Abstract
Wound healing is essential for survival. This is a multistep process involving a number of different cell types. In the skin wounding triggers an acute inflammatory response, with the innate immune system contributing both to protection against invasive organisms and to triggering the invasion of inflammatory cells into the wounded area. These cells release a variety of cytokines and growth factors that stimulate the proliferation and migration of dermal and epidermal cells to close the wound. In particular, wounding activates stem cells in the interfollicular epidermis (IFE) and hair follicles (HF) to proliferate and send their progeny to re-epithelialize the wound. β-catenin and calcium signaling are important for this activation process. Mice lacking the VDR when placed on a low calcium diet have delayed wound healing. This is associated with reduced β-catenin transcriptional activity and proliferation in the cells at the leading edge of wound closure. These data suggest that vitamin D and calcium signaling are necessary components of the epidermal response to wounding, likely by regulating stem cell activation through increased β-catenin transcriptional activity.
Keywords: Vitamin D receptor, calcium, keratinocytes, epidermis, stem cells, wound repair
1. Introduction
Chronic skin wounds are estimated to affect 6.5 million patients in the US at a cost of over $25 billion (1). Moreover, skin wound repair leads to the additional burden of scarring, a $12 billion annual market (1). This does not take into account the psychologic damage caused by disfiguring lesions that such skin wounds can cause.
Wound healing is a multistep process involving a number of cell types and processes (2, 3). The initial stages involve blood clotting and an inflammatory response. The innate immune system plays an important role in initiating the inflammatory response. Cells null for VDR or CYP27B1 (the enzyme producing 1,25(OH2D3) cannot mount this innate immune response (4), and we have recently shown that cells null for the calcium sensing receptor (CaSR) are likewise deficient (5). Wounding also leads to the activation of a number of signaling pathways (review in (6)). In particular, developmental pathways including wnt/β-catenin, TGFβ1, notch, and sonic hedgehog have all been implicated in the repair of skin wounds.
Following the initial inflammatory reaction, and in response to the cytokines and growth factors expressed following wounding, the epidermal and dermal cells proliferate and migrate to fill the wound with matrix enabling the keratinocytes to re-epithelialize and subsequently reform the epidermal barrier. Stem cells in the bulge region and infundibulum of the hair follicle (HF) and in the interfollicular epidermis (IFE) play critical roles in this process. Under normal circumstances the stem cells in the IFE maintain the epidermis, the stem cells in the infundibulum maintain the infundibulum and sebaceous gland, and the stem cells in the bulge and secondary hair germ maintain the cycling portion of the hair follicle (7, 8). However, when the skin is wounded the progeny of stem cells from all regions of the HF and IFE contribute at least initially (9, 10), although to variable extent. Ito et al. (11) labeled the stem cells in the bulge of the adult mouse using an inducible K15-crePR/R26R transgenic and found that after wounding approximately 25% of the cells in the newly formed epidermis originated from the bulge. However, these cells did not persist. Levy et al. (12) labeled the stem cells throughout the follicle including the infundibulum with a Shh cre/R26R transgenic and confirmed that cells from other regions of the follicle also contributed to re-epithelialization after wounding, and noted that these cells persisted in the regenerated epidermis. However, stem cells in the IFE make the greatest and most lasting contribution (10). Moreover, the stem cells from the HF are not absolutely required for re-epithelialization. Langton et al. (13) evaluated wound healing in a mouse model lacking HF, and observed that although healing was delayed, re-epithelialization did eventually occur.
Alopecia is a well described characteristic of mice and humans lacking VDR (14–16) due to failure to regenerate the cycling lower portion of the hair follicle after the initial developmental cycle is completed (5). Cianferotti et al. (17) attributed this to a gradual loss of the proliferative potential in the bulge stem cells, which they attribute in part to the loss of VDR. However, this conclusion has been challenged by Palmer et al. (18), who attributed the failure of HF cycling in the VDR null mouse in part to a failure of the bulge stem cells’ progeny to migrate out of the bulge rather than their loss of proliferative potential suggesting a loss of activation.
