Abstract
Brain-derived neurotrophic factor (BDNF) release to nerve terminals in the central nervous system is crucial in synaptic transmission and neuronal plasticity. However, BDNF release peripherally from primary afferent neurons has not been investigated. In the present study, we show that BDNF is synthesized by primary afferent neurons located in the dorsal root ganglia (DRG), and releases to spinal nerve terminals in response to depolarization or visceral inflammation. In two-compartmented culture that separates DRG neuronal cell bodies and spinal nerve terminals, application of 50 mM K+ to either the nerve terminal or the cell body evokes BDNF release to the terminal compartment. Inflammatory stimulation of the visceral organ (e.g. the urinary bladder) also facilitates an increase in spontaneous BDNF release from the primary afferent neurons to the axonal terminals. In the inflamed viscera, we show that BDNF immunoreactivity is increased in nerve fibers that are immuno-positive to the neuronal marker PGP9.5. Both BDNF and pro-BDNF levels are increased, however, pro-BDNF immunoreactivity is not expressed in PGP9.5-positive nerve-fiber like structures. Determination of receptor profiles in the inflamed bladder demonstrates that BDNF high affinity receptor TrkB and general receptor p75 expression levels are elevated, with an increased level of TrkB tyrosine phosphorylation/activity. These results suggest a possibility of pro-proliferative effect in the inflamed bladder. Consistently we show that the proliferation marker Ki67 expression levels are enhanced in the inflamed organ. Our results imply that in vivo BDNF release to the peripheral organ is an important event in neurogenic inflammatory state.
Keywords: BDNF, release, receptor, periphery, inflammation
INTRODUCTION
Bidirectional communication between sensory neurons and peripheral organs is an important physiological process. Signals emanating from the target organ flow into sensory neurons located in the dorsal root ganglia (DRG) to ensure correct sensation of environmental cues. In response to peripheral stimulation, neurotransmitters are produced by sensory neurons, which carries the signals into the central nervous system, i.e. spinal cord, where signals are further organized. Neurotransmitters can also release along the distal branch of the sensory neuron axon into the peripheral organs where a paracrine action is undertaken to affect the cytological and physiological properties of the organ. Under pathological condition such as peripheral inflammation or nerve injury, abnormal levels of neurotransmitters are generated by sensory neuronal cell bodies (Tonra et al., 1998, Obata and Noguchi, 2006, Qiao and Grider, 2007, Qiao et al., 2008, Lin et al., 2011). Neurotransmitters release to the spinal dorsal horn under these conditions often generate central sensitization contributing to painful sensation (Tonra et al., 1998, Lever et al., 2001, Kay et al., 2013). Excessive neurotransmitter release to the peripheral organs and their effects on the cellular changes in the organ have not been well characterized.
Brain-derived neurotrophic factor (BDNF) is one of the nerve growth factor (NGF) family members. As the same as all other neurotrophins, BDNF is initially generated as an unprocessed precursor, pro-BDNF (Mowla et al., 2001). In the cytoplasm, pro-BDNF can be cleaved within the endoplasmic reticulum or regulated secretory vesicles to generate mature BDNF (also called BDNF) (Greenberg et al., 2009). Pro-BDNF can also secret extracellularly where in the matrix pro-BDNF is either cleaved by plasmin to become BDNF, or functions as a ligand to facilitate cellular signals (Greenberg et al., 2009). Three cell surface receptors are responsible for BDNF and pro-BDNF action. The high affinity receptor TrkB is predominantly binding to BDNF. The low affinity receptor p75 can bind both pro-BDNF and BDNF. Sortilin binds a number of unrelated ligands including the pro-domain of the pro-neurotrophins (Nykjaer and Willnow, 2012). In neurons, BDNF binding to TrkB supports neuronal growth, survival and differentiation. Coupling of TrkB and p75 enhances BDNF action in growth and survive (Ho et al., 2011). On the other hand, pro-BDNF binding to p75 and Sortilin leads to apoptosis (Teng et al., 2005).
Different from NGF which is target-derived and is generated by the peripheral organs (Zhang and Qiao, 2012), BDNF is enriched in the sensory neuronal cell body in the DRG and is implicated to participate in sensory neuronal activation in a variety of states (Mannion et al., 1999, Obata and Noguchi, 2006, Cao et al., 2012, Xia et al., 2012, Yu et al., 2012, Qiao et al., 2013). In the DRG, BDNF is synthesized by a subpopulation of unmyelinated primary afferents and is packaged in large dense-core vesicles for anterograde transport to the axon terminals (Conner et al., 1997, Michael et al., 1997). It is extensively reported that anterograde transport of BDNF to the central terminals in the spinal cord dorsal horn (Conner et al., 1997, Michael et al., 1997) can interact with receptors and facilitate excitatory (glutamatergic) neurotransmission (Kerr et al., 1999), or modulate inhibitory (GABAergic/glycinergic) signaling in spinal neurons (Carrasco et al., 2007). BDNF release is often accompanied with other neurotransmitters including glutamate, substance P (SP), somatostatin, calcitonin gene related peptide (CGRP), etc. in facilitation of spinal cord plasticity (Tonra et al., 1998, Lever et al., 2001, Luo et al., 2001, Ng et al., 2007, Ha et al., 2008). Peripheral release of CGRP and SP has been reported (White and Helme, 1985, Kilo et al., 1997). However, few information is known about peripheral release of BDNF.
The present study combined in vitro and in vivo approaches and investigated the possibility of BDNF release to the peripheral terminals of the DRG axons. We used a DRG-spinal nerve preparation along with an in vivo rat model of visceral inflammation to better understand the nature of BDNF peripheral release and its possible effect on peripheral organs. This is clinically significant because neurotransmitter release from primary afferent neurons at the peripheral terminals in response to the increase in the axonal terminal excitability may participate in the generation and maintenance of neurogenic inflammation.
