Abstract
Toxic industrial chemicals are used throughout the world to produce everyday products such as household and commercial cleaners, disinfectants, pesticides, pharmaceuticals, plastics, paper, and fertilizers. These chemicals are produced, stored, and transported in large quantities, which poses a threat to the local civilian population in cases of accidental or intentional release. Several of these chemicals have no known medical countermeasures for their toxic effects. Phosgene is a highly toxic industrial chemical which was used as a chemical warfare agent in WWI. Exposure to phosgene causes latent, non-cardiogenic pulmonary edema which can result in respiratory failure and death. The mechanisms of phosgene-induced pulmonary injury are not fully identified, and currently there is no efficacious countermeasure. Here, we provide a proposed mechanism of phosgene-induced lung injury based on the literature and from studies conducted in our lab, as well as provide results from studies designed to evaluate survival efficacy of potential therapies following whole-body phosgene exposure in mice. Several therapies were able to significantly increase 24 hr survival following an LCt50–70 exposure to phosgene; however, no treatment was able to fully protect against phosgene-induced mortality. These studies provide evidence that mortality following phosgene toxicity can be mitigated by neuro- and calcium-regulators, antioxidants, phosphodiesterase and endothelin receptor antagonists, angiotensin converting enzymes, and transient receptor potential cation channel inhibitors. However, because the mechanism of phosgene toxicity is multifaceted, we conclude that a single therapeutic is unlikely to be sufficient to ameliorate the multitude of direct and secondary toxic effects caused by phosgene inhalation.
Keywords: phosgene, inhalation, whole-body, mechanism, survival, treatment, toxicity, mice, exposure
2.0 Introduction
2.1
Toxic chemicals are a potential threat to unprotected populations, particularly in urban environments where residential, commercial, and industrial areas are intermingled. The possibility exists for portions of the civilian population to be exposed to chemical agents through industrial accidents, natural disasters, and terrorist attacks. The potential of mass casualties by an industrial accident was brought to the forefront in the 1984 Bhopal, India, disaster when approximately 3,600 people died as a result of an accidental release of 40 tons of methyl isocyanate, and possibly phosgene and hydrogen cyanide [1]. For many of these toxic chemicals, inhalation is the primary route of exposure and typically, there are no identified medical countermeasures. Supportive care usually is the only option for treatment.
Phosgene (COCl2) is a highly reactive and extensively produced toxic industrial gas that poses a substantial risk to public health. In the US, phosgene is used primarily as a chemical intermediate in the production of dyes, pesticides, plastics, polyurethanes, isocyanates, and pharmaceuticals. In 2002, the estimated annual usage in the US was over 1 million metric tons with over 10,000 workers at risk [2]. The manufacture of phosgene is mostly captive, where phosgene is produced and consumed on site; however, some phosgene is still transported by rail. For industrial applications, phosgene is produced by reacting carbon monoxide with chlorine gas in the presence of activated charcoal. In addition, phosgene can be formed by the thermal decomposition of chlorinated hydrocarbons during fires [3, 4]. As a result of this extensive usage, thousands of chemical and industrial workers, welders, and firemen are potentially at risk of exposure. Phosgene was used as a chemical warfare agent during WWI and proved to be extremely effective and lethal. Phosgene exposure has been implicated as the cause of roughly 80% of all chemical-related deaths in WWI [5]. Several characteristics of phosgene make it viable as a potential chemical weapon for terror groups: phosgene is cheap and easily produced, it can elude detection by human olfactory senses, and it has been demonstrated to be an effective weapon of mass destruction.
At low doses, odor and/or irritation by phosgene is of little use for exposure avoidance. The smell of phosgene is described as that of musty, moldy or new mown hay, and/or green corn. The odor recognition threshold ranges from 0.4 ppm to 1.5 ppm [6]. Although phosgene has an odor, it is not effective as a deterrent to exposure because olfactory fatigue occurs rapidly, and the odor may be masked by other smells [7]. While phosgene exposure can cause irritation of mucosal membranes, physiological effects can manifest from concentrations well below the threshold concentration (>3 ppm) for phosgene-induced mucosal irritation. Thus, mild or delayed irritation can result in a lack of avoidance and subsequent prolonged exposure. At higher concentrations (>150 ppm·min) phosgene exposure can cause life-threatening and latent noncardiogenic pulmonary edema that is seen 6 to 24 hr post-exposure [8]. According to the Medical Management Guidelines for Phosgene issued by the Agency for Toxic Substances Disease Registry, there is currently no effective medical countermeasure for phosgene exposure, and emergency medical treatment consists of support of cardiopulmonary functions via supplemental oxygen and possibly bronchodilators and corticosteroids [9]. Corticosteroids are known to depress several biological processes that are involved in phosgene-induced lung injury such as cytokine production/release, inflammation, and vascular leakage; there is little supportive evidence that corticosteroid treatment following phosgene exposure has any positive effect on the outcome of the injury [10–12].
Inhalation of phosgene results in distal lung damage and life-threatening pulmonary edema. Chemically, the slow rate of hydrolysis and low solubility of phosgene favor penetration into the deep lung regions, where pathophysiological changes occur. The diffusion length of phosgene in an aqueous solution has been measured to be about 8.8 μm, which is 4–8 times the thickness of the air/blood barrier [13]. The ability of phosgene to enter the capillary circulation following inhalation has been shown [14], thus providing evidence that phosgene can exert toxic effects on tissues, blood, and cellular components throughout the lung. Biologically, acylation and free radical-mediated reactions are the most relevant reactions that occur between phosgene and important cellular constituents. Acylation reactions involving phosgene occur with biological molecules containing sulfhydryl, amino, and hydroxyl moieties [15]. In addition, phosgene can undergo heterolytic and homolytic dissociation to form a highly reactive carbamoyl monochloride radical [16]. Taken together, these reactions are responsible for the alteration and dysfunction of proteins and phospholipids and the generation of pernicious reactive oxygen and nitrogen species (ROS). In laboratory animals, exposure to phosgene causes edema, affects type I pneumocytes [17, 18], and alters energy metabolism [18, 19], gene transcription, and expression of proteins involved in the glutathione (GSH) redox cycle [20–22], enhances leukotriene production [23–25], stimulates ET-1 release [26], increases lipid peroxidation [27], and decreases 3′-5′-cyclic adenosine monophosphate (cAMP) levels [25]. Based on these observations we have developed a proposed mechanism for phosgene-induced lung injury and have used this model to select and evaluate potential therapeutics.
3.0 Methods and Materials
3.1 ANIMALS
Male CD-1 mice weighing 25 to 30 grams were purchased from Charles River Laboratory (Wilmington, MA).
3.2 EXPERIMENTAL DESIGN
Groups of 40 CD-1 male mice (Charles River, Wilmington, MA) were placed in a Plexiglas cylinder (25 cm in height, 28 cm in diameter), 15.4 L in volume and exposed whole-body to a concentration time amount of 32–40.5 mg/m3 (8–10 ppm) × 20 min (640–810 mg × min/m3) phosgene and filtered clean house air at a rate of 20 L/min followed by a 5-minute room air washout. Phosgene was metered through a Brooks mass flow controller (Brooks Instruments, Fremont, CA) at a rate dependent upon the desired concentration, mixed with air, and then passed through an infrared spectrometer (Miran 1A, Foxboro Co, Sharon, MA) equipped with a real-time analog output prior to entering the exposure chamber. Out-flowing gas from the chamber was passed through a Gasmet Fourier transform infrared spectrometer (FTIR) to determine the concentration of phosgene exiting the chamber. Exhaust from the FTIR is passed through an M18 charcoal canister before being passed through a standard chemical agent fume hood. Under these conditions, the coefficient of variation of exposure to phosgene has been calculated to be 4±0.5% (n 25–30). Exposures were run utilizing a randomized block design where each treatment and treatment dose was represented. For each exposure, mice received phosgene with no treatment or phosgene with one of three selected treatment doses. Therapeutics were administered ~15–20 minutes post-exposure (PE) via intraperitoneal (ip), intramuscular (im), subcutaneous (sc) injection, or oral gavage. Although there is a latent period for frank phosgene-induced pulmonary edema, injury processes (i.e. molecular alterations, vasoconstriction, epithelial damage, and vascular leakage) are rapidly initiated (< 1hr) following exposure [22, 26, 28]. Therefore, it seems that administration of therapeutics that aim to prevent vascular leakage and edema formation should begin as soon as possible following exposure. In addition, it was determined that the quickest treatment time that could be expected by emergency personnel, i.e. first responders, would be ~15–20 minutes. Therefore, the initial dose of treatment of was set at 15 min post-exposure. Since both molecular and physiological processes become altered shortly after exposure to phosgene we consider any post-exposure treatment to be therapy rather than post-exposure prophylaxes. The dosing schedule following the 15–20-minute PE administration time was dependent on the published half-life of the drug. For each drug evaluation, three to four exposures were conducted for a total of 30–40 mice/treatment group. The number of animals used, the administration type, dosing, and dosing schedule for each treatment can be found in Table 1.
Table 1.
