Abstract
Heightened emotional reactivity to peer feedback is predictive of adolescents’ depression risk. Examining variation in emotional reactivity within currently depressed adolescents may identify subgroups that struggle the most with these daily interactions. We tested whether trait rumination, which amplifies emotional reactions, explained variance in depressed adolescents’ physiological reactivity to peer feedback, hypothesizing that rumination would be associated with greater pupillary response to peer rejection and diminished response to peer acceptance. Twenty currently depressed adolescents (12–17) completed a virtual peer interaction paradigm where they received fictitious rejection and acceptance feedback. Pupillary response provided a time-sensitive index of physiological arousal. Rumination was associated with greater initial pupil dilation to both peer rejection and acceptance, and diminished late pupillary response to peer acceptance trials only. Results indicate that depressed adolescents high on trait rumination are more reactive to social feedback regardless of valence, but fail to sustain cognitive-affective load on positive feedback.
Keywords: Peer relationships, Depression, Adolescence, Emotional reactivity, Pupil
Introduction
Social feedback from peers plays a central role in adolescent emotional adjustment as peers take on a greater influence in adolescents’ lives. Depressive symptoms and diagnoses have been associated with altered responses to peer feedback on multiple levels of analysis including: heightened neural reactivity [1–3], use of poorer behavior coping strategies [4] and greater sadness [2], for review see [5]. Given that depression has a heterogeneous presentation and etiology, a subset of depressed youth are likely to be more reactive to peer feedback, which may predispose them to worse outcomes, such as longer and more severe episodes of depression [6]. Identifying this subset of depressed youth is critical for informing interventions to bolster their psycho-social functioning. The tendency to ruminate, or perseverate on dysphoric mood, symptoms, and reactions to ongoing events, is linked to the magnification of emotional states, increased physiological reactivity to social evaluative threat, and longer, more severe depressive episodes [7, 8]. As such, rumination may be a characteristic that can differentiate which depressed adolescents are more reactive to social feedback. Thus, we examined whether rumination predicts differences in physiological reactivity to peer feedback in a sample of currently depressed adolescents.
As adolescents begin to emphasize social networks outside the parental relationships, they increasingly rely on peers to meet their social and emotional needs, making peers a primary source of support and daily companionship [9, 10]. However, adolescents’ reliance on peers can be costly. Adolescents’ growing social networks are characterized by a complex social hierarchy and friendship instability [11]. A vital determinant of adolescents’ social success, and thus emotional functioning, is the capacity to react and respond to social feedback from peers. Peer feedback not only signals potential changes in social status, but also influences youths’ intrapersonal processing, including evaluation of one’s own self-worth [12, 13]. Despite the developmental relevance of peer feedback, it is only recently that technological advances (i.e., devising interaction paradigms with virtual peers) have enabled ecologically valid assessments in laboratory settings with physiological and neural indices in order to better delineate alterations in adolescents’ emotional reactivity to these salient events.
Initial studies support that adolescent depression is associated with greater reactivity to both positive and negative forms of peer feedback. Specifically, compared to healthy controls, currently depressed youth exhibit greater activation in brain regions implicated in emotional reactivity in response to positive social feedback [1] and negative peer rejection [2]. Further, neural activation in response to social exclusion has also been found to predict adolescents’ depressive symptom trajectories [3]. Taken together, these findings indicate that adolescents who require greater physiological or neural resources to respond to peer feedback are at heightened risk for depression, or are currently depressed. However, given the heterogeneity in depression, it is quite likely that there are subsets of depressed youth for whom altered reactivity to social feedback also impacts depression maintenance and prognosis. Understanding variation within depressed adolescents’ response to peer feedback may identify which youth struggle more with daily social interactions [14].
The tendency to ruminate is a likely candidate for explaining variation in depressed adolescents’ physiological reactivity to peer feedback. There is evidence to suggest that individuals’ high on trait rumination experience greater physiological arousal to social stimuli than nonruminators. Specifically, trait rumination has been linked with greater cortisol reactivity and delayed recovery in response to social stress and rejection in adults [15–17]; and cardiovascular reactivity to interpersonal stress in children [18]. Therefore, depressed adolescents’ higher on trait rumination may be more negatively impacted by peer feedback as the tendency to perseverate on past social interactions places them at greater risk for a longer and more severe course of depression. Rumination has indeed been implicated in depression maintenance in adolescence (longer episode duration) [19], and a host of poorer treatment response indices in adults [20–23]. Thus, we examined whether rumination explains variance in depressed adolescents’ physiological responses to peer feedback.
In the current study, adolescents completed a laboratory assessment where they received peer acceptance and rejection feedback in real-time from virtual peers, while pupil dilation was assessed continuously during 10 s trials (Chatroom Interact Task) [2, 24]. Task evoked-pupillary response is a temporally sensitive physiological index that is ideal for assessing emotional reactivity and sustained cognitive processes. The pupil is directly innervated by neural circuitry implicated in arousal (locus coeruleus) and emotional reactivity (limbic regions) as well as emotion regulation (prefrontal cortex) [25–29]. Pupil dilation increases with both cognitive load and emotional intensity [30, 31], and persists if processing is sustained [32]. Thus, the magnitude and timing of pupil dilation provides a summary index of the intensity and duration of task-evoked neural activity. In this study, initial dilation peaks (which occur during the first 3 s) provided a proxy for initial reactivity; the remaining time was used as a proxy for sustained processing.