In the studies to be described we observed that deletion of VDR from keratinocytes of mice also fed a low calcium diet resulted in delayed wound healing, that we conclude is due to failure to activate β-catenin signaling in the epidermal stem cells, limiting their proliferative and migratory response to wounding.
2. Materials and Methods
2.1. Animals
Mice expressing VDR floxed in exon 3 (Vdrtm1Pcn) gift from Dr. Shigeaki Kato) are bred with mice expressing Tg(KRT14-cre)amc (Krt14 cre) (Jackson Laboratory). The final breeding (females homozygous for the floxed gene bred with males homozygous for the floxed gene and transgenic for the cre) produces litters homozygous for the floxed gene, but only half have the cre and thus the keratinocyte specific KO (epiVDRKO). The non cre expressing mice serve as controls. All mice were raised post weaning on a diet containing 0.02% calcium. These mice have been bred into the C57Bl/6J background.
2.2. Wounding protocol
3 month old epiVDRKO mice and their control littermates (CON) underwent full thickness skin biopsies 10 days after hair depilation by wax stripping to shift the skin to anagen. The size of the wound (3mm or 6mm) varied depending on the assay. To quantitate the wounding repair rate photographs were taken of the wounds daily through 10 days. The wound area was calculated by measurement of wound size each day, and shown as percent of 0 time control. In addition wounds at day 3 post wounding were examined histologically to evaluate re-epithelization. Percentage re-epithelialization was defined as the distance traveled by both epithelial margins divided by the distance needed to travel to fully re-epithelialize the wound. Six wounds from at least 3 mice per genotype (KO and control) were evaluated. All experiments were approved by the Institutional Animal care and Ethics Committee at the San Francisco VA Medical Center.
2.3. Proliferation studies
Full thickness wounds (3mm) were made on the backs of KO mice and their controls (CON). After 3d, the skin wound was excised and sectioning was performed with HFs arranged in a longitudinal orientation. The sections were immunostained for PCNA to assess cell proliferation. Cell proliferation was quantitatively evaluated by counting PCNA positive cells at the leading edge on multiple wound sections using Bioquant software and expressed as % of control. Six sections from each of 3 KO and 3 control mice were evaluated.
2.4. Immunohistochemistry
VDR expression in the hair follicle and epidermis was determined using antibody D-6 from Santa Cruz as previously described (19). Axin 2 expression was assessed by the axin 2 antibody from Abcam.
2.5. Gene expression
The skin wounds and control tissues were excised from epiVDRKO and CON mice. Total RNA was prepared using combination of Stat60 and RNA easy mini RNA purification kit (Qiagen). The quantity and quality of RNA were verified using the Aligent Bioanalyer. QPCR was used to evaluate gene expression using GAPDH to normalize data, and. The primer sequences are available upon request. Gene expression profile was evaluated by Illumina microarray (Mouse Ref 8 v 2.0). Pathway analysis through IPA software (Ingenuity Systems).was performed to determine signaling pathways affected by the deletion of VDR.
3. Results
3.1. VDR deletion delays wound closure
At 3 mo of age the epiVDRKO mice and their controls were subjected to 6mm full thickness skin biopsies on the left side of their backs, the right side serving as non wounded control tissue (figure 1A). Pictures were taken daily of the wounds to determine rates of closure (figure 1C). The epiVDRKO mice failed to undergo anagen after the first hair follicle cycle, whereas the control mice were in anagen at the time of the experiment, hence the darker skin due to the melanin in the hair shaft. The epiVDRKO mice show a retardation in wound closure (figure 1B). This is especially apparent at day 3. With time the wounds of the control mice show contraction, which is most notable in the dorsal ventral dimension. Re-epithelialization was then measured histologically by determining the ratio of the distance between the epithelial edges and the edges of the wound. These results [39.0+4.7 % (CON) compared to 29.7+8.1% (KO) (n=9–10 p<0.02)]confirmed the delay of wound closure on day 3 (figure 1D). On the other hand we did not observe significant changes in collagen deposition in VDR KO by Mason staining, nor did we find differences in myofibroblast numbers as assessed by α-smooth muscle actin (αSMA) immunohistochemistry and myoblast differentiation gene expression (data not shown).
