Abstract
Objective
To evaluate the prognostic performance of a new biomarker, plasma bioactive adrenomedullin (bio-ADM), for short-term clinical outcomes in acute heart failure.
Methods
A multicenter prospective cohort study of adult emergency department (ED) patients suspected of having acute heart failure was conducted to evaluate the association between plasma bio-ADM concentration and clinical outcomes. The primary outcome was a composite of the following within 30 days: death, cardiac arrest with resuscitation, respiratory failure, emergency dialysis, acute coronary syndrome, hospitalization >5 days, and repeat ED visit or hospitalization. Prognostic accuracy was evaluated with a non-parametric receiver operating characteristic (ROC) curve. Additionally, a multivariable logistic regression model was constructed to assess the additive prognostic performance of bio-ADM while adjusting for other biomarkers routinely used clinically, including BNP, cardiac troponin I, creatinine, and sodium concentration.
Results
Two hundred forty-six patients were enrolled, including 85 (34.6%) patients with the primary outcome. Plasma bio-ADM concentrations were higher among patients who experienced the primary outcome (median: 80.5 pg/ml; IQR: 53.7 pg/ml, 151.5 pg/ml) compared to those who did not (median: 54.4 pg/ml; IQR: 43.4 pg/ml, 78.4 pg/ml) (p < 0.01). Area under the ROC curve was 0.70 (95% CI: 0.63, 0.75). After adjusting for the other biomarkers, plasma bio-ADM remained a strong predictor of the primary outcome (adjusted odds ratio per IQR change: 2.68; 95% CI: 1.60, 4.51).
Conclusions
Bio-ADM concentrations at the time of ED evaluation for acute heart failure were predictive of clinically-important 30-day outcomes, suggesting bio-ADM is a promising prognostic marker for further study.
1. INTRODUCTION
Acute heart failure (AHF) is one of the most common illnesses treated in emergency departments (EDs) in the United States (US).1 After establishing an AHF diagnosis and initiating treatment, a major clinical decision facing emergency physicians is whether to pursue hospitalization for in-patient management.2 Approximately 80% of patients diagnosed with AHF in US EDs are hospitalized.1 Hospitalization allows for close observation and titration of intravenous medications, but is also associated with substantially higher cost than outpatient management and places patients at risk for nosocomial complications, such as infections, delirium, and falls.2-5 Ideally, hospitalization would be reserved for patients at high risk for short-term severe complications of AHF, such as respiratory and renal failure, and those needing specific in-patient therapies, such as intravenous vasoactive medications.2-3 However, ensuring clinical stability and estimating the risk of severe short-term outcomes is problematic with existing prognostic tools. This prognostic uncertainty plays a significant role in the high admission rate for AHF patients.1-3. 6
A prognostic biomarker that accurately identified AHF patients at high risk for short-term complications would be invaluable for guiding management decisions, particularly decisions regarding hospitalization. Natriuretic peptides, such as B-type natriuretic peptide (BNP), are widely used as diagnostic biomarkers for AHF, but their prognostic performance is suboptimal.7,8 While patients with an elevated cardiac troponin, renal dysfunction, and hyponatremia are at increased risk for severe outcomes, a subset of patients without these risk factors also experiences adverse events, suggesting additional biomarkers are needed.9-12
Adrenomedullin is a vasodilatory peptide that is elevated in patients with chronic heart failure and may also acutely rise in AHF.13-15 Elevated levels of mid-region pro-adrenomedullin (MR-proADM), a stable fragment derived from the same precursor peptide as adrenomedullin, have previously been shown to predict 90-day mortality among patients presenting with acute shortness of breath, including those with AHF.8,16 MR-proADM measurement does not distinguish between biologically-active amidated adrenomedullin and the non-functional adrenomedullin variant containing a glycine-extended C-terminal residue. Recently, a sandwich immunoassay has been developed to specifically measure the biologically-active form of adrenomedullin—bio-active adrenomedullin (bio-ADM)—making it feasible to use it as a biomarker in clinical medicine.17-19 Due to the need for better prognostic markers in AHF and a biologically-plausible association between bio-ADM and AHF severity, we evaluated the prognostic accuracy of plasma bio-ADM for predicting severe, short-term clinical outcomes in patients evaluated in the ED for AHF.
2. METHODS
2.1. Study Design
We conducted a multicenter prospective cohort study to evaluate plasma bio-ADM as a prognostic biomarker in adults presenting to the ED with signs and symptoms consistent with AHF at two university-affiliated tertiary care EDs and two community EDs in the US. We studied single measurements of bio-ADM in plasma collected at the time of initial ED presentation. This study utilized a subset of patients recruited for the STRATIFY and DECIDE studies.20 Methodological details of patient recruitment and enrollment have been reported previously.20 The local Institutional Review Boards of the participating sites approved the study. All enrolled patients provided written informed consent for participation.
