Abstract
The beneficial effects of physical activity on brain health (synaptogenesis, neurogenesis, enhanced synaptic plasticity, improved learning and memory) appear to be mediated through changes in region-specific expression of neurotrophins, transcription factors, and postsynaptic receptors, though investigations of sex differences in response to long-term voluntary wheel running are limited.
Purpose
To examine the effect of five months of voluntary wheel running on hippocampal mRNA and protein expression of factors critical for exercise-induced structural and functional plasticity in male and female adult mice.
Methods
At 8 weeks of age, male and female C57BL/6 mice were individually housed with (PA; n=20; 10 male) or without (SED; n=20; 10 male) access to a computer monitored voluntary running wheel. At 28 weeks, all mice were sacrificed and hippocampi removed. Total RNA was isolated from the hippocampus and expression of total Bdnf, Bdnf transcript IV, tPA, Pgc-1a, GluR1, NR2A, and NR2B were assessed with quantitative RT-PCR and total and mature Bdnf protein were assessed with ELISA.
Results
We found significantly higher Bdnf IV mRNA expression in PA males (p=0.03) and females (p=0.03) compared to SED animals. Total Bdnf mRNA expression was significantly greater in PA males compared to SED males (p=0.01), but there was no difference in females. Similarly, we observed significantly higher mature Bdnf protein in PA males compared to SED males (p=0.04), but not in females.
Conclusion
These findings indicate that the impact of long-term voluntary wheel running on transcriptional and post-translational regulation of Bdnf may be sex-dependent, though the activity-dependent Bdnf IV transcript is sensitive to exercise independent of sex.
Keywords: Brain-Derived Neurotrophic Factor, Sex-Differences, Exercise, Physical Activity, Hippocampus
1. Introduction
Chronic exercise training and physical activity have remarkable effects on the human and rodent hippocampus (66). Structural adaptations observed in the hippocampus in response to exercise training and physical activity include synaptogenesis, dendritic arborization, and neurogenesis (15, 35, 51, 55) while functional adaptations include enhanced learning and memory, increased amplitude of long-term potentiation (LTP), and reduced threshold for LTP (17, 31, 59, 61). Moreover, hippocampal neurons show increased mitochondrial biogenesis in response to chronic exercise training (54). These structural and functional adaptations are believed to be the result of increased expression of important neurotrophins, transcription factors, and postsynaptic receptors.
Elevated expression (mRNA and/or protein) and downstream signaling of brain-derived neurotrophic factor (BDNF) has been identified as a primary exercise-induced regulator of functional and structural plasticity since blocking Bdnf expression or signaling attenuates improvement in learning, memory, and expression of genes important for synaptic plasticity in the hippocampus following exercise training (23, 62). Circulating BDNF is elevated in humans following acute exercise and exercise training (16, 18, 22, 64). In the rodent hippocampus, Bdnf protein and mRNA are elevated following brief exercise exposures (≤ 7 days) (4, 12, 28, 43–45, 53, 63) and longer exercise exposures (>7 days) (4, 5, 10, 12, 17, 33, 37, 41, 43, 53, 67). Importantly, most studies in rodents that attempt to address mechanisms mediating the cognitive enhancing effects of chronic exercise have focused on exercise training ranging from ~7 days to 3 months and how longer voluntary wheel exposures influence mRNA expression of plasticity-associated genes is not fully understood. When mice are exposed to a voluntary running wheel, activity decreases over time (52, 65) and focusing on short-term chronic exercise favors plasticity by highlighting the response to the high wheel activity and the novelty of activity. This might present a biased view of the benefits of chronic exercise training on plasticity-associated gene and protein expression. However, Marlatt et al. (41) showed that eight months of voluntary wheel running increased Bdnf protein expression in 17-month old female C57BL/6J mice that began running at nine months of age. This suggests that long-term voluntary exercise maintains elevated Bdnf expression that is normally observed following short-term exercise exposure. It is not fully understood how long-term voluntary wheel exposure influences young-adult male and female hippocampal plasticity-associated gene and protein expression.
Importantly, the Bdnf gene is highly complex, containing eight non-coding exons with individual promoters that all splice to one 3′ protein coding exon (exon IX). Though Bdnf is considered an activity-regulated gene, promoter IV-driven Bdnf transcription is especially sensitive to neuronal activity (57, 58) and environmental stimuli (29, 38). Interestingly, all Bdnf transcripts are translated into the same protein (proBDNF), which is then cleaved to produce the mature plasticity-associated protein. It is not fully understood how long-term voluntary exercise influences the transcription and post-translational processing of Bdnf.
The hippocampus is a sexually dimorphic structure (40) and environmental stimuli result in sex-dependent hippocampal adaptations (8). In fact, many stimuli will result in similar behavioral responses in males and females, but the mechanisms by which these responses are mediated may be different between the sexes (8). Exercise results in beneficial adaptations to the hippocampus in both males (17) and females (61), though it is not known if exercise is stimulating the same signaling pathways in both sexes. Further, research in adolescent rats supports that sex differences exist in the hippocampal response to exercise (59). The purpose of the present investigation was to examine how five months of voluntary wheel running influences hippocampal mRNA and protein expression in adult male and female C57Bl/6J mice. We hypothesized that long-term chronic voluntary wheel running would have small or no effects on hippocampal mRNA expression of plasticity-associated genes due to reduced wheel running over time and that any observed differences in mRNA expression would be sex-dependent. We focused our investigation on Bdnf mRNA and protein as well as other genes important for the effect of exercise on structural and functional plasticity, mitochondrial biogenesis, and synaptic transmission.
2. Methods
2.1. Animals and Voluntary Wheel Running
Male and female C57Bl/6J mice were used in this investigation. All animals were cared for by University of Maryland veterinary staff and kept on 12hr light/12hr dark cycle and provided standard rodent chow ad libitum. All protocols were IACUC approved. At eight weeks of age, male and female C57Bl/6J mice were individually housed with (n=20; 10 male) or without (n=20; 10 male) continuous access to a computer-monitored voluntary running wheel (Lafayette Instruments, Lafayette IN). Mice were sacrificed at 28 weeks of age.
2.2. Tissue Collection & Processing
All mice were exposed to intraperitoneal glucose tolerance testing (IPGTT) 24 hours before sacrifice. Mice were fasted (ad libitum water access) for 6 hours prior to IPGTT. Baseline blood glucose measurements were made and then each mouse was injected intraperitoneally with 2.0 mg of D-glucose (Sigma-Aldrich, St. Louis, MO) per gram of body mass. Blood glucose was measured 15, 30, 60, 90, and 120 minutes after injection in all animals. All blood glucose measurements were made on blood removed from a single tail snip. Following the glucose tolerance test animals were returned to ad libitum food and water access. On the day of sacrifice, total body mass of anesthetized mice was recorded and mice underwent euthanasia by exsanguination followed by removal of the heart under isoflurane anesthesia. The hippocampus was isolated, halved, and immediately frozen in liquid nitrogen.
