Abstract
The process of repair of wounded skin involves intricate orchestration not only between the epidermal and dermal compartments but also between the resident and immigrant cells and the local microenvironment. Only now are we beginning to appreciate the complex roles played by the matrix in directing the outcome of the repair processes, and how this impacts the signals from the various cells. Recent findings speak of dynamic and reciprocal interactions that occurs among the matrix, growth factors, and cells that underlies this integrated process. Further confounding this integration are the physiologic and pathologic situations that directly alter the matrix to impart at least part of the dysrepair that occurs. These topics will be discussed with a call for innovative model systems of direct relevance to the human situation.
Keywords: Extracellular matrix, wound healing, chronic wound, skin organ cultures, matrikine signaling, tenascin-C, decorin, collagen
Introduction
Wound healing is a highly orchestrated process that involves multiple developmental lineages, cell types, and local and systemic effects. Not only do the resident parenchymal cells and their stromal counterparts need to be replaced, but the support structures of the vascular, nervous and immune systems must be re-established. The process has been extensively studied in the skin and mucosal surfaces as these sites are the most often wounded both traumatically and iatrogenically. While most surface wounds heal with near regenerative repair, regaining the vast majority of pre-wound functionality, the ubiquity of such insults, particularly in individuals with comorbidities and advanced age, means that wounds that ‘fail to heal’ or heal excessively (scarring) remain major medical issues.
It should be noted that the discussion herein focuses on excisional wound repair, i.e. healing that replaces lost tissue. Incisional repair, encompassing surgical wound repair, is both qualitatively and quantitatively distinct in that the major process is a re-integration of the separated tissue sections, rather than a regeneration of tissue mass. Thus, the granulation tissue response that marks excisional repair is largely absent during incisional repair. While some of the processes are common, such as stromal production of a collagen-rich matrix, even in these situations, the extent of these processes is dramatically different to constitute a significant difference. Furthermore, scarring occurs in all tissues, but such a discussion would be excessively extensive. To maintain the focus and comprehensibility, we are limiting our discussion to excisional/regenerative repair of the skin.
Non-healing wounds and pressure ulcers present significant morbidity, and even mortality in the US, with elderly and diabetic and neuropathic patients at the greatest risk. In diabetics alone, non-healing wounds result in over 70,000 amputations annually according to the CDC. At the other end of the spectrum is scarring and keloids. What combines these two different aspects is that these wounds do not progress from the tissue replacement phase to a competent resolving phase and thus remain in an immature state of cellular proliferation and matrix deposition/remodeling. Immature wounds are significantly weaker and prone to dehiscence. Hypervascular wound beds are also at increased risk of re-ulceration. Both events predispose to infection and chronic wounding, and ultimately failure to heal such wounds is the major cause of amputation in the US today (1, 2).
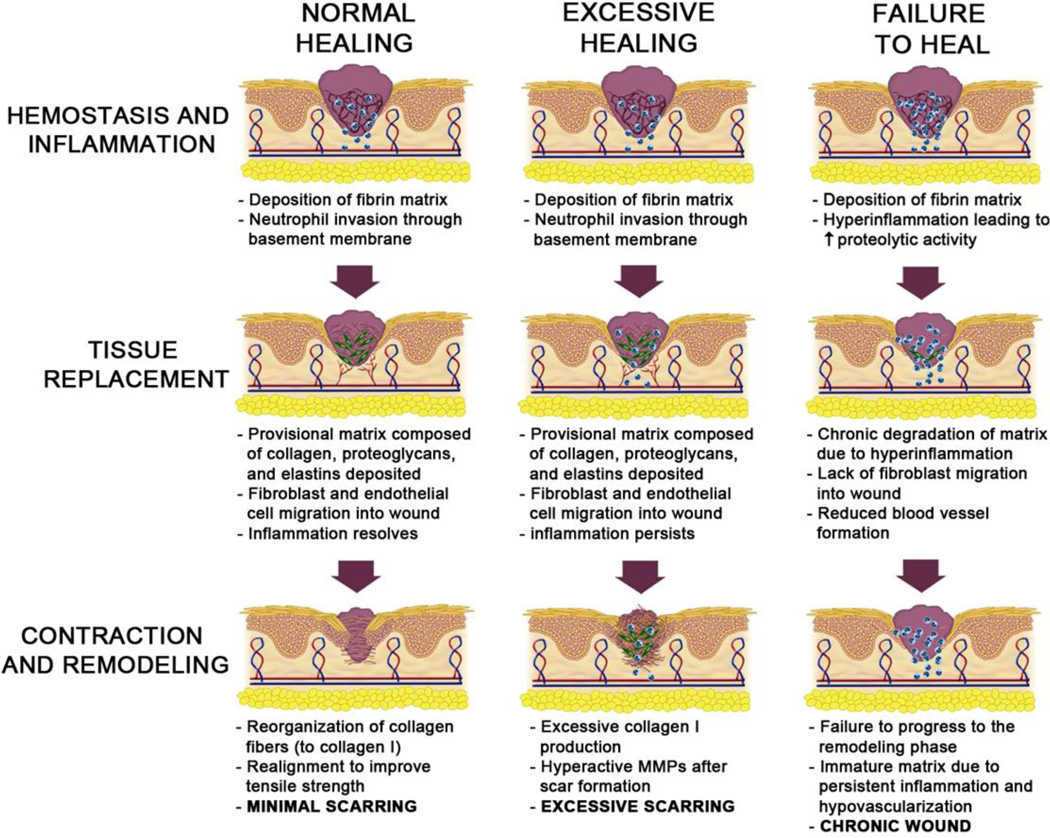
Repair of this tissue system is also the best-described as the skin is readily accessible for both wounding and longitudinal observation with easy, repeated sampling. As most wounds heal with little to no complication, such studies have been undertaken in human volunteers. What has emerged is a process that has been parsed into overlapping stages: initial hemostasis to quickly seal the breach and prevent desiccation and infection (hemostatic phase), tissue regeneration to replace the lost cells (tissue replacement phase), and finally wound resolution to restore the diverse functions of the skin and remodel the new matrix (resolving phase) (Figure 1) (3).
Figure 1. Phase transitions in excisional wound healing and dysrepair.
Wound healing proceeds from the initial homeostatic phase through tissue regeneration and into resolution. While these phases overlap both temporally and spatially within a wound, the orchestrated progression leads to re-established functioning with minimal scarring (left sequence). If the resolution phase has excessive cellularity and matrix from an over exuberant tissue replacement phase that lacks stop signals, this is not a stable phenotype. The renewal of an active immature matrix in the resolution phase results in excessive tissue and hypertrophic scars or even keloids (middle sequence). When the homeostatic phase does not transition towards regeneration, the healing is stalled and the initial, tissue-destructive inflammation persists. This situation leads to a chronic wound or ulcer (right sequence).