1. The impact of keratinocyte specific deletion of VDR on wound healing.
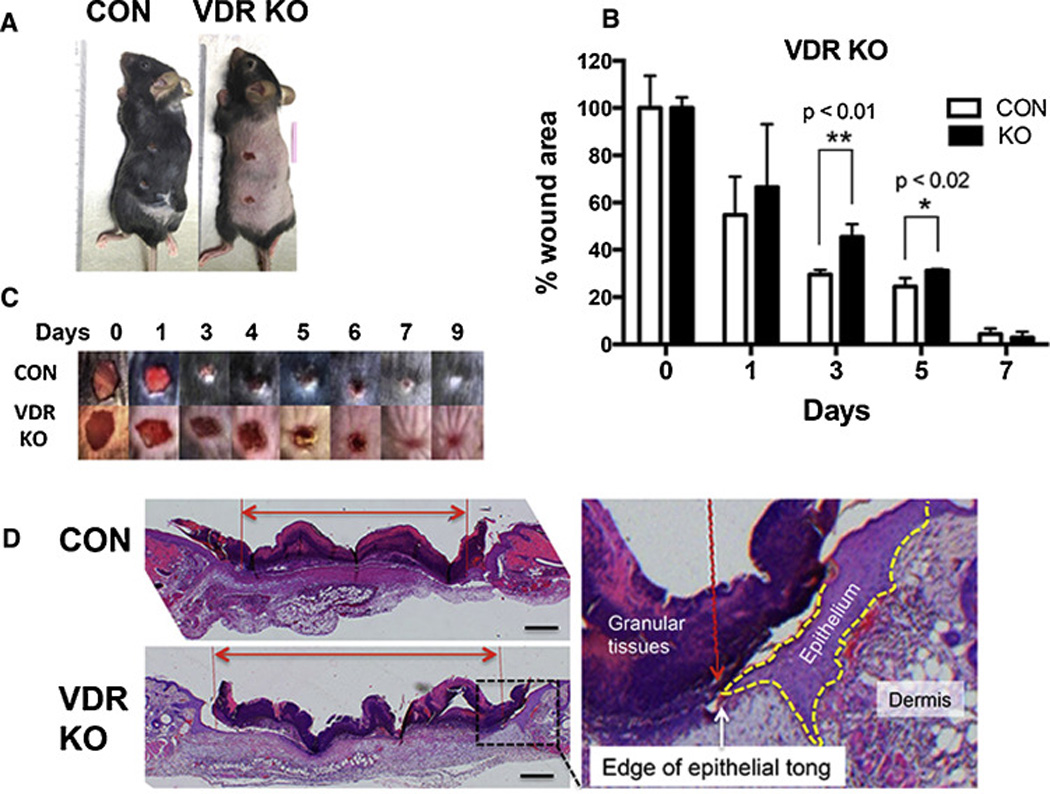
A. 3 month old epiVDRKO mice and their control littermates (CON) underwent 6mm full thickness skin biopsies. B. The wound area was calculated by measurement of wound size each day, and shown as percent of 0 time control. The bars enclose mean +/− SD, *p<0.05 (n=7–8). C. Photographs were taken of the wounds daily through 9 days. Representative photographs from KO and control mice are shown. D. 3 mm wounds excised at day 3 were examined histologically to evaluate re-epithelization. The representative H&E stained sections across the anterior/posterior diameter of the wounds are shown. The yellow dotted line outlines the epidermal tongue. The red lines show the edges of the epidermal tongues crossing the wound, and the red double headed arrow shows the distance to be traveled to close the wound. Percentage re-epithelialization was defined as the distance traveled by both epithelial tongues divided by the distance needed to travel to fully re-epithelialize the wound. Bar=400µm.
3.2. Localization of the VDR in the skin
Immunohistochemistry was used to localize the VDR in the epidermis and hair follicles (figure 2). The VDR was nuclear in location and found in the cells of the bulge, hair germ, and outer root sheath of the hair follicle (HF), and basal layer of the interfollicular epidermis (IFE). Although not specific for stem cells in these locations, these are also the locations for the stem cell populations in the skin. Deletion of VDR with the keratinocyte specific cre recombinase (K14-cre) ablated the VDR from all these locations (data not shown).
