Abstract
Fetal growth restriction (FGR) is a significant complication of pregnancy describing a fetus that does not grow to full potential due to pathological compromise. FGR affects 3–9% of pregnancies in high‐income countries, and is a leading cause of perinatal mortality and morbidity. Placental insufficiency is the principal cause of FGR, resulting in chronic fetal hypoxia. This hypoxia induces a fetal adaptive response of cardiac output redistribution to favour vital organs, including the brain, and is in consequence called brain sparing. Despite this, it is now apparent that brain sparing does not ensure normal brain development in growth‐restricted fetuses. In this review we have brought together available evidence from human and experimental animal studies to describe the complex changes in brain structure and function that occur as a consequence of FGR. In both humans and animals, neurodevelopmental outcomes are influenced by the timing of the onset of FGR, the severity of FGR, and gestational age at delivery. FGR is broadly associated with reduced total brain volume and altered cortical volume and structure, decreased total number of cells and myelination deficits. Brain connectivity is also impaired, evidenced by neuronal migration deficits, reduced dendritic processes, and less efficient networks with decreased long‐range connections. Subsequent to these structural alterations, short‐ and long‐term functional consequences have been described in school children who had FGR, most commonly including problems in motor skills, cognition, memory and neuropsychological dysfunctions.
Abbreviations
- ACA
anterior cerebral artery
- AGA
appropriate for gestational age
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- FGR
fetal growth restriction
- IQ
intelligence quotient
- IUGR
intrauterine growth restriction
- IVH
intraventricular haemorrhage
- MCA
middle cerebral artery
- MRI
magnetic resonance imaging
- PI
pulsatility index
- RD
radial diffusivity
- SGA
small for gestational age
Introduction
Many neurodevelopmental disorders of motor and cognitive function have their origins in the antenatal period. Suboptimal fetal growth is likely to be a key factor underlying altered brain development. Specifically, fetal growth restriction (FGR), broadly describing the fetus that does not grow to its genetically destined potential (Kingdom & Smith, 2000; Resnik, 2002), is a critical pregnancy compromise that is strongly linked to neurodevelopmental deficits. Depending on the definition used, FGR complicates 3–9% of all pregnancies in high‐income countries, but the incidence is reportedly sixfold greater in low‐income countries such that worldwide FGR may affect up to 30 million infants per year (de Onis et al. 1998; Bernstein et al. 2000; Lackman et al. 2001; Fang, 2005; Chauhan & Magann, 2006; Figueras & Gratacos, 2014). FGR is associated with preterm birth, perinatal death and, for survivors, an increased risk of motor and sensory neurodevelopmental deficits, cognitive and learning impairments, and cerebral palsy (Blair, 2000; Marlow, 2000; Gagnon, 2003; Yanney & Marlow, 2004; van Wassenaer, 2005; Guellec et al. 2011; von Beckerath et al. 2013). The implementation of neuroprotective treatments can only occur in response to careful characterisation of the abnormalities in brain development that arise due to FGR, first requiring the identification of infants at greatest risk for neurodevelopmental impairment secondary to poor fetal growth.
There is no internationally recognised clinical definition for intrauterine or fetal growth restriction (IUGR or FGR). This is unfortunate, as it does not allow appropriate consideration of the distinction between poor fetal growth due to pathological compromise (true FGR), and those infants who are constitutionally small. Traditionally, the diagnosis of FGR described an infant with birth weight below the 10th percentile for their sex and gestational age (Kingdom & Smith, 2000), with more recent definitions incorporating measure of placental pathology with abnormal umbilical artery Doppler flow velocimetry during pregnancy (Figueras & Gratacos, 2014). Abnormality of Doppler flow in the umbilical arteries is clinically significant as it reflects placental compromise, where placental insufficiency is considered the principal cause of FGR (Cetin & Alvino, 2009; Figueras & Gardosi, 2011; Story et al. 2013). The Doppler cerebroplacental ratio may also be utilised, calculated using the umbilical artery pulsatility index (PI) divided by the middle cerebral artery PI (umbilical/cerebral ratio), and thereby providing a more sensitive marker of growth restriction (Figueras & Gratacos, 2014). In contrast, small for gestational age (SGA) is a term often used to describe infants with birth weight below the 10th percentile, and this is likely to include FGR infants, in addition to infants who are naturally small but healthy. Clinically, the evaluation of placental function using umbilical artery Doppler allows a distinction between SGA and FGR (Figueras & Gardosi, 2011). Many published papers use the term fetal growth restriction or intrauterine growth restriction where in fact SGA would be the more appropriate term. For the purpose of this review we have included any work that describes their cohort as FGR or IUGR.
The causes of FGR are diverse, broadly incorporating factors that may be fetal (chromosomal or congenital abnormalities, multiples, infection), maternal (malnutrition, vascular disease, lifestyle factors including smoking or drug use) or placental in origin (Resnik, 2002). Poor placental function is the greatest contributor to FGR, causing placental insufficiency (Pollack & Divon, 1992; Gagnon, 2003; Figueras & Gardosi, 2011). Placental insufficiency results in chronic fetal hypoxaemia, and reduced nutrient availability including altered amino acid transfer and fetal hypoglycaemia (Soothill et al. 1987; McMillen et al. 2001; Cetin & Alvino, 2009). In turn, chronic fetal hypoxaemia and reduced nutrient availability directly cause decreased fetal growth rate. The growth‐restricted fetus responds to chronic hypoxia by slowing its growth rate, and redistributing cardiac output to favour essential organs (brain, heart and adrenals) (Kamitomo et al. 1993; Miller et al. 2009; Damodaram et al. 2012; Poudel et al. 2015). This redistribution of fetal cardiac output tends to protect brain growth relative to other organs, resulting in asymmetric fetal growth, or so‐called brain sparing (McMillen et al. 2001; Resnik, 2002; Geva et al. 2006 b). However, brain sparing does not ensure normal brain development. Here, we will describe the neuropathological consequences that are observed in human FGR, and examine the experimental animal literature in an attempt to reveal the complex cellular responses and mechanisms of brain injury secondary to placental insufficiency and chronic hypoxia.
The human brain in FGR: structure and function
Placental insufficiency and FGR have neuropathological consequences for the developing brain. The spectrum of brain abnormalities associated with FGR is heterogeneous, reflecting spread in the timing and severity of in utero compromise, whether the FGR infant is born preterm or at term, and whether other co‐existing complications are present (see Abstract figure). Two broad factors appear critical to neurodevelopmental outcome – the severity of placental dysfunction together with the gestational age at onset; and the gestational age at delivery (Baschat, 2011). Thus, the management of FGR represents a careful balance between antenatal in utero compromise that is likely to contribute to brain injury, and the risks associated with preterm delivery and postnatal intensive care (Baschat, 2011). The contribution of antenatal compromise versus postnatal complications, or potential interactions between the two, towards the development of neurodevelopmental sequelae is not yet known.
Current dogma of FGR describes the condition as either early‐onset or late‐onset, reflecting heterogeneity of underlying causes and clinical features. Early‐onset FGR is typically diagnosed by the second trimester, is strongly associated with severe placental dysfunction and chronic fetal hypoxia, is present with preeclampsia in up to 50% of cases, and tends to describe the more severe cases of FGR (Baschat, 2014; Figueras & Gratacos, 2014). Late‐onset FGR is the more common form, present in 70–80% of FGR, and typically becoming apparent in the third trimester of pregnancy. In late‐onset FGR the umbilical artery Doppler may be normal, reflecting milder placental dysfunction, but advancing fetal deterioration is evidenced by changing umbilical/cerebral ratio (Baschat, 2014; Figueras & Gratacos, 2014). In both early‐ and late‐onset FGR, progressive fetal hypoxia caused by placental dysfunction induces cardiovascular redistribution, and when this is sufficiently severe or prolonged, asymmetric fetal growth and brain sparing occur. The haemodynamic adaptation of brain sparing is considered a mechanism to protect brain development when oxygen availability is low. Unfortunately, however, normal brain development does not necessarily follow cardiac output redistribution to favour the brain (Fig. 1). Late‐onset FGR infants born preterm or at term and with evidence of brain sparing have abnormal neurobehaviour in the neonatal period and at 2 years of age (Tolsa et al. 2004; Eixarch et al. 2008; Oros et al. 2010). Studies of neurodevelopment in early‐onset FGR offspring also reveal adverse motor, cognitive and behaviour outcomes; however, these may be somewhat confounded by preterm birth (Baschat, 2014). FGR infants with altered umbilical artery Doppler demonstrate significantly poorer motor and cognitive outcomes at 2 years of age, and at school age, compared with appropriately grown age‐matched preterm or term offspring (Vossbeck et al. 2001; Morsing et al. 2011). Scherjon and colleagues showed that fetal brain sparing with elevated umbilical/cerebral ratio was not associated with adverse neurodevelopmental outcome at 3 years of age, but at 5 years of age there was a negative association with mean intelligence quotient (IQ) score such that infants with brain sparing had an IQ score 9‐points lower than expected (Scherjon et al. 1998, 2000). Motor ability, cognition and behaviour scores remain lower in older children and young adolescents who demonstrated reversed end‐diastolic velocity in utero and were born preterm (Schreuder et al. 2002). Both early‐ and late‐onset FGR are a significant risk factor for the development of cerebral palsy (McIntyre et al. 2013; Baschat, 2014; Blair & Nelson, 2014).
Figure 1. The characteristics, cause, diagnostic use and neuropathology associated with brain sparing in human infants and children .
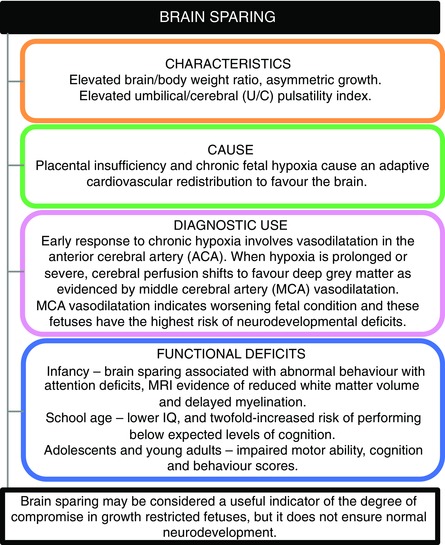
Brain sparing occurs as an adaptive cardiovascular response to fetal hypoxia and may protect against worse brain damage, but does not spare the brain from injury.