2.2. Study Population
We randomly selected 250 patients enrolled in the STRATIFY/DECIDE cohort between July 20, 2007 and February 4, 2011.20 Enrolled patients presented to the ED with acute cardiopulmonary symptoms, met the Modified Framingham Criteria for AHF,20 and were clinically suspected of having AHF. To ascertain if AHF was truly the cause of each patient’s acute symptoms, a panel of three cardiologists reviewed the medical record of each enrolled patient’s ED visit and subsequent hospitalization. Each cardiologist classified the primary cause of symptoms as AHF or not AHF. Two cardiologists initially reviewed each case. If classification by the initial two cardiologists were discordant, a third cardiologist reviewed the case, with the final diagnosis based on majority. Inter-rater agreement between the initial two reviewers was calculated with Cohen’s Kappa. The prognostic accuracy of bio-ADM was evaluated both in the full study population (Suspected AHF Population) and separately in the subset of patients who had AHF confirmed based on the cardiologists’ review (Confirmed AHF Population).
2.3. Biomarker Measurement
Trained research personnel collected and banked plasma from patients in the ED. Samples were frozen within two hours and stored at −80°C. Plasma bio-ADM concentrations were measured by investigators blinded to clinical data at Sphingotec GmbH (Hennigsdorf, Germany). The bio-ADM assay has been previously described.19 In brief, it is a one-step sandwich chemiluminescence immunoassay, based on Acridinium NHS-ester labeling for the detection of human ADM in unprocessed, neat plasma. It employs two mouse monoclonal antibodies, one directed against the midregion (solid phase), and the other directed against the amidated C-terminal moiety of ADM (labeled antibody). The assay employs 50 μL of plasma samples/calibrators and 200 μL of labeled detection antibody. The analytical assay sensitivity is 2 pg/ml. In prior work,19 the median bio-ADM concentration of 200 healthy adults was 20.7 pg/mL; the 99th percentile was 43.0 pg/mL.
Patients also had additional, standard-of-care biomarkers measured while in the ED, including BNP, cardiac troponin I, creatinine, and sodium concentration.
2.4. Outcomes
Research personnel ascertained outcomes 30 days (+/− 2 days) after the index ED visit via phone interviews and medical record review. Two categories were evaluated: severe clinical outcomes and healthcare utilization outcomes. A severe clinical outcome was defined as the occurrence of ≥ 1 of the following events: death; cardiac arrest with return of spontaneous circulation; respiratory failure with intubation; emergency dialysis; and acute coronary syndrome. 21 Healthcare utilization outcomes included: hospital length of stay (LOS) greater than 5 days, return ED visit for AHF within 30 days, and repeat hospitalization for AHF within 30 days. Five days was chosen to denote prolonged LOS because this represents the median hospital LOS for AHF in the US.1
The primary study outcome was a composite of all severe clinical and healthcare utilization outcomes. In secondary analyses, we separately evaluated severe clinical outcomes without the healthcare utilization outcomes.
2.5. Data Analysis
2.5.1. Unadjusted Analyses
In the primary analysis, we evaluated the prognostic accuracy of plasma bio-ADM concentration in the Suspected AHF Population (all enrolled patients) for a composite of all 30-day outcomes. Bio-ADM concentrations in patients who experienced ≥ 1 outcome were compared to those who did not experience any outcomes with the Wilcoxon Rank Sum Test. A non-parametric receiver operating characteristic (ROC) curve was constructed to display the performance of bio-ADM to discriminate between patients who did and did not experience a 30-day outcome. We also used the 25th, 50th, and 75th percentile of bio-ADM concentration in the study population as cut-points, and calculated the proportion of patients with bio-ADM levels above and below these cut-points who experienced the primary outcome.
Secondary analyses were also performed after limiting study outcomes to the severe clinical outcomes and limiting the study population to the Confirmed AHF Population. Therefore, three secondary analyses were conducted: 1) severe clinical outcomes in the Suspected AHF Population; 2) all 30-day outcomes in the Confirmed AHF Population; and 3) severe clinical outcomes in the Confirmed AHF Population.
2.5.2. Multivariable Biomarker Model
A multivariable logistic regression model was constructed to assess the additive prognostic performance of bio-ADM while adjusting for other biomarker results, including BNP, cardiac troponin I, creatinine, and sodium concentration. Data for bio-ADM, BNP, cardiac troponin I and creatinine were logarithmically transformed (log-10) due to highly skewed distributions. The study population for this model included Suspected AHF patients who had non-missing data for all five biomarkers. The dependent variable was a composite of all 30-day outcomes. Independent variables included: bio-ADM log-10 transformed, BNP log-10 transformed, cardiac troponin I log-10 transformed, creatinine log-10 transformed, and serum sodium concentration. To allow for comparisons of odds ratios (ORs) among the biomarkers, we reported ORs associated with one interquartile range (IQR) change in each biomarker level. To quantify the relative strength of association for each of the biomarkers with the outcome, we removed one biomarker from the full regression model at a time and calculated the added value of each biomarker based on the likelihood ratio chi-square test for nested models. A higher likelihood ratio chi-square statistic for a particular biomarker indicated greater contribution to the overall model.