List of treatment compounds, functions, doses, routes, and dosing time(s). intraperitoneal (ip), intramuscular (im), subcutaneous (sc). tBHQ, tertbutylhydoquinone; VPA, valproic acid; SS-31, Szeto-Schiller-31; ETA, endothelin receptor A; GABA, gamma-aminobutyric acid; ACE, angiotensin converting enzyme; AMPK; AMP-activated protein kinase; TRPA1, Transient receptor potential cation channel, member A1
| Therapeutic | Mode of Action | Doses (mg/kg) | Route of Administration | Dosing Schedule (Post-Exposure) | N/Dose |
|---|---|---|---|---|---|
| AEOL 10150 | Antioxidant | 0, 10, 15, 20 | sc | 15 min, 6, 12, & 18 hr | 32 |
| Ambrisentan | ETA receptor blocker | 0, 10, 30, 100 | gavage | 15 min | 40 |
| Bio300 | Radioprotectant/antioxidant | 0, 100, 200, 400 | im | 15 min | 40 |
| Captopril | ACE inhibitor | 0, 30, 60, 120 | ip | 15 min, 3, 6, 9 hr | 40 |
| CP-80633 | PDE4 inhibitor | 0, 0.5, 1, 2 | gavage | 15 min, 3, 6, 9 hr | 40 |
| Cyproheptadine | Antihistamine, anticholinergic, antiseratonergic | 0, 2.5, 5, 10 | ip | 15 min | 30 |
| HC-030031 | TRPA1 inhibitor | 0, 7.5, 15, 30 | ip | 15 min, 4 hr | 40 |
| Memantine | NMDA receptor antagonist | 0, 5, 10, 20 | gavage | 15 min | 30 |
| Metformin | AMPK activator | 0, 10, 35, 100 | gavage | 15 min, 6 hr | 20 |
| RR* | Pan TRP channel & Ca2+ inhibitor | 0, 3, 6, 9 | ip | 15 min | 30 |
| SS31 | Mitochondrial Antioxidant | 0, 0.1, 0.3, 1.0 | ip | 15 min | 40 |
| Scopolamine | muscarinic receptor antagonist | .008, .02, .05 | ip | 15 min | 40 |
| Sildenafil | PDE5 inhibitor | 0, 12.5, 25, 50 | gavage | 15 min | 40 |
| SKF 96365 | SOCE & TRP Channel inhibitor | 0, 10 | ip | 15 min | 30 |
| Valproic Acid | GABA transaminase inhibitor | 0, 15, 30, 60 | ip | 15 min | 40 |
| Vigabatrin | GABA transaminase inhibitor | 0, 37.5, 75, 150 | ip | 15 min | 30 |
| Zileuton | 5-lipoxygenase inhibitor | 0, 15, 30, 60 | ip | 15 min, 3, 6, 9 hr | 40 |
RR mentioned here and elsewhere in the text stands for a drug name that cannot be disclosed due to a potential patent restriction.
3.3 THERAPEUTICS
SKF 96365, captopril, cyproheptidine, scopolamine, tert-butylhydroquinone (tBHQ), and memantine (Sigma Aldrich; St. Louis, MO); sildenafil, zileuton, and CP-80633 (Cayman Chemical; Ann Arbor, MI); valproic acid (VPA; Qualitest Pharmaceuticals; Huntsville, AL), vigabatrin (Lundbeck; Deerfield, IL), metformin (Ranbaxy Laboratories Inc.; Jacksonville, FL), Szeto-Schiller-31 (SS-31; GenScript; Piscataway, NJ); HC-030031 (Hydra Biosciences; Cambridge, MA); ambrisentan (Gilead; Foster City, CA), and RR (Acros Organics; Pittsburgh, PA).
3.4 DATA ANALYSIS
Survival data for individual therapeutics was analyzed in GraphPad Prism 5 (ver. 5.04; La Jolla, CA). Each therapeutic was evaluated against its own set of non-treated controls. Significance for each dose compared to its respective untreated phosgene control was determined using a one-way Fisher’s exact test. Significance was acknowledged if p≤ 0.05.
4.0 Results and Discussion
4.1 Putative mechanism of phosgene-induced lung injury
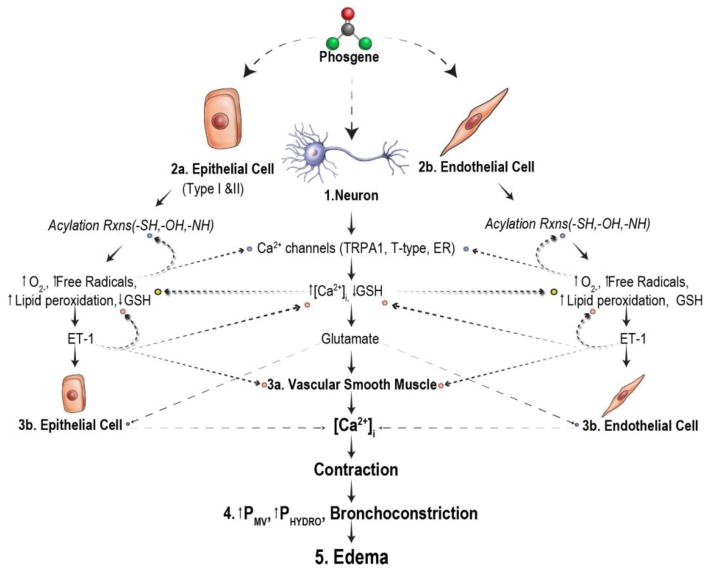
A proposed mechanism of phosgene-induced lung injury has been devised based on the extensive literature and studies conducted in our labs. A schematic of this work in progress is shown in Figure 1. At low concentrations, phosgene mainly reacts with alveolar surfactant, a mixture of lipids and proteins whose function is to lower surface tension and prevent atelectasis. Phosgene has been shown to form adducts with phosphotidylcholine (PC), the main lipid constituent of surfactant [29]. As a result, the structure and function of both PC and surfactant are altered. In addition, reactions involving carbamoyl monochloride radicals and surfactant lipids may lead to the formation of free radicals, increased lipid peroxidation, and elevated glutathione (GSH) activity. At sufficiently high concentrations, phosgene is able to penetrate the surfactant layer and deplete surfactant GSH activity, leading to elevated ROS production and diffusion into the tissue layer. At this point phosgene is capable of interacting with all components of the tissue layer (i.e., epithelial cells [Figure 1; 2a], endothelial cells [Figure 1; 2b], neurons [Figure. 1; 1], and blood constituents).
Figure 1.
Putative mechanism of phosgene-induced lung injury. GSH, glutathione; TRPA1, transient receptor potential cation channel, member A1; NMDA, N-Methyl-D-aspartic acid; Met, metabotropic; ET-1, endothelin 1; ETA, endothelin receptor A; ETB, endothelin receptor B; Pmv, pulmonary microvascular pressure; SOCE, store-operated calcium entry; Rxns, reactions
Once phosgene penetrates into the deep lung tissue and ROS levels are sufficiently high, neurogenic stimulation (Figure 1; 1 and Figure 2) occurs, resulting in early onset (within 30 min) vasoconstriction [26], hemolysis [30], and mild edema [28]. Histopathologic evaluation of phosgene-induced toxicity indicates that the initial pulmonary response to phosgene is centered around the pulmonary microvasculature and occurs without evidence of airway epithelial damage or markers of inflammation [17]. In addition to vasoconstriction, hemolysis occurs, causing vascular congestion, blood stasis, and engorging of the vasculature. Neuronal-induced changes often precede pathologic changes in studies of pulmonary edema [31]. Therefore, early phosgene-induced pulmonary responses (i.e., vasoconstriction) may be meditated by neurogenic stimulation.
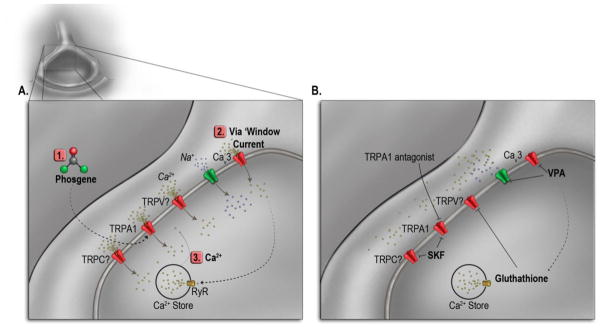
Figure 2.
Phosgene-induced neurogenic stimulation and therapeutic intervention; A) activation of neuron receptors and release of intracellular calcium stores by phosgene; B) inhibition of phosgene-induced ion channels by transient receptor potential (TRPA1) antagonist, SKF 96365, and valproic acid (VPA). TRP-, transient receptor potential channel, -C, -A1, -V.
Neurogenic activation following phosgene exposure may act through the initiation of oxygen radicals by covalently modifying cysteine residues on the intracellular -NH2 domains (Figure 2; 1) [32–37]. This covalent modification leads to a weak activation of the transient receptor potential ankyrin-1 (TRPA1) channel [38–41]. For complete activation of the TRPA1 channel, calcium is required [38–43]. This Ca2+ could be contributed from activation of T-type Ca2+ channels or through a phenomenon known as ‘window current’ (Figure 2; 2), in which voltage-activated channels exhibit both activation (open channel state) and inactivation (closed channel state) states. Each of these states can be illustrated by plotting the probability vs. voltage. Between the overlap in voltages from this plot exists the probability that a small percentage of the channels do not inactivate but remain activated allowing for a small but persistent current known as window current. The weak activation of the TRPA1 channel could depolarize the neuron to the point at which window current or activation of T-type Ca2+ channels occurs. There is evidence that T-type-mediated window current can modulate [Ca2+]i [44, 45], provide the ‘Ca2+ spark’ required for cells to progress from G1 to S phase [46–49], and contribute the Ca2+ necessary for differentiation [50, 51].
T-type-mediated window current and weakly activated TRPA1 could cause a sufficient increase in [Ca2+]i and activate ryanodine receptors on the ER, leading to a further rise in [Ca2+]i (Figure 2; 3), which would completely activate the TRPA1 channel. Indeed, there is evidence of direct involvement for T-type Ca2+ channels in capacitive calcium entry [52–55]. The depletion of the ER Ca2+ stores would then induce TRPC and TRPV channels to open. All of these acting together would lead to a significant rise in [Ca2+]i and activation of the Ca2+ dependent processes that accompany phosgene exposure, such as the release of glutamate and/or other neurotransmitters. Glutamate receptors have been found in airway neurons [56], alveolar walls, bronchiolar smooth muscle cells, and epithelial cells [57, 58]. Binding of glutamate to N-Methyl-D-aspartic acid or N-Methyl-D-aspartate (NMDA) receptors on the vascular smooth muscle cells (VSMC, Figure 1; 3a) results in the influx of Ca2+ into the intracellular space, leading to cell contraction and vasoconstriction of the pulmonary microvasculature, apoptosis, and necrosis (Figure 1; 3a-4) [59].