MATERIALS AND METHODS
Experimental animals
Adult male rats (200–250 g) from Harlan Sprague Dawley, Inc. (Indianapolis, IN) were used. All experimental protocols involving animal use were approved by the Institutional Animal Care and Use Committee at the Virginia Commonwealth University. Animal care was in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) and National Institutes of Health guidelines. All efforts were made to minimize the potential for animal pain, stress or distress as well as to reduce the number of animals used.
Immunoprecipitation and western blot
After determination of protein concentration, protein extracts from tissues were separated on a 10 % SDS-PAGE gel and transferred to a nitrocellulose membrane directly for western blot. Antibodies used for western blot include rabbit anti-TrkB (1:500, Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti-BDNF/pro-BDNF (1:500, Santa Cruz Biotechnology, Santa Cruz, CA), mouse monoclonal antibody against p75 (1 μg/mL, Chemicon-Millipore), rabbit anti-Sortilin (1 μg/mL, Chemicon-Millipore), and mouse antibody against β-actin antibody (1:3000, Sigma-Aldrich, St. Louis, MO). To examine phospho-TrkB, protein extracts were pre-incubated with rabbit anti-TrkB primary antibody (1:200) overnight at 4 °C and then immunoprecipitated by Protein A/G PLUS-Agarose (Santa Cruz Biotechnology, Santa Cruz, CA). The precipitates were boiled in laemmili loading buffer and loaded for western blot against phospho-tyrosine with a mouse monoclonal antibody (1:1000, Cell Signaling Technology). Horseradish peroxidase (HRP)-conjugated secondary antibody/enhanced chemiluminescence (ECL) or IRDye/ODYSSEY infrared imaging was used to detect immunoreactive bands. Internal loading control used for normalization was obtained by re-probing the same membrane with anti-β-actin antibody after thorough stripping procedure. In immunoprecipitation, the supernatant after precipitation of TrkB was used to examine β-actin as loading control. Densitometric analysis was achieved by scanning and quantifying the density of the immunoreactive bands with software FluorChem 8800 (Alpha Innotech, San Leabdro, CA).
BDNF release assay
BDNF release was tested in ex vivo preparation of DRG-spinal nerve complex as described previously by us (Yu et al., 2012). Briefly, the Campenot chamber was used to separate the ganglion and the nerve terminals. After initial equilibration in Dulbecco’s Modified Eagle Medium (DMEM) (~2h), the chambers were filled with fresh medium. The amount of DMEM used in both chambers kept consistently among experimental groups. In some of these preparations, a final concentration of 50 mM K+ was added to either the terminal-containing chamber or the cell body-containing chamber. The relative amount of BDNF release into the nerve terminal-containing chamber was determined by slot blot (Bio-Rad). All of medium from the terminal-containing chamber was collected and added to the designated slot. After all medium passed through, the membrane was subjected to primary antibody incubation followed by secondary antibody similar to the western blot procedure.
Quantitative real-time PCR
Total RNA was extracted using a RNA extraction kit RNAqueous (Ambion, TX). RNA concentration was determined spectrophotometrically. cDNA was obtained by reverse transcription. Quantitative real-time PCR was performed on a 7300 real-time PCR system (Applied Biosystems). Taqman probes (Applied Biosystems) were used for quantification of BDNF. β-actin expression in the same sample with the same amount of cDNA was used as internal control for normalization.
Enzyme-linked immunosorbent assay
BDNF ELISA kit (Promega Corporation) was used for examination of BDNF content. Briefly, polystyrene microtiter plates (96-well) were pre-coated according to manufacturer’s instruction. The protein extracts were sequentially treated with 1 N HCl and then neutralized with 1 N NaOH. The treated samples and BDNF standard were incubated sequentially with chicken anti-human BDNF pAb (1:500), anti-IgY HRP conjugate (1:200) and TMB One Solution (3,3′,5,5′-tetramethylbenzidine). The absorbance at 450 nm was measured within 30 min of stopping the reaction and the BDNF levels were expressed as pg BDNF per mg total protein calculated against the standard curve.
Immunohistochemistry
Cryostat sections were incubated with specific primary antibodies followed by secondary antibody conjugated to fluorescence or peroxidase enzyme used for 3,3′-Diaminobenzidine (DAB) stain. Sagittal sections of the urinary bladder were obtained to visualize fiber-like structures in the urinary bladder examined by guinea pig anti-PGP9.5 primary antibody (1:1000, Chemicon-Millipore) and co-stained by rabbit anti-BDNF (1:500, Santa Cruz, CA), or rabbit anti-pro-BDNF (1:400, Millipore), followed by incubation with Alexa 488 (green)-, or Cy3 (red)-conjugated species-specific secondary antibody. Transverse sections were obtained to determine cellular proliferation visualized by Ki67 immunostaining with DAB method. Transverse sections were also stained by 4’,6-Diamidino-2-Phenylindole (DAPI), a DNA-specific fluorescent probe (Thermo Fisher Scientific Inc.). A Zeiss fluorescent microscope equipped with a black-white camera, and a Nikon brightfield microscope equipped with a color camera were used to obtain microscopic photographs. For analysis of the Ki67 immunoreactivity in the tissue, three random microscopy fields were chosen from each section with caution to avoid field overlap. We focused on the detrusor muscle layers. The number of Ki67 positive cells in each field was counted. Results were averaged. The size of the area in the microscopic field that contained cells were measured, for the purpose of normalization, with free-tool software installed with the microscopy. The Ki67 expression level was expressed as number of immuno-positive cell per mm2 area. Similarly, randomly selected detrusor muscle areas were also measured for DAPI intensity with the built-in analysis software that was attached to the microscope. For BDNF, pro-BDNF and PGP9.5 immuno-densitometry, the fluorescent images were converted to a gray scale that ranged in intensity from 0 (black) to 255 (white) for the purpose of densitometry. Intensity measured, after subtracting the background stain, was averaged from each animal as one point. The results in the inflamed animals were presented as percentage of those in control animals.