Statistical analyses were conducted with STATA 12.0 (College Station, TX).
3. RESULTS
3.1. Patient Characteristics
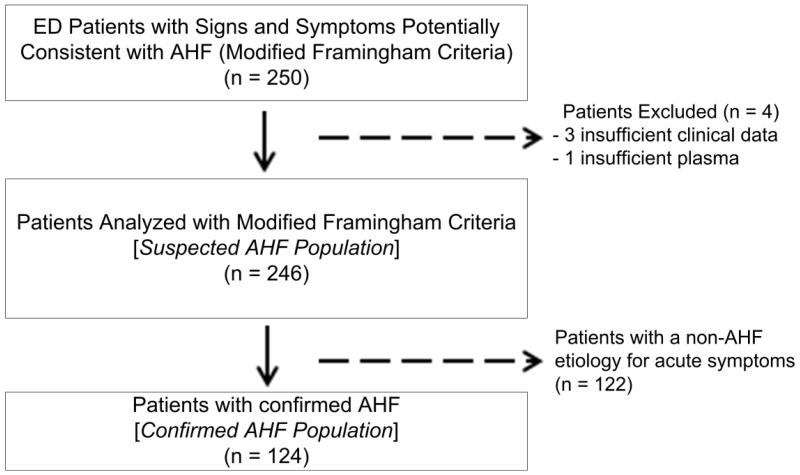
Two hundred fifty patients were randomly selected from the STRATIFY/DECIDE cohort20 for this study. Four patients were excluded, including three patients with insufficient data to classify whether their symptoms were due to AHF and one patient who had insufficient plasma for bio-ADM measurement; the remaining 246 patients were included as the Suspected AHF Population (Figure 1; Table 1). Of these 246 patients, 124 (50.4%) were confirmed to have AHF based on the cardiologist review and were considered the Confirmed AHF Population. The initial two cardiologists agreed on whether AHF was the cause of symptoms in 224 (91.1%) cases [Kappa = 0.82 (standard error: 0.060)].
Figure 1.
Flow diagram of patient enrollment.
Table 1.
Patient characteristics.
| Characteristic | Suspected AHF Population (n = 246) |
Confirmed AHF Population (n = 124) |
|---|---|---|
| Age, median (IQR), years | 62 (53, 74) | 65 (56, 76.5) |
| Female, n (%) | 106 (43.1) | 43 (34.7) |
|
| ||
| Race / Ethnicity | ||
| White, non-Hispanic | 160 (65.0) | 72 (58.1) |
| Hispanic | 1 (0.4) | 0 |
| Black | 84 (35.2) | 52 (41.9) |
| Other | 1 (0.4) | 0 |
|
| ||
| Medical Insurance (primary) | ||
| Private insurance | 62 (25.2) | 32 (25.8) |
| Medicare | 127 (51.6) | 66 (53.2) |
| Medicaid | 33 (13.4) | 16 (12.9) |
| None | 24 (9.8) | 10 (8.1) |
|
| ||
| Presenting Vital Signs | ||
| Systolic BP, median (IQR) | 144 (127, 172) | 148 (131, 179) |
| Heart Rate, median (IQR) | 89 (77, 104) | 94 (78, 109) |
| Respiratory Rate, median (IQR) | 20 (18, 24) | 20 (18, 24) |
| Oxygen Saturation, median (IQR) | 96 (94, 98) | 96 (92, 98) |
|
| ||
| Presenting Symptoms | ||
| Shortness of breath | 147 (59.8) | 73 (58.9.) |
| Chest pain | 54 (22.0) | 18 (14.5) |
| Orthopnea | 167 (68.2) | 83 (67.5) |
| Fatigue | 203 (82.5) | 89 (79.0) |
|
| ||
| Medical History | ||
| Body Mass Index, median (IQR) | 30.7 (25.5, 38.1) | 30.0 (24.9, 36.1) |
| Current smoker, n (%) | 41 (16.7) | 20 (16.1) |
| Chronic heart failure, n (%) | 145 (58.9) | 85 (68.6) |
| Prior myocardial infarction, n (%) | 75 (30.5) | 40 (32.3) |
| Valvular heart disease, n (%) | 51 (20.7) | 25 (20.2) |
| Hypertension, n (%) | 188 (76.4) | 99 (79.8) |
| Diabetes Mellitus, n (%) | 106 (43.1) | 57 (46.0) |
| Chronic pulmonary disease, n (%) | 79 (32.1) | 27 (21.8) |
| Chronic renal disease, n (%) | 45 (18.3) | 26 (21.0) |
|
| ||
| Laboratory Results | ||
| Sodium, median (IQR) [meq/L] | 139 (137, 141) | 140 (138, 141) |
| Creatinine, median (iQr) [mg/dL] | 1.2 (0.92, 1.70) | 1.40 (1.13, 1.90) |
| Blood urea nitrogen, median (IQR) [mg/dL] | 20 (12, 30) | 22 (15, 34) |
| Hemoglobin, median (IQR) [g/dL] | 12.4 (11.0, 13.6) | 12.4 (10.8, 13.7) |
| Cardiac Troponin I, median (IQR) [ng/ml] | 0.01 (0, 0.04) | 0.03 (0.01, 0.06) |
| B-type natriuretic peptide, median (IQR) [pg/ml] | 329 (82, 1005) | 863 (410, 1718) |
|
| ||
| Admitted to Hospital, n (%) | 199 (80.9) | 119 (96.0) |
AHF: acute heart failure
IQR: interquartile range
3.2. Suspected AHF Population
Among the 246 patients in the Suspected AHF Population, 85 (34.6%) met the primary outcome by experiencing at least one of the 30-day outcomes; 18 (7.3%) patients had a severe clinical outcome (Table 2).