Multiple studies support the utility of pupillometry for assessing alterations associated with depression in adolescence. In comparison to healthy peers, high risk youth (with mothers who have a history of depression) exhibit greater pupillary response to sad faces [33]. Further, within these high-risk youth, greater pupillary response is predictive of prospective depressive symptoms and episode onsets [34]. In contrast, currently depressed youth exhibit diminished sustained pupillary response to negative words [35, 36]. Taken together these results may indicate that different alterations in emotion reactivity are implicated in depression risk versus maintenance. To date though, pupillary assessments on the effects of rumination on emotional reactivity have been limited to adult samples and word identification tasks. It is worth highlighting though, that initial adult versus adolescent findings appear in conflict. Depressed adolescents as a group have been found to display diminished sustained pupillary response when compared to healthy youth [35, 36], whereas rumination in adults has been associated with greater initial [37] and sustained pupil dilation [38, 39]. We propose that examining variance within depressed youth may identify important variance in emotional reactivity that accounts for this discrepancy.
In developing hypotheses, we anticipated that the effect of trait rumination would differ according to condition (positive peer acceptance vs. negative rejection). That is, both adult studies on the effects of rumination [37–39] and youth studies assessing depression status [33, 35, 36] have consistently identified alterations in pupillary response that were specific to negative stimuli. Since rumination is characterized by both the tendency to react strongly to, and also have difficulty disengaging from negative events, we hypothesized that in response to peer rejection, rumination would be associated with greater initial pupil dilation that is sustained for a longer duration, corresponding with higher reactivity and sustained cognitive-affective load. Regarding peer acceptance, the majority of research supports that depressed ruminators do not react differently to positive stimuli, but have preferential memory for negative events [40] and tend to dampen or diminish responses to positive events [41]. Thus in response to peer acceptance, we hypothesized that rumination would not be associated with initial dilation peaks, but would be associated with diminished sustained dilation, indicating a lack of sustained cognitive-affective load.
Method
Participants
Participants were 20 of 22 adolescents (14 female; ages 12—17, M = 14.70) with a current, primary diagnosis of Major Depressive Disorder (MDD) and intact pupillary data, participating in a larger study on emotional development [2]. Exclusion criteria included current psychoactive medications except for SSRI’s (n = 2), and a current diagnosis of obsessive–compulsive disorder, post-traumatic stress disorder, conduct disorder, substance abuse or dependence and ADHD combined type or predominantly hyperactive–impulsive type, or a lifetime diagnosis of bipolar disorder, psychotic depression, schizophrenia, schizoaffective disorder, or a pervasive developmental disorder. For the current analyses, inclusion also required intact pupillary response data to the Chatroom Interact task, which was administered during the second visit. Thus, of 22 potential participants, 2 were removed due to lack of viable pupillary data with less than 50 % of intact trials per condition. Regarding co-morbidity, of the final sample of 20 adolescents, 8 also met criteria for a current or past diagnosis of one or more anxiety disorders (panic disorder, n = 2; specific phobia, n = 2; social phobia, n = 1; separation anxiety disorder, n = 2; generalized anxiety disorder, n = 6; agoraphobia = 1). Three participants met criteria for a current or past behavioral disorder: oppositional defiant disorder n = 2; ADHD inattentive only subtype, n = 1.
Procedure
Participants were recruited from community advertisements or were referred from mental health clinics and pediatricians’ offices. Parents completed a phone screen, and then were invited to complete two laboratory visits with their child. On Day 1, informed consent and assent were obtained. Then both parents and adolescents each completed diagnostic interviews, and self-report surveys. Adolescents also selected peers to interact with online on a future visit. Two weeks later, on Day 2, adolescents completed the computer-based Chatroom Interact task during a neuroimaging assessment with concurrent pupillometry. The present report focuses only on the pupillary data because it provides greater temporal resolution than other indices, making it ideal for capturing changes in physiological arousal implicated in emotional reactivity. All research procedures were approved by the University’s Institutional Review Board.
Diagnostic Assessment
The Schedule for Affective Disorders and Schizophrenia in School Age Children—Present and Lifetime version (K-SADS-PL) [42] was used to assess adolescents’ psychopathology. Parents and adolescents were interviewed separately with results synthesized by the clinician. Inter-rater reliability of MDD diagnoses based on 15 % of the interviews was excellent (κ = 1.00).
Self-Report Measures
Adolescents completed the Mood and Feelings Questionnaire (MFQ) [43] as a widely used measure of youths’ current depressive symptoms, with excellent psychometric properties (α = 0.95). The Children’s Response Styles Scale (CRSS) [44] rumination subscale was used to measures youths’ tendency to ruminate. Youth rated on a 10-point Likert scale how often they do things when they feel sad (e.g., I think back to other times when I felt this way), and scores are calculated by summing the responses. The 10-item rumination subscale has demonstrated reliability and validity [44]. Internal reliability in the current study was excellent, (α = 0.89).
Pupil Assessment
Participants completed the Chatroom Interact task while undergoing a functional magnetic resonance imaging assessment. As participants lay in the moderately lit 3T Siemens Trio scanner, stimuli were presented on a back-projection screen approximately 127 cm from a mirror placed approximately 12 cm above their eye (varied slightly by head size). Pupil dilation was acquired using an ASL Model 504 eye-tracker. This device consisted of a video camera and infrared light source positioned outside the magnet’s bore. The pupil was automatically tracked through a mirror anchored to the headcoil. Pupil size was recorded at 60 Hz (every 16.7 ms) along with signals marking the beginning of trials, and stimulus onset time.