Induction of urinary bladder inflammation
Bladder acute inflammation was used as an in vivo model. Bladder inflammation was induced by intraperitoneal injection of cyclophosphamide (CYP) at a single dose of 150 mg/kg as described by us previously (Chung et al., 2010). Control rats received volume-matched injections of saline. All injections were performed under isoflurane (2 %) anesthesia.
Statistical analysis
The results from each study were presented as mean ± SD. Comparison between control and experimental groups was made by using student t-test. Differences between means at a level of p<0.05 were considered to be significant.
RESULTS
BDNF release to periphery was enhanced in response to activity and inflammation
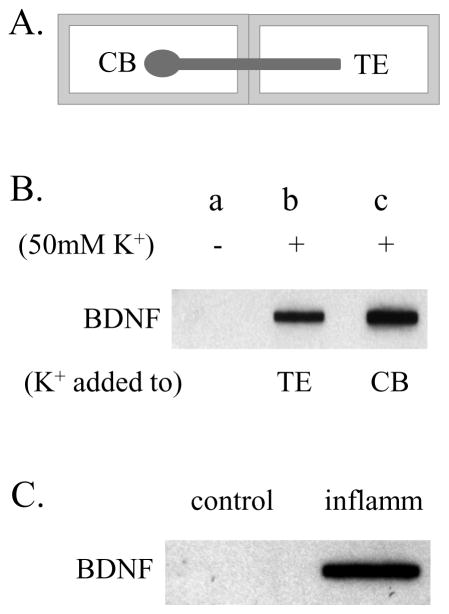
Extensive studies have suggested that primary afferent neurons in DRG produce high levels of BDNF which participates in sensory sensitization (Mannion et al., 1999, Obata and Noguchi, 2006, Cao et al., 2012, Xia et al., 2012, Yu et al., 2012, Qiao et al., 2013). Anterograde release of BDNF from primary afferent neurons to spinal cord dorsal horn is critical in spinal plasticity (Conner et al., 1997, Michael et al., 1997). The ability of BDNF peripheral release has not been examined. Here we showed that in a two-compartmented culture that separated the cell bodies and the axonal terminals (Figure 1A), depolarization (application of 50 mM KCl) to either the nerve terminal compartment (Figure 1B: TE), or the ganglion compartment (Figure 1B: CB) for 30 min evoked BDNF release to the terminal compartment. BDNF content in the medium after K+ treatment was robustly increased when compared to baseline BDNF release level (Figure 1B).
Figure 1. BDNF release in response to stimulation.
Ganglion-spinal nerve complex was cultured in two-compartmented chambers with ganglion and nerve terminals separated (A). Stimulation of either terminals or cell bodies evoked BDNF release to the terminal compartment (B). Inflammatory stimulation of visceral organ that contained nerve terminals also resulted in an increase in spontaneous BDNF release to the terminal compartment (C). TE: terminals; CB: cell body.
Ganglion-nerve preparations from healthy animal and animal with urinary bladder inflammation were also compared. These preparations were not treated with any chemical for examination of spontaneous BDNF release. After parallel culture in fresh medium for 30 min, BDNF content in the terminal compartment containing preparation from the inflamed animal demonstrated higher BDNF release when compared to those from the control animal (Figure 1C).
It was noteworthy that the level of BDNF release to the medium in the terminal compartment was not detectable by ELISA when a commercial kit was used. This may be due to low concentration of BDNF that was diluted by the volume of medium in the chamber. To examine whether the released BDNF contained pro-BDNF, we used a specific pro-BDNF antibody and performed the same experiments, however, we did not observe any pro-BDNF content in the medium.
BDNF protein was expressed in peripheral nerve fibers
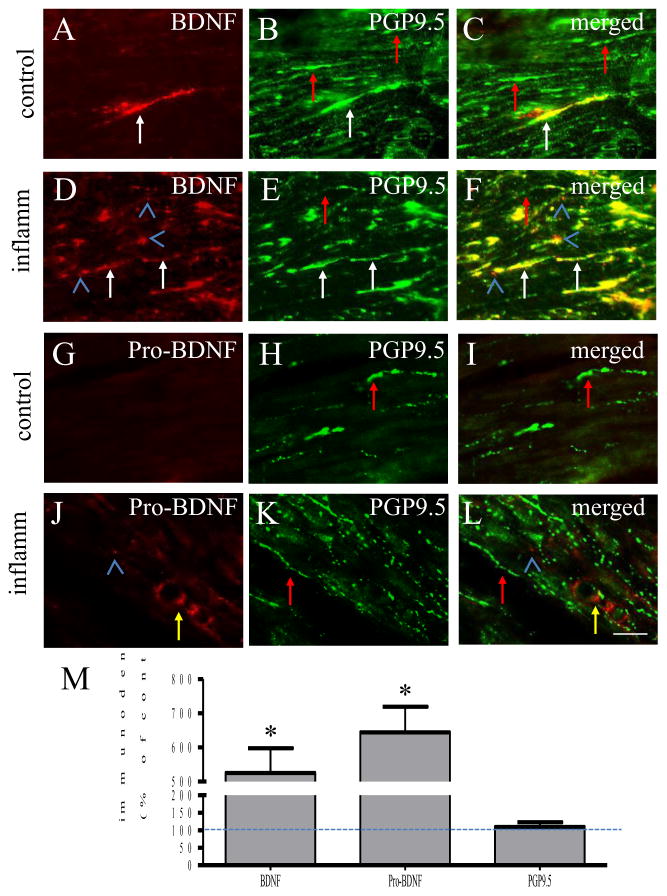
We next examined whether there was endogenous BDNF release in intact animals. We compared the urinary bladder in control (Figure 2A–2C) and inflammatory state (Figure 2D–2F). Immunostaining with a BDNF antibody (also recognizes pro-BDNF due to shared mature BDNF sequence) showed that BDNF immunoreactivity (Figure 2A, 2D: red stains) co-expressed with neuronal marker PGP9.5 (Figure 2B, 2E: green stains; 2C, 2F: merged yellow stains). The intensity of fiber-like structures expressing BDNF was higher in the inflamed organ when compared to control (compare Figure 2D to 2A, Figure 2M); while the intensity of fiber-like structures expressing PGP9.5 appeared similar in both animals (compare Figure 2E to 2B, Figure 2M). It was noted that BDNF immunoreactivity was not exclusively expressed in PGP9.5-positive structures (Figure 2D, F: blue arrowheads).