Table 2.
Patient outcomes.
| 30-Day Outcome | Suspected AHF Population (n = 246) |
Confirmed AHF Population (n = 124) |
|---|---|---|
| Severe Clinical Outcomes | ||
| Death | 14 (5.7) | 10 (8.1) |
| Cardiac Arrest with ROSC | 4 (1.6) | 4 (3.2) |
| Respiratory Failure | 2 (0.8) | 1 (0.8) |
| Emergency Dialysis | 2 (0.8) | 2 (1.6) |
| Acute Coronary Syndrome | 2 (0.8) | 1 (0.8) |
|
| ||
| ≥ 1 severe clinical outcome (secondary outcome) |
18 (7.3) | 13 (10.5) |
|
| ||
| Healthcare Utilization Outcomes | ||
| Hospital Length of Stay > 5 days | 65 (26.4) | 54 (43.6) |
| Return ED Visit for AHF | 20 (8.1) | 19 (15.3) |
| Return Hospitalization for AHF | 19 (7.7) | 18 (14.5) |
| ≥ 1 healthcare utilization outcome | 78 (31.7) | 66 (53.2) |
|
| ||
| ≥ 1 Any 30-Day Outcome (primary outcome) |
85 (34.6) | 71 (57.3) |
AHF: acute heart failure
ROSC: return of spontaneous circulation
ED: emergency department
3.2.1. All 30-Day Outcomes (Primary Outcome)
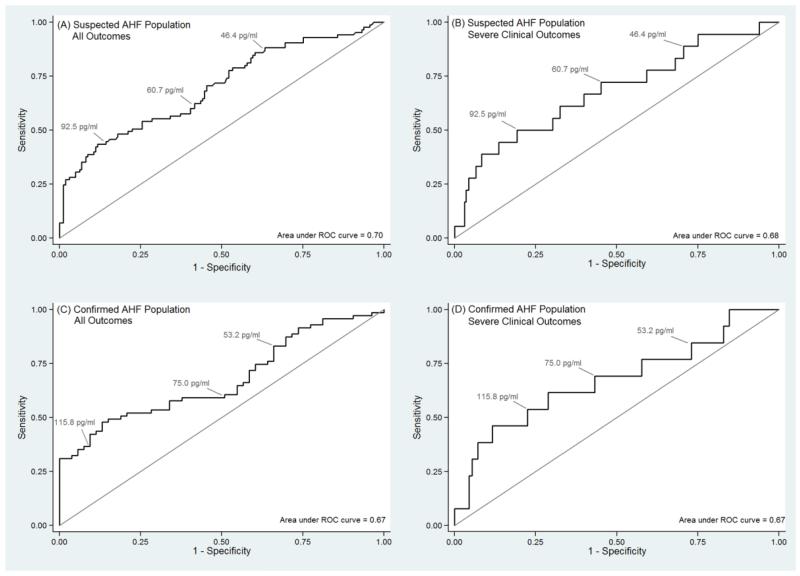
Plasma bio-ADM concentration was higher in patients who experienced at least one 30-day outcome (median: 80.5 pg/ml; IQR: 53.7 pg/ml, 151.5 pg/ml) compared to those who did not (median: 54.4 pg/ml; IQR: 43.4 pg/ml, 78.4 pg/ml) (p < 0.01). Area under the ROC curve was 0.70 (95% CI: 0.63, 0.75) (Figure 2a). The 25th, 50th, and 75th percentile of plasma bio-ADM concentration in the Suspected AHF population was 46.4 pg/ml, 60.7 pg/ml, and 92.5 pg/ml, respectively. Proportions of patients with bio-ADM concentrations above and below each of these cut-points who experienced the composite outcome are shown in Table 3.
