Abstract
Mammals typically display alternating active and resting phases and, in most species, these rhythms follow a circadian pattern. The active and resting phases often are accompanied by corresponding physiological changes. In humans, blood pressure decreases during the resting phase of the activity cycle, and the magnitude of that “nocturnal dipping” has been used to stratify patients according to the risk for cardiovascular disease. However, in contrast to most mammals, prairie voles (Microtus ochrogaster) have periods of activity and rest that follow an ultradian rhythm with period lengths significantly less than 24h. While rhythmic changes in blood pressure across a circadian activity cycle have been well-documented, blood pressure patterns in species that display ultradian rhythms in activity is less well-studied. In the current study, we implanted pressure-sensitive radiotelemetry devices in male prairie voles and recorded activity, mean arterial pressure (MAP), and heart rate (HR) continuously for 3 days. Visualization of the ultradian rhythms was enhanced using a 1hr running average to filter the dataset. Positive correlations were found between activity and MAP and between activity and HR. During the inactive period of the ultradian cycle, blood pressure decreased by about 15%, which parallels the nocturnal dipping pattern seen in healthy humans. Further, the duration of inactivity did not affect any of the cardiovascular measures, so the differences in blood pressure values between the active and inactive periods are likely driven by ultradian oscillations in hormones and autonomic function. Finally, specific behavioral patterns also were examined. Both the instrumented animal and his non-instrumented cagemate appeared to show synchronized activity patterns, with both animals displaying sleep-like behavior for more than 90% of the inactive period. We propose that the prairie vole ultradian rhythm in blood pressure is an analogue for circadian blood pressure variability and can be used to study the long-term effects of commonly prescribed drugs on blood pressure dipping.
Keywords: Microtus, nocturnal dipping, biological rhythms, running average, data smoothing
Graphical Abstract
1. Introduction
Assessment of blood pressure throughout the day and night has facilitated the characterization of blood pressure rhythms in clinical and healthy populations [1, 2]. Short-term and long-term rhythms have been identified; and among the most clinically relevant, is the circadian blood pressure rhythm. During sleep, blood pressure decreases 10–20% from the average waking value. The absence of this “nocturnal dipping” has emerged as a predictor of cardiovascular morbidity and mortality. Abnormal blood pressure rhythms, including attenuated dipping and non-dipping patterns, have been linked to higher risks of adverse cardiovascular events, such as ventricular hypertrophy and microalbuminuria[3, 4]. Consequently, addressing circadian blood pressure variability, and not simply mean BP, may maximize the effectiveness of clinical interventions.
The mechanisms that regulate such blood pressure variations have been examined in human and animal models. Previous research has demonstrated that a variety of factors, including respiration, autonomic tone, and hormone levels, contribute to blood pressure dipping [1, 2, 4, 5]. These physiological functions adapt to the metabolic demands associated with periods of activity and periods of rest. As a result, the blood pressure rhythm remains tightly linked to physical activity rhythms [6–8]. Diurnal mammals, for instance, will display a greater average blood pressure during the day, when the animal is most active, compared to during the night.
Understanding the strong connection between physical activity and cardiovascular function has been advanced by implantable telemetry in laboratory animals [6]. Importantly, these technologies have the ability to acquire blood pressure data during sleep [9, 10], allowing for comparisons to be made between the active and resting periods. In addition to recording during sleep, the activity data from these devices can be used to identify the sleep/wake cycle. Recently, telemetry-derived activity measures were shown to correlate strongly with the sleep/wake cycle, which was estimated by recordings from electroencephalograms and electromyograms [11]. Accordingly, radiotelemetry could be used to track BP across the sleep/wake cycle.
Radiotelemetry in rat and mouse models of cardiovascular disease have been used to understand the mechanisms that contribute to disturbances in blood pressure dipping. [12–17]. Nevertheless, traditional rodent models are not without their limitations[18]. Rats and mice are nocturnal, and thus, show only one BP dipping event every 24h. Examination of long-term effects of experimental manipulations on blood pressure dipping could be better assessed in a model organism with an accelerated activity cycle that is largely independent of the time of day.
In contrast to traditional laboratory rodents, many of the microtine rodents, including voles, do not show circadian rhythms in activity. Rather, these animals exhibit ultradian activity rhythms in which activity cycles occur throughout the day and night with period lengths of 1 to 4 hours [19–21]. The period lengths of the ultradian rhythms in activity are resistant to changes in the environment [21]. Recently, radiotelemetry has been used to identify ultradian rhythms in core body temperature and heart rate in microtine rodents [22, 23]. However, no one has examined blood pressure dipping in a species that shows ultradian rhythms in activity.
Here, we performed a detailed analysis of blood pressure across an ultradian activity cycle. In studies of biological rhythms, data often are collected at scheduled intervals [24] with the major advantage being that the amount of ‘noise’ in the dataset is reduced. However, accurate detection of the period length of a rhythm depends on the frequency with which data are acquired [25]. If the frequency or phase of an ultradian rhythm is variable or unknown, then a scheduled interval may not capture each peak for each animal [25]. In contrast, we acquired cardiovascular and activity data continuously and then filtered the data set with a one-hour running average. While commonly used for financial forecasting in business[26–28], running averages are rarely used in physiology. Consistent with the effects on financial data, we predicted that the running average filter would facilitate the visualization of each animal’s ultradian rhythms. Also, because of the link between activity and cardiovascular function in species that show circadian rhythms, we hypothesized that prairie voles would display ultradian rhythms in HR and BP and that those rhythms would correlate positively with activity. Lastly, we hypothesized that prairie voles would show an ultradian blood pressure dipping pattern that parallels blood pressure dipping in animals with circadian rhythms.
2. Methods
2.1 Animals
Subjects were adult male prairie voles from a captive breeding-colony at Oklahoma State University’s Center for Health Sciences and were of the F3-F4 generation relative to the most recent outcrossing with wild stock. After weaning (20 ± 0.5 days of age), animals were housed as same-sex pairs in shoebox style cages (10 × 17 x 28 cm). Animals had ad libitum access to food (Purina rabbit chow supplemented with black oil sunflower seeds) and water. The colony is kept on a 14:10 light/dark cycle in a temperature-controlled room (about 21 °C) throughout the year. All subjects were sexually naïve adults (86.1 ± 9.5 days of age) with an average mass of 48.4 ± 3.0g at the beginning of the experiment. All experimental procedures were approved by the Oklahoma State University Center for Health Sciences Institutional Animal Care and Use Committee.
2.2 Surgery
Radiotelemetry devices (PA-C10, Data Sciences International) were implanted in male prairie voles under isoflurane anesthesia. These devices are designed for use in mice, which typically are about ½ the size of voles. Thus, the weight of the device (1.4g) is about 3% of the mass of the voles used in this study. Neither this small amount of added mass, nor the presence of the device per se appeared to impede the animals’ performance in any way.
Body temperatures were maintained by placing the animals on a heating pad during the surgery. A small incision was made in the throat, and a sub-cutaneous pocket was created on each animal’s dorsum, near the right scapula, into which the body of the telemetry device was placed. The left common carotid artery was exposed by blunt dissection, after which the artery was carefully isolated from the vagus nerve. The pressure-sensitive tip of the device was inserted into the left common carotid artery and advanced 14–14.5 mm relative to the carotid bifurcation to place the tip in the aortic arch. The carotid artery was selected as the implantation site because it is associated with a higher survival rate than is implantation of the abdominal aorta [29] A subcutaneous injection of isotonic saline (0.5 mL) was given to animals that lost more than a minimal amount of blood. Each animal received perioral administration of 0.05 to 0.1 mL of meloxicam (1.5mg/mL) on the day of surgery. All animals were within about 95% of their pre-surgery weights at the time surgery and were allowed six days to recover with data recording commencing on day 7 after surgery. This recovery period is consistent with recovery periods used in other studies [29, 30]. Further, normal circadian rhythms return by day 6 post-surgery in CD-1 mice [31].
2.3 Overview of the experimental design
Surgical implantation of the telemetry devices was performed on day 0. Animals were returned to their home cages with their same-sex cagemates. Animals were given 6 days post-surgery to recover. For days 0–5, the subjects were separated from their cagemates using a wire mesh barrier, which permitted visual, auditory, and olfactory stimulation but prevented physical contact while the surgical wounds healed. On day 6 post-surgery, the wire mesh barrier was removed. On day 7, the subjects were moved into the testing room and then activity and cardiovascular data were collected continuously from the telemetry device for 72 hours in the presence of their same-sex cagemates. The telemetric data were then filtered with a 1 hour running average.