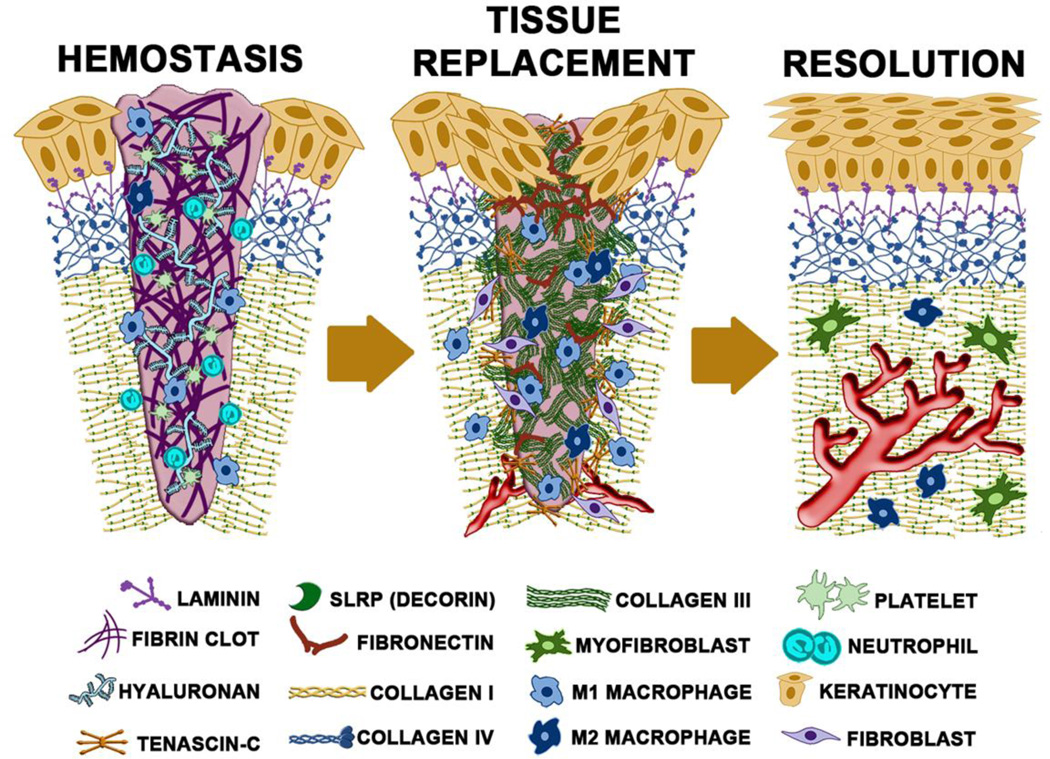
These phases, which occur at different rates across the wound, have been considered from multiple angles (Figure 2). Many conceptions of wound healing focus on either the cell types, soluble signals, or structures that predominate during each phase. However, the reality is that each of these not only are present but impact each other. For instance, the hemostatic stage includes both the initial platelet plug to prevent bleeding and form a barrier eschar and the lysate-attracted inflammatory infiltrate of hematopoietically-derived immune cells to prevent infection and limit colonization. The soluble signals present late in this stage herald the invasion and expansion of the formed elements that mark the tissue replacement phase, during which the tissue is reformed, but only once the appropriate and supportive matrix is generated. Subsequent to this, various signals released by the maturing structures (functional vessels and redifferentiated epidermis) induce both the quiescence of the cells in the tissue and involution of excess cells (keratinocytes and dermal fibroblasts) and structures (immature vessels), to revert to a relatively avascular and quiescent skin that is reinforced by a matrix suppressive to cell proliferation and migration. This parallels the concept of ‘dynamic reciprocity’, first described by Bissell et al in 1982 (4), describing the critical interaction of key components of the healing process that ultimately drives resolution. Specifically, in skin healing the dynamic reciprocity between cells and the surrounding microenvironment has been shown to play a role in many aspects of healing, including differentiation, migration, proliferation, and others (reviewed recently in (5)). It is this interwoven connectivity that is being emphasized herein with a focus on the matrix aspects.
Figure 2. Key players during different stages of wound repair.
The hemostasis (day 2), tissue replacement (day 10) and resolving (day 30) phases of healing are schematized here. Highlighted in each stage (and identified below) are the main cells and matrix/matricellular components that constitute that stage. (please note that the basement membrane is greatly exaggerated in size).
A changing matrix during repair and regeneration
Many investigations have discerned the cellular and soluble signal changes during the stages of wound repair (for reviews see (3, 6–9)); select key cells and signals are schematized in Fig 2. However, fewer studies have focused on the matrix changes, not because the matrix is considered unimportant, but rather it is generally assumed to be consequence of the cellular changes rather than a driver of phenotype in its own right. At one level, it is inarguable that other than the initial fibrin-rich clot formed during the initial hemostasis, the matrix does derive from the cells resident in various healing phases. For instance, the hematopoietic inflammatory cells attracted early on release the extracellular proteases that degrade the fibrin- and vitronectin-rich clot and allow it to be replaced by the macrophage-produced fibronectin. Later in the process, the keratinocytes contribute much of the basement membrane laminins (10) and collagenIV (11).
In contrast to this classical view a new conception places the matrix as central to controlling the cell phenotypes during the repair process (12). In this approach to thinking about healing, the nature of the extracellular matrix impacts the skin both through specific signaling/attachment domains and via physical aspects (stiffness). The iterative nature of matrix/matricellular components impacting the resident cells to produce specific matrix elements is proposed to control the outcome of healing – complete repair versus dysfunctional healing (Table 1). It should be noted that most of these matrix and matricellular molecules are multifunctional with both overt and cryptic signaling elements. Thus, discussion of specific downstream signaling pathways in cells is not included herein, as both the relevant signaling events change with time, and wound repair status and key elements likely remain to be established for these events. For details on many of the molecules noted, please see current reviews ((13–21)).
Table 1.
Matrix and matricellular proteins during wound repair.
| Matrix/Matri- cellular Protein |
Phase(s) | Main Producer Cell(s) | Cellular Effects | Cellular Receptors (major ones) |
|---|---|---|---|---|
| Collagen I |
Tissue Formation Tissue Remodeling |
Epithelial, Endothelial Fibroblasts, Smooth Muscle | Tissue architecture, Tensile strength Suppresses cell immigration Transitions fibroblasts to myofibroblasts | Integrins (mainly α2β1, α11β1), DDR1 and DDR2, LAIR1, Glycoprotein VI |
| Collagen III |
Tissue Formation |
Epithelial, Endothelial Fibroblasts | Tissue architecture Promotes fibroblast migration Supports angiogenesis | Integrins (mainly α2β1, α11β1), Glycoprotein VI, LAIR1, DDR1 and DDR2 |
| Fibrinogen/Fibrin |
Homeostasis Tissue Formation |
Platelets Leukocytes, Endothelial (from plasma) | Cell-Cell interaction, Cell-Matrix Platelet -protein, Promotes cell migration | αIIbβ3 integrin, VE- cadherin |
| Hyaluronan |
Tissue Formation Tissue Remodeling |
Epithelial Fibroblasts Smooth Muscle | Limits matrix stiffness Decreases cell proliferation Induces fibroblast differentiation | CD44, ICAM-1 |
| Fibronectin |
Homeostasis Tissue Formation Tissue Remodeling |
Epithelial, Endothelial Fibroblasts, Leukocytes, | Tissue architecture Promotes cell proliferation and migration Increases opsoninization | Several Integrins (mainly α5β1, avβ3) |
| Tenascin C |
Tissue Formation |
Epithelial, Fibroblasts | Modulates cell-matrix Anti-adhesive, Promotes migration Promotes proliferation, Angiogenesis | αvβ3 and α9β3 Integrins, annexin II, EGFR |
| Decorin |
Tissue Formation Tissue Remodeling |
Fibroblasts | Stabilizes collagen matrix Limits cell proliferation Negates growth factor signaling | EGFR, Met, IGF-IR, TLR2/4 |
| Entactin (or nidogen) |
Homeostasis Tissue Formation |
Epithelial,Fibroblasts Neutrophil | Basement membrane component Stabilizes basement membrane | α3β1 Integrin |
| Laminin |
Tissue Formation Tissue Remodeling |
Epithelial, Endothelial Fibroblasts, Smooth Muscle, Platelets, Monocyte | Basement membrane component Limits cell compartmentalization | 67LR, various integrins (α2β1, α3β1, α7β1, α6β4) |
| Thrombospondin |
Homeostasis Tissue Formation |
Platelets, Fibroblasts, Smooth Muscle cells | Angiogenesis Promotes cell proliferation and migration | CD36, CD47, β1 integrins, IAP- mediated interactions |
| SPARC |
Tissue Formation |
Fibroblasts | Promotes cell proliferation and migration | α5β1, stabilin-1, V- CAM |
An outline of major matrix elements known to be involved in or produced during wound healing can be found in Figure 2. The initial fibrinogen-cleaved fibrin clot contains the plasma proteins entrapped during hemostasis. The highly pliant fibrin, accompanied by more adhesive vitronectin, supports the invasion of macrophages and other leukocytes. In addition to serving to limit infection, the proteases produced by the cells breaks down the suppressive boundary matrices that limit subsequent tissue ingrowth. The leukocyte elastases released into the wound bed degrade molecules suppressive to angiogenesis (22, 23) while also affecting fibronectin degradation and protease inhibitor activity in more chronic wounds (ref). In addition, matrix metalloproteinases (MMPs) degrade collagen I that limit stromal migration (24, 25). In this manner, the suppressive small leucine-rich glycoproteins (SLRP) decorin and lumican are untethered and removed from the adjacent matrix. MMPs also provide other functions to the wound healing process, such as regulating mediators of inflammation via establishment and modification of chemokine gradients (26) .