Figure 2.
Receiver operating characteristic (ROC) curves showing the predictive performance of bioactive adrenomedullin (bio-ADM) to identify patients with the study outcomes: (A) Suspected Acute Heart Failure (AHF) Population with a composite of all 30-day outcomes; (B) Suspected AHF Population with only severe clinical outcomes; (C) Confirmed AHF Population with a composite of all 30-day outcomes; (D) Confirmed AHF Population with only severe clinical outcomes. The 25th, 50th, and 75th percentiles of bio-ADM concentration for each population are displayed on the ROC plots.
Table 3.
Proportion of patients who experienced a composite of all 30-day outcomes based on bio-ADM concentration cut-points. Selected bio-ADM cut-points represent the 25th, 50th, and 75th percentile of bio-ADM concentration in the study populations.
| bio-ADM cut-point | bio-ADM < cut-point |
bio-ADM ≥ cut-point |
||
|---|---|---|---|---|
| Patients, n | Patients with Outcome, n (%) |
Patients, n | Patients with Outcome, n (%) |
|
| Suspected AHF Population (N=246) | ||||
| 46.4 pg/ml [25th %tile] | 61 | 10 (16.4) | 185 | 75 (40.5) |
| 60.7 pg/ml [50th %tile] | 123 | 32 (26.0) | 123 | 53 (43.1) |
| 92.5 pg/ml [75th %tile] | 185 | 47 (25.4) | 61 | 38 (62.3) |
|
| ||||
| Confirmed AHF Population (N=124) | ||||
| 53.2 pg/ml [25th %tile] | 31 | 13 (41.9) | 93 | 58 (62.4) |
| 75.0 pg/ml [50th %tile] | 62 | 29 (46.8) | 62 | 42 (67.7) |
| 115.8 pg/ml [75th %tile] | 93 | 45 (48.4) | 31 | 26 (83.9) |
bio-ADM: bioactive adrenomedullin
CI: confidence interval
3.2.2. Severe Clinical Outcomes
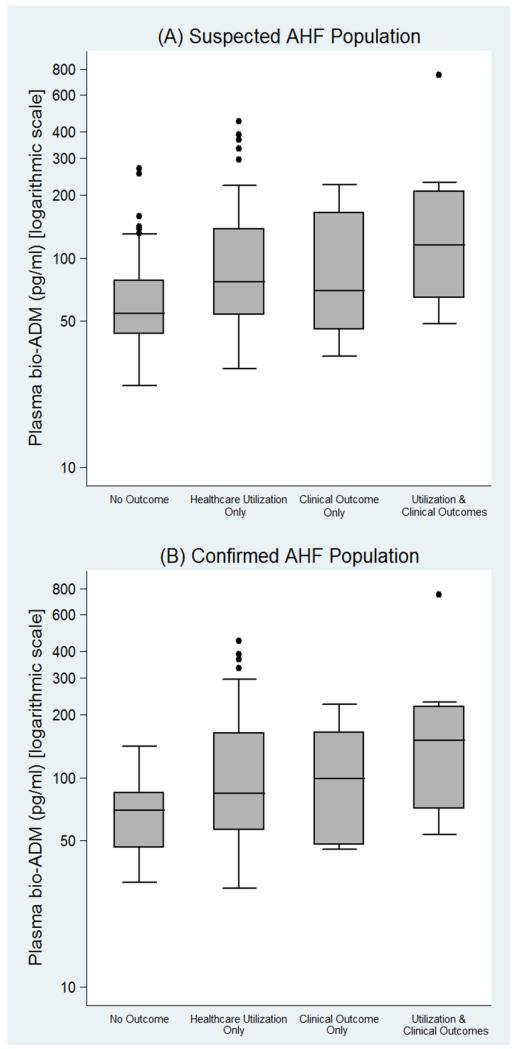
After limiting the outcome of interest to only severe clinical outcomes, plasma bio-ADM concentration was significantly higher in patients who experienced the outcome (median: 89.9; IQR: 53.5 pg/ml, 186.6 pg/ml) compared to those who did not (median: 59.5 pg/ml; IQR: 45.8 pg/ml, 89.7 pg/ml) (p = 0.01). Area under the ROC curve was 0.68 (95% CI: 0.62, 0.74) (Figure 2b). Bio-ADM levels for patients with no outcomes, healthcare utilization outcomes, and severe clinical outcomes are illustrated in Figure 3.
Figure 3.