2. Expression of VDR in the HF and IFE of control mice.
Immunohistochemistry (IHC) was performed on the skin of 3mo control mice. VDR was detected in the nuclei of cells in the basal, suprabasal, and infundibular regions of the HF and basal layer of the IFE.
3.3. Impact of VDR deletion on gene expression
Deletion of VDR from the epidermis resulted in decreased expression of genes critical for a number of signaling pathways as determined by PCR (figure 3). These genes included Tgfb1, Shh, Gli1, Bmpr1a, Bmp4, Lef1, and a number of targets of β-catenin signaling such as Padi1, Dix3, and Tubb3. Decreased expression of several hair keratin genes is also shown for comparison. To evaluate the response of these pathways to wounding mRNA was prepared from the skin at the edge of the wound 3d after wounding of the epiVDRKO mice and their controls and analyzed by microarray. The results are available as accession number GSE68727 under super-series GSE68730 in public data base (GEO/NCBI/NIH http://www.ncbi.nlm.nih.gov/geo). The results are expressed as a ratio of KO:control. Pathway analysis was used to examine patterns most affected by the deletion of VDR. The effect on the β-catenin pathway was most striking (figure 4) with down regulation of β-catenin itself, LEF/TCF, and a number of β-catenin target genes such as Cyclin D1 and D2, Axin 2, and Wif1. To confirm the decrease in β-catenin signaling in the epiVDRKO skin following wounding the mRNA levels of the β-catenin target genes Ccnd1 and Axin2 in the skin prior to and 3d after wounding were analyzed. These results are shown in figure 5. Wounding stimulated the expression of Ccnd1 and Axin 2 in control skin, but this stimulation was not observed in the epiVDRKO skin, although the basal levels were comparable.
3. The decreased expression of signaling pathways promoting proliferation and HF differentiation in mice null for VDR in their keratinocytes.
The mRNA levels in the skin of epiVDRKO mice and their controls were measured by QPCR. The PCR results are normalized to the expression of L19 and expressed as a ratio of KO to control (WT) littermates.
4. β-catenin signaling is down regulated in epiVDRKO skin wounds that are delayed in their closure rate compared to the control wounds.
RNA was extracted from skin wounds and in epiVDRKO and littermate control mice 3 days post wounding. The RNA was evaluated by Illumina microarray (Mouse Ref 8 v 2.0), and fold changes were calculated. Pathway analysis by Ingenuity IPA software demonstrated that the β-catenin signaling pathway is decreased in epiVDRKO wounds as indicated by the genes in green. The TCF/β-catenin transcriptional complex is shown by the white double circle in the center. The β-catenin target genes are aligned under the complex. They are categorized by their function such as cell cycle or stem cell fate. The arrows denote the regulation through β-catenin signaling found in the IPA database. The crosses symbolize DNA. The potential location of VDR to regulate β-catenin signaling is added. Green represents down regulation, red represents up regulation, and the intensity of the hue indicates the degree of change.
5. The injury induced expression of β-catenin target genes is blunted in mice lacking VDR.
To confirm the microarray results skin biopsies were taken from 3mo epiVDRKO and control littermates (CON). RNA was extracted from wounded skin and adjacent control skin. The expression of β-catenin target genes Axin2 and Ccnd1 (cyclin D1) was measured by QPCR. The results are expressed as percentage over the expression in the non-wounded skin (CON mice) (mean + SD), and statistically significant decreases in wounded skin in VDR KO compared to control wounded skin are shown by asterisks. *p<0.05 (n=3). The unwounded and wounded skin are shown by open bars and grey or black bars, respectively. Lower magnification (upper panels) Bar=100µm; higher magnification upper panels and lower panels Bar=25µm
3.4. Proliferation and axin 2 expression in situ
To further explore the impact of VDR deletion on the epidermal response to wounding we examined by immunohistochemistry the number of PCNA positive and axin 2 positive cells in the epithelium at the edge of the wound 3 days after wounding. These results are shown in figure 6. The red bolts mark the edge of the wound. In the controls proliferation of the cells in the basal layer of the epidermis is markedly increased in the leading edge of the epithelium as shown by the number of cells expressing PCNA. This response was substantially blunted in the epiVDRKO mice. Similarly, axin 2 expression in the cells in the basal layer of the epidermis was also markedly increased in the cells forming the leading edge of the epithelium in the control mice, but not in the epiVDRKO mice. Further away from the wound, PCNA and axin 2 expression in the epidermis are much reduced in both epiVDRKO and control mice (data not shown).