2.3. Gene Expression
Prior to nucleic acid isolation, hippocampi were homogenized in TRIzol reagent (Life Technologies, Grand Island, NY, USA) using a glass Dounce homogenizer. Total RNA was isolated with TRIzol reagent following manufacturers instructions and quantified via spectrophotometry. Reverse transcription was performed with 1 μg of total RNA with the High-Capacity cDNA RT kit (Life Technologies). Real-time quantitative PCR (qPCR) was used to assess mRNA expression of total Bdnf (exon IX); Bdnf exon IV (Bdnf IV); peroxisome proliferator-activated receptor γ coactivator 1 alpha (Pgc-1α); tissue plasminogen activator (tPa); glutamate receptor, ionotropic, AMPA 1 (GluR1); glutamate receptor, ionotropic, NMDA2A (NR2A), glutamate receptor, ionotropic, NMDA2B (NR2B); and glyceraldehyde-3-phosphate dehydrogenase (Gapdh; expression control; primer sequences listed in Supplemental Table 1). Primer:probe assays were purchased pre-made (Pgc-1α, tPa, GluR1, NR2A, NR2B, Gapdh) or designed (Bdnf IX, Bdnf IV) for the mRNA sequence of each gene using Integrated DNA Technologies’ PrimeTime qPCR Assay designer. All primer pairs except Bdnf total spanned exons to prevent amplification of genomic DNA. Because Bdnf total is represented by amplification of only exon IX, this primer pair could not span exons. Efficiency for each primer:probe assay was determined prior to use.
2.4. Bdnf Protein
Total and mature Bdnf protein levels were measured using the E-Max Bdnf ELISA kit (Promega, WI, USA) according to manufacturer’s instructions. Tissues were homogenized on ice in lysis buffer [137mM NaCl, 20mM Tris-HCl (pH 8.0), 1% NP40, 10% glycerol, 0.5mM sodium vanadate, and protease inhibitor cocktail (complete mini EDTA-free protease inhibitors, Roche, 1 tablet/10ml)]. Homogenized samples were diluted in two volumes DPBS containing calcium and magnesium (Life Technologies, NY, USA) and centrifuged for 3 min. at 13,500 rpm at 4°C (4). Supernatant was collected and total protein concentration determined by a Bicinchoninic acid (BCA) assay following manufacturer’s instructions (Pierce Biotechnology, IL, USA). Samples were then diluted in 1× block and sample buffer. For determination of total Bdnf, samples were acidified with 1N HCl for 15 minutes to pH 2–3 and neutralized with 1N NaOH to pH 7–8. The standard curve produced from Bdnf standard dilutions produced an R-value of >0.99.
2.5. Statistics
T-tests were used to test for differences in body mass and IPGTT area under the curve (AUC). Running wheel activity was analyzed with a repeated measures ANOVA. Protein and mRNA data were analyzed by two-way ANOVA (exercise × sex) and pre-planned LSD post hoc contrasts to compare exercise vs. sedentary within sexes and male vs. female within exercise conditions. A p≤0.05 was considered statistically significant.
3. Results
3.1 Wheel Running
Wheel running data are shown in Figure 1. The repeated measures ANOVA revealed a significant effect of time (F(3,12)=14.80; p=0.0002) and a tendency for an interaction between sex and time (F(3,12)=3.22; p=0.06) for average distance ran per 24 hours. Females ran significantly more during week 1 than males (t(10.6)=2.24; p<0.05); however, during week 20, males ran significantly more than females (t(16)=−2.94; p<0.01).
Figure 1. Average daily running distance for male and female C57Bl/6J mice.
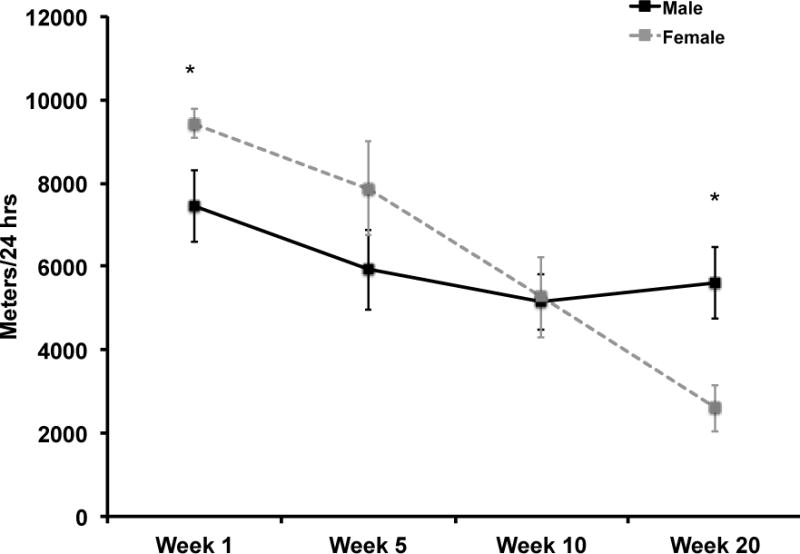
Females ran significantly more per day during week one (p=0.03) and significantly less per day during week twenty (p<0.01) compared to males. Error bars represent SEM
3.2. Body Mass and GTTs
Female runners weighed significantly less than their sedentary counterparts after five months of running wheel exposure (t(18)=−2.37; p=0.03; Fig. 2). There was a tendency for male runners to weigh significantly less than sedentary males (t(17)=−1.92; p=0.07; Fig. 2). There was no significant effect of wheel running on blood glucose response (AUC) to an IP injection of glucose (data not shown).
Figure 2. Male and female body mass.
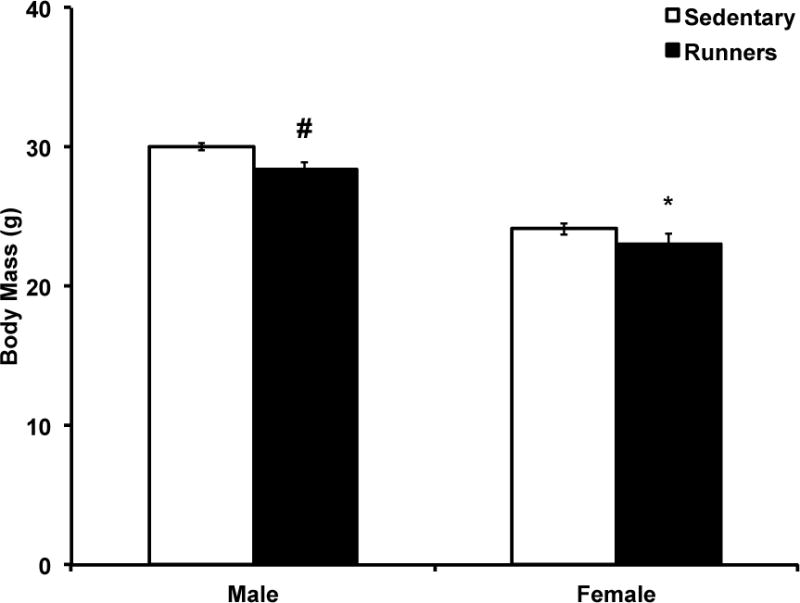
Females with access to voluntary running wheels had significantly lower body mass compared to sedentary females (p=0.03). There was a tendency for males with access to voluntary running wheels to have lower body mass compared to sedentary males (p=0.07). *p<0.05; #p<0.1. Error bars represent SEM.
3.3. Gene Expression
Gene expression data are shown in Figures 3 and 4. The two-way ANOVA revealed a main effect of exercise (F(1,33)=10.89; p=0.002) but no main effect of sex or an exercise by sex interaction on Bdnf IV (Fig. 3). Five months of voluntary wheel running led to significantly greater Bdnf IV gene expression compared to sedentary living conditions (Fig. 3a) and this effect remained when sexes were separated in the analysis (males: t(16)=2.41, p=0.03, Fig. 3b; females: t(17)=2.32, p=0.03, Fig. 3c). The two-way ANOVA revealed a significant interaction between exercise and sex for total Bdnf mRNA (F(1,33)=4.98; p=0.03). There was no main effect of exercise or sex on total Bdnf mRNA levels. Post-hoc analysis revealed that when the sexes were separated, exercise males had significantly higher total Bdnf mRNA expression compared to sedentary males (t(16)=2.76; p=0.01, Fig. 3b) and this was not observed in females (Fig. 3c). There was no significant effect of five months of voluntary wheel running or sex on Pgc-1a, tPa, or glutamate receptor subunit expression in either sex (Figs. 3 and 4).