The resident cells prime the adjacent ECM allowing for immigration and proliferation of stromal cells, while also producing fibronecton, tenascin, entactin and thrombospondin. The latter matrix elements come together to form a provisional ECM with both adhesive and anti-adhesive properties allowing for optimal locomotion of the stromal cellular components (fibroblasts, endothelial cells and keratinocytes).(27). Tenascin and other proteins are mainly at the edge of the advancing wound front wherein they establish a pro-migratory environment, serving also to enhance inflammatory cell adhesion and migration early in the healing process (28). These matricellular proteins in turn effect growth factor signaling through cryptic receptor binding motifs within the proteins themselves (matrikines), which signal via the EGF receptor to induce motility (15, 29–31) In the same vein, these components also may contain growth factor binding sites that similarly regulate signaling, such as those found in fibronectin (32). As the fibroblasts immigrate and establish in the wound site, they produce collagen III and collagen I to provide further structural integrity to the matrix, with fibronectin acting in this important case as a scaffold for collagen deposition (33). Along with these collagens, SLRPs become present again, limiting the signaling through motility-triggering growth factor receptors (18, 34). At the skin surface, dedifferentiated keratinocytes re-epithelialize the wound by ingrowth on top of this matrix. Behind the leading edge, the contact-inhibited keratinocytes are induced by unknown signals to contribute collagen IV and laminins to the delineating basement membrane, which in turn anchors the keratinocytes by hemidesmosomes, further driving maturation (6, 35, 36).
The transition to the resolving phase is marked by dramatic involution of the excess cells of all types that appear during the tissue replacement phase. This coincides with the change in the matrix from a supportive, tenascin-C and collagen III-predominant matrix to one in which collagen I and SLRPs constitute the dominant matrix proteins. This stiffer matrix in turn drives the stromal cells to take on a myofibroblast phenotype and further produce fibrillar collagen (37). This combination of collagen fibrils and myofibroblasts results in wound contraction, as the motility signals are channeled to transcellular contractility (24). This ultimately results in a scar with overly aligned collagen fibrils that regains only about 80% of the strength of the unwounded skin. Still, as the skin regains most functionality and is not overly cellular or collagenous, this is considered as normal wound healing (Figure 1).
Regenerative healing
Wounds in the fetus or on mucous membranes present an altered resolution phase leading to more regenerative or complete healing. Cutaneous wound healing within the first 24 weeks of human gestation results in scarless regeneration (38). We now know that this remarkable healing capacity is due to more than just an aseptic environment or amniotic fluid, as when the fetus approaches the third trimester this mode of regeneration is lost (9) and differences from adult healing persist in explanted fetal tissue when the skin is damaged (39). The mechanisms underlying the transition from fetal to adult healing are not well defined, and may relate to the interplay of signals and cellular inflammation as related below. Still, it does appear that both the cellular and matrix remodeling events are distinct in fetal healing and responsible for driving this perfect regeneration.
In fetal healing, there is minimal acute inflammation as the cellular infiltrate is mainly macrophages, with relatively few leukocytes and lymphocytes (40). This results in little to no fetal platelet degranulation and aggregation in addition to a lower level of fibrogenic PDGF and TGF-β. This key difference is evidenced by the induction of inflammation in fetal wounds after 24 weeks leading to scar formation (40). In the absence of neutrophils, other cells must clear the debris of the devitalized tissue; this is accomplished by macrophages and wound fibroblasts (39, 41). While these differences might be contributory, they are only a part of the answer. The balance between pro- and anti-inflammatory activity from the inflammatory cells that is coordinated by chemokines, cytokines, growth factors, and other soluble mediators is critical in normal healing (42). Yet, in fetal wounds, this balance is shifted and anti-inflammatory cytokines, such as IL-10, are highly expressed and pro-inflammatory chemokines, such as interleukin (IL)-8, are diminished. The other cellular element that differs in fetal healing is the presence of stem-like cells. Although they are a minority of the cells, they are thought to be educating the stromal cells and directing their behaviors toward regeneration rather than repair with scarring. For example, the presence of mesenchymal stem cells in the wound bed may in fact direct macrophages from a pro-inflammatory phenotype towards a pro-regeneration phenotype, characterized by increased anti-inflammatory cytokines including IL-10 with reduced TNF-α (43, 44). Concurrently, mesenchymal stem cells may also play a key role in directing regeneration through paracrine direction of macrophages and endothelial cells to migrate into the wound space, another key ‘educating’ step for such stromal cells in the healing process (43, 44).
Matrix production and remodeling are the other main distinctions between fetal and adult wound healing and one that persists past the initial hemostatic phase. Fetal fibroblasts proliferate at a faster rate and have migratory properties that are both intrinsically enhanced and more responsive to exogenous cues (45). Fetal fibroblast ECM synthesis and deposition is more robust as the fetal fibroblasts have enhanced synthetic and secretory processes. Fetal fibroblasts differ from adult fibroblasts in collagen synthesis in terms of speed of deposition, variation in collagen type ratios, and quantity of collagen. Fibronectin in fetal wounds also shows an earlier expression, which may contribute to the migratory properties of fetal fibroblasts (46). Tenascin-C shows earlier deposition in fetal wounds as well, and may further explain their ability to re-epithelize rapidly with a reduced presence of inflammatory cells. With respect to the fetal wound environment itself, compared to normal adult wounds there is an increase in proteoglycans, including the SLRP decorin, which suppresses pro-proliferative and –migratory signaling via many growth factor receptors (47–49), while also having inhibitory or stimulatory effects on angiogenesis, depending on the other components present in the microenvironment (50). Furthermore, the increase in levels of glycosaminoglycans such as hyaluronic acid (HA) changes the rheology of the matrix toward a more elastic one (51), concurrent with decreased HA degradation from a lower level of hyaluronidase in the wound bed {West, 1997 #5858; Overall, the intrinsic ability to synthesize a mature, well-organized dermal matrix is superior in fetal fibroblast to adult fibroblast.
In line with our central hypothesis, a regenerative matrix occurs in the fetus wound secondary to the shift in composition towards early expression a collagen I-rich matrix but in a less stiff dermis, due to higher hyaluronic acid levels, coupled with a shortened provisional matrix allowing for fewer fibroblasts to immigrate. This suggests that the matrix flows from lessened and altered inflammatory infiltrate impacts the resident cells to synthesize a mature, well-organized dermal matrix that synchronizes the scarless outcome.
Matrix in dysrepair
Dysrepair falls mainly at the two ends in the process – inability to repair the wound (failure to heal) and over-exuberant healing (scarring). Counter-intuitively, both of these medical issues can be traced to excessive matrix turnover. Chronic ulcers are found to have increased activity of MMPs and other matrix-degrading proteases {Raffetto, 2009 #5874}. This has even led to suggestions that protease inhibition be a part of chronic wound treatment (52). At the other end, we and others have found persistence of MMP activity in hypertrophic scars (12, 53–55). This does not take into account the failure to initiate healing in persons whose skin is highly compromised due to advanced age and severe metabolic derangements (including persons with malnutrition and widespread metastatic cancer), nor select genetic defects. Furthermore, vascular compromise including advanced arteriosclerotic conditions, such as those found in extremities in long-standing type II diabetes, present barriers to healing that lie outside the matrix issues, and thus require reestablishing adequate blood flow to enable any healing to occur.