Figure 2. Co-expression of BDNF with PGP9.5 in the urinary bladder.
In control animals, few fiber-like structure expressed BDNF immunoreactivity in the urinary bladder (A, red stain). The number of fibers expressing BDNF was increased in the viscera during inflammation (D, red stain). BDNF-positive fibers were immunoreactive to PGP9.5 which was not changed during inflammation (B and E, green stain; C and F, yellow fibers indicated by white arrows). Not all PGP9.5-potitive fibers expressed BDNF (C and F, green stain indicated by red arrows). Some of BDNF was not expressed in PGP9.5-potive structures (C and F, blue arrowheads). Pro-BDNF immunoreactivity was also increased in the viscera during inflammation (compare J to G, red stain). Pro-BDNF did not express in PGP9.5-positive structures (H, K, green stain). Immuno-intensity analysis was performed and compared by student t-test between treated group and control and expressed by percentage (M). Bar = 30 μm. n=4. *, p<0.05.
Since the BDNF antibody used recognizes both mature form (called BDNF) and pro-form (called pro-BDNF) of the peptide due to their overlapped domain, a pro-BDNF antibody was further used and demonstrated that these non-neuronal BDNF (or pro-BDNF) immunoreactivity was likely pro-BDNF-positive (Figure 2J, L: blue arrowhead). In addition, pro-BDNF also expressed in tube-like structures appearing like blood vessels (Figure 2J, L: yellow arrow). Pro-BDNF immunoreactivity was increased in the inflamed organ (compare Figure 2J to 2G, Figure 2M), however, pro-BDNF did not express in PGP9.5-positve nerve fibers (Figure 2G–L, red arrows).
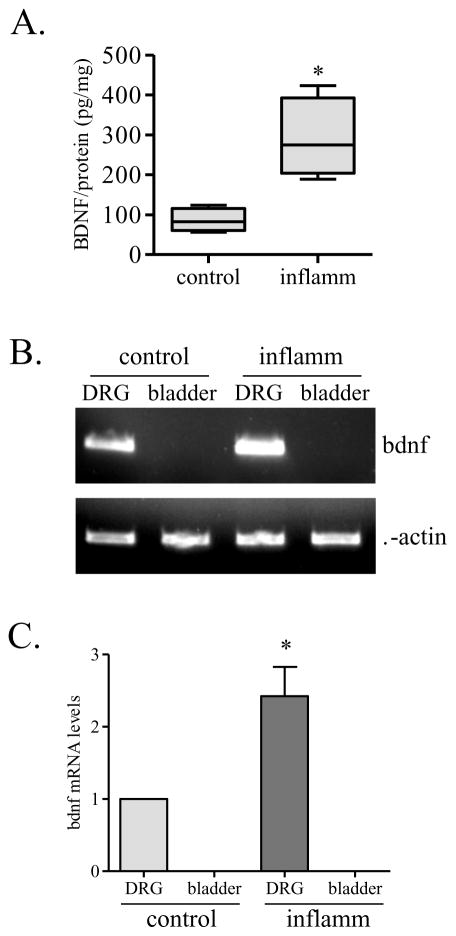
The BDNF content in the organ was further examined by ELISA showing that BDNF protein levels were indeed higher in the inflamed state when compared to control (Figure 3A). However, BDNF mRNA level in the inflamed organ was not detectable when examined by conventional PCR and compared to that in L6 DRG where BDNF mRNA levels were increased in inflammation (Figure 3B and 3C). We also used real-time PCR to confirm these findings. The CT value of BDNF in the urinary bladder from control and inflammation were greater than 40. A CT value bigger than 40 was often considered as a threshold of undetectable level.
Figure 3. BDNF expression in the visceral organ.
BDNF protein content was significantly increased in the inflamed organ (A). However, BDNF transcripts was increased in the L6 DRG but not detectable in the viscera (B and C). *, p<0.05; n=4.
Both BDNF and pro-BDNF levels were increased in the inflamed organ
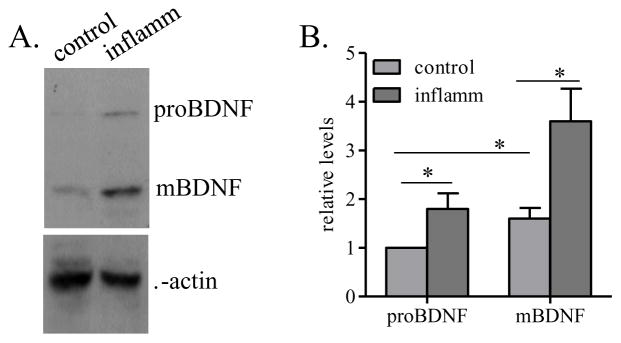
To confirm the above immunohistochemistry results and further differentiate BDNF and pro-BDNF expression profiles, we used western blot to differentiate these two isoforms by the differences of their molecular weight and also to compare their relative levels (Figure 4A). Results showed that both pro-BDNF and BDNF levels were elevated in the inflamed organ (Figure 4B). The levels of pro-BDNF were lower than BDNF levels in both control and inflamed animals (Figure 4B).
Figure 4. Expression of BDNF isoforms in the organ.
Western blot characterized both BDNF and pro-BDNF (A) showing that the levels of both isoforms were elevated in the urinary bladder during inflammation, with the BDNF having a relative higher level than pro-BDNF (B). The relative level of pro-BDNF in control animals was designated as 1 and was used for comparison of the expression levels of rest pro-BDNF and mature BDNF. *, p<0.05; n=4.