Figure 3. Five months of voluntary wheel running increases Bdnf transcription in a transcript and sex-dependent manner.
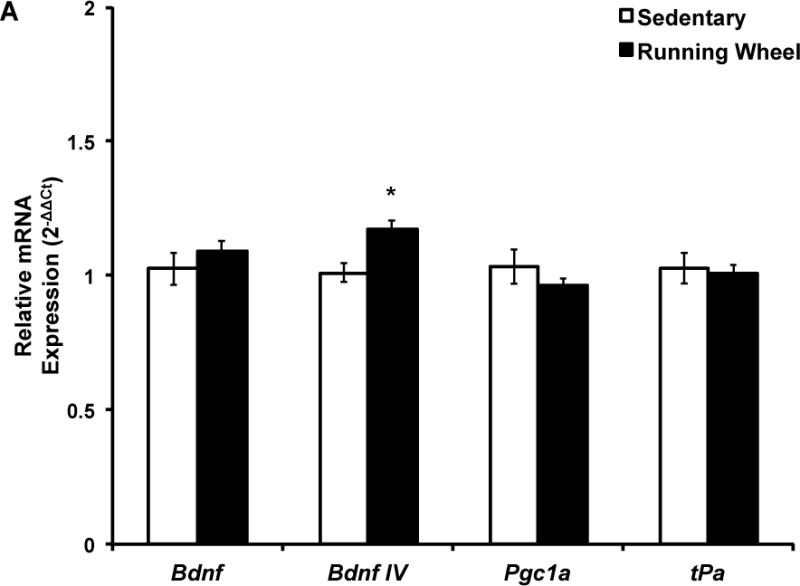
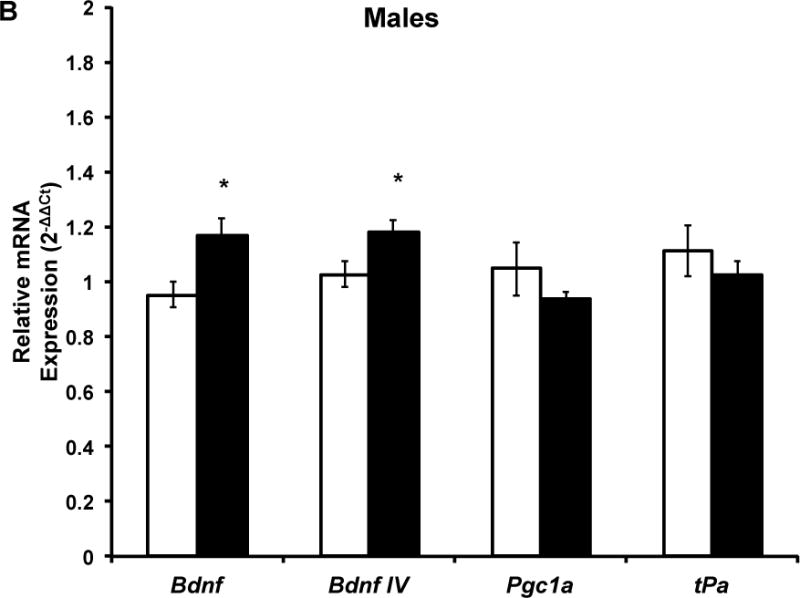
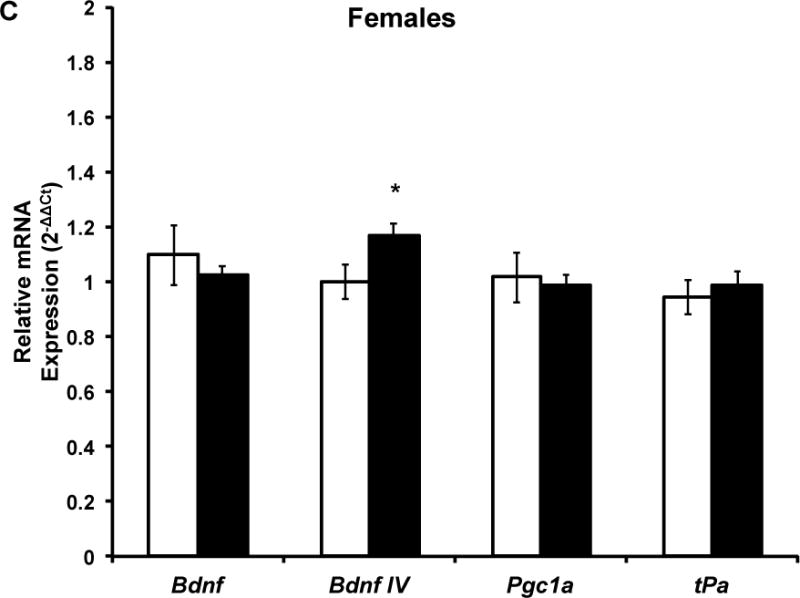
Target mRNA expression is presented as ddCt relative to Gapdh. A) qPCR analysis indicated that five months of wheel exposure increased Bdnf IV in the combined sample (p=0.004) but did not influence Bdnf total, Pgc1a, or tPa expression. B) Five months of wheel exposure increased Bdnf IV (p=0.03) and total Bdnf (p=0.01) expression but had no effect on Pgc1a or tPa expression in males. C) Five months of wheel exposure increased Bdnf IV (p=0.03) expression but not total Bdnf, Pgc1a, or tPa in females. Error bars represent SEM.
Figure 4. Five months of voluntary wheel running does not influence hippocampal GluR1, NR2A, NR2B, and NR2B/NR2A mRNA expression.