Both failure to heal the wound and scarring are marked by matrix turnover disrupting the normal processes. Non-healing ulcers are stalled in matrix generation and maturation. The open wound becomes compromised as it is colonized by the skin microbiome (56, 57). Signals from microbiome products maintains a level of hematopoietically-derived immune cell infiltration. Both the leukocytes and microbes produce proteases that degrade the provisional matrix. These protein fragments further attract leukocytes and keep the stromal cells in a synthetic mode, generating matricellular proteins. The initiating event is still unclear, whether it is colonization/infection, excessive inflammatory infiltrate, or matrix turnover, though the ongoing failure to heal clearly has a matrix component that is critical to the pathological feed-forward loop.
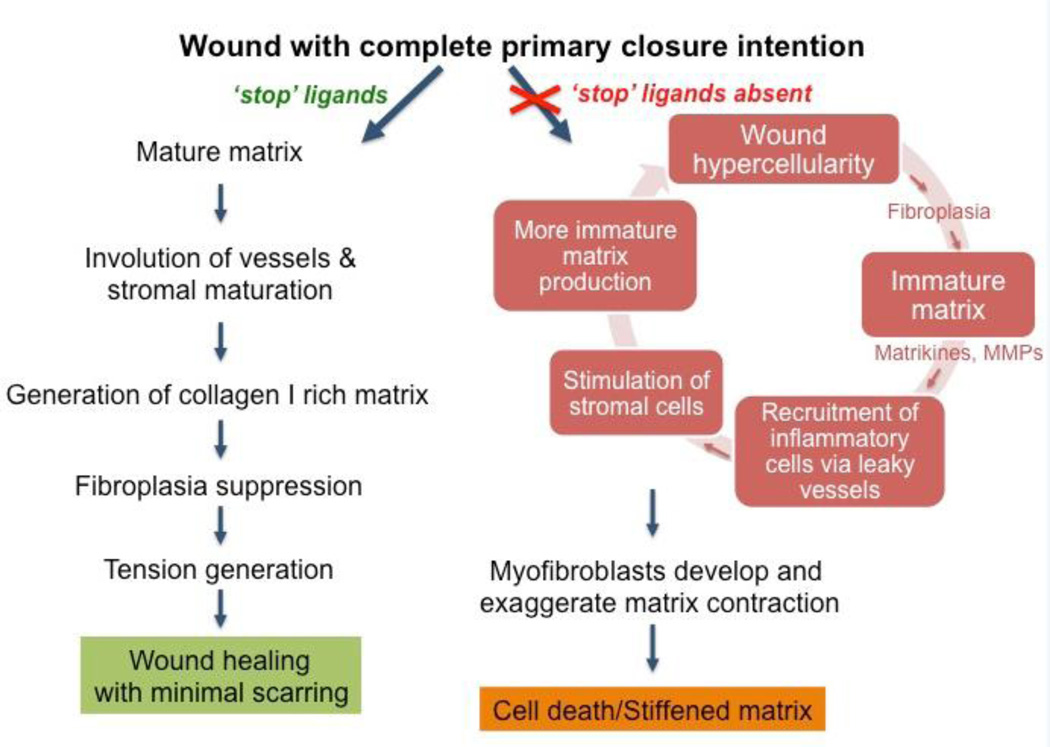
Scarring results from the failure to appropriately terminate the healing process (Figure 3). The presence of excess fibrillar collagen in both hypertrophic scars and keloids belies the active turnover that led to the accumulation. Proteases are found to persist in scar tissues. MMP-2 in particular, strangely in conjunction with its inhibitors TIMP-1 and TIMP-2, is found in human burn and hypertrophic scars (58), whereas MMP-9 appears to correlate with scar resolution (59). Other MMPs, particularly MMP-1 have been proposed as therapeutics to break down the fibrillar collagen to reduce scars. The reasons for this excessive accumulation of collagen I are uncertain, but once started, the process may be cyclical (12). Excessive tissue transglutaminase not only leads to the cross-linking of the collagen fibrils, but also directly or indirectly protect the stromal cells from apoptosis, thereby increasing the synthetic period of scars (60).
Figure 3. The ‘scarring cycle’ schematized.
Hypertrophic scars are regenerated from a persistence of the tissue replacement phase, in the absence of ‘stop signals’ such as ligands for CXCR3 or angiopoietins even after full re-epithelialization and seeming ‘wound closure’ (right arm). This hypercellularity with active matrix turnover re-initiates an chronic inflammatory milieu which in turns drive more immature matrix production, eventually leading to excessive but poorly functional fibrillar collagen. Adapted from ref 10.
To better investigate the mechanisms underlying matrix accumulation in scarring, animal models have been probed. However, such wounds do not often arise in animal models (61), limiting our understanding beyond the descriptive nature of examination of human wounds. The female Duroc/Yorkshire pig undergoes scarring after full thickness wounding or third degree burns (62); however, the role of specific signals and matrix has not been discerned in this genetically-predisposed model as molecular and cellular interventions are more convoluted in the porcine model. A more malleable, albeit genetically engineered, model of hypertrophic scarring in model animals is that in which the CXCR3 chemokine receptor is deleted in mice (63, 64). This receptor transmits ‘stop’ signals from the CXCL10 (IP-10) and CXCL11 (IP-9) chemokines that are produced when small vessels mature and reparative keratinocytes reach confluence and re-differentiate, respectively (65, 66). While the most obvious consequence of CXCR3 signaling is vascular involution (67, 68) and channeling fibroblasts towards matrix compaction (69), the lack of CXCR3 signaling also leads to the persistence of an immature dermal matrix with high levels of tenascin-C and fibronectin (54, 63). Thus, during their formation, hypertrophic scars are marked by a hyperactive regenerative matrix rather than simply more and more accumulation of fibrillar collagen. Interestingly, the transition between the tenascin-rich regenerative matrix and collagen I-dominant mature matrix is blurred with both being present in the same temporo-spatial domain. Key to the role of matrix in driving the scarring phenotype is the finding that transient presence of normal fibroblasts corrects this defect (42). A mature matrix that ushers in the resolving phase is produced during this short period and appears to suppress the ongoing scarring. This strongly implicates the matrix as the main regulator of dermal phenotype, with a tenascin-C-, SPARC- and fibronectin-rich matrix maintaining a synthetic dermis that accumulates excessive and misaligned collagen fibrils, but with a collagen I matrix rendering the dermis inactive to prevent this scar buildup (12).
In between these two bookends is the issue of re-ulceration of healed wounds. Outside of the problems of repeated trauma (the most common cause, especially in pressure ulcers) or failure to correct the underlying cause (for venous stasis ulcers), this dysrepair results from limited matrix involvement, rather than excessive deposition and turnover as in the above situations. This is not dissimilar to surgical dehiscence, wherein many of the failures relate to insufficient collagen deposition and cross-linking. (It should be noted that while surgical wound dehiscence is particularly problematic in persons with collagen disorders, these same patients suffer from a primary failure to heal excisional wounds (70)). In excisional healing, overly rapid re-epithelialization communicates with the underlying dermis to cause premature transition to the resolution phase (66, 71, 72). This would be expected to result in a thin and weaker dermis that would be predisposed to re-ulceration in the face of renewed insult, just as the skin of the aged or persons suffering from inanition is ripe for ulcers. The implications of such crosstalk, with the closing epidermis signaling ‘stop repair’ signals to the dermis, must be considered when using new re-epithelialization technologies such as keratinocyte transplantation (73).