Box plots of plasma bioactive adrenomedullin (bio-ADM) concentration, by outcome category among the (A) Suspected Acute Heart Failure (AHF) Population, and (B) Confirmed AHF Population. The y-axis of each plot is displayed on a logarithmic scale. The center of each box plot represents the median, with the box denoting the interquartile range (IQR), the upper and lower whiskers representing 1.5-times the IQR above and below the 75% percentile and 25% percentile, respectively, and dots noting outliners beyond the whiskers.
3.3. Confirmed AHF Population
Among the 124 patients in the Confirmed AHF Population, 71 (57.3%) experienced at least one of the 30-day outcomes, including 13 (10.5%) with a severe clinical outcome (Table 2). Plasma bio-ADM concentration was higher in those who experienced at least one of the 30-day outcomes (median: 91.8 pg/ml; IQR: 56.0 pg/ml, 167.8 pg/ml) than those who did not (median: 69.7 pg/ml; IQR: 46.4 pg/ml, 84.6 pg/ml) (p < 0.01). Area under the ROC curve was 0.67 (95% CI: 0.59, 0.76) (Figure 2c). When only considering severe clinical outcomes, bio-ADM remained a predictor of outcomes (Figure 2d).
3.4. Multivariable Biomarker Model
The multivariable logistic regression model included 151 patients in the Suspected AHF Population who had ED measurements for all five biomarkers; 61(40.4%) of these patients experienced the composite 30-day outcome. Both bio-ADM (aOR per one IQR change: 2.68; 95% CI: 1.60, 4.51) and BNP (aOR per one IQR change: 3.06; 95% CI: 1.34, 7.00) were significantly associated with the outcome in the multivariable model, with bio-ADM (likelihood ratio chi-square: 17.70) contributing more to overall model fit than BNP (likelihood ratio chi-square: 7.56) (Table 4).
Table 4.
Results of univariate and multivariate logistic regression models evaluating five biomarkers as predictors of the composite 30-day outcome in patients evaluated for acute heart failure. Adjusted odds ratio (OR): OR adjusted for all other biomarkers. Added likelihood ratio (LR) chi-square: added value analysis based on nested regression models.
| Variable | df | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|---|
| LR Chi- square |
p | OR (95% CI) | Added LR Chi-square |
p | Adjusted OR (95% CI) |
||
| Log10 bio-ADM (per IQR change) |
1 | 20.52 | <0.001 | 2.62 (1.65, 4.17) | 17.70 | <0.001 | 2.68 (1.60, 4.51) |
|
| |||||||
| Log10 BNP (per IQR change) |
1 | 12.75 | <0.001 | 3.24 (1.62, 6.52) | 7.56 | 0.006 | 3.06 (1.34, 7.00) |
|
| |||||||
| Log10 Troponin I (per IQR change) |
1 | 2.20 | 0.138 | 1.45 (0.88, 2.40) | 0.15 | 0.70 | 1.12 (0.63, 1.98) |
|
| |||||||
| Log10 Creatinine (per IQR change) |
1 | 1.73 | 0.188 | 1.25 (0.89, 1.76) | 0.42 | 0.52 | 0.88 (0.58, 1.31) |
|
| |||||||
| Sodium (per IQR change) |
1 | 0.59 | 0.444 | 1.16 (0.78, 1.74) | 1.70 | 0.19 | 1.34 (0.86, 2.08) |
Log10: data transformed logarithmically using base-10
df: degrees of freedom
LR: likelihood ratio
OR: odds ratio
CI: confidence interval
bio-ADM: bioactive adrenomedullin
BNP: B-type naturetic peptide
4. DISCUSSION
This is the first study to evaluate plasma bio-ADM in patients with AHF and suggests it may be a useful prognostic biomarker. Our study has two important findings. First, higher bio-ADM levels were associated with increased risk for important 30-day outcomes. Second, bio-ADM was an independent risk factor for clinical outcomes in a multivariable model including other biomarkers clinicians currently use when evaluating AHF patients, including BNP, cardiac troponin I, creatinine, and sodium concentration. This suggests potential complementary roles for bio-ADM and BNP in the risk stratification of AHF patients.
We evaluated the predictive performance of plasma bio-ADM for several short-term outcomes. Initially, we used a composite of a broad range of outcomes clinicians find important when deciding on disposition for a patient in the ED.2,3,6,22-24 These outcomes included mortality, morbidity and healthcare utilization, such as prolonged hospitalization and repeat presentations for AHF. The rationale for including healthcare utilization outcomes stems from the concept that ED AHF patients who undergo a prolonged hospitalization or rapidly return to the hospital after an ED visit are likely poor candidates for outpatient management. Acknowledging hospital length of stay and repeat presentations may be driven by factors other than AHF severity, we also evaluated the prognostic performance of bio-ADM for an outcome limited to severe clinical events, including 30-day mortality, cardiac arrest, respiratory failure, emergency dialysis and acute coronary syndrome. Bio-ADM maintained similar prognostic performance both with (ROC AUC = 0.70) and without (ROC AUC = 0.68) healthcare utilization endpoints included in the composite outcome of interest.