Throughout the 72 hour recording period, subjects also were video recorded, so that specific patterns of behavior could be analyzed. A red light illuminated the animals during the lights-off period. A 1hr running average activity graph was used to identify active and inactive stages of the activity cycle for each animal, and specific patterns of behavior were compared between the active and inactive stages. Social contact has been reported to entrain activity rhythms in microtine rodents[30], so we predicted that the instrumented and non-instrumented animals would display synchronized activity patterns (i.e., simultaneous mobility and immobility). Further, we quantified the amount of time in which both animals remained in quiet, physical contact (i.e., side-by-side sleep-like behavior).
2.4 Data acquisition and analysis
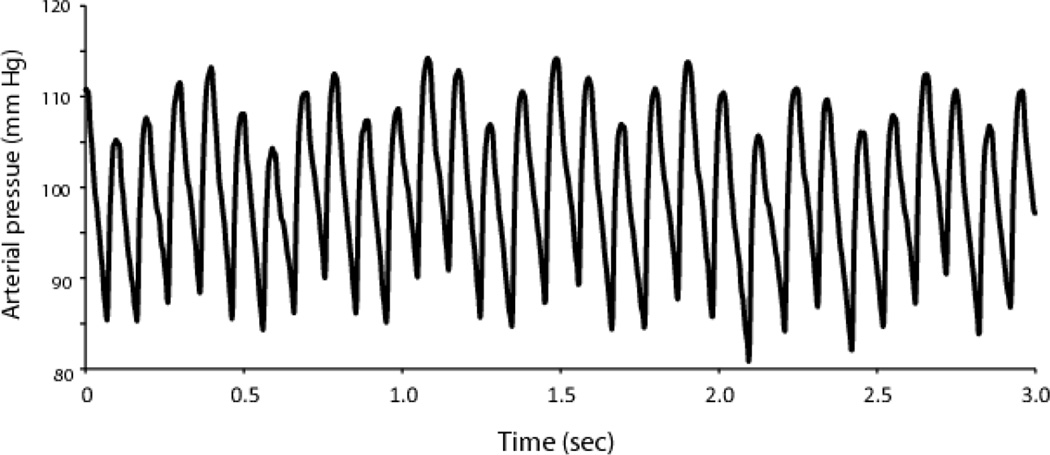
Dataquest ART software was used to acquire activity and BP measurements. Beginning during the lights-on period on day 7 after surgery, BP and activity data were sampled continuously at 500 Hz for 72 hours, with measurements for each parameter generated in 10 second epochs. Data were collected in each animal’s home cage and in the presence of its original same-sex cagemate. Heart rate was derived from the blood pressure data (Figure 1). An ambient pressure monitor (APR-1) was used to correct for atmospheric pressure.
Figure 1.
Arterial blood pressure record from an adult male prairie vole. Three seconds of unprocessed blood pressure telemetry data from a probe placed in the aortic arch. Heart rate data were derived from such traces.
2.5 Data smoothing
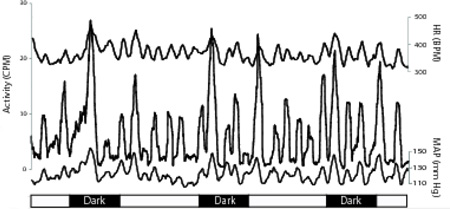
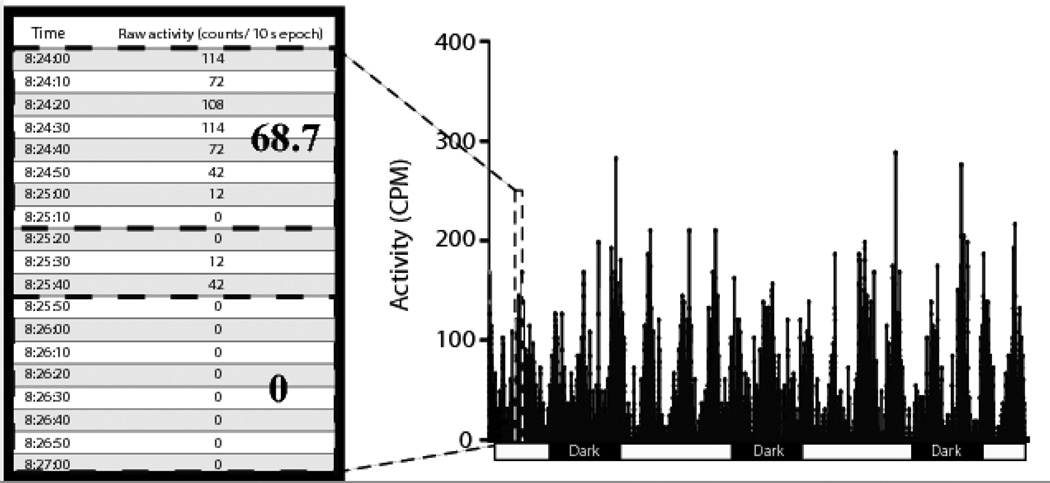
Activity data were highly variable (Figure 2) making it difficult to accurately identify specific components of behavioral rhythms. To more accurately detect long-term rhythms, we filtered out short-term oscillations using a one-hour running average. In a running average, each data point is replaced by an average of x number of data points that immediately precede it and x number of data points that immediately follow it. Consequently, each data point is replaced by the average of 2x +1 data points in the time series. In the current study, 30 minutes prior and 30 minutes after each data point were used to calculate the running average. A filtered dataset of 10 second epochs was used strictly to visualize the rhythm because the number of data points (>26,000) rendered every regression analysis statistically significant.
Figure 2.
Noise and variability in a continuously acquired dataset. Activity counts are extremely variable, especially during a period of high activity. The dashed box outlines the area of the graph from which the data in the table were collected. The table illustrates the variability in activity count within an activity bout – an 80 second segment with an average of nearly 70 activity counts per minute separated by only 30 seconds from an 80 second period in which no activity was recorded.
2.6 Descriptive statistics and rhythm characterization
Descriptive statistics for the cardiovascular data were performed on the raw data from the entire 72-hour period. Averages were based on the calculation of a grand mean, in which the mean for each individual animal was determined and the overall mean was calculated from the means of the individual animals. The measures of variability for the overall data were also based on the means of the individual animals.
Active and inactive periods were identified by visual inspection of the filtered activity graphs (Figure 3). The active period was defined as the 1.5 minutes before and 1.5 minutes after the peak of an activity cycle, and the inactive period was similarly defined as the 3 minutes surrounding the trough of an activity cycle. The active and inactive parameters were determined for each animal by considering two active periods during the day and during the night, and two inactive periods during the day and the during the night for each animal. Although the filtered dataset was used to identify active and inactive periods, the statistics were performed on the raw data. The average percent change in cardiovascular measures was calculated from the percent change for each individual animal.