Concurrent with neuron activation, the constituents of the phospholipid membranes (i.e., lipids and proteins) of epithelial type I and II cells (Figure 1; 2a) and endothelial cells (Figure 1; 2b) undergo acylation reactions with phosgene and free radical-mediated reactions with reactive carbamoyl monochloride radicals, which are formed through hetero and/or homolytic cleavage of phosgene [16]. As mentioned above, this causes the gradual loss of structure and function of the cellular membranes, leading to an increase in membrane fluidity, heightened ROS formation, and the depletion of tissue GSH. Evidence of this has been demonstrated in studies where inhalation of phosgene caused a reduction in both bronchoalveolar lavage fluid superoxide dismutase activity [21] and tissue GSH levels [21, 27], while lipid peroxidation in lung was elevated [27, 60]. This increase in ROS may stimulate the induction of endothelin 1 (ET-1) mRNA [61–63], followed by the release of ET-1 peptide, a potent inducer of vasoconstriction [64]. Studies have also shown that angiotensin-converting enzyme activity and angiotensin II production are elevated in phosgene-induced pulmonary damage [65]. Angiotensin II, along with norepinephrine, is a vasoactive peptide that increases vascular permeability and induces vasoconstriction. Furthermore, both angiotensin II and norepinephrine stimulate the induction of ET-1 [66, 67]. Early (within 1 hr) interstitial edema and protein transudation [27] following phosgene exposure may be the result of the angiotensin II and norepinephrine release, while the ET-1 signaling cascade may play a vital role in the latent fulminating edema stages (4–8 hr PE).
Induction of ET-1 mRNA has been seen as early as 30 min PE [68] with elevations in ET-1 ligand within 3 hr post-exposure [26]. Soluble ET-1 binds to endothelin receptor A and endothelin receptor B (ETA or ETB) on the VSMC and endothelial cells (Figure 3) [69]. Based on pathophysiological outcomes (e.g., vasoconstriction), ET-1 signal transduction through ETA may have the most prominent physiological effects following phosgene exposure. Activation of this pathway results in the biphasic release of Ca2+ and the activation of a Gαq protein, which in turn activates phospholipase C and the production of inositol trisphosphate (IP3) [69, 70]. IP3 then binds to a receptor-mediated channel in the endoplasmic reticulum, causing the transient release of intracellular Ca2+ stores [69, 70]. Following the transient release of Ca2+, voltage-gated (e.g., T-type) or receptor-operated Ca2+ channels (e.g., TRP channels) are opened, providing the sustained Ca2+ flux required to produce long-term contraction of VSMC (Figure 1; 3a) [69, 70]. However, L-type Ca2+ channels have been determined to not be involved in intracellular Ca2+ mobilization in endothelial cells [69], which is of importance when discussing constriction of the microvasculature. Furthermore, ET-1 receptor signaling is not fully understood, and there is evidence that VSMC contraction induced by ET-1 may be the result of ETA/ETB coupled signal transduction rather than ETA alone [67, 71]. This must be taken into consideration when testing ET-1 receptor antagonists to attenuate vasoconstriction in different pathologies.
Figure 3.
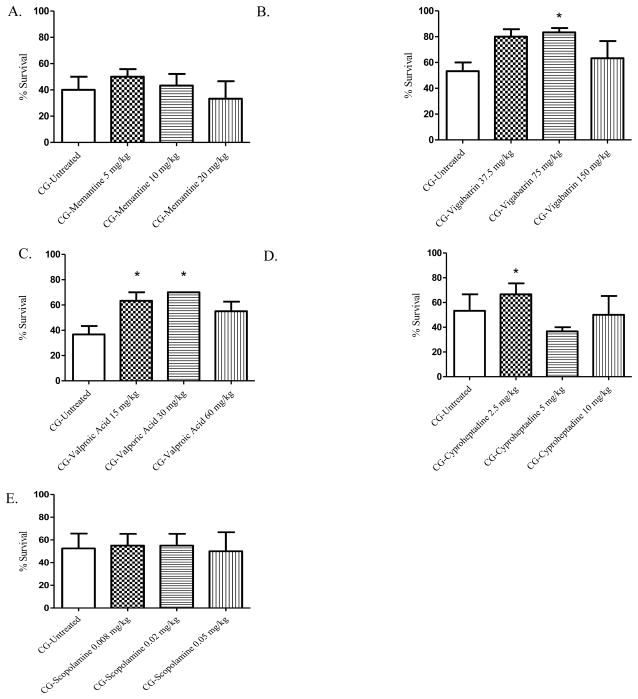
A–E. Modulators of neurogenic signaling and 24-hour survival percentages. Animals were exposed whole-body to phosgene (CG) at a concentration of 8–10 ppm for 20 min and 15–20 min post-exposure animals received either no treatment (untreated phosgene exposed control) or one of three doses of treatment drug; A) memantine; B) vigabatrin; C) valproic acid; D) cyproheptadine; and E) scopolamine. Data shown as mean ± standard error of the mean; *p<0.05; therapeutic dose vs respective untreated phosgene exposed group
Phosgene toxicity has been shown to stimulate the production of additional mediators of vasoconstriction (e.g., leukotrienes C4, D4, and E4) [24, 60] and suppress mediators of vasodilation (e.g., prostaglandins and 3′-5′-cyclic adenosine monophosphate (cAMP) [24, 27, 72], all of which can occur via ETA/ETB coupled signal transduction [69, 73]. Activation of this pathway results in Gα activation of phospholipase A2 and the subsequent deacylation of phospholipids, leading to the release of arachidonic acid (AA) [73]. Liberated AA is then metabolized by lipoxygenase-producing leukotrines C4, D4, and E4. The release of these cysteinyl leukotrienes (LTC4, LTD4 and LTE4) leads to cellular contraction by stimulating the release of intracellular Ca2+ stores. The type of receptors involved in leukotriene-induced Ca2+ release is not fully understood; however, receptor-operated Ca2+ channels are theorized to be involved [74]. Metabolism of AA can also result in the production of prostaglandins through cyclooxygenase activity, which in turn can stimulate cAMP synthesis inducing vasodilatation. However, activation of ETB has been demonstrated to have no effect on cyclooxygenase activity [75] and to have an inhibitory effect on adenylate cyclase [76] and cAMP synthesis [77]. These findings are in agreement with this proposed mechanism of phosgene toxicity, as there is no evidence of altered cyclooxagenase activity [24] and levels of cAMP are diminished [60] following phosgene exposure.
Endothelial ETB stimulation, on the other hand, increases eNOS activity and the subsequent production of NO. The activation of NO synthesis by ETB may occur early following phosgene exposure as evidenced by the elevation of NO levels in plasma from 1–3 hr PE [26]. However, ET-1 and NO production is controlled in paracrine fashion, in which an imbalance in the synthesis of one will inhibit the production of the other [78, 79]. NO–induced effects are most likely blunted by phosgene-induced ROS formation at early timepoints PE, as vasoconstriction has been seen within 1 hr PE. In addition, activation of ETA has been shown to significantly increase superoxide production, which may further inhibit NO signaling and synthesis [80]. This creates an imbalance between mediators of vasoconstriction and vasodilatation, resulting in prolonged and intense contraction of both VSMC and the endothelium (Fig 1; 3a & 3b).
Contraction of both VSMC and endothelium of the pulmonary microvasculature creates a rise in pressure (Pmv, Fig 1; 4) that exceeds the threshold value for edema formation, causing the transudation of fluid, proteins, and erythrocytes (RBC) from the capillaries into the interstitial space and eventually into the alveolar space. As ET-1 signal transduction becomes more prominent, vasoconstriction becomes more intense, causing pressure to rise in the larger airways. As a result, hydrostatic stress occurs which leads to fulminating and life-threatening pulmonary edema (Fig 1; 5).
Phosgene-induced hemolysis may also play a role in increased Pmv, fluid extravasation and the formation of reactive oxygen species. Hemolysis causes cellular hyperaggregation, which leads to the blocking of microcirculation in constricted areas [81] and increases blood flow in areas adjacent to capillary constriction. Increased blood flow in areas of already heightened capillary pressure will cause pressure to further increase, thus surpassing the ability of alveolar capillaries to maintain proper fluid balance. In addition, free hemoglobin released from damaged RBC can act as a Fenton reagent and produce highly reactive hydroxyl radicals, further stimulating ROS formation in the alveolar space [14].
4.2 Survival evaluation of selected therapies for phosgene-induced lung injury
4.2.1 Therapeutic compounds that may mediate neuronal responses
While neurogenic responses may play a role in phosgene-induced pulmonary edema [30, 82], very few studies have been conducted. To assess the ability of neuro-regulators to improve survival following phosgene inhalation, we evaluated memantine, VPA, vigabatrin, scopolamine, cyproheptadine, and HC-030031 (Figure 3A–E).
4.2.1.1 Compounds affecting neurotransmission
Glutamate toxicity and NMDA receptor activation have been indicated in the pathogenesis of acute lung injury, wherein overstimulation of the NMDA receptor has been shown to cause acute pulmonary edema, airway hyper-responsiveness, and inflammation [83]. To determine if modulating the NMDA receptor would improve survival following phosgene challenge, we tested memantine (Figure 3A), an NMDA receptor blocker used clinically for the treatment of Alzheimer’s disease. Memantine did not improve 24 hr survival at any of the three doses administered (5, 10, 20 mg/kg). In addition, we tested vigabatrin (Figure 3B) and VPA (Figure 3C), which prevent the catabolism of the neurotransmitter gamma-aminobutyric acid (GABA) by inhibiting GABA transaminase. Both vigabatrin and VPA had increased 24 hr survival rates when administered 20 min PE when compared to non-treated, phosgene-exposed mice. At doses of 75 and 150 mg/kg, vigabatrin treatment had significantly increased survival rates (83.3 and 80%, respectively) compared to non-treated exposed animals, 53.3%. VPA had a significantly increased survival rate at doses of 15 and 30 mg/kg compared to 35% for the non-treated, exposed group, 67.5 and 70%, respectively.