Brain structure
Deficits in brain structure and function are commonly observed in FGR offspring (Fig. 2). Despite the adaptation of brain sparing, a number of studies report that FGR infants have smaller head circumference than age‐matched appropriately grown infants (Harel et al. 1985; Tolsa et al. 2004; Padilla et al. 2010). This is clinically important, since small head size during infancy is a strong predictor for poor neurodevelopmental outcome (Gale et al. 2006). Human FGR is associated with decreased total brain and cortical grey matter volumes (Tolsa et al. 2004) with significantly smaller brain volume first evident in utero (Businelli et al. 2015). Relative cortical grey matter volume is decreased by FGR, indicating that there is a specific vulnerability of grey matter to FGR, independent of overall brain volume reduction (Tolsa et al. 2004), and these structural abnormalities are paralleled by neonatal neurobehavioural deficits such as less well developed attention capacity. Postmortem studies of human infants with FGR confirm a decrease in the total number of cells within the brain (Samuelsen et al. 2007) and decreased myelin content (Chase et al. 1972). Head circumference often remains lower in 6‐year‐old children and adolescents that were SGA at birth (Hadders‐Algra & Touwen, 1990; Pryor et al. 1995).
Figure 2. Deficits in brain structure and function commonly observed in FGR offspring .
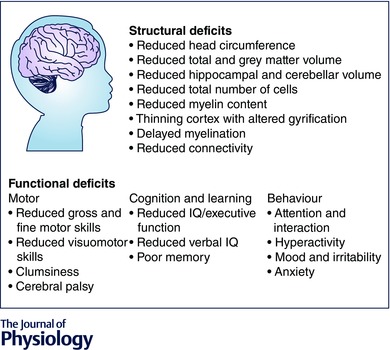
Listed at the top are structural deficits within the total brain, grey matter and white matter of FGR human infants. The bottom describes deficits in motor function, cognition and learning, and behaviour that have been observed in children that were FGR.
Soon after birth, FGR infants display significant differences in morphological neurostructure, evident as altered cortical gyrification, which is likely to be due to a thinner cortical thickness compared to appropriately grown infants (Dubois et al. 2008), and at 12 months of age FGR infants have reduced structural complexity of brain grey and white matter (Esteban et al. 2010). Preterm FGR infants with brain sparing and who underwent MRI at term‐equivalent age further demonstrate a delay in myelination and reduced posterior white matter volume in the absence of white matter lesions (Ramenghi et al. 2011). A number of studies note decreased volume in the hippocampus and the cerebellum, contributing to the discussion that these areas are very vulnerable to chronic hypoxia during periods of accelerated growth (Lodygensky et al. 2008; Padilla et al. 2011, 2014). One particularly useful imaging advancement is the use of diffusion tensor imaging (DTI) assessment of fractional anisotropy (FA), which provides microstructural information on the organisation and integrity of white matter tracts. In healthy, myelinated white matter, FA values are high and radial diffusivity (RD) is low, since water molecules preferentially diffuse in the direction of the tracts rather than perpendicular to fibre tracts (Huppi et al. 1998). In neonatal white matter, the FA increases and the RD decreases with age, as fibre organisation, axonal coherence and myelination progress (van de Looij et al. 2015). In FGR infants, DTI imaging of the white matter at 12 months of age suggests a complex set of altered white matter organisation, with clustered areas of decreased FA in the corpus callosum, but increased FA in fronto‐occipital, internal and external capsule white matter tracts (Padilla et al. 2014). Recently, developments in whole brain connectome analysis show reduced global and local network efficiency using graph model measures (Batalle et al. 2012) and reduced connectivity in long range cortico‐basal ganglia connections predominantly in the prefrontal and limbic networks in preterm children born FGR, compared with preterm children with appropriate birth weight. These structural brain network measures also correlate with neurobehavioral impairments such as hyperactivity or cognitive deficits, in the executive function domain at school age (Fischi‐Gomez et al. 2014). Notably, suboptimal brain development in utero has profound long‐term consequences, with deficits in total brain volume and white matter integrity underlying reduced IQ and cognitive impairment particularly in preterm infants with additional FGR (Chiang et al. 2009; Guellec et al. 2011). Interestingly, in the large French cohort study EPIPAGE, the rate of neurocognitive deficits in moderately preterm infants with FGR is around 40%, and is identical to the incidence of neurocognitive deficits in extremely preterm infants (Guellec et al. 2011), indicating that there is a common period of vulnerability in brain development, both for intra‐uterine and extra‐uterine adverse conditions.
In addition to providing clinical information about the presence or degree of fetal brain sparing during conditions of fetal growth restriction, Doppler ultrasound can also give an insight into haemodynamic changes in cerebral perfusion associated with FGR. The middle cerebral artery (MCA) has been most often examined, and reduced Doppler pulsatility index in the MCA is commonly reported as evidence of brain vasodilatation (Hernandez‐Andrade et al. 2012). However, recent studies in FGR fetuses suggest that vasodilatation of the MCA may only detect an advanced stage of brain blood flow redistribution, reflecting an association between progressive fetal demise and regional changes in cerebral perfusion (Figueroa‐Diesel et al. 2007; Rossi et al. 2011). In FGR fetuses, vasodilatation is first evident in the anterior cerebral artery (ACA) before being observed in the MCA (Rossi et al. 2011; Hernandez‐Andrade et al. 2012). This is indicative of preferential perfusion to the frontal region of the brain as an early response to chronic hypoxia. As chronic hypoxia continues, or becomes worse and the fetus deteriorates, perfusion changes to favour (in an attempt to protect) the basal ganglia at the expense of the frontal lobe, and reduced pulsatility index in the MCA become apparent (Hernandez‐Andrade et al. 2012). Supporting this, decreasing MCA pulsatility index is correlated with worsening fetal hypoxaemia (Akalin‐Sel et al. 1994), and an increased prevalence of brain injury (Spinillo et al. 2009). The presence of MCA vasodilatation is also associated with significantly increased risk for neonatal acidosis, indicative of fetal distress and poor fetal reserve in the face of intrapartum challenge (Cruz‐Martinez et al. 2011). Vasodilatation in the MCA also predicts poor neonatal behaviour, and more than half of fetuses with abnormal Dopplers and growth restriction will subsequently have neurological deficits at 2 years of age (Eixarch et al. 2008). While abnormal MCA flow detects advanced haemodynamic changes in the FGR brain, ACA vasodilation, and therefore preferential blood flow to the frontal region, is also associated with deficits in neurodevelopmental behaviour soon after birth, and increased incidence of emotional and attention problems at 18 months of age (Roza et al. 2008; Cruz‐Martinez et al. 2009). Combined, these data confirm that brain sparing is a characteristic FGR response to chronic placental insufficiency and hypoxia, aimed at conserving energy and preserving normal cell processes in critical brain regions (Pearce, 2006). It is now evident that with advancing fetal hypoxia and compromise, the cerebral haemodynamic response involves two components – firstly an initial stage aimed at protecting the brain, followed by a second stage of decompensation that is associated with brain injury (Hernandez‐Andrade et al. 2012). Certainly the observation of brain sparing is a very useful indicator of compromise in growth‐restricted fetuses, and may act to moderate against greater injury to the developing brain. In general, however, it must be considered that the term brain sparing is a misnomer and the brain is not spared from injury (Fig. 1).
FGR is widely considered a risk factor for perinatal brain injury including intraventricular haemorrhage (IVH), albeit clinical studies document varied results with elevated, reduced, or unchanged rates of IVH in FGR infants compared to appropriately grown counterparts (Bernstein et al. 2000; Gilbert & Danielsen, 2003; von Beckerath et al. 2013; Malhotra et al. 2015). Placental insufficiency with abnormal umbilical artery Doppler was linked to the presence of IVH (Marsoosi et al. 2012). When considering that brain sparing is a characteristic response to placental insufficiency and chronic hypoxia, it is not surprising that alterations in blood flow to the brain may be both indicative of the clinical severity of FGR, and associated with neurodevelopmental impairments (Hernandez‐Andrade et al. 2012). The adaptive response of brain sparing necessitates remodelling of the fetal brain circulation, structurally (altered number of blood vessels) and functionally, where chronic hypoxia induces vasodilatation of the cerebral circulation that can be detected via Doppler ultrasound as a decreased pulsatility index in the cerebral arteries (Roza et al. 2008; Hernandez‐Andrade et al. 2012). This brain circulatory adaptation differs according to the severity of chronic hypoxia, and the gestational age of the fetus, reflecting the stage of cerebral circulatory development (Hernandez‐Andrade et al. 2012). In SGA preterm infants, a significantly lower rate of IVH has been observed compared to AGA infants, and the authors hypothesised that intrauterine stress may be protective (Procianoy et al. 1980). Conversely, Bernstein et al. (2000) showed a non‐significant trend towards increasing rates of IVH in FGR infants born between 25 and 30 weeks’ gestation. When a large cohort of babies were separated into weekly birth intervals, it was found that IVH rates were significantly lower in FGR versus non‐FGR infants born at 28 weeks, suggesting a protective effect of growth restriction, but that IVH rates became significantly elevated in late‐preterm FGR births > 34 weeks (Gilbert & Danielsen, 2003). This result has been confirmed by a more recent study (Ortigosa Rocha et al. 2010) showing that IVH was more common in late‐preterm FGR infants compared to appropriately grown infants. This finding is of concern since late preterm births, > 34 week and < 37 weeks, account for the vast majority of preterm births, and the incidence of late‐preterm births is increasing (Loftin et al. 2010).