6. epiVDRKO reduces the proliferative and axin 2 responses to wounding.
Three days after wounding sections of the wound were made and evaluated for PCNA and axin 2 expression by immunohistochemistry as markers of proliferation and β-catenin signaling, respectively. At the edge of the wound the epidermis was hyperplastic in both genotypes. PCNA and axin 2 expression was limited to the basal layer of the epidermis. However, PCNA and axin 2 expression was markedly reduced in the epiVDRKO mice compared to their littermate controls. *p<0.05 (n=3).
4. Discussion
Although vitamin D is well known as a regulator of epidermal and HF differentiation, its role in wound repair has received considerably less attention. Tian et al. (20) observed that topical 1,25(OH2D enhanced wound healing. Luderer et al. (21) observed that in the global VDR knockout mouse, there was a reduction in TGFβ signaling in the dermis, although re-epithelialization was not impaired. Although our initial studies with epiVDRKO mice did not show a significant delay in re-epithelialization, comparable to the results obtained by Luderer et al. (21) in their global VDRKO mouse, we have observed that re-epithelialization is impaired when the deletion of VDR is accompanied by a low calcium diet. The low calcium diet by itself did not produce this delay suggesting that calcium signaling can compensate for defective vitamin D signaling, and vice versa, but disrupting both produces the phenotype. We (22) have observed this type of synergism previously in keratinocyte cultures. Although deletion of the VDR in the keratinocyte regardless of the diet does result in decreased expression of genes in a number of signaling pathways expected to be involved in wound healing such as TGFβ1, sonic hedgehog and Gli 1, BMP, and components of the β-catenin pathway, it required the low calcium diet to make the phenotype grossly manifest as delayed wound healing.
The delay in wound closure was observed only during the first 5 days. In this model closure of the wound also involves the myofibroblasts in the dermis. The deletion of the VDR was specific for the keratinocyte, and we did not observe a reduction in myofibroblasts that formed by three days following wounding (data not shown),
We focused on the disruption of β-catenin signaling as this is known to be critical for stem cell activation in both the HF and IFE. In the HF β-catenin signaling and HF stem cell activation are dependent on the presence of the VDR (23). Moreover, LEF1, the transcription factor involved with canonical β-catenin signaling, binds to the first zinc finger in the DNA binding domain of VDR (24), and cells lacking LEF1 do not support ligand independent VDR binding to β-catenin target genes such as Axin2 and Gli1. That the same may be true for epidermal stem cells comes from our observations that the epidermal response to wounding shows a blunted increase in proliferation and β-catenin signaling in the keratinocytes at the edge of the wound in epiVDRKO mice Nuclear translocation of β-catenin results in binding to transcription factors of the T-cell factor (TCF) and lymphoid enhancer factor (LEF) families to promote expression of genes such as Cnnd1 and Myc(25) important for proliferation. A considerable body of literature has developed focused on the role of β-catenin in HF stem cell function (26, 27), but comparably less on its role in IFE stem cell function, the primary focus of this study. However, sensitive markers of this transcriptional role of β-catenin in the IFE using an axin 2 reporter have also indicated a role for β-catenin signaling in IFE stem cells (26). Deletion of β-catenin from keratinocytes using Krt14 cre recombinase leads to loss of HF cycling but sebocyte hypertrophy, dermal cysts lined with epithelia expressing epidermal markers, and epidermal hyperplasia, a phenotype similar to that in VDR null mice (28, 29). These results were initially interpreted as demonstrating not only a role of β-catenin (and VDR) in regulating HF stem cell fate but also a role for β-catenin in suppressing IFE proliferation, an action opposite to its role in the HF. However, deletion of β-catenin during telogen with the appropriate timing of tamoxifen to mice expressing tamoxifen regulated axin 2 cre, thus limiting the deletion to the IFE, indicated that β-catenin is also required for epidermal proliferation (27, 30).