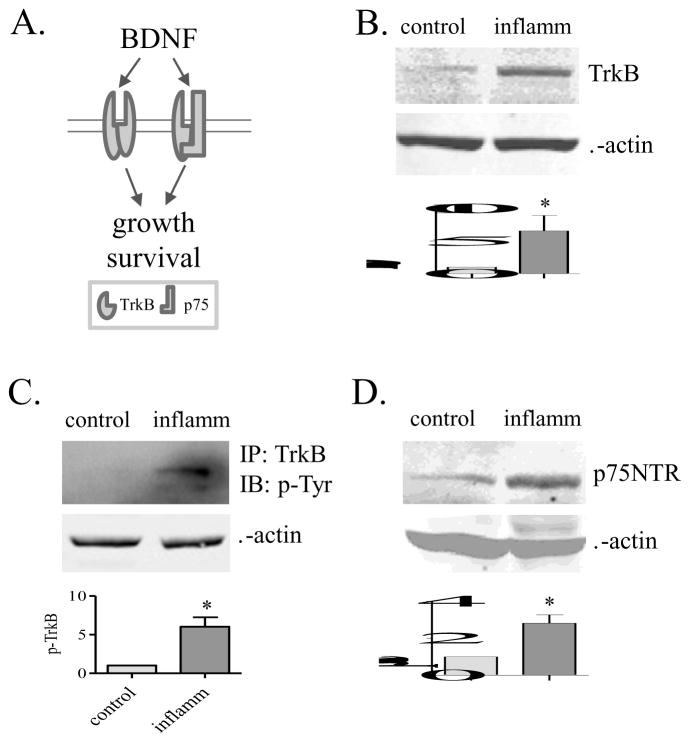
Up-regulation of BDNF receptors in the inflamed organ
Two receptors are responsive to BDNF, namely TrkB that has high affinity binding to BDNF, and p75 that is a general receptor for both BDNF and pro-BDNF (Figure 5A). Examination of these receptors showed that TrkB expression level (Figure 5B) and activity (phosphorylation) (Figure 5C), as well as p75 expression level (Figure 5D) were increased in the inflamed organ.
Figure 5. BDNF receptor profiles.
Two receptors including TrkB and p75 (A) were characterized. The expression levels of TrkB (B), the phosphorylation (activity) level of TrkB (C), and the expression level of p75 (D) were increased in the inflamed organ. *, p<0.05; n=4–5.
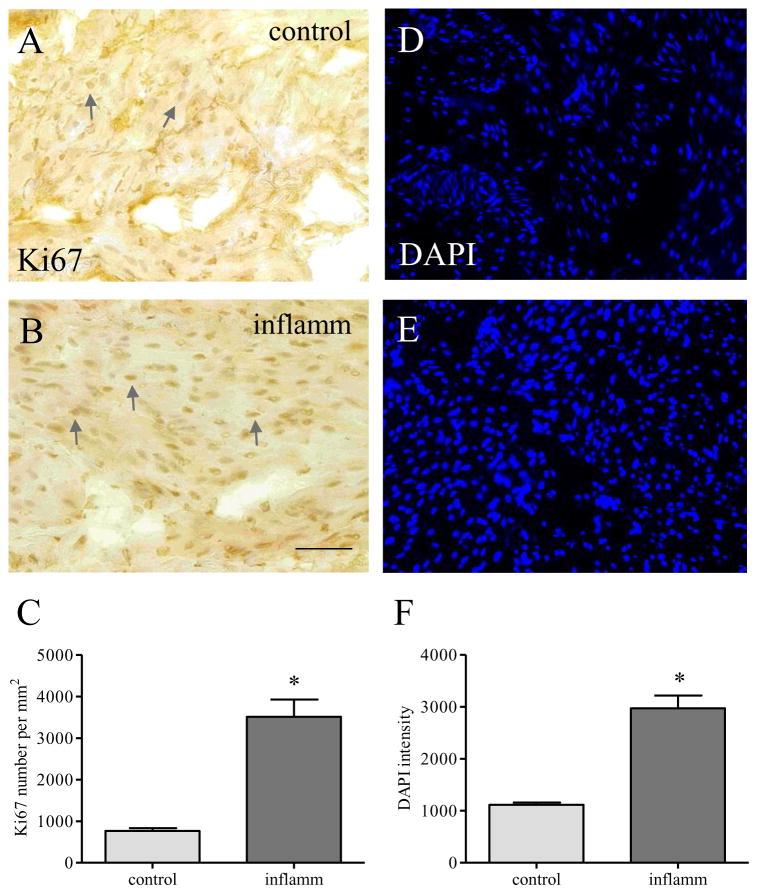
Enhanced cellular proliferation in the inflamed organ
BDNF binding to TrkB and p75 increases cellular proliferation and survival. Since BDNF, TrkB and p75 expression levels were increased in the inflamed bladder, we examined the level of proliferation marker Ki67. Immunostaining of the bladder section (7 μm) showed that there was a greater number of cells positive to Ki67 per unit area during inflammation when compared to control (compare Figure 6B to 6A, arrows; Figure 6C), suggesting an enhancement of cellular proliferation in the inflamed bladder. DAPI stain of the bladder sections showed that the density of cell profiles revealed by DAPI was greater in the inflamed organ (Figure 6E) when compared to control (Figure 6D; 6F).
Figure 6. Increases in cellular proliferation in the bladder.
The expression profile of proliferation marker Ki67 was examined in the viscera before (A) and during inflammation (B). The number of Ki67-positive cells was increased during inflammation (C). DAPI stain was performed to measure the density of detrusor cell profiles (D–F). *, p<0.05; n=4–5.