Brain function
The adverse effects of FGR on brain structure have a variety of consequences for function. FGR infants born preterm and assessed at term equivalent age demonstrate functional deficits in neurobehavioural score for attention and responsivity, compared to appropriately grown preterm infants, with cerebral cortex grey matter volume correlated to attention–interaction score (Tolsa et al. 2004). At 7 months of age, FGR infants perform more poorly on a visual recognition memory task than age‐matched appropriately grown infants (Gotlieb et al. 1988). Preterm FGR infants followed‐up at 1, 2 and 3 years of age showed deficits in developmental and behavioural outcomes, compared to preterm age‐matched appropriately grown infants, but it is interesting to note that preterm FGR infants were not different from birth weight matched controls (that is, infants who were born at an earlier gestation but were not growth restricted) (Sung et al. 1993). Leitner and co‐workers undertook a longitudinal study from birth to middle school age (age 9–10 years) in children born with late‐onset FGR with evidence of brain sparing. This study showed that FGR children have a complex set of neurodevelopmental deficits compared to age‐matched appropriately grown children (Leitner et al. 2007). While suboptimal cognitive performance (IQ < 85) was apparent in 15% of FGR children, they were also more likely to have specific learning disabilities such as reduced memory performance and visuomotor functions, attention and behavioural deficits (Leitner et al. 2007). Where brain sparing (elevated umbilical/cerebral ratio) was apparent, IQ at 5 years of age was 9 points lower (87 versus 96) compared to children with a normal umbilical/cerebral ratio (Scherjon et al. 2000). Multiple follow‐up studies of FGR infants into school‐age childhood find deficits in gross and fine motor skills, cognition, memory and academic ability, as well as neuropsychological dysfunctions encompassing poor attention, hyperactivity and altered mood (Low et al. 1992; Kok et al. 1998; Geva et al. 2006 a,b; Fischi‐Gomez et al. 2014).
It is apparent from the literature that determining the neurodevelopmental consequences of FGR is complicated by the severity of FGR, early or late onset, and the gestational age at delivery (Baschat, 2014). Early‐onset severe FGR is considered high risk for deficits in outcome, and indeed at school age, severe FGR children perform worse on assessment tasks for cognition, motor function, behaviour and educational achievements than children who had mild to moderate FGR, or preterm appropriately grown children (Schreuder et al. 2002). The combination of early‐onset FGR, fetal cardiovascular compromise and preterm delivery is linked with the most significant neurodevelopmental deficits (Baschat, 2014). Preterm birth is likely to be an exacerbating factor when describing the neurological outcomes associated with FGR, and Yanney & Marlow (2004) suggest that preterm birth overrides the effects of FGR per se. This is supported by a large prospective French study that examined neurological outcomes in school‐age children that were born AGA or SGA at 24–28 weeks or 29–32 weeks gestation, and found cognitive deficits, inattention–hyperactivity, and school difficulties in AGA and SGA infants born at 24–28 weeks gestation, and similarly in those SGA infants born in the 29–32 week group, but much less in infants born appropriate for gestational age at 29–32 weeks. Interestingly, even mild‐SGA infants (10th–19th centile) born at 29–32 weeks also showed mild cognitive and behavioural difficulties (Guellec et al. 2011). Finally, it has also been shown that serious neurodevelopmental consequences are more prevalent in SGA infants who demonstrate perinatal acidosis (Soothill et al. 1995), suggesting that a secondary acute (birth) compromise has profound additive adverse effects in fetuses that have experienced chronic antenatal hypoxia. Recent studies find that impaired fetal growth increases the risk for low intellectual performance across all stages of gestation. Indeed this has been confirmed by comparing monozygotic twin pairs at school age or in adulthood, where the growth‐restricted twin is at increased risk for low intellectual performance with reduced verbal IQ compared to the appropriately grown twin (Bergvall et al. 2006; Edmonds et al. 2010). In addition, a further study in twins has quantified the effects of low birth weight, showing that a 500 g increase in (term) birth weight results in a 2% increase in total brain volume, grey matter volume and white matter volume, and a 2‐point increase in IQ (Raznahan et al. 2012).
A number of studies have analysed results based on the child's sex, where sex differences may influence neurodevelopmental outcomes for FGR infants. Parkinson and colleagues reported that poor school achievement and behavioural problems were more frequently observed in boys with early‐onset growth restriction (Parkinson et al. 1981). This is supported by another study showing that FGR boys born very preterm (24–29 weeks) were at the greatest risk of cognitive impairment examined at school age, compared to FGR preterm girls and appropriately grown preterm offspring (Morsing et al. 2011). In girls, severe growth restriction is a strong indicator for impaired neurocognition, whereas mild‐ to moderate‐growth restriction is not (Streimish et al. 2012). The pattern of adverse behaviours may also differ according to the child's sex; girls at school age that had been FGR at birth were more likely to be irritable and cry than non‐FGR girls, whereas FGR boys tended to display anxiety, clumsiness and an inability to concentrate in class (Parkinson et al. 1981).
Animal experimental induction of FGR
FGR has been experimentally induced in rats, guinea pigs, rabbits and sheep, and the general physiology and advantages and disadvantages for each model have been reviewed previously (McMillen et al. 2001; Morrison, 2008; Basilious et al. 2015; Swanson & David, 2015) (Table 1). These animal models have been used experimentally to characterise altered brain development, and other organ and systemic pathologies associated with fetal growth restriction. While no single animal model can encompass the multifactorial causes and consequences of FGR, each animal model brings insight into the brain's developmental adaptations, cellular and microstructural responses to chronic hypoxia and hypoglycaemia, and potential causal pathways that contribute to brain injury (Table 1 and Fig. 3). These same animal models provide valuable and informative tools to examine the short‐ and longer‐term benefits of potential neuroprotective therapies for use in FGR.
Table 1.
Summary of animal experimental models of FGR and their neuropathology
| General description | Brain structure and microstructure in FGR offspring | References |
|---|---|---|
| Guinea pig; unilateral uterine artery ligation; onset 0.45 gestation (30 of 67 days gestation) | ||
|
|
Nitsos & Rees, 1990; Dieni & Rees, 2003; Rehn et al. 2004; Mallard et al. 2000; Tolcos et al. (2011) |
| Rabbit; ligation of 40–50% of uteroplacental vessels; onset 0.8 gestation (25 of 31 days gestation) | ||
| Birth weight reduced by 26–40% |
|
Eixarch et al. 2012; Illa et al. 2013 |
| Rats; uterine artery ligation; onset 0.86 gestation (19 of 22 days gestation) | ||
|
|
Tashima et al. 2001; Reid et al. 2003; Olivier et al. 2005, 2007; Liu et al. 2011; Delcour et al. 2012; Fung et al. 2012; Reid et al. 2012; Basilious et al. 2015 |
|
||
| Rats; chronic hypoxia; 10% O2 from day 5 to 19 of gestation | ||
| Body weight reduced by 15% at postnatal day 3 |
|
Pham et al. 2015 |
| Sheep; placental embolisation with microspheres; onset 0.8 gestation (120 of 145 days gestation) | ||
|
|
Mallard et al. 1998 |
| Sheep; single umbilical artery ligation (SUAL); onset 0.7 gestation (105 of 145 days gestation) | ||
|
|
Miller et al. 2014 |
| Sheep; carunclectomy; prior to mating | ||
|
|
Bisignano & Rees 1988; Rees et al. 1998 |
Figure 3. A summary of human and animal experimental results showing the principal adverse mechanisms contributing to grey matter and white matter pathology in FGR .
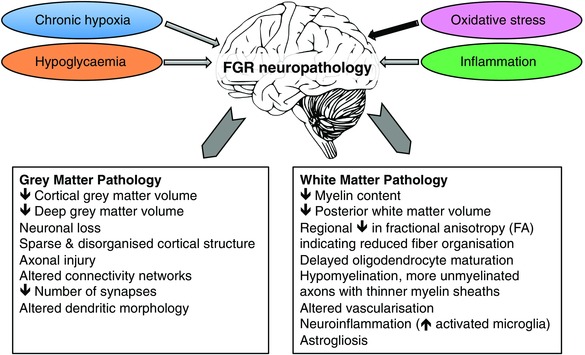
Chronic fetal hypoxia, hypoglycaemia, oxidative stress and inflammation are the likely causes of adverse neurodevelopment. Gross changes in brain volume are observed in both grey and white matter of the FGR brain, contributed by cell loss and sparsity of neuropil layers with altered axons, dendrites, synapses and myelination.
The most commonly used methods to induce FGR in fetal sheep are removal of the endometrial caruncles in the ewe prior to mating (Alexander, 1964; Robinson et al. 1979), by injecting microspheres in the placental circulation (Gagnon et al. 1994; Mallard et al. 1998; Louey et al. 2000) and by ligation of one of the umbilical arteries (Emmanouilides et al. 1968; Supramaniam et al. 2006). While carunclectomy results in placental insufficiency throughout gestation, the other methods reflect late onset FGR. In pregnant rodents, guinea pigs and rabbits, FGR is often induced by uterine artery ligation at different gestational ages (Wigglesworth, 1974; van Marthens et al. 1975). In precocial species such as the guinea pig, FGR uterine artery ligation is typically performed at mid‐gestation (Lafeber et al. 1984), while in altricial species, such as the rat, FGR is induced at the end of pregnancy to mimic early third‐trimester onset FGR in humans (Basilious et al. 2015). It is, however, useful to consider that the timing of brain development and maturation varies markedly across the experimental animal models studied. For example, sheep display a relatively mature brain at the time of birth compared to humans, while the rat brain is more immature at birth (Dobbing & Sands, 1979). Principally, the FGR animal models described here cause variable periods of chronic fetal hypoxia and hypoglycaemia to reflect human placental insufficiency, and in turn cause subsequent asymmetric FGR. Maternal nutrient restriction, maternal corticosteroid exposure and FGR are also important causes of compromised brain growth and growing areas of investigation (Woodall et al. 1996; Antonow‐Schlorke et al. 2011; Cottrell et al. 2012; Somm et al. 2012, 2014; Soo et al. 2012; Swanson & David, 2015).