Our results confirm these earlier studies. Wounding activates proliferation of the keratinocytes at the leading edge of the wound, and this is associated with an increase in β-catenin signaling. Deletion of VDR from these keratinocytes blocks the increased proliferation and blunts the activation of β-catenin signaling following wounding. We postulate that this reduction in β-catenin signaling and its subsequent effects on stem cell activation underlies the reduction in wound healing in the epiVDRKO mouse. At this point we do not exclude the role of other signaling pathways including those of TGF βand sonic hedgehog, but the impact of VDR on β-catenin signaling clearly plays a major role in the response of the skin to wounding.
Highlights.
Deleting the vitamin D receptor (VDR)) from keratinocytes (epiVDRKO) in combination with a low calcium diet delays skin wound healing.
The delay in wound healing is accompanied by a reduction in β-catenin signaling.
Proliferation and migration of the keratinocytes at the edge of the wound are reduced in the epiVDRKO as is the expression of axin 2, indicating the role of β-catenin signaling in the epidermal response to wounding and its dependence on the VDR.
Acknowledgements
This project was supported by VA Merit Review IBX001066 and NIH grant RO1 AR050023.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenhalgh DG. Models of wound healing. J Burn Care Rehabil. 2005;26(4):293–305. doi: 10.1097/01.bcr.0000169885.66639.b5. [DOI] [PubMed] [Google Scholar]
- 3.Braiman-Wiksman L, Solomonik I, Spira R, Tennenbaum T. Novel insights into wound healing sequence of events. Toxicol Pathol. 2007;35(6):767–779. doi: 10.1080/01926230701584189. [DOI] [PubMed] [Google Scholar]
- 4.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zugel U, Bikle DD, Modlin RL, Gallo RL. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117(3):803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tu CL, Crumrine DA, Man MQ, Chang W, Elalieh H, You M, Elias PM, Bikle DD. Ablation of the calcium-sensing receptor in keratinocytes impairs epidermal differentiation and barrier function. J Invest Dermatol. 2012;132(10):2350–2359. doi: 10.1038/jid.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bielefeld KA, Amini-Nik S, Alman BA. Cutaneous wound healing: recruiting developmental pathways for regeneration. Cell Mol Life Sci. 2013;70(12):2059–2081. doi: 10.1007/s00018-012-1152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Page ME, Lombard P, Ng F, Gottgens B, Jensen KB. The epidermis comprises autonomous compartments maintained by distinct stem cell populations. Cell Stem Cell. 2013;13(4):471–482. doi: 10.1016/j.stem.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alcolea MP, Jones PH. Lineage analysis of epidermal stem cells. Cold Spring Harb Perspect Med. 2014;4(1):a015206. doi: 10.1101/cshperspect.a015206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plikus MV, Gay DL, Treffeisen E, Wang A, Supapannachart RJ, Cotsarelis G. Epithelial stem cells and implications for wound repair. Semin Cell Dev Biol. 2012;23(9):946–953. doi: 10.1016/j.semcdb.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mascre G, Dekoninck S, Drogat B, Youssef KK, Brohee S, Sotiropoulou PA, Simons BD, Blanpain C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489(7415):257–262. doi: 10.1038/nature11393. [DOI] [PubMed] [Google Scholar]
- 11.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, Cotsarelis G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med. 2005;11(12):1351–1354. doi: 10.1038/nm1328. [DOI] [PubMed] [Google Scholar]
- 12.Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. Faseb J. 2007;21(7):1358–1366. doi: 10.1096/fj.06-6926com. [DOI] [PubMed] [Google Scholar]