Model systems and matrix interventions
Advances in skin repair and disruptions of such rely on robust and representative models (74). Unfortunately, truly relevant models are lacking for a number of reasons. First, human skin presents an infrequently used architecture that is shared only with other primates and pigs; the tight attachment of the skin to the underlying integum, at least in the absence of a thick layer of insulating fat, is rarely noted in mammals with the exception of faces and palms/soles. Second, the skin adnexia of few hair follicles, sun exposure, and many dermal sweat glands are found by and large only in the pig, and not even in other primates that are hairy. Third, the chronic diseases that afflict humans and are recalcitrant to current therapies are not readily recreated in animal models. These two major issues confound the usual challenges that confront most biomedical research in model animals, differences in size, lifespan, and genetics, and result in subtle but important biological distances between all species, and especially humans. Of note, scarring of other organs appears to be better recapitulated in animal models, however, these organs fall outside the considering of the current communication
The pig is a preferred model animal due to the similarity in the architecture and the presence of auxillary structures of the skin. While many insights have been garnered, the challenges of genetic manipulations in the porcine model have promoted the use of suboptimal rodent models, wherein genetically altered animals and tissues are more easily generated. As the skin in rodents is only loosely connected to the underlying fascias, the largest part of healing of full thickness wounds occurs by primary contraction; partial thickness wounds are a challenge due to the thinness of the skin and are thus not representative given the small amounts of tissue to regenerate. To overcome this major role of contraction, wounds may be splinted in rodents, but again such a situation artificially imposes constraints absent in human wounds with the tension leading to excessive collagen deposition and thus a hypertrophic scar (75, 76). An alternate approach is to wound the ear (often in rabbits) or the tail (usually of rats) as the covering of the underlying cartilage is thin and juxtaposed, but these wounds do not reflect the human situation as such surfaces are few.
As for dysrepair, few animals fully scar in a manner comparable to human hypertrophic scars or frank neoplastic keloids (61). Keloids do occur spontaneously in the shins of horses, though some differences in these wounds exist (77). The female Duroc/Yorkshire pig does form hypertrophic scars (62), though the occurrence in only this breed, and mainly just the females, strongly suggests an unknown genetic alteration or variant that is hormonally modified. Rodents will form hypertrophic scars in a distracted situation, as noted above (75, 76), possibly due to decreased apoptosis (78) that occurs in the transition to wound resolution. In the mouse, ablation of the CXCR3 stop signals does result in hypertrophic scarring becoming prominent some 6 months and more after wounding, in a manner that mirrors the human situation (63). In these case, the dermal matrix reflects that of human hypertrophic scars with increased but disorganized fibrillar collagen, and persistence of fibronectin and tenascin-C. MMPs are found even after 6 months in these scars, while absent from healed skin (63, 79). Of interest, this scarring phenotype can be prevented by even just transient transplantation of allograft/xenograft fibroblasts that produce a mature matrix to start the resolving phase (12, 42). Still, the use of a defined genetic defect that is not found to be present in human scars renders the model more useful for anti-scarring interventions than in discerning fundamental biology of scarring. To circumvent this absence in animal models, human skin has been grafted onto rodents, but the vascular, neuronal, and lymph connections are absent and the immune system that is critical during the inflammatory phase is abrogated.
Chronic wounds represent even greater challenges. First, the main causes of human chronic wounds are vascular compromise and repeated trauma. For the latter, pressure ulcers and blister ulcers, the standard therapy of off-loading leads to healing unless significant comorbidities are present; these comorbidities are not faithfully recreated in model animals. Diabetes is a major confounding diseases that leads to non-healing ulcers, but here too the proximate cause of long-standing arteriolosclerosis is not present in the animal models that can present the hyperglycemia and advanced glycosylated end products; these short term perturbations in themselves do not prevent healing in human wounds in the absence of small vessel disease. Thus, the wounds in diabetic mice and other animals (either genetic variants or by killing of beta cells) do heal well even though with a slight delay compared to normal littermates. As such, an oft-used chronic wound model remains the porcine skin flap model, which maintains the similar architecture to the human skin while generating avascular/ischemic regions to mimic a chronic wound (80). However, ultimately surgical generation of avascular flaps does not represent these wounds so much as compromised pedicles and muscle flaps in humans. Rather, in human diabetic and chronic wounds the vascular compromise occurs at the small arteriole level, and not usually from limited arterial supply.
For chronic wounds, those therapies that have made it through these limited animal models and into human use have often focused on antimicrobial treatment and/or matrix-based interventions, such as collagen scaffolds or comparable treatments seeded with fibroblasts. The immediate goal of such products is to ameliorate the lack of fibroblast migration and collagen deposition in a chronic lesion. However, these treatments (beyond the scope of negative pressure therapy) have shown limited clinical success. Venous stasis ulcers, that plague millions of persons in the US alone, have not been successfully modeled in animals.
Further compounding these representative models in animals are some unique differences in the biology of the skin. For instance, wound healing in rodents is dependent on resident gamma-delta T-cells in the dermis (81, 82), but this subset of T-cells is a very minor subpopulation in the human skin.
Due to these limitations, there is a push to move quickly to human skin as the model system. Skin organ cultures are quite advanced and have been used for over a decade (83, 84). These constructs can be generated for cellular reseeding of decellularized human skin, or more elegantly can be established using human fibroblast-seeded collagen gels overlaid with human keratinocytes and melanocytes. While the decellularized skin constructs contain the rete plugs and a more physiological dermal matrix and basement membrane at the start, barriers to stromal cell penetration and inability to interventionally modify the dermis are limitations. The de novo generation of the organ constructs allows for designed cells and matrices to contribute to the skin. Further, the ready access to discarded human skin allows for large genetic diversity to be represented in these ex vivo constructs. While key cellular and molecular events in wound healing responses continue to be discerned with these (85), the lack of the vascular and immune systems limits investigation of a fuller response, as the entire initial homeostatic phase is absent (Figure 1). Until microfluidic support can provide for this, these models will remain limited (http://www.ncats.nih.gov/research/reengineering/tissue-chip/tissue-chip.html).
Interestingly, live skin organ cultures and cells have moved quickly into clinical use as treatments for large area losses and nonhealing wounds. These constructs have been reviewed recently (86, 87), and will not be discussed herein. However, they provide unwittingly for tests of basic mechanisms of repair. Many of these provide temporary coverings rather than integrated tissues, in that the pre-formed dermal or combined dermal-epidermal constructs fail to be incorporated into the healed skin (88). The reasons for this failure to become part of the healed skin likely relates to lack of vascularization of the bi-layer constructs possibly due to a mature epidermal layer limiting either the turnover of the dermal matrix or even vascular ingrowth. While it would interesting to speculate that CXCR3 ligands could be responsible (67, 68), this is unlikely as mature keratinocytes express little if any cognate ligands, and even the pre-formed dermal matrices also have limited incorporation. While it is more likely that the fibrillar collagen of these constructs are repressive to potentially invading stromal cells, that needs to be ascertained. This view is supported by a very recent report that decellularized dermal constructs limit collagen I levels and subsequent scarring in human volunteers (89).
More recently, cellular transplants often in the form of keratinocytes have been proposed as a rapid covering for burn wounds (73) and venous stasis ulcers (90). While both compassionate use and a phase II trial have suggested some benefit, that even persists at six months post closure (91), the healed wounds have not been studied in detail. In these situations wherein the applied keratinocytes would be exposed to the wound environment, one may expect the expression of CXCL11 from these cells to prematurely limit the generation and maturation of the dermal matrix and the vascularization therein, resulting in a weakened wound. This would be noted as a quantitatively diminished but mature matrix rich in fibrillar collagen I. As these approaches move beyond limited trials, such an adverse outcome, predisposing to dehiscence due to a weakened matrix, would need to be studied.
Directed studies on human wounds are ongoing, and face lower barriers that other organ systems. The ready access to the skin and near regenerative repair of most small skin wounds allows for even purposeful wounding studies with informed consent (92). Therapeutic interventional studies are readily available and often follow from quite limited animal studies. It will be from careful review of such trials that a fuller understanding of the changing matrix will arise to offer new approaches healing.