Effect of experimental FGR on brain development
Morphological alterations in grey matter are commonly seen in FGR animal models including reduced volume of motor and visual cortices, hippocampus, basal ganglia and cerebellum (Rees et al. 1988; Mallard et al. 1998, 2000; Rehn et al. 2004). Ventriculomegaly is also noted in the guinea pig model of FGR (Rehn et al. 2004). Neuronal loss is observed within the FGR brain across a number of regions, including the hippocampus (Fung et al. 2012; Miller et al. 2014), and surviving neurons demonstrate selective changes in the morphology of hippocampal cell dendrites (Dieni & Rees, 2003). Indeed the hippocampus appears particularly susceptible to injury in growth‐restricted offspring, with reduced volume and total number of neurons, and with surviving neurons demonstrating abnormal axonal and dendritic morphology, as well as altered connectivity (Mallard et al. 2000; Dieni & Rees, 2003; Delcour et al. 2012; Illa et al. 2013; Miller et al. 2014; Basilious et al. 2015). These cellular disturbances in the hippocampus are, however, complex with results obtained in rats showing that FGR induces neuronal, astrocytic and immature oligodendrocyte deficits in a region‐ and sex‐specific manner (Fung et al. 2012). Neuronal cells of the cerebral cortex are also vulnerable, with significant cell loss, and disturbance in proliferation and migration of existing neurons that is first evident antenatally and persists for at least 10 weeks postnatally in FGR rats (Tashima et al. 2001; Liu et al. 2011). Deficits in neuronal connectivity are also apparent, with term‐equivalent FGR fetal sheep demonstrating a 17% decrease in synaptic density within the cerebral cortex (Bisignano & Rees, 1988). Furthermore, cell proliferation zones show decreased expression of anti‐apoptotic proteins (Bcl‐2), while p53 (pro‐apoptotic) immunoreactivity is increased (Uysal et al. 2008).
Reduced volume of the cerebellar white matter (Mallard et al. 2000) and subcortical white matter tracts (Mallard et al. 1998) including up to 20% reduction of myelin density in parts of the cerebral white matter are seen in FGR sheep (Miller et al. 2014). Further, axonal injury (β‐APP immunoreactivity) and lipid peroxidation are increased in the white matter in FGR newborn sheep (Miller et al. 2014), and axonal swelling is observed postnatally in FGR rats (Olivier et al. 2007). Growth‐restricted guinea pig fetuses also demonstrate a reduction in the total number of myelinated axons and, where axons were myelinated, the myelin sheath thickness was reduced (Nitsos & Rees, 1990). The delay in oligodendrocyte maturation and myelination in utero is transient and myelin is restored postnatally in the FGR guinea pig (Tolcos et al. 2011; Pham et al. 2015). As with observations in humans, the effects of FGR on white matter appears to be dependent on the severity of the initial growth restriction, as rats with severe FGR exhibit white matter damage that persisted to adulthood (Olivier) while moderate FGR is associated with diffuse white matter lesions, transient hypomyelination, microglial activation and astrogliosis (Olivier et al. 2007). The moderate deficits within the white matter may still have long‐term effects, with myelination abnormalities resolving after birth, but behavioural deficits still found in 8‐week‐old FGR female rats (Reid et al. 2012). Interestingly, it was recently reported in the FGR rabbit model that regional fractional anisotropy changes, indicative of white matter disorganisation, were correlated with worse outcome in neurobehavioural tests (Eixarch et al. 2012) and FGR‐induced brain reorganisation persisted up to 70 days of age in the rabbit (equivalent to pre‐adolescence human age) (Batalle et al. 2014). Indeed, FGR rabbits at 70 days postnatal age demonstrate a number of persistent neurostructural abnormalities consistent with delayed white matter development and poor connectivity. FGR rabbits show changes in grey matter using DTI imaging indicative of altered dendritic architecture, and decreased FA in the hippocampus and subventricular white matter reflecting less organised fibre tracts. These MRI changes were correlated with neurobehavioural and cognitive impairments in FGR rabbits, principally indicative of compromised short‐term memory and attention and anxiety behaviour (Illa et al. 2013).
FGR effects on cerebral metabolites, the vascular network and cerebral blood flow
Animal studies have not shown evidence that FGR results in reduced total blood flow to the brain (Clapp et al. 1984); rather, higher blood flow is seen to some brain areas (Poudel et al. 2015), while reduced regional blood flow is observed in other studies, including decreased blood flow to periventricular white matter (Miller et al. 2009). Neither cerebral oxidative metabolites nor glucose appears to be altered in FGR rats (Brown & Vannucci, 1978). On the other hand, more recent studies using mass spectrometry suggest that FGR in rabbits leads to metabolic alterations in the fetal brain, affecting neuronal viability, energy metabolism, amino acid levels, fatty acid profiles and oxidative stress mechanisms (van Vliet et al. 2013). There is also evidence to show that while central catecholaminergic pathways are not affected by FGR (Oyama et al. 1992), both serotonin metabolism and cholinergic functions may be altered (Represa et al. 1989; Thordstein & Hedner, 1992; Manjarrez‐Gutierrez et al. 2010).
Normal brain development and function require appropriate networks of blood vessels to supply high energy demands, and a highly integrated communication system between cells. The neurovascular unit comprises vascular endothelial cells ensheathed by a basal lamina membrane, in close association with surrounding astrocytes and pericytes. FGR fetal sheep and newborn lambs show evidence of altered cerebrovascular growth, with a relatively late onset period of chronic fetal hypoxaemia inducing increased capillary vessel size but no change to the number of vessels within the cortex (Rees et al. 1998), while FGR lambs demonstrate reduced vessel density within the brain's white matter, accompanied by decreased endothelial cell proliferation (Castillo‐Melendez et al. 2015). Astrogliosis, characterised by enlarged astrocytes and with more processes is described in the fetus or neonatal growth‐restricted rat, guinea pig and sheep (Nitsos & Rees, 1990; Rees et al. 1998; Olivier et al. 2007), and may or may not resolve after birth (Tolcos et al. 2011; Delcour et al. 2012). The attachment of astrocytes and pericytes to blood vessels is reduced in FGR lambs, which may impact the integrity of the blood–brain barrier (Castillo‐Melendez et al. 2015). Functional differences are also observed, with reactivity of cerebral blood vessels altered in FGR, likely to be mediated by decreased endothelium‐dependent relaxation (Herrera et al. 2014). It has also been shown that the cerebrovascular response to exogenous antenatal glucocorticoids, administered to mature the preterm lung, is quite different in human and sheep growth‐restricted fetuses compared to their appropriately grown counterparts (Miller & Wallace, 2013).
The observation of astrogliosis in FGR is interesting, as it is a characteristic pathology in white matter injury (Back & Rosenberg, 2014), and may provide the basis for a brain injury biomarker in FGR. S100B is an astroglial protein that is released in response to glial injury, and human cord blood S100B concentrations are associated with the later diagnosis of cerebral palsy (Costantine et al. 2011). Umbilical cord blood levels of S100B are elevated in FGR newborns (Gazzolo et al. 2002). During pregnancy, S100B levels in maternal blood are elevated with a growth‐restricted fetus that is then diagnosed with IVH after birth (Gazzolo et al. 2006). Furthermore, brain sparing (umbilical artery PI/middle cerebral artery PI ratio > 1) was apparent in all FGR fetuses with elevated maternal S100B, and cord blood S100B is negatively correlated with decreased PI in the middle cerebral artery, indicative of blood flow redistribution towards the cerebral circulation (Gazzolo et al. 2002).
Summary
It is clear that growth‐restricted infants represent a high‐risk population for antenatal, perinatal and postnatal complications. Moderate to severe FGR infants are at increased risk of stillbirth and likely to be born preterm, and FGR imposes additional risks for birth complications and neonatal morbidities than preterm infants who are not growth restricted. These risk factors are all exacerbated when evidence of brain sparing is apparent. Brain sparing results from the chronically hypoxic fetal environment imposed by placental insufficiency, and it is likely that this haemodynamic adaptation involves two stages, with the first aimed at protecting the brain with increased oxygen supply, followed by a second stage of decompensation. Brain sparing is therefore considered a useful indicator of the degree of compromise in growth‐restricted fetuses, but it does not ensure normal brain development (Fig. 1). Indeed it is clear that growth‐restricted infants present a high‐risk subgroup of infants with a complex and distinct set of microstructural brain abnormalities not observed in appropriately grown infants (Fig. 3).
In human FGR, decreased total brain volume is first apparent in utero, along with reduced total cell number, and a specific and independent vulnerability of the cortical grey matter to volume loss. Recent imaging advances demonstrate more complex microstructural changes in the FGR brain, which include altered fibre organisation and impaired connectivity networks such as the long range cortico‐basal ganglia thalamic tracts. Consequently, neurological impairments are described in response to FGR, and may be immediately evident in the newborn with altered attention and alertness. Neurological impairments in school age children who were born with FGR have now been well characterised comprising gross and fine motor deficits, and specific learning disabilities encompassing cognitive, memory, and academic performance. Neurobehavioural dysfunctions are also widely described in FGR children, including poor attention, hyperactivity and irritability. It is, however, evident from the wide range of observations and outcomes that many studies describing structural or functional pathologies (or their absence) subsequent to FGR do not clearly control for the clinical phenotype – early‐ or late‐onset FGR, severity, and gestational age at birth. It is apparent that early‐onset severe FGR infants with evidence of brain sparing (MCA vasodilatation) and born preterm are the infants with the most pronounced neuropathologies.
Animal models to induce placental insufficiency, chronic hypoxia and FGR provide an opportunity to examine the microstructural, functional and biochemical consequences for the developing growth‐restricted brain. The controlled experimental setting allows us to distinguish between the effects of early‐ or late‐onset placental insufficiency, chronic hypoxia and/or hypoglycaemia. As with human findings, it is the most severely affected FGR offspring with brain sparing that demonstrate significant brain structural deficits. At the cellular level there is a fundamental but region‐specific loss of neuronal cells, and altered developmental progression of oligodendrocytes and myelination, leading to deficits in white matter organisation and axonal injury. Indeed there appears an imbalance between the regulation of cellular proliferation and apoptosis within the developing brain of growth‐restricted fetuses. The morphology of remaining neurons is also affected, with a number of studies reporting decreased dendritic outgrowth and reduced cellular connectivity. Given that brain sparing is a key adaptation in response to chronic hypoxia, it is not surprising that cerebral vascular development is altered in FGR offspring including reduced structural integrity of the neurovascular unit, which may in turn cause an altered susceptibility to intraventricular haemorrhage, with these effects also dependent on the severity and duration of hypoxia. Reactive astrogliosis is noted in a number of animal models of FGR, which is important since astrogliosis is a principal pathology in white matter injury. FGR animal models can be used to examine the mechanisms that contribute to neuropathologies identified in FGR, and accordingly have identified the direct effects of chronic hypoxia, and additional indirect effects such as oxidative stress, or inflammatory mediators. With advances in the identification of neuropathologies in growth‐restricted fetuses and newborns, and particularly the complex microstructural changes in brain development, we must now look towards targeted therapies to restore normal brain development in response to placental insufficiency, chronic hypoxia and FGR.