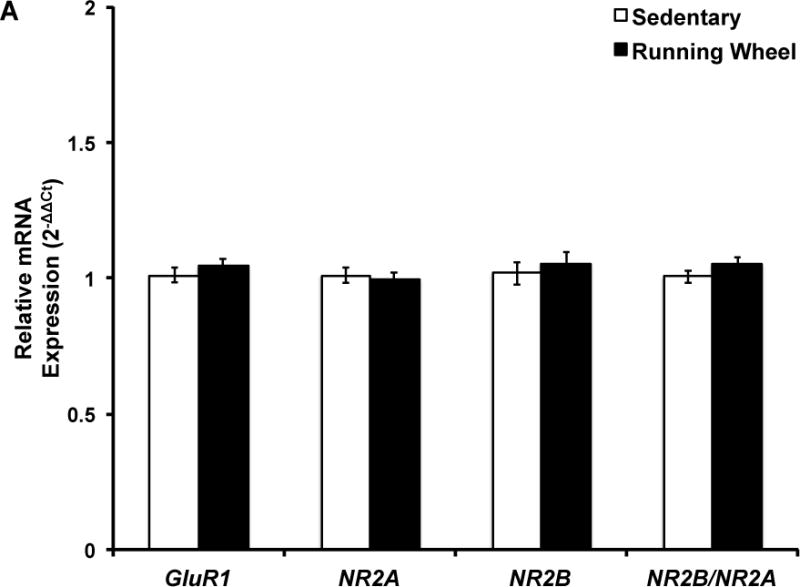
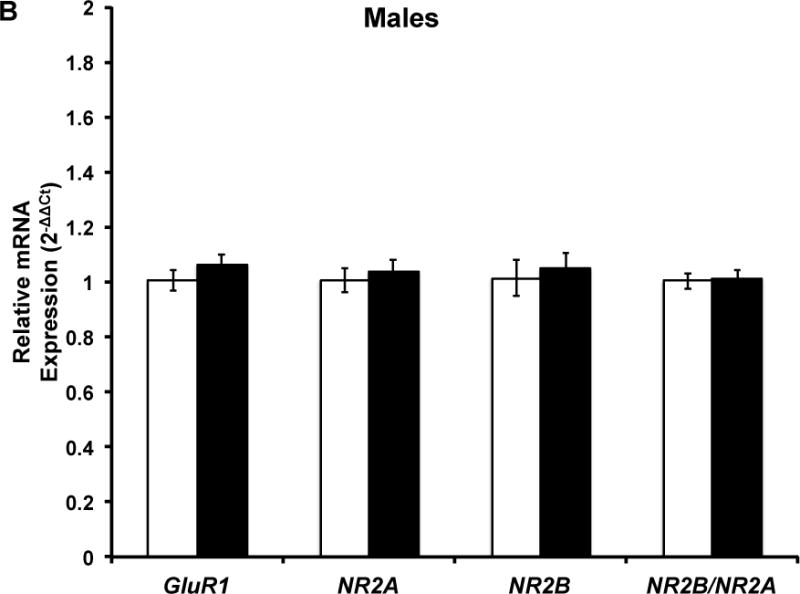
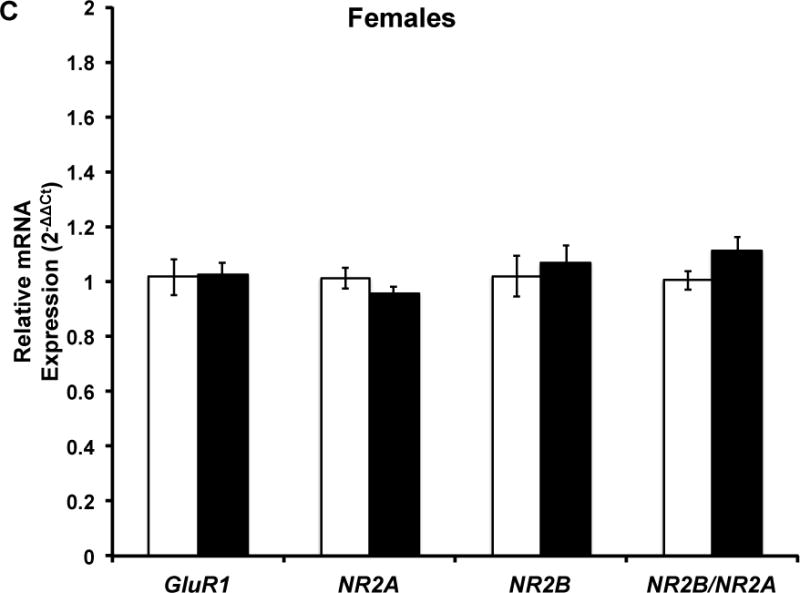
Target mRNA expression is presented as ddCt relative to Gapdh. qPCR analysis indicated that there was no significant effect of chronic wheel exposure on GluR1, NR2A, or NR2B mRNA expression in males, females, or the combined sample. Error bars represent SEM.
3.4. Bdnf Protein
The two-way ANOVA revealed no significant effects of exercise or sex or an interaction between exercise condition and sex on total (Fig. 5) or mature (Fig. 6) Bdnf protein. However, based on our mRNA data, we analyzed by sex and found that mature Bdnf levels were significantly higher in exercise males compared to sedentary males (t(15)=2.31, p=0.04, Fig. 6b), an effect not observed in females. Moreover, sedentary males had significantly lower mature Bdnf protein compared to sedentary females (t(16)=2.25, p=0.04, Fig. 6b).
Figure 5. Five months of voluntary wheel running does not influence hippocampal total Bdnf protein levels.
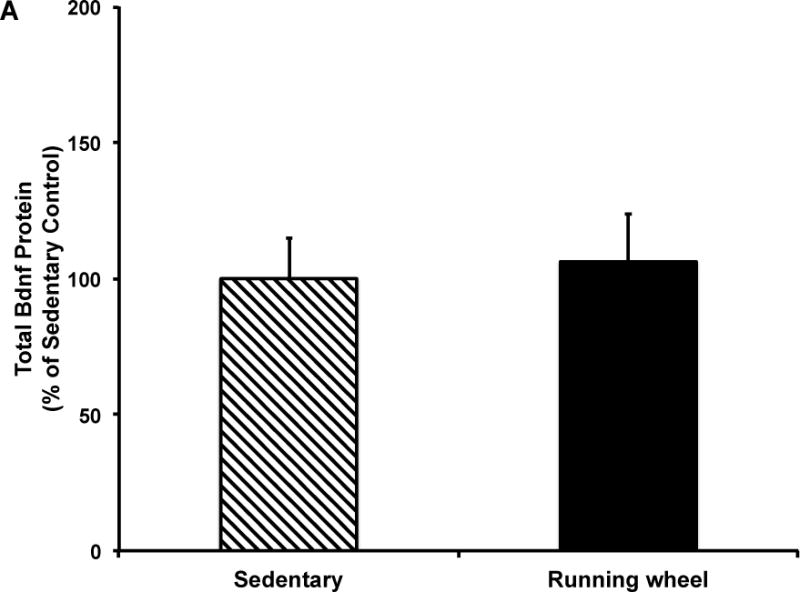
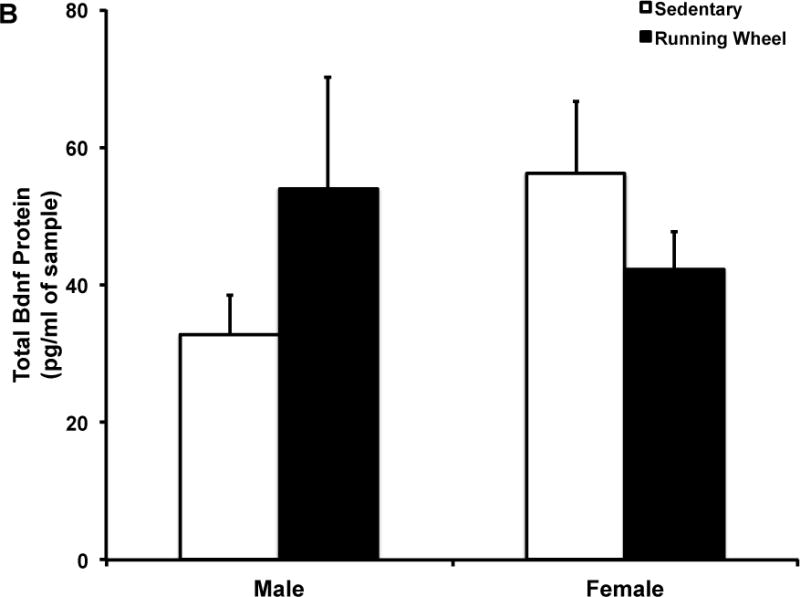
A) Total Bdnf protein in combined sample of males and females. Data presented as percent of sedentary controls. five months of wheel exposure did not influence total Bdnf protein expression. B) five months of wheel exposure did not significantly affect total Bdnf protein expression in males or females. Error bars represent SEM.