Pupil Data Selection, Cleaning, and Reduction
Data were cleaned using our laboratory’s standard procedures [45] modified for fMRI-based pupil dilation assessments [26], including interpolation through blinks, removal of trials with over 50 % blinks and smoothing. Because absolute dilation could not be calculated for data collected in the scanner, an index representing the proportion of maximal dilation was used as a proxy for diameter. Pupil values were first range-corrected to standardize according to the 95 % maximally dilated pupil diameter and the 95 % maximally constricted pupil diameter [(current pupil diameter—minimum pupil diameter)/(maximum pupil diameter—minimum pupil diameter)] [26]. Next, baselines were calculated using the first 10 samples (167 ms) of each trial [2, 24]. Final pupil data reflect changes in dilation from baseline. The data were down-sampled to 5 Hz yielding a decreased number of parameters (65 vs. 800 time points) to facilitate moderation tests with rumination as a continuous covariate.
Data Analysis Strategy
A hierarchical growth model with Four Fourier basis functions comprised of sine and cosine waves of different frequencies (see Eqs. 1–4; Fig. 1) was run to test hypotheses. This model was selected in order to model the non-linear changes in pupillary response that were task relevant (to affective processing), while simultaneously removing known sources of task-irrelevant variance (light-reflex in response to social feedback). In Model 1, the intercept and basis functions were modeled as random effects, with each sine and cosine pair modeled to have the same variance according to Toeplitz (1) covariance structure. In Model 2, the main effects of rumination and condition (acceptance vs. rejection) were added as level 2 predictors of the intercept and each basis function. In Model 3, the primary model of interest, the rumination-by-condition interaction were added as level 2 predictors of the intercept and each basis function (see Eqs. 5–10). Significant Chi squared comparison tests indicated if the addition of predictors improved model fit. Contrasts test were then conducted to identify significant correlations between rumination and pupil dilation along the waveform. To control for Type I error, we used Guthrie and Buchwald’s (1991) strategy to account for data autocorrelation: regions of the waveform were considered statistically significant (p < .05), when 1.80 s, or 10 consecutive contrast tests were significant at p < .10.
| (1) |
| (2) |
| (3) |
| (4) |
Fig. 1.
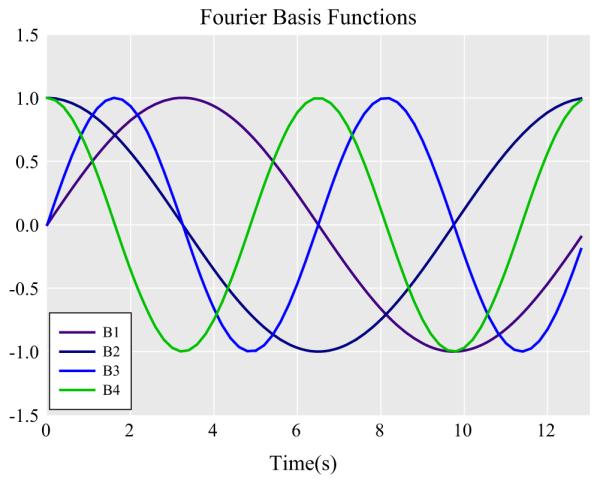
Fourier Basis functions were used to model theoretically relevant aspects of the pupillary waveforms
Level 1
| (5) |
Level 2
| (6) |
| (7) |
| (8) |
| (9) |
| (10) |
Task
Chatroom Interact Task [24]: on Day 1, adolescents selected 10 peers (from fictitious profiles) to interact with online during the next visit, and they also created their own profile. On Day 2, participants were told that they had been matched with four selected peers, who were ready to participate in an interaction online. While in the scanner, the participant and virtual peers took turns selecting who they would rather talk to about various teen interests (e.g., music, friends). Stimuli were presented using E-prime 1.0 software. The photograph of one peer (the agent choosing) was shown at the bottom left corner of the screen while the other two players’ photographs (participant and other peer) were shown in the middle of the screen (Fig. 2). Each trial began with a 3.34 s ‘choice phase’ when the agent is asked to select who they would rather discuss a developmentally relevant topic with: for example, “Who would you rather talk to about music”. In rejection trials, the participants’ photo was superimposed with an ‘X’ while the virtual peer’s photo was highlighted across the 10.02 s ‘feedback phase’. The opposite pattern occurred during peer acceptance trials. The order of trials was randomized within block. Participants were debriefed afterwards. One participant reported suspicion that the virtual peers were not real.1
Fig. 2.
Depiction of a trial on the Chatroom Interact Task
Results
Two participants were missing self-report data on rumination. Given the small sample size, in order to minimize Type II errors we examined whether their data could be imputed via maximum likelihood estimation (from depressive symptoms and demographic variables). To justify data imputation we first examined if their data was missing at random [46]. Little’s missing completely at random (MCAR) test was non-significant (χ2(6) = 5.05, p = .54), supporting the imputation of missing values [47]. In no instance did results differ with the inclusion of estimated data. As expected, participants reported elevated depressive symptoms (M = 34.40, SD = 14.91) and moderately high levels of rumination (M = 66.24, SD = 17.74), which were highly correlated (r = .61, p < .01). Neither variable differed according to participant age (MFQ: r = .12, p = .61; CRSS: r = .24, p = .30) or gender (MFQ: t(18) = −0.46, p = .65; CRSS: t(18) = 0.25, p = .81).