Additional information
Competing interests
The authors declare no conflicts of interest.
Author contributions
All authors have approved the final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
This research was funded by an Australian Research Council Future Fellowship to S.L.M. (FT130100650), The National Health and Medical Research Council of Australia (546005), the Cerebral Palsy Alliance Australia and the Victorian Government's Operational Infrastructure Support Program. C.M.’s research was supported by the Swedish Research Council (2012‐2992), Sahlgrenska University Hospital (ALFGBG‐432291), Leducq Foundation (DSRR_P34404), Åhlens Foundation and the Swedish Brain Foundation (FO2014‐0080). P.S.H.’s research was funded by the Swiss National Science Foundation (SNF) 32003B‐113632, 32473B_135817, EU framework CONNECT project.
Biographies
Suzanne Miller leads the Fetal and Neonatal Health, Neurodevelopment and Neuroprotection Theme at The Ritchie Centre. She received her PhD from Monash University in 1999, and undertook postdoctoral training at University College London studying antenatal compromise and fetal brain development, before returning to a postdoctoral appointment at Monash University. Her research group aims to understand, and inhibit, the mechanisms that contribute to perinatal brain injury, with the goal of reducing the incidence and/or severity of cerebral palsy.

Petra Huppi is a clinician in paediatrics, leading the Child Development Unit and the Division of Development and Growth, Department of Pediatrics, Geneva University Hospitals, Switzerland. She is also a full professor at the University of Geneva and has international research activities with a visiting scientist position at Harvard Medical School, Boston, USA. She has directed numerous research projects both at Harvard Medical School and University of Geneva, aimed at better understanding early human brain development. Her research has contributed towards understanding brain deficits in preterm infants.
Carina Mallard directs the Perinatal Research group at The Institute for Neuroscience and Physiology, Sahlgrenska Academy, University of Gothenburg, Sweden. Carina received her PhD from University of Auckland in 1995 and undertook postdoctoral training at University of Melbourne as a SIDS Research Fellow. In 1999 she received a Swedish Research Council Award. The goal of her research is to understand mechanisms that lead to brain damage in preterm infants and to develop neuroprotective therapies for this vulnerable population.
References
- Akalin‐Sel T, Nicolaides KH, Peacock J & Campbell S (1994). Doppler dynamics and their complex interrelation with fetal oxygen pressure, carbon dioxide pressure, and pH in growth‐retarded fetuses. Obstet Gynecol 84, 439–444. [PubMed] [Google Scholar]
- Alexander G (1964). Studies on the placenta of the sheep (Ovis aries L.). Effect of surgical reduction in the number of caruncles. J Reprod Fertil 7, 307–322. [DOI] [PubMed] [Google Scholar]
- Antonow‐Schlorke I, Schwab M, Cox LA, Li C, Stuchlik K, Witte OW, Nathanielsz PW & McDonald TJ (2011). Vulnerability of the fetal primate brain to moderate reduction in maternal global nutrient availability. Proc Natl Acad Sci USA 108, 3011–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SA & Rosenberg PA (2014). Pathophysiology of glia in perinatal white matter injury. Glia 62, 1790–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baschat AA (2011). Neurodevelopment following fetal growth restriction and its relationship with antepartum parameters of placental dysfunction. Ultrasound Obstet Gynecol 37, 501–514. [DOI] [PubMed] [Google Scholar]
- Baschat AA (2014). Neurodevelopment after fetal growth restriction. Fetal Diagn Ther 36, 136–142. [DOI] [PubMed] [Google Scholar]
- Basilious A, Yager J & Fehlings MG (2015). Neurological outcomes of animal models of uterine artery ligation and relevance to human intrauterine growth restriction: a systematic review. Dev Med Child Neurol 57, 420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalle D, Eixarch E, Figueras F, Munoz‐Moreno E, Bargallo N, Illa M, Acosta‐Rojas R, Amat‐Roldan I & Gratacos E (2012). Altered small‐world topology of structural brain networks in infants with intrauterine growth restriction and its association with later neurodevelopmental outcome. Neuroimage 60, 1352–1366. [DOI] [PubMed] [Google Scholar]
- Batalle D, Munoz‐Moreno E, Arbat‐Plana A, Illa M, Figueras F, Eixarch E & Gratacos E (2014). Long‐term reorganization of structural brain networks in a rabbit model of intrauterine growth restriction. Neuroimage 100, 24–38. [DOI] [PubMed] [Google Scholar]
- Bergvall N, Iliadou A, Tuvemo T & Cnattingius S (2006). Birth characteristics and risk of low intellectual performance in early adulthood: are the associations confounded by socioeconomic factors in adolescence or familial effects? Pediatrics 117, 714–721. [DOI] [PubMed] [Google Scholar]
- Bernstein IM, Horbar JD, Badger GJ, Ohlsson A & Golan A (2000). Morbidity and mortality among very‐low‐birth‐weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am J Obstet Gynecol 182, 198–206. [DOI] [PubMed] [Google Scholar]
- Bisignano M & Rees S (1988). The effects of intrauterine growth retardation on synaptogenesis and mitochondrial formation in the cerebral and cerebellar cortices of fetal sheep. Int J Dev Neurosci 6, 453–460. [DOI] [PubMed] [Google Scholar]
- Blair E (2000). Paediatric implications of IUGR with special reference to cerebral palsy In: Intrauterine Growth Restriction Aetiology and Management, ed. Kingdom J. & Baker P, pp 351–366. Springer, London. [Google Scholar]
- Blair EM & Nelson KB (2014). Fetal growth restriction and risk of cerebral palsy in singletons born after at least 35 weeks' gestation. Am J Obstet Gynecol 212, 520.e1–520.e7. [DOI] [PubMed] [Google Scholar]
- Brown JD & Vannucci RC (1978). Cerebral oxidative metabolism during intrauterine growth retardation. Biol Neonate 34, 170–173. [DOI] [PubMed] [Google Scholar]
- Businelli C, de Wit C, Visser GH & Pistorius LR (2015). Ultrasound evaluation of cortical brain development in fetuses with intrauterine growth restriction. J Matern Fetal Neonatal Med 28, 1302–1307. [DOI] [PubMed] [Google Scholar]
- Castillo‐Melendez M, Yawno T, Allison BJ, Jenkin G, Wallace EM & Miller SL (2015). Cerebrovascular adaptations to chronic hypoxia in the growth restricted lamb. Int J Dev Neurosci 45, 55–65. [DOI] [PubMed] [Google Scholar]
- Cetin I & Alvino G (2009). Intrauterine growth restriction: implications for placental metabolism and transport. A review. Placenta 30(Suppl A), S77–S82. [DOI] [PubMed] [Google Scholar]
- Chase HP, Welch NN, Dabiere CS, Vasan NS & Butterfield LJ (1972). Alterations in human brain biochemistry following intrauterine growth retardation. Pediatrics 50, 403–411. [PubMed] [Google Scholar]
- Chauhan SP & Magann EF (2006). Screening for fetal growth restriction. Clin Obstet Gynecol 49, 284–294. [DOI] [PubMed] [Google Scholar]
- Chiang MC, Barysheva M, Shattuck DW, Lee AD, Madsen SK, Avedissian C, Klunder AD, Toga AW, McMahon KL, de Zubicaray GI, Wright MJ, Srivastava A, Balov N & Thompson PM (2009). Genetics of brain fiber architecture and intellectual performance. J Neurosci 29, 2212–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp JF 3rd, McLaughlin MK, Gellis J, Hemphill C & Catalano PM (1984). Regional distribution of cerebral blood flow in experimental intrauterine growth retardation. Am J Obstet Gynecol 150, 843–846. [DOI] [PubMed] [Google Scholar]
- Costantine MM, Weiner SJ, Rouse DJ, Hirtz DG, Varner MW, Spong CY, Mercer BM, Iams JD, Wapner RJ, Sorokin Y, Thorp JM Jr, Ramin SM, O'Sullivan MJ, Peaceman AM, Simhan HN; Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Maternal‐Fetal Medicine Units Network (2011). Umbilical cord blood biomarkers of neurologic injury and the risk of cerebral palsy or infant death. Int J Dev Neurosci 29, 917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell EC, Holmes MC, Livingstone DE, Kenyon CJ & Seckl JR (2012). Reconciling the nutritional and glucocorticoid hypotheses of fetal programming. FASEB J 26, 1866–1874. [DOI] [PubMed] [Google Scholar]
- Cruz‐Martinez R, Figueras F, Hernandez‐Andrade E, Oros D & Gratacos E (2011). Fetal brain Doppler to predict cesarean delivery for nonreassuring fetal status in term small‐for‐gestational‐age fetuses. Obstet Gynecol 117, 618–626. [DOI] [PubMed] [Google Scholar]
- Cruz‐Martinez R, Figueras F, Oros D, Padilla N, Meler E, Hernandez‐Andrade E & Gratacos E (2009). Cerebral blood perfusion and neurobehavioral performance in full‐term small‐for‐gestational‐age fetuses. Am J Obstet Gynecol 201, 474.e1–474.e7. [DOI] [PubMed] [Google Scholar]
- Damodaram MS, Story L, Eixarch E, Patkee P, Patel A, Kumar S & Rutherford M (2012). Foetal volumetry using magnetic resonance imaging in intrauterine growth restriction. Early Hum Dev 88(Suppl 1), S35–S40. [DOI] [PubMed] [Google Scholar]