Figure 6. Five months of voluntary wheel running significantly increases mature Bdnf protein levels in the male hippocampus.
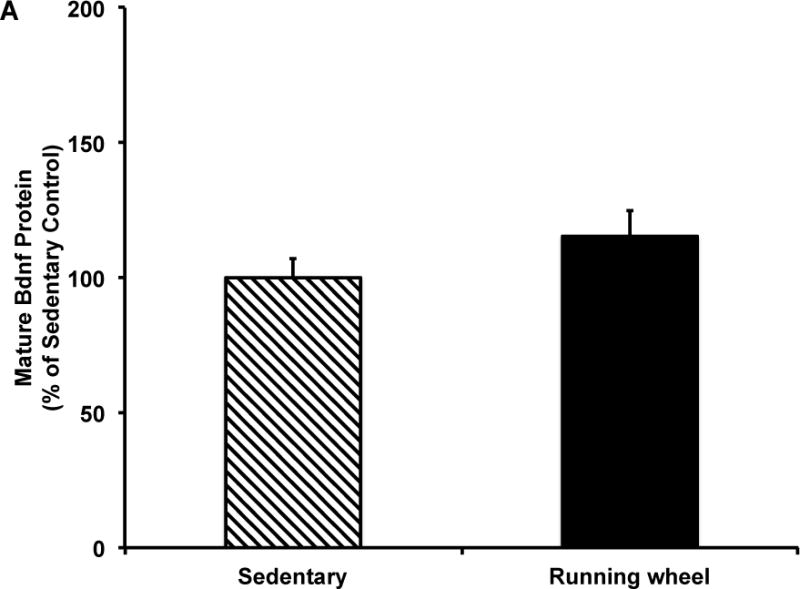
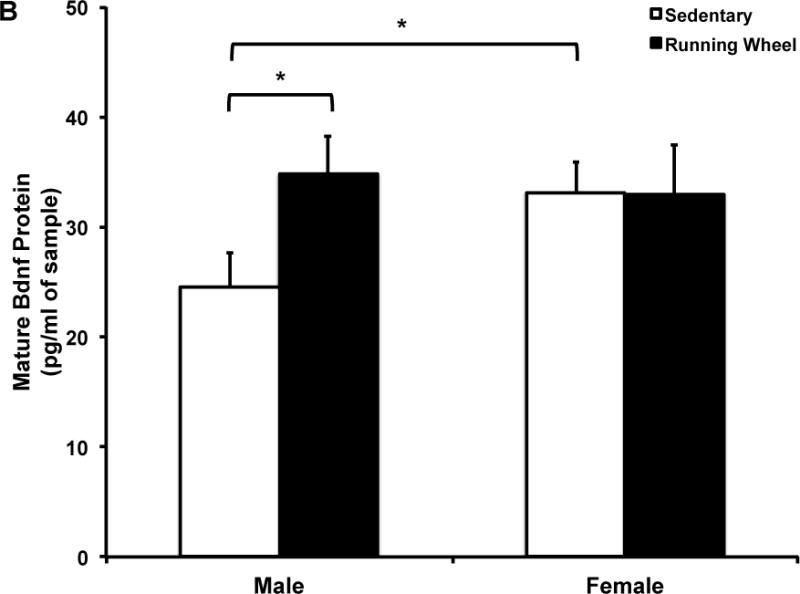
A) Mature Bdnf protein in combined sample of males and females. Data presented as percent of sedentary controls. five months of wheel exposure did not influence mature Bdnf protein expression. B) five months of wheel exposure increased mature Bdnf protein expression in males (p=0.04) but had no effect in females. Sedentary females had significantly higher mature Bdnf protein expression compared to sedentary males (p=0.04). Error bars represent SEM.
4. Discussion
We found a sex- and transcript-dependent effect of long-term voluntary wheel running on Bdnf transcription. Five months of voluntary wheel running increased Bdnf IV gene expression but had no effect on total Bdnf expression in the combined sample (male & female). Interestingly, when males and females were separated for analysis, we observed an increase in Bdnf IV in both males and females and an increase in total Bdnf in males only. Moreover, we found that five months of voluntary wheel running increased mature Bdnf protein in males but had no effect in females, which is consistent with total Bdnf mRNA and provides strong evidence for sex-dependent effects of long term exercise training on Bdnf expression and processing. These are interesting observations because voluntary wheel running enhances hippocampal plasticity in both males (17) and females (61).
Numerous studies have demonstrated that both brief and longer exercise training exposures increase hippocampal Bdnf mRNA and protein expression (4, 5, 12, 43, 44, 53, 62, 63). In addition, human studies have demonstrated that peripheral BDNF levels are elevated with aerobic exercise training (56). However, we report here that five months of voluntary wheel running increases total Bdnf mRNA and mature Bdnf protein only in male mice. When males and females were combined for analysis, there were no significant effects of exercise on Bdnf mRNA or mature protein expression. The majority of research to date has primarily used only males or only females, making our results difficult to compare to the literature. However, Gallego et al. (21) reported that 21 days of voluntary running wheel access increased Bdnf protein and mRNA expression in the hippocampus of both male and female adolescent C57Bl/6J mice. Both age and duration of wheel exposure have been reported to influence Bdnf expression (1), which might explain the difference between the results reported in Gallego et al. (21) and this investigation. A recent meta-analysis concluded that exercise training increases peripheral levels of BDNF in humans, though effect sizes were smaller for studies that included females in the sample (56). Titterness et al. (59) reported sex differences in hippocampal LTP following two weeks of voluntary wheel running in adolescent rats, though there were no differences in Bdnf protein expression in either males or females. Other research has demonstrated that voluntary wheel running does increase Bdnf mRNA expression in females but the expression is dependent on sex hormones (6). There is strong evidence that sex hormones are important regulators of Bdnf expression (9, 49). In humans, plasma BDNF fluctuates during the menstrual cycle and women who experience normal ovulatory cycles have higher plasma BDNF compared to amenorrhoeic or postmenopausal women (3). Further, in male-to-female transsexuals, 12-months of hormone therapy results in reduced serum BDNF (20). These studies suggest a complex relationship between sex hormones and BDNF in humans. Further research with long-term exercise training in ovariectomized mice is necessary. Potentially, a non-Bdnf pathway plays a more important role in exercise-induced hippocampal plasticity in females compared to males, whereas males may rely more heavily on Bdnf-mediated plasticity.
There is evidence of differential hippocampal Bdnf expression between males and females following acute and chronic stress (36). Females have a higher prevalence of mental disorders such as clinical depression and post-traumatic stress disorder, though animal research suggests that chronic stress leads to more structural damage to the male hippocampus (8). The literature suggesting sex differences in hippocampal adaptations to stress offers another potential explanation for the findings reported here. The animals in this investigation underwent glucose tolerance testing one-day prior to sacrifice. This was done to determine if any whole body metabolic adaptations occurred following five months of voluntary wheel running. The IPGTT was novel to the rodents and required handling and a tail snip and, though we took every precaution to minimize the stress response, the procedure was undoubtedly novel and presented an opportunity for stress. Lin et al. (36) reported that in response to an acute footshock, female rats responded with greater Bdnf protein expression in the dentate gyrus whereas stressed and control male rats showed no difference in Bdnf expression. Moreover, rodents that are chronically exercised have a lower stress response to stressful stimuli (13, 14, 25, 26) and therefore Bdnf expression in sedentary females may have been greater compared to exercise females in response to the IPGTT stress, masking any observable effect of the chronic physical activity. Though there was no difference between sedentary male and sedentary female total Bdnf mRNA expression, there was a significant difference in mature Bdnf protein and a tendency for a difference in total Bdnf protein (p=0.08) between sedentary males and sedentary females.