The full model predicting the pupil dilation waveforms as a function of rumination and condition is presented in Table 1, Model 3. First, we examined the significant main effect of condition on intercept and basis functions, which suggested that the pupil dilation model fits differed between acceptance versus rejection trials. Figure 3 depicts both the raw data as well as the model fits used in the analyses. In general the model fits closely tracked the raw data, and appeared to remove known sources of variance unrelated to affectively meaningful aspects of the design (light reflexes in the second following stimulus and feedback onset). Condition comparisons revealed that depressed adolescents displayed greater pupil dilation to peer rejection than acceptance from 2.4 to 12.8 s (Cohen’s d = 3.26). Among rejection trials, pupil dilation across the final contiguous region (11.0–12.8 s) was significantly greater than baseline, (model M = 0.0494; Raw M = 0.0624), t(49) = 3.78, p < .001, indicating that dilation was sustained across the feedback period.
Table 1.
Fixed effects and variance estimates of pupillary response to Chatroom Interact Task
| Parameter | Model 1 |
Model 2 |
Model 3 |
||||
|---|---|---|---|---|---|---|---|
| β | SE | β | SE | β | SE | ||
| Level 1 | |||||||
| β 00 | Intercept | 0.01* | (0.096) | 0.21* | (0.095) | 0.21* | (0.096) |
| β 10 | B1 | 0.23* | (0.089) | 0.13 | (0.080) | 0.13 | (0.080) |
| β 20 | B2 | −0.54*** | (0.089) | −0.52*** | (0.080) | −0.52*** | (0.080) |
| β 30 | B3 | −0.29*** | (0.048) | −0.42*** | (0.051) | −0.42*** | (0.051) |
| β 40 | B4 | −0.11* | (0.048) | −0.14** | (0.051) | −0.14** | (0.051) |
| Level 2 | |||||||
| β 01 | Rumination | 0.11 | (0.094) | 0.14 | (0.096) | ||
| β 02 | Condition | −0.42*** | (0.022) | −0.43*** | (0.021) | ||
| β 03 | Rumination × condition | −0.07*** | (0.021) | ||||
| β 11 | B1 × rumination | 0.14 | (0.079) | 0.06 | (0.080) | ||
| β 12 | B1 × condition | 0.20*** | (0.031) | 0.21*** | (0.030) | ||
| β 13 | B1 × rumination × condition | 0.17*** | (0.030) | ||||
| β 21 | B2 × rumination | −0.24** | (0.079) | −0.20* | (0.080) | ||
| β 22 | B2 × condition | −0.05 | (0.031) | 0.05 | (0.030) | ||
| β 23 | B2 × rumination × condition | −0.09** | (0.030) | ||||
| β 31 | B3 × rumination | −0.08 | (0.049) | −0.11* | (0.051) | ||
| β 32 | B3 × condition | 0.27** | (0.030) | 0.27*** | (0.030) | ||
| β 33 | B3 × rumination × condition | 0.07* | (0.030) | ||||
| β 41 | B4 × rumination | 0.00 | (0.049) | −0.03 | (0.051) | ||
| β 42 | B4 × condition | 0.07 | (0.030) | 0.08* | (0.030) | ||
| β 43 | B4 × rumination × condition | 0.07* | (0.030) | ||||
| Random parameters | |||||||
| roi | Intercept | 0.181** | (0.060) | 0.177** | (0.060) | 0.178** | (0.060) |
| r1i | B1, B2 | 0.154*** | (0.040) | 0.120*** | (0.030) | 0.119*** | (0.030) |
| r2i | B3, B4 | <0.041* | (0.011) | 0.043*** | (0.012) | 0.044*** | (0.012) |
| eti | Level 1-error | 0.348* | (0.010) | 0.288*** | (0.008) | 0.282*** | (0.008) |
| −2 LL | 4832.2 | 4397.3 | 4362.5 | ||||
| Χ2(5) | 869.8*** | 69.6*** | |||||
p < .05;
p < .01;
p < .001
Fig. 3.
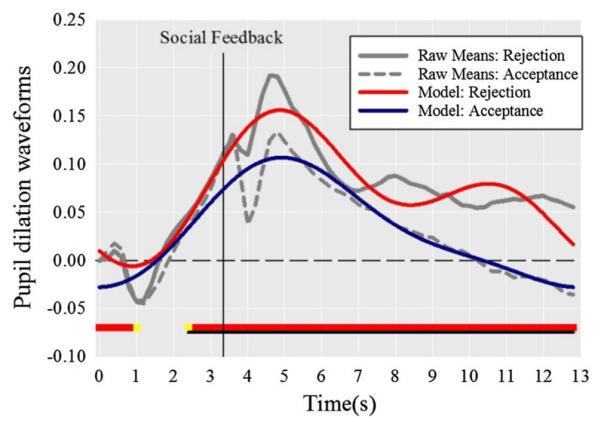
Pupillary response differed according to peer rejection versus acceptance feedback. Statistically significant contrast tests are highlighted along the x axis in yellow (p < .10) and red (p < .05), with underlined areas identifying contiguously significant regions (Color figure online)
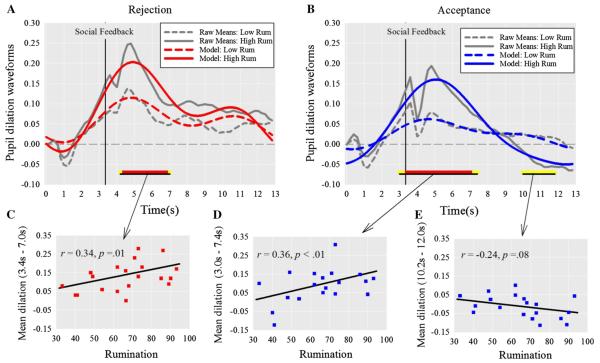
As anticipated, there were significant rumination × condition interactions at each basis function. Therefore we proceeded to examine hypotheses, testing associations between rumination and the model fits for pupillary waveforms within rejection and acceptance trials individually. In Fig. 4a, b, the effect of rumination on the pupil dilation waveforms is depicted via graphing high vs. low ruminative tendency (±1SD) for rejection and acceptance conditions respectively.