- de Onis M, Blossner M & Villar J (1998). Levels and patterns of intrauterine growth retardation in developing countries. Eur J Clin Nutr 52(Suppl 1), S5–S15. [PubMed] [Google Scholar]
- Delcour M, Russier M, Amin M, Baud O, Paban V, Barbe MF & Coq JO (2012). Impact of prenatal ischemia on behavior, cognitive abilities and neuroanatomy in adult rats with white matter damage. Behav Brain Res 232, 233–244. [DOI] [PubMed] [Google Scholar]
- Dieni S & Rees S (2003). Dendritic morphology is altered in hippocampal neurons following prenatal compromise. J Neurobiol 55, 41–52. [DOI] [PubMed] [Google Scholar]
- Dobbing J & Sands J (1979). Comparative aspects of the brain growth spurt. Early Hum Dev 3, 79–83. [DOI] [PubMed] [Google Scholar]
- Dubois J, Benders M, Borradori‐Tolsa C, Cachia A, Lazeyras F, Ha‐Vinh Leuchter R, Sizonenko SV, Warfield SK, Mangin JF & Huppi PS (2008). Primary cortical folding in the human newborn: an early marker of later functional development. Brain 131, 2028–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds CJ, Isaacs EB, Cole TJ, Rogers MH, Lanigan J, Singhal A, Birbara T, Gringras P, Denton J & Lucas A (2010). The effect of intrauterine growth on verbal IQ scores in childhood: a study of monozygotic twins. Pediatrics 126, e1095–e1101. [DOI] [PubMed] [Google Scholar]
- Eixarch E, Batalle D, Illa M, Munoz‐Moreno E, Arbat‐Plana A, Amat‐Roldan I, Figueras F & Gratacos E (2012). Neonatal neurobehavior and diffusion MRI changes in brain reorganization due to intrauterine growth restriction in a rabbit model. PLoS One 7, e31497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eixarch E, Meler E, Iraola A, Illa M, Crispi F, Hernandez‐Andrade E, Gratacos E & Figueras F (2008). Neurodevelopmental outcome in 2‐year‐old infants who were small‐for‐gestational age term fetuses with cerebral blood flow redistribution. Ultrasound Obstet Gynecol 32, 894–899. [DOI] [PubMed] [Google Scholar]
- Emmanouilides GC, Townsend DE & Bauer RA (1968). Effects of single umbilical artery ligation in the lamb fetus. Pediatrics 42, 919–927. [PubMed] [Google Scholar]
- Esteban FJ, Padilla N, Sanz‐Cortes M, de Miras JR, Bargallo N, Villoslada P & Gratacos E (2010). Fractal‐dimension analysis detects cerebral changes in preterm infants with and without intrauterine growth restriction. Neuroimage 53, 1225–1232. [DOI] [PubMed] [Google Scholar]
- Fang S (2005). Management of preterm infants with intrauterine growth restriction. Early Hum Dev 81, 889–900. [DOI] [PubMed] [Google Scholar]
- Figueras F & Gardosi J (2011). Intrauterine growth restriction: new concepts in antenatal surveillance, diagnosis, and management. Am J Obstet Gynecol 204, 288–300. [DOI] [PubMed] [Google Scholar]
- Figueras F & Gratacos E (2014). Update on the diagnosis and classification of fetal growth restriction and proposal of a stage‐based management protocol. Fetal Diagn Ther 36, 86–98. [DOI] [PubMed] [Google Scholar]
- Figueroa‐Diesel H, Hernandez‐Andrade E, Acosta‐Rojas R, Cabero L & Gratacos E (2007). Doppler changes in the main fetal brain arteries at different stages of hemodynamic adaptation in severe intrauterine growth restriction. Ultrasound Obstet Gynecol 30, 297–302. [DOI] [PubMed] [Google Scholar]
- Fischi‐Gomez E, Vasung L, Meskaldji DE, Lazeyras F, Borradori‐Tolsa C, Hagmann P, Barisnikov K, Thiran JP & Huppi PS (2014). Structural brain connectivity in school‐age preterm infants provides evidence for impaired networks relevant for higher order cognitive skills and social cognition. Cerebral cortex 25, 2793–2805. [DOI] [PubMed] [Google Scholar]
- Fung C, Ke X, Brown AS, Yu X, McKnight RA & Lane RH (2012). Uteroplacental insufficiency alters rat hippocampal cellular phenotype in conjunction with ErbB receptor expression. Pediatr Res 72, 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon R (2003). Placental insufficiency and its consequences. Eur J Obstet Gynecol Reprod Biol 110, S99–S107. [DOI] [PubMed] [Google Scholar]
- Gagnon R, Challis J, Johnston L & Fraher L (1994). Fetal endocrine responses to chronic placental embolization in the late‐gestation ovine fetus. Am J Obstet Gynecol 170, 929–938. [DOI] [PubMed] [Google Scholar]
- Gale CR, O'Callaghan FJ, Bredow M & Martyn CN (2006). The influence of head growth in fetal life, infancy, and childhood on intelligence at the ages of 4 and 8 years. Pediatrics 118, 1486–1492. [DOI] [PubMed] [Google Scholar]
- Gazzolo D, Marinoni E, di Iorio R, Lituania M, Bruschettini PL & Michetti F (2002). Circulating S100β protein is increased in intrauterine growth‐retarded fetuses. Pediatr Res 51, 215–219. [DOI] [PubMed] [Google Scholar]
- Gazzolo D, Marinoni E, Di Iorio R, Lituania M, Marras M, Bruschettini M, Bruschettini P, Frulio R, Michetti F, Petraglia F & Florio P (2006). High maternal blood S100B concentrations in pregnancies complicated by intrauterine growth restriction and intraventricular hemorrhage. Clin Chem 52, 819–826. [DOI] [PubMed] [Google Scholar]
- Geva R, Eshel R, Leitner Y, Fattal‐Valevski A & Harel S (2006. a). Memory functions of children born with asymmetric intrauterine growth restriction. Brain Res 1117, 186–194. [DOI] [PubMed] [Google Scholar]
- Geva R, Eshel R, Leitner Y, Valevski AF & Harel S (2006. b). Neuropsychological outcome of children with intrauterine growth restriction: a 9‐year prospective study. Pediatrics 118, 91–100. [DOI] [PubMed] [Google Scholar]
- Gilbert WM & Danielsen B (2003). Pregnancy outcomes associated with intrauterine growth restriction. Am J Obstet Gynecol 188, 1596–1599; discussion 1599–1601. [DOI] [PubMed] [Google Scholar]
- Gotlieb SJ, Biasini FJ & Bray NW (1988). Visual recognition memory in IUGR and normal birth‐weight infants. Infant Behav Dev 11, 223–228. [Google Scholar]
- Guellec I, Lapillonne A, Renolleau S, Charlaluk ML, Roze JC, Marret S, Vieux R, Monique K, Ancel PY & Group ES (2011). Neurologic outcomes at school age in very preterm infants born with severe or mild growth restriction. Pediatrics 127, e883–e891. [DOI] [PubMed] [Google Scholar]
- Hadders‐Algra M & Touwen B (1990). Body measurements, neurological and behavioural development in six‐year‐old children born preterm and/or small‐for‐gestational‐age. Early Hum Dev 22, 1–13. [DOI] [PubMed] [Google Scholar]
- Harel S, Tomer A, Barak Y, Binderman I & Yavin E (1985). The cephalization index: a screening device for brain maturity and vulnerability in normal and intrauterine growth retarded newborns. Brain Dev 7, 580–584. [DOI] [PubMed] [Google Scholar]
- Hernandez‐Andrade E, Serralde JA & Cruz‐Martinez R (2012). Can anomalies of fetal brain circulation be useful in the management of growth restricted fetuses? Prenat Diagn 32, 103–112. [DOI] [PubMed] [Google Scholar]
- Herrera EA, Macchiavello R, Montt C, Ebensperger G, Diaz M, Ramirez S, Parer JT, Seron‐Ferre M, Reyes RV & Llanos AJ (2014). Melatonin improves cerebrovascular function and decreases oxidative stress in chronically hypoxic lambs. J Pineal Res 57, 33–42. [DOI] [PubMed] [Google Scholar]
- Huppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA & Volpe JJ (1998). Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res 44, 584–590. [DOI] [PubMed] [Google Scholar]
- Illa M, Eixarch E, Batalle D, Arbat‐Plana A, Munoz‐Moreno E, Figueras F & Gratacos E (2013). Long‐term functional outcomes and correlation with regional brain connectivity by MRI diffusion tractography metrics in a near‐term rabbit model of intrauterine growth restriction. PLoS One 8, e76453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamitomo M, Alonso JG, Okai T, Longo LD & Gilbert RD (1993). Effects of long‐term, high‐altitude hypoxemia on ovine fetal cardiac output and blood flow distribution. Am J Obstet Gynecol 169, 701–707. [DOI] [PubMed] [Google Scholar]
- Kingdom J & Smith G (2000). Diagnosis and management of IUGR In: Intrauterine Growth Restriction Aetiology and Management, ed. Kingdom J. & Baker P, pp. 257–273. Springer, London. [Google Scholar]
- Kok JH, den Ouden AL, Verloove‐Vanhorick SP & Brand R (1998). Outcome of very preterm small for gestational age infants: the first nine years of life. Br J Obstet Gynaecol 105, 162–168. [DOI] [PubMed] [Google Scholar]
- Lackman F, Capewell V, Richardson B, daSilva O & Gagnon R (2001). The risks of spontaneous preterm delivery and perinatal mortality in relation to size at birth according to fetal versus neonatal growth standards. Am J Obstet Gynecol 184, 946–953. [DOI] [PubMed] [Google Scholar]
- Lafeber HN, Rolph TP & Jones CT (1984). Studies on the growth of the fetal guinea pig. The effects of ligation of the uterine artery on organ growth and development. J Dev Physiol 6, 441–459. [PubMed] [Google Scholar]