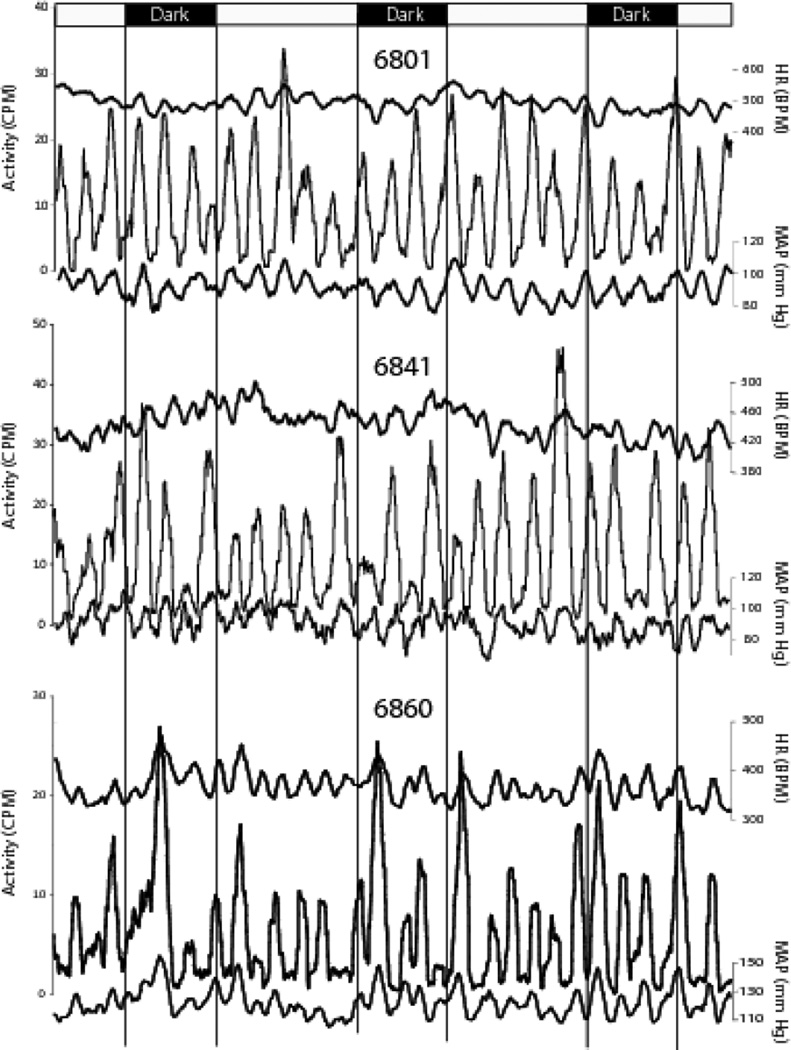
Figure 3.
Data smoothing with a 1 hr running average. Data smoothing renders activity cycles readily apparent. One-hour running averages were applied to HR, activity, and BP data. The temporal correspondence between activity (left ordinate), and HR and MAP (right ordinates) is clearly illustrated. Each set of traces illustrates 72 hours of data from one animal (identified by numbers above traces). Vertical lines delineate the 14:10 (lights on: lights off) photoperiod. Activity peaks from these and similar graphs from the remaining animals in the study were used to identify specific temporal epochs for detailed analysis of the unfiltered HR and MAP data.
Analyses of period lengths were done using the first peak after lights-off as time zero. Period lengths were identified using visual inspection of the running averages graphs to determine the approximate time-range within which the event occurred followed by identification of the exact ten second epoch in which the event occurred. Period lengths were measured by determining the time interval between adjacent peak maxima. The number of peaks for each parameter for each animal over 24 hours also was counted. The temporal stability of the rhythm was visualized by plotting each of the three 24 hour periods on the same x-axis.
2.7 Assessment of circadian rhythms
2.7.1 Comparison of peak magnitudes of the activity rhythm between day and night
Several animals appeared to show circadian rhythms in activity, so individual activity rhythms were also assessed by comparing peak maxima between the day and night using a repeated measures ANOVA. Since the animals were under a 14:10 light: dark cycle, the number of nighttime peaks was the limiting factor. The peak maximum for each nighttime peak was identified from the running averages graph of the 10 second epochs. A corresponding number of daytime peaks were selected. If there were 3 peaks during the night, then the last peak before the dark period and the first two peaks following lights-on were used. If 4 peaks occurred during the night, then the 2 peaks preceding the dark period and the two peaks following lights-on were used. No peak was used more than once.
2.7.2 Comparison of grand means of activity and cardiovascular measures between day and night
In addition to peak magnitudes of the activity rhythm, grand means for activity and cardiovascular measures were also compared between day and night, again using a repeated measures ANOVA.
2.8 Comparison of cardiovascular function between active and inactive periods
The running averages graphs were used to identify active and inactive periods of the activity cycle. Raw cardiovascular parameters were compared between active and inactive periods. Two active periods were selected from the lights-on, and two were selected from the lights-off period. Similarly, two corresponding inactive periods were selected during the lights-on period, and two were selected during the lights-off period. The three minutes surrounding the peak of an activity cycle and the three minutes surrounding the trough were used to compare the active and inactive periods.
2.9 Assessment of behavioral patterns
2.9.1. Assessment of the behavioral synchrony between the instrumented animals and non-instrumented cage-mates
Each pair of animals was video-recorded throughout the 72-hour period during which activity and cardiovascular data were recorded. The running averages graph was used to identify peaks and troughs in the activity cycle. Two representative peaks during the day and two during the night were selected, and two representative troughs during the day and two during the night also were selected. Ten minutes of video before and after each peak and trough were examined. The amount of time that both animals displayed synchrony in their behavior (i.e., both animals moving about or remaining immobile at the same time) was determined. The amount of time spent in which both animals displayed immobile, side-by-side contact (i.e., sleep-like behavior) also was quantified.
2.10 Assessment of the relationship between duration of inactivity and cardiovascular parameters
Animals often remain motionless for extended periods of time even during the active period (see Figure 2), potentially influencing cardiovascular measurements. Thus, differences in cardiovascular measurements between active and inactive periods may be explained, in part, by differences in the duration of inactivity. To examine the relationships between the duration of inactivity and cardiovascular measurements, activity peaks were visually identified from the running averages graph, and the thirty minutes of raw data before and the thirty minutes of raw data after each peak were analyzed. We identified “pauses” during the active period – times when the animal was motionless for some period of time (seconds to minutes). Regression analyses were performed on the duration of pauses and the cardiovascular measures for that time segment. In addition to the typical cardiovascular measures, we also added a measure of the change in MAP between the beginning and the end of the behavioral pause. A single activity cycle during the day was analyzed for each animal.
2.11 Statistics
All statistical analyses were performed using Statistica software (Statsoft, Tulsa OK). One-way ANOVAs followed by Student-Neuman-Keuls tests for pair-wise comparisons were performed to compare period lengths. Regression analyses were performed on data sorted in 15 minute bins and processed with a one-hour running average, because the dataset in 10 second bins was too large to yield meaningful statistical results (number of data points >26,000). Regression analyses were done to describe the relationship between activity and cardiovascular function. Comparisons of cardiovascular and behavioral measures between active and inactive periods were made using the raw data, via two-way ANOVA with repeated measures, with activity cycle (i.e., active vs. inactive periods) and time of day (i.e., day vs. night) as factors. The behavioral synchrony data were analyzed with a two-way ANOVA with repeated measures. Stage of the activity cycle (active vs. inactive) and time of day (day vs. night) were used as factors. Assessment of circadian rhythms in the group was made using a repeated measures ANOVA. All values are presented as mean ± SEM unless otherwise indicated. A p-value < 0.05 was considered to be statistically significant.
3. Results
3.1 Period lengths obtained after application of 1 hr running averages filter
During the peak of an activity cycle, the raw activity counts are highly variable (Figure 2). A one-hour running average renders activity rhythms readily apparent (Figure 3). The number of peaks, the magnitude of each peak, and the temporal difference between adjacent peaks thus can readily be determined. Repeated measures ANOVA showed that the average period lengths for activity (171.3±7.0 min.), MAP (167.4±7.0 min.), and HR (168.9±6.9 min.) were not statistically different from each other (F(2,14)= 0.14; p=0.870). In a 24-hour period, the average number of peaks for activity (8.3± 0.5), MAP (8.5 ± 0.5), and HR (8.4 ± 0.3) were not different from each other (F(2,14)= 0.13; p=0.876) (F(2,21)= 0.08; p=0.92). Descriptive statistics for the cardiovascular parameters are shown in Table 1.
Table 1.
Overall descriptive statistics for prairie vole activity and cardiovascular measures across a 72 hour sampling period
| Blood Pressure (mmHg) | |||||
|---|---|---|---|---|---|
| Activity (cpm) | HR (bpm) | MAP | Systolic | Diastolic | |
| Overall | 9.1 ± 1.0 | 451 ± 20 | 95 ± 5 | 108 ± 7 | 84 ± 16 |
| Day | 9.2 ± 1.2 | 483 ± 16 | 89 ± 3 | 101 ± 5 | 78 ± 3 |
| Night | 8.8 ± 0.8 | 471 ± 17 | 84 ± 4 | 96 ± 5 | 78 ± 3 |
| %change | −2.4 | −5.7 | −5.5 | 0.3 | |
| Active | 19.1 ± 2.3 | 465 ± 14 | 102 ± 4 | 117 ± 5 | 90 ± 3 |
| Inactive | 2.3 ± 0.3* | 431 ± 15* | 88 ± 4* | 100 ± 5* | 77 ± 3* |
| %change | −7.3 | −14.9 | −14.9 | −14.5 | |
indicates significant differences between cardiovascular measures from active and inactive periods
3.2 Assessment of circadian rhythms
3.2.1 Comparison of peak magnitudes of the activity rhythm between day and night
Repeated measures comparisons of equal numbers of peaks for each individual were made between the daytime and nighttime raw activity, and no statistically significant differences were found between the day and night (F(1,47)=0.56; p=0.46).