Interestingly, Bdnf IV mRNA was greater in exercised mice compared to sedentary mice, and this effect remained when analyzing sexes separately. Bdnf IV mRNA expression is stimulated with neural activity (42), exercise (24, 29), and other external stimuli (39). Remarkably, Bdnf IV promoter methylation is reduced with fear learning (39) and short-term exercise (24), and decreased promoter methylation suggests greater transcriptional activity (42). This is a potential mechanism mediating the effects of long-term exercise training on Bdnf IV transcription. The finding that both males and females had increased expression of Bdnf IV, though only males had higher total Bdnf suggests that exercise stimulates sex-specific up- and/or down-regulation of transcript-specific Bdnf gene expression.
The elevation in Bdnf protein in males was limited to mature Bdnf with no difference in total Bdnf, suggesting that five months of voluntary wheel exposure selectively increases expression of the mature plasticity-promoting Bdnf isoform. Sartori et al. (53) reported that 28 days of voluntary wheel running selectively increased mature Bdnf with no difference in the immature proBdnf in male C57/Bl6 mice. In contrast, Ding et al. (12) reported that seven days of wheel exposure increased both mature and proBdnf in the rat hippocampus. Differences in animal model and exercise duration likely explain the differences between our findings and those of Ding and colleagues (12).
Curiously, five months of voluntary wheel running did not impact the other mRNA targets measured in the present study. tPa and Pgc-1a mRNA are reportedly increased with voluntary wheel running (53, 54), an effect we did not observe. tPa has been shown to influence the beneficial effects of exercise on hippocampal function and is known to be an important enzyme in the cleavage of apoptotic proBdnf to generate the mature and plasticity-promoting mature Bdnf (47, 53). Interestingly, Sartori et al. (53) also used C57Bl/6J mice and qPCR to demonstrate that voluntary wheel running increases tPa expression in the hippocampus. Animals in the Sartori et al. (53) investigation were only provided access to a voluntary running wheel for 28 days. Longer exposure to a voluntary running wheel may result in a return to control levels of tPa. Pgc-1a is a co-transcription factor that regulates mitochondrial biogenesis and when co-expressed with other tissue- and temporal-specific transcription factors, Pgc-1α stimulates the transcription of genes necessary for mitochondrial biogenesis (19). Mitochondrial biogenesis in the rodent hippocampus has been observed following exercise training (54) and we recently reported that in utero exercise exposure increases Pgc-1α expression in offspring hippocampus (65). In the current investigation, we observed no effect of long-term wheel running on Pgc-1α. Steiner et al. (54) reported an increase in hippocampal Pgc-1α following an eight-week treadmill exercise protocol. Importantly, forced and voluntary exercise are distinct forms of exercise, generally associated with different levels of stress hormones (27, 30, 37, 68), and volume and intensity of exercise (27, 34). Indeed, voluntary and forced exercise induce both similar and distinct structural and functional adaptations to the rodent brain (7, 27, 30, 32, 34, 37, 48, 60), which might explain the discrepancy between our data and Steiner et al (54). We also observed no influence of chronic wheel running on glutamate receptor subunit expression. Short-term exposure to a voluntary running wheel increases mRNA expression of the NR2B subunit of the NMDA glutamate receptor and higher expression of this subunit is associated with a more plastic synapse (17, 43). There is limited and inconsistent (increases, decreases, no effect) data on the influence of exercise on GluR1 and NR2A subunit mRNA expression (11, 43, 46, 50). Potentially, due to the long duration of running and the steady decline in wheel activity over the course of the five months, the stimulus was not intense enough to maintain elevated mRNA expression of plasticity-associated genes. Further, differences in mRNA expression of plasticity-associated genes may have been observed if we investigated specific hippocampal subfields (dentate gyrus, CA1, and CA3) instead of whole hippocampal homogenates. Hippocampal subfields contain specific cell types and varying levels of sensitivity and adaptations to stimuli including exercise (2, 66). Future investigations should utilize additional methods of mRNA detection such as in situ hybridization.
A limitation of our investigation was that the control group was not housed with a locked running wheel. The differences observed in Bdnf mRNA and protein expression were potentially due to enriched housing or the combined effects of enriched housing and running. Sartori et al. (53) observed greater mature Bdnf protein in mice housed with a locked running wheel compared to mice housed in standard cages without a wheel, demonstrating that the presence of a wheel can influence Bdnf expression independent of running. Importantly, our data still demonstrate that long-term housing with a freely rotating voluntary running wheel influences Bdnf expression and processing differently between sexes.
4.1 Summary
The present data suggest that long-term voluntary exercise has limited and sex-dependent effects on hippocampal mRNA and Bdnf protein expression. Due to the limited effects of long-term voluntary wheel running observed in our investigation, we speculate that voluntary wheel running volume and/or intensity might need to be maintained or even manipulated (i.e., increase intensity) to most effectively maintain hippocampal plasticity-associated gene and protein expression. This might explain why we saw a greater benefit of exercise in males, which maintained their running volume better than females. Voluntary wheel running is a good model for unstructured leisure-time physical activity but might not be the best model for exercise training, which is generally associated with structured frequency, intensity, and duration components. Forced exercise, though associated with elevated stress hormones (27, 30, 37, 48, 68), might be an alternative to enhance brain health and plasticity (27, 32, 34, 35, 37, 60) in the long-term if declining voluntary running volume and intensity is limiting plasticity, which is yet to be determined. Future research should examine the effectiveness of long-term forced exercise due to the high levels of stress associated with this model of exercise.
Supplementary Material
Highlights.
We investigate influence of long-term exercise on male and female hippocampus
Five months of voluntary wheel running increases hippocampal Bdnf mRNA in male mice
Voluntary wheel running increases hippocampal mature Bdnf protein in male mice
Voluntary wheel running increases hippocampal Bdnf IV in male and female mice
Acknowledgments
Funding
This work was supported by NIH grant HD062868, The College of Health and Human Performance Public Health Research Seed Money Program award (S.M.R. and E.E.S.), NIH T32 AG000268 (A.C.V. and L.M.G.), and NIH F31 MH103951-01A1 (A.C.V.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interest
None declared
Authors’ contributions.
ACV, LMG, EES, and SMR designed the study; ACV, LMG, and RMS collected the data; data analysis, preparation of figures, and drafting the manuscript was done by ACV; ACV, LMG, RMS, EES, and SMR edited and revised this manuscript; and all authors approved the final version.