Highlights.
-
➢
Matrix composition dynamically changes through the phases of wound healing
-
➢
Matrix elements educate the cells to dictate cellular phenotypes
-
➢
Aberrant matrix progression leads to stalled healing or scarring
Acknowledgements
These studies were supported by grants from National Institute for General Medical Science (NIH) (GM63569 and GM69668 to AW), a fellowship from the National Cancer Institute (NIH) (T32CA175294 to AN), and the University of Pittsburgh Center for Research and Evaluation (School of Nursing). We thank members of the Wells and Yates laboratories for discussions through the years that have shaped our view of healing and led to any insights thereof. We also thank Erin Steer at the University of Pittsburgh for her assistance in preparing the figures and schematics for this review.
Abbreviations
- MMP
Matrix metalloproteinase
- SLRP
Small leucine-rich glycoprotein
- CXCR3
chemokine receptor 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Margolis DJ, Allen-Taylor L, Haffstad O, Berlin JA. Diabetic neuropathic foot ulcers and amputation. Wound Repair and Regeneration. 2005;13:230–236. doi: 10.1111/j.1067-1927.2005.130303.x. [DOI] [PubMed] [Google Scholar]
- 2.Cavanagh PR, Lipsky BA, Bradbury A, Botek G. Treatment for diabetic foot ulcers. Lancet. 2005;366:1725–1735. doi: 10.1016/S0140-6736(05)67699-4. [DOI] [PubMed] [Google Scholar]
- 3.Yates CC, Hebda P, Wells A. Skin wound healing and scarring: fetal wounds and regenerative restitution. Birth Defects Research. 2012;96:325–333. doi: 10.1002/bdrc.21024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bissell M, Hall HG, Parry GJ. How does the extracellular matrix direct gene expression. Theoretical Biology. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 5.Schultz GS, Davidson JM, Kirsner RS, Bornstein P, Herman IM. Dynamic reciprocity in the wound microenvironment. Wound Repair and Regeneration. 2011;19:134–148. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singer AJ, Clark RAF. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 7.Babu M, Wells A. Dermal-epidermal communication in wound healing. Wounds. 2001;13:183–189. [Google Scholar]
- 8.Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair and Regeneration. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 9.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiology Reviews. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- 10.D’Alessio S, Gerasi L, Blasi F. uPAR-deficient mouse keratinocytes fail to produce EGF-dependent laminin-5, affecting migration in vivo and in vitro. J Cell Sci. 2008;121:3922–3932. doi: 10.1242/jcs.037549. [DOI] [PubMed] [Google Scholar]
- 11.McMillan JR, Akiyama M, Nakamura H, Shimizu H. Colocalization of multiple laminin isoforms predominantly beneath hemidesmosomes in the upper lamina densa of the epidermal basement membrane. Journal of Histochemistry and Cytochemistry. 2006;54:109–118. doi: 10.1369/jhc.5A6701.2005. [DOI] [PubMed] [Google Scholar]
- 12.Yates CC, Bodnar R, Wells A. Matrix control of scarring. Cellular and Molecular Life Sciences. 2011;68:1871–1881. doi: 10.1007/s00018-011-0663-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoffels JM, Zhao C, Baron W. Fibronectin in tissue regeneration: timely disassembly of the scaffold is necessary to complete build. Cellular and Molecular Life Sciences. 2013;70:4243–4253. doi: 10.1007/s00018-013-1350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Voigt J, Driver VR. Hyaluronic acid derivatives and their healing effect on burns, epithelial surgical wounds, and chronic wounds: a systematic review and meta-analysis of randomized controlled trials. Wound Repair and Regeneration. 2012;20:317–331. doi: 10.1111/j.1524-475X.2012.00777.x. [DOI] [PubMed] [Google Scholar]
- 15.Grahovac J, Wells A. Matricellular proteins as regulators of cancer cell invasion. Lab Invest. 2014;94:31–40. doi: 10.1038/labinvest.2013.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Midwood KS, Hussenet T, Langlois B, Orend G. Advances in tenascin-C biology. Cellular and Molecular Life Sciences. 2011;68:3175–3199. doi: 10.1007/s00018-011-0783-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merline R, Schaefer RM, Schaefer L. The matricellular functions of small leucine-rich proteoglycans (SLRPs) Journal of Cell Communication and Signaling. 2009;3:323–335. doi: 10.1007/s12079-009-0066-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neill T, Schaefer L, Iozzo RV. Decorin: a guardian from the matrix. Am J Pathol. 2012;181:380–387. doi: 10.1016/j.ajpath.2012.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aguilera KY, Brekken RA. Recruitment and retention: factors that affect pericyte migration. Cellular and Molecular Life Sciences epub ahead of print. 2013 doi: 10.1007/s00018-013-1432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Halper J, Kjaer M. Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenasins, and thrombospondins. Adv Exp Med Biol. 2014;802:31–47. doi: 10.1007/978-94-007-7893-1_3. [DOI] [PubMed] [Google Scholar]
- 21.Murphy-Ullrich JE, Sage EH. Revisting the matricellular concept. Matrix Biology. 2014;37:1–14. doi: 10.1016/j.matbio.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cowan KN, Jones PL, Rabinovitch M. Elastase and matrix metalloproteinase inhibitors induce regression, and tenascin-C antisense prevents progression, of vascular disease. J Clin Invest. 2000;105:21–34. doi: 10.1172/JCI6539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 24.Allen FD, Asnes CF, Chang P, Elson EL, Lauffenburger DA, Wells A. EGF-induced matrix contraction is modulated by calpain. Wound Repair and Regeneration. 2002;10:67–76. doi: 10.1046/j.1524-475x.2002.10701.x. [DOI] [PubMed] [Google Scholar]
- 25.Smith KD, Wells A, Lauffenburger DA. Multiple signaling pathways mediate compaction of the collagen matrices by EGF-stimulated fibroblasts. Exp Cell Res. 2006;312:1970–1982. doi: 10.1016/j.yexcr.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez D, Morrison CJ, Overall CM. Matrix metalloproteinases: what do they not do? new substrates and biology identified by murine models and proteomics. Bioch Bioph Acta. 2010;1803:39–54. doi: 10.1016/j.bbamcr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 27.Murphy-Ullrich J. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state. J Clin Invest. 2001;107:785–790. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chiquet-Ehrismann R, Orend G, Chiquet M, Tucker RP, Midwood KS. Tenascins in stem cell niches. Matrix Biology. 2014;37:112–123. doi: 10.1016/j.matbio.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 29.Shao H, Li S, Watkins SC, Wells A. α-Actinin-4 is required for amoeboid-type invasiveness of melanoma cells. J Biol Chem. 2014;289:32717–32728. doi: 10.1074/jbc.M114.579185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iyer AKV, Tran KT, Griffith L, Wells A. Cell surface restriction of EGFR by a Tenascin cytotactin-encoded EGF-like repeat is preferential for motility-related signaling. J Cell Physiol. 2008;214:504–512. doi: 10.1002/jcp.21232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swindle CS, Tran K, Johnson TD, Banerjee P, Mayes AM, Griffith LG, Wells A. Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. J Cell Biol. 2001;154:459–468. doi: 10.1083/jcb.200103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu J, Clark RAF. Fibronectin at select sites binds multiple growth factors and enhances their activity: expansion of the collaborative ECM-GF paradigm. J Invest Dermatol. 2014;134:895–901. doi: 10.1038/jid.2013.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadler KE, Hill A, Canty-Laird EG. Collagen fibrillogenesis: fibronectin, integrin, and minor collagens as organizers and nucleators. Current Opinion in Cell Biology. 2008;20:495–501. doi: 10.1016/j.ceb.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reed CC, Iozzo RV. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconjugate Journal. 2002;19:249–255. doi: 10.1023/A:1025383913444. [DOI] [PubMed] [Google Scholar]