3.2.2 Comparison of grand means of activity and cardiovascular measures between day and night
Similarly, the question of circadian rhythms in activity and cardiovascular measures was addressed using a repeated measures ANOVA to compare average daytime and average nighttime values. No statistically significant result was found (activity: F(1,7)=0.28; p=0.61; MAP: F(1,7)=0.005; p=0.94; HR: F(1,7)=0.33; p=0.58).
3.3 Relationship between activity and cardiovascular rhythms
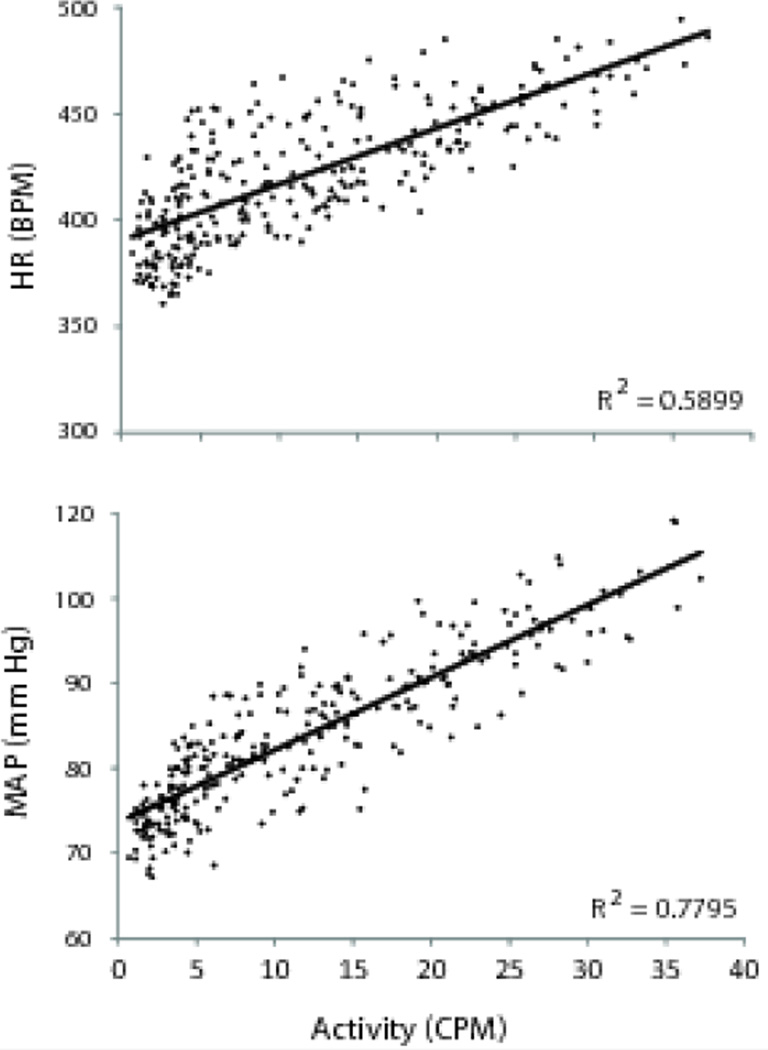
Similar to the activity pattern, cardiovascular measures also showed ultradian rhythms. The graphs of the running averages data (Figure) show that the timing of the peaks for the cardiovascular rhythms typically coincided with activity peaks, suggesting that each of the cardiovascular rhythms (i.e., MAP, HR) was in phase with the activity cycle. The relationship between the activity and cardiovascular rhythms was quantified using regression analyses, which were performed on the filtered data in 15 minute bins (Figure 4). Both HR (r=0.4±0.09; r2=0.26±0.07; slope=1.93±0.4) and MAP (r=0.63±0.09;r2=0.44±0.1;slope=0.61±0.1) were positively correlated with activity.
Figure 4.
Activity and cardiovascular rhythms. HR and MAP (black points) were positively correlated with activity. Gray lines indicate the lines of best fit. Graphs portray data from a single animal, but are representative of the animals as a group. Both relationships were statistically significant (p < 0.05).
3.4 Comparison of cardiovascular function between active and inactive periods
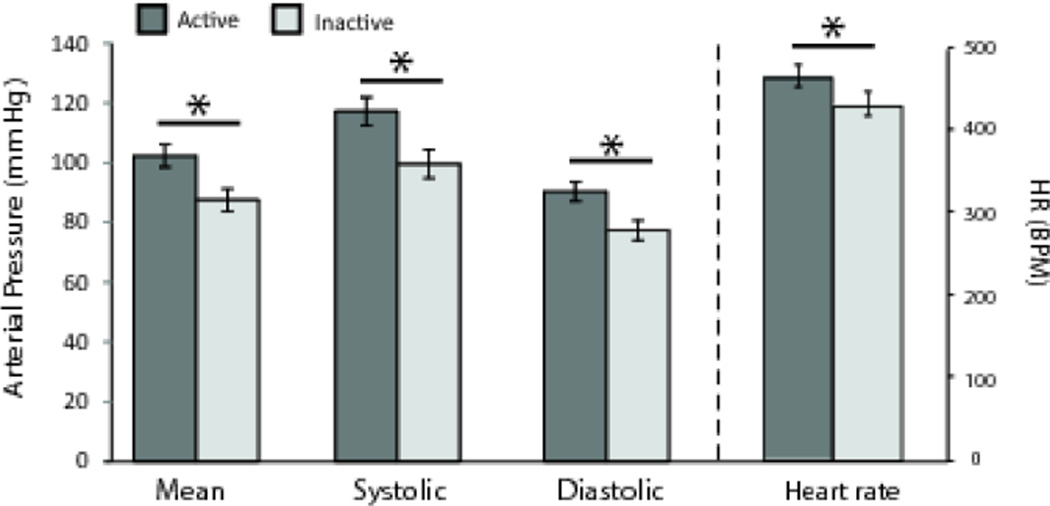
We used the running averages graphs to identify active and inactive periods and then compared raw activity and cardiovascular parameters between day and night, and between active and inactive periods (Table 1). No main effects of time of day were found for MAP (F(1,14)=0.002; p=0.96), systolic pressure (F(1,14)=0.005; p=0.98), diastolic pressure (F(1,14)=0.007; p=0.93), or HR (F(1,14)=0.041; p=0.84). However, there were main effects of activity period for each cardiovascular measure (Figure 5). MAP (F(1,14)=95.25; p<0. 001), systolic pressure (F(1,14)=92.36; p<0. 001), diastolic pressure (F(1,14)=135.26; p<0. 001), and HR (F(1,14)=18.25; p<0.001) all were higher during the active periods compared to those during the inactive periods (Figure 5). No interactions between time of day and activity levels were found (all p-values > 0.30). As expected, main effects for activity period (F(1,14)=43.76; p=0.0001) but not time of day (F(1,14)=0.06; p=0.81) were seen in activity with no interaction (F(1,14)=0.77; p=0.40).
Figure 5.
Cardiovascular dipping during the inactive period. HR and BP measures all were lower during the inactive period. Systolic and diastolic BP, as well as the MAP (left ordinate), and HR (right ordinate) all were significantly lower during the resting phase than they were during the active phase of the activity cycle . The ∼15% difference for BP measures and ∼7% difference for HR between active and inactive measures reflects inactive dipping, an ultradian analogue to nocturnal dipping in humans. Asterisks (*) indicate statistically significant differences (p < 0.05).
