mitochondria. Arch Biochem Biophys. 2015;568:64–70. doi: 10.1016/j.abb.2015.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai CF, Hsu CC, Hung JN, Wang YT, Choong WK, Zeng MY, Lin PY, Hong RW, Sung TY, Chen YJ. Sequential Phosphoproteomic Enrichment through Complementary Metal-Directed Immobilized Metal Ion Affinity Chromatography. Anal Chem. 2014;86:685–693. doi: 10.1021/ac4031175. [DOI] [PubMed] [Google Scholar]
- 45.Ye J, Zhang X, Young C, Zhao X, Hao Q, Cheng L, Jensen ON. Optimized IMAC-IMAC protocol for phosphopeptide recovery from complex biological samples. J Proteome Res. 2010;9:3561–3573. doi: 10.1021/pr100075x. [DOI] [PubMed] [Google Scholar]
- 46.Fíla J, Honys D. Enrichment techniques employed in phosphoproteomics. Amino Acids. 2012;43:1025–1047. doi: 10.1007/s00726-011-1111-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu R, Haas W, Dephoure N, Huttlin EL, Zhai B, Sowa ME, Gygi SP. A large-scale method to measure absolute protein phosphorylation stoichiometries. Nat Methods. 2011;8:677–683. doi: 10.1038/nmeth.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, Gnad F, Cox J, Jensen TS, Nigg EA. Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal. 2010;3:ra3. doi: 10.1126/scisignal.2000475. [DOI] [PubMed] [Google Scholar]
- 49.Wohlrab H. Transport proteins (carriers) of mitochondria. IUBMB Life. 2009;61:40–46. doi: 10.1002/iub.139. [DOI] [PubMed] [Google Scholar]
- 50.Palmieri F, Pierri CL. Mitochondrial metabolite transport. Essays Biochem. 2010;47:37–52. doi: 10.1042/bse0470037. [DOI] [PubMed] [Google Scholar]
- 51.Arco AD, Satrústegui J. New mitochondrial carriers: an overview. Cell Mol Life Sci. 2005;62:2204–2227. doi: 10.1007/s00018-005-5197-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmieri L, Lasorsa FM, Vozza A, Agrimi G, Fiermonte G, Runswick MJ, Walker JE, Palmieri F. Identification and functions of new transporters in yeast mitochondria. Biochim Biophys Acta. 2000;1459:363–369. doi: 10.1016/s0005-2728(00)00173-0. [DOI] [PubMed] [Google Scholar]
- 53.Palmieri F, Agrimi G, Blanco E, Castegna A, Di Noia MA, Iacobazzi V, Lasorsa FM, Marobbio CM, Palmieri L, Scarcia P, Todisco S, Vozza A, Walker J. Identification of mitochondrial carriers in Saccharomyces cerevisiae by transport assay of reconstituted recombinant proteins. Biochim Biophys Acta. 2006;1757:1249–1262. doi: 10.1016/j.bbabio.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 54.Whittaker MM, Whittaker JW. Expression and Purification of Recombinant Saccharomyces cerevisiae Mitochondrial Carrier Protein YGR257Cp (Mtm1p) Prot Exp Purif. 2014;93:77–86. doi: 10.1016/j.pep.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Varnier A, Kermarrec F, Blesneac I, Moreau C, Liguori L, Lenormand JL, Picollet-D’hahan N. A simple method for the reconstitution of membrane proteins into giant unilamellar vesicles. J Membr Biol. 2010;233:85–92. doi: 10.1007/s00232-010-9227-8. [DOI] [PubMed] [Google Scholar]
- 56.Walde P, Cosentino K, Engel H, Stano P. Giant vesicles: preparations and applications. Chembiochem. 2010;11:848–865. doi: 10.1002/cbic.201000010. [DOI] [PubMed] [Google Scholar]
- 57.Xu X, Costa A, Burgess DJ. Protein encapsulation in unilamellar liposomes: high encapsulation efficiency and a novel technique to assess lipid-protein interaction. Pharm Res. 2012;29:1919–1931. doi: 10.1007/s11095-012-0720-x. [DOI] [PubMed] [Google Scholar]
- 58.Walde P, Ichikawa S. Enzymes inside lipid vesicles: preparation, reactivity and applications. Biomol Eng. 2001;18:143–177. doi: 10.1016/s1389-0344(01)00088-0. [DOI] [PubMed] [Google Scholar]
- 59.Suelter CH, Wang J, Snell EE. Direct spectrophotometric assay of tryptophanase. FEBS Lett. 1976;66:230–232. doi: 10.1016/0014-5793(76)80510-8. [DOI] [PubMed] [Google Scholar]
- 60.Suelter CH, Snell EE. Assay of pyridoxal phosphate and pyridoxamine phosphate, employing S-o-nitrophenyl-L-cysteine, a chromogenic substrate of tryptophanase. Methods Enzymol. 1979;62:561–568. doi: 10.1016/0076-6879(79)62262-0. [DOI] [PubMed] [Google Scholar]
- 61.Linn CP, Mize PD, Hoke RA, Quante JM, Pitner JB. Synthesis of serine-AMC-carbamate: a fluorogenic tryptophanase substrate. Anal Biochem. 1992;200:400–404. doi: 10.1016/0003-2697(92)90486-q. [DOI] [PubMed] [Google Scholar]
- 62.di Salvo ML, Safo MK, Contestabile R. Biomedical aspects of pyridoxal 5′-phosphate availability. Front Biosci. 2012;4:897–913. doi: 10.2741/E428. [DOI] [PubMed] [Google Scholar]
- 63.Ansari R, Mahta A, Mallack E, Luo JJ. Hyperhomocysteinemia and neurologic disorders: a review. J Clin Neurol. 2014;10:281–288. doi: 10.3988/jcn.2014.10.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhao YJ, Zhang WH, Xu CQ, Li HZ, Wang LN, Li H, Sun YH, Lin Y, Han LP, Zhang L, Tian Y, Wang R, Yang BF, Li WM. Involvement of the ornithine decarboxylase/polyamine system in precondition-induced cardioprotection through an interaction with PKC in rat hearts. Mol Cell Biochem. 2009;332:135–144. doi: 10.1007/s11010-009-0183-5. [DOI] [PubMed] [Google Scholar]
- 65.Palmieri F. Diseases caused by defects of mitochondrial carriers: a review. Biochim Biophys Acta. 2008;1777:564–578. doi: 10.1016/j.bbabio.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 66.Pieczenik SR, Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol. 2007;83:84–92. doi: 10.1016/j.yexmp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 67.Schapira AH. Mitochondrial disease. Lancet. 2006;368:70–82. doi: 10.1016/S0140-6736(06)68970-8. [DOI] [PubMed] [Google Scholar]