Serotonin is a monoamine neurotransmitter that can act on endothelial cells of the pulmonary microvasculature and cause vasoconstriction [84] that may lead to vascular permeability and edema formation. Histamine, released by mast cells and basophils, can act upon vascular smooth muscle cells and perivascular autonomic nerve terminals, causing an increase in capillary permeability and bronchoconstriction. Moreover, when nitric oxide formation is inhibited, as may be the case with phosgene [85], the release of acetylcholine from parasympathetic cholinergic neurons can cause smooth muscle contraction through smooth muscle muscarinic, M2 and M3, receptor signaling. The antihistamine, anticholinergic, and antiserotonergic compound cyproheptadine (Figure 3D) had an significantly increased 24 hr survival rate following phosgene exposure from 53.3% (non-treated) to 66.7% when administered at a dose of 2.5 mg/kg. To further assess if phosgene-induced cholinergic signaling plays a major role in mortality, we administered a cholinergic muscarinic antagonist, scopolamine (Figure 3E), at 0.008, 0.02, and 0.05 mg/kg; however, scopolamine failed to alter phosgene-induced mortality at any of the three doses tested. The increased survival rates achieved through the administration of the GABAergic compounds VPA and vigabatrin or the anticholinergic/antiserotonergic compound cyproheptadine suggests that phosgene toxicity and mortality are partially mediated by neurogenic signaling mechanisms.
4.2.1.2 Compounds affecting TRP channel signaling
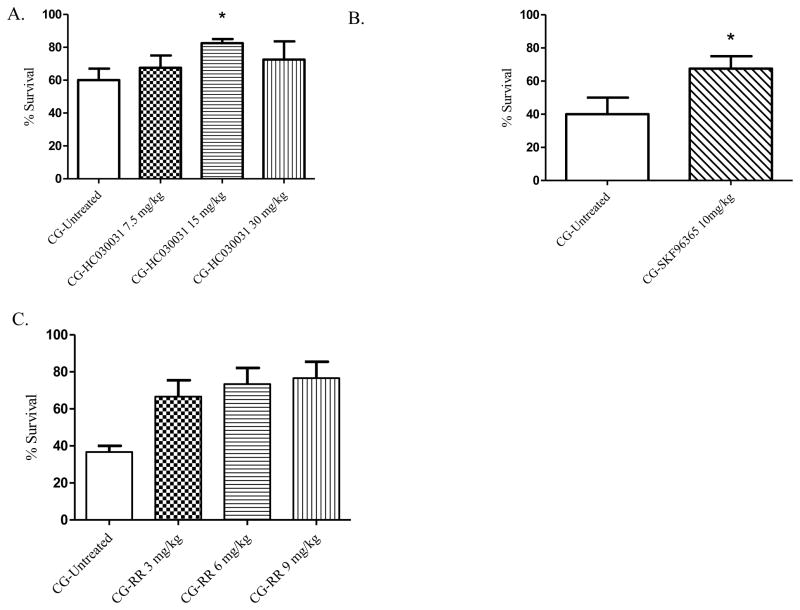
TRP channels are expressed on neurons of the respiratory tract and the pulmonary epithelium and endothelium. These chemosensory channels are activated by potential harmful inhalants [86], which leads to neurogenic inflammation, alterations of vascular blood flow, and edema formation. TRPA1 is highly expressed on the trigeminal and vagal nerves throughout the lung to include the respiratory bronchioles, alveolar ducts and alveoli [87] and have been found in human lung fibroblast and alveolar epithelial cell lines [88]. Activation of TRPA1 causes airway irritation and inflammation. TRPA1 may be activated by phosgene, resulting in the release of intracellular Ca2+ stores as described above. The TRPA1 inhibitor HC-030031 at a dose of 15 mg/kg had a significantly (p=0.047) increased 24 hr survival rate from 60% in non-treated controls to 82.5% with treatment (Figure 4A). SKF 96365 is a general TRP antagonist [89] and an inhibitor of store-operated calcium entry channels (SOCE) that has been shown to block TRP channel activity in the airway [90]. In this model of phosgene-induced lung injury, SKF had a significantly increased 24 hr survival rate from 43% in phosgene-exposed, non-treated mice to 70% when SKF was administered at 10 mg/kg (Figure 4B). In addition to SKF, we also evaluated the pan-TRP inhibitor RR. When administered 20 min after phosgene exposure, all three doses of RR (3, 6, and 9 mg/kg) had significantly increased 24 hr survival rates from 36.7% in exposed, non-treated animals to 66.7, 73.3 and 76.7%, respectively (Figure 4C). The increased survival rates were found to be highly significant at the 6 and 9 mg/kg doses (p=0.004 and p=0.002).
Figure 4.
A–C. Modulators of TRP channel signaling and 24-hour survival percentages. Animals were exposed whole-body to phosgene (CG) at a concentration of 8–10 ppm for 20 min and 15–20 min post-exposure animals received either no treatment (untreated phosgene exposed control) or one of three doses of treatment drug; A) HC-030031 (subsequent dose administered at 4 hr post-exposure); B) SKF96365; and C) RR. Data shown as mean ± standard error of the mean; *p<0.05; therapeutic dose vs respective untreated phosgene exposed group
4.3 Compounds affecting vascular tone (targeting endothelial and smooth muscle cell signaling)
4.3.1 Phosphodiesterase inhibitors
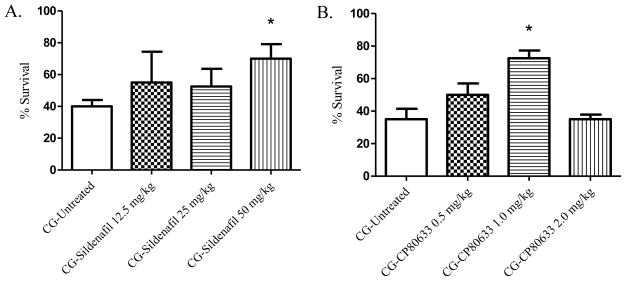
cAMP levels have been demonstrated to decrease following phosgene exposure, and treatment with selected phosphodiesterase inhibitors has been shown to reduce phosgene-induced edema formation [23, 91, 92]. cAMP and cGMP promote smooth muscle relaxation and help maintain vascular permeability. Sciuto et al., (2004) postulate that PDE activity is increased following phosgene exposure due to a rise in intracellular free Ca2+. In turn, PDEs degrade cAMP and cGMP resulting in barrier loss and edema formation. Sildenafil is a commonly used PDE 5 inhibitor for the treatment of pulmonary hypertension and erectile dysfunction that blocks the breakdown of cGMP, a signaling molecule that stimulates vasodilation and increases blood flow. Oral administration by gavage of sildenafil (50 mg/kg) at 20 min, 3, 6 and 9 hr PE resulted in a 30% (p=0.006, 40 to 70%) increase in survival following phosgene exposure (Figure 5A). Treatment with the phosphodiesterase 4 inhibitor CP-80633 had a slight increase in survival rate over sildenafil and had an increased survival rate from 35% in the phosgene non-treated group to 72.5% with CP treatment (p=0.008, Figure 5B). Thus, both cGMP and cAMP seem to have a significant role in phosgene-induced mortality, and PDE inhibitors are viable treatment options that should be investigated further.
Figure 5.
A–B. Phosphodiesterase inhibitors and 24-hour survival percentages. Animals were exposed whole-body to phosgene (CG) at a concentration of 8–10 ppm for 20 min and 15–20 min post-exposure animals received either no treatment (untreated phosgene exposed control) or one of three doses of treatment drug; A) sildenafil; and B) CP-80633 (subsequent doses administered at 3, 6, and 9hr post-exposure). Data shown as mean ± standard error of the mean; *p<0.05; therapeutic dose vs respective untreated phosgene exposed group
4.3.2 Leukotriene, ACE, and Endothelin-1 Inhibition
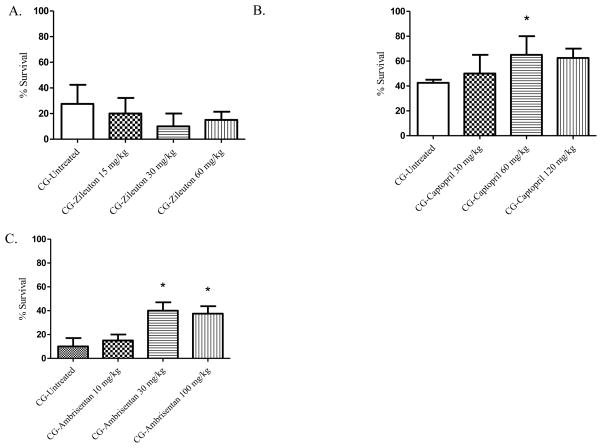
Phosgene exposure has been shown to enhance potent mediators of vasculature permeability and vascular tone to include leukotrienes, endothelin, and angiotensin [24, 26, 65]. In addition, animal studies have shown that inhibition of leukotriene synthesis or leukotriene receptor signaling by treatment compounds such as methylprednisolone, FPL 55712, and LY 171883 can reduce phosgene-induced pulmonary edema [24]. Based on these data, we evaluated the following FDA-approved therapeutics for their ability to improve survival following phosgene intoxication (Figure 6A–C): zileuton, a 5-lipoxygenase inhibitor; captopril, an ACE inhibitor; and ambrisentan, an endothelin A receptor antagonist. Zileuton prevents the synthesis of vasoactive leukotrienes (LTB 4, LTC 4, LTD 4, and LTE 4) and is used clinically as maintenance therapy for treatment of asthma. While other studies have shown the benefit of leukotriene inhibition in reducing phosgene-induced edema formation, zileuton administered 20 min PE failed to improve 24 hr survival at any of the three doses tested (15, 30, 60 mg/kg) (Figure 6A). Captopril is an inhibitor of ACE activity which in turn blocks the conversion of angiotensin I to angiotensin II. Clinical indications for captopril include hypertension, heart failure, and diabetic nephropathy. Angiotensin II promotes constriction of the pulmonary vasculature primarily by acting on vascular smooth muscle cells. Captopril, at a dose of 60 mg/kg, had an increase in survival rate in mice following phosgene exposure from 42.5% in non-treated control animals to 65% with treatment (p = 0.036) (Figure 6B). At a dose of 120 mg/kg, captopril treatment had an increase in survival rate over the non-treated control group (62.5% vs 42.5%, respectively); however, it was not significant (p =0.0583). Angiotensin is also capable of stimulating the production of ET-1 following activation. ET-1 is potent vasoconstrictor that has the ability to promote long-term contraction of the microvasculature. Ambrisentan is an ETA receptor antagonist which is clinically used to treat pulmonary artery hypertension. When tested in our phosgene inhalation model, administration of 30 and 100 mg/kg ambrisentan 20 min after phosgene exposure had an increased 24 hr survival rate of 30% and 27%, respectively, over non-treated, exposed mice. Both of these increases were found to be significant, p=0.004 and p=0.008, respectively (Figure 6C).