3.5 Stability across days
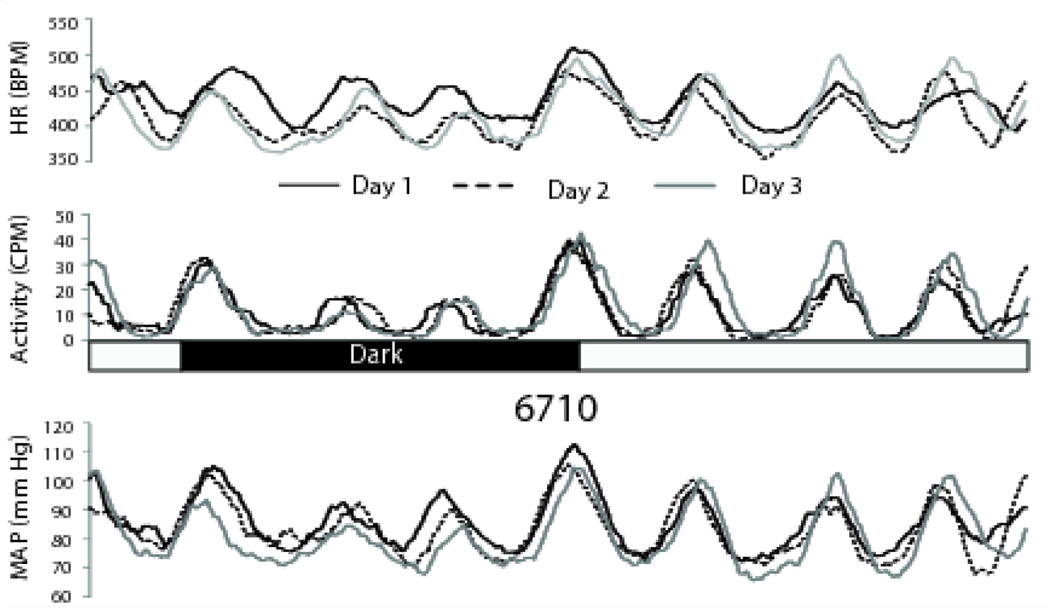
The timing and often the amplitude of each peak were consistent across days for activity, MAP, and HR (Figure 6).
Figure 6.
Activity and cardiovascular cycles are highly consistent across days. Both the patterns and amplitudes of activity and cardiovascular measures were remarkably consistent from day to day. Smoothed data from a single animal are presented in three overlaid 24 hour segments to illustrate the day-to-day consistency of the voles’ ultradian rhythms. This animal appears to show a circadian pattern (lower values during the lights-off period) but as a group, the voles did not display significant light-dark differences. Slightly greater activity was seen at the lights-on and lights-off transitions also were seen in some, but not all, animals. Light and dark periods are indicated by the black and white bar. 6710 is the animal number
3.6 Assessment of the relationship between duration of behavioral pauses and cardiovascular parameters
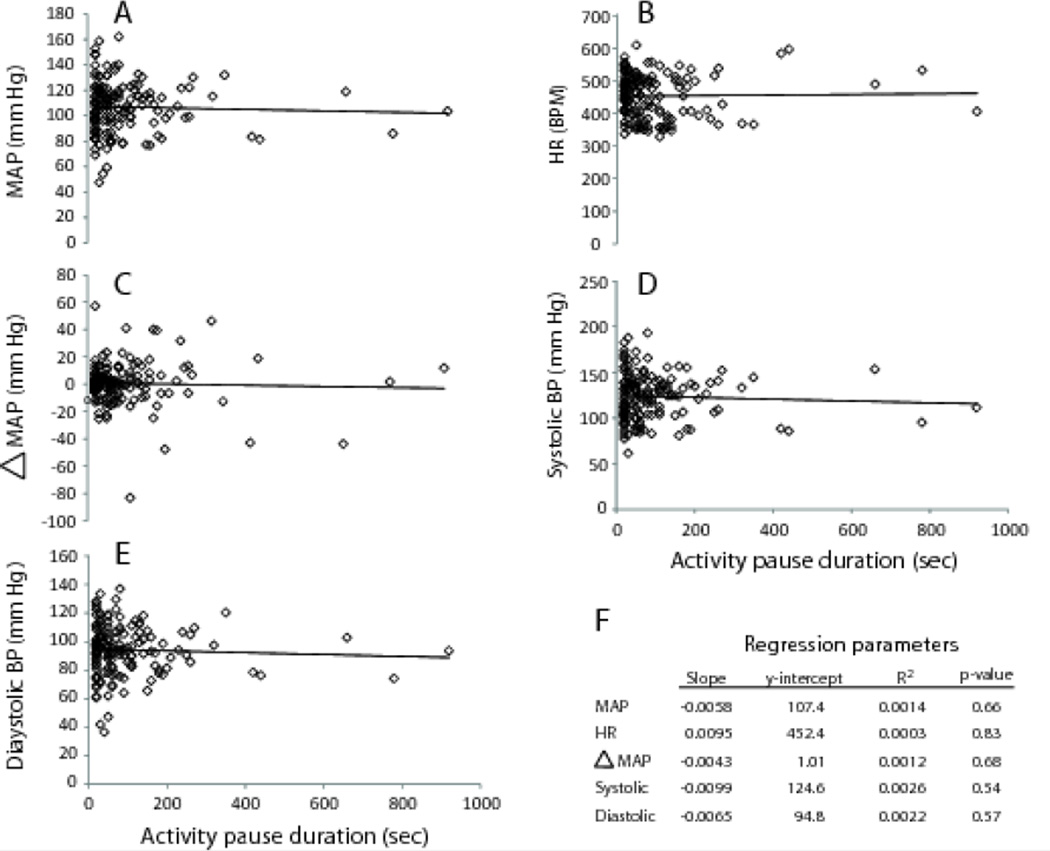
The mean and modal pause durations during the active period were 95.2 seconds and 20 seconds, respectively. None of the cardiovascular measurements varied with duration of pauses in activity during the active periods (Figure 7).
Figure 7.
Duration of immobility and cardiovascular measures. There were no statistically significant correlations between duration of activity pauses and any of the cardiovascular measures (A–E). The regression parameters are compiled in tabular form (F) to facilitate comparisons. None of the regressions were statistically significant.
3.7 Assessment of behavioral synchrony between the instrumented animals and their non-instrumented cage-mates
3.7.1 Day-Night comparisons of synchronized behavior
Behavioral synchrony between instrumented and non-instrumented cage-mates (i.e., both animals active or immobile at the same time) was assessed from 20 minute video records captured during peaks and troughs. During the day, the instrumented animal and the non-instrumented cagemate spent 72.8 ± 4.2% of the time displaying simultaneous activity or simultaneous immobility with 57.2± 7.0% spent engaging in side-by-side sleep-like behavior. Similarly, during the night, 73.0 ± 4.8% of the time the animals displayed behavioral synchrony with 56.6 ± 8.0% of the time spent in side-by-side sleep-like behavior. No differences were found between day and night measures of behavioral synchrony (simultaneous activity/immobility (F(1,26)=0.005; p= 0.95); sleeplike behavior (F(1,26)=0.01; p= 0.91).
3.7.2 Active-Inactive comparisons of synchronized behavior
During the active stage, the instrumented and non-instrumented animals displayed behavioral synchrony 54.4 ± 2.9% of the time with both animals displaying side-by-side sleep-like behavior 22.7± 4.5% of the time. During the inactive period, the animals spent 91.5 ±2.4% in behavioral synchrony with 91.0±2.6% of the time being spent in side-by-side sleep-like behavior. Differences in behavioral synchrony were observed between active and inactive periods (simultaneous activity/immobility (F(1,26)=125.76; p<0.001); and sleep-like behavior (F(1,26)=188.89; p<0.001). There were no interactions between stage of the activity cycle and time of day for any of the dependent variables (simultaneous activity/immobility (F(1,26)=3.18; p= 0.09); sleep-like behavior (F(1,26)=1.91; p= 0.18).
4. Discussion
4.1. Blood pressure dipping
Male prairie voles show cyclic changes in HR and MAP that parallel the ultradian activity cycle. MAP during the inactive periods was about 15% lower than that during the active periods. The decrease in MAP was accompanied by similar decreases in both systolic and diastolic pressures. Similarly, HR was significantly lower during the inactive periods, although the magnitude of the decrease (∼7%) was about half of that seen for the BP parameters. As a group, prairie voles in this study did not show circadian rhythms in either activity or cardiovascular function. Although they did not display circadian blood pressure dipping, they did exhibit a dipping pattern, but on an ultradian scale. The magnitude of the blood pressure decrease corresponds to the nocturnal dipping seen in patients with low cardiovascular risk. [1, 3, 31], suggesting similar mechanisms that link the activity cycle with blood pressure.