Fig. 4.
Pupillary response to peer rejection (a) and acceptance trials (b) differed by ruminative tendency. Significant contrast tests are highlighted along the x-axis in yellow (p < .10) and red (p < .05), with contiguously significant regions underlined. Scatterplots depict the association between rumination and pupillary response for each contiguous region in response to rejection trials (c), and acceptance trials (d, e) (Color figure online)
We found partial support for our hypothesis that rumination would be associated with greater initial pupil dilation that is sustained for longer duration in response to peer rejection. Rumination was indeed associated with greater initial pupil dilation from 3.4 to 7.0 s (r = .34, p = .01; see Fig. 4c), but there was no effect on dilation duration. Examining peer acceptance trials next, we had hypothesized that rumination would not be associated with initial pupil dilation peaks but would be negatively associated with sustained dilation. Analyses revealed two contiguously significant regions. Rumination was associated with greater initial dilation 3.0–7.4 s (r = .36, p < .01; Fig. 4d), and diminished pupillary response from 10.2 to 12.0 s (r = −.24, p = .08; Fig. 4e). Analysis of the late window revealed that higher ruminators (at +1SD) exhibited less dilation to acceptance compared to baseline (Model M = −0.0356; Raw M = −0.0481), t(52) = −1.88, p = .07, (Cohen’s d = 0.49).
Finally, we examined whether the associations were specific to rumination versus attributable to depression severity. First, we tested whether depressive symptom levels independently predicted differences in pupillary response, using the same model described for rumination. No region met the 1.8 s contiguity threshold. We then re-ran Model-3, regressing out the variance in rumination overlapping with depressive symptoms. None of the magnitude of the associations found (regions depicted in Fig. 4c, e) significantly differed (largest Z = −0.72, p = .47).
Discussion
Given the importance of peer feedback on adolescents’ psychosocial functioning, we examined whether rumination explained variation in depressed adolescents’ physiological response to peer acceptance and rejection feedback, as a means of identifying youth most sensitive to social stimuli. Adolescents higher on trait rumination displayed greater initial pupil dilation in response to both peer rejection and acceptance trials, than those lower on trait rumination. Importantly, results did not differ when controlling for depression severity, which suggests that effects were specific to rumination. Pupil dilation is associated with activation of brain regions implicated in emotional reactivity and regulation [25, 26, 29]. Therefore, depressed-ruminators’ larger peak pupil dilation suggests that they experience greater reactivity and or require more resources to regulate their responses to both positive and negative feedback than depressed adolescents with lower ruminative tendency. Thus in adolescence, depressed ruminators appear to be especially sensitive to peer interactions.
The current findings extend earlier research examining valence differences in pupillary response to peer feedback among healthy adolescents, which showed greater initial dilation to peer rejection than acceptance trials [24]. Although it was not a primary aim of the current analyses, we also found a significant condition effect whereby depressed adolescents exhibited greater initial pupil dilation to peer rejection than acceptance. To our knowledge this is the first pupillary assessment on peer feedback conducted in a clinical population. Thus it is noteworthy that the initial condition effect exhibited in healthy youth [24] replicates, and extends to clinically depressed youth.
The patterns of sustained pupillary response to peer rejection were surprising. Contrary to hypothesis, sustained dilation to peer rejection trials did not differ according to rumination. All of our depressed participants, regardless of ruminative tendency, maintained pupil dilation significantly greater than baseline across the 10 s feedback period. This null finding suggests that all depressed youth sustained cognitive-affective load in response to negative social feedback. However, it is possible that differences in sustained processing of negative feedback may emerge with longer assessment periods, or with paradigms that are designed to elicit state-rumination. That is, with the current focus on trait-rumination, it is not known whether participants were actively ruminating during the Chatroom Interact task. Future research that incorporates paradigms with larger inter-stimulus intervals (that do not require participants to immediately prepare for the next feedback trial) may be vital in identifying physiological correlates of state rumination. Additionally, it is striking that the sustained dilation exhibited to peer rejection in the current results contrasts with prior findings that depressed youth exhibit diminished sustained pupillary response to negative words [35, 36]. It may be that depressed youth find social feedback more emotionally salient compared to affective words; however, future research is needed to test this possibility.
As hypothesized, rumination was associated with a distinct pattern in sustained pupillary response to peer acceptance trials, which may have implications for the role of rumination on positive affective functioning. High-ruminators’ greater initial pupil dilation sharply declined to lower than baseline across the feedback period, compared to low-ruminators’ slow return to baseline. To the extent that rumination was associated with diminished sustained pupillary response (which suggests less brain activity), this finding may be consistent with prior research that ruminators fail to sustain positive affect and their reactions to positive events.
The current study extends prior work examining the role of rumination in emotional reactivity in several ways. Although research supported that rumination is associated with greater reactivity to negative social stimuli in adults and early childhood [17, 18], the current study is the first to examine how rumination affects physiological reactivity to both positive and negative social interactions in adolescence. This is notable since results indicate that rumination is associated with differences in both positive and negative affective processes. Further, the current study is also the first to examine pupillary response to social feedback in a clinical youth sample, and current results are consistent with a prior pupillometry study linking rumination with greater dilation to both positive and negative words in depressed and healthy adults [38].