DISCUSSION
Three pieces of evidence are presented in the present study in support of each other to demonstrate BDNF release to the peripheral terminals of the primary afferent neurons upon activity and inflammatory stimulation. Firstly we show that depolarization of nerve terminals or neuronal cell body increases BDNF release to the terminal compartment in two-compartmented culture. We then demonstrate that BDNF transcripts are present in the primary afferent neurons but not in the peripheral organ (i.e. urinary bladder in the present study); while BDNF protein levels are elevated in the viscera upon inflammatory challenge. Furthermore, we find that BDNF immunoreactivity is present in fiber-like structures, presumably neuronal axons, which are immuno-positive to the neuronal marker PGP9.5. Western blot shows that the BDNF detected in the organ include both isoforms namely pro-BDNF and mature BDNF (also called BDNF). However, pro-BDNF, unlike mature BDNF, is not released by the nerve terminals but is likely produced by the visceral organ in a relatively lower level than BDNF. BDNF and pro-BDNF receptors are further examined showing that the expression and activity levels of BDNF high affinity receptor TrkB is increased in the inflamed organ. The expression level of the general neurotrophin receptor p75 is also increased in the inflamed organ, suggesting a possibility of an enhanced growth/survival signal because coupling of TrkB and p75 can strengthen BDNF action in promoting cellular growth. This is in line with further findings that the expression level of Ki67, a proliferation marker, is also increased in the inflamed organ. These results implicate that activity and inflammation are able to facilitate anterograde release of BDNF from neuronal cell bodies to the nerve terminals located at the periphery, which may contribute, in part, to cellular growth and neurogenic inflammation.
It is well documented that in the peripheral nervous system BDNF is primarily synthesized by primary afferent neurons located in the DRG. The present study using PCR and ELISA obtains consistent results showing that BDNF transcription mainly occurs in the ganglia with less detectable amount of BDNF mRNA in the visceral organ (In our hands, both real-time PCR and conventional PCR confirm that BDNF mRNA in the urinary bladder is undetectable). However, BDNF protein levels are increased in the viscera during inflammation. These results are consistent with studies by others that BDNF/TrkB system prefers anterograde transport away from the neuronal cell bodies toward the axonal terminals (Tonra et al., 1998, Ng et al., 2007, Ha et al., 2008). Indeed BDNF protein largely co-expresses with PGP9.5 in the neuronal axons located in the urinary bladder. In culture, BDNF release to the sensory neuron nerve terminals is also detected in response to depolarization or inflammation. The DRG neurons are of the pseudo-unipolar type with a split two axonal branches referring to as a distal process in the periphery and a proximal process at the terminals in the spinal dorsal horn. BDNF release to the spinal cord has been extensively studied where BDNF acts on spinal neurons facilitates spinal cord plasticity and long-term potentiation (LTP)-like activities, and is believed to contribute to spinal “central sensitization” (Tonra et al., 1998, Lever et al., 2001, Luo et al., 2001, Ng et al., 2007, Ha et al., 2008). BDNF is also able to act on microglia and facilitates tumor necrosis factor-α (TNF-α) release (Zhang et al., 2014), and TNF-α-potentiated N-methyl-D-aspartate (NMDA) receptor activation (Xu et al., 2010). BDNF release along the distal (peripheral) axonal branch has not been characterized. The present study proves that BDNF indeed is able to release to the peripheral organ, which is enhanced under the condition of peripheral organ inflammation. This does not preclude the possibility of local BDNF translation which can increase BDNF protein level independent of mRNA up-regulation. It may also explain the up-regulation of pro-BDNF protein but not mRNA in non-neuronal structures that an enhanced protein synthesis may occur in the inflamed viscera. Our previous study shows that the activity of Akt is increased in the inflamed bladder (Chung et al., 2010); this can activate the pathway that governs protein synthesis.
BDNF release by non-neuronal tissues has also been suggested (Vohra et al., 2013). For example, BDNF secretion by smooth muscle cells have been demonstrated in culture (Vohra et al., 2013). In the present study, BDNF protein is found not to exclusively express in PGP9.5-positive structures in the urinary bladder. Some cells appearing in muscle bundles also express BDNF protein. Originated from the same transcripts, pro-BDNF and BDNF share the same sequence of the mature domain. The BDNF antibody used in this study and those commercially available recognizes the mature domain of the peptide thus detects both BDNF and pro-BDNF. In fact, western blot shows both isoforms are elevated in the inflamed organ. Using a specific pro-BDNF antibody that does not recognize the mature BDNF, we find that the immunoreactivity detected in the non-neuronal structures by BDNF antibody is likely pro-BDNF. Pro-BDNF immunoreactivity appears in the cells within the muscle bundles that are not PGP9.5-positive. Pro-BDNF immunoreactivity also appears in tube-like structures, presumably blood-vessels (needs further characterization in future studies). These results suggest that mature BDNF and pro-BDNF in the visceral organ are likely generated differentially, with mature BDNF emanating from a neuronal origin while pro-BDNF is produced locally.
To examine the cellular physiology of BDNF in the urinary bladder, we checked the expression levels of receptors including TrkB and p75. In neurons, BDNF binds to high affinity receptor TrkB and sometimes binds to TrkB/p75 complex to enhance growth and survival promoting signals (Ho et al., 2011). In this study we show that the protein levels of TrkB and p75 are increased. The level of TrkB tyrosine phosphorylation is also elevated in the inflamed organ, suggesting a possible growth-promoting signal in the inflamed bladder. Elevation of Ki67 expression and the enhanced intensity of cell profiles examined by DAPI in the inflamed organ support the effects of BDNF-TrkB/p75 action in vivo. BDNF binding to TrkB/p75 can lead to the activation of the extracellular signal regulated pathway (ERK) and/or the phosphatidylinositide 3-kinases (PI3K)/Akt pathway (Binder and Scharfman, 2004). Both of these two pathways are reported to be activated in the inflamed bladder (Chung et al., 2010). The pro-proliferative effect of BDNF on smooth muscle in culture is also mediated by TrkB and ERK1/2 (Kwapiszewska et al., 2012). It is noted that although the proliferative effects of BDNF are correlated to Ki67 expression in the inflamed organ, it is not certain yet whether BDNF/TrkB/p75 system is a dominant pathway in regulating proliferation in the inflamed urinary bladder. Other growth factors such as NGF, insulin-like growth factor (IGF) etc. and cytokines may also have roles in promoting cellular proliferation and growth. With a decreased level of Sortilin by gene deletion, cell death can be inhibited even though in the presence of pro-BDNF (Jansen et al., 2007). It is possible that excessive pro-BDNF in the inflamed bladder may also bind to TrkB (Mowla et al., 2001). The biological function of this interaction reminds to be elucidated.