References
- 1.Adlard PA, Perreau VM, Cotman CW. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiology of Aging. 2005;26(4):511–20. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Andersen P, Morris R, Amaral D, Bliss T, O’Keefe J. The Hippocampus Book. Oxford University Press; USA: 2006. p. 1. [Google Scholar]
- 3.Begliuomini S, Casarosa E, Pluchino N, Lenzi E, Centofanti M, Freschi L, Pieri M, Genazzani AD, Luisi S, Genazzani AR. Influence of endogenous and exogenous sex hormones on plasma brain-derived neurotrophic factor. Human Reproduction. 2007;22(4):995–1002. doi: 10.1093/humrep/del479. [DOI] [PubMed] [Google Scholar]
- 4.Berchtold N, Chinn G, Chou M, Kesslak J, Cotman C. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience. 2005;133(3):853–61. doi: 10.1016/j.neuroscience.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 5.Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167(3):588–97. doi: 10.1016/j.neuroscience.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berchtold NC, Kesslak JP, Pike CJ, Adlard PA, Cotman CW. Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus. Eur J Neurosci. 2001;14(12):1992–2002. doi: 10.1046/j.0953-816x.2001.01825.x. [DOI] [PubMed] [Google Scholar]
- 7.Burghardt PR, Fulk LJ, Hand GA, Wilson MA. The effects of chronic treadmill and wheel running on behavior in rats. Brain Research. 2004;1019(1–2):84–96. doi: 10.1016/j.brainres.2004.05.086. [DOI] [PubMed] [Google Scholar]
- 8.Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7(6):477–84. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 9.Carbone DL, Handa RJ. Sex and stress hormone influences on the expression and activity of brain-derived neurotrophic factor. Neuroscience. 2013;239:295–303. doi: 10.1016/j.neuroscience.2012.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darlington TM, McCarthy RD, Cox RJ, Ehringer MA. Mesolimbic transcriptional response to hedonic substitution of voluntary exercise and voluntary ethanol consumption. Behavioral Brain Research. 2014;259:313–20. doi: 10.1016/j.bbr.2013.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietrich MO, Mantese CE, Porciúncula LO, Ghisleni G, Vinade L, Souza DO, Portela LV. Exercise affects glutamate receptors in postsynaptic densities from cortical mice brain. Brain Research. 2005;1065(1–2):20–5. doi: 10.1016/j.brainres.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 12.Ding Q, Ying Z, Gómez-Pinilla F. Exercise influences hippocampal plasticity by modulating brain-derived neurotrophic factor processing. Neuroscience. 2011;192:773–80. doi: 10.1016/j.neuroscience.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dishman RK, Bunnell BN, Youngstedt SD, Yoo HS, Mougey EH, Meyerhoff JL. Activity wheel running blunts increased plasma adrenocorticotrophin (ACTH) after footshock and cage-switch stress. Physiology & Behavior. 1998;63(5):911–7. doi: 10.1016/s0031-9384(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 14.Dishman RK, Renner KJ, Youngstedt SD, Reigle TG, Bunnell BN, Burke KA, Yoo HS, Mougey EH, Meyerhoff JL. Activity wheel running reduces escape latency and alters brain monoamine levels after footshock. Brain Research Bulletin. 1997;42(5):399–406. doi: 10.1016/s0361-9230(96)00329-2. [DOI] [PubMed] [Google Scholar]
- 15.Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486(1):39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- 16.Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proceedings of the National Academy of Sciences. 2011;108(7):3017–22. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male sprague–dawley rats in vivo. Neuroscience. 2004;124(1):71–9. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 18.Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Medicine and science in sports and exercise. 2007 doi: 10.1249/mss.0b013e31802f04c7. [DOI] [PubMed] [Google Scholar]
- 19.Finck BN. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. Journal of Clinical Investigation. 2006;116(3):615–22. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuss J, Hellweg R, Van Caenegem E, Briken P, Stalla GK, T’Sjoen G, Auer MK. Cross-sex hormone treatment in male-to-female transsexual persons reduces serum brain-derived neurotrophic factor (BDNF) European Neuropsychopharmacology. 2015;25(1):95–9. doi: 10.1016/j.euroneuro.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 21.Gallego X, Cox RJ, Funk E, Foster RA, Ehringer MA. Voluntary exercise decreases ethanol preference and consumption in C57BL/6 adolescent mice: Sex differences and hippocampal BDNF expression. Physiology & Behavior. 2015;138:28–36. doi: 10.1016/j.physbeh.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gold SM, Schulz K-H, Hartmann S, Mladek M, Lang UE, Hellweg R, Reer R, Braumann KM, Heesen C. Basal serum levels and reactivity of nerve growth factor and brain-derived neurotrophic factor to standardized acute exercise in multiple sclerosis and controls. J Neuroimmunol. 2003;138(1–2):99–105. doi: 10.1016/s0165-5728(03)00121-8. [DOI] [PubMed] [Google Scholar]
- 23.Gomez-Pinilla F, Vaynman S, Ying Z. Brain-derived neurotrophic factor functions as a metabotrophin to mediate the effects of exercise on cognition. European Journal of Neuroscience. 2008;28(11):2278–87. doi: 10.1111/j.1460-9568.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomez-Pinilla F, Zhuang Y, Feng J, YING Z, Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. European Journal of Neuroscience. 2010;33(3):383–90. doi: 10.1111/j.1460-9568.2010.07508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenwood BN, Foley TE, Burhans D, Maier SF, Fleshner M. The consequences of uncontrollable stress are sensitive to duration of prior wheel running. Brain Research. 2005;1033(2):164–78. doi: 10.1016/j.brainres.2004.11.037. [DOI] [PubMed] [Google Scholar]
- 26.Greenwood BN, Kennedy S, Smith TP, Campeau S, Day HEW, Fleshner M. Voluntary freewheel running selectively modulates catecholamine content in peripheral tissue and c-fos expression in the central sympathetic circuit following exposure to uncontrollable stress in rats. Neuroscience. 2003;120(1):269–81. doi: 10.1016/s0306-4522(03)00047-2. [DOI] [PubMed] [Google Scholar]
- 27.Hayes K, Sprague S, Guo M, Davis W, Friedman A, Kumar A, Jimenez DF, Ding Y. Forced, not voluntary, exercise effectively induces neuroprotection in stroke. Acta Neuropathol. 2008;115(3):289–96. doi: 10.1007/s00401-008-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang AM, Jen CJ, Chen HF, Yu L, Kuo YM, Chen HI. Compulsive exercise acutely upregulates rat hippocampal brain-derived neurotrophic factor. J Neural Transm. 2005;113(7):803–11. doi: 10.1007/s00702-005-0359-4. [DOI] [PubMed] [Google Scholar]
- 29.Intlekofer KA, Berchtold NC, Malvaez M, Carlos AJ, McQuown SC, Cunningham MJ, Wood MA, Cotman CW. Exercise and Sodium Butyrate Transform a Subthreshold Learning Event into Long-Term Memory via a Brain-Derived Neurotrophic factor-Dependent Mechanism. Neuropsychopharmacology. 2013;38(10):2027–34. doi: 10.1038/npp.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ke Z, Yip SP, Li L, Zheng X-X, Tong K-Y. The Effects of Voluntary, Involuntary, and Forced Exercises on Brain-Derived Neurotrophic Factor and Motor Function Recovery: A Rat Brain Ischemia Model. PLoS ONE. 2011;6(2):e16643. doi: 10.1371/journal.pone.0016643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kida S, Josselyn SA, de Ortiz SP, Kogan JH, Chevere I, Masushige S, Silva AJ. CREB required for the stability of new and reactivated fear memories. Nat Neurosci. 2002;5(4):348–55. doi: 10.1038/nn819. [DOI] [PubMed] [Google Scholar]
- 32.Kinni H, Guo M, Ding JY, Konakondla S, Dornbos D, III, Tran R, Guthikonda M, Ding Y. Cerebral metabolism after forced or voluntary physical exercise. Brain Research. 2011;1388:48–55. doi: 10.1016/j.brainres.2011.02.076. [DOI] [PubMed] [Google Scholar]