The current results have potential clinical implications for advancing treatment for depressed adolescents, although prospective research supporting directional effects is needed to bolster the interpretation of these preliminary, cross-sectional associations. First, the results indicate that ruminators are more reactive to peer feedback, which suggests that targeting rumination in treatment may be helpful in bolstering these depressed adolescents’ psychosocial functioning. The diminished sustained pupillary response to positive peer feedback associated with rumination may also be informative. That is, although it is not possible to discern whether the diminished pupillary response reflects an inability to sustain positive affective arousal versus a dampening response, this finding is consistent with the idea that helping ruminators develop skills for sustaining positive affective processing could be a fruitful target for intervention.
Several study limitations should be noted. First, because the data are cross-sectional, it is not possible to discern whether pupillary responses are correlates or consequences of chronic rumination or ongoing depression. Future research in high-risk samples could better delineate the course of physiological alterations implicated in depression onset versus maintenance. The current study employed a self-report measure of trait rumination to explain differences in physiological reactivity. Future analyses may benefit from paradigms that assess induced state-ruminative processes. Another consideration is that healthy youth’s visual attention allocation has been found to differ in response to peer rejection vs. peer acceptance [24], which likely reflects different regulation processes. Incorporating eye-tracking data in future work may further qualify the links between rumination, attention, and physiological reactivity to social feedback in depressed youth. Finally, there are several considerations to note given the small sample size. First, it is critical that results replicate in larger, more representative clinical adolescent samples. Secondly, sample size also limited our capacity to test moderating effects on the link between rumination and peer feedback. Although the current results were maintained when covarying for gender and age, prior studies have found that gender, pubertal development, and maternal affection are significant predictors of youths’ reactivity to peer feedback [2, 48, 49]. Future research is needed to determine how rumination may interact with established predictors of adolescents’ reactivity to peer rejection and acceptance.
Despite these limitations, the current study benefits from several strengths including a clinical sample of currently depressed youth, and a virtual peer interaction paradigm that provided ecologically valid social stimuli. In assessing pupillary response to social feedback, we targeted the role of rumination as a particularly maladaptive emotion regulation strategy that may warrant greater emphasis in treatment. Findings highlight that rumination is associated with greater sensitivity to social feedback regardless of valence, as well as diminished sustained processing of peer acceptance.
Summary
Alterations in adolescents’ emotional reactivity to peer feedback is implicated in depression risk [1–3, 5]. The current study examined variation within currently depressed adolescents’ physiological reactivity to peer feedback as a means of identifying vulnerable subsamples who struggle with heightened reactions to these salient daily events, which may serve to maintain or worsen depressive episodes. Rumination is a maladaptive emotional regulation strategy that serves to magnify emotional reactions [7,8], and is associated with greater reactivity to interpersonal stress [17] and poorer depression treatment response [20–23]. Thus the current study tested whether rumination explained variation in depressed adolescents’ physiological reactivity to peer feedback. Rumination was associated with greater initial pupillary response to both positive peer acceptance and negative peer rejection. This indicates that depressed adolescents high on trait rumination are more sensitive to peer feedback regardless of valence. Rumination was also associated with diminished sustained pupillary response specifically to positive peer feedback. Results align with initial work indicating that ruminators experience greater physiological reactivity to interpersonal stimuli [18], and fail to sustain processing of positive stimuli [41].
Acknowledgments
This research was supported by National Institute of Drug Abuse Grant R21DA024144 (J.S.S./R.E.D., PI’s), the Clinical and Translational Science Institute at the University of Pittsburgh (NIH/NCRR/CTSA Grant UL1 RR024153) and the National Institute of Mental Health intramural research program. Dr. Jones is supported by MH086811.The authors are grateful to Daniel Pine, MD for his input and assistance on this project; Marcie Walker, Katie Burkhouse and Karen Garelik for their assistance in data acquisition; Harvey Iwamoto for task related computer programming; and Ruth Stroud and Jennifer Sears for their assistance with photography. We also thank the participants and their families.
Footnotes
Analyses were rerun: (1) removing the two subjects on SSRI’s (n = 18) (2) removing the subject who reported suspicion (n = 19), and (3) covarying for gender and age. The magnitude of the associations found between rumination and pupillary response did not significantly differ after considering these multiple sources of influence.