Figure 6.
A–C. Inhibitors of potent pulmonary vasoconstrictors and 24-hour survival percentages. Animals were exposed whole-body to phosgene (CG) at a concentration of 8–10 ppm for 20 min and 15–20 min post-exposure animals received either no treatment (untreated phosgene exposed control) or one of three doses of treatment drug; A) zileuton (subsequent doses administered at 3, 6, and 9 hr post-exposure); B) captopril (subsequent doses administered at 3, 6, and 9 hr post-exposure); and C) ambrisentan. Data shown as mean ± standard error of the mean; *p<0.05; therapeutic dose vs respective untreated phosgene exposed group
4.4 AMP-activated protein kinase (AMPK) agonist
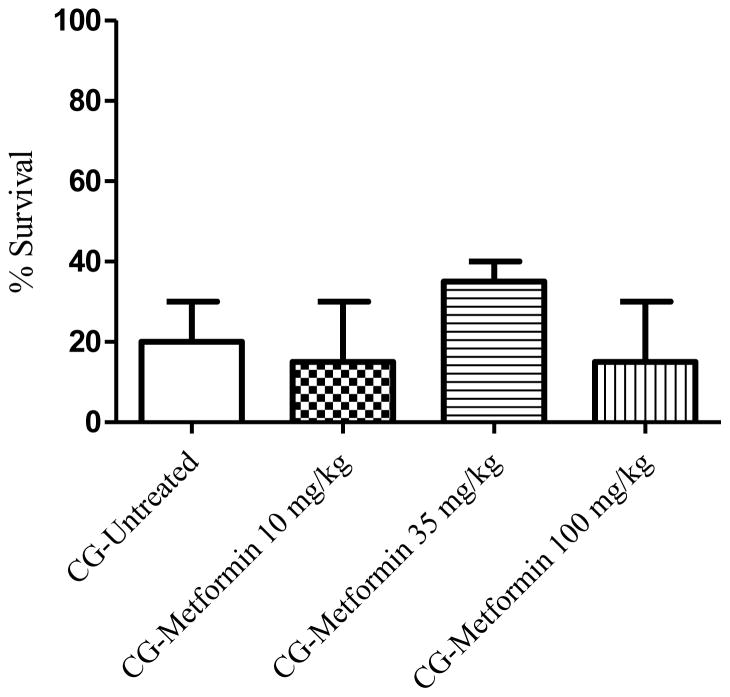
The metabolic sensor AMPK has been shown to play a role in vascular endothelial repair and modulation of vascular permeability [93]. AMPK has two known isoforms, a1 and a2. AMPK a1 is expressed in the microvasculature and is important in lung microvasculature endothelial repair, whereas a2 is found primarily in large extra alveolar vessels and may contribute to endothelial dysfunction [94]. Activation of AMPK by metformin, which is FDA-approved for the treatment of type 2 diabetes, was able to significantly improve LPS-induced lung injury, as indicated by a reduction in neutrophil accumulation in the lungs, a decrease in interstitial pulmonary edema, and a reduction in the accumulation of Evans Blue dye in the lung tissue [95]. In addition, metformin is known to affect a plethora of markers, such as cell viability, apoptosis, generation of reactive oxygen species, intracellular antioxidant enzymes and reduced glutathione, regulation of Nrf2 genes and genes related to the antioxidant response element, inflammation, and mitochondrial redox states, that are a common with phosgene-induced pulmonary toxicity [96–98]. However, in our model of phosgene-induced lung injury, oral administration of metformin (10, 35 or 100 mg/kg) at 20 min and 6 hr PE did not significantly increase survival (Figure 7). A dose of 35 mg/kg of metformin had an increased survival rate of 15% as compared to the non-treated, exposed animals. Therefore, additional doses between 35 and 100 mg/kg should be considered for further investigation.
Figure 7.
Modulator of AMP-activated protein kinase and 24-hour survival percentage. Animals were exposed whole-body to phosgene (CG) at a concentration of 8–10 ppm for 20 min and 15–20 min post-exposure animals received either no treatment (untreated phosgene exposed control) or one of three doses of metformin. At six hours post-exposure an additional injection of each dose of metformin was administered to the respective treatment group. Data shown as mean ± standard error of the mean
4.5 Antioxidants
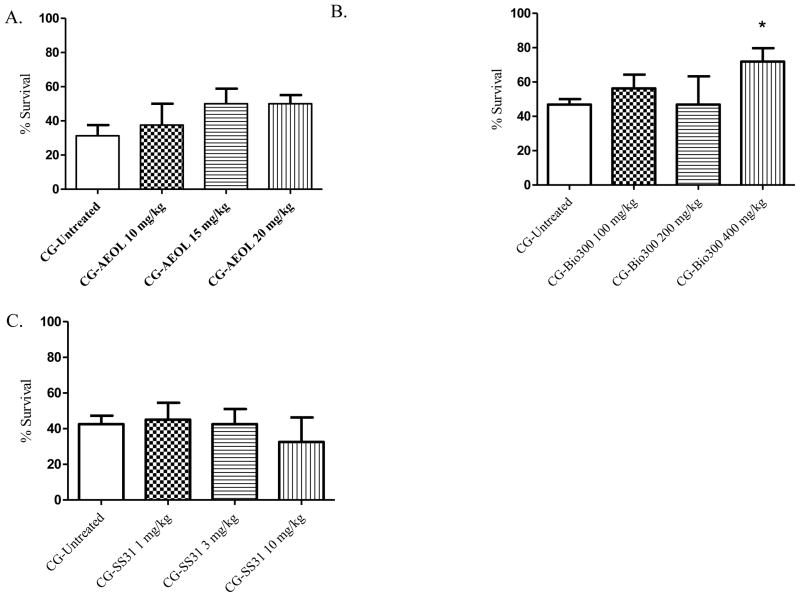
Oxidative stress plays a significant role in phosgene-induced lung injury and is likely to cause damage to all constituents of the deep lung. Several studies have evaluated the therapeutic value of antioxidant compounds against phosgene toxicity [20, 21, 99–102], and while it seems that pre-exposure administration of antioxidants may provide substantial protection against phosgene toxicity, protection is rarely afforded if administered PE. The lack of PE efficacy may be attributed to the inability of these antioxidant compounds to reach a body-burden level that is sufficient to detoxify phosgene-induced oxidative radicals at a sufficiently high rate and within a short duration so as to prevent oxidant-induced pulmonary injury. Prophylactic treatment, on the other hand, is not a viable option for protecting against phosgene exposure considering the unpredictability of an exposure. However, these studies provide substantial evidence that oxidative stress plays an important role in phosgene toxicity and demonstrate the limitations of many antioxidants as viable treatment options. Two antioxidant compounds, n-acetylcysteine (NAC) and caffeic acid phenethyl ester (CAPE), have been shown to reduce phosgene toxicity when administered PE. Both NAC and CAPE were able to reduce lung edema and improve biochemical markers of lung injury, such as glutathione, superoxide dismutase, and lipid peroxidation, following phosgene inhalation [20, 101, 102]. The mechanism by which these compounds provide protection against phosgene toxicity may be due to their ability to act directly as an antioxidant as well as their ability to activate the Nrf2 pathway. The activation of Nrf2 may be important to the mitigation of phosgene toxicity as it is responsible for regulating the production of endogenous phase 2 and antioxidant proteins. Based on this success, we evaluated three novel antioxidants (Figure 8A–C): AEOL 10150, Bio300 (Genistein), and SS-31. AEOL 10150 is a metalloporphyrin catalytic antioxidant that possesses superoxide-, hydrogen peroxide-, peroxynitrite-, and lipid peroxide-scavenging activities [103]. Bio300 is an isoflavone that has antioxidant properties and is capable of inducing Nrf2 expression. SS31 is a small peptide antioxidant that scavenges reactive oxygen species in the inner membrane of mitochondria. Of these three drugs, Bio300 was the only compound to demonstrate a significant increase in 24 hr survival following phosgene exposure. With Bio300 treatment administered intramuscularly at 400 mg/kg, survival increased from 47% in non-treated, exposed mice to 72% with treatment (p=0.037) (Figure 8B). Intraperitoneal injection of SS31 did not produce a positive effect on survival at doses of 1 mg/kg; however, at 0.1 mg/kg, SS31 had an increased survival rate (55.5%) when compared to non-treated, exposed mice (42.5)(Figure 8C). AEOL 10150 did not change 24 hr survival with the three doses tested; however, all three doses did improve survival when compared to non-treated, exposed animals (Figure 8A).
Figure 8.