- 33.Kobilo T, Liu Q-R, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011;18(9):605–9. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leasure JL, Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156(3):456–65. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 35.Lin T-W, Chen S-J, Huang T-Y, Chang C-Y, Chuang J-I, Wu F-S, Kuo Y-M, Jen C-J. Different types of exercise induce differential effects on neuronal adaptations and memory performance. Neurobiology of learning and memory. 2012;97(1):140–7. doi: 10.1016/j.nlm.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 36.Lin Y, Horst Ter GJ, Wichmann R, Bakker P, Liu A, Li X, Westenbroek C. Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cerebral Cortex. 2009;19(9):1978–89. doi: 10.1093/cercor/bhn225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y-F, Chen H-I, Wu C-L, Kuo Y-M, Yu L, Huang A-M, Wu F-S, Chuang J-I, Jen CJ. Differential effects of treadmill running and wheel running on spatial or aversive learning and memory: roles of amygdalar brain-derived neurotrophic factor and synaptotagmin I. The Journal of Physiology. 2009;587(13):3221–31. doi: 10.1113/jphysiol.2009.173088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lubin FD, Roth TL, Sweatt JD. Epigenetic Regulation of bdnf Gene Transcription in the Consolidation of Fear Memory. Journal of Neuroscience. 2008;28(42):10576–86. doi: 10.1523/JNEUROSCI.1786-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lubin FD. Epigenetic gene regulation in the adult mammalian brain: Multiple roles in memory formation. Neurobiology of learning and memory. 2011;96(1):68–78. doi: 10.1016/j.nlm.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Madeira MD, Lieberman AR. Sexual dimorphism in the mammalian limbic system. Progress in Neurobiology. 1995;45(4):275–333. doi: 10.1016/0301-0082(94)00052-j. [DOI] [PubMed] [Google Scholar]
- 41.Marlatt MW, Potter MC, Lucassen PJ, van Praag H. Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Devel Neurobio. 2012;72(6):943–52. doi: 10.1002/dneu.22009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, Fan G, Sun YE. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302(5646):890–3. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- 43.Molteni R, Ying Z, Gomez-Pinilla F. Differential effects of acute and chronic exercise on plasticity-related genes in the rat hippocampus revealed by microarray. European Journal of Neuroscience. 2002;16(6):1107–16. doi: 10.1046/j.1460-9568.2002.02158.x. [DOI] [PubMed] [Google Scholar]
- 44.Neeper SA, Gomez-Pinilla F, Choi J, Cotman CW. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Research. 1996;726(1–2):49–56. [PubMed] [Google Scholar]
- 45.Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995 doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- 46.Ni H, Li C, Tao L-Y, Cen J-N. Physical exercise improves learning by modulating hippocampal mossy fiber sprouting and related gene expression in a developmental rat model of penicillin-induced recurrent epilepticus. Toxicology Letters. 2009;191(1):26–32. doi: 10.1016/j.toxlet.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 47.Pawlak R, Rao BSS, Melchor JP, Chattarji S, McEwen B, Strickland S. Tissue plasminogen activator and plasminogen mediate stress-induced decline of neuronal and cognitive functions in the mouse hippocampus. Proc Natl Acad Sci USA. 2005;102(50):18201–6. doi: 10.1073/pnas.0509232102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ploughman M, Granter-Button S, Chernenko G, Tucker BA, Mearow KM, Corbett D. Endurance exercise regimens induce differential effects on brain-derived neurotrophic factor, synapsin-I and insulin-like growth factor I after focal ischemia. Neuroscience. 2005;136(4):991–1001. doi: 10.1016/j.neuroscience.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 49.Pluchino N, Russo M, Santoro AN, Litta P, Cela V, Genazzani AR. Steroid hormones and BDNF. Neuroscience. 2013;239:271–9. doi: 10.1016/j.neuroscience.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 50.Real CC, Ferreira AFB, Hernandes MS, Britto LRG, Pires RS. Exercise-induced plasticity of AMPA-type glutamate receptor subunits in the rat brain. Brain Research. 2010;1363:63–71. doi: 10.1016/j.brainres.2010.09.060. [DOI] [PubMed] [Google Scholar]
- 51.Redila VA, Christie BR. Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. NSC. 2006;137(4):1299–307. doi: 10.1016/j.neuroscience.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 52.Richter SH, Gass P, Fuss J. Resting Is Rusting: A Critical View on Rodent Wheel-Running Behavior. The Neuroscientist. 2014;20(4):313–25. doi: 10.1177/1073858413516798. [DOI] [PubMed] [Google Scholar]
- 53.Sartori CR, Vieira AS, Ferrari EM, Langone F, Tongiorgi E, Parada CA. The antidepressive effect of the physical exercise correlates with increased levels of mature BDNF, and proBDNF proteolytic cleavage-related genes, p11 and tPA. Neuroscience. 2011;180:9–18. doi: 10.1016/j.neuroscience.2011.02.055. [DOI] [PubMed] [Google Scholar]
- 54.Steiner JL, Murphy EA, McClellan JL, Carmichael MD, Davis JM. Exercise training increases mitochondrial biogenesis in the brain. Journal of Applied Physiology. 2011;111(4):1066–71. doi: 10.1152/japplphysiol.00343.2011. [DOI] [PubMed] [Google Scholar]
- 55.Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17(11):1017–22. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. Journal of Psychiatric Research. 2015;60:56–64. doi: 10.1016/j.jpsychires.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ Influx Regulates BDNF Transcription by a CREB Family Transcription Factor-Dependent Mechanism. Neuron. 1998;20(4):709–26. doi: 10.1016/s0896-6273(00)81010-7. [DOI] [PubMed] [Google Scholar]
- 58.Tao X, West AE, Chen WG, Corfas G, Greenberg ME. A calcium-responsive transcription factor, CaRF, that regulates neuronal activity-dependent expression of BDNF. Neuron. 2002;33(3):383–95. doi: 10.1016/s0896-6273(01)00561-x. [DOI] [PubMed] [Google Scholar]
- 59.Titterness AK, Wiebe E, Kwasnica A, Keyes G, Christie BR. Voluntary exercise does not enhance long-term potentiation in the adolescent female dentate gyrus. Neuroscience. 2011;183:25–31. doi: 10.1016/j.neuroscience.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 60.Toscano-Silva M, da Silva SG, Scorza FA, Bonvent JJ, Cavalheiro EA, Arida RM. Hippocampal mossy fiber sprouting induced by forced and voluntary physical exercise. Physiology & Behavior. 2010;101(2):302–8. doi: 10.1016/j.physbeh.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 61.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96(23):13427–31. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. European Journal of Neuroscience. 2004;20(10):2580–90. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- 63.Vaynman S, YING Z, Gómez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. NSC. 2003;122(3):647–57. doi: 10.1016/j.neuroscience.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 64.Vega SR, Strüder HK, Wahrmann BV. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Research. 2006 doi: 10.1016/j.brainres.2006.08.105. [DOI] [PubMed] [Google Scholar]
- 65.Venezia AC, Guth LM, Spangenburg EE, Roth SM. Lifelong parental voluntary wheel running increases offspring hippocampal Pgc-1α mRNA expression but not mitochondrial content or Bdnf expression. Neuroreport. 2015;26(8):467–72. doi: 10.1097/WNR.0000000000000371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends in Cognitive Sciences. 2013;17(10):525–44. doi: 10.1016/j.tics.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wrann CD, White JP, Salogiannnis J, Laznik-Bogoslavski D, Wu J, Ma D, Lin JD, Greenberg ME, Spiegelman BM. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metabolism. 2013;18(5):649–59. doi: 10.1016/j.cmet.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yanagita S, Amemiya S, Suzuki S, Kita I. Effects of spontaneous and forced running on activation of hypothalamic corticotropin-releasing hormone neurons in rats. Life Sciences. 2007;80(4):356–63. doi: 10.1016/j.lfs.2006.09.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