References
- 1.Davey CG, Allen NB, Harrison BJ, Yucel M. Increased amygdala response to positive social feedback in young people with major depressive disorder. Biol Psychiatry. 2011;69:734–741. doi: 10.1016/j.biopsych.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Silk JS, Siegle GJ, Lee KH, Nelson EE, Stroud LR, Dahl RE. Increased neural response to peer rejection associated with adolescent depression and pubertal development. Soc Cogn Affect Neurosci. 2014;9:1798–1807. doi: 10.1093/scan/nst175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masten CL, Eisenberger NI, Borofsky LA, McNealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: a marker of adolescents’ risk for depression. Dev Psychopathol. 2011;23:283–292. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reijntjes A, Stegge H, Terwogt MM, Kamphuis JH, Telch MJ. Children’s coping with in vivo peer rejection: an experimental investigation. J Abnorm Child Psychol. 2006;34:877–889. doi: 10.1007/s10802-006-9061-8. [DOI] [PubMed] [Google Scholar]
- 5.Platt B, Cohen KK, Lau JY. The role of peer rejection in adolescent depression. Depress Anxiety. 2013;30:809–821. doi: 10.1002/da.22120. [DOI] [PubMed] [Google Scholar]
- 6.La Greca AM, Davila J, Siegel R. Peer relations, friendships, and romantic relationships: implications for the development and maintenance of depression in adolescents. In: Allen NB, Sheeber LB, editors. Adolescent emotional development and the emergence of depressive disorders. Cambridge University Press; Cambridge: 2008. pp. 318–336. [Google Scholar]
- 7.Nolen-Hoeksema S. Responses to depression and their effects on the duration of depressive episodes. J Abnorm Psychol. 1991;100:569–582. doi: 10.1037//0021-843x.100.4.569. [DOI] [PubMed] [Google Scholar]
- 8.Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspect Psychol Sci. 2008;3:400–424. doi: 10.1111/j.1745-6924.2008.00088.x. [DOI] [PubMed] [Google Scholar]
- 9.Furman W, Buhrmester D. Age and sex differences in perceptions of networks of personal relationships. Child Dev. 1992;63:103–115. doi: 10.1111/j.1467-8624.1992.tb03599.x. [DOI] [PubMed] [Google Scholar]
- 10.Hartup WW. Adolescents and their friends. New Dir Child Adolesc Dev. 1993;60:3–22. doi: 10.1002/cd.23219936003. [DOI] [PubMed] [Google Scholar]
- 11.Bukowski WM, Newcomb AF, Hartup WW. The company they keep: friendship in childhood and adolescence. Cambridge University Press; New York: 1996. [Google Scholar]
- 12.Bukowski WM, Velasquez AM, Brendgen M. Variation in patterns of peer influence: considerations of self and other. In: Dodge MJPKA, editor. Understanding peer influence in children and adolescents. Guilford Press; New York: 2008. pp. 125–140. [Google Scholar]
- 13.Gibb BE, Stone LB, Crossett SE. Peer victimization and prospective changes in children’s inferential styles. J Clin Child Adolesc Psychol. 2012;41:561–569. doi: 10.1080/15374416.2012.703124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larson RW, Raffaelli M, Richards MH, Ham M, Jewell L. Ecology of depression in late childhood and early adolescence: a profile of daily states and activities. J Abnorm Psychol. 1990;99:92–102. doi: 10.1037//0021-843x.99.1.92. [DOI] [PubMed] [Google Scholar]
- 15.McCullough ME, Orsulak P, Brandon A, Akers L. Rumination, fear, and cortisol: an in vivo study of interpersonal transgressions. Health Psychol. 2007;26:126–132. doi: 10.1037/0278-6133.26.1.126. [DOI] [PubMed] [Google Scholar]
- 16.Zoccola PM, Quas JA, Yim IS. Salivary cortisol responses to a psychosocial laboratory stressor and later verbal recall of the stressor: the role of trait and state rumination. Stress. 2010;13:435–443. doi: 10.3109/10253891003713765. [DOI] [PubMed] [Google Scholar]
- 17.Zoccola PM, Dickerson SS. Assessing the relationship between rumination and cortisol: a review. J Psychosom Res. 2012;73:1–9. doi: 10.1016/j.jpsychores.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Borelli JL, Hilt LM, West JL, Weekes NY, Gonzalez MC. School-aged children’s depressive rumination is associated with their reactivity to sadness but not fear. J Clin Child Adolesc Psychol. 2014;43:799–812. doi: 10.1080/15374416.2013.814542. [DOI] [PubMed] [Google Scholar]
- 19.Abela JRZ, Hankin BL. Rumination as a vulnerability factor to depression during the transition from early to middle adolescence: a multiwave longitudinal study. J Abnorm Psychol. 2011;120:259–271. doi: 10.1037/a0022796. [DOI] [PubMed] [Google Scholar]
- 20.Ciesla JA, Roberts JE. Self-directed thought and response to treatment for depression: a preliminary investigation. J Cogn Psychother. 2002;16:435–453. [Google Scholar]
- 21.Jones NP, Siegle GJ, Thase ME. Effects of rumination and initial severity on remission to cognitive therapy for depression. Cognit Ther Res. 2008;32:591–604. doi: 10.1007/s10608-008-9191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmaling KB, Dimidjian S, Katon W, Sullivan M. Response styles among patients with minor depression and dysthymia in primary care. J Abnorm Psychol. 2002;111:350–356. doi: 10.1037//0021-843x.111.2.350. [DOI] [PubMed] [Google Scholar]
- 23.Siegle GJ, Carter CS, Thase ME. Use of fmri to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- 24.Silk JS, Stroud LR, Siegle GJ, Dahl RE, Lee KH, Nelson EE. Peer acceptance and rejection through the eyes of youth: pupillary, eyetracking and ecological data from the chatroom interact task. Soc Cogn Affect Neurosci. 2012;7:93–105. doi: 10.1093/scan/nsr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegle GJ, Steinhauer SR, Friedman ES, Thompson WS, Thase ME. Remission prognosis for cognitive therapy for recurrent depression using the pupil: utility and neural correlates. Biol Psychiatry. 2011;69:726–733. doi: 10.1016/j.biopsych.2010.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siegle GJ, Steinhauer SR, Stenger VA, Konecky R, Carter CS. Use of concurrent pupil dilation assessment to inform interpretation and analysis of fMRI data. NeuroImage. 2003;20:114–124. doi: 10.1016/s1053-8119(03)00298-2. [DOI] [PubMed] [Google Scholar]