- Leitner Y, Fattal‐Valevski A, Geva R, Eshel R, Toledano‐Alhadef H, Rotstein M, Bassan H, Radianu B, Bitchonsky O, Jaffa AJ & Harel S (2007). Neurodevelopmental outcome of children with intrauterine growth retardation: a longitudinal, 10‐year prospective study. J Child Neurol 22, 580–587. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu L & Chen H (2011). Antenatal taurine supplementation for improving brain ultrastructure in fetal rats with intrauterine growth restriction. Neuroscience 181, 265–270. [DOI] [PubMed] [Google Scholar]
- Lodygensky GA, Seghier ML, Warfield SK, Tolsa CB, Sizonenko S, Lazeyras F & Huppi PS (2008). Intrauterine growth restriction affects the preterm infant's hippocampus. Pediatr Res 63, 438–443. [DOI] [PubMed] [Google Scholar]
- Loftin RW, Habli M, Snyder CC, Cormier CM, Lewis DF & Defranco EA (2010). Late preterm birth. Rev Obstet Gynecol 3, 10–19. [PMC free article] [PubMed] [Google Scholar]
- Louey S, Cock ML, Stevenson KM & Harding R (2000). Placental insufficiency and fetal growth restriction lead to postnatal hypotension and altered postnatal growth in sheep. Pediatr Res 48, 808–814. [DOI] [PubMed] [Google Scholar]
- Low JA, Handley‐Derry MH, Burke SO, Peters RD, Pater EA, Killen HL & Derrick EJ (1992). Association of intrauterine fetal growth retardation and learning deficits at age 9 to 11 years. Am J Obstet Gynecol 167, 1499–1505. [DOI] [PubMed] [Google Scholar]
- McIntyre S, Taitz D, Keogh J, Goldsmith S, Badawi N & Blair E (2013). A systematic review of risk factors for cerebral palsy in children born at term in developed countries. Dev Med Child Neurol 55, 499–508. [DOI] [PubMed] [Google Scholar]
- McMillen IC, Adams MB, Ross JT, Coulter CL, Simonetta G, Owens JA, Robinson JS & Edwards LJ (2001). Fetal growth restriction: adaptations and consequences. Reproduction 122, 195–204. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Yahya Z, Sasi A, Jenkin G, Ditchfield M, Polglase GR & Miller SL (2015). Does fetal growth restriction lead to increased brain injury as detected by neonatal cranial ultrasound in premature infants? J Paediatr Child Health 51, 1103–1108. [DOI] [PubMed] [Google Scholar]
- Mallard C, Loeliger M, Copolov D & Rees S (2000). Reduced number of neurons in the hippocampus and the cerebellum in the postnatal guinea‐pig following intrauterine growth‐restriction. Neuroscience 100, 327–333. [DOI] [PubMed] [Google Scholar]
- Mallard EC, Rees S, Stringer M, Cock ML & Harding R (1998). Effects of chronic placental insufficiency on brain development in fetal sheep. Pediatr Res 43, 262–270. [DOI] [PubMed] [Google Scholar]
- Manjarrez‐Gutierrez G, Gonzalez‐Ramirez M, Boyzo‐Montes de Oca A & Hernandez‐Rodriguez J (2010). Dihydropteridine reductase activity in the brainstem of intrauterine growth‐restricted rats. Int J Dev Neurosci 28, 621–624. [DOI] [PubMed] [Google Scholar]
- Marlow N (2000). Paediatric implications–neonatal complications In Intrauterine Growth Restriction Aetiology and Management, ed. Kingdom J. & Baker P, pp. 337–349. Springer, London. [Google Scholar]
- Marsoosi V, Bahadori F, Esfahani F & Ghasemi‐Rad M (2012). The role of Doppler indices in predicting intra ventricular hemorrhage and perinatal mortality in fetal growth restriction. Med Ultrason 14, 125–132. [PubMed] [Google Scholar]
- Miller SL, Supramaniam VG, Jenkin G, Walker DW & Wallace EM (2009). Cardiovascular responses to maternal betamethasone administration in the intrauterine growth‐restricted ovine fetus. Am J Obstet Gynecol 201, 613.e1–613.e8. [DOI] [PubMed] [Google Scholar]
- Miller SL & Wallace EM (2013). Effect of antenatal steroids on haemodynamics in the normally grown and growth restricted fetus. Curr Pediatr Rev 9, 67–74. [Google Scholar]
- Miller SL, Yawno T, Alers NO, Castillo‐Melendez M, Supramaniam VG, Vanzyl N, Sabaretnam T, Loose JM, Drummond GR, Walker DW, Jenkin G & Wallace EM (2014). Antenatal antioxidant treatment with melatonin to decrease newborn neurodevelopmental deficits and brain injury caused by fetal growth restriction. J Pineal Res 56, 283–294. [DOI] [PubMed] [Google Scholar]
- Morrison JL (2008). Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol 35, 730–743. [DOI] [PubMed] [Google Scholar]
- Morsing E, Asard M, Ley D, Stjernqvist K & Marsal K (2011). Cognitive function after intrauterine growth restriction and very preterm birth. Pediatrics 127, e874–e882. [DOI] [PubMed] [Google Scholar]
- Nitsos I & Rees S (1990). The effects of intrauterine growth retardation on the development of neuroglia in fetal guinea pigs. An immunohistochemical and an ultrastructural study. Int J Dev Neurosci 8, 233–244. [DOI] [PubMed] [Google Scholar]
- Olivier P, Baud O, Bouslama M, Evrard P, Gressens P & Verney C (2007). Moderate growth restriction: deleterious and protective effects on white matter damage. Neurobiol Dis 26, 253–263. [DOI] [PubMed] [Google Scholar]
- Olivier P, Baud O, Evrard P, Gressens P & Verney C (2005). Prenatal ischemia and white matter damage in rats. J Neuropathol Exp Neurol 64, 998–1006. [DOI] [PubMed] [Google Scholar]
- Oros D, Figueras F, Cruz‐Martinez R, Padilla N, Meler E, Hernandez‐Andrade E & Gratacos E (2010). Middle versus anterior cerebral artery Doppler for the prediction of perinatal outcome and neonatal neurobehavior in term small‐for‐gestational‐age fetuses with normal umbilical artery Doppler. Ultrasound Obstet Gynecol 35, 456–461. [DOI] [PubMed] [Google Scholar]
- Ortigosa Rocha C, Bittar RE & Zugaib M (2010). Neonatal outcomes of late‐preterm birth associated or not with intrauterine growth restriction. Obstet Gynecol Int 2010, 231842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama K, Padbury J, Chappell B, Martinez A, Stein H & Humme J (1992). Single umbilical artery ligation‐induced fetal growth retardation: effect on postnatal adaptation. Am J Physiol Endocrinol Metab 263, E575–E583. [DOI] [PubMed] [Google Scholar]
- Padilla N, Falcon C, Sanz‐Cortes M, Figueras F, Bargallo N, Crispi F, Eixarch E, Arranz A, Botet F & Gratacos E (2011). Differential effects of intrauterine growth restriction on brain structure and development in preterm infants: a magnetic resonance imaging study. Brain Res 1382, 98–108. [DOI] [PubMed] [Google Scholar]
- Padilla N, Junque C, Figueras F, Sanz‐Cortes M, Bargallo N, Arranz A, Donaire A, Figueras J & Gratacos E (2014). Differential vulnerability of gray matter and white matter to intrauterine growth restriction in preterm infants at 12 months corrected age. Brain Res 1545, 1–11. [DOI] [PubMed] [Google Scholar]
- Padilla N, Perapoch J, Carrascosa A, Acosta‐Rojas R, Botet F & Gratacos E (2010). Twelve‐month neurodevelopmental outcome in preterm infants with and without intrauterine growth restriction. Acta Paediatr 99, 1498–1503. [DOI] [PubMed] [Google Scholar]
- Parkinson CE, Wallis S & Harvey D (1981). School achievement and behaviour of children who were small‐for‐dates at birth. Dev Med Child Neurol 23, 41–50. [DOI] [PubMed] [Google Scholar]
- Pearce W (2006). Hypoxic regulation of the fetal cerebral circulation. J Appl Physiol 100, 731–738. [DOI] [PubMed] [Google Scholar]
- Pham H, Duy AP, Pansiot J, Bollen B, Gallego J, Charriaut‐Marlangue C & Baud O (2015). Impact of inhaled nitric oxide on white matter damage in growth‐restricted neonatal rats. Pediatr Res 77, 563–569. [DOI] [PubMed] [Google Scholar]
- Pollack RN & Divon MY (1992). Intrauterine growth retardation: definition, classification, and etiology. Clin Obstet Gynecol 35, 99–107. [DOI] [PubMed] [Google Scholar]
- Poudel R, McMillen IC, Dunn SL, Zhang S & Morrison JL (2015). Impact of chronic hypoxemia on blood flow to the brain, heart, and adrenal gland in the late‐gestation IUGR sheep fetus. Am J Physiol Regul Integr Comp Physiol 308, R151–R162. [DOI] [PubMed] [Google Scholar]
- Procianoy RS, Garcia‐Prats JA, Adams JM, Silvers A & Rudolph AJ (1980). Hyaline membrane disease and intraventricular haemorrhage in small for gestational age infants. Arch Dis Child 55, 502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor J, Silva PA & Brooke M (1995). Growth, development and behaviour in adolescents born small‐for‐gestational‐age. J Paediatr Child Health 31, 403–407. [DOI] [PubMed] [Google Scholar]
- Ramenghi LA, Martinelli A, De Carli A, Brusati V, Mandia L, Fumagalli M, Triulzi F, Mosca F & Cetin I (2011). Cerebral maturation in IUGR and appropriate for gestational age preterm babies. Reprod Sci 18, 469–475. [DOI] [PubMed] [Google Scholar]
- Raznahan A, Greenstein D, Lee NR, Clasen LS & Giedd JN (2012). Prenatal growth in humans and postnatal brain maturation into late adolescence. Proc Natl Acad Sci USA 109, 11366–11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees S, Bocking AD & Harding R (1988). Structure of the fetal sheep brain in experimental growth retardation. J Dev Physiol 10, 211–225. [PubMed] [Google Scholar]
- Rees S, Mallard C, Breen S, Stringer M, Cock M & Harding R (1998). Fetal brain injury following prolonged hypoxemia and placental insufficiency: a review. Comp Biochem Physiol A Mol Integr Physiol 119, 653–660. [DOI] [PubMed] [Google Scholar]