- 35.Gipson IK, Spurr-Michaud SJ, Tisdale AS. Hemidesmosomes and anchoring fibril collagen appear synchronously during development and wound healing. Developmental Biology. 1988;126:253–262. doi: 10.1016/0012-1606(88)90136-4. [DOI] [PubMed] [Google Scholar]
- 36.Clark RAF. The Molecular and Cellular Biology of Wound Repair. 1996 [Google Scholar]
- 37.Georges PC, Hui JJ, Gombos Z, McCormick ME, Wang AY, Uemura M, Mick R, Janmey PA, Furth EE, Wells RG. Increased stiffness of the rat liver precedes matrix deposition: implications for fibrosis. American Journal of Physiology -Gastrointestinal and Liver Physiology. 2007;293:G1147–G1154. doi: 10.1152/ajpgi.00032.2007. [DOI] [PubMed] [Google Scholar]
- 38.Rowlatt U. Intrauterine healing in a 20-week human fetus. Virchows Archives. 1979;381:353–361. doi: 10.1007/BF00432477. [DOI] [PubMed] [Google Scholar]
- 39.Coolen NA, Schouten KC, Boekema BK, Middlekoop E, Ulrich MM. Wound healing in a fetal, adult, and scar tissue model: a comparative study. Wound Repair and Regeneration. 2010;18:291–301. doi: 10.1111/j.1524-475X.2010.00585.x. [DOI] [PubMed] [Google Scholar]
- 40.Hopkinson-Woolley J, Hughes D, Gordon S, Martin P. Macrophage recruitment during limb development and wound healing in the embryonic and foetal mouse. J Cell Sci. 1994;107:1159–1167. doi: 10.1242/jcs.107.5.1159. [DOI] [PubMed] [Google Scholar]
- 41.Coolen NA, Schouten KC, Middlekoop E, Ulrich MM. Comparison between human fetal and adult skin. Archives of Dermatological Research. 2010;302:47–55. doi: 10.1007/s00403-009-0989-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yates CC, Whaley D, Wells A. Transplanted fibroblasts prevent dysfunctional repair in a murine CXCR3-deficient scarring model. Cell Transplantation. 2012;21:919–931. doi: 10.3727/096368911X623817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J, Hematti P. Mesenchymal stem cell-educated macrophages: a novel type of alternatively activated macrophages. Experimental Hematology. 2009;37:1445–1453. doi: 10.1016/j.exphem.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3:e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nodder S, Martin P. Wound healing in embryos: a review. Anatomy and Embryology. 1997;195:215–228. doi: 10.1007/s004290050041. [DOI] [PubMed] [Google Scholar]
- 46.Carter R, Jain K, Sykes V, Lanning D. Differential expression of procollagen genes between mid- and late-gestational fibroblasts. Journal of Surgical Research. 2009;156:90–94. doi: 10.1016/j.jss.2009.03.056. [DOI] [PubMed] [Google Scholar]
- 47.Zhu J-X, Goldoni S, Bix G, Owens RT, McQuillan DJ, Reed CC, Iozzo RV. Decorin evokes protracted internalization and degradation of the EGF receptor via caveolar endocytosis. J Biol Chem. 2005;280:32468–32479. doi: 10.1074/jbc.M503833200. [DOI] [PubMed] [Google Scholar]
- 48.Goldoni S, Humphries A, Nystrom A, Sattar S, Owens RT, McQuillan DJ, Ireton K, Iozzo RV. Decorin is a novel antagonistic ligand of the Met receptor. J Cell Biol. 2009;185:743–754. doi: 10.1083/jcb.200901129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krishna P, Regner M, Palko J, Abramowitch S, Jiang J, Wells A. The effects of decorin and HGF-primed vocal fold fibroblasts in vitro and ex vivo in a porcine model of vocal fold scarring. The Laryngoscope. 2010;120:2247–2257. doi: 10.1002/lary.21087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jarvelainen H, Sainio A, Wight TN. Pivotal role for decorin in angiogenesis. Matrix Biology. 2015;43:15–26. doi: 10.1016/j.matbio.2015.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mast BA, Diegelmann RF, Krummel TM, Cohen IK. Hyaluronic acid modulates proliferation, collagen and protein synthesis of cultured fetal fibroblasts. Matrix. 1993;13:441–446. doi: 10.1016/s0934-8832(11)80110-1. [DOI] [PubMed] [Google Scholar]
- 52.Raffetto JD. Dermal pathology, cellular biology, and inflammation in chronic venous disease. Thrombosis Research. 2009;123:s66–s71. doi: 10.1016/S0049-3848(09)70147-1. [DOI] [PubMed] [Google Scholar]
- 53.Djafarzadeh R, Notohamiprodjo S, Rieth N, Hofstetter M, Noessner E, Nelson PJ. Treatment of dermal fibroblasts with GPI-anchored human TIMP-1 protein moderates processes linked to scar formation. J Invest Dermatol. 2013;133:803–811. doi: 10.1038/jid.2012.375. [DOI] [PubMed] [Google Scholar]
- 54.Yates CC, Whaley D, Kulasekeran P, Hancock WW, Lu B, Bodnar R, Newsome J, Hebda PA, Wells A. Delayed and deficient dermal maturation in mice lacking the CXCR3 ELR-negative CXC chemokine receptor. Am J Pathol. 2007;171:484–495. doi: 10.2353/ajpath.2007.061092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rohani MG, Parks WC. Matrix remodeling by MMPs during wound repair. Matrix Biology in press; 2015. [DOI] [PubMed] [Google Scholar]
- 56.Grice EA, Segre JA. The skin microbiome. Nature Reviews - Microbiology. 2011;9:244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grice EA, Segre JA. Interactions of the microbiome with the innate immune response in chronic wounds. Adv Exp Med Biol. 2012;946:55–68. doi: 10.1007/978-1-4614-0106-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ulrich D, Ulrich F, Unglaub F, Piatkowski A, Pallua N. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in patients with different types of scars and keloids. Journal of Plastic and Reconstructive Aesthetic Surgery. 2010;63:1015–1021. doi: 10.1016/j.bjps.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 59.Reno F, Grazianetti P, Stella M, Magliacani G, Pezzuto C, Cannas M. Release and activation of matrix metalloproteinase-9 during in vitro mechanical compression in hypertrophic scars. Archives of Dermatology. 2002;138:475–478. doi: 10.1001/archderm.138.4.475. [DOI] [PubMed] [Google Scholar]
- 60.Linge C, Richardson J, Bifor C, Clayton E, Hardas B, Rolfe K. Hypertrophic scar cells fail to undergo a form of apoptosis specific to contractile collagen-the role of tissue transglutaminase. J Invest Dermatol. 2005;125:72–82. doi: 10.1111/j.0022-202X.2005.23771.x. [DOI] [PubMed] [Google Scholar]
- 61.Ramos ML, Gragnani A, Ferreira LM. Is there an ideal animal model to study hypertrophic scarring. Journal of Burn Care Research. 2008;29:363–368. doi: 10.1097/BCR.0b013e3181667557. [DOI] [PubMed] [Google Scholar]