A–C. Evaluated antioxidants and 24-hour survival percentages. Animals were exposed whole-body to phosgene (CG) at a concentration of 8–10 ppm for 20 min and 15–20 min post-exposure animals received either no treatment (untreated phosgene exposed control) or one of three doses of treatment drug; A) AEOL (subsequent doses administered at 6, 12, and 18 hr post-exposure); B) Bio-300; and C) SS31. Data shown as mean ± standard error of the mean; *p<0.05; therapeutic dose vs respective untreated phosgene exposed group
5.0 Conclusion
In summary, the mechanisms of phosgene injury are complex, and not fully understood. For these reasons, the identification of a practical therapeutic has eluded researchers. The chemical properties of phosgene allow for its penetration into the deep lung where it undergoes both acylation and free radical-mediated reactions. These reactions occur with important biological constituents, altering their structures and functions and generating destructive ROS. In sufficient concentrations, phosgene is able to penetrate the tissue layer and stimulate pulmonary neurons and react with important cellular constituents of epithelial and endothelial cells. Phosgene, either directly or indirectly, stimulates Ca2+ channels in pulmonary neurons, resulting in cell depolarization and neurotransmitter release, and leading to VSMC constriction, resulting in early phosgene-induced vasoconstriction. Concurrent with neuronal stimulation, phosgene-induced ROS formation stimulates the induction and release of potent mediators of vasculature tone and vascular permeability (leukotrienes, angiotensin, and ET-1) from epithelial and endothelial cells, promoting both short- and long-term constriction of the microvasculature. Signal transduction from these mediators is dependent on the release of intracellular Ca2+ stores into the intracellular space of VSMC and endothelial cells, causing prolonged and intense cellular contractions. This leads to heightened capillary pressures, hydrostatic stress, and fulminating pulmonary edema.
Survival outcomes from our therapeutic mouse screening model provide supportive evidence for the idea that neuromodulation may be a viable treatment option for phosgene-induced lung injury. In addition, this study further demonstrates that therapeutics which inhibit the formation of and/or interrupt the signaling pathways of potent mediators of vascular tone and permeability, like PDEs, angiotensin, and endothelin, may also enhance survival following phosgene exposure. Moreover, these studies provide evidence that phosgene-induced ROS formation plays a critical role in phosgene-induced mortality and that antioxidant administration may increase survival following phosgene exposure. Survival is an important endpoint and may well be the most important endpoint when considering the effectiveness of a therapeutic against a highly toxic compound that triggers a multitude of injurious pathways. For this reason we selected survival as the endpoint for the initial screening of the potential therapeutics discussed. However, little is gained on the role these treatments played in mediating such pathways. More in-depth studies must be conducted to gain a better understanding of the potential mechanisms that provide such survival benefits, which may be extrapolated to humans. While progress has been made a single therapeutic seems unlikely to be sufficient to ameliorate the multitude of direct and secondary toxic effects caused by phosgene inhalation.
Highlights.
Describes proposed mechanisms of phosgene induced-lung injury
Provides rational treatment strategies for phosgene inhalation injury
Several therapeutics significantly increased 24 hr survival
Acknowledgments
Funding: This research was supported by an interagency agreement between NIH/NIAID (Y1-A1-6179-04) and the USAMRICD (A120-B.P2009-03).
We thank Hydra Biosciences and Gilead for their generous donations of HC-030031 and ambrisentan, respectively.
We thank James Abraham for the artistic illustrations of figure 1 and 2.
Abbreviations
- FTIR
Fourier transform infrared spectrometer
- PE
post-exposure
- ip
intraperitoneal
- im
intramuscular
- tBHQ
tertbutylhydoquinone
- VPA
valproic acid
- SS-31
Szeto-Schiller-31
- PC
phosphotidylcholine
- GSH
glutathione
- ROS
reactive oxygen species
- TRP(A1)
transient receptor potential cation channel, member A1
- NMDA
N-Methyl-D-aspartic acid or N-Methyl-D-aspartate
- VSMC
vascular smooth muscle cells
- ET-1
endothelin 1
- ETA
endothelin receptor A
- ETB
endothelin receptor B
- IP3
inositol trisphosphate
- AA
arachidonic acid
- cAMP
cyclic adenosine monophosphate
- RBC
erythrocytes
- Pmv
pulmonary microvascular pressure
- SOCE
store-operated calcium entry
- GABA
gamma-aminobutyric acid
- ACE
angiotensin converting enzyme
- cGMP
cyclic guanosine monophosphate
- PDE
phosphodiesterase
- AMPK
AMP-activated protein kinase
- NAC
n-actylcysteine
- CAPE
caffeic acid phenethyl ester
Footnotes
Disclaimer
The views expressed in this abstract are those of the authors and do not reflect the official policy of the Army, DoD, or the U.S. Government. The experimental protocol was approved by the Animal Care and Use Committee at the USAMRICD and all procedures were conducted in accordance with the principles stated in the most current Guide for the Care and Use of Laboratory Animals; Animal Welfare Act and implementing Animal Welfare Regulations, as amended; and Public Health Service Policy on Humane Care and Use of Laboratory Animals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dhara VR. On the bioavailability of methyl isocyanate in the Bhopal gas leak. 1992 doi: 10.1080/00039896.1992.9938379. [DOI] [PubMed] [Google Scholar]
- 2.Council NR. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 2. Washington, DC: The National Academies Press; 2002. p. 292. [PubMed] [Google Scholar]
- 3.Brown JE, Birky MM. Phosgene in the thermal decomposition products of poly (vinyl chloride): Generation, detection and measurement. Journal of Analytical Toxicology. 1980;4(4):166–174. doi: 10.1093/jat/4.4.166. [DOI] [PubMed] [Google Scholar]
- 4.Gerritsen W, Buschmann C. Phosgene poisoning caused by the use of chemical paint removers containing methylene chloride in ill-ventilated rooms heated by kerosene stoves. British Journal of Industrial Medicine. 1960;17(3):187–189. doi: 10.1136/oem.17.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winternitz MC, Yale U. Collected studies on the pathology of war gas poisoning from the Department of Pathology and Bacteriology, Medical Science Section, Chemical Warfare Service, under the direction of M.C. Winternitz. xxi. New Haven: Yale University Press; 1920. p. 165.p. 1. [Google Scholar]
- 6.Association, A.I.H. Odor thresholds for chemicals with established occupational health standards. American Industrial Hygiene Association; 1989. [Google Scholar]
- 7.Borak J, Diller WF. Phosgene exposure: mechanisms of injury and treatment strategies. Journal of Occupational and Environmental Medicine. 2001;43(2) doi: 10.1097/00043764-200102000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Diller WF. Medical phosgene problems and their possible solution. Journal of Occupational Medicine. 1978;20(3):189–93. doi: 10.1097/00043764-197803000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Agency for Toxic Substances and Disease Registry. Toxic Substances Portal - Phosgene. 2014 Oct 21; cited 2015 13 May; Available from: http://www.atsdr.cdc.gov/mmg/mmg.asp?id=1201&tid=182.
- 10.Smith A, et al. The effect of steroid treatment with inhaled budesonide or intravenous methylprednisolone on phosgene-induced acute lung injury in a porcine model. Military Medicine. 2009;174(12):1287–1294. doi: 10.7205/milmed-d-09-00050. [DOI] [PubMed] [Google Scholar]
- 11.Liu F, et al. Single high-dose dexamethasone and sodium salicylate failed to attenuate phosgene-induced acute lung injury in rats. Toxicology. 2014;315:17–23. doi: 10.1016/j.tox.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Mautone AJ, Katz Z, Scarpelli EM. Acute responses to phosgene inhalation and selected corrective measures (including surfactant) Toxicology and Industrial Health. 1985;1(2):37–57. doi: 10.1177/074823378500100205. [DOI] [PubMed] [Google Scholar]
- 13.Nash T, Pattle RE. The absorption of phosgene by aqueous solutions and its relation to toxicity. Ann Occup Hyg. 1971;14(3):227–233. doi: 10.1093/annhyg/14.3.227. [DOI] [PubMed] [Google Scholar]
- 14.Sciuto AM, et al. Changes in absorbance at 413 nm in plasma from three rodent species exposed to phosgene. Biochemical and Biophysical Research Communications. 1996;226(3):906–911. doi: 10.1006/bbrc.1996.1448. [DOI] [PubMed] [Google Scholar]
- 15.Babad H, Zeiler AG. Chemistry of phosgene. Chemical Reviews. 1973;73(1):75–91. [Google Scholar]
- 16.Arroyo CM, et al. Autoionization reaction of phosgene (OCCl2) studied by electron paramagnetic resonance/spin trapping techniques. Journal of Biochemical Toxicology. 1993;8(2):107–110. doi: 10.1002/jbt.2570080208. [DOI] [PubMed] [Google Scholar]
- 17.Diller WF, Bruch J, Dehnen W. Pulmonary changes in the rat following low phosgene exposure. Archives of Toxicology. 1985;57(3):184–190. doi: 10.1007/BF00290885. [DOI] [PubMed] [Google Scholar]
- 18.Frosolono MF, Pawlowski R. Effect of phosgene on rat lungs after single high-level exposure. Arch Environ Health. 1977;32(6):271–7. doi: 10.1080/00039896.1977.10667294. [DOI] [PubMed] [Google Scholar]
- 19.Currie WD, Pratt PC, Frosolono MF. Response of pulmonary energy metabolism to phosgene. Toxicology and Industrial Health. 1985;1(2):17–27. doi: 10.1177/074823378500100203. [DOI] [PubMed] [Google Scholar]
- 20.Sciuto AM, et al. Protective effects of n-acetylcysteine treatment after phosgene exposure in rabbits. American Journal of Respiratory and Critical Care Medicine. 1995;151(3):768–772. doi: 10.1164/ajrccm.151.3.7881668. [DOI] [PubMed] [Google Scholar]