Providing clinicians with rapid and accurate prognostic information for AHF patients at the time of ED evaluation has the potential to improve clinical care.2,20,23,24 Currently, approximately 80% of patients with suspected AHF in US EDs are hospitalized with little attention to an individual patient’s risk for clinical decompensation or potential benefit of hospitalization.1,2,20,23,24 A reliable prognostic biomarker could help guide clinicians to an aggressive management strategy with intravenous medications and hospitalization selectively for AHF patients most likely to benefit from it.
Due to the heterogeneity of AHF, involvement of multiple organ systems, and diverse precipitants leading to acute decompensation, no single biomarker provides comprehensive risk stratification.22 Accounting for this broad range of physiological disturbances with a multi-biomarker panel for AHF prognosis may be an approach to improve risk stratification. Bio-ADM is a promising new candidate for an AHF biomarker panel. It is a mediator of vascular control and marker of vascular dysregulation, which is a hallmark of AHF exacerbations.10 While it has similar biological activity to the natriuretic peptides, bio-ADM responds to different stimuli in the peripheral circulation, and therefore likely provides complementary information to BNP.10 Prior work suggests cardiac troponin, renal function, and serum sodium concentration may also help differentiate high- and low-risk patients with AHF.9-11 Initial evaluation in this study suggests bio-ADM provides predictive information independent of these other markers. Additional work with larger cohorts is indicated to explore how bio-ADM may be incorporated into a panel of multiple biomarkers for AHF risk stratification.
4.1. Limitations
This study was a secondary analysis of the a multicenter cohort study.20 This was a relatively small study conducted at a four hospitals in the US. Additional study with larger sample sizes and diverse settings is needed to further adjust for potential confounders and more thoroughly evaluate the additive prognostic information provided by bio-ADM above other markers. Single measurements of bio-ADM were evaluated in this study; evaluation of serial measurements is planned.
5. CONCLUSIONS
In this study of 246 ED patients with suspected AHF, single measurements of plasma bio-ADM were significantly associated with clinically-important 30-day outcomes. Bio-ADM is a promising new biomarker that may add important prognostic information for patients with AHF. Further study is indicated to robustly evaluate bio-ADM in larger sample sizes, both as a single biomarker and as a component of multi-marker panels.
6. ACKNOWLEDGEMENTS
We would like to thank Karen F. Miller, RN and Susan K. Roll, RN for their dedication to this project.
Funding/Support: This work was supported by NIH grants K23GM110469 (to WHS) from the National Institute of General Medical Sciences, and 1R01HL088459 (to ABS), K23 HL085387 (to SPC), and K23HL102069 (to TWB) from the National Heart, Lung and Blood Institute. Sphingotec GmbH supplied assays for biomarker measurement.
Role of the Sponsors: NIH had no role in the design and conduct of the study, or the collection, management, analysis or interpretation of data. Sphingotec GmbH supplied assays for biomarker measurement; additionally, employees of Sphingotec (OH, JS, AB) participated in the study as investigators and authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Storrow AB, Jenkins CA, Self WH, et al. The burden of acute heart failure on U.S. Emergency departments. JACC Heart Fail. 2014;2:269–77. doi: 10.1016/j.jchf.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fermann GJ, Collins SP. Initial management of patient with acute heart failure. Heart Fail Clin. 2013;9:291–301. doi: 10.1016/j.hfc.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kransdorf EP, Kittlesson MM. Dissecting the CHF admission: An evidence-based review of the evaluation and management of acute decompensated heart failure for the hospitalist. J Hosp Med. 2012;7:439–45. doi: 10.1002/jhm.1919. [DOI] [PubMed] [Google Scholar]
- 4.Bereket W, Hemalatha K, Getenet B, et al. Update on bacterial nosocomial infections. Eur Rev Med Pharm Sci. 2012;16:1039–1044. [PubMed] [Google Scholar]