Visceral inflammation is often accompanied by organ hypertrophy which impairs normal physiology. Neuronal components from the extrinsic pathway have been suggested to have roles in the generation and maintenance of neurogenic inflammation and subsequent organ dysfunction (Liu et al., 2015). The present study implicates that neurogenic inflammation and inflammation-induced organ hypertrophy may also associate to BDNF release to the periphery, and subsequent hyperplasia.
Highlights.
Depolarization evoked BDNF release to nerve terminals
BDNF released to the visceral organ in vivo
Both BDNF and pro-BDNF content were increased in the viscera in response to inflammation
TrkB and p75 expression/activity were increased during inflammation
Acknowledgments
This work is supported by grant: NIH DK077917 to LYQ
A list of abbreviations
- BDNF
brain-derived neurotrophic factor
- CGRP
calcitonin gene related peptide
- DAB
diaminobenzidine
- DRG
dorsal root ganglion
- ELISA
enzyme-linked immunosorbent assay
- ERK
extracellular signal-regulated kinases
- IGF
insulin-like growth factor
- NGF
nerve growth factor
- NMDA
N-methyl-D-aspartate
- PI3K
phosphatidylinositide 3-kinases
- SP
substance P
- TNF-α
tumor necrosis factor-α
Footnotes
Conflict of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–131. doi: 10.1080/08977190410001723308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao XH, Chen SR, Li L, Pan HL. Nerve injury increases brain-derived neurotrophic factor levels to suppress BK channel activity in primary sensory neurons. J Neurochem. 2012;121:944–953. doi: 10.1111/j.1471-4159.2012.07736.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco MA, Castro P, Sepulveda FJ, Tapia JC, Gatica K, Davis MI, Aguayo LG. Regulation of glycinergic and GABAergic synaptogenesis by brain-derived neurotrophic factor in developing spinal neurons. Neuroscience. 2007;145:484–494. doi: 10.1016/j.neuroscience.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Chung CW, Zhang QL, Qiao LY. Endogenous Nerve Growth Factor Regulates Collagen Expression and Bladder Hypertrophy through Akt and MAPK Pathways during Cystitis. Journal of Biological Chemistry. 2010;285:4206–4212. doi: 10.1074/jbc.M109.040444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JM, Lauterborn JC, Yan Q, Gall CM, Varon S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: Evidence for anterograde axonal transport. J Neurosci. 1997;17:2295–2313. doi: 10.1523/JNEUROSCI.17-07-02295.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg ME, Xu BJ, Lu B, Hempstead BL. New Insights in the Biology of BDNF Synthesis and Release: Implications in CNS Function. J Neurosci. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha J, Lo KW, Myers KR, Carr TM, Humsi MK, Rasoul BA, Segal RA, Pfister KK. A neuron-specific cytoplasmic dynein isoform preferentially transports TrkB signaling endosomes. J Cell Biol. 2008;181:1027–1039. doi: 10.1083/jcb.200803150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho R, Minturn JE, Simpson AM, Iyer R, Light JE, Evans AE, Brodeur GM. The effect of P75 on Trk receptors in neuroblastomas. Cancer Lett. 2011;305:76–85. doi: 10.1016/j.canlet.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen P, Giehl K, Nyengaard JR, Teng K, Lioubinski O, Sjoegaard SS, Breiderhoff T, Gotthardt M, Lin F, Eilers A, Petersen CM, Lewin GR, Hempstead BL, Willnow TE, Nykjaer A. Roles for the pro-neurotrophin receptor sortilin in neuronal development, aging and brain injury. Nat Neurosci. 2007;10:1449–1457. doi: 10.1038/nn2000. [DOI] [PubMed] [Google Scholar]
- Kay JC, Xia CM, Liu M, Shen S, Yu SJ, Chung C, Qiao LY. Endogenous PI3K/Akt and NMDAR act independently in the regulation of CREB activity in lumbosacral spinal cord in cystitis. Exp Neurol. 2013;250:366–375. doi: 10.1016/j.expneurol.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr BJ, Bradbury EJ, Bennett DLH, Trivedi PM, Dassan P, French J, Shelton DB, McMahon SB, Thompson SWN. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. J Neurosci. 1999;19:5138–5148. doi: 10.1523/JNEUROSCI.19-12-05138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilo S, HardingRose C, Hargreaves KM, Flores CM. Peripheral CGRP release as a marker for neurogenic inflammation: a model system for the study of neuropeptide secretion in rat paw skin. Pain. 1997;73:201–207. doi: 10.1016/S0304-3959(97)00108-5. [DOI] [PubMed] [Google Scholar]
- Kwapiszewska G, Chwalek K, Marsh LM, Wygrecka M, Wilhelm J, Best J, Egemnazarov B, Weisel FC, Osswald SL, Schermuly RT, Olschewski A, Seeger W, Weissmann N, Eickelberg O, Fink L. BDNF/TrkB Signaling Augments Smooth Muscle Cell Proliferation in Pulmonary Hypertension. Am J Pathol. 2012;181:2018–2029. doi: 10.1016/j.ajpath.2012.08.028. [DOI] [PubMed] [Google Scholar]