- 21.Sciuto AM, et al. The fate of antioxidant enzymes in bronchoalveolar lavage fluid over 7 days in mice with acute lung injury. Inhal Toxicol. 2003;15(7):675–85. doi: 10.1080/08958370390197245. [DOI] [PubMed] [Google Scholar]
- 22.Sciuto AM, et al. Genomic analysis of murine pulmonary tissue following carbonyl chloride inhalation. Chemical Research in Toxicology. 2005;18(11):1654–1660. doi: 10.1021/tx050126f. [DOI] [PubMed] [Google Scholar]
- 23.Sciuto AM, et al. Intratracheal administration of DBcAMP attenuates edema formation in phosgene-induced acute lung injury. Journal of Applied Physiology. 1996;80(1):149–157. doi: 10.1152/jappl.1996.80.1.149. [DOI] [PubMed] [Google Scholar]
- 24.Guo YL, et al. Mechanism of phosgene-induced lung toxicity: role of arachidonate mediators. J Appl Physiol. 1990;69(5):1615–1622. doi: 10.1152/jappl.1990.69.5.1615. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy TP, et al. Dibutyryl cAMP, aminophylline, and beta-adrenergic agonists protect against pulmonary edema caused by phosgene. J Appl Physiol. 1989;67(6):2542–52. doi: 10.1152/jappl.1989.67.6.2542. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X-d, et al. Time course for expression of vegf and its receptor and regulator levels of contraction and relaxation in increased vascular permeability of lung induced by phosgene. Inhalation Toxicology: International Forum for Respiratory Research. 2008;20(9):805–812. doi: 10.1080/08958370802015091. [DOI] [PubMed] [Google Scholar]
- 27.Sciuto AM. Assessment of early acute lung injury in rodents exposed to phosgene. Archives of Toxicology. 1998;72(5):283–288. doi: 10.1007/s002040050503. [DOI] [PubMed] [Google Scholar]
- 28.Duniho SM, et al. Acute changes in lung histopathology and bronchoalveolar lavage parameters in mice exposed to the choking agent gas phosgene. Toxicologic Pathology. 2002;30(3):339–349. doi: 10.1080/01926230252929918. [DOI] [PubMed] [Google Scholar]
- 29.Guastadisegni C, et al. Characterization of a phospholipid adduct formed in sprague dawley rats by chloroform metabolism: Nmr studies. Journal of Biochemical and Molecular Toxicology. 1998;12(2):93–102. doi: 10.1002/(sici)1099-0461(1998)12:2<93::aid-jbt4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 30.Prentiss AM, Fisher GJB. Chemicals in war. 1937 [Google Scholar]
- 31.Beckman DLMKF, Bean JW. Sympathetic factors in acute pulmonary edema. Chest. 1972;65:38s–40s. doi: 10.1378/chest.65.4_supplement.38s. [DOI] [PubMed] [Google Scholar]
- 32.Bessac BF, et al. TRPA1 is a major oxidant sensor in murine airway sensory neurons. J Clin Invest. 2008;118(5):1899–910. doi: 10.1172/JCI34192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinman A, et al. TRP channel activation by reversible covalent modification. Proc Natl Acad Sci U S A. 2006;103(51):19564–8. doi: 10.1073/pnas.0609598103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macpherson LJ, et al. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445(7127):541–5. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 35.Escalera J, et al. TRPA1 mediates the noxious effects of natural sesquiterpene deterrents. J Biol Chem. 2008;283(35):24136–44. doi: 10.1074/jbc.M710280200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trevisani M, et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104(33):13519–24. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bessac BF, Jordt SE. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 2008;23:360–70. doi: 10.1152/physiol.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jordt SE, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427(6971):260–5. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 39.Nagata K, et al. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25(16):4052–61. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cavanaugh EJ, Simkin D, Kim D. Activation of transient receptor potential A1 channels by mustard oil, tetrahydrocannabinol and Ca2+ reveals different functional channel states. Neuroscience. 2008;154(4):1467–76. doi: 10.1016/j.neuroscience.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 41.Wang YY, et al. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem. 2008;283(47):32691–703. doi: 10.1074/jbc.M803568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doerner JF, et al. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282(18):13180–9. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- 43.Zurborg S, et al. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10(3):277–9. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- 44.Keyser BM, Soo-Kyoung TJT, Lu Y, Bhattacharjee A, Huang L, Pottle J, Matrougui K, Xu Z, Li M. Role of T-type calcium channel in basal [Ca2+]i regulation and basal insulin secretion. Current Trends in Endocrinology. 2014;7(4):35–44. [Google Scholar]
- 45.Chemin J, et al. Overexpression of T-type calcium channels in HEK-293 cells increases intracellular calcium without affecting cellular proliferation. FEBS Lett. 2000;478(1–2):166–72. doi: 10.1016/s0014-5793(00)01832-9. [DOI] [PubMed] [Google Scholar]
- 46.Kuga T, et al. Cell cycle-dependent expression of L-and T-type Ca2+ currents in rat aortic smooth muscle cells in primary culture. Circulation Research. 1996;79(1):14–19. doi: 10.1161/01.res.79.1.14. [DOI] [PubMed] [Google Scholar]
- 47.Li M, et al. T-type Ca2+ channels are involved in high glucose-induced rat neonatal cardiomyocyte proliferation. Pediatr Res. 2005;57(4):550–6. doi: 10.1203/01.PDR.0000155756.89681.3C. [DOI] [PubMed] [Google Scholar]
- 48.Guo W, et al. Cell cycle-related changes in the voltage-gated Ca2+ currents in cultured newborn rat ventricular myocytes. J Mol Cell Cardiol. 1998;30(6):1095–103. doi: 10.1006/jmcc.1998.0675. [DOI] [PubMed] [Google Scholar]
- 49.Taylor JT, et al. Calcium signaling and T-type calcium channels in cancer cell cycling. World J Gastroenterol. 2008;14(32):4984–91. doi: 10.3748/wjg.14.4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bijlenga P, et al. T-type alpha 1H Ca2+ channels are involved in Ca2+ signaling during terminal differentiation (fusion) of human myoblasts. Proc Natl Acad Sci U S A. 2000;97(13):7627–32. doi: 10.1073/pnas.97.13.7627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mariot P, et al. Overexpression of an alpha 1H (Cav3.2) T-type calcium channel during neuroendocrine differentiation of human prostate cancer cells. J Biol Chem. 2002;277(13):10824–33. doi: 10.1074/jbc.M108754200. [DOI] [PubMed] [Google Scholar]
- 52.Zhou C, Wu S. T-type calcium channels in pulmonary vascular endothelium. Microcirculation. 2006;13(8):645–56. doi: 10.1080/10739680600930289. [DOI] [PubMed] [Google Scholar]
- 53.Gray LS, et al. The role of voltage gated T-type Ca2+ channel isoforms in mediating “capacitative” Ca2+ entry in cancer cells. Cell Calcium. 2004;36(6):489–97. doi: 10.1016/j.ceca.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Nilius B, et al. Inhibition by mibefradil, a novel calcium channel antagonist, of Ca(2+)- and volume-activated Cl- channels in macrovascular endothelial cells. Br J Pharmacol. 1997;121(3):547–55. doi: 10.1038/sj.bjp.0701140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yazawa K, Ono K, Iijima T. Modulation by mibefradil of the histamine-induced Ca2+ entry in human aortic endothelial cells. Jpn J Pharmacol. 2002;90(2):125–30. doi: 10.1254/jjp.90.125. [DOI] [PubMed] [Google Scholar]
- 56.Robertson BS, et al. N-Methyl--aspartate receptors are expressed by intrinsic neurons of rat larynx and esophagus. Neuroscience Letters. 1998;244(2):77–80. doi: 10.1016/s0304-3940(98)00130-x. [DOI] [PubMed] [Google Scholar]
- 57.Shen L, et al. Inhibition of pulmonary surfactants synthesis during n-methyl-d-aspartate-induced lung injury. Basic & Clinical Pharmacology & Toxicology. 2010;107(3):751–757. doi: 10.1111/j.1742-7843.2010.00572.x. [DOI] [PubMed] [Google Scholar]
- 58.Gill SS, et al. Potential target sites in peripheral tissues for excitatory neurotransmission and excitotoxicity. Toxicologic Pathology. 2000;28(2):277–284. doi: 10.1177/019262330002800207. [DOI] [PubMed] [Google Scholar]
- 59.Said SI. Glutamate receptors and asthmatic airway disease. Trends in Pharmacological Sciences. 1999;20(4):132–134. doi: 10.1016/s0165-6147(98)01275-9. [DOI] [PubMed] [Google Scholar]
- 60.Sciuto AM, Hurt HH. Therapeutic treatments of phosgene-induced lung injury. Inhalation Toxicology. 2004;16(8):565–580. doi: 10.1080/08958370490442584. [DOI] [PubMed] [Google Scholar]
- 61.Hughes AK, et al. Effect of reactive oxygen species on endothelin-1 production by human mesangial cells. Kidney Int. 1996;49(1):181–189. doi: 10.1038/ki.1996.25. [DOI] [PubMed] [Google Scholar]
- 62.Kähler J, et al. Oxidative stress increases endothelin-1 synthesis in human coronary artery smooth muscle cells. Journal of Cardiovascular Pharmacology. 2001;38(1) doi: 10.1097/00005344-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Ruef J, et al. Induction of endothelin-1 expression by oxidative stress in vascular smooth muscle cells. Cardiovasc Pathol. 2001;10(6):311–5. doi: 10.1016/s1054-8807(01)00095-3. [DOI] [PubMed] [Google Scholar]
- 64.Yanagisawa M, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332(6163):411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 65.Jaskot RH, Grose EC, Stead AG. Increase in angiotensin-converting enzyme in rat lungs following inhalation of phosgene. Inhalation Toxicology: International Forum for Respiratory Research. 1989;1(1):71–78. [Google Scholar]
- 66.Imai T, et al. Induction of endothelin-1 gene by angiotensin and vasopressin in endothelial-cells. Hypertension. 1992;19(6):753–757. doi: 10.1161/01.hyp.19.6.753. [DOI] [PubMed] [Google Scholar]
- 67.Miyauchi T, Masaki T. Pathophysiology of endothelin in the cardiovascular system. Annual Review of Physiology. 1999;61(1):391–415. doi: 10.1146/annurev.physiol.61.1.391. [DOI] [PubMed] [Google Scholar]
- 68.Dillman JF. Personal Communication. 2010.