- 27.Murphy PR, Redmond G, O’Sullivan M, Robertson IH, Balsters JH. Pupil diameter covaries with bold activity in human locus coeruleus. Hum Brain Mapp. 2014;35:4140–4154. doi: 10.1002/hbm.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alnaes D, Sneve MH, Espeseth T, Endestad T, van de Pavert SH, Laeng B. Pupil size signals mental effort deployed during multiple object tracking and predicts brain activity in the dorsal attention network and the locus coeruleus. J Vis. 2014;14:1–20. doi: 10.1167/14.4.1. [DOI] [PubMed] [Google Scholar]
- 29.Sterpenich V, D’Argembeau A, Desseilles M, Balteau E, Albouy G, Vandewalle G, et al. The locus ceruleus is involved in the successful retrieval of emotional memories in humans. J Neurosci. 2006;26:7416–7423. doi: 10.1523/JNEUROSCI.1001-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beatty J. Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychol Bull. 1982;91:276–292. [PubMed] [Google Scholar]
- 31.Granholm E, Asarnow RF, Sarkin AJ, Dykes KL. Pupillary responses index cognitive resource limitations. Psychophysiology. 1996;33:457–461. doi: 10.1111/j.1469-8986.1996.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 32.Beatty J. Phasic not tonic pupillary responses vary with auditory vigilance performance. Psychophysiology. 1982;19:167–172. doi: 10.1111/j.1469-8986.1982.tb02540.x. [DOI] [PubMed] [Google Scholar]
- 33.Burkhouse KL, Siegle GJ, Gibb BE. Pupillary reactivity to emotional stimuli in children of depressed and anxious mothers. J Child Psychol Psychiatry. 2014;55:1009–1016. doi: 10.1111/jcpp.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burkhouse KL, Siegle GJ, Woody ML, Kudinova AY, Gibb BE. Pupillary reactivity to sad stimuli as a biomarker of depression risk: evidence from a prospective study of children. J Abnorm Psychol. 2015;124:498–506. doi: 10.1037/abn0000072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones NP, Siegle GJ, Proud L, Silk JS, Hardy D, Keljo DJ, et al. The impact of inflammatory bowel disease and high dose steroid exposure on pupillary responses to negative information in pediatric depression. Psychosom Med. 2011;73:151–157. doi: 10.1097/PSY.0b013e318207ffea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silk JS, Dahl RE, Ryan ND, Forbes EE, Axelson DA, Birmaher B, et al. Pupillary reactivity to emotional information in child and adolescent depression: links to clinical and ecological measures. Am J Psychiatry. 2007;164:1873–1880. doi: 10.1176/appi.ajp.2007.06111816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duque A, Sanchez A, Vazquez C. Gaze-fixation and pupil dilation in the processing of emotional faces: the role of rumination. Cogn Emot. 2014;28:1347–1366. doi: 10.1080/02699931.2014.881327. [DOI] [PubMed] [Google Scholar]
- 38.Siegle GJ, Granholm E, Ingram RE, Matt GE. Pupillary and reaction time measures of sustained processing of negative information in depression. Biol Psychiatry. 2001;49:624–636. doi: 10.1016/s0006-3223(00)01024-6. [DOI] [PubMed] [Google Scholar]
- 39.Siegle GJ, Steinhauer SR, Carter CS, Ramel W, Thase ME. Do the seconds turn into hours? Relationships between sustained pupil dilation in response to emotional information and self-reported rumination. Cognit Ther Res. 2003;30:365–382. [Google Scholar]
- 40.Whitmer AJ, Gotlib IH. An attentional scope model of rumination. Psychol Bull. 2013;139:1036–1061. doi: 10.1037/a0030923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feldman GC, Joormann J, Johnson SL. Responses to positive affect: a self-report measure of rumination and dampening. Cognit Ther Res. 2008;32:507–525. doi: 10.1007/s10608-006-9083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 43.Angold A, Erkanli A, Silberg J, Eaves L, Costello EJ. Depression scale scores in 8–17 year-olds: effects of age and gender. J Child Psychol Psychiatry. 2002;43:1052–1063. doi: 10.1111/1469-7610.00232. [DOI] [PubMed] [Google Scholar]
- 44.Ziegert DI, Kistner JA. Response styles theory: downward extension to children. J Clin Child Adolesc Psychol. 2002;31:325–334. doi: 10.1207/S15374424JCCP3103_04. [DOI] [PubMed] [Google Scholar]
- 45.Siegle GJ, Ichikawa N, Stienhauer S. Blink before and after you think: blinks occur prior to and following cognitive load indexed by pupillary responses. Psychophysiology. 2008;45:679–687. doi: 10.1111/j.1469-8986.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- 46.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- 47.Little RJA, Rubin DB. Statistical analysis with missing data. Wiley; New York: 1987. [Google Scholar]
- 48.Silk JS, Siegle GJ, Whalen DJ, Ostapenko LJ, Ladouceur CD, Dahl RE. Pubertal changes in emotional information processing: pupillary, behavioral, and subjective evidence during emotional word identification. Dev Psychopathol. 2009;21:7–26. doi: 10.1017/S0954579409000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tan PZ, Lee KH, Dahl RE, Nelson EE, Stroud LJ, Siegle GJ, et al. Associations between maternal negative affect and adolescent’s neural response to peer evaluation. Dev Cogn Neurosci. 2014;8:28–39. doi: 10.1016/j.dcn.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]