- Rehn AE, Van Den Buuse M, Copolov D, Briscoe T, Lambert G & Rees S (2004). An animal model of chronic placental insufficiency: relevance to neurodevelopmental disorders including schizophrenia. Neuroscience 129, 381–391. [DOI] [PubMed] [Google Scholar]
- Reid MV, Murray KA, Marsh ED, Golden JA, Simmons RA & Grinspan JB (2012). Delayed myelination in an intrauterine growth retardation model is mediated by oxidative stress upregulating bone morphogenetic protein 4. J Neuropathol Exp Neurol 71, 640–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Represa A, Chanez C, Flexor MA & Ben‐Ari Y (1989). Development of the cholinergic system in control and intra‐uterine growth retarded rat brain. Brain Res Dev Brain Res 47, 71–79. [DOI] [PubMed] [Google Scholar]
- Resnik R (2002). Intrauterine growth restriction. Obstet Gynecol 99, 490–496. [DOI] [PubMed] [Google Scholar]
- Robinson JS, Kingston EJ, Jones CT & Thorburn GD (1979). Studies on experimental growth retardation in sheep. The effect of removal of a endometrial caruncles on fetal size and metabolism. J Dev Physiol 1, 379–398. [PubMed] [Google Scholar]
- Rossi A, Romanello I, Forzano L, Fachechi G & Marchesoni D (2011). Evaluation of fetal cerebral blood flow perfusion using power Doppler ultrasound angiography (3D‐PDA) in growth‐restricted fetuses. Facts Views Vis Obgyn 3, 175–180. [PMC free article] [PubMed] [Google Scholar]
- Roza SJ, Steegers EA, Verburg BO, Jaddoe VW, Moll HA, Hofman A, Verhulst FC & Tiemeier H (2008). What is spared by fetal brain‐sparing? Fetal circulatory redistribution and behavioral problems in the general population. Am J Epidemiol 168, 1145–1152. [DOI] [PubMed] [Google Scholar]
- Samuelsen GB, Pakkenberg B, Bogdanovic N, Gundersen HJ, Larsen JF, Graem N & Laursen H (2007). Severe cell reduction in the future brain cortex in human growth‐restricted fetuses and infants. Am J Obstet Gynecol 197, 56.e1–56.e7. [DOI] [PubMed] [Google Scholar]
- Scherjon S, Briet J, Oosting H & Kok J (2000). The discrepancy between maturation of visual‐evoked potentials and cognitive outcome at five years in very preterm infants with and without hemodynamic signs of fetal brain‐sparing. Pediatrics 105, 385–391. [DOI] [PubMed] [Google Scholar]
- Scherjon SA, Oosting H, Smolders‐DeHaas H, Zondervan HA & Kok JH (1998). Neurodevelopmental outcome at three years of age after fetal ‘brain‐sparing’. Early Hum Dev 52, 67–79. [DOI] [PubMed] [Google Scholar]
- Schreuder AM, McDonnell M, Gaffney G, Johnson A & Hope PL (2002). Outcome at school age following antenatal detection of absent or reversed end diastolic flow velocity in the umbilical artery. Arch Dis Child Fetal Neonatal Ed 86, F108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somm E, Larvaron P, van de Looij Y, Toulotte A, Chatagner A, Faure M, Metairon S, Mansourian R, Raymond F, Gruetter R, Wang B, Sizonenko SV & Huppi PS (2014). Protective effects of maternal nutritional supplementation with lactoferrin on growth and brain metabolism. Pediatr Res 75, 51–61. [DOI] [PubMed] [Google Scholar]
- Somm E, Vauthay DM, Guerardel A, Toulotte A, Cettour‐Rose P, Klee P, Meda P, Aubert ML, Huppi PS & Schwitzgebel VM (2012). Early metabolic defects in dexamethasone‐exposed and undernourished intrauterine growth restricted rats. PLoS One 7, e50131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soo PS, Hiscock J, Botting KJ, Roberts CT, Davey AK & Morrison JL (2012). Maternal undernutrition reduces P‐glycoprotein in guinea pig placenta and developing brain in late gestation. Reprod Toxicol 33, 374–381. [DOI] [PubMed] [Google Scholar]
- Soothill PW, Ajayi RA, Campbell S, Ross EM & Nicolaides KH (1995). Fetal oxygenation at cordocentesis, maternal smoking and childhood neuro‐development. Eur J Obstet Gynecol Reprod Biol 59, 21–24. [DOI] [PubMed] [Google Scholar]
- Soothill PW, Nicolaides KH & Campbell S (1987). Prenatal asphyxia, hyperlacticaemia, hypoglycaemia, and erythroblastosis in growth retarded fetuses. Br Med J (Clin Res Ed) 294, 1051–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinillo A, Montanari L, Roccio M, Zanchi S, Tzialla C & Stronati M (2009). Prognostic significance of the interaction between abnormal umbilical and middle cerebral artery Doppler velocimetry in pregnancies complicated by fetal growth restriction. Acta Obstet Gynecol Scand 88, 159–166. [DOI] [PubMed] [Google Scholar]
- Story L, Damodaram MS, Supramaniam V, Allsop JM, McGuinness A, Patel A, Wylezinska M, Kumar S & Rutherford MA (2013). Myo‐inositol metabolism in appropriately grown and growth‐restricted fetuses: a proton magnetic resonance spectroscopy study. Eur J Obstet Gynecol Reprod Biol 170, 77–81. [DOI] [PubMed] [Google Scholar]
- Streimish IG, Ehrenkranz RA, Allred EN, O'Shea TM, Kuban KC, Paneth N & Leviton A; ELGAN Study Investigators (2012). Birth weight‐ and fetal weight‐growth restriction: impact on neurodevelopment. Early Hum Dev 88, 765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung IK, Vohr B & Oh W (1993). Growth and neurodevelopmental outcome of very low birth weight infants with intrauterine growth retardation: comparison with control subjects matched by birth weight and gestational age. J Pediatr 123, 618–624. [DOI] [PubMed] [Google Scholar]
- Supramaniam VG, Jenkin G, Loose J, Wallace EM & Miller SL (2006). Chronic fetal hypoxia increases activin A concentrations in the late‐pregnant sheep. BJOG 113, 102–109. [DOI] [PubMed] [Google Scholar]
- Swanson AM & David AL (2015). Animal models of fetal growth restriction: Considerations for translational medicine. Placenta 36, 623–630. [DOI] [PubMed] [Google Scholar]
- Tashima L, Nakata M, Anno K, Sugino N & Kato H (2001). Prenatal influence of ischemia‐hypoxia‐induced intrauterine growth retardation on brain development and behavioral activity in rats. Biol Neonate 80, 81–87. [DOI] [PubMed] [Google Scholar]
- Thordstein M & Hedner T (1992). Cerebral and adrenal monoamine metabolism in the growth‐retarded rat fetus under normoxia and hypoxia. Pediatr Res 31, 131–137. [DOI] [PubMed] [Google Scholar]
- Tolcos M, Bateman E, O'Dowd R, Markwick R, Vrijsen K, Rehn A & Rees S (2011). Intrauterine growth restriction affects the maturation of myelin. Exp Neurol 232, 53–65. [DOI] [PubMed] [Google Scholar]
- Tolsa CB, Zimine S, Warfield SK, Freschi M, Sancho Rossignol A, Lazeyras F, Hanquinet S, Pfizenmaier M & Huppi PS (2004). Early alteration of structural and functional brain development in premature infants born with intrauterine growth restriction. Pediatr Res 56, 132–138. [DOI] [PubMed] [Google Scholar]
- Uysal A, Oktem G, Yilmaz O, Uslu S, Aktug H & Yurtseven ME (2008). Quantitative immunohistochemical analysis of nitric oxide synthases and apoptosis regulator proteins in the fetal rat brain following maternal uterine artery ligation. Int J Neurosci 118, 891–901. [DOI] [PubMed] [Google Scholar]
- van de Looij Y, Dean JM, Gunn AJ, Huppi PS & Sizonenko SV (2015). Advanced magnetic resonance spectroscopy and imaging techniques applied to brain development and animal models of perinatal injury. Int J Dev Neurosci 45, 29–38. [DOI] [PubMed] [Google Scholar]
- van Marthens E, Harel S & Zamenshof S (1975). Experimental intrauterine growth retardation. Biol Neonate 26, 221–231. [DOI] [PubMed] [Google Scholar]
- van Vliet E, Eixarch E, Illa M, Arbat‐Plana A, Gonzalez‐Tendero A, Hogberg HT, Zhao L, Hartung T & Gratacos E (2013). Metabolomics reveals metabolic alterations by intrauterine growth restriction in the fetal rabbit brain. PLoS One 8, e64545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wassenaer A (2005). Neurodevelopmental consequences of being born SGA. Pediatr Endocrinol Rev 2, 372–377. [PubMed] [Google Scholar]
- von Beckerath AK, Kollmann M, Rotky‐Fast C, Karpf E, Lang U & Klaritsch P (2013). Perinatal complications and long‐term neurodevelopmental outcome of infants with intrauterine growth restriction. Am J Obstet Gynecol 208, 130.e1–130.e6. [DOI] [PubMed] [Google Scholar]
- Vossbeck S, de Camargo OK, Grab D, Bode H & Pohlandt F (2001). Neonatal and neurodevelopmental outcome in infants born before 30 weeks of gestation with absent or reversed end‐diastolic flow velocities in the umbilical artery. Eur J Pediatr 160, 128–134. [DOI] [PubMed] [Google Scholar]
- Wigglesworth JS (1974). Fetal growth retardation. Animal model: uterine vessel ligation in the pregnant rat. Am J Pathol 77, 347–350. [PMC free article] [PubMed] [Google Scholar]
- Woodall SM, Breier BH, Johnston BM & Gluckman PD (1996). A model of intrauterine growth retardation caused by chronic maternal undernutrition in the rat: effects on the somatotrophic axis and postnatal growth. J Endocrinol 150, 231–242. [DOI] [PubMed] [Google Scholar]
- Yanney M & Marlow N (2004). Paediatric consequences of fetal growth restriction. Semin Fetal Neonatal Med 9, 411–418. [DOI] [PubMed] [Google Scholar]