- 69.Pollock DM, Keith TL, Highsmith RF. Endothelin receptors and calcium signaling. The FASEB Journal. 1995;9(12):1196–1204. doi: 10.1096/fasebj.9.12.7672512. [DOI] [PubMed] [Google Scholar]
- 70.Hyvelin J-M, et al. Cellular mechanisms and role of endothelin-1-induced calcium oscillations in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol. 1998;275(2):L269–282. doi: 10.1152/ajplung.1998.275.2.L269. [DOI] [PubMed] [Google Scholar]
- 71.Haynes WG, Strachan FE, Webb DJ. Endothelin ET(A) and ET(B) and receptors cause vasoconstriction of human resistance and capacitance vessels in-vivo. Circulation. 1995;92(3):357–363. doi: 10.1161/01.cir.92.3.357. [DOI] [PubMed] [Google Scholar]
- 72.Sciuto AM, Stotts RR, Hurt HH. Efficacy of ibuprofen and pentoxifylline in the treatment of phosgene-induced acute lung injury. J Appl Toxicol. 1996;16(5):381–4. doi: 10.1002/(SICI)1099-1263(199609)16:5<381::AID-JAT355>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 73.Resink TJ, Scott-Burden T, Bühler FR. Activation of multiple signal transduction pathways by endothelin in cultured human vascular smooth muscle cells. European Journal of Biochemistry. 1990;189(2):415–421. doi: 10.1111/j.1432-1033.1990.tb15504.x. [DOI] [PubMed] [Google Scholar]
- 74.Gorenne I, et al. Leukotriene D4 contractions in human airways are blocked by SK&F 96365, an inhibitor of receptor-mediated calcium entry. Journal of Pharmacology and Experimental Therapeutics. 1998;284(2):549–552. [PubMed] [Google Scholar]
- 75.Sauvageau S, et al. Endothelin-1-induced pulmonary vasoreactivity is regulated by ET A and ET B receptor interactions. Journal of Vascular Research. 2007;44(5):375–381. doi: 10.1159/000102534. [DOI] [PubMed] [Google Scholar]
- 76.Kent A, Keenan AK. Evidence for signaling by endothelin ET-A and ET-B receptors in bovine pulmonary-artery smooth-muscle cells. British Journal of Pharmacology. 1994;112:U62–U62. [Google Scholar]
- 77.Takagi Y, et al. Structural basis of G protein specificity of human endothelin receptors. Journal of Biological Chemistry. 1995;270(17):10072–10078. doi: 10.1074/jbc.270.17.10072. [DOI] [PubMed] [Google Scholar]
- 78.Boulanger C, Luscher TF. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J Clin Invest. 1990;85(2):587–90. doi: 10.1172/JCI114477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wiley KE, Davenport AP. Nitric oxide-mediated modulation of the endothelin-1 signalling pathway in the human cardiovascular system. Br J Pharmacol. 2001;132(1):213–20. doi: 10.1038/sj.bjp.0703834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wedgwood S, Dettman RW, Black SM. ET-1 stimulates pulmonary arterial smooth muscle cell proliferation via induction of reactive oxygen species. Am J Physiol Lung Cell Mol Physiol. 2001;281(5):L1058–67. doi: 10.1152/ajplung.2001.281.5.L1058. [DOI] [PubMed] [Google Scholar]
- 81.Durussel JJ, et al. Effects of red blood cell hyperaggregation on the rat microcirculation blood flow. Acta Physiologica Scandinavica. 1998;163(1):25–32. doi: 10.1046/j.1365-201x.1998.00342.x. [DOI] [PubMed] [Google Scholar]
- 82.Ivanhoe F, Meyers FH. Phosgene poisoning as an example of neuroparalytic acute pulmonary edema: the sympathetic vasomotor reflex involved. Chest. 1964;46(2):211–218. doi: 10.1378/chest.46.2.211. [DOI] [PubMed] [Google Scholar]
- 83.Said SI, Berisha HI, Pakbaz H. Excitotoxicity in the lung: N-methyl-D-aspartate-induced, nitric oxide-dependent, pulmonary edema is attenuated by vasoactive intestinal peptide and by inhibitors of poly(ADP-ribose) polymerase. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(10):4688–4692. doi: 10.1073/pnas.93.10.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Said SI. Metabolic functions of the pulmonary circulation. Circ Res. 1982;50(3):325–33. doi: 10.1161/01.res.50.3.325. [DOI] [PubMed] [Google Scholar]
- 85.Li W, et al. Novel insights into phosgene-induced acute lung injury in rats: role of dysregulated cardiopulmonary reflexes and nitric oxide in lung edema pathogenesis. Toxicological Sciences: An Official Journal Of The Society Of Toxicology. 2013;131(2):612–628. doi: 10.1093/toxsci/kfs317. [DOI] [PubMed] [Google Scholar]
- 86.Büch T, et al. Reviews of Physiology, Biochemistry and Pharmacology. Vol. 165. Springer; 2013. Chemosensory TRP channels in the respiratory tract: role in toxic lung injury and potential as “sweet spots” for targeted therapies; pp. 31–65. [DOI] [PubMed] [Google Scholar]
- 87.Shapiro D, et al. Activation of transient receptor potential ankyrin-1 (TRPA1) in lung cells by wood smoke particulate material. Chemical research in toxicology. 2013;26(5):750–758. doi: 10.1021/tx400024h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mukhopadhyay I, et al. Expression of functional TRPA1 receptor on human lung fibroblast and epithelial cells. Journal Of Receptor And Signal Transduction Research. 2011;31(5):350–358. doi: 10.3109/10799893.2011.602413. [DOI] [PubMed] [Google Scholar]
- 89.Pena F, Ordaz B. Non-selective cation channel blockers: potential use in nervous system basic research and therapeutics. Mini Rev Med Chem. 2008;8(8):812–9. doi: 10.2174/138955708784912166. [DOI] [PubMed] [Google Scholar]
- 90.Beech DJ, Muraki K, Flemming R. Non-selective cationic channels of smooth muscle and the mammalian homologues of Drosophila TRP. J Physiol. 2004;559(Pt 3):685–706. doi: 10.1113/jphysiol.2004.068734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kennedy TP, et al. Dibutyryl cAMP, aminophylline, and beta-adrenergic agonists protect against pulmonary-edema caused by phosgene. Journal of Applied Physiology. 1989;67(6):2542–2552. doi: 10.1152/jappl.1989.67.6.2542. [DOI] [PubMed] [Google Scholar]
- 92.Sciuto AM, et al. Postexposure treatment with aminophylline protects against phosgene-induced acute lung injury. Exp Lung Res. 1997;23(4):317–32. doi: 10.3109/01902149709039229. [DOI] [PubMed] [Google Scholar]
- 93.Jian M-Y, et al. Metformin-stimulated AMPK-α1 promotes microvascular repair in acute lung injury. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2013;305(11):L844–L855. doi: 10.1152/ajplung.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Judy C, Karen L, MingYuan J. D27. SIGNALING MOLECULES INVOLVED IN PULMONARY ENDOTHELIAL INJURY AND REPAIR. American Thoracic Society; 2012. AMP-activated kinase (ampk) isoforms selectively contribute to lung vascular repair; pp. A5520–A5520. [Google Scholar]
- 95.Xing J, et al. Inhibition of AMP-activated protein kinase accentuates lipopolysaccharide-induced lung endothelial barrier dysfunction and lung injury in vivo. The American journal of pathology. 2013;182(3):1021–1030. doi: 10.1016/j.ajpath.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.JANG A-H, KIM YW. Metformin reduces inflammation and lung fibrosis in a bleomycin-induced lung injury model (lb505) The FASEB Journal. 2014;28(1 Supplement):LB505. [Google Scholar]
- 97.Madiraju AK, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014 doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ramachandran R, Saraswathy M. Up-regulation of nuclear related factor 2 (NRF2) and antioxidant responsive elements by metformin protects hepatocytes against the acetaminophen toxicity. Toxicology Research. 2014;3(5):350–358. [Google Scholar]
- 99.Sciuto AM, Moran TS. Bha diet enhances the survival of mice exposed to phosgene: The effect of bha on glutathione levels in the lung. Inhalation Toxicology: International Forum for Respiratory Research. 1999;11(9):855–871. doi: 10.1080/089583799196772. [DOI] [PubMed] [Google Scholar]
- 100.Sciuto AM, Moran TS. Effect of dietary treatment with n-propyl gallate or vitamin E on the survival of mice exposed to phosgene. Journal of Applied Toxicology. 2001;21(1):33–39. doi: 10.1002/jat.729. [DOI] [PubMed] [Google Scholar]
- 101.Ji L, et al. N-acetylcysteine attenuates phosgene-induced acute lung injury via up-regulation of Nrf2 expression. Inhalation Toxicology. 2010;22(7):535–542. doi: 10.3109/08958370903525183. [DOI] [PubMed] [Google Scholar]
- 102.Wang P, et al. Mechanism of acute lung injury due to phosgene exposition and its protection by cafeic acid phenethyl ester in the rat. Exp Toxicol Pathol. 2011 doi: 10.1016/j.etp.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 103.Gould NS, White CW, Day BJ. A role for mitochondrial oxidative stress in sulfur mustard analog 2-chloroethyl ethyl sulfide-induced lung cell injury and antioxidant protection. Journal of Pharmacology and Experimental Therapeutics. 2009;328(3):732–739. doi: 10.1124/jpet.108.145037. [DOI] [PMC free article] [PubMed] [Google Scholar]