Heart rate and BP were positively correlated with activity, which on the surface might suggest that the lower cardiovascular measures during the inactive periods might simply reflect decreased cardiovascular demand associated with low physical activity. In healthy humans, systolic BP returns to baseline levels within 6–7 minutes after exercise that was rated, both objectively and subjectively, as “severe” [32]. HR returns more slowly to the resting baseline, although a sharp decline in HR does occur within the first 2–3 minutes after cessation of exercise [33]. To further evaluate the relationship between activity and cardiovascular output, we examined changes in BP and HR in relation to durations of pauses in activity. We expected that, if the BP and HR changes we observed were due to changes in activity per se, decreases in MAP and HR would be correlated with the duration of activity pauses. In fact, none of the cardiovascular measures varied with the amount of time spent immobile.
Since cardiovascular measures did not vary with the duration of immobility, the BP differences between the active and inactive periods are not simply a function of differences in activity. Rather, ultradian blood pressure dipping appears to reflect varying underlying physiological processes, such as those seen across the sleep/wake cycle[6, 34, 35]. In fact, several lines of evidence suggest that the dipping pattern seen in prairie voles during the inactive period likely occurs during sleep. First, activity data obtained from telemetry devices correlates strongly with the stages of the sleep/wake cycle [11]. Second, during the inactive period, both of the animals in a cage displayed high levels of behavioral synchrony with both animals displaying sleep-like behavior over 90% of the time. The observed behavioral patterns coupled with the extended periods of inactivity obtained from the telemetric recordings suggest that the inactive period corresponds to the sleep phase of the activity cycle. Thus, the activity rhythm displayed throughout the day and night comprises multiple sleep-wake cycles, demonstrating that voles display approximately 8 cardiovascular dipping events within a single 24h period.
These ultradian blood pressure cycles may be useful in the study of blood pressure rhythms. Model organisms with, for example, short life cycles are commonly used to understand other cyclic processes [36, 37]. In a similar fashion, the short period length of cardiovascular rhythms in the prairie vole may prove to be a useful model in which to investigate long-term effects of medications and other manipulations on blood pressure dipping.
4.2 Data collection and processing
Accurate detection of a biological rhythm depends on the sampling frequency and minimizing the noise in the dataset [25]. Typically, biological rhythms are studied by acquiring data at scheduled intervals. While the noise is considerably less than that in continuously acquired data, inter- and intra-individual variability may obscure the exact periodicity of the rhythm [38]. Consequently, peak-to-peak intervals would be miscalculated, leading to an inaccurate characterization of the frequency of the rhythm. Continuous acquisition of data overcomes the limitations of scheduled recording but introduces the problem of noise and high variability. Note that in Figure 2, the activity quickly decreased from an average of 66 activity counts/minute in an 80 second epoch to an average of 0 activity counts in another 80 second epoch. Such extreme fluctuations make it difficult to ascertain where the peak and trough of each activity cycle occurs [38]. However, in the current study, data were collected continuously but then processed with a 1hr running average, which reduced the noise and enhanced the visualization of the rhythm.
We found that this method of data collection and processing allows for clear visualization of each animal’s ultradian rhythm. Inspection of graphs of the filtered data provides reliable detection of peaks and troughs (Figure 3), allowing for peak-to-peak intervals to be used to determine period lengths. Using this method, we found that the period lengths for activity, HR, and MAP did not differ from each other. The similar period lengths and the positive correlation between activity and cardiovascular function suggest that ultradian rhythms in cardiovascular function are synchronized with the activity cycle. These results show the utility of using a running average to manage large noisy data sets. We used a one hour running average as we felt that window would best reduce noise while still retaining the ability to detect major patterns. Shorter or longer windows may be useful depending on the measure of interest.
4.3 Comparative cardiovascular physiology
This study is among the first to examine BP in the socially monogamous prairie vole (see also [39]). The 72 hour average for MAP is similar to values reported for other species [2, 40, 41]. Interestingly, the MAP we measured in prairie voles (95 mmHg) is somewhat lower than that (107 mmHg)in the closely related meadow vole (M. pennsylvanicus) [42]. This difference may be due to the methods used to obtain the measurements (implantable telemetry in conscious prairie voles vs. external catheterization in anesthetized meadow voles), or to the ages of the animals (circa 90 days in the current study vs animals as old as 482 days for the meadow voles). These methodological differences between the former and the current study permit only speculation regarding differences in arterial pressure between prairie voles and meadow voles. Meadow voles display a promiscuous mating system as opposed to the monogamous mating system of prairie voles. Lower BP in prairie voles would be consistent with the suggestion that the parasympathetic branch of the autonomic nervous system dominates cardiovascular control in monogamous species [43]
Pulse pressure was somewhat low (∼25mmHg) in the current study compared to previous studies using other rodents. [44, 45]. While it is possible that prairie voles have unusually low pulse pressure, it is more likely that the sensitivity to systolic pressure was reduced. Placement of the catheter tip is a critically important aspect in the implantation of pressure-sensitive telemetry devices [46]. However, morphological variability and even slight differences in size among subjects may produce slight differences in catheter placement relative to the aortic arch. In order to maximize the accuracy of pressure recordings, future studies may include detailed assessments of the distance between an anatomical landmark, such as the carotid bifurcation, and the aortic arch, as well as how such differences distance relate to the mass of the animal.
Ultimately, direct comparisons of monogamous and promiscuous vole species using a common method will be necessary to determine whether the BP difference between the two species is real or is simply a methodological artifact.
We chose to instrument the animals via the carotid artery as this approach improves survival rate [29]. Although the survival rate is better when the common carotid artery is used, one caveat is that baroreceptor function may be impaired. Comparison with blood pressure patterns from animal with sinoaortic denervation suggest that the baroreflex was not completely abolished in our animals [47, 48]. However, the relatively narrow range of blood pressures and the presence of activity-induced increases in blood pressure suggest some level of baroreflex function in instrumented voles. Further, recent research suggests that the arterial baroreflex not only buffers blood pressure changes but may also contribute to the blood pressure increase seen during physical activity as was seen I the this study, again suggesting functional baroreflex[47]. Nonetheless, we recognize that, with blood flow moving only through the right carotid, our implanted voles may have had reduced baroreflex sensitivity. Further testing will be needed to determine if and how much baroreflexes may be impaired when a carotid approach is used in voles.
The HR in our prairie voles (∼450 bpm) differs from that observed in other laboratory rodents such as rats and mice, likely due to the allometric relationship between HR and body mass [49]. However, the average HR of males in the current study is quite similar to the 24-hour averages of ∼430–460 bpm reported previously from electrocardiogram (ECG) data from female prairie voles [23, 50] and from male common voles (M. arvalis) [51]. Thus, at a minimum, these results suggest that male and female prairie voles do not differ in their average HRs, and that the HR in a monogamous species is not fundamentally differently from that in a promiscuous species.
The allometric relationship between HR and body mass was further examined. Previous studies have mathematically modeled the relationship using 33 mammalian species, ranging from the mouse to the humpback whale [49]. When the average vole mass of 48.4 grams from the current study is used, the predicted HR ranges from 395–469 bpm. The actual HR in the current study was 450±20.4 bpm, and the average heart rates observed during the active and inactive periods also fall within this range (Table 1). Thus, it appears that the prairie vole HR can be accurately predicted using allometric scaling methods.
4.4 Behavioral patterns
Animals that synchronize their activity rhythms with members of their social group gain survival advantages [52]. Among microtine rodents, activity rhythms can be entrained by social contact [30] as well as by photoperiod [53, 54]. The voles in the current study displayed evidence of synchronized activity with their non-instrumented cagemates. Overall, our male prairie voles spent over 70% of their time in synchronized activity/immobility with their cagemate and showed side-by-side sleeplike behavior slightly under 60% of the time. Animals continued similar degrees of behavioral synchrony during the night as they did during the day; however, differences were found between the active and inactive stages of the activity cycle. During the active stage, slightly over half of the time was spent in synchronized mobility/immobility while during the inactive stage over 90% of the time was spent in synchronized activity. Since the animals likely were sleeping during the inactive period (see 4.1), greater synchrony was observed during the inactive period. Thus, whether during the day or at night, the activity rhythms of prairie vole cagemates appear to be well-synchronized. However, further study is needed to determine if behavioral synchrony in prairie voles is driven by environmental cues, such as social contact, or by an endogenous pacemaker.