- Lever IJ, Bradbury EJ, Cunningham JR, Adelson DW, Jones MG, McMahon SB, Marvizon JCG, Malcangio M. Brain-derived neurotrophic factor is released in the dorsal horn by distinctive patterns of afferent fiber stimulation. J Neurosci. 2001;21:4469–4477. doi: 10.1523/JNEUROSCI.21-12-04469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YT, Ro LS, Wang HL, Chen JC. Up-regulation of dorsal root ganglia BDNF and trkB receptor in inflammatory pain: an in vivo and in vitro study. J Neuroinflammation. 2011;8:126. doi: 10.1186/1742-2094-8-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Shen SW, Kendig DM, Mahavadi S, Murthy KS, Grider JR, Qiao LY. Inhibition of NMDAR Reduces Bladder Hypertrophy and Improves Bladder Function in Cyclophosphamide Induced Cystitis. J Urology. 2015;193:1676–1683. doi: 10.1016/j.juro.2014.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo XG, Rush RA, Zhou XF. Ultrastructural localization of brain-derived neurotrophic factor in rat primary sensory neurons. Neurosci Res. 2001;39:377–384. doi: 10.1016/s0168-0102(00)00238-8. [DOI] [PubMed] [Google Scholar]
- Mannion RJ, Costigan M, Decosterd I, Amaya F, Ma QP, Holstege JC, Ji RR, Acheson A, Lindsay RM, Wilkinson GA, Woolf CJ. Neurotrophins: peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc Natl Acad Sci U S A. 1999;96:9385–9390. doi: 10.1073/pnas.96.16.9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael GJ, Averill S, Nitkunan A, Rattray M, Bennett DLH, Yan Q, Priestley JV. Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. Journal of Neuroscience. 1997;17:8476–8490. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowla SJ, Farhadi HF, Pareek S, Atwal JK, Morris SJ, Seidah NG, Murphy RA. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. Journal of Biological Chemistry. 2001;276:12660–12666. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]
- Ng BK, Chen L, Mandemakers W, Cosgaya JM, Chan JR. Anterograde transport and secretion of brain-derived neurotrophic factor along sensory axons promote Schwann cell myelination. J Neurosci. 2007;27:7597–7603. doi: 10.1523/JNEUROSCI.0563-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A, Willnow TE. Sortilin: a receptor to regulate neuronal viability and function. Trends Neurosci. 2012;35:261–270. doi: 10.1016/j.tins.2012.01.003. [DOI] [PubMed] [Google Scholar]
- Obata K, Noguchi K. BDNF in sensory neurons and chronic pain. Neurosci Res. 2006;55:1–10. doi: 10.1016/j.neures.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Qiao LY, Grider JR. Up-regulation of calcitonin gene-related peptide and receptor tyrosine kinase TrkB in rat bladder afferent neurons following TNBS colitis. Exp Neurol. 2007;204:667–679. doi: 10.1016/j.expneurol.2006.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao LY, Gulick MA, Bowers J, Kuemmerle JF, Grider JR. Differential changes in brain-derived neurotrophic factor and extracellular signal-regulated kinase in rat primary afferent pathways with colitis. Neurogastroenterol Motil. 2008;20:928–938. doi: 10.1111/j.1365-2982.2008.01119.x. [DOI] [PubMed] [Google Scholar]
- Qiao LY, Yu SJ, Kay JC, Xia CM. In Vivo Regulation of Brain-Derived Neurotrophic Factor in Dorsal Root Ganglia Is Mediated by Nerve Growth Factor-Triggered Akt Activation during Cystitis. Plos One. 2013;8 doi: 10.1371/journal.pone.0081547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin. J Neurosci. 2005;25:5455–5463. doi: 10.1523/JNEUROSCI.5123-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonra JR, Curtis R, Wong V, Cliffer KD, Park JS, Timmes A, Nguyen T, Lindsay RM, Acheson A, DiStefano PS. Axotomy upregulates the anterograde transport and expression of brain-derived neurotrophic factor by sensory neurons. J Neurosci. 1998;18:4374–4383. doi: 10.1523/JNEUROSCI.18-11-04374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vohra PK, Thompson MA, Sathish V, Kiel A, Jerde C, Pabelick CM, Singh BB, Prakash YS. TRPC3 regulates release of brain-derived neurotrophic factor from human airway smooth muscle. Bba-Mol Cell Res. 2013;1833:2953–2960. doi: 10.1016/j.bbamcr.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DM, Helme RD. Release of Substance-P from Peripheral-Nerve Terminals Following Electrical-Stimulation of the Sciatic-Nerve. Brain Res. 1985;336:27–31. doi: 10.1016/0006-8993(85)90412-3. [DOI] [PubMed] [Google Scholar]
- Xia CM, Gulick MA, Yu SJ, Grider JR, Murthy KS, Kuemmerle JF, Akbarali HI, Qiao LY. Up-regulation of brain-derived neurotrophic factor in primary afferent pathway regulates colon-to-bladder cross-sensitization in rat. J Neuroinflammation. 2012;9:30. doi: 10.1186/1742-2094-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–U129. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SJ, Grider JR, Gulick MA, Xia CM, Shen SW, Qiao LY. Up-regulation of brain-derived neurotrophic factor is regulated by extracellular signal-regulated protein kinase 5 and by nerve growth factor retrograde signaling in colonic afferent neurons in colitis. Experimental Neurology. 2012;238:209–217. doi: 10.1016/j.expneurol.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QL, Qiao LY. Regulation of IGF-1 but not TGF-beta 1 by NGF in the smooth muscle of the inflamed urinary bladder. Regulatory Peptides. 2012;177:73–78. doi: 10.1016/j.regpep.2012.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zeng L, Yu T, Xu Y, Pu S, Du D, Jiang W. Positive feedback loop of autocrine BDNF from microglia causes prolonged microglia activation. Cell Physiol Biochem. 2014;34:715–723. doi: 10.1159/000363036. [DOI] [PubMed] [Google Scholar]