- 62.Zhu KQ, Carrougher GJ, Gibran NS, Isik FF, Engrav LH. Review of the female Duroc/Yorkshire pig model of human fibroproliferative scarring. Wound Repair and Regeneration. 2007;15:S32–s39. doi: 10.1111/j.1524-475X.2007.00223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yates CC, Krishna P, Whaley D, Bodnar R, Turner T, Wells A. Lack of CXC chemokine receptor 3 (CXCR3) signaling leads to hypertrophic and hypercellular scarring. Am J Pathol. 2010;176:1743–1755. doi: 10.2353/ajpath.2010.090564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davidson JM. Can scarring be turned off? Am J Pathol. 2010;176:1588–1591. doi: 10.2353/ajpath.2010.100064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luster AD, Greenberg SM, Leder P. The IP-10 chemokine binds to a specific cell surface heparan sulfate site shared with platelet factor 4 and inhibits endothelial cell proliferation. Journal of Experimental Medicine. 1995;182:219–231. doi: 10.1084/jem.182.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Satish L, Yager D, Wells A. ELR-negative CXC chemokine IP-9 as a mediator of epidermal-dermal communication during wound repair. J Invest Dermatol. 2003;120:1110–1117. doi: 10.1046/j.1523-1747.2003.12230.x. [DOI] [PubMed] [Google Scholar]
- 67.Bodnar RJ, Rodgers ME, Chen W, Wells A. Pericyte regulation of vascular remodeling through the CXC Receptor 3. Arteriosclerosis, Thrombosis and Vascular Biology. 2013;33:2818–2829. doi: 10.1161/ATVBAHA.113.302012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bodnar RJ, Yates CC, Du X, Wells A. ELR-negative chemokine IP-10/CXCL10 induces dissociation of newly-formed vessels secondary to calpain cleavage of beta3 integrin. J Cell Sci. 2009;122:2064–2077. doi: 10.1242/jcs.048793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shiraha H, Gupta K, Glading A, Wells A. IP-10 inhibits epidermal growth factor-induced motility by decreasing epidermal growth factor receptor-mediated calpain activity. J Cell Biol. 1999;146:243–253. doi: 10.1083/jcb.146.1.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fogel S. Surgical failures: is it the surgeon or the patient? the all too often missed diagnosis of Ehlers-Danlos syndrome. The American Surgeon. 2013;79:608–613. [PubMed] [Google Scholar]
- 71.Clark RAF. Epithelial-mesenchymal networks in wounds: a hierarchical view. J Invest Dermatol. 2003;120:x–xi. doi: 10.1046/j.1523-1747.2003.12264.x. [DOI] [PubMed] [Google Scholar]
- 72.Gallant-Behm CL, Du P, Lin SM, Marucha PT, DiPietro LA, Mustoe TA. Epithelial regulation of mesenchymal tissue behavior. J Invest Dermatol. 2011;131:892–899. doi: 10.1038/jid.2010.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gerlach JC, Johnen C, McCoy E, Brautigam K, Plettig J, Corcos A. Autologous skin cell spray-transplantation for a deep dermal burn patient in an ambulant treatment room setting. Burns. 2011;37:e19–e23. doi: 10.1016/j.burns.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 74.Domergue S, Jorgensen C, Noel D. Advances in research in animal models of burn-related hypertrophic scarring. Journal of Burn Care Research epub ahead of print. 2014 doi: 10.1097/BCR.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 75.Guo F, Carter DE, Leask A. Mechanical tension increases CCN2/CTGF expression and proliferation in gingival fibroblasts via a TGFbeta-dependent mechanism. PLoS One. 2011;6:e19756. doi: 10.1371/journal.pone.0019756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suarez E, Syed F, Rasgado TA, Walmsley A, Mandal P, Bayat A. Skin equivalent tensional force alters keloid fibroblast behavior and phenotype. Wound Repair and Regeneration. 2014;22:557–568. doi: 10.1111/wrr.12215. [DOI] [PubMed] [Google Scholar]
- 77.Theoret CL, Olutoye OO, Parnell LK, Hicks J. Equine exuberant granulation tissue and human keloids: a comparative histopathologic study. Veterinary Surgery. 2013;42:783–789. doi: 10.1111/j.1532-950X.2013.12055.x. [DOI] [PubMed] [Google Scholar]
- 78.Aarabi S, Bhatt KA, Shi Y, Paterno J, Change EI, Loh SA, Holmes JW, Longaker MT, Yee H, Gurtner GC. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB Journal. 2007;21:3250–3261. doi: 10.1096/fj.07-8218com. [DOI] [PubMed] [Google Scholar]
- 79.Yates CC, Whaley D, Hooda S, Hebda PA, Bodnar RJ, Wells A. Delayed re-epithelialization and basement membrane regeneration after wounding in mice lacking CXCR3. Wound Repair and Regeneration. 2009;17:34–41. doi: 10.1111/j.1524-475X.2008.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boateng JS, Matthews KH, Stevens HN, Eccleston GM. Wound healing dressing and drug delivery systems: a review. Journal of Pharmacological Sciences. 2008;97:2892–2923. doi: 10.1002/jps.21210. [DOI] [PubMed] [Google Scholar]
- 81.Jameson J, Ugarte K, Chen N, Yachi P, Fuchs E, Boismenu R, Havran WL. A role for skin gd T cells in wound repair. Science. 2002;296:747–749. doi: 10.1126/science.1069639. [DOI] [PubMed] [Google Scholar]
- 82.Havran WL, Jameson JM. Epidermal T cells and wound healing. Journal of Immunology. 2010;184:5423–5428. doi: 10.4049/jimmunol.0902733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moll I. Human skin organ culture. Methods in Molecular Medicine. 2003;78:305–310. doi: 10.1385/1-59259-332-1:305. [DOI] [PubMed] [Google Scholar]
- 84.Moll I, Houdek P, Schmidt H, Moll R. Characterization of epidermal wound healing in a human skin organ culture model: acceleration by transplanted keratinocytes. J Invest Dermatol. 1998;111:251–258. doi: 10.1046/j.1523-1747.1998.00265.x. [DOI] [PubMed] [Google Scholar]
- 85.Safferling K, Sutterlin T, Westphal K, Ernst C, Breuhahn K, James M, Jager D, Halama N, Grabe N. Wound healing revised: a novel reepithelialization mechanism revealed by in vitro and in silico models. J Cell Biol. 2013;203:691–709. doi: 10.1083/jcb.201212020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nathoo R, Howe N, Cohen G. Skin substitutes: an overview of the key players in wound management. The Journal of Clinical and Aesthetic Dermatology. 2014;7:44–48. [PMC free article] [PubMed] [Google Scholar]
- 87.Nyame TT, Chiang HA, Orgill DP. Clinical applications of skin substitutes. Surgical Clinics of North American. 2014;94:839–850. doi: 10.1016/j.suc.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 88.Griffiths M, Ojeh N, Livingstone R, Price R, Navsaria H. Survival of Apligraf in acute human wounds. Tissue Engineering. 2004;10:1180–1195. doi: 10.1089/ten.2004.10.1180. [DOI] [PubMed] [Google Scholar]
- 89.Greaves NS, Iqbal SA, Hodgkinson T, Morris J, Benatar B, Alonso-Rasgado T, Baguneid M, Bayat A. Skin substitute-assisted repair shows reduced dermal fibrosis in acute human wounds validated simultaneously by histology and optical coherence tomography. Wound Repair and Regeneration epub ahead of print. 2015 doi: 10.1111/wrr.12308. [DOI] [PubMed] [Google Scholar]
- 90.Kirsner RS, Marston WA, Snyder RJ, Lee TD, Cargill DI, Slade HB. Spray-applied cell therapy with human allogeneic fibroblasts and keratinocytes for the treatment of chronic venous leg ulcers: a phase 2, multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2012;380:977–985. doi: 10.1016/S0140-6736(12)60644-8. [DOI] [PubMed] [Google Scholar]
- 91.Kirsner RS, Marston WA, Snyder RJ, Lee TD, Cargill DI, Zhang Y, Dickerson JE, Slade HB. Durability of healing from spray-applied cell therapy with human allogeneic fibroblasts and keratinocytes for the treatment of chronic venous leg ulcers: a 6-month follow-up. Wound Repair and Regeneration. 2013;21:682–687. doi: 10.1111/wrr.12076. [DOI] [PubMed] [Google Scholar]
- 92.Nwomeh BC, Liang HX, Diegelmann RF, Cohen IK, Yager DR. Dynamics of the matrix metalloproteinases MMP-1 and MMP-8 in acute open human dermal wounds. Wound Repair and Regeneration. 1998;6:127–134. doi: 10.1046/j.1524-475x.1998.60206.x. [DOI] [PubMed] [Google Scholar]