Prairie voles are highly social, and social isolation is stressful to them [55–61]. As such, instrumented voles must be housed with another animal, which can result in excessive grooming around the area where the device is implanted. In some cases, we observed evidence of excessive allogrooming behavior by the cagemate on the instrumented animal. Longer term studies may benefit from the placement of barriers to prevent such behavior.
4.5 Conclusion
To our knowledge, the current study is the first detailed examination of rhythmic changes in BP and HR, in addition to activity, in a species that displays ultradian activity rhythms. We found that filtering the data with a running average enhances the visualization of the ultradian rhythms. We also found that the magnitude of the “dip” in BP across an ultradian activity cycle is quite similar to that displayed during human circadian cycles, suggesting that his may be a valuable model for studying blood pressure dipping over multiple activity cycles. Although all of the voles in this study displayed some extent of cardiovascular dipping, there was some variability in individual responses (range 7–23%), suggesting that this measure may be responsive to manipulation. The true utility of this measure will be found when voles are subjected to surgical or pharmacological interventions that are designed to induce or mimic cardiovascular disorders[62].
Highlights.
A running average facilitates visualization of rhythmic patterns in a data set
Male prairie voles display ultradian rhythms in MAP and HR as well as in activity
Activity and cardiovascular function retain the same temporal patterns across days
Prairie voles display ultradian dipping patterns in MAP, HR
The prairie vole may be a useful model in which to study blood pressure dipping
Acknowledgments
This study was supported in part by an Oklahoma State University Graduate College Summer Dissertation Scholarship to RL and by NIH grant HD48462 to JTC. The authors would like to thank Dr. Kathleen Curtis for her critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors declare that they have no conflicts of interest.
Literature Cited
- 1.Parati G, et al. Assessment and management of blood-pressure variability. Nature Reviews Cardiology. 2013;10(3):143–155. doi: 10.1038/nrcardio.2013.1. [DOI] [PubMed] [Google Scholar]
- 2.Janssen BJ, Leenders PJ, Smits JF. Short-term and long-term blood pressure and heart rate variability in the mouse. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2000;278(1):R215–R225. doi: 10.1152/ajpregu.2000.278.1.R215. [DOI] [PubMed] [Google Scholar]
- 3.Yano Y, Kario K. Nocturnal blood pressure and cardiovascular disease: a review of recent advances. Hypertension Research. 2012;35(7):695–701. doi: 10.1038/hr.2012.26. [DOI] [PubMed] [Google Scholar]
- 4.Floras JS. Blood pressure variability: a novel and important risk factor. The Canadian Journal of Cardiology. 2013;29(5):557–563. doi: 10.1016/j.cjca.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 5.O'brien E, et al. European Society of Hypertension Position Paper on Ambulatory Blood Pressure Monitoring. Journal of Hypertension. 2013;31(9):1731–1768. doi: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- 6.Hermida RC, Ayala DE, Portaluppi F. Circadian variation of blood pressure: The basis for the chronotherapy of hypertension. Advanced Drug Delivery Reviews. 2007;59(9–10):904–922. doi: 10.1016/j.addr.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Miki K, Yoshimoto M. Sympathetic nerve activity during sleep, exercise, and mental stress. Autonomic Neuroscience : Basic & Clinical. 2013;174(1–2):15–20. doi: 10.1016/j.autneu.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Miki K, Yoshimoto M. Differential effects of behaviour on sympathetic outflow during sleep and exercise. Experimental Physiology. 2005;90(2):155–158. doi: 10.1113/expphysiol.2004.029033. [DOI] [PubMed] [Google Scholar]
- 9.Pickering TG, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697–716. doi: 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- 10.Kurtz TW, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 2: blood pressure measurement in experimental animals: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(3):e22–e33. doi: 10.1161/01.ATV.0000158419.98675.d7. [DOI] [PubMed] [Google Scholar]
- 11.Igarashi A, et al. Effect of estradiol replacement on diurnal sleep/wake pattern in ovariectomized rats measured with a subcutaneously implanted acceleration sensor. The FASEB Journal. 2015;29(1 Supplement) [Google Scholar]
- 12.Kuo TB, et al. The role of autonomic and baroreceptor reflex control in blood pressure dipping and nondipping in rats. Journal of Hypertension. 2014;32(4):806–816. doi: 10.1097/HJH.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 13.Katayama T, et al. Long-term renal denervation normalizes disrupted blood pressure circadian rhythm and ameliorates cardiovascular injury in a rat model of metabolic syndrome. Journal of the American Heart Association. 2013;2(4):e000197. doi: 10.1161/JAHA.113.000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuo TB, et al. Unstable sleep and higher sympathetic activity during late-sleep periods of rats: implication for late-sleep-related higher cardiovascular events. Journal of Sleep Research. 2013;22(1):108–118. doi: 10.1111/j.1365-2869.2012.01046.x. [DOI] [PubMed] [Google Scholar]
- 15.Kuo TB, Yang CC. Sleep-related changes in cardiovascular neural regulation in spontaneously hypertensive rats. Circulation. 2005;112(6):849–854. doi: 10.1161/CIRCULATIONAHA.104.503920. [DOI] [PubMed] [Google Scholar]
- 16.Silvani A, et al. Control of cardiovascular variability during undisturbed wake-sleep behavior in hypocretin-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2012;302(8):R958–R964. doi: 10.1152/ajpregu.00668.2011. [DOI] [PubMed] [Google Scholar]
- 17.Su DF. Treatment of hypertension based on measurement of blood pressure variability: lessons from animal studies. Current Opinion in Cardiology. 2006;21(5):486–491. doi: 10.1097/01.hco.0000240587.14463.58. [DOI] [PubMed] [Google Scholar]
- 18.Doggrell SA, Brown L. Rat models of hypertension, cardiac hypertrophy and failure. Cardiovascular Research. 1998;39(1):89–105. doi: 10.1016/s0008-6363(98)00076-5. [DOI] [PubMed] [Google Scholar]
- 19.Madison DM. Activity rhythms and spacing. In: Tamarin RH, editor. Biology of New World Microtus. Special Publication No. 2. Shippensburg PA: American Society of Mammalogists; 1985. pp. 373–419. [Google Scholar]
- 20.Gerkema MP, Groos GA, Daan S. Differential elimination of circadian and ultradian rhythmicity by hypothalamic lesions in the common vole, Microtus arvalis. Journal of Biological Rhythms. 1990;5(2):81–95. doi: 10.1177/074873049000500201. [DOI] [PubMed] [Google Scholar]
- 21.Gerkema MP, van der Leest F. Ongoing ultradian activity rhythms in the common vole, Microtus arvalis, during deprivations of food, water and rest. Journal of Comparative Physiology. A, Sensory, Neural, and Behavioral Physiology. 1991;168(5):591–597. doi: 10.1007/BF00215081. [DOI] [PubMed] [Google Scholar]
- 22.Nieminen P, Hohtola ESA, Mustonen A-M. Body temperature rhythms in Microtus voles during feeding, food deprivation, and winter acclimatization. Journal of Mammalogy. 2013;94(3):591–600. [Google Scholar]
- 23.Grippo AJ, et al. Cardiac regulation in the socially monogamous prairie vole. Physiology & Behavior. 2007;90(2–3):386–393. doi: 10.1016/j.physbeh.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oosting J, Struijker-Boudier HAJ, Janssen BJA. Circadian and ultradian control of cardiac output in spontaneous hypertension in rats. American Journal of Physiology-Heart and Circulatory Physiology. 1997;273(1):H66–H75. doi: 10.1152/ajpheart.1997.273.1.H66. [DOI] [PubMed] [Google Scholar]
- 25.Refinetti R, Cornelissen G, Halberg F. Procedures for numerical analysis of circadian rhythms. Biological Rhythm Research. 2007;38(4):275–325. doi: 10.1080/09291010600903692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brännäs KH, Andreia Estimation in integer-valued moving average models. Applied Stochastic Models in Business and Industry. 2007;17(3):277–291. [Google Scholar]
- 27.Brännäs KGJ. Autoregressive-asymmetric moving average models for business cycle data. Journal of Forcasting. 1994;13(6):529–544. [Google Scholar]
- 28.Wang L, Haizhong A, Xiaohua X, Xiaojia L, Xiaoqi S, Huang X. Generating Moving Average Trading Rules on the Oil Futures Market with Genetic Algorithms. Mathematical Problems in Engineering. 2014 [Google Scholar]
- 29.Kaidi S, et al. Comparison of two methods (left carotid artery and abdominal aorta) for surgical implantation of radiotelemetry devices in CD-1 mice. Laboratory Animals. 2007;41(3):388–402. doi: 10.1258/002367707781282839. [DOI] [PubMed] [Google Scholar]
- 30.Korslund L. Activity of root voles (Microtus oeconomus) under snow: social encounters synchronize individual activity rhythms. Behavioral Ecology and Sociobiology. 2006;61(2):255–263. [Google Scholar]
- 31.Mancia G. Short- and long-term blood pressure variability: present and future. Hypertension. 2012;60(2):512–517. doi: 10.1161/HYPERTENSIONAHA.112.194340. [DOI] [PubMed] [Google Scholar]
- 32.Uchechukwu DU, Andrew C. Determination of systolic blood pressure recovery time after exercise in apparently healthy, normotensive, nonatheltic adults the effects of age gender and exercise intensity. International Journal of Exercise Science. 2009;2(2):115–130. [Google Scholar]
- 33.Kannankeril PJ, et al. Parasympathetic effects on heart rate recovery after exercise. Journal of Investigative Medicine. 2004;52(6):394–401. doi: 10.1136/jim-52-06-34. [DOI] [PubMed] [Google Scholar]
- 34.Nisbet LC, et al. Blood pressure regulation, autonomic control and sleep disordered breathing in children. Sleep Medicine Reviews. 2013 doi: 10.1016/j.smrv.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 35.Tobaldini E, et al. Heart rate variability in normal and pathological sleep. Frontiers in Physiology. 2013;4:294. doi: 10.3389/fphys.2013.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arias AM. Drosophila melanogaster and the development of biology in the 20th century. Methods in Molecular Biology. 2008;420:1–25. doi: 10.1007/978-1-59745-583-1_1. [DOI] [PubMed] [Google Scholar]
- 37.Corsi AK, Wightman B, Chalfie M. A transparent window into biology: A primer on Caenorhabditis elegans. Genetics. 2015;200(2):387–407. doi: 10.1534/genetics.115.176099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leise TL. Wavelet analysis of circadian and ultradian behavioral rhythms. Journal of Circadian Rhythms. 2013;11(1):5. doi: 10.1186/1740-3391-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNeal N, et al. Exploring social stress though pharmacological manipulation of pair bonds in prairie voles (1171.2) The FASEB Journal. 2014;28(1 Supplement) [Google Scholar]
- 40.Just A, Faulhaber J, Ehmke H. Autonomic cardiovascular control in conscious mice. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2000;279(6):R2214–R2221. doi: 10.1152/ajpregu.2000.279.6.R2214. [DOI] [PubMed] [Google Scholar]
- 41.Molcan L, et al. The long-term effects of phase advance shifts of photoperiod on cardiovascular parameters as measured by radiotelemetry in rats. Physiological Measurement. 2013;34(12):1623–1632. doi: 10.1088/0967-3334/34/12/1623. [DOI] [PubMed] [Google Scholar]
- 42.Blaine EH. Elevated arterial blood pressure in an asymptotic population of meadow voles (Microtus pennsylvanicus) Nature. 1973;242(5393):135. doi: 10.1038/242135a0. [DOI] [PubMed] [Google Scholar]
- 43.Porges SW. The polyvagal perspective. Biological Psychology. 2007;74(2):116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Van Vliet BN, Chafe LL, Montani JP. Characteristics of 24 h telemetered blood pressure in eNOS-knockout and C57Bl/6J control mice. The Journal of Physiology. 2003;549(Pt 1):313–325. doi: 10.1113/jphysiol.2003.041897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mills PA, et al. A new method for measurement of blood pressure, heart rate, and activity in the mouse by radiotelemetry. Journal of Applied Physiology. 2000;88(5):1537–1544. doi: 10.1152/jappl.2000.88.5.1537. [DOI] [PubMed] [Google Scholar]
- 46.Zhao X, et al. Arterial pressure monitoring in mice. Current Protocols in Mouse Biology. 2011;1:105–122. doi: 10.1002/9780470942390.mo100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nishida Y, et al. Sinoaortic denervation abolishes pressure resetting for daily physical activity in rabbits. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2002;282(3):R649–R657. doi: 10.1152/ajpregu.00160.2001. [DOI] [PubMed] [Google Scholar]
- 48.Mancia G, et al. Effect of sinoaortic denervation on frequency-domain estimates of baroreflex sensitivity in conscious cats. The American Journal of Physiology. 1999;276(6 Pt 2):H1987–H1993. doi: 10.1152/ajpheart.1999.276.6.H1987. [DOI] [PubMed] [Google Scholar]
- 49.Noujaim SF, et al. From mouse to whale: a universal scaling relation for the PR Interval of the electrocardiogram of mammals. Circulation. 2004;110(18):2802–2808. doi: 10.1161/01.CIR.0000146785.15995.67. [DOI] [PubMed] [Google Scholar]
- 50.Stewart AM, et al. Acoustic features of prairie vole (Microtus ochrogaster) ultrasonic vocalizations covary with heart rate. Physiology & Behavior. 2015;138:94–100. doi: 10.1016/j.physbeh.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishii K, et al. Autonomic nervous function in mice and voles (Microtus arvalis): Investigation by power spectral analysis of heart rate variability. Laboratory Animals. 1996;30(4):359–364. doi: 10.1258/002367796780739880. [DOI] [PubMed] [Google Scholar]
- 52.Gerkema MP, Verhulst S. Warning against an unseen predator: a functional aspect of synchronous feeding in the Common Vole, Microtus arvalis. Animal Behaviour. 1990;40:1169–1178. [Google Scholar]
- 53.Cranford JA. Effect of photoperiod on activity patterns in pine voles (Microtus pinetorum) Eastern Pine and Meadow Vole Symposia. 1981 [Google Scholar]
- 54.Lehmann U, Halle S. Circadian activity patterns, photoperiodic responses and population-cycles in voles .2. Photoperiodic responses and population-cycles. Oecologia. 1987;71(4):573–576. doi: 10.1007/BF00379300. [DOI] [PubMed] [Google Scholar]
- 55.Klein SL, et al. Social environment and steroid hormones affect species and sex differences in immune function among voles. Hormones and Behavior. 1997;32(1):30–39. doi: 10.1006/hbeh.1997.1402. [DOI] [PubMed] [Google Scholar]
- 56.Grippo AJ, et al. 24-Hour autonomic dysfunction and depressive behaviors in an animal model of social isolation: Implications for the study of depression and cardiovascular disease. Psychosomatic Medicine. 2011;73(1):59–66. doi: 10.1097/PSY.0b013e31820019e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peuler JD, et al. Chronic social isolation in the prairie vole induces endothelial dysfunction: implications for depression and cardiovascular disease. Physiology & Behavior. 2012;106(4):476–484. doi: 10.1016/j.physbeh.2012.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosomatic Medicine. 2007;69(2):149–157. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McNeal N, et al. Disruption of social bonds induces behavioral and physiological dysregulation in male and female prairie voles. Autonomic Neuroscience. 2014;(0) doi: 10.1016/j.autneu.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grippo AJ, et al. Peripheral oxytocin administration buffers autonomic but not behavioral responses to environmental stressors in isolated prairie voles. Stress. 2012;15(2):149–161. doi: 10.3109/10253890.2011.605486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grippo AJ, et al. Social isolation disrupts autonomic regulation of the heart and influences negative affective behaviors. Biological Psychiatry. 2007;62(10):1162–1170. doi: 10.1016/j.biopsych.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zaragoza C, et al. Animal models of cardiovascular diseases. Journal of Biomedicine & Biotechnology. 2011;2011:497841. doi: 10.1155/2011/497841. [DOI] [PMC free article] [PubMed] [Google Scholar]