- 13.Langton AK, Herrick SE, Headon DJ. An extended epidermal response heals cutaneous wounds in the absence of a hair follicle stem cell contribution. J Invest Dermatol. 2008;128(5):1311–1318. doi: 10.1038/sj.jid.5701178. [DOI] [PubMed] [Google Scholar]
- 14.Malloy PJ, Pike JW, Feldman D. The vitamin D receptor and the syndrome of hereditary 1,25-dihydroxyvitamin D-resistant rickets. Endocr Rev. 1999;20(2):156–188. doi: 10.1210/edrv.20.2.0359. [DOI] [PubMed] [Google Scholar]
- 15.Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997;94(18):9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16(4):391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 17.Cianferotti L, Cox M, Skorija K, Demay MB. Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc Natl Acad Sci U S A. 2007;104(22):9428–9433. doi: 10.1073/pnas.0702884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palmer HG, Martinez D, Carmeliet G, Watt FM. The vitamin D receptor is required for mouse hair cycle progression but not for maintenance of the epidermal stem cell compartment. J Invest Dermatol. 2008;128(8):2113–2117. doi: 10.1038/jid.2008.43. [DOI] [PubMed] [Google Scholar]
- 19.Xie Z, Chang S, Oda Y, Bikle DD. Hairless suppresses vitamin D receptor transactivation in human keratinocytes. Endocrinology. 2006;147(1):314–323. doi: 10.1210/en.2005-1111. [DOI] [PubMed] [Google Scholar]
- 20.Tian XQ, Chen TC, Holick MF. 1,25-dihydroxyvitamin D3: a novel agent for enhancing wound healing. J Cell Biochem. 1995;59(1):53–56. doi: 10.1002/jcb.240590107. [DOI] [PubMed] [Google Scholar]
- 21.Luderer HF, Nazarian RM, Zhu ED, Demay MB. Ligand-dependent actions of the vitamin D receptor are required for activation of TGF-beta signaling during the inflammatory response to cutaneous injury. Endocrinology. 2013;154(1):16–24. doi: 10.1210/en.2012-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su MJ, Bikle DD, Mancianti ML, Pillai S. 1,25-Dihydroxyvitamin D3 potentiates the keratinocyte response to calcium. J Biol Chem. 1994;269(20):14723–14729. [PubMed] [Google Scholar]
- 23.Lisse TS, Saini V, Zhao H, Luderer HF, Gori F, Demay MB. The Vitamin D Receptor Is Required for Activation of cWnt and Hedgehog Signaling in Keratinocytes. Mol Endocrinol. 2014;28(10):1698–1706. doi: 10.1210/me.2014-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luderer HF, Gori F, Demay MB. Lymphoid enhancer-binding factor-1 (LEF1) interacts with the DNA-binding domain of the vitamin D receptor. J Biol Chem. 2011;286(21):18444–18451. doi: 10.1074/jbc.M110.188219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281(5382):1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 26.Lien WH, Polak L, Lin M, Lay K, Zheng D, Fuchs E. In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators. Nat Cell Biol. 2014;16(2):179–190. doi: 10.1038/ncb2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi YS, Zhang Y, Xu M, Yang Y, Ito M, Peng T, Cui Z, Nagy A, Hadjantonakis AK, Lang RA, Cotsarelis G, Andl T, Morrisey EE, Millar SE. Distinct functions for Wnt/beta-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell. 2013;13(6):720–733. doi: 10.1016/j.stem.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xie Z, Komuves L, Yu QC, Elalieh H, Ng DC, Leary C, Chang S, Crumrine D, Yoshizawa T, Kato S, Bikle DD. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118(1):11–16. doi: 10.1046/j.1523-1747.2002.01644.x. [DOI] [PubMed] [Google Scholar]
- 29.Bikle DD, Elalieh H, Chang S, Xie Z, Sundberg JP. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207(2):340–353. doi: 10.1002/jcp.20578. [DOI] [PubMed] [Google Scholar]
- 30.Lim X, Tan SH, Koh WL, Chau RM, Yan KS, Kuo CJ, van Amerongen R, Klein AM, Nusse R. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science. 2013;342(6163):1226–1230. doi: 10.1126/science.1239730. [DOI] [PMC free article] [PubMed] [Google Scholar]