- 5.Joosten E, Demuynck M, Detroyer E, Milisen K. Prevalence of frailty and its ability to predict hospital delirium, falls and 6-month mortality in hospitalized older patients. BMC Geriatr. 2014;14:1. doi: 10.1186/1471-2318-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCausland JB, Machi MS, Yealy DM. Emergency physicians’ risk attitudes in acute decompensated heart failure patients. Acad Emerg Med. 2010;17:108–110. doi: 10.1111/j.1553-2712.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 7.Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–7. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 8.Maisel A, Mueller C, Nowak R, et al. Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. J Am Coll Cardiol. 2010;55:2062–76. doi: 10.1016/j.jacc.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Peacock WF, DeMarco T, Fonarow GC, et al. Cardiac troponin and outcome in acute heart failure. N Engl J Med. 2008;358:2117–26. doi: 10.1056/NEJMoa0706824. [DOI] [PubMed] [Google Scholar]
- 10.Fonarow GC, Adams KF, Abraham WT, et al. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. 2005;293:572–80. doi: 10.1001/jama.293.5.572. [DOI] [PubMed] [Google Scholar]
- 11.Gheorghiade M, Abraham WT, Albert NM, et al. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J. 2007;28:980–988. doi: 10.1093/eurheartj/ehl542. [DOI] [PubMed] [Google Scholar]
- 12.Gheorghiade M, Abraham WT, Albert NM, et al. Systolic blood pressure at admission, clinical characteristics and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217–26. doi: 10.1001/jama.296.18.2217. [DOI] [PubMed] [Google Scholar]
- 13.Jougasaki M, Burnett JC., Jr Adrenomedullin: potential in physiology and pathophysiology. Life sciences. 2000;66:855–72. doi: 10.1016/s0024-3205(99)00358-6. [DOI] [PubMed] [Google Scholar]
- 14.Jougasaki M, Wei CM, McKinley LJ, Burnett JC., Jr Elevation of circulating and ventricular adrenomedullin in human congestive heart failure. Circulation. 1995;92:286–9. doi: 10.1161/01.cir.92.3.286. [DOI] [PubMed] [Google Scholar]
- 15.Adlbrecht C, Hulsmann M, Strunk G, et al. Prognostic value of plasma midregional pro-adrenomedullin and C-terminal-pro-endothelin-1 in chronic heart failure outpatients. Eur J Heart Fail. 2009;11:361–6. doi: 10.1093/eurjhf/hfp004. [DOI] [PubMed] [Google Scholar]
- 16.Maisel A, Mueller C, Nowak RM, Peacock WF, et al. Midregion prohormone adrenomedullin and prognosis in patients presenting with acute dyspnea: results from the BACH (Biomarkers in Heart Failure) trial. J Am Coll Cardiol. 2011;58:1057–67. doi: 10.1016/j.jacc.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Ohta H, Tsuji T, Asai S, Tanizaki S, Sasakura K, Teraoka H, et al. A simple immunoradiometric assay for measuring the entire molecules of adrenomedullin in human plasma. Clinica chimica acta; international journal of clinical chemistry. 1999;287:131–43. doi: 10.1016/s0009-8981(99)00128-x. [DOI] [PubMed] [Google Scholar]
- 18.Eguchi S, Hirata Y, Iwasaki H, Sato K, Watanabe TX, Inui T, et al. Structure-activity relationship of adrenomedullin, a novel vasodilatory peptide, in cultured rat vascular smooth muscle cells. Endocrinology. 1994;135:2454–8. doi: 10.1210/endo.135.6.7988431. [DOI] [PubMed] [Google Scholar]
- 19.Marino R, Struck J, Maisel AS, Magrini L, Bergmann A, Di Somma S. Plasma adrenomedullin is associated with short-term mortality and vasopressor requirement in patients admitted with sepsis. Critical Care. 2014;18:R34. doi: 10.1186/cc13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins SP, Lindsell CJ, Jenkins CA, et al. Risk stratification in acute heart failure: rationale and design of the STRATIFY and DECIDE studies. Am Heart J. 2012;164:825–34. doi: 10.1016/j.ahj.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jneid H, Anderson JL, Wright RS, et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2012;60:645–8. doi: 10.1016/j.jacc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Travaglino F, Russo V, De Berardinis B, et al. Thirty and ninety days mortality predictive value of admission and in-hospital procalcitonin and mid-regional pro-adrenomedullin testing in patients with dyspnea. Results from the VERyfing DYspnea trial. Am J Emerg Med. 2014;32:334–41. doi: 10.1016/j.ajem.2013.12.045. [DOI] [PubMed] [Google Scholar]
- 23.Collins SP, Storrow AB. Acute heart failure risk stratification: Can we define low risk? Heart Fail Clin. 2009;5:75–83. doi: 10.1016/j.hfc.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 24.Stiell IG, Clement CM, Brison RJ, et al. A risk scoring system to identify emergency department patients with heart failure at high risk for serious adverse events. Acad Emerg Med. 2013;20:17–26. doi: 10.1111/acem.12056. [DOI] [PubMed] [Google Scholar]
- 25.Collins SP, Storrow AB. Moving toward comprehensive acute heart failure risk assessment in the emergency department: The importance of self-care and shared decision making. JACC Heart Fail. 2013;1:273–80. doi: 10.1016/j.jchf.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad T, Fiuzat M, Pencina MJ, et al. Charting a roadmap for heart failure biomarker studies. JACC Heart Fail. 2014;2:477–488. doi: 10.1016/j.